Abstract
Fetal lymphoid tissue inducer (LTi) cells are required for lymph node and Peyer’s patch (PP) organogenesis, but where these specialized group 3 innate lymphoid cells (ILC3s) develop remains unclear. Here, we identify extrahepatic arginase-1+, Id2+ fetal ILC precursors that express a transitional developmental phenotype (ftILCPs) and differentiate into ILC1s, ILC2s, and ILC3s in vitro. These cells populate the intestine by embryonic day (E) 13.5, and prior to PP organogenesis (E14.5-E15) are broadly dispersed in the proximal gut, correlating with regions where PPs first develop. At E16.5, after PP development begins, ftILCPs accumulate at PP anlagen in a lymphotoxin-α-dependent manner. Thus, ftILCPs reside in the intestine during PP development, where they aggregate at PP anlagen after stromal cell activation and become a localized source of ILC populations.
Peyer’s patches (PPs) are aggregated lymphoid follicles in the small intestine that sample intestinal luminal antigens and facilitate mucosal immune responses. In the mouse, the formation of the primordial PP, or PP anlage, is induced during fetal development by hematopoietic lineage (Lin)−c-kit+ interleukin 7 receptor-α (IL-7Rα)+ CD4+/−RORγt+ lymphoid tissue inducer (LTi) cells1,2, which activate lymphotoxin-β receptor (LTβR)-signaling in stromal cells at the developing site with the LTβR ligand LTα1β2 (refs. 3–6). Lymphotoxin-activated stromal cells express chemokines that attract additional LTi cells to the developing PP, initiating a positive feedback loop that enhances stromal activation at the anlage5,7. This specific interaction between LTi and stromal cells is required for PP organogenesis, since neither LTα1β2-deficient Lta−/− mice nor LTi cell-deficient Rorc(γt)−/− animals develop these structures1.
LTi cells belong to a family of innate lymphoid cells (ILCs), which are dependent on common gamma chain (γc) cytokines, but lack most lineage markers and do not require the recombination activating genes Rag1 and Rag2 for development8. ILCs participate in a wide range of immune responses, and have been divided into groups based on transcription factor and cytokine expression. Group 1 ILCs (ILC1s) express the transcription factor T-bet and produce the cytokine interferon-γ (IFN-γ); group 2 ILCs (ILC2s) highly express the transcription factor GATA-3 and produce the cytokines IL-5 and IL-13; and group 3 ILCs (ILC3s), which include fetal LTi cells, require the transcription factor RORγt and produce the cytokines IL-22 and IL-17A. In contrast to other innate lymphoid populations, LTi cells are abundant in the fetal intestine and are the only described ILCs in the fetal mouse that function in organ development.
How these innate lymphoid subsets develop is a topic under active investigation. LTi cells and other ILC subsets require the E2A transcriptional inhibitor Id2, indicating a shared developmental pathway for ILC lineages9−11. Indeed, a common precursor to multiple ILC subsets was recently described in fetal liver and adult bone marrow (BM), the major sites of hematopoiesis in fetuses after embryonic day (E) 10.5 and adults, respectively12. These Lin−Id2+α4β7+Flt3−CD25− cells differentiate into NK1.1+IL-7Rα+T-bet+ ILC1s, GATA-3hi ILC2s, and RORγt+ ILC3s, but not T cells, B cells or conventional NK cells. A subset of Id2+ ILC progenitors also expresses the transcription factor PLZF, and appears to have restricted lineage potential12,13.
Although ILC precursors have been described at sites of hematopoiesis, little is known about these cells in peripheral tissues. In the fetal mouse, there is evidence that precursor activity exist outside of the liver, since LTi cells have been derived in vitro from Lin−c-kit+IL-7Rα+α4β7+ RORγtGFP− cells from the intestines of E14 Rorc(γt)GFP ‘knock in’ reporter mice14. Although these data suggest that undifferentiated ILC precursors can migrate to the fetal intestine and continue their development in tissue, the location and lineage potential of these extrahepatic ILC precursors remain unclear.
Arginase-1 (Arg1) is a urea cycle enzyme that is induced in macrophages during type 2 immune responses and wound repair15–18. We recently reported that in the immune system, Arg1 is not only expressed by activated myeloid populations, but is also constitutively expressed by ILC2s in adult mice19. Here, we demonstrate that Arg1 expression additionally marks fetal ILC precursors that are in transitional developmental stages (fetal transitional ILC precursors, or ftILCPs) and their progeny. Arg1+ ftILCPs are capable of differentiating into functional ILC1, ILC2, and ILC3 populations and are present in the fetal intestine during PP organogenesis. These cells are dispersed in proximal portions of the fetal small intestine prior to PP development, and accumulate at the developing PP in an LTα-dependent manner once intestinal lymphoid tissue organogenesis is initiated. These results indicate that fetal ILC precursors leave the liver and continue differentiating in intestinal tissues during active lymphoid tissue organogenesis.
Results
Adult LTi-like cells express the enzyme Arginase-I
We previously reported that ILC2s constitutively express Arg1, and that ILC2s are >95% of all Arg1-expressing hematopoietic cells in the naïve mouse lung19. We next investigated Arg1 expression in the enteric system to determine whether additional cell populations express the enzyme. Using Arg1YFP reporter mice, in which a construct containing IRES-YFP was inserted after exon 8 of the Arg1 gene without disrupting enzyme expression20, we determined that YFP+ cells made up less than 1% of hematopoietic cells isolated from the small intestine (lamina propria and intraepithelial cells combined) (Fig. 1a). These cells were identified as ILCs based on their expression of Thy-1 and IL-7Rα, and lack of common myeloid and lymphoid lineage surface markers CD11b, CD11c, CD3, B220, NK1.1 and NKp46 (Fig. 1b). In wild-type and Rag2−/− mice, Arg1-expressing cells were present in cryptopatches (Fig. 1c,d), tertiary lymphoid structures that contain LTi-like cells and CD11c+ dendritic cells21,22. To test whether YFP+ cells express LTi-like cell surface markers, cryptopatches were dissected from the small intestine and analyzed by flow cytometry. YFP+ cells isolated from cryptopatches were IL-7Rα+c-kit+CD4+/−CD3−, consistent with the profile of LTi-like cells (Fig. 1e). Furthermore, YFP+ cells in cryptopatches expressed the LTi transcription factor RORγt as determined by RORγ(t) antibody staining in Rag2−/− mice (Fig. 1d). RORγt+ cells that did not express Arg1 were also present in cryptopatches, indicating heterogeneity among cryptopatch ILCs. These results indicate that in addition to ILC2s, Arg1 marks a subset of intestinal LTi-like cells in adult cryptopatches.
