Abstract
Background
There is increasing interest in using neurobiological measures to inform psychiatric nosology. At present, it is unclear whether anxiety and depression are neurobiologically distinct or similar processes. Moreover, it is unknown if the best way to examine these disorders neurobiologically is by contrasting categorical definitions or by examining symptom dimensions.
Methods
We conducted a cross-sectional neuroimaging study of patients with generalized anxiety disorder (GAD), major depressive disorder (MDD), comorbid (GAD/MDD) or neither (controls). Participants included 90 medication-free individuals (17 GAD, 12 MDD, 23 GAD/MDD and 38 controls). Diagnosis/category was assessed along with dimensions/symptoms to determine the best fit for neurobiological data. Symptoms included general distress, common to anxiety/depression, as well as anxiety-specific (anxious arousal) or depression-specific (anhedonia) measures. Low frequency (0.008–0.1 Hz) signal amplitude and functional connectivity analyses of resting fMRI data focused on a priori cortical/subcortical regions of interest.
Results
Support was found for effects of diagnosis above and beyond those related to symptom levels, as well as for effects of symptom levels above and beyond effects of diagnostic categories. The specific dimensional factors of general distress and anxious arousal as well as a diagnosis of MDD explained unique proportions of variance in signal amplitude or functional connectivity.
Conclusions
Using resting fMRI, our data show that a single conceptual model alone (i.e. categorical diagnoses or symptom dimensions) provides an incomplete mapping of psychopathology to neurobiology. Instead, they support an additive model that best captures abnormal neural patterns in anxious and depressed patients
Keywords: anxiety, depression, fMRI, neuroimaging, anhedonia, resting state
INTRODUCTION
A key issue for understanding the pathophysiology of mental illness, as well as its nosology, is the need to determine how symptomatology relates to abnormal brain processes (i.e. putative mechanisms) (1). This issue is particularly salient for mood and anxiety disorders, as comorbidity between disorders is the normative clinical course (2, 3). One view, which treats anxiety and depression as reflecting the same core process, is supported by concordance studies indicating a shared genetic diathesis between GAD and MDD (4, 5). Symptom-based evidence also suggests a general class of ‘anxious-misery’ disorders, including GAD and MDD (6–8). Yet, these disorders can be differentiated with respect to illness predictors and symptoms (3, 9–11). Comparisons between GAD and MDD indicate greater emotion intensity and goal motivation in GAD as well as lower positive affect in MDD, among other factors (10).
It is not only unclear to what degree GAD and MDD are similar or different as disorders, but even whether categorical definitions of GAD and MDD best capture abnormalities. Many have argued for understanding anxiety and depression as a set of distinct and overlapping dimensions of dysfunction (12, 13). One of the most well-known of these models (13–15) proposes a tripartite organization: a shared general factor (general negative affect or distress), as well as two specific factors – anxious arousal (more central to anxiety) and low positive affect or anhedonia (more central to depression).
Complicating a clearer understanding of the neurobiology of anxiety and depressive disorders is the fact that few studies directly compare patients across these diagnostic groups (16–18). In a recent study, we found that only GAD patients (with or without MDD) showed a behavioral deficit in emotion regulation, not present for MDD only patients (19). This deficit reflected an abnormality in cingulate-amygdala circuitry normally required for this task (20), as well as a unique (compensatory) pattern of activation in MDD-only patients. In a study of anxious and depressed adolescents, Beesdo and colleagues found both common and disorder-specific abnormalities in amygdala activation during emotional face processing (21). All patients had greater amygdala activation to fear faces when focused on their own emotions but group by facial affect interactions divided patients into complex patterns during passive viewing.
Broadly comparing anxiety and depression, it may be advantageous to examine task-independent brain activity, allowing assessment across brain regions that may not be involved in a particular task. A powerful tool for doing so is resting-state fMRI, in which intrinsic activity and connectivity of brain circuitry can be examined across many brain systems and regions (22). Separate resting-state studies of GAD and MDD have implicated abnormalities in a number of structures, including the amygdala, hippocampus, ventral striatum, insula, dorsal and subgenual anterior cingulate (ACC), as well as dorsolateral and medial prefrontal cortices (DLPFC, MPFC) (23–32). It is yet unclear how these brain areas covary in patients as a function of pathophysiology. Analyses of the relationship between anxiety symptoms in healthy subjects and resting-state brain activity have demonstrated effects in many of these same regions (33–35). To our knowledge, however, anxiety and depression have never been directly compared using task-independent resting-state methods, nor have categorical and dimensional conceptualizations been evaluated in a single cohort.
In this study we sought to answer three questions: 1) Are neural signatures of anxiety and depression consistent with their being common or distinct neurobiological processes? 2) What are the relative contributions of categorical and dimensional formulations of anxiety and depression on neural processes? 3) Which brain regions are most strongly related to anxiety and depression? We analyzed models of combined categorical and dimensional factors according to the amplitude of the low-frequency resting-state signal within each region, as well as functional connectivity (FC) between regions, measured as time series correlations between region pairs. All participants were medication-free when scanned.
