Abstract
Enzymatic depletion of the non-essential amino acid L-Arginine (L-Arg) in cancer patients by the administration of a pegylated form of the catabolic enzyme arginase I (peg-Arg I) has shown some promise as a therapeutic approach. However, L-Arg deprivation also suppresses T-cell responses in tumors. In this study, we sought to reconcile these observations by conducting a detailed analysis of the effects of peg-Arg I on normal T-cells. Strikingly, we found that peg-Arg I blocked proliferation and cell cycle progression in normal activated T-cells without triggering apoptosis or blunting T-cell activation. These effects were associated with an inhibition of aerobic glycolysis in activated T-cells, but not with significant alterations in mitochondrial oxidative respiration, which thereby regulated survival of T-cells exposed to peg-Arg I. Further mechanistic investigations showed that addition of citrulline, a metabolic precursor for L-Arg, rescued the anti-proliferative effects of peg-Arg I on T-cells in vitro. Moreover, serum levels of citrulline increased after in vivo administration of peg-Arg I. In support of the hypothesis that peg-Arg I acted indirectly to block T-cell responses in vivo, peg-Arg I inhibited T-cell proliferation in mice by inducing accumulation of myeloid-derived suppressor cells (MDSC). MDSC induction by peg-Arg I occurred through the general control non-repressed-2 eIF2α kinase. Moreover, we found that peg-Arg I enhanced the growth of tumors in mice in a manner that correlated with higher MDSC numbers. Taken together, our results highlight the risks of the L-Arg-depleting therapy for cancer treatment and suggest a need for co-targeting MDSC in such therapeutic settings.
Introduction
Therapeutic modulation of specific cellular metabolic pathways in solid and hematological malignancies represents a major strategy for the development of new treatments (1-4). Metabolism of the non-essential amino acid L-Arginine (L-Arg) plays a central role in several biological systems including the modulation of immune responses and tumor growth (5). We and others described the therapeutic benefit of depleting L-Arg in hematological and solid tumors through a pegylated form of the human L-Arg-metabolizing enzyme arginase I (peg-Arg I) (6-10). Pegylation of arginase I increased its stability in vivo, without altering its activity (7). Moreover, peg-Arg I was effective against malignant cells that depended upon exogenous L-Arg for growth (auxotrophic) and did not induce significant toxic side effects (3). The anti-tumor effect induced by peg-Arg I was mediated by the induction of malignant cell apoptosis (6) and controlled through the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α) and the expression of general control non-repressed 2 eIF2α kinase (GCN2) (11). Although peg-Arg I was highly effective in controlling the growth of leukemic T-cells (6, 11), its effect on normal T-cells remains unclear. Initial in vitro studies showed that L-Arg starvation blocked proliferation of activated normal T-cells (12-14). In addition, we found that peg-Arg I delayed development of graft vs. host disease (GVHD) and increased burden of Listeria monocytogenes (15, 16), both conditions linked to impaired T-cell function. However, the mechanisms by which peg-Arg I could impair T-cell responses in vivo and how normal activated T-cells maintain survival under L-Arg starvation remain unknown.
Specific energy metabolic pathways regulate the activation and proliferation of normal T-cells. Production of ATP and reactive oxygen species (ROS) from the mitochondria control the initial T-cell-activation phase, while aerobic glycolysis modulates proliferation and effector T-cell functions (17-21). Although specific energy metabolic programming regulates global function of T-cells, it remains unknown the effect of L-Arg in the modulation of energy metabolism.
Accumulation of myeloid-derived suppressor cells (MDSC), a heterogeneous population of immature myeloid cells expressing CD11b+ Gr1+, is a hallmark of chronic inflammation and a major mediator for the induction of T-cell suppression in various tumors (22, 23). MDSC block T-cell responses through the metabolism of L-Arg by the enzymes arginase I and inducible nitric oxide synthase (iNOS), which promote L-Arg depletion and production of peroxynitrite, respectively (24, 25). Although the role of L-Arg metabolism on the T-cell suppression induced by MDSC is well understood, the effect of the deprivation of L-Arg in the accumulation and function of MDSC remains unknown.
Because the potential contradictory effect of L-Arg depletion as an anti-tumor therapy and as a mechanism for inhibition of immune responses, we aimed to understand the effects of peg-Arg I on normal T-cells. Our results show the regulatory effect of peg-Arg I on T-cell proliferation and the ability of T-cells to resist peg-Arg I through de novo L-Arg synthesis. Moreover, L-Arg deprivation induced the accumulation of MDSC, which inhibited T-cell proliferation in mice. These results support the novel role of MDSC in the regulation of T-cell responses by L-Arg starvation and suggest the need to therapeutically target MDSC in peg-Arg I-based therapies.
Material and methods
Mice and cells
C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN). CD45.1+, GCN2-/-, and anti-OVA257-264 (siinfekl) OT-1 mice were from the Jackson Laboratories (Bar Harbor, ME). Lewis lung carcinoma cells (3LL) were obtained in 2012 from the American Type Culture Collection (ATCC, Manassas, VA) and injected s.c. into the mice (26). 3LL cells were periodically tested (last-test May 2014) and validated to be mycoplasma-free, using an ATCC kit. All mice studies were achieved using an approved IACUC protocol from LSU-HSC. T-cells were isolated from spleens and lymph nodes of mice using T-cell negative isolation kits (Dynal, Life Technologies). Then, T-cells were activated using 0.5 μg/ml plate bound anti-CD3 plus anti-CD28 (26). MDSC were isolated from spleens of mice using Gr-1 selection kits (Stem Cell Technologies, Vancouver, BC). Purity for cell isolations ranged from 90–99%.