Figure 1.

Adult LTi-like cells express the enzyme Arginase-I. (a) YFP+ cells in the adult wild-type (WT) and Arg1YFP small intestine. Plots show live CD45+ cells. (b) Surface markers expressed by YFP+ cells in the adult small intestine. (c) Histological sections of WT and Arg1YFP cryptopatches. Sections are counterstained with DAPI. (d) RORγt antibody staining in an Arg1YFPRag2−/− cryptopatch. (e) Surface markers expressed by YFP+ cells isolated from dissected cryptopatches. Data are representative of three independent experiments (a–e).
Innate lymphoid cells express Arg1 in the fetal gut
LTi-like cells in the adult intestinal cryptopatch have been proposed to be analogous to fetal LTi cells at the developing PP, since both of these cell types are RORγt-dependent and cluster at VCAM-1+ intestinal sites that support B cell accumulation1,21−23. We therefore set out to determine whether Arg1 is expressed in the fetal intestine during PP organogenesis. In the mouse embryo, LTi cells first begin forming PP anlagen between E15.5–E16.5 in the proximal small intestine24. At E15.5, hematopoietic YFP+ cells were detected in the intestine that expressed α4β7, IL-7Rα, c-Kit, and low amounts of CD11b, but not CD11c, CD3, CD19, or NKp46 (Fig. 2a,b). To determine whether these were LTi cells, Arg1YFP reporter mice were crossed to Rorc(γt)creRosa26floxSTOP-RFP fate-mapping (fm) mice. Intestinal flow cytometry confirmed that RORγt(fm)+ LTi cells expressed Arg1 (Fig. 2c). Smaller subsets of RORγt(fm)+ cells were Arg1YFP+NK1.1+ and Arg1YFP−CD11b+ (Fig. 2c and data not shown). Our results indicate that RORγt+ LTi cells express Arg1 in both the fetal and adult intestine.
Figure 2.
Innate lymphoid cells express Arg1 in the fetal gut. (a) YFP expression in E15.5 WT and Arg1YFP intestines. (b) Surface markers expressed by YFP+ cells isolated from E15.5 intestines. (c) RORγt(fm), NK1.1, and ST2 expression in distinct Arg1YFP+ populations in E15.5 intestines. Arg1YFP+RORγt(fm)−NK1.1−ST2− (Arg1YFP+RNT−) cells are also present. (d) Expression of T-bet, GATA-3, and RORγ(t) in Arg1YFP+ST2+ and Arg1YFP+NK1.1+ cells. Plots were previously gated on CD45+Arg1YFP+ cells. (e) Arg1YFP+RORγtGFP− populations from E15.5 Arg1YFPRorc(γt)GFP double-reporter intestines. (f) Arg1YFP+RORγtGFP−NK1.1−ST2− cells isolated from E15-E15.5 Rorc(γt)−/− and Rorc(γt)+/− intestines (n = 4 mice per group). P>0.05 (unpaired Student’s t-test). (g) RFP expression in intestinal Arg1YFP+RORγtGFP+ LTi cells from E15.5 and E16.5 Arg1YFP Rorc(γt)GFPRorc(γt)creRosa26floxSTOP-RFP triple-reporter mice. (h) GFP expression in Arg1YFP+RNT− cells (identified using fate mapping) isolated from an E15.5 Arg1YFPRorc(γt)GFPRorc(γt)creRosa26floxSTOP-RFP intestine (left). Right, GFP expression in all Arg1+ cells in an Arg1YFPRorc(γt)GFP littermate as a positive control. (i) Arg1YFP+NK1.1−ST2−CD4− counts in E15.5 Il2rg−/− and Ilr2g+/− intestines (n = 4–6 mice per group). ***P 0.0001 (unpaired Student’s t-test) (j) Left, Id2 (GFP) expression in Arg1YFP+NK1.1−ST2−CD4− cells isolated from E15.5 Arg1YFPId2GFP double-reporter intestines. Right, Arg1YFP+NK1.1−ST2−CD4− cells in E15.5 Id2+/− and Id2−/− intestines. Right plots were previously gated on CD45+NK1.1−ST2−CD4− cells. Data are representative of four (a,c,e) or three (b,d,j) or two (f–i) independent experiments.
In addition to marking RORγt(fm)+ LTi cells, Arg1YFP also marked RORγt(fm)− innate lymphoid cells in the fetal intestine (Fig. 2c). These RORγt(fm)− cells were grouped into three populations based on surface marker and transcription factor expression: NK1.1+T-bet+ cells, ST2+GATA-3hi ILC2s, and a population that lacked NK1.1 and ST2 expression, which we abbreviate here as Arg1YFP+RNT− (Arg1YFP+RORγt(fm)−NK1.1−ST2−) cells (Fig. 2c,d). YFP expression in fetal ILC2s validated our previous finding that Arg1 expression is a constitutive feature of this cell type19. To determine whether the NK1.1+T-bet+ cells were ILC1s, we characterized these cells in neonatal and adult animals. Arg1YFP+NK1.1+ cells were present in the liver and spleen after birth, although the percent of NK1.1+ cells that expressed YFP decreased with age (Supplementary Fig. 1a). These cells lacked expression of RORγt and Eomesodermin, and were present in 20-day-old Rag2−/− mice, indicating that they were neither ILC3s, classical NK cells, nor iNKT cells (Supplementary Fig. 1b–d). In the adult liver and spleen, Arg1YFP+NK1.1+ cells were IL-7Rα+NKp46+NKG2D+ and did not express high amounts of CD11b (Supplementary Fig. 1a,e). These data indicate that fetal Arg1YFP+NK1.1+ cells are early IL-7Rα+NK1.1+ ILC1s12.
