Abstract
Retinal pigmented epithelium (RPE) secretes transforming growth factor beta 1 and 2 (TGF-β1 and -β2) cytokines involved in fibrosis, immune privilege, and proliferative vitreoretinopathy (PVR). Since RPE cell polarity may be altered in various disease conditions including PVR and age-related macular degeneration, we determined levels of TGF-β from polarized human RPE (hRPE) and human stem cell derived RPE (hESC-RPE) as compared to nonpolarized cells. TGF-β2 was the predominant isoform in all cell culture conditions. Nonpolarized cells secreted significantly more TGF-β2 supporting the contention that loss of polarity of RPE in PVR leads to rise of intravitreal TGF-β2. Active TGF-β2, secreted mainly from apical side of polarized RPE, represented 6–10% of total TGF-β2. In conclusion, polarity is an important determinant of TGF-β2 secretion in RPE. Low levels of apically secreted active TGF-β2 may play a role in the normal physiology of the subretinal space. Comparable secretion of TGF-β from polarized hESC-RPE and hRPE supports the potential for hESC-RPE in RPE replacement therapies.
Keywords: Retinal pigment epithelium, TGF-β, embryonic stem cell, proliferative vitreoretinopathy, polarity
INTRODUCTION
The retinal pigmented epithelium (RPE) comprises a monolayer of polarized cells that is critical for the health of the retina, in part by providing bidirectional transport of nutrients and waste products between the photoreceptors and choroid, by phagocytosis of photoreceptor outer segments, and by production of neurotrophic growth factors.1 RPE polarization is known to be altered in diseases such as proliferative vitreoretinopathy (PVR) and age-related macular degeneration.2, 3 Our laboratory has long-standing interest in studies on cytokine metabolism of RPE cells particularly at various stages of polarization and differentiation. We reported that highly differentiated polarized cells secrete significantly higher VEGF and PEDF than less differentiated nonpolarized RPE and a significant difference was found in apical and basal cytokine secretion by polarized RPE.4
Transforming growth factor-beta (TGF-β) is elevated in states of pathological fibrosis in the eye, including PVR. Of the three TGF-β isoforms, TGF-β1 and -β2 but not -β3 are known to be secreted by the RPE.5 The RPE secretes both TGF-β isoforms mainly as latent proteins, which then become activated. In addition to its association with fibrogenesis, TGF-β also plays a role in subretinal immune privilege.6 Clinical trials to implant human stem cell derived RPE cells (hESC-RPE) in macular degenerative diseases are under way.7 Since secretion of TGF-β by RPE is a major component of the subretinal immune privilege status, production of this family of cytokines by hESC-RPE is considered a potential advantage for hESC-RPE transplantation. To our knowledge, no direct comparison of TGF-β secretion between hESC-RPE and human RPE (hRPE) has been recorded. The aim of this study was to determine the effect of RPE cell polarization on the secretion of the bioactive cytokines TGF-β1 and -β2 and to compare TGF-β secretion by hRPE and hESC-RPE.
MATERIAL and METHODS
Cell culture media and fetal bovine serum (FBS) were obtained from Lonza BioWhittaker, Walkersville, MD. Cell culture dishes and Transwell filters were purchased from Corning Costar, Lowell, MA. Primary anti-TGF-β2 antibody was from Abcam, Cambridge, MA and secondary antibody was from Millipore, Billerica, MA. All experiments were conducted in compliance with the Declaration of Helsinki and with approval from the Institutional Review Board of the University of Southern California. The USC Stem Cell Research Oversight Committee approved the use of the H9 hESC line. RPE cells isolated from human fetal eyes were utilized at passage 2–3.8 hESC were differentiated into a polarized RPE monolayer.9 Three hRPE culture conditions were used: (1) highly differentiated polarized hRPE grown on fibronectin-coated Transwell filters (transepithelial resistance >350 ohms.cm2); (2) nonpolarized confluent hRPE; (3) nonpolarized 70–80% subconfluent hRPE. All cultures contained >95% viable cells.