We recruited participants on the basis of GAD, MDD, both or neither diagnosis to ensure a comprehensive categorical analysis. Moreover, since these three groups overlapped by symptom profiles, we were similarly able to conduct dimensional analyses. For the categorical analyses, separate predictors corresponding to a diagnosis of GAD or MDD allowed us to examine disorder-specific effects, while a single predictor corresponding to either diagnosis tested for a general patient deficit relative to controls. By having partially overlapping patient groups and modeling diagnoses together we allowed for the possibility that abnormalities in one diagnostic group (e.g. MDD) could be best explained by its frequent comorbid diagnosis (e.g. GAD) or the possibility that individual diagnoses could explain independent neural abnormalities even after accounting for the presence of the other diagnosis. For dimensional analyses, we used the Mood and Anxiety Symptom Questionnaire (MASQ) (36), developed to assess the common symptom domain of general distress elevated across anxiety and depressive disorders, and specific domains of anxious arousal and anhedonia in accordance with the dimensional tripartite model of anxiety and depression. Three separate models were run for each metric (signal amplitude and functional connectivity): individual categorical and dimensional models were first run to establish possible relationships between these factors and brain measures. Next, to further understand the primacy of either categorical or dimensional measures for explaining variability in brain measures, we combined categorical and dimensional measures in a simultaneous regression. This strategy allowed for uncovering additive or overlapping predictors across categories and dimensions depending on which measures were the strongest predictors and which explained unique brain variability after accounting for the other factors.
METHODS AND MATERIALS
Participants
90 subjects participated in this study, after providing informed consent, and largely overlapped those reported in our task-based fMRI papers (19, 23, 37). Current-episode DSM-IV-based psychiatric diagnoses (38) were determined with the MINI structured diagnostic interview (39, 40). Participants included 38 healthy controls, 17 with a primary diagnosis of GAD and no MDD, 12 with a primary diagnosis of MDD and no GAD, and 23 with both diagnoses (see Supplemental Table S1 for other comorbidities). Exclusion criteria were substance abuse or post-traumatic stress disorders; a history of a neurological disorder or severe mental illness (psychosis or bipolar), head trauma or loss of consciousness; claustrophobia or regular use of benzodiazepines, opiate or thyroid medications. All controls were free of current or past Axis I conditions or psychiatric medications. No patient took a benzodiazepine within 48 hours of the scan and all patients were free of antidepressant medication for >6 wks.
fMRI Data Acquisition and Processing
Neuroimaging data were acquired on a 3T GE Signal scanner using a custom-built 8-channel head coil. Functional data were acquired in 29 axial slices (4.0mm thickness, 0.5mm gap) across the whole brain using a T2*-weighted gradient echo spiral (in/out) sequence (TR=2s, TE=30ms, flip angle=80°, 1 interleaf, FOV=22cm, 64×64 matrix, 236 volumes) (41). Instructions for the 8-min resting scan asked participants to keep still, keep their eyes closed, and let their mind wander. An automated high-order shim for spiral acquisitions was used before acquiring fMRI data (42). A high-resolution T1-weighted 3D inversion recovery SPGR anatomical scan (inversion time=300ms, TR=8ms, TE=3.6ms, flip angle=15°, FOV=22cm, 124 coronal plane slices, matrix=256×192, 2 excitations, acquired resolution=1.5 × 0.9 × 1.1mm) was acquired in the same session as fMRI data.
Physiological variability recorded in respiration and heart rate pulse oximetry was used for fMRI data correction as an initial preprocessing step (image based using acquisition timing relative to phases of cardiac and respiratory cycles) (43). Motion (middle volume reference; 12 dof affine, FSL FLIRT defaults) and then slice timing correction was applied to functional images; no participant had movement >3mm translation or 3 deg of rotation (cumulative, absolute value). Affine registration of the functional to structural images was combined with nonlinear normalization between T1 and MNI152 space for functional images using standard settings for the FSL 5.0 FNIRT tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Functional images were smoothed with a 5mm FWHM Gaussian filter. Time series data were bandpass filtered (0.008–0.1 Hz) and entered in a first-level fixed-effects GLM model for functional connectivity calculation, covarying for white matter and CSF signal (see supplemental materials for analyses of this noise estimate) as well as residual motion parameters. Fisher’s r-to-z calculation on FC images was performed before average connectivity values were extracted across each ROI. The DPARSF toolbox (44) was used on unfiltered data to generate spectral power amplitudes in the low frequency (LF) band (0.008–0.1 Hz). The root mean square of the power spectrum density in the chosen band was then standardized to the average amplitude of the full gray matter mask (voxel/average rest of brain). Then fractional amplitude was calculated relative to the full power spectrum (low frequency/full band) defined by the fMRI sampling rate (0~0.25 Hz for TR=2.0) and was used for ROI extraction and analysis, referred to hereafter as ‘signal amplitude’.