To validate the previously undescribed fetal Arg1YFP+RNT− population, YFP+ cells were characterized using Rorc(γt)GFP ‘knock in’ reporter mice1. Arg1YFP+RORγtGFP−NK1.1−ST2− cells were detected in the fetal intestine at E15.5, confirming the existence of this population (Fig. 2e). Additionally, these cells were present in LTi-deficient Rorc(γt)−/− E15-E15.5 intestines, indicating that they were not LTi cells (Fig. 2f). To directly compare the two RORγt reporters, Arg1YFPRorc(γt)GFPRorc(γt)creRosa26floxSTOP-RFP triple reporter mice were generated. In these mice, over 95% of intestinal Arg1YFP+RORγtGFP+ LTi cells were marked by RFP at E15.5 and E16.5 (Fig. 2g), and over 95% of Arg1YFP+RNT− cells identified using fate mapping were GFP− at E15.5 (Fig. 2h), indicating that the two RORγt reporters have similar efficiency in marking Arg1YFP+ LTi cells and in identifying Arg1YFP+RNT− cells in the fetal intestine. Similar to other ILC populations, Arg1YFP+RNT− cells (estimated by staining for Arg1YFP+NK1.1−ST2−CD4− cells) were Il2rg-dependent, expressed the transcription factor Id2, and were absent in Id2−/− animals (Fig. 2i,j). Thus, Arg1YFP+RNT− cells are an ILC population in the fetal mouse intestine with unknown lineage specificity. Taken together, our results indicate that NK1.1+IL-7Rα+ ILC1s, ST2+ ILC2s, RORγt+ ILC3s, and an uncategorized RNT− ILC population express Arg1 in the fetal intestine.
Fetal Arg1YFP+RNT− cells aggregate at the developing PP
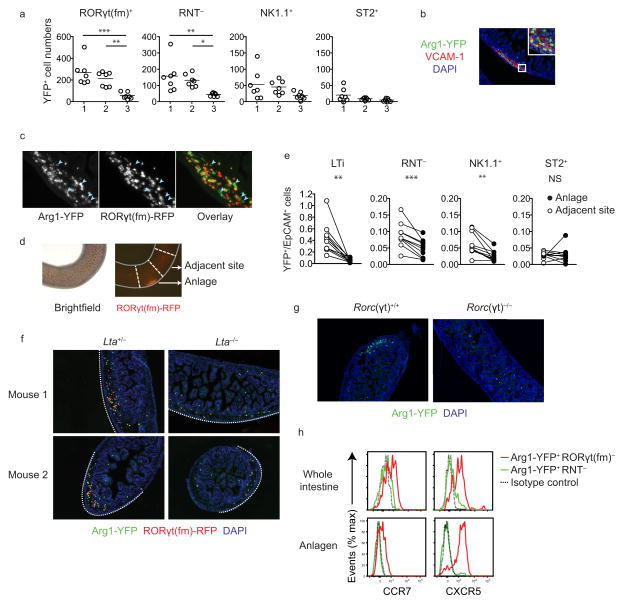
Since Arg1YFP+RNT− cells were present in the fetal intestine during PP development, we next determined whether these cells were interacting with developing lymphoid organs. In the fetus, the most proximal PP begins to develop between E15.5-E16.5, with PPs distal to the first site developing sequentially over the next few days24. At E14.5-E15, before PP development begins, Arg1YFP+ LTi and RNT− cells were most abundant in the upper and middle portions of the small intestine, indicating that these ILCs are locally positioned prior to the development of proximal PP anlagen (Fig. 3a and Supplementary Fig. 2). At E16.5, after PP development starts, Arg1YFP+ cells were present at the first developing VCAM-1+ PP anlage (Fig. 3b), and consisted of both RORγt(fm)+ LTi cells and RORγt(fm)− ILCs (Fig. 3c). To quantify Arg1YFP+RORγt(fm)− populations at the anlage, LTi clusters were identified by RFP expression in whole intestines and dissected for flow cytometric analysis (Fig. 3d). At E16.5, the first developing PP contained significantly more Arg1YFP+RNT− and Arg1YFP+NK1.1+ cells than sites immediately adjacent to the developing organ based on the ratio of YFP cells to CD45−EpCAM+ cells (Fig. 3e). In contrast, Arg1YFP+ST2+ ILC2s did not accumulate at the anlage. These data indicate that multiple ILC populations, including Arg1YFP+RNT− cells, accumulate with LTi cells at the developing PP.
Figure 3.
Fetal Arg1YFP+RNT− cells aggregate at the developing PP anlage. (a) YFP+ cell counts from upper (1), middle (2), and lower (3) sections of the E14.5-E15 small intestine (n = 7 mice per group) *P < 0.05, ** P ≤ 0.01, *** P ≤ 0.001 (one-way ANOVA followed by Tukey’s test). (b) YFP+ cells at the PP anlage in the E16.5 intestine. VCAM-1+ marks activated stromal cells, and sections were counterstained with DAPI. (c) Arg1 (YFP) and RORγt(fm) (RFP) expression at the anlage of E16.5 Arg1YFPRorc(γt)creRosa26floxSTOP-RFP double reporter mice. Light blue arrowheads point at examples of YFP+RFP− cells. (c) Identification of the anlage in intact E16.5 Arg1YFPRorc(γt)creRosa26floxSTOP-RFP intestines. (e) Ratio of YFP+ populations to EpCAM+ cells in dissected anlagen and adjacent sites (n = 10 mice per group) ** P ≤ 0.01, *** P ≤ 0.001, NS P>0.05 (paired Student’s t-test). (f) Arg1 (YFP) and RORγt(fm) (RFP) expression in sections of E16.5 intestines from Lta+/− (left) and Lta−/− littermates (right). Lta+/− images are of PP anlagen, while Lta−/− images are representative of sections throughout the proximal half of the intestine (n = 3-4 mice per group). Dotted white lines indicate the anti-mesenteric side of each intestine. (g) Arg1 (YFP) expression in sections of E16.5 intestines from Rorc(γt)+/+ (left) and Rorc(γt)−/− (right) littermates (n = 3-4 mice per group). (h) Expression of CCR7 and CXCR5 in Arg1YFP+RNT− cells and Arg1YFP+RORγt(fm)+ LTi cells from whole intestines (left) or dissected anlagen (right). Data are representative of three (b–d,f) or two (g–h) independent experiments, or are pooled from two independent experiments (a,e)
The PP anlage is formed when stromal cells at the anti-mesenteric side of the intestine are activated at discrete sites by LTα1β2+ hematopoietic cells5. To test whether fetal Arg1YFP+RNT− accumulation at the anlage was dependent on stromal activation, intestines from E16.5 Lta−/− animals, which are unable to form the LTα1β2 heterotrimer, were assessed for YFP+ aggregates in consecutive sections throughout the proximal half of the small intestine. RFP+ LTi cells and YFP+RFP− cells were enriched along the anti-mesenteric side of the intestine in both Lta−/− and Lta+/− littermates (Fig. 3f). However, aggregated clustering of YFP+ cells was dependent on Lta, indicating that factors induced by LTα1β2 in stromal cells are required for Arg1YFP+RNT− cell accumulation at the PP anlage. LTi cells express LTα1β2, and to test whether this population was required for Arg1YFP+RNT− aggregates, sections of LTi-deficient Rorc(γt)−/− intestines were assessed for YFP+ clusters. E16.5 Rorc(γt)−/− intestines lacked YFP+ aggregates in consecutive sections throughout the small intestine (Fig. 3g), indicating that Arg1YFP+RNT− cells cluster after LTi cells activate stromal cells at the anlage. Finally, since LTα1β2 activates expression of the chemokines CXCL13 and CCL19 in intestinal stromal cells in situ, and LTi cells migrate towards these chemokines in vitro, we tested whether these factors could also recruit Arg1+RNT− cells5,7. Compared to LTi cells, which express the chemokine receptors CXCR5 and CCR7, Arg1YFP+RNT− cells did not express these receptors even after restricting our analysis to cells isolated from PP anlagen (Fig. 3h). We conclude that Arg1YFP+RNT− cells accumulate at the PP anlage in a CCR7- and CXCR5-independent manner after LTβR signaling is activated in stromal cells.