To measure protein secretion, supernatants from the culture dishes of nonpolarized cells and from the apical and basal compartments of the Transwells were collected after incubating cells in serum free medium for 48 hours. Supernatants were analyzed by TGF-β 1 and -β 2 enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Total cellular protein was measured using Protein Assay Kit II (Bio-Rad, Hercules, CA). All human RPE assays were performed on cell cultures from at least 3 donors. hESC-RPE samples were assayed in triplicate in 3 independent experiments. All cytokine concentrations were compared in units of cytokine normalized to pg/µg of cell protein. TGF-β present in supernatants was considered as the active form whereas TGF-β detected following acid activation was considered as the total (active+latent) TGF-β. Intracellular TGF-β 2 in polarized hRPE was immunolocalized on a spinning disc confocal microscope (Perkin-Elmer). Generalized estimating equations were run to obtain analysis of variance (ANOVA) P-values and t-test P-values for pairwise comparisons. Differences between two sub groups (polarized apical vs. polarized basal) were analyzed by paired t-test, and those among multiple groups were analyzed by ANOVA followed by pairwise comparisons with Bonferonni adjusted significance levels. Overall significance level for all tests considered <0.05.
RESULTS and DISCUSSION
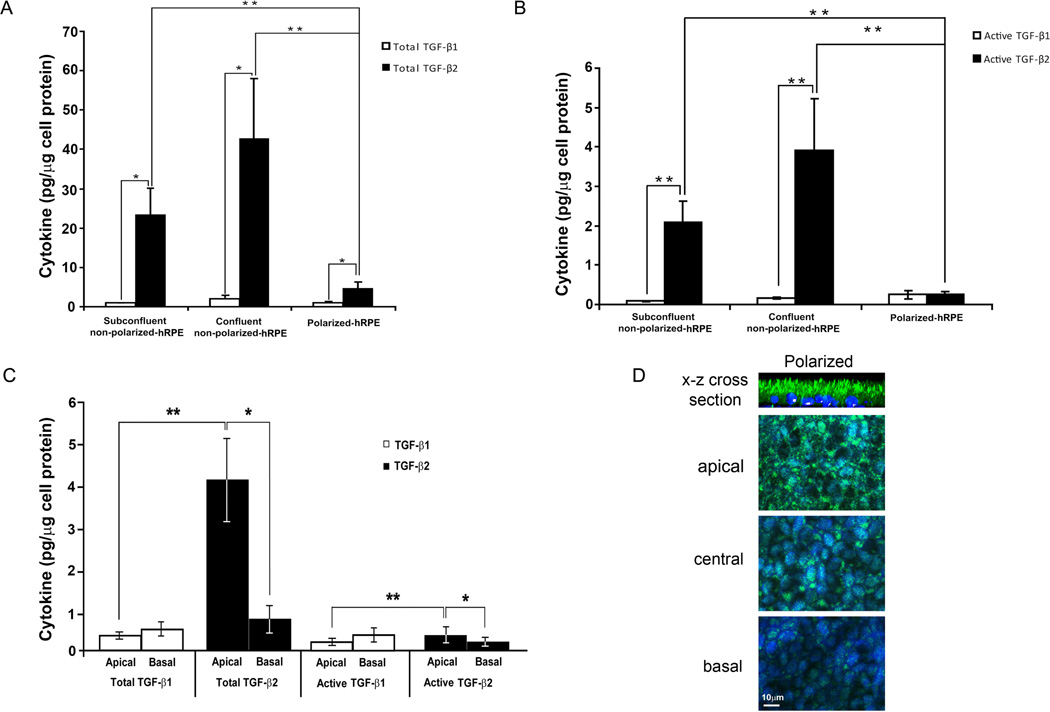
TGF-β1 and -β2 were measured in the medium from polarized, nonpolarized, confluent and subconfluent hRPE. TGF-β2 is the major isoform secreted by the hRPE and hESC-RPE (Fig. 1A and Fig. 2). At every stage of cell maturity, total TGF-β 2 is secreted in significantly higher concentrations than total TGF-β1 (P<0.01). Total TGF-β is the sum of both latent and active forms and in polarized cells, it is the sum of total TGF-β secreted by apical and basal domains. Nonpolarized RPE secreted significantly more TGF-β2 than polarized RPE, for both total and active -β2 (P<0.005, Figs. 1A & 1B). In contrast, TGF-β1 secretion was low and not significantly different between polarized and nonpolarized RPE.