Antibodies and reagents
Detailed description of antibodies, methodologies for flow cytometry and fluorescence, and statistical analysis are in the Supplemental Methods. O′-methylpolyethylene-glycol (PEG) 5000 mw (Sigma-Aldrich) was covalently attached to human-recombinant arginase I (AbboMax, San Jose, CA) or bovine serum albumin (BSA, Sigma-Aldrich) in a 50:1 molar ratio (7). Pegylated-BSA (peg-BSA) was used as control for peg-Arg I.
Adoptive T-cell transfer
Mice were treated with peg-Arg I or peg-BSA every 2 days starting the day before the T-cell transfer. CD45.2+ mice were adoptively transferred with 5×106 CD45.1+/OT-1 T-cells, followed by immunization s.c. with 0.5 μg siinfekl peptide in incomplete Freud's Adjuvant (IFA). Four days later, mice were injected i.p with 200 μg 5-bromo-2-deoxyuridine (BrdU) (BD Bioscience), and BrdU incorporation measured 24 hours later using the APC-BrdU kit (BD Bioscience). For studies using depletion of MDSC, mice were treated with 200 μg anti-Gr-1 antibody (RB6-8C5) or IgG control twice a week, starting the day before the adoptive transfer. For MDSC proliferation, mice were treated with peg-Arg I every other day for 7 days, after which BrdU uptake into CD11b+ Gr-1+ cells was tested. Analysis of nuclear DNA content was achieved using CycleTEST-DNA kit (BD Biosciences). T-cell apoptosis was tested using annexin V-FITC apoptosis detection kit (BD Bioscience). Staurosporine (1 μM) was added 24 hours prior the apoptosis analysis as positive control.
[3-3H]-Glucose uptake
Glucose uptake was tested after pulsing activated T-cells cultured with peg-BSA or peg-Arg I (48 hours) with 1 μCi/ml Glucose-[3-3H] (Perkin Elmer Life Sciences, MA). Eight hours later, T-cells were washed in PBS, collected, and tested for radioactivity (counts per minute, cpm).
Extracellular flux analysis
Oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were measured using the XF-24-extracellular flux analyzer (Seahorse Bioscience). In brief, 5×105 activated T-cells treated for 24-72 hours with peg-Arg I or peg-BSA were plated in XF-24 cell culture plates coated with 15 μg CellTak (BD Biosciences) in XF media (Dulbeccos Modified Eagle's Medium containing 11 mM glucose and 100 mM sodium pyruvate). For OCR, T-cells were analyzed under basal conditions and in response to 10 μM oligomycin, 1 μM fluorocarbonyl-cyanide-phenylhydrazone (FCCP), and 1 μM rotenone (Sigma-Aldrich). For ECAR, T-cells were plated in XF media lacking glucose and monitored under basal conditions and in response to 10 mM glucose, 10 μM oligomycin, and 100 mM 2-Deoxy-D-glucose (2-DG, Sigma-Aldrich).
siRNA transfection of primary T-cells
Argininosuccinate synthetase (ASS-1) siRNA, non-targeting control siRNA, and FAM-labeled non-targeting control siRNA were obtained from Thermo Scientific (Accell-SMARTpool, Waltham, MA). Activated-T-cells (2×105) were transfected (2 μM siRNA) using Accell siRNA delivery media plus 2% FBS. Transfection efficiency ranged from 90-95% and viability from 95-99%. Peg-Arg I (1 μg/mL) or peg-BSA (1 μg/mL) were added 12 hours after transfection.
Results
Effects of peg-Arg I on T-cell proliferation and activation
Our previous results showed that peg-Arg I blocked malignant T-cell proliferation through the induction of cellular apoptosis; however, the effect of peg-Arg I on normal T-cells remains unclear (6, 11). To test this, we first investigated the effect of peg-Arg I on the proliferation of CFSE-labeled activated T-cells. A dose-dependent inhibition of T-cell proliferation was found after the addition of peg-Arg I, but not with control peg-BSA (Figure 1A). The anti-proliferative effect triggered by peg-Arg I was similarly noted in both CD4+ and CD8+ T-cells and was associated with a lower IFNγ production (Supplemental Figure 1A-B). To validate our results in vivo, we tested the effect of peg-Arg I in the proliferation of anti-OVA257-264 (siinfekl) transgenic CD8+ OT-1 cells. Thus, CD45.2+ mice were adoptively transferred with CD45.1+ CD8+ OT-1 cells, followed by immunization with siinfekl peptide. Mice were injected i.p. with peg-Arg I or peg-BSA the day before T-cell transfer and then every two days. A significant decrease in CD45.1+ OT-1 cell proliferation, as tested by BrdU uptake, was found in immunized mice treated with peg-Arg I, compared to those injected with peg-BSA (Figure 1B). To evaluate a potential T-cell toxicity side effect, we measured T-cell subsets in spleens of naïve mice treated for a week with increasing concentrations of peg-Arg I or peg-BSA. Peg-Arg I treatment was well tolerated and did not significantly alter the percentages of CD3+, CD4+, and CD8+ T-cells in spleen (Supplemental Figure 2A-C). Therefore, peg-Arg I blocked proliferation of activated T-cells, but did not affect homeostatic naïve T-cell numbers.