Although multiple ILC populations expressed Arg1 at the developing PP, Arg1 expression by hematopoietic cells was not required for normal numbers of PP or PP follicles as assessed in VavcreArg1fl/fl adult mice (Supplementary Fig. 3). Mice deficient in hematopoietic Arg1 also had normal B and T cell compartmentalization in the PP, inguinal lymph node, and spleen (data not shown). Thus, hematopoietic Arg1 expression is not required for secondary lymphoid tissue development.
Arg1YFP+RNT− cells differentiate into mature ILCs
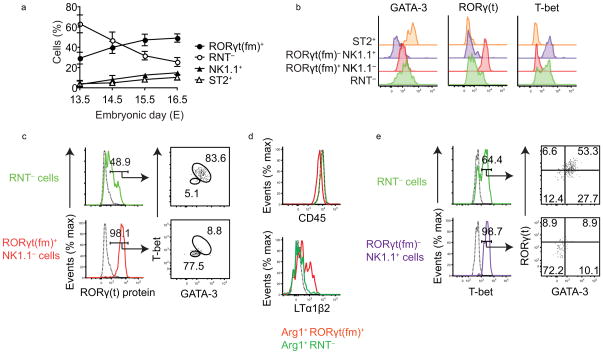
Before PP development, at E13.5, Arg1YFP+RNT− cells were the most abundant YFP+ ILC lineage in the fetal intestine (Fig. 4a). As the frequency of YFP+ cells that were RORγt(fm)+ LTi cells increased over the next 3 days, the percent of RNT− cells decreased, suggesting that Arg1YFP+RNT− cells in the fetal intestine are ILC precursors that undergo differentiation into ILC lineages. To test this possibility, we assessed transcription factor protein expression in Arg1YFP+RNT− cells by flow cytometry. In comparison to adult BM ILC precursors, which express GATA-3 but lack T-bet and RORγt12, Arg1YFP+RNT− cells in the E15.5 intestine expressed all three transcription factors at varying amounts (Fig. 4b). Since the anti-RORγ(t) antibodies used in these experiments (B2D clone) can also bind to the RORγ isoform, we confirmed that in Arg1YFP+ILCs these antibodies were specifically interacting with RORγt using cells from fetal Rorc(γt)−/− intestines (Supplementary Fig. 4).
Figure 4.
Characterization of Arg1YFP+RNT− cells. (a) The frequency of each ILC population as a percent of total YFP+ cells (n = 5–7). Shown are the mean+/-s.d. P<0.001 for all comparisons with RNT− cells at E13.5 (one-way ANOVA followed by Tukey’s test). (b) Transcription factor expression in Arg1YFP+RNT− cells compared to other Arg1YFP+ ILC populations. (c) GATA-3 and T-bet expression in RORγt protein-expressing RNT− cells compared to RORγt(fm)+ cells. (d) CD45 and LTα1β2 expression in Arg1YFP+RNT− cells and Arg1YFP+RORγt(fm)+ LTi cells. The dotted black line represents ILC2s in the CD45 plot, and an Ig control in the LTα1β2 plot. (e) RORγt and GATA-3 protein expression in T-bet-expressing RNT− cells compared to RORγt(fm)−NK1.1+ cells. Data are representative of three independent (b–e) or two independent experiments (a).
Even though the transcription factors expressed by Arg1YFP+RNT− cells indicated that this population was heterogeneous, these cells lacked the distinct protein expression profiles associated with mature ILC lineages. Arg1YFP+RNT− cells that expressed RORγt protein were distinct from RORγt(fm)+ LTi cells based on increased protein abundance of GATA-3 and T-bet in the Arg1YFP+RNT− population (Fig. 4c). Additionally, fetal Arg1YFP+RNT− cells expressed more CD45 than RORγt(fm)+ LTi cells and did not express the surface trimer LTα1β2 (Fig. 4d). Similarly, Arg1YFP+RNT− cells that expressed T-bet protein were distinct from Arg1YFP+NK1.1+ ILC1s based on increased protein expression of GATA-3 and RORγ(t) in the Arg1YFP+RNT− population (Fig. 4e). Therefore, Arg1YFP+RNT− cells in the fetal intestine express multiple transcription factors associated with known ILC lineages, but do not consist of mature ILC1s or ILC3s based on their surface markers and transcription factor expression profiles. Because we found that a small percentage of Arg1YFP+RNT− cells expressed CD25 (Supplementary Fig. 5a), and may represent an ILC2 precursor-like cell, we tested whether this could account for variation in transcription factor expression. Although Arg1+RNT−CD25+ cells were T-bet− and RORγ(t)−, this did not account for the heterogeneity in total Arg1YFP+RNT− cells (Supplementary Fig. 5b,c). Collectively, these data indicate that intestinal Arg1YFP+RNT−CD25− cells are a heterogeneous population of cells that does not consist of known mature ILC subsets.
It recently was shown that E14 PLZFhi ILC progenitors in the fetal liver express RNA for RORγt and T-bet. To determine whether intestinal Arg1YFP+RNT− cells are similar to liver ILC progenitors in terms of transcription factor expression, we assessed RORγt and T-bet expression in E14.5 Lin−IL-7Rα+α4β7+ Flt3−CD25−ST2− fetal liver cells, a population that consists of progenitors and early committed ILCs. The GATA-3+ fraction of these cells, which contains ILC progenitors, was RORγt− and T-bet−, indicating that ILC progenitors from the fetal liver are more homogenous than Arg1YFP+RNT− cells from the fetal intestine (Supplementary Fig. 6).