ROI Selection
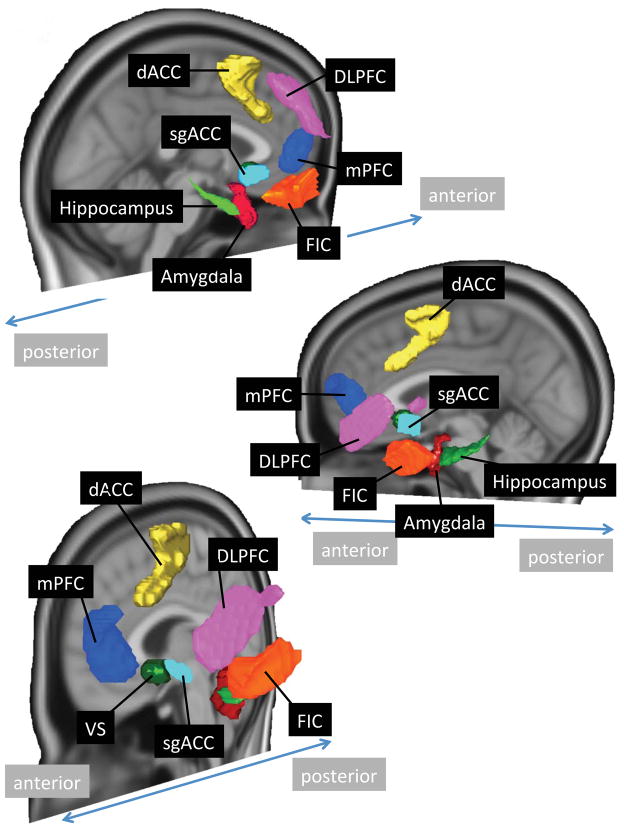
A priori regions of interest (ROIs) were defined (see Figure 1) where possible by anatomical boundaries, including the hippocampus (70% probability threshold in the Harvard-Oxford atlas) (45), basolateral and centromedial amygdala (maximum probability defined in our prior paper, from a cytoarchitectonically-defined atlas) (23, 46), and the ventral striatum (based on subcortical anatomical segmentation atlas (47) with a small overlap in the left hemisphere with the subgenual ACC ROI removed). Amygdala extractions used a combined basolateral and centromedial mask, as separating the amygdala by subregion did not alter findings. The subgenual ACC ROI was a 8mm gray matter-masked sphere around a peak coordinate (x, y, z: 2, 18, −8) associated with MDD abnormalities averaged from 14 fMRI studies (48). ROIs for the dorsal ACC, DLPFC, MPFC and fronto-insular cortex (FIC) were derived by thresholding resting-state independent component maps from a separate cohort of healthy subjects to yield 1000 voxel clusters for each region to allow them approximately equal weight as seeds for cross region comparisons, in accordance with the topography of typical resting state networks (49). For non-midline regions, we averaged signal from left and right-sided ROIs given no laterality predictions and an exploratory PCA indicating left and right hemisphere signal amplitude and connectivity loading consistently on the same factor.
Figure 1.
Brain image depicts surface reconstructions of regions of interest (ROIs) used in functional connectivity and intrinsic signal amplitude analyses. DLPFC=dorsolateral prefrontal cortex, dACC=dorsal anterior cingulate cortex, sgACC=subgenual anterior cingulate cortex, mPFC=medial prefrontal cortex, FIC=fronto-insular cortex, VS=ventral striatum.
Statistical analyses
Mean LF signal amplitudes and functional connectivity (z-score) values for each ROI were determined in MNI space. Using SPSS software (SPSS, Inc., Chicago, IL) we conducted principal components analyses with quartimax rotation for data reduction to limit multiple comparisons and to recognize that dimensional and categorical constructs related to affective disorders probably involve several distributed neural processes. For specific component interactions with categorical or dimensional variables, stepwise regressions were included to determine standardized betas (sβ) and significance values for individual predictors (stepping criteria: entry .05, removal .10). The optimal number of extracted components was determined by eigenvalues >1. Identical analyses were run separately for signal amplitude and functional connectivity z-scores. Resting-state data were analyzed using general linear models, including principal components as repeated measures with a Greenhouse-Geisser correction, as described in the results section. Chi-square and t-tests were used where noted.
RESULTS
Sample characteristics (see Supplemental Table S1)
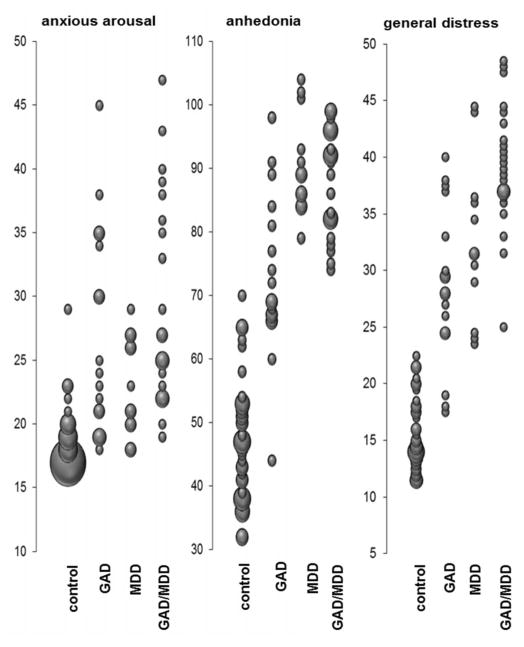
No significant differences in age, education, gender or average head motion (x, y, z directions) were found using either categorical or dimensional models (ps>.08). As shown in Figure 2, there was partial overlap in symptom scores between control subjects and patients, motivating our primary analytic approach of including data from all participants, supplemented by a follow-up analysis restricted to patients (see supplemental text).
Figure 2.
Distribution of the dimensional Mood and Anxiety Symptom Questionnaire (MASQ) subscale scores across each of the diagnostic categories.
Low Frequency Signal Amplitudes
To minimize the number of comparisons, our principal components data reduction across ROIs yielded three principal components. As shown by rotated component loadings in table 1, these involve primarily limbic/paralimbic regions (amygdala, hippocampus, ventral striatum, subgenual ACC), the DLPFC/MPFC, and cingulo-opercular regions (dorsal cingulate and fronto-insular cortex). We entered these three components as repeated measures into three general linear models examining: 1) categorical predictors only (GAD, MDD), 2) dimensional predictors only (anxious arousal, anhedonia, general distress), then 3) categorical and dimensional predictors together to determine the strongest independent predictors of signal amplitude.