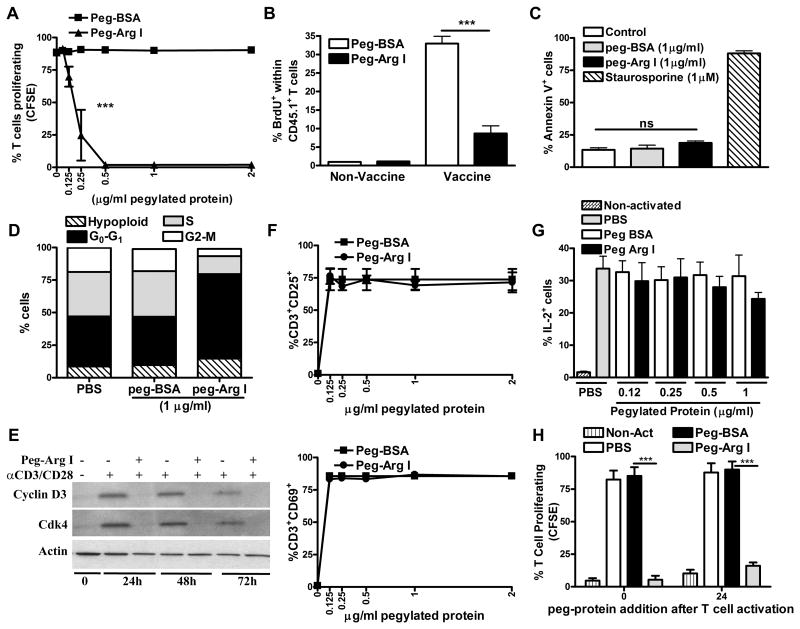
Figure 1. Peg-Arg I arrest T-cell proliferation, without inducing apoptosis or altering activation.
(A) CFSE-labelled T-cells were stimulated with anti-CD3/CD28 in the presence of peg-Arg I or peg-BSA. Percentages of proliferating T-cells were determined 72 hours later by flow cytometry. Values are from 3 experiments. (B) CD45.1+/OT-1 cell proliferation was monitored in spleens of mice treated with peg-Arg I or peg-BSA (5 mg/mouse, n=10) using BrdU, as described in the Methods. (C-D) Activated T-cells were treated with 1 μg/ml peg-Arg I or peg-BSA for 72 hours, after which they were stained with annexin V (C) or propidium iodide (D). T-cells treated with 1 μM staurosporine were used as positive apoptosis controls. (E) Representative experiment showing the expression of cyclin D3 and Cdk4 in T-cells treated with 1 μg/ml peg-Arg I. (F-G) Percentages of T-cells having CD25, CD69 (F), or IL-2 (G) after activation and culture for 48 hours with increasing levels of peg-Arg I or peg-BSA. (H) Peg-Arg I or peg-BSA (1 μg/mL) were added to stimulated T-cells labelled with CFSE at 0 or 24 hours after plating, and proliferation detected after 72 hours by flow cytometry. All data are expressed as mean +/- SEM from 3 experiments. * ** p < 0.001
To better understand the mechanisms by which peg-Arg I blocked T-cell proliferation, we tested the effect of peg-Arg I in the induction of T-cell apoptosis and cell cycle progression. A similar low expression of the apoptosis marker annexin V was found in activated T-cells treated with peg-Arg I or peg-BSA (Figure 1C). Conversely, upregulation of annexin V was noted in T-cells treated with the apoptosis-inducer staurosporine. Furthermore, an arrest in the G0-G1 phase of the cell cycle, which correlated with a decreased expression of cyclin D3 and cdk4, was found in activated T-cells cultured with peg-Arg I, compared to peg-BSA-treated T-cells (Figure 1D-E). We next evaluated the effect of peg-Arg I on T-cell activation by testing the expression of the early activation T-cell markers CD25 and CD69 and the production of IL-2. Similar upregulation of CD25 and CD69 and comparable percentages of IL-2+ cells were found in activated T-cells treated with increasing doses of peg-Arg I or peg-BSA (Figure 1F-G). Also, in agreement with the results showing the activation-independent anti-proliferative effect induced by peg-Arg I, we found that addition of peg-Arg I after 24 hours of activation still inhibited T-cell proliferation (Figure 1H). Therefore, peg-Arg I blocks proliferation and cell cycle progression of T-cells, without leading to apoptosis or impairing the expression of early activation mediators.
Effects of peg-Arg I on T-cell energy metabolic pathways
Specific energy metabolic pathways control the proliferation and activation of normal T-cells. Proliferating T-cells are highly dependent on the production of energy through aerobic glycolysis, while mitochondrial oxidative respiration fuels T-cell activation (20). Thus, we tested the effect of peg-Arg I on T-cell energy metabolism using extracellular flux analysis. A significant decrease in glycolysis, as measured by extracellular acidification rate (ECAR), was noted in activated T-cells treated with peg-Arg I, compared to T-cells cultured with peg-BSA (Figure 2A, Supplemental Figure 3A-B). Moreover, a lower glucose-[3-3H] uptake and decreased expression of the glucose transporter Glut-1 were found in T-cells cultured in the presence of peg-Arg I (Figure 2B-C), compared to peg-BSA-treated T-cells, suggesting that peg-Arg I blocked glycolysis in normal activated T-cells.
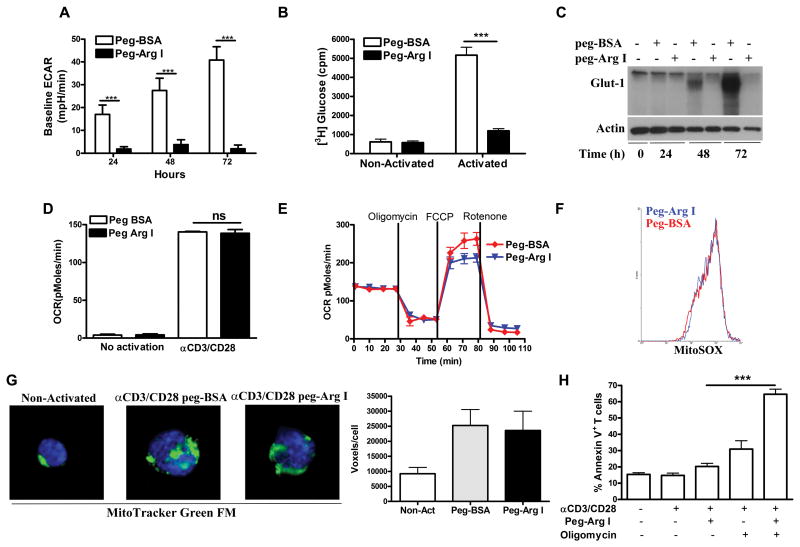
Figure 2. Effects of peg-Arg I on T-cell energy metabolic pathways.