To test whether Arg1YFP+RNT− cells could differentiate into mature ILCs, cells from E15.5 intestines were isolated and cultured in vitro with recombinant mouse IL-7 (Fig. 5a). By 20 h, Arg1YFP+RNT− cells gave rise to RORγt(fm)+, RORγt(fm)−NK1.1+ and ST2+ cells (Fig. 5b). RORγt(fm)+ cells that developed in culture did not express CD3 or NKp46 at day 6 (Fig. 5c), consistent with these cells being NK receptor-negative ILC3s. Since a subset of Arg1YFP+RNT− cells express CD25 (Supplementary Fig. 5a), we excluded these cells by sorting and culturing Arg1YFP+RNT−CD25− cells from E15.5 intestines in subsequent experiments. An analysis of transcription factors after 6 days of culture with OP9 cells indicated that Arg1YFP+RNT−CD25− cells gave rise to NK1.1+RORγt(fm)−T-bet+GATA-3− ILC1s, CD25+ICOShiRORγt(fm)−T-bet−GATA-3+ ILC2s, RORγt(fm)+T-bet−GATA-3− ILC3s, and a small population of RORγt(fm)+T-bet+GATA-3− ex-RORγt cells (Fig. 5d,e and data not shown). Day 6 cultures did not contain CD5, CD19, or CD11b+ populations (Fig. 5f). Although YFP and ST2 were expressed by cultured cells after 20 h, these proteins were not detected at day 6, indicating that additional factors are required to maintain expression of Arg1 and IL-33R in fetal cells (data not shown). However, ILCs that developed in culture were functional, since these cells expressed signature cytokines associated with mature ILC subsets after 3 h of stimulation with PMA and Ionomycin (Supplementary Fig. 7a). Additionally, ILC3s from day 10 cultures expressed high amounts of LTα1β2, indicating that this population derived from Arg1YFP+RNT− cells has the potential to function as LTi cells by activating stromal cells (Supplementary Fig. 7b).
Figure 5.
Arg1YFP+RNT− cells differentiate into mature ILCs. (a) Purity of sorted Arg1YFP+RNT− cells (right) compared to unsorted cells (left). (b) Populations detected after culturing Arg1YFP+RNT− cells for 20 h. Left, cultured YFP+ cells from Arg1YFP single-reporter animals; right, YFP+RNT− cells from Arg1YFPRorc(γt)creRosa26floxSTOP-RFP double-reporter mice. (c) Expression of CD3 and NKp46 by RORγt(fm)+ cells after 6 days of culture. (d) Transcription factors expressed by RORγt(fm)+, NK1.1+ and RORγt(fm)−NK1.1−CD25+ cells after 6 days of culturing Arg1YFP+RNT−CD25− cells with OP9 cells. (e) ICOS expression in NK1.1−RORγt(fm)−CD25+ cells (red) compared to NK1.1+ (green) and RORγt(fm)+ (blue) populations at day 6 of culture. (f) CD5, CD19, and CD11b expression at day 6 of culture. (g) Examples of gates used to identify populations in single cell cultures at day 6. Left plots are combined files of single wells from a 96-well plate. Right plots are examples of individual wells from single cell cultures. The top left, bottom left, and right plots show different ILC populations from 3 separate wells. (h) Cell populations isolated from wells from single cell cultures at day 6 (left) and breakdown of wells that contained 2 populations (right). Undetermined (UD) cells did not express markers used to identify other lineages. Data are representative of four (a–b), three (g), or two (c–f) independent experiments, or are pooled from three independent experiments of 96 wells each (h).
To determine the frequency of E15.5 intestinal Arg1YFP+RNT−CD25− cells that are capable of differentiating into Arg1YFP+ ILC lineages, we cultured single cells with the OP9 cell line. At 6 days, the outgrowth frequency of live, CD45+ cells in 96-well plates was 50.4 ± 5.4% (mean ± standard deviation) as assessed by 5 independent experiments. The majority of CD45+ wells contained homogeneous populations of RORγt(fm)−NK1.1+ (35.7%), RORγt(fm)−NK1.1−CD25+ICOS+ (16.6%), or RORγt(fm)+NK1.1− cells (23.6%), indicating that Arg1YFP+RNT−CD25− cells can become ILC1s, ILC2s, and ILC3s (Fig. 5g,h and Supplementary Fig. 8). Ex-RORγt cells that were NK1.1+RORγt(fm)+ and expressed T-bet were present in <2% of wells (Fig. 5h and data not shown)25,26. Five percent of wells contained an undetermined CD45+ population that did not reside in the gates used to score these experiments, but may represent incompletely differentiated ILCs. Wells that contained 2 populations (18.5%) consisted primarily of the undetermined population with RORγt(fm)+NK1.1− cells (23.3%) or RORγt(fm)−NK1.1+ ILC1s (60%). These data indicate that Arg1YFP+RNT− cells in the intestine are extrahepatic cells that give rise to Arg1+ ILC lineages found in vivo.
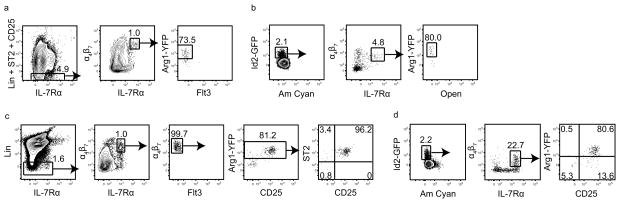
To determine whether Arg1 also marks ILC precursors in the adult mouse, adult BM was assessed for YFP expression. Adult Lin−Id2+IL-7Rα+α4β7+ Flt3−CD25− ILC progenitors in BM are distinguished from CLP by Flt3 and from ILC2 precursors by CD25 (ref. 12). While 80% of Lin−Id2GFP+IL-7Rα+α4β7+ CD25−ST2−Flt3− cells from E14.5-E15 fetal livers were Arg1+ (Fig. 6a,b), Lin−IL-7Rα+α4β7+ Flt3− Arg1YFP+ cells in adult BM consisted of only CD25+ and/or ST2+ ILC2 cells (Fig. 6c). Arg1YFP and Arg1YFPId2GFP mice confirmed that Lin−IL-7Rα+α4β7+ cells in adult BM were not CLPs since they did not express Flt3 (Fig. 6c), and that the majority of Lin−Id2GFP+IL-7Rα+α4β7+ CD25− adult BM ILC precursors did not express Arg1 (Fig. 6d). Thus, Arg1 is not a marker for adult BM ILC precursors, although this enzyme is expressed by CD25+ BM ILC2 precursors. However, in the adult small intestine, a population of cells that was Arg1+, but did not fit known ILC subsets due to lack of NK1.1, KLRG1, CD25 and RORγt(fm), was present in lamina propria and Peyer’s patches (Supplementary Fig. 9). These data suggest that ILC precursors may have the potential to express Arg1 in adult animals, although whether these adult intestinal cells have precursor potential requires further study.