Table 1.
Data reduction for low frequency signal amplitude and functional connectivity data*
| Signal Amplitude Principal components | |||
|---|---|---|---|
| Region | Limbic/paralimbic | DLPFC/MPFC | Cingulo-opercular |
| sgACC | .848 | .236 | .082 |
| ventral striatum | .826 | .278 | .059 |
| amygdala | .702 | −.211 | −.217 |
| hippocampus | .376 | −.145 | .028 |
| MPFC | .146 | .868 | .092 |
| DLPFC | −.135 | .840 | −.110 |
| dorsal ACC | −.245 | .175 | .791 |
| FIC | .309 | −.253 | .749 |
| Functional Connectivity Principal components | ||||||
|---|---|---|---|---|---|---|
| Connectivity pair | cortical | sgACC/VS | amygdala-sub | amyg-pfc | fronto-operc | amyg-fronto-operc |
| hippocampus <-> dACC | .880 | −.025 | .041 | .033 | −.114 | .226 |
| hippocampus <-> FIC | .852 | .017 | .027 | −.198 | .023 | .344 |
| hippocampus <-> DLFPC | .827 | .035 | −.026 | .275 | −.080 | −.014 |
| VS <-> dACC | .802 | .061 | .118 | −.205 | −.190 | −.174 |
| MPFC <-> dACC | .798 | −.008 | .011 | −.107 | .465 | .038 |
| sgACC <-> dACC | .798 | .098 | .059 | −.373 | −.109 | −.184 |
| VS <-> DLPFC | .794 | .184 | .020 | .088 | −.078 | −.395 |
| FIC <-> MPFC | .793 | .134 | −.143 | −.135 | .398 | .127 |
| MPFC <-> DLPFC | .768 | −.210 | .053 | .022 | .285 | −.226 |
| VS <-> FIC | .755 | .218 | −.005 | −.436 | −.164 | .082 |
| FIC <-> DLPFC | .755 | .083 | −.097 | .146 | .489 | −.112 |
| sgACC <-> FIC | .739 | .249 | −.051 | −.510 | −.073 | .102 |
| amygdala <-> DLPFC | .736 | −.051 | .178 | .434 | .039 | .224 |
| sgACC <-> DLPFC | .733 | .088 | .004 | −.146 | −.120 | −.475 |
| FIC <-> dACC | .728 | .010 | .067 | .096 | .126 | −.111 |
| amygdala <-> dACC | .716 | −.065 | .179 | .223 | −.232 | .441 |
| hippocampus <-> MPFC | .685 | .379 | −.053 | .391 | −.169 | −.008 |
| amygdala <-> FIC | .676 | −.163 | .396 | −.086 | −.096 | .448 |
| DLPFC <-> dACC | .656 | −.026 | −.026 | .092 | .625 | .032 |
| amygdala <-> MPFC | .643 | .154 | .413 | .472 | .031 | .128 |
| sgACC <-> VS | .295 | .797 | .075 | −.105 | .181 | .071 |
| hippocampus <-> sgACC | .390 | .686 | .207 | −.043 | .092 | .009 |
| hippocampus <-> VS | .497 | .655 | .108 | .034 | −.192 | .036 |
| VS <-> MPFC | .557 | .608 | −.054 | .219 | −.063 | −.153 |
| sgACC <-> MPFC | .444 | .580 | .042 | −.031 | −.253 | −.354 |
| amygdala <-> VS | .464 | .247 | .742 | .025 | −.060 | .014 |
| amygdala <-> sgACC | .350 | .371 | .718 | −.106 | .176 | −.025 |
| amygdala <-> hippo | .371 | −.053 | .703 | .102 | −.111 | .022 |
Column labels refer to brain areas or connectivity pairs with higher loadings (shown in bold font) that especially contribute to individual factors.