(A) T-cells were treated with 1 μg/mL peg-Arg I or peg-BSA for 24-72 hours. Then, ECAR was measured via an extracellular flux analyser. (B) Glucose-[3-3H] uptake in activated T-cells treated with peg-Arg I or peg-BSA for 48 hour. (C) Expression of Glut-1 was tested in T-cells cultured in the presence of 1 μg/mL peg-Arg I or peg-BSA. (D-E) OCR in activated T-cells cultured with peg-Arg I or peg-BSA for 24 hours was analyzed under basal conditions (D) and in response to oligomycin, FCCP and rotenone (E). (F) Activated T-cells treated with peg-Arg I or peg-BSA for 48 hours were stained with MitoSOX and analyzed by flow cytometry. (G) Activated T-cells treated with peg-Arg I or peg-BSA for 72 hours were stained with Mitotracker Green-FM and DAPI and images were captured at equal exposure. Voxel quantification was achieved using Mask analysis. (H) Annexin V staining in activated T-cells treated with peg-Arg I +/- oligomycin (100 nM) for 48 hours. Results are expressed as mean +/- SEM from 3 experiments. Non-statistical significant differences (ns): p > 0.05. *** p < 0.001.
Next, we evaluated the effect of peg-Arg I on mitochondrial oxidative respiration by measuring oxygen consumption rate (OCR). Activated T-cells were cultured in the presence of peg-Arg I or peg-BSA for 24 hours, after which basal OCR was tested. A similar upregulation of OCR levels was noted in activated T-cells cultured with peg-Arg I or peg-BSA, compared to non-activated T-cells (Figure 2D). We then tested mitochondrial function by evaluating ATP-linked respiration using the mitochondrial ATP synthase inhibitor oligomycin, maximal respiration using the uncoupling ionophore FCCP, and non-mitochondrial OCR using the electron transport chain inhibitor rotenone. ATP-linked and non-mitochondrial OCR were similar in the peg-Arg I and peg-BSA-treated T-cells, though the peg-Arg I-treated cells did have a slightly lower OCR reserved capacity (Figure 2E). Furthermore, in agreement with the lack of effect of peg-Arg I on mitochondrial respiration, we noticed a similar production of mitochondrial ROS (MitoSOX) and mitochondrial biogenesis (MitoTracker) in activated T-cells cultured with peg-Arg I or peg-BSA (Figure 2F-G). Next, we elucidated whether energy production by mitochondrial respiration is needed for the survival of T-cells to peg-Arg I. Addition of oligomycin resulted in a significant increase of apoptosis in peg-Arg I-treated T-cells, but not in controls (Figure 2H), suggesting the importance of the mitochondrial ATP production in the survival of normal T-cells to peg-Arg I.
L-Arg synthesis from citrulline and T-cell resistance to peg-Arg I
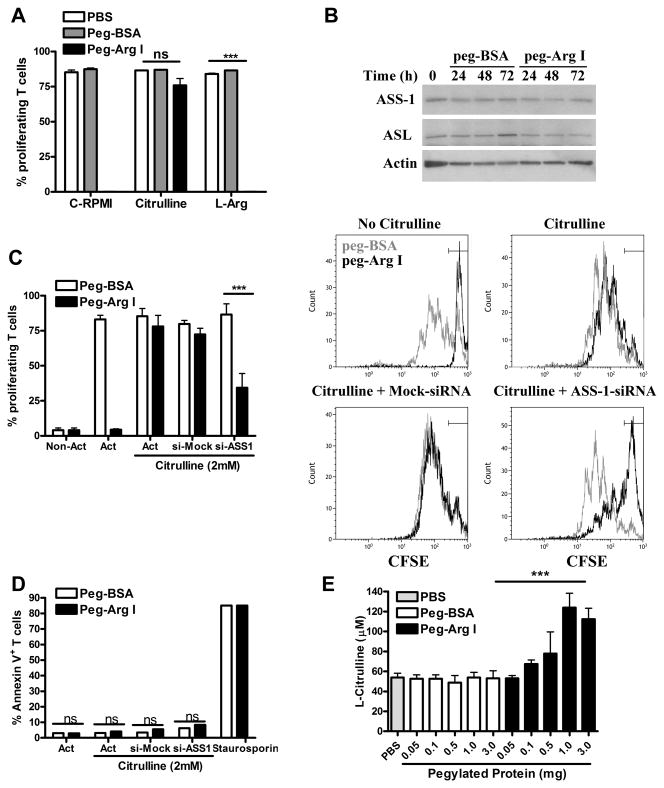
De novo synthesis of L-Arg occurs from citrulline and is reliant upon enzymes ASS-1 and argininosuccinate lyase (ASL) (23). Because complete culture media lacks citrulline, we asked whether the addition of exogenous citrulline or L-Arg rescued the proliferation arrest induced by peg-Arg I. Addition of citrulline, but not L-Arg, restored proliferation of T-cells cultured in the presence of peg-Arg I (Figure 3A). The lack of effect of the supplemented L-Arg can be explained by its immediate metabolism by peg-Arg I (6). Then, we compared the expression of ASS-1 and ASL in activated T-cells cultured with peg-Arg I or peg-BSA. A similar expression of both enzymes was observed in T-cells independent of the activation or the presence of peg-Arg I (Figure 3B). To understand the role of L-Arg synthesis in the proliferation of T-cells cultured with peg-Arg I and citrulline, we silenced the expression of ASS-1 using siRNA (Supplemental Figure 4). Silencing of ASS-1 significantly impaired the ability of peg-Arg I-treated T-cells to proliferate in citrulline-supplemented media (Figure 3C), without resulting in T-cell apoptosis (Figure 3D). Since normal T-cells were resistant to peg-Arg I in the presence of citrulline, we evaluated the availability of citrulline in the serum of peg-Arg I-treated mice. A dose-dependent increase in citrulline levels was noted in the serum of mice treated with peg-Arg I, but not in peg-BSA controls (Figure 3E), indicating the elevation of citrulline as a systemic response to acute L-Arg deprivation in vivo.