Figure 6.
Arg1YFP expression by Lin−Id2+IL-7Rα+α4β7+ Flt3− cells in fetal liver and adult bone marrow. (a) YFP expression in E14.5-E15 CD45+Lin−ST2−CD25−IL-7Rα+α4β7+ Flt3− fetal liver cells from Arg1YFP reporter mice. Plots were previously gated on CD45+ cells. (b) Arg1 expression in CD45+Lin−ST2−CD25−Id2GFP+IL-7Rα+α4β7+ fetal liver cells from Arg1YFPId2GFP double-reporter mice. Plots were previously gated on CD45+Lin−ST2−CD25− cells. (c) Expression of CD25 and ST2 in Lin−IL-7Rα+α4β7+ Flt3−YFP+ cells from Arg1YFP adult bone marrow. (d) YFP expression in Lin−Id2GFP+IL-7Rα+α4β7+ cells from Arg1YFPId2GFP adult bone marrow. Plots were previously gated on Lin− cells. Data are representative of three (a,c) or two (b,d) independent experiments.
Discussion
ILCs have critical functions in organogenesis, homeostasis and immunity, but their development in the fetal mouse is not fully characterized. Here, we show that Id2-dependent Arg1+ ftILCPs are present in the fetal intestine, and have the capacity to develop into ILC1s, ILC2s and ILC3s. These intestinal Arg1YFP+RNT− cells are heterogeneous and express varying amounts of GATA-3, T-bet and RORγt, but do not consist of mature ILC populations based on their surface markers and transcription factors expression profiles. In contrast, Id2+ multipotent ILC progenitors described in adult BM do not express RORγt or T-bet12, and fetal liver ILC progenitors are more homogenous than intestinal Arg1YFP+RNT− cells. These data suggest that Arg1+ ftILCPs in the intestine may occupy an intermediate developmental step between undifferentiated GATA-3+T-bet−RORγt− ILC precursors and mature ILC populations. Indeed, intestinal Arg1YFP+RNT− cells appear to have more restricted potential at the single cell level than BM and fetal liver progenitors12,13. We found that most intestinal precursors give rise to single lineages, while BM and fetal liver progenitors have been reported to produce multiple lineages at the single cell level. These results indicate that Arg1YFP+RNT− intestinal cells in the fetus are further along in development than their hepatic counterparts, and support a model in which ILC precursors leave the fetal liver and enter other organs, where they exist in a transitional developmental stage before completing their differentiation into mature ILC lineages in tissues. Whether differential abundance of transcription factors expressed by Arg1YFP+RNT− are associated with differentiation into specific lineages is unknown, since these intracellular proteins were only identified in fixed cells. Further studies are required to determine whether intestinal Arg1YFP+RNT− cells consist of committed subsets, or whether at this stage they are undergoing terminal lineage differentiation in response to local tissue factors.
During the analysis of Arg1+ lineages in the fetal intestine, we found that RORγt reporter and protein expression did not completely overlap. This incomplete reporting is not due to inefficiency of Cre-induced flox excision in Rorc(γt)creRosa26floxSTOP-RFP animals, since Rorc(γt)GFP ‘knock in-knock out’ reporter mice show similar patterns of expression. Instead, the lack of fluorescent protein expression in Arg1+RNT− cells in these reporter mice may be due to low Rorc(γt) gene expression, or temporal delays as RNT− cells begin expressing this gene. This restricted reporting proved to be useful in distinguishing two distinct populations of RORγt protein-expressing cells in the fetal intestine. RORγt(fm)+ are LTi cells based on transcriptional and functional definitions. These T-bet−RORγthiGATA-3lo cells express CXCR5 and CCR7, receptors used for chemotaxis to the anlage, and LTα1β2, which is required for stromal activation at the developing PP. In comparison, RORγt protein-expressing RORγt(fm)− cells express more T-bet and GATA-3 than LTi cells, and lack LTi-associated chemokine receptors and LTα1β2. These data reveal that incomplete RORγt detection by available reporter mice is useful in differentiating between LTi and transitional ILC precursors in the fetal intestine.
Here, we found that intestinal ILC precursors express Arg1, while the majority of BM ILC progenitors do not express this enzyme. Whether Arg1 expression is activated in precursors that have begun to undergo differentiation, or whether this enzyme marks different precursor populations based on their fetal or adult origin is unclear. Determining how Arg1 expression is regulated in ILC populations should provide insight into these differences. While Arg1 expression in myeloid cells is induced by STAT6 or MyD88 activation, Arg1 expression in ILC2s occurs under homeostatic conditions16–19,27. Recently, Arg1 was identified as one of many GATA-3-dependent genes in ILC2s and ILC3s in vitro28. However, type 2 CD4+ T cells in the helminth-infected lung and ILC precursors in adult BM are GATA-3+ but do not express Arg1, indicating that this transcription factor is not sufficient for expression of this enzyme in lymphoid cells19. Further studies will be required to identify additional factors that regulate Arg1 in different ILC populations.
In contrast to ILC precursors in the fetal liver, Arg1+ ILC precursors in the fetal intestine are a uniquely localized source of LTi cells at sites that require their rapid accumulation. Here, we show that ILC precursors preferentially accumulate at the developing PP at E16.5 as compared to adjacent areas. Recently, CD45+IL-7Rα+SCA-1+CD4− cells have been found at the lymph node anlage as early as E13.5 (ref. 29). Whether this population contains precursors that give rise to multiple ILC lineages during lymph node development remains unknown. In the intestine, we determined that Arg1YFP+RNT− accumulation at the PP was dependent on Lta and Rorc(γt), indicating that this event occurs after LTi cells initiate organogenesis. LTα1β2 induces expression of adhesion molecules and chemokines at the PP anlage, and identifying which factors are required for accumulation will be critical to test the effects of cell localization. Intestinal Arg1+ ILC precursors are unable to initiate the development of lymphoid organs because they lack LTα1β2 expression, but may enhance the positive feedback loop once at the PP anlage by providing additional LTi cells on site. We suggest that this system reinforces stromal cell activation at each PP while maintaining normal numbers of lymphoid organs. By this model, stochastically differentiated LTi cells that induce PP development at E15.5–E16.5 initiate a LTβR-signaling program in stromal cells necessary for the aggregation of ftILCPs at the PP, thus providing a localized source of additional LTi cells as well as other ILC populations resident in the fetal gut. The fate of ILC precursors and the functions of their progeny during early life are intriguing areas for further investigation.