Figure 1.
Total TGF-β1 and -β2 (A) and active TGF-β1 and -β2 (B) secretion by polarized, nonpolarized confluent, and nonpolarized subconfluent hRPE were measured by ELISA in supernatants of cell cultures. Active TGF-β is a protein that became activated in the native cell culture environment. This assay excludes the latent (inactive) TGF-β from the measurement. Total TGF-β is detected after acid activation of the samples (active+latent). C. Apical and basal secretions of TGF-β1 and -β2 were measured in their total and active forms. Apical production of total and active TGF-β2 is greater than their basal production. TGF-β1 is not secreted in significantly different concentrations between apical and basal compartments of polarized hRPE (*P < 0.05, **P < 0.005). D: Confocal images were taken at apical, central and basal levels of the polarized RPE monolayer. TGF-β2 was predominantly localized in the apical domain as confirmed by the x-z cross section stack image shown as the top panel.
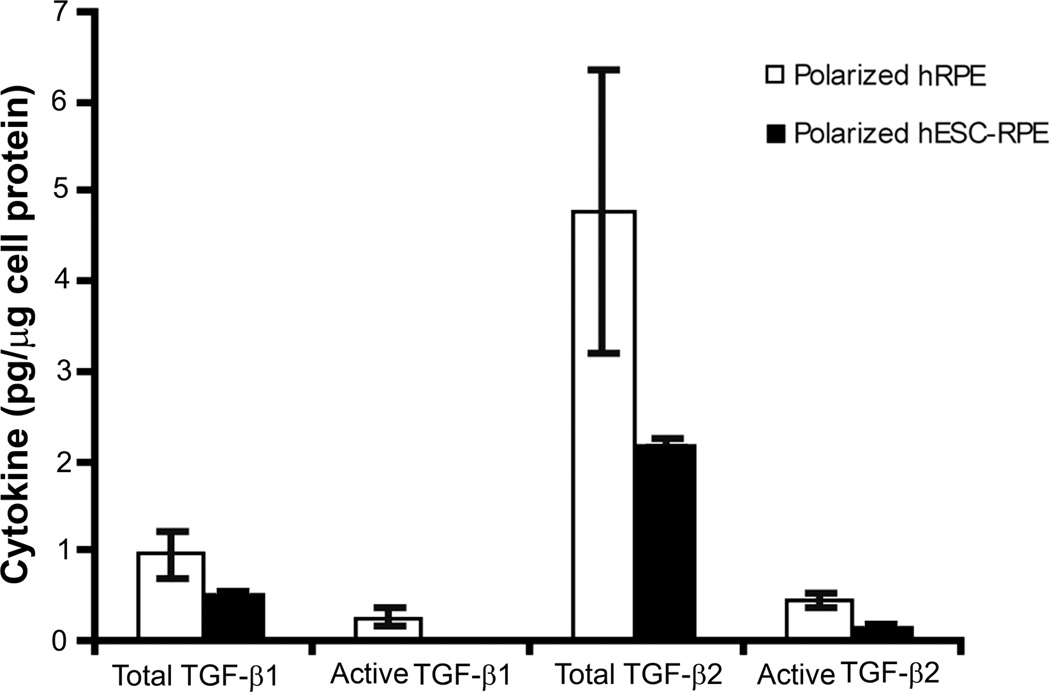
Figure 2.