Figure 3. Role of L-Arg synthesis in T-cells in the effects induced by peg-Arg I.
(A) Activated CFSE-labelled T-cells were treated with peg-Arg I or peg-BSA (1 μg/mL) in media supplemented with 2 mM citrulline or L-Arg. Percentages of proliferating T-cells were evaluated by flow cytometry at 72 hours. (B) Representative experiment for the expression of ASS-1 and ASL in T-cells cultured in the presence of peg-Arg I or peg-BSA. (C) Activated CFSE-labelled T-cells were transfected with ASS-1 or non-targeting control siRNA (2 μM) +/- peg-Arg I or peg-BSA in media supplemented with citrulline for 72 hours. T-cell proliferation was measured by flow cytometry, with pooled results from three experiments (left) and a representative result (right). (D) T-cells from (C) were tested for annexin V expression by flow cytometry. (E) Serum collected from mice (n=5) treated with peg-Arg I or peg-BSA for 12 hours. Levels of citrulline were established by HPLC. Findings are expressed as mean +/- SEM from 3 experiments. Non-statistical significant differences (ns): p > 0.05. *** p < 0.001.
MDSC mediate anti-proliferative effects of peg-Arg I in vivo
Because the elevation of citrulline in peg-Arg I-treated mice and the ability of T-cells to produce L-Arg from citrulline, we sought to determine whether an indirect mechanism was mediating the impaired T-cell proliferation after peg-Arg I treatment. To test the potential role of MDSC, CD8+ T-cells from CD45.1+ OT-1 mice were adoptively transferred into cohorts of CD45.2+ mice treated with peg-Arg I, peg-BSA, or PBS, which received repeated injections with anti-Gr-1 or control IgG antibodies and were immunized with siinfekl. A significant restoration in OT-1 cell proliferation was detected in peg-Arg I-treated mice receiving anti-Gr-1, but not in those receiving IgG (Figure 4A), suggesting that MDSC regulated T-cell suppression induced by peg-Arg I in vivo. In addition, a dose-dependent accumulation of CD11b+ Gr1+ cells occurred in spleens of mice treated with peg-Arg I, but not in those treated with peg-BSA or PBS (Figure 4B), which correlated with a higher CD11b+ Gr1+ cell proliferation, as tested by BrdU (Figure 4C). Moreover, the increase in MDSC by peg-Arg I treatment was the result of the expansion of granulocytic-MDSC (G-MDSC, CD11b+ Ly6G+ Ly6Clow), but not monocytic-MDSC (M-MDSC, CD11b+ Ly6Gneg Ly6Chigh) (Figure 4D). To test whether the increase in MDSC was also induced by deprivation of other amino acids, we studied the effect of asparagine-metabolizing enzyme peg-asparaginase. A similar increase in G-MDSC, but not M-MDSC, was found in peg-asparaginase-treated mice compared to controls (Supplemental Figure 5), supporting the effect of amino acid starvation on MDSC accumulation.
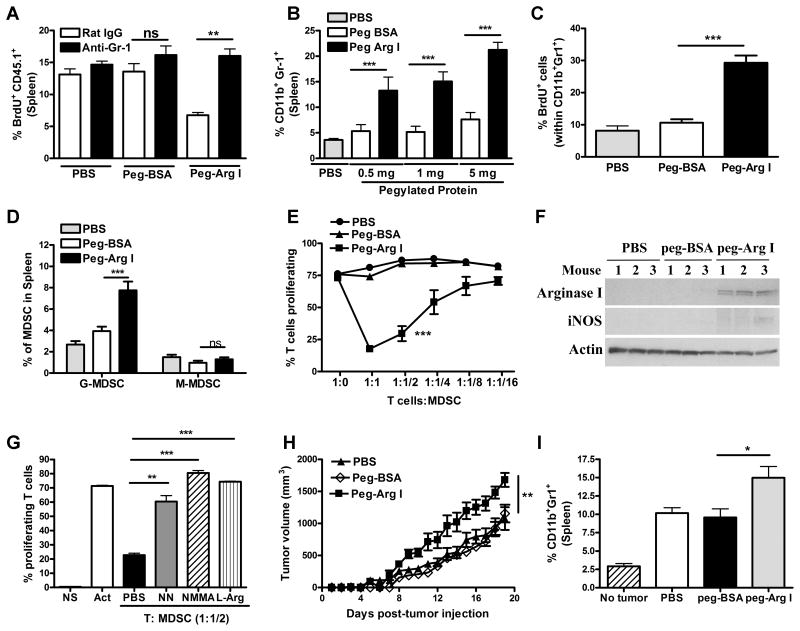
Figure 4. MDSC mediate anti-proliferative effects of peg-Arg I in mice.