Online Methods
Mice
Arg1YFP (YARG) mice have been previously described20. Rorc(γt)GFP, Id2GFP, Il2rg−/−, Arg1flox, and wild-type C57BL/6 mice were purchased from the Jackson Laboratory. Rag2−/− mice were purchased from Taconic. D. Sheppard (University of California, San Francisco) and D. Kioussis (MRC National Institute for Medical Research, London, UK) kindly provided Vavcre mice30. Rorc(γt)cre Tg mice were provided by D. Littman (New York University)22. Rosa26floxSTOP-RFP mice were provided by E. Robey (University of California, Berkeley), H. Luche, and H. Fehling (University Clinics Ulm, Germany)31. In Arg1YFP x Rorc(γt)cre x Rosa26floxSTOP-RFP crosses, Arg1YFP and Rosa26floxSTOP-RFP were kept homozygous in both male and female breeders. We found that using male breeders that carried both Rorc(γt)cre and Rosa26floxSTOP-RFP alleles led to sporadic germline transmission of RFP to progeny. Thus, in all experiments Rorc(γt)cre was carried only by female breeders to prevent germline RFP expression. Fetal mice were genotyped by tail PCR and flow cytometry of spleens. In experiments with Vavcre mice, Cre was also only carried by female breeders. All mice were backcrossed to the C57BL/6 background for at least 7 generations. All experiments were conducted according to protocols approved by the UCSF Institutional Animal Care and Use Committee.
Tissue dissociation
Small intestines from adult mice were flushed with PBS and PPs were removed. Intestines were filleted, rinsed, chopped into 5 mm pieces and digested with collagenase VIII (Sigma) and DNase I (Roche Diagnostics) at 37 °C for four rounds of 35-min incubations. Final digested samples were then centrifuged on a 40–90% Percoll gradient (GE Healthcare Biosciences).
Detection of c-kit and CD4 was reduced by the intestinal cell isolation protocol, and were thus assayed on cells obtained by directly dissecting cryptopatches. Briefly, intestines were filleted and laid flat on glass slides. Cryptopatches were identified under a microscope and biopsied out of the tissue with the use of a flexible plastic needle. Biopsies were gently crushed through a 70-micron filter to obtain a single cell suspension.
To obtain cells from the fetal mouse gut, intestines were first isolated from embryos under a dissecting microscope, and then further dissected under magnification to remove the associated mesenteric tissue. In experiments where the intestine was segmented into proximal, middle, and distal regions, the small intestine was divided into three equal portions using a ruler as a guide. Intestines were digested with dispase (Gibco) and DNase I (Roche Diagnostics) at 37 °C for 25 min and mechanically dissociated using a gentleMACS Dissociator (Miltenyi Biotec). Tissue homogenates were then passed through a 70-micron filter.
PP anlagen were dissected from embryonic intestines under a fluorescent dissecting microscope. RORγt(fm)+ cell aggregates were used as markers for developing PPs. Anlagen were digested, mechanically dissociated, and filtered as described above.
Flow cytometry
Rat anti-mouse CD4 (RM4-5), rat anti-mouse CD11b (M1/70), Armenian hamster anti-mouse CD11c (HL3), rat anti-mouse CD19 (ID3), rat anti-mouse CD25 (7D4), rat anti-mouse B220 (RB6-8C5), rat anti-mouse CXCR5 (2G8), rat anti-mouse/human IL-5 (TRFK5), and rat anti-mouse IFN-γ (XMG1.2) antibodies were purchased from BD Pharmingen; rat anti-mouse c-kit (2B8), rat anti-mouse CD3 (17A2), rat anti-mouse CD5 (53-7.3), rat anti-mouse CD25 (eBio7D4), rat anti-mouse CD127 (A7R34), rat anti-mouse NKp46 (29A1.4), rat anti-mouse LPAM (DATK32), rat anti-mouse CCR7 (4B12), rat anti-human/mouse GATA-3 (TWAJ), rat anti-mouse RORγ(t), mouse anti-mouse NK1.1 (PK136), rat anti-mouse IL-17A (eBio17B7), rat anti-mouse IL-22 (IL22JOP), and rat anti-mouse EOMES (Dan11mag) antibodies were purchased from eBioscience; rat anti-mouse CD45 (30-F11), rat anti-mouse Ter119 (TER-119), mouse anti-human/mouse T-bet (2B10), Armenian hamster anti-human/mouse/rat ICOS (C398.4A), rat anti-mouse CD25 (PC61), and rat anti-mouse Flt3 (A2F10) antibodies were purchased from Biolegend; and rat anti-mouse ST2 (DJ8) antibodies were purchased from MD Bioproducts. LTβR-Ig fusion protein and Ig control was purchased from R&D Systems. PE-cy7-conjugated streptavidin was purchased from BD Biosciences and APC-conjugated streptavidin was purchased from eBioscience. Live/dead (Invitrogen) or DAPI was used to exclude dead cells. Cells were sorted with an Aria II or MoFlo prior to intracellular staining due to loss of YFP after fixation and reduced RFP detection after permeabilization. In experiments where transcription factors were assessed in Arg1YFP+RNT− cells, Arg1YFP+RFP− cells (consisting of RNT−, NK1.1+ and ST2+ cells) were sorted to 99% purity into a single tube prior to intracellular staining. Arg1YFP+RORγt(fm)+ cells were sorted in parallel. In fetal liver experiments, Lin− cells were defined as lacking CD3, CD4, CD5, CD19, NK1.1, Ter119, Gr-1, and CD11b. In adult BM experiments, Lin− cells were defined as lacking CD3, CD4, CD5, CD19, NK1.1, Ter119, Gr-1, and B220. Transcription factors were analyzed using the Foxp3/Transcription Factor Staining Buffer Set from eBioscience. In day 6 culture experiments where RFP detection was required after intracellular staining for transcription factors, cells were fixed in 2% paraformaldehyde (PFA) for 2 min and washed with PBS prior to fixation with reagents from the Transcription Factor Staining Buffer set. Cytokine production was assessed using Cytofix/Cytoperm reagents from BD Biosciences. For LTα1β2 detection, sorted or cultured cells were blocked with donkey anti-mouse Fab fragments (Jackson ImmunoResearch) prior to staining with LTβR-Ig. Cells were stained with biotin-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch) and blocked with mouse serum before incubating cells with antibodies and APC-conjugated streptavidin. Counting beads were used to determine total cell numbers (CountBright absolute counting beads, Invitrogen). Flow cytometry was performed using a LSR II (BD Biosciences).