Total and active TGF-β1 and -β2 secretion by polarized hRPE and hESC-RPE. No significant difference could be found between polarized hRPE and hESC-RPE for both cytokine isoforms.
While a previous study10 measured actual concentration of cytokines in the media, we normalized our data to picograms per microgram of protein in cell lysates. By doing so, we were able to control for cell number, and therefore, accurately compare polarized RPE cells to nonpolarized confluent and subconfluent cultures, where cell numbers varied considerably.
RPE also secreted significantly more active TGF-β2 than -β1 during nonpolarized stages of cell maturity, both confluent and subconfluent (P<0.001, Fig. 1B). Active TGF-β2 represented 6–10% of total -β2, which presumably allows for function of -β2 in vivo in the quiescent nonpathologic retina.
In polarized cells, TGF-β2 was secreted at significantly higher concentrations apically for both total and active -β2 (P < 0.05, Fig. 1C). TGF-β1 level was low and not significantly different between apical and basal compartments of polarized hRPE (Fig. 1C). Consistent with this secretion patterns, intense fluorescence of TGF-β2 was observed at the apical side of the polarized hRPE cells by confocal microscopy (Fig. 1D). Confocal microscopy detected TGF-β1 (data not shown) and -β2 in all three stages of hRPE cell maturity, namely, polarized, nonpolarized confluent, and nonpolarized subconfluent. Compared to TGF-β1, TGF-β2 was observed at significantly higher concentrations in the apical compartments in both hRPE and hESC-RPE (P < 0.005), for both total and active isoforms. However, there was no significant difference between the basolateral secretion of TGF-β1 and -β2.
Production of total TGF-β1 and TGF-β2 by polarized hRPE and polarized hESC-RPE was comparable (Figure 2). Although the level of secretion of TGF-β1 and -β2 was higher in hRPE cultures, the difference did not reach statistical significance (P=0.24 and P=0.07, respectively) (Figure 2). The lack of a statistical difference may be related to the inherent variability among primary RPE cells derived from multiple human donors as opposed to the uniformity in multiple derivations of RPE cells from a single embryonic stem cell line.
TGF-β2 secretion from the apical surface may play a role in the maintenance of immune privilege in the subretinal space by converting T-cells into regulatory T-cells, decreasing T-cell proliferation, and inhibiting interferon-gamma induced expression of major histocompatibility complex class II on human RPE cells.11 Thus, TGF-β2 levels that are not at a level sufficient to stimulate fibrosis may still be able to suppress inflammation. Maintaining the immune suppressive status of the subretinal space is a major consideration in the development of successful cell therapies using stem cell derived RPE.12 In the present study, the secretion of TGF-β2 in hESC-RPE was similar to that in hRPE. This may imply a comparable immunosuppressive function provided by hESC- RPE.
The role of TGF-β2 in PVR is important because elevated levels have been demonstrated in the vitreous of patients with PVR.3 Our results imply that loss of RPE cell polarity contributes to the intraocular TGF-β2 increase observed during PVR. TGF-β2 stimulates RPE-derived fibrosis through EMT2, 13 and stimulates other ocular fibroblasts to proliferate, contract, and produce collagen.14 Anti-TGF-β2 antibodies have been shown to inhibit RPE-mediated contraction of epiretinal membranes in a model of PVR.15
In our study, TGF-β1 was found at very low levels and was not preferentially secreted from the apical surface. Although TGF-β1 is less associated with ocular pathology, it has been shown to stimulate ocular fibrogenesis, to inhibit RPE proliferation, and to promote ocular immune privilege. Further investigation is needed to determine whether low levels of RPE-derived TGF-β1 have a physiologic or pathologic role in the retina.