‘DLPFC’=dorsolateral prefrontal cortex; ‘MPFC’=medial prefrontal cortex; ‘ACC’=anterior cingulate cortex; ‘FIC’=fronto-insular cortex; ‘sgACC’=subgenual ACC; ‘VS’=ventral striatum; ‘amyg’=amygdala; ‘pfc’=prefrontal cortex; ‘operc’=operculum; ‘hippo’=hippocampus; ‘<->‘ indicates functional connectivity between brain regions listed to the left and right of the symbol
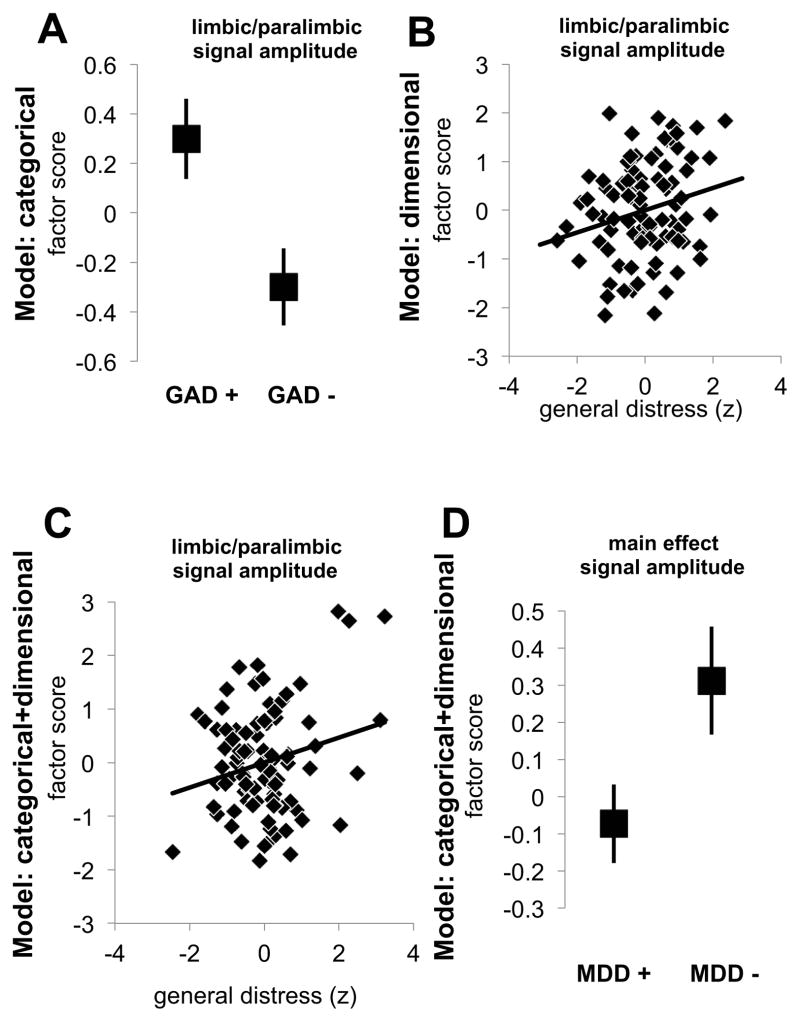
For categorical predictors only, we found a GAD by principal component interaction (F2, 172=1.4, p=.036) and a marginal GAD by MDD interaction (F1, 86=2.8, p=.096; other ps>0.12). As shown in figure 3A, the GAD by principal component interaction reflected a GAD effect specifically on limbic/paralimbic regions, wherein greater LF signal amplitudes were seen for participants with GAD compared to those without (t88=2.5, p=.015; other ps>0.28). In a stepwise model, only GAD was a significant predictor of limbic/paralimbic signal (sβ=.255, p=.015). We also examined in a repeated measures model whether patients as a whole differed from controls in LF signal amplitudes, but found no main effect of patient versus control, or interaction with principal component (ps>.40).
Figure 3.
Signal amplitude results from models of only categorical, dimensional, or both categorical and dimensional models of anxiety and depression for all participants, plotting estimated marginal means and their standard errors. For the categorical model, (A) a specific effect of GAD on limbic/paralimbic signal amplitude was found. For the dimensional only model, there was a significant positive relationship between the broad symptom of general distress and limbic/paralimbic signal amplitude (B) that remained significant in the combined categorical and dimensional model (C) after accounting for other symptoms and diagnostic categories. In the combined model, a main effect of MDD was driven by overall lower signal amplitudes for MDD patients compared to individuals without MDD (D).
For the dimensional predictors only model, we found interactions of principal component with both anhedonia (F2, 172=3.5, p=.034) and general distress (F2, 172=5.3, p=.006). The interaction with anhedonia was driven by correlations that differed slightly by component (slightly positive, slightly negative, and nil) but without approaching significance (ps>0.21). General distress was specifically positively related to limbic/paralimbic LF signal amplitudes in the stepwise model (sβ=.248, p=.019; see figure 3B; other factors ps>0.13), driving the full model interaction.
Despite the fact that symptom scores were continuously distributed and partially overlapping between patients and controls, effects of dimensional predictors across participants may still reflect higher symptom scores in patients. We thus tested a model that included the three dimensional predictors, a categorical predictor for patient versus control, and the interactions between the categorical patient predictor and dimensional symptom predictors. Nonetheless, we found still the significant interaction between principal component and general distress (F2, 170=6.33, p=.002), no significant interaction with anhedonia (F2, 170=1.11, p=.331), and no interactions or main effects for the patient predictor (ps>.29).
Finally, we tested the combination of categorical and dimensional predictors to determine which predictors account for the greatest variance in LF signal. Since GAD and general distress were each associated with greater limbic/paralimbic signal in the separate categorical and dimensional models, respectively, we were particularly interested in which was the stronger predictor in a combined model. We found that the principal component by general distress interaction remained significant (F2, 168=5.0, p=.008), again driven by a positive relationship with limbic/paralimbic signal (Figure 3C), whereas the GAD main effect was reduced to non-significance (F1, 84=0.1, p=.824) indicating that distress substantially overlapped with the GAD effect and was an even better predictor of limbic/paralimbic LF signal amplitude (forced entry GAD first block, sβ=.255, p=.015; 2nd block stepwise remaining variables, ps>0.29). There was a marginal component by MDD interaction (F2, 168=2.76, p=.066) and also a significant MDD main effect (F1, 84=5.62, p=.020) driven by lower signal amplitudes for MDD (see Figure 3D and Supplemental Figure S1). Thus, after accounting for relationships between signal amplitude and all other factors from each of the three symptom scales and GAD diagnosis (estimated marginal means), MDD was the only factor associated with reduced signal amplitudes.
Parallel analyses as above on only the 52 patients revealed highly similar findings (see supplement), demonstrating consistency and robustness of findings.