Immunohistochemistry
Samples were fixed with 4% PFA (Electron Microscopy Sciences) in PBS for 2 h, and then left in PBS overnight. Tissues were then incubated in 30% sucrose for 2 h before they were frozen in OCT compound (Sakura). Frozen tissue blocks were sectioned at 8 microns (adult gut) or 7 microns (fetal gut) using a Leica CM3050-S cryostat. In experiments with Lta−/− and Rorc(γt)−/− animals, intestines from both genotypes were frozen in the same block for direct comparison. Serial sections were taken of the proximal half of the small intestine (where the first PP develops), and every section was analyzed for YFP+ aggregates. Animals were genotyped before tissues were frozen, and thus the investigator was not blinded to sample genotypes.
Sections were incubated with 3% H2O2, 0.1% NaN3 in PBS for 45 min to quench endogenous peroxidase, and then blocked with rat anti-mouse CD16/CD32, 1% mouse serum, and 1% rat serum for 1 h. Endogenous biotin and avidin-binding sites were blocked with a Biotin/Avidin Blocking Kit (Vector Labs). Slides were incubated with biotin-conjugated goat anti-GFP (Abcam), biotin-conjugated rabbit anti-RFP (Abcam), or biotin-conjugated rat anti-mouse VCAM-1 biotin (eBioscience) for 1–2 h followed by HRP-conjugated streptavidin (Perkin Elmer) and FITC- (Perkin Elmer) or A555-conjugated tyramide (Invitrogen). Sections that were stained with two biotinylated antibodies were treated with H2O2, NaN3 and avidin/biotin blocking reagents prior to each antibody incubation. In other experiments, sections were stained with rat anti-mouse RORγ(t) (B2D, eBioscience). DAPI was added to sections for 5 min to visualize nuclei.
Cell culture
Cells were sorted on an Aria II (BD Biosciences) with doublets excluded, and cultured with 10 ng/ml recombinant mouse IL-7 (R&D Systems) in RPMI 1640 medium (supplemented with HEPES, pH?, fetal calf serum, sodium pyruvate, 2-mercaptoethanol, streptomycin/penicillin, and L-glutamine) for 20 h, 6 days, or 10 days at 2–5 × 103 cells/well. In other experiments, 1.5 × 103 cells or single cells (doublets excluded) were sorted into 96-well plates containing 1.2 × 104 irradiated OP9 cells/well (American Type Culture Collection), 10 ng/ml recombinant IL-7, and 10 ng/ml rSCF (R&D Systems). Media was replenished on the third day and wells were analyzed by flow cytometry on day 6. In experiments where cytokine production by ILCs was assessed, day 10 cultured ILCs were removed from OP9 co-cultures and stimulated for 3 h with 50 ng/ml PMA (Sigma) and 500 ng/ml ionomycin (Sigma). Brefeldin A (Biolegend) was added to the culture during the last 1.5 h. After activation, ILC1s were identified as CD45+RORγt(fm)−NK1.1+ cells, ILC2s as CD45hiRORγt(fm)−NK1.1−ICOShi cells, ILC3s as CD45+RORγt(fm)+NK1.1− cells, and ex-RORγt cells as CD45+RORγt(fm)+NK1.1+ cells.
Statistics
Data were analyzed with Prism 4 (GraphPad Software) using the two-tailed unpaired or paired Student’s t-test, or one-way ANOVA followed by Tukey’s test. The unpaired Student’s t-test was used to compare cell numbers from two different mouse genotypes, the paired Student’s t-test analysis was used to compare cell counts from paired samples originating from the same mouse, and one-way ANOVA analysis was used in the comparison of three or more samples. Each independent experiment was conducted with three to seven animals per group. In experiments determining cell localization within the intestine, repeats were pooled to determine reproducibility. Mice were not given different experimental treatments, and thus were not subjected to randomization.
Supplementary Material
Acknowledgments
The authors thank V. Nguyen from the UCSF Flow Cytometry Core and Z. Wang from the Sabre Sorting Facility for cell sorting, and D. Sheppard, D. Kioussis (MRC National Institute for Medical Research, London, UK), D. Littman (New York University), E. Robey (University of California, Berkeley), H. Luche and H. Fehling (University Clinics Ulm, Germany) for providing mice for these studies. We also thank J. Cyster and L. Lanier for critical reading of the manuscript. This work was supported by Howard Hughes Medical Institute; grants AI026918, AI030663, and AI119944 from the NIH; and the Sandler Asthma Basic Research Center at UCSF. J.K.B received support from the Biomedical Sciences (BMS) Graduate Program.
Footnotes
AUTHOR CONTRIBUTIONS
J.K.B. and R.M.L designed experiments and wrote the manuscript. J.K.B conducted experiments and H.-E.L. provided reagents.
COMPETING FINANCIAL INTERESTS
The authors have no competing financial interests.
References
- 1.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H, et al. IL-7 receptor alpha+ CD3(−) cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 3.De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 4.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 5.Honda K, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koni PA, et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 7.Okuda M, Togawa A, Wada H, Nishikawa S. Distinct activities of stromal cells involved in the organogenesis of lymph nodes and Peyer’s patches. J Immunol. 2007;179:804–811. doi: 10.4049/jimmunol.179.2.804. [DOI] [PubMed] [Google Scholar]
- 8.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 9.Yokota Y, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 10.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 11.Satoh-Takayama N, et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klose CS, et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med. 2012;209:729–740. doi: 10.1084/jem.20111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 16.Herbert DR, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 17.Hesse M, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 18.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 19.Bando JK, Nussbaum JC, Liang HE, Locksley RM. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J Leukoc Biol. 2013;94:877–884. doi: 10.1189/jlb.0213084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanamori Y, et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 23.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 24.Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol. 1997;9:507–514. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- 25.Klose CS, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2012;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 26.Vonarbourg C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Kasmi KC, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagi R, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer J, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 31.Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.