(A) Proliferation, as tested by BrdU, in CD45.1+/OT-1 T-cells adoptively transferred into CD45.2+ mice that received peg-Arg I or peg-BSA (1 mg/mouse), immunization with siinfekl, and treatments with anti-Gr-1 antibody or IgG, as described in the Methods (n=10 for each group). (B) Mice (n=10) were injected i.p. with peg-Arg I, peg-BSA, or PBS every 2 days for 7 days. Then, percentages of CD11b+ Gr-1+ were tested in spleens by flow cytometry. (C) Proliferation of MDSC was determined by BrdU, as described in the Methods. (D) Samples from B (0.5 mg/mouse) were tested for G-MDSC (CD11b+ Ly6G+ Ly6Clow) and M-MDSC (CD11b+ Ly6Gneg Ly6Chigh). (E) Proliferation of CFSE-labeled T-cells was measured 72 hours after co-culture with different ratios of splenic CD11b+ Gr-1+ cells from peg-Arg I, peg-BSA or PBS-treated mice. (F) Arginase I and iNOS in splenic CD11b+ Gr-1+ cells from mice treated with peg-Arg I, peg-BSA or PBS. (G) Proliferation of CFSE-labeled T-cells co-cultured with splenic peg-Arg I-induced MDSC (1:1/2 ratio) after addition of NN (200 μM), L-NMMA (500 μM) or L-Arg (2 mM). (H) 1×106 3LL cells were injected s.c. into mice (n=5), followed by peg-Arg I, peg-BSA or PBS injections i.p. (1 mg) every 3 days. (I) CD11b+ Gr-1+ within spleens of peg-Arg I, peg-BSA or PBS-treated 3LL-bearing mice (17 days). Results are expressed as mean +/- SEM from 3 experiments. Non-statistical significant differences (ns): p > 0.05;** p<0.01; *** p < 0.001.
Next, we tested the ability of peg-Arg I-induced CD11b+ Gr1+ cells to block T-cell responses in vitro. CD11b+ Gr1+ cells isolated from peg-Arg I-treated mice, but not from those treated with peg-BSA or PBS, blocked proliferation of activated T-cells in a dose-dependent manner (Figure 4E). In addition, MDSC from peg-Arg I-treated mice displayed increased expression of MDSC-inhibitory protein arginase I (Figure 4F) and higher arginase activity (Supplemental Figure 6). Also, the addition of arginase inhibitor nor-NOHA, iNOS inhibitor L-NMMA, or exogenous L-Arg blocked the increased regulatory effect of peg-Arg I-induced MDSC (Figure 4G), suggesting a role of L-Arg metabolism in the inhibitory function of MDSC induced by peg-Arg I. Next, we tested the effect of peg-Arg I in the growth of 3LL tumors, a model that depends upon MDSC (27, 28). An accelerated tumor growth was found in 3LL-bearing mice treated with peg-Arg I, compared to mice treated with peg-BSA or PBS (Figure 4H), which correlated with higher numbers of MDSC (Figure 4I). Thus, our results support the primary role of MDSC in the T-cell suppression induced by L-Arg starvation in vivo and warrants caution in the use of peg-Arg I as an anti-tumor therapy without targeting MDSC accumulation.
Peg-Arg I-induced MDSC accumulation is mediated by GCN2
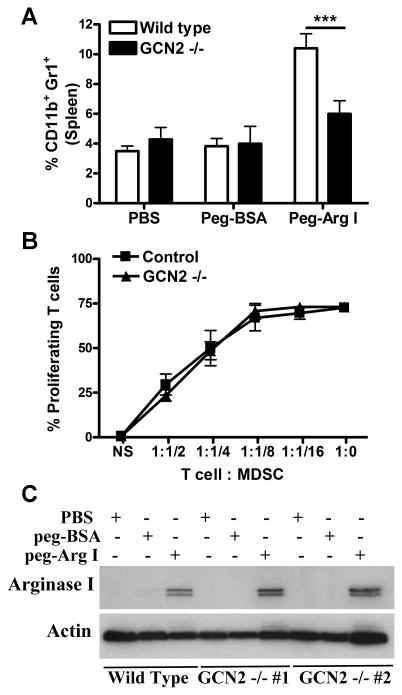
We aimed to determine the molecular mediators by which peg-Arg I induced the accumulation of MDSC. Kinase GCN2 is a key mediator of the effects induced by amino acid starvation (11, 29, 30, 30-33). Therefore, we sought to examine the effect of GCN2 in the MDSC induction by peg-Arg I. An impaired accumulation of MDSC was detected in peg-Arg I-treated GCN2-deficient mice compared to controls (Figure 5A), suggesting the key role of GCN2 in the accumulation of MDSC by peg-Arg I. To determine whether GCN2 also mediated the inhibitory function of peg-Arg I-induced MDSC, we compared on a per cell basis the ability of CD11b+ Gr1+ cells from wild type and GCN2-deficient mice to impair T-cell proliferation and to express arginase I. An identical ability to suppress T-cell proliferation and a similar expression of arginase I were found in sorted MDSC from wild type and GCN2-deficient mice treated with peg-Arg I (Figure 5B-C), suggesting that GCN2 is involved in the accumulation of MDSC, but not in the per-cell function of peg-Arg I-induced MDSC.
Figure 5. Peg-Arg I induced MDSC accumulation is mediated by GCN2.
(A) Wild type and GCN2-deficient mice were injected i.p. with 1 mg of peg-Arg I, peg-BSA, or PBS every 2 days for 7 days. Then, splenic CD11b+ Gr+ cells were determined by flow cytometry. (B) Proliferation of CFSE-labeled T-cells after co-culture with different ratios of splenic MDSC from control or GCN2-/- peg-Arg I-treated mice. (C) Arginase I in splenic CD11b+ Gr+ cells from wild type or GCN2-/- mice treated with peg-Arg I, peg-BSA, or PBS. Results are expressed as mean +/- SEM from 3 experiments. *** p < 0.001.
Discussion
Previous studies established the therapeutic benefit of peg-Arg I in various pre-clinical malignancies (6-10). Moreover, peg-Arg I is undergoing evaluation in phase I/II trials for adults with liver carcinoma, with favorable safety profiles (34). Because L-Arg depletion is also an important mechanism for suppression of T-cell responses in tumors (22), we sought to reconcile these observations by conducting a detailed analysis of the effects of peg-Arg I on normal T-cells. Our findings show the regulatory effect of peg-Arg I on T-cell proliferation and the ability of T-cells to resist peg-Arg I through L-Arg synthesis. In addition, we show the new role of MDSC in the inhibition of T-cell responses by L-Arg starvation.