Functional Connectivity
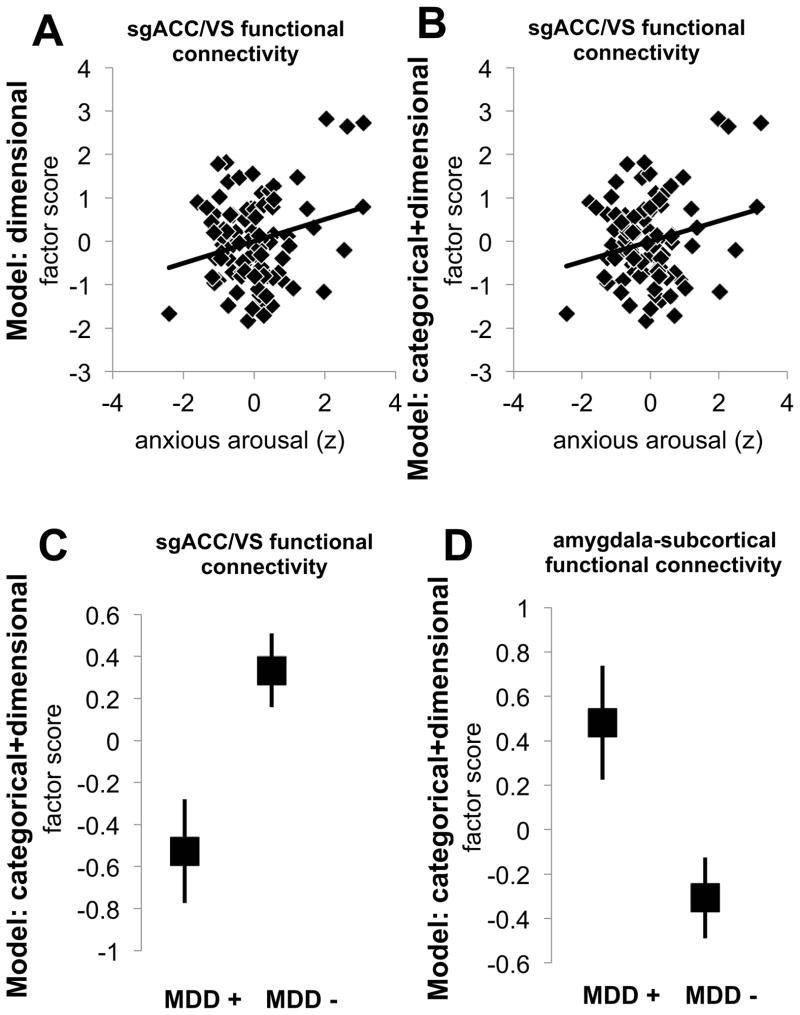
Principal component data reduction on all ROI pairwise functional connectivity correlations yielded six components (see Table 1). The first component captured cortical connectivity (non-subcortical), the second subgenual cingulate and ventral striatal connectivity, the third amygdala subcortical connectivity, the fourth positive amygdala prefrontal (and negative fronto-opercular subcortical) connectivity, the fifth prefrontal fronto-opercular connectivity, and the sixth amygdala to fronto-opercular connectivity. Entering these into a repeated measures model with only categorical predictors did not produce any significant findings (ps>0.23). With the dimensional only model, there was a component main effect (F5, 430=2.5, p=.029) and a factor by anxious arousal interaction (F5, 430=2.6, p=.027; other ps>.20). In the follow up stepwise regressions, the only significant model was for sgACC/ventral striatal connectivity (F1, 89=7.33, p=.008) driven by a positive relationship with anxious arousal (sβ=.277, p=.008; excluded variable ps>0.81; see Figure 4A). We were then especially interested in whether this relationship held when controlling for diagnoses in the same model.
Figure 4.
Functional connectivity results from models of only categorical, dimensional, or both categorical and dimensional models of anxiety and depression for all participants, plotting estimated marginal means and their standard errors. (A) For the dimensional model, there was a significant relationship between anxious arousal and connectivity with the sgACC/ventral striatum that was again significant in the combined categorical and dimensional model (B). In the combined model, MDD was associated with reduced sgACC/VS connectivity and increased amygdala-subcortical connectivity.
In the model with both categorical and dimensional predictors, the component by anxious arousal interaction was marginally significant (F5, 420=2.1, p=.065). The positive relationship between anxious arousal and sgACC/VS connectivity remained significant (Figure 4B) whereas the other variables did not contribute additional explanatory power (forced entry anxious arousal first block, sβ=.277, p=.008; 2nd block stepwise remaining variables, ps>0.35). There was also a marginal component by anhedonia interaction (F5, 420=2.0, p=.077) and a significant interaction between component and MDD diagnosis (F5, 420=2.5, p=.029). To follow up the MDD interaction, regressions for each component testing marginal means covarying the other factors suggested that the effect was driven by lower sgACC/VS connectivity (F1, 84=5.4, p=.022; Figure 4C) and greater amygdala subcortical connectivity (F1, 84=4.2, p=.043; Figure 4D) for MDD patients (other ps>.24). An overlap in findings across the models thus suggests that anxious arousal drives up sgACC/VS connectivity independent of an MDD diagnosis influence that drives down connectivity in the same circuit. Findings in patients indicated the same patterns (see supplemental materials).