In conclusion, we showed that attainment of polarity is an important determinant of the level of TGF-β2 secretion from RPE raising the possibility that loss of polarity of RPE in PVR may lead to the rise of intravitreal TGF-β2. Low levels of apically secreted active TGF-β2 may play a role in the normal physiology of the subretinal space. Further, our data show that hRPE and hESC-RPE are similar in their secretion of TGF-β1 and TGF-β2 suggesting that transplantation of hESC-RPE to replace damaged RPE might regenerate secretion of this key cytokine involved in retinal immune privilege.
HIGHLIGHTS.
TGF-β2 is the main TGF-β isoform secreted by human retinal-derived and stem cell-derived retinal pigment epithelial cell cultures.
Non-polarized hRPE cells secrete significantly higher amounts of TGF-β2 compared to polarized hRPE.
TGF-β2 secretion is predominantly from the apical side of polarized RPE.
Comparable secretion of TGF-β 2 from hRPE and hESC-RPE supports the use of hESC-RPE in RPE replacement therapies.
Acknowledgements
The California Institute for Regenerative Medicine DR1- 01444 and TG2-01161; NIH Grant EYO1545 and Core Grant EY03040, Research to Prevent Blindness, New York; The Arnold and Mabel Beckman Foundation; The Keck School of Medicine Dean’s Research Scholars Program. The authors thank Christine Spee for maintenance of RPE cultures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rizzolo LJ. Polarity and the development of the outer blood-retinal barrier. Histology and histopathology. 1997;12:1057–1067. [PubMed] [Google Scholar]
- 2.Parapuram SK, Chang B, Li L, et al. Differential effects of TGFbeta and vitreous on the transformation of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:5965–5974. doi: 10.1167/iovs.09-3621. [DOI] [PubMed] [Google Scholar]
- 3.Connor TB, Jr, Roberts AB, Sporn MB, et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannan R, Sreekumar PG, Hinton DR. VEGF and PEDF secretion in ARPE-19 and fhRPE cells. Investigative ophthalmology & visual science. 2011;52:9047. doi: 10.1167/iovs.11-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvanta A. Expression and secretion of transforming growth factor-beta in transformed and nontransformed retinal pigment epithelial cells. Ophthalmic research. 1994;26:361–367. doi: 10.1159/000267502. [DOI] [PubMed] [Google Scholar]
- 6.Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW. Thrombospondin plays a vital role in the immune privilege of the eye. Investigative ophthalmology & visual science. 2005;46:908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 7.Bharti K, Rao M, Hull SC, et al. Developing cellular therapies for retinal degenerative diseases. Investigative ophthalmology & visual science. 2014;55:1191–1202. doi: 10.1167/iovs.13-13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nature protocols. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu D, Deng X, Spee C, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Investigative ophthalmology & visual science. 2011;52:1573–1585. doi: 10.1167/iovs.10-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Wen R, Banzon T, Maminishkis A, Miller SS. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PloS one. 2011;6:e23148. doi: 10.1371/journal.pone.0023148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugita S, Futagami Y, Smith SB, Naggar H, Mochizuki M. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Exp Eye Res. 2006;83:1459–1471. doi: 10.1016/j.exer.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Investigative ophthalmology & visual science. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saika S, Yamanaka O, Okada Y, et al. TGF beta in fibroproliferative diseases in the eye. Frontiers in bioscience. 2009;1:376–390. doi: 10.2741/S32. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-beta1, -beta2, and -beta3 in vitro: biphasic effects on Tenon's fibroblast contraction, proliferation, and migration. Investigative ophthalmology & visual science. 2000;41:756–763. [PubMed] [Google Scholar]
- 15.Carrington L, McLeod D, Boulton M. IL-10 and antibodies to TGF-beta2 and PDGF inhibit RPE-mediated retinal contraction. Investigative ophthalmology & visual science. 2000;41:1210–1216. [PubMed] [Google Scholar]