Our findings showed the ability of T-cells to synthesize L-Arg de novo and indicated the elevation of serum citrulline as a systemic response to acute depletion of L-Arg. The increased citrulline synthesis occurring under systemic low arginine levels has been shown to be mediated by enterocytes using diet-based glutamine (35-39). Also, in agreement with the role of citrulline after L-Arg deprivation, injection of citrulline ameliorated toxic effects after arginase therapy (40). Although our data suggest the role of L-Arg synthesis in the effects induced by peg-Arg I, we could not specifically test the effect of citrulline on T cells or MDSC from peg-Arg I-treated mice because the lack of pharmacological inhibitors against ASS-1 or ASL, the toxicity of ASS-1 deletion in mice (41, 42), and the unavailability of conditional ASS-1 or ASL deficient mice.
Peg-Arg I blocked malignant T-cell proliferation through the induction of apoptosis (6), while it failed to trigger cell death in normal T-cells. Survival of normal T-cells to peg-Arg I was mediated by ATP production in the mitochondria. The lack of this adaptive pathway in malignant cells could explain the differences in apoptosis observed in malignant vs. normal T-cells treated with peg-Arg I. In fact, tumor cells are mostly dependent on aerobic glycolysis, while normal cells retain the ability to sense stress and maintain survival through mitochondrial-mediated pathways (43). Our results also showed that peg-Arg I did not impair activation processes or mitochondrial respiration in T-cells. However, the role of T-cell activation in the low proliferation induced by peg-Arg I is still unknown, in part because of the difficulty to separating proliferation and activation processes. Previous research showed the importance of fatty acid oxidation and mitochondrial respiration on the proliferation of regulatory T-cells (2). Therefore, it is possible that regulatory T-cells are less susceptible to peg-Arg I, which could also contribute to the tolerogenic effects induced by L-Arg starvation.
We found that in addition to the potential suppression of T-cells by MDSC-mediated L-Arg deprivation, L-Arg starvation also promotes accumulation of MDSC, further suppressing anti-tumor T-cell responses. Accordingly, peg-Arg I mimicked T-cell suppression induced by physical injury, a process also mediated by MDSC (16). Although immune suppression could be a major limitation for the use of peg-Arg I as a therapy in cancer, it may also lead to novel therapies in transplantation and autoimmune diseases. In these settings, peg-Arg I could maintain a permanent influx of MDSC to provide T-cell suppression. As such, peg-Arg I prolonged mice survival after bone marrow transplantation and prevented development of GVHD similar to mice receiving adoptive transfer of MDSC (15). With regards to the use of peg-Arg I in different cancers, peg-Arg I could be combined with chemotherapy agents that block MDSC accumulation and function, such as gemcitabine (44), 5-fluorouracil (45), and sunitinib (46). Furthermore, we found a role of GCN2 in the accumulation of MDSC in peg-Arg I-treated mice. Accordingly, previous studies indicated the role of GCN2 in the immune effects induced by L-asparaginase (30, 47). However, the intracellular mechanisms by which the depletion of L-Arg and its sensing pathways promote MDSC accumulation remain unknown. Possible pathways could include the induction of factors that promote MDSC accumulation, including vascular endothelial growth factor (VEGF), S100A8/A9, granulocyte-macrophage colony stimulating factor (GM-CSF), and granulocyte colony stimulating factor (G-CSF). Also, in agreement with the role of cellular stress in the regulation of myeloid cells function and accumulation, recent studies showed the effect of L-Arg starvation on macrophage responses to infections (42) and the specific role of stress mediators in the induction of VEGF, arginase I (48), and MDSC accumulation (49) in tumors.
In summary, our results show for the first time the role of MDSC in the suppression of T-cell function caused by L-Arg starvation. This increases the understanding of the effects of L-Arg deprivation in the regulation of anti-tumor immunity and suggests caution for the use of peg-Arg I in malignancies without targeting MDSC accumulation.
Supplementary Material
Acknowledgments
We are very thankful to Dr. Arnold Zea, PhD (Stanley S. Scott Cancer Center HPLC-facilities) for testing the citrulline levels in the sera from treated mice.
Financial support: This work was partially supported by Hope on wheels Hyundai award to MF; National Institutes of Health (NIH) grant P20GM103501 subproject #3 and NIH-R21CA162133 to P.C.R.; and R01OD016990, R01CA107974, and U54GM104940 to ACO.
Abbreviations
- L-Arg
L-Arginine
- MDSC
Myeloid-derived suppressor cells
- peg-Arg I
Pegylated Arginase I
- peg-BSA
Pegylated bovine serum albumin
Footnotes
Authorship contributions: MF, MER: Planned, developed, and analyzed experiments. Wrote manuscript
RAS, PR, PT, AAA, DS, CH, DDW: Performed experiments.
ACO: Provide reagents and advise for experiments.
PCR: Planned, developed, and analyzed experiments. Wrote manuscript.
Disclosure of conflict of interest: Authors do not have conflict of interest to disclose.