DISCUSSION
This is the first study, to our knowledge, to directly compare anxiety and depressive disorders with resting-state fMRI, and to explicitly explore both categorical and dimensional conceptualizations of these conditions together relative to one another. Our findings lend support for both commonalities and distinctions between anxiety and depression. These findings suggest that neither a categorical nor a dimensional model of anxiety and depression explains neurobiology to the exclusion of the other. Rather, mapping the affective pathology onto neurobiological substrates requires combining categorical and dimensional specifications of anxiety and depressive disorders. The combination accounted for patterns of overlapping as well as independent predictors of resting fMRI brain measures. More specifically, our data argue that general distress increases limbic/paralimbic low frequency signal amplitudes whereas MDD diagnosis drives signal down across the brain areas we studied. In terms of functional connectivity between ROIs, anxious arousal was associated with greater sgACC/ventral striatum connectivity whereas MDD was associated with reduced connectivity in this same circuit when covarying symptoms and GAD diagnosis. The anxiety and distress correlations remained significant with or without covarying other symptoms and diagnoses. Both relationships were also significant in the patient group alone indicating relevance to affective pathology. The regions most important for understanding both categorical and dimensional aspects of anxiety and depression were the amygdala, ventral striatum, hippocampus and subgenual ACC. These regions are consistently implicated in anxiety and depression-relevant processes, such as threat processing, reward and motivation, affect regulation and memory.
With regard to whether anxiety and depression are components of a single psychopathological process or reflect distinct processes, we found that categorical diagnoses MDD and GAD are associated with distinct resting-state alterations. However, the GAD effect (greater limbic/paralimbic signal) is better attributed to the symptom dimension of general distress. General distress can be considered a measure of disorder-nonspecific broad negative affect, and similar results (not shown) are found by replacing this measure with the first principal component from a PCA across a many depression and anxiety measures (Beck Anxiety and Depression Inventories (50, 51), Penn State Worry Questionnaire (52), Spielberger Trait Anxiety Inventory (53)). The categorical effect of MDD, however, was especially strong in patients and represented a non-overlapping suppression of signal amplitude across ROIs. In functional connectivity, anxious arousal emerged as a strong independent predictor of increased sgACC/VS connectivity in the opposite direction of an MDD effect (covarying GAD diagnosis and other symptoms). Hence, we found diagnosis-specific perturbations related to MDD, as well as diagnosis-nonspecific perturbations related to general distress and anxious arousal. While there were small differences between models, results were strikingly consistent at multiple levels (full group and patients alone) and with or without covarying other categorical/dimensional factors.
Our findings hold implications not only for how neurobiology can inform psychopathology and its nosology, but also for emerging theoretical models of mental illness. A DSM-type approach relies on specific symptom criteria for establishing a categorical diagnosis, whereas an approach such as the Research Domain Criteria (RDoC) from the National Institute of Mental Health, advocates for a dimensional organization. Our data suggest that use of only a single conceptual framework (i.e. categorical diagnoses or symptom dimensions) provides an incomplete answer. While it may, in principle, be possible to establish dimensions that capture the MDD diagnosis effect, it is apparent that one of the best-described attempts to do so (i.e. the tripartite model) was insufficient for this application. GAD was subsumed under general distress as a predictor for our brain measures. Also, MDD emerged primarily as an effect only when accounting for GAD and dimensional scales (combined model). One possibility is that symptoms are especially strong predictors of resting brain abnormalities but could partially obscure a weaker but distinct MDD categorical abnormality. The functional relevance of both dimensional and categorical predictors should thus be carried forward as an investigation for future research. The patient only correlation between distress and DLPFC/mPFC connectivity did not reflect an abnormality in models with healthy controls but nevertheless may reflect important within-patient clinical phenomena given especially the importance of these regions for mapping individual differences in MDD response to transcranial magnetic stimulation (48, 54).
Methodologically, it is notable that our findings suggest unique sources of pathology across domains of low frequency signal amplitude and low frequency correlation (functional connectivity) between brain regions implicated in clinical anxiety and depression. Nevertheless, theoretical overlaps can be seen across the two measures. For example, anxious arousal was associated with greater sgACC/VS connectivity and general distress was associated with greater signal for a factor that included these brain areas (limbic/paralimbic factor). Also, an MDD diagnosis was associated broadly with lower signal amplitudes consistent with lower sgACC/VS connectivity findings for MDD. However, anxious arousal was also associated with lower amygdala connectivity to a specific targets (fronto-opercular) and MDD was associated with greater amygdala to subcortical connectivity which represent a seemingly unrelated set of abnormalities. LF signal amplitude is a reliable (55) fMRI BOLD derivative sensitive to both stable individual differences (56) and to transient brain fluctuations (49). However, it is used far less frequently than ICA or functional connectivity to study resting brain networks and thus the degree of complementarity between signal amplitude and these traditional measures deserves added attention including in the domain of psychopathology.