Reference List
- 1.Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014;28:1784–1792. doi: 10.1038/leu.2014.108. [DOI] [PubMed] [Google Scholar]
- 2.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips MM, Sheaff MT, Szlosarek PW. Targeting Arginine-Dependent Cancers with Arginine-Degrading Enzymes: Opportunities and Challenges. Cancer Res Treat. 2013;45:251–262. doi: 10.4143/crt.2013.45.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szlosarek PW, Luong P, Phillips MM, Baccarini M, Stephen E, Szyszko T, et al. Metabolic response to pegylated arginine deiminase in mesothelioma with promoter methylation of argininosuccinate synthetase. J Clin Oncol. 2013;31:e111–e113. doi: 10.1200/JCO.2012.42.1784. [DOI] [PubMed] [Google Scholar]
- 5.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez CP, Morrow K, Lopez-Barcons LA, Zabaleta J, Sierra R, Velasco C, et al. Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood. 2010;115:5214–5221. doi: 10.1182/blood-2009-12-258822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, et al. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67:309–317. doi: 10.1158/0008-5472.CAN-06-1945. [DOI] [PubMed] [Google Scholar]
- 8.Lam TL, Wong GK, Chow HY, Chong HC, Chow TL, Kwok SY, et al. Recombinant human arginase inhibits the in vitro and in vivo proliferation of human melanoma by inducing cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2011;24:366–376. doi: 10.1111/j.1755-148X.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 9.Tsui SM, Lam WM, Lam TL, Chong HC, So PK, Kwok SY, et al. Pegylated derivatives of recombinant human arginase (rhArg1) for sustained in vivo activity in cancer therapy: preparation, characterization and analysis of their pharmacodynamics in vivo and in vitro and action upon hepatocellular carcinoma cell (HCC) Cancer Cell Int. 2009;9:9. doi: 10.1186/1475-2867-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal V, Woo JH, Mauldin JP, Jo C, Stone EM, Georgiou G, et al. Cytotoxicity of human recombinant arginase I (Co)-PEG5000 in the presence of supplemental L-citrulline is dependent on decreased argininosuccinate synthetase expression in human cells. Anticancer Drugs. 2012;23:51–64. doi: 10.1097/CAD.0b013e32834ae42b. [DOI] [PubMed] [Google Scholar]
- 11.Morrow K, Hernandez CP, Raber P, Del VL, Wilk AM, Majumdar S, et al. Anti-leukemic mechanisms of pegylated arginase I in acute lymphoblastic T-cell leukemia. Leukemia. 2013;27:569–577. doi: 10.1038/leu.2012.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez PC, Hernandez CP, Morrow K, Sierra R, Zabaleta J, Wyczechowska DD, et al. L-arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression. J Immunol. 2010;185:5198–5204. doi: 10.4049/jimmunol.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, et al. l-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Pribis JP, Rodriguez PC, Morris SM, Jr, Vodovotz Y, Billiar TR, et al. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg. 2014;259:171–178. doi: 10.1097/SLA.0b013e31828611f8. [DOI] [PubMed] [Google Scholar]
- 17.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 19.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green DR, Rathmell J. Sweet nothings: sensing of sugar metabolites controls T cell function. Cell Metab. 2013;18:7–8. doi: 10.1016/j.cmet.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peranzoni E, Marigo I, Dolcetti L, Ugel S, Sonda N, Taschin E, et al. Role of arginine metabolism in immunity and immunopathology. Immunobiology. 2007;212:795–812. doi: 10.1016/j.imbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of Myeloid-Derived Suppressor Cells (MDSC) impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2013 doi: 10.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo G, Marrero L, Rodriguez P, Del VL, Ochoa A, Cui Y. Trp53 inactivation in the tumor microenvironment promotes tumor progression by expanding the immunosuppressive lymphoid-like stromal network. Cancer Res. 2013;73:1668–1675. doi: 10.1158/0008-5472.CAN-12-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem. 2009;284:32742–32749. doi: 10.1074/jbc.M109.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 32.Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, et al. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med. 2012;4:118ra11. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Yau T, Cheng PN, Chan P, Chan W, Chen L, Yuen J, et al. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2013;31:99–107. doi: 10.1007/s10637-012-9807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugan ME, Knabe DA, Wu G. The induction of citrulline synthesis from glutamine in enterocytes of weaned pigs is not due primarily to age or change in diet. J Nutr. 1995;125:2388–2393. doi: 10.1093/jn/125.9.2388. [DOI] [PubMed] [Google Scholar]
- 36.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974;249:5070–5079. [PubMed] [Google Scholar]
- 37.Windmueller HG. Glutamine utilization by the small intestine. Adv Enzymol Relat Areas Mol Biol. 1982;53:201–237. doi: 10.1002/9780470122983.ch6. [DOI] [PubMed] [Google Scholar]
- 38.Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772–776. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 39.Ligthart-Melis GC, van de Poll MC, Vermeulen MA, Boelens PG, van den Tol MP, van SC, et al. Enteral administration of alanyl-[2-(15)N]glutamine contributes more to the de novo synthesis of arginine than does intravenous infusion of the dipeptide in humans. Am J Clin Nutr. 2009;90:95–105. doi: 10.3945/ajcn.2008.26399. [DOI] [PubMed] [Google Scholar]
- 40.Mauldin JP, Zeinali I, Kleypas K, Woo JH, Blackwood RS, Jo CH, et al. Recombinant human arginase toxicity in mice is reduced by citrulline supplementation. Transl Oncol. 2012;5:26–31. doi: 10.1593/tlo.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patejunas G, Bradley A, Beaudet AL, O'Brien WE. Generation of a mouse model for citrullinemia by targeted disruption of the argininosuccinate synthetase gene. Somat Cell Mol Genet. 1994;20:55–60. doi: 10.1007/BF02257486. [DOI] [PubMed] [Google Scholar]
- 42.Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, DeFreitas AA, et al. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe. 2012;12:313–323. doi: 10.1016/j.chom.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 46.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 47.Bunpo P, Cundiff JK, Reinert RB, Wek RC, Aldrich CJ, Anthony TG. The eIF2 kinase GCN2 is essential for the murine immune system to adapt to amino acid deprivation by asparaginase. J Nutr. 2010;140:2020–2027. doi: 10.3945/jn.110.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.