Limitations include a relatively small sample of patients with MDD alone (see also power calculations in supplemental materials regarding MDD only effects). Nonetheless, sample sizes were substantial across disorders for comparing effects for anxiety and depression as general groupings. Also, our groups were well-educated, on average, which may have influenced findings. Allowing some comorbidity increased the representativeness of our sample but may also have influenced results. A limitation of resting-state fMRI is that the nature of the measures in isolation precludes insight about specific mental processes. Future work will need to extend these findings using tasks more directly tapping functions subserved by key regions identified here and with appropriate manipulations/assessments of mental states related to psychopathology at rest. Resting-state networks are largely stable across behavioral states, levels of consciousness and species (57) which suggests that resting measures may capture consistent mental state differences between patients and healthy individuals. Thus, our data likely speak to broad baseline neuropathology that may be superimposed on or interact with brain signatures of specific mental states (that may also differ by patient group). Additional descriptions of patients using other factors (cognitive symptoms, perseverative thinking, functional impairment, etc.) may explain additional variance compared with these initial models. Similarly, additional brain regions or networks could contribute further evidence of patient abnormalities related to dimensions and categories. Methodologically, increasing the number of transmit/receive coils could improve SNR and lead to additional findings (perhaps better resolution of amygdala subregional effects). Typical psychiatric medications influence the neural circuitry studied here and, as many patients regularly take these medications, a follow up study might include medicated patients to understand how the metrics observed in the present study are influenced by specific medications.
In summary, our results provide support for a combination of categorical and dimensional conceptualizations of anxiety and depression, and illustrate both the condition-specific perturbations and those related more broadly to affective distress. Use of a broadly applicable neurobiological measure, resting-state fMRI, has thus provided a powerful brain-based test of dominant conceptual models of anxiety and depression developed through the study of symptoms and diagnostic categories.
Supplementary Material
Acknowledgments
This research was supported by the War Related Illness and Injury Study Center (WRIISC) Special Fellowship Program at the VA Palo Alto and the Medical Research Service of the Department of Veterans Affairs, VA Palo Alto awarded to DJO as well as by National Institute of Mental Health Grants P30MH089888 to AFS and R01MH091860 to AE, and the Sierra-Pacific Mental Illness Research Education and Clinical Center at the Palo Alto VA HealthCare System.
Footnotes
FINANCIAL DISCLOSURES
Dr. Schatzberg has served as a consultant to BrainCells, CeNeRx, CNS Response, Corcept, Eli Lilly, Forest Labs, GSK, Innapharma, Lundbeck, Merck, Neuronetics, Novartis, Pathway Diagnostics, Pfizer, PharmaNeuroBoost, Quintiles, Sanofi-Aventis, Synosis, Takeda, Xytis and Wyeth. Dr. Schatzberg has equity in Amnestix, BrainCells, CeNeRx, Corcept (co-founder), Forest, Merck, Neurocrine, Pfizer, PharaNeuroBoost, Somaxon and Synosis, and was named an inventor on pharmacogenetic use patents on prediction of antidepressant response. Dr. Schatzberg has also received speaking fees from GlaxoSmithKline and Roche. Drs. Oathes, Patenaude, and Etkin report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 2.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- 5.Roy MA, Neale MC, Pedersen NL, Mathe AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychol Med. 1995;25:1037–1049. doi: 10.1017/s0033291700037533. [DOI] [PubMed] [Google Scholar]
- 6.Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- 7.Grant DM, Beck JG, Marques L, Palyo SA, Clapp JD. The structure of distress following trauma: posttraumatic stress disorder, major depressive disorder, and generalized anxiety disorder. J Abnorm Psychol. 2008;117:662–672. doi: 10.1037/a0012591. [DOI] [PubMed] [Google Scholar]
- 8.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, et al. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 9.Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67:47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- 10.Aldao A, Mennin DS, Linardatos E, Fresco DM. Differential patterns of physical symptoms and subjective processes in generalized anxiety disorder and unipolar depression. J Anxiety Disord. 2010;24:250–259. doi: 10.1016/j.janxdis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence AE, Liverant GI, Rosellini AJ, Brown TA. Generalized anxiety disorder within the course of major depressive disorder: examining the utility of the DSM-IV hierarchy rule. Depress Anxiety. 2009;26:909–916. doi: 10.1002/da.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- 13.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 14.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Aldao A, Nolen-Hoeksema S. When are adaptive strategies most predictive of psychopathology? J Abnorm Psychol. 2012;121:276–281. doi: 10.1037/a0023598. [DOI] [PubMed] [Google Scholar]
- 17.Ionescu DF, Niciu MJ, Mathews DC, Richards EM, Zarate CA., Jr Neurobiology of anxious depression: a review. Depress Anxiety. 2013;30:374–385. doi: 10.1002/da.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 20.Maier ME, di Pellegrino G. Impaired conflict adaptation in an emotional task context following rostral anterior cingulate cortex lesions in humans. J Cogn Neurosci. 2012;24:2070–2079. doi: 10.1162/jocn_a_00266. [DOI] [PubMed] [Google Scholar]
- 21.Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etkin A, Prater K, Schatzberg A, Menon V, Greicius M. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of general psychiatry. 2009;66:1361. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 24.Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299. e292. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao H, Guo S, Ge T, Kendrick KM, Xue Z, Liu Z, et al. Depression uncouples brain hate circuit. Mol Psychiatry. 2013;18:101–111. doi: 10.1038/mp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baur V, Hanggi J, Langer N, Jancke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Dennis EL, Gotlib IH, Thompson PM, Thomason ME. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain connectivity. 2011;1:245–254. doi: 10.1089/brain.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson D, Clark LA. The mood and anxiety symptom questionnaire. Iowa City, IA: University of Iowa; 1991. [Google Scholar]
- 37.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.APA. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 40.Sheehan DV, YL, KH-S, JJ, EW, LIB, et al. Reliability and Validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): According to the SCID-P. European Psychiatry. 1997;12:232–241. [Google Scholar]
- 41.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 42.Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- 43.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 51.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 52.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 53.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 54.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.