Abstract
Background
Longitudinal studies have implicated both marital distress and depression in the development of the metabolic syndrome, a risk factor for diabetes and cardiovascular disease. This study addressed the impact of hostile marital interactions and a mood disorder history on obesity-related metabolic responses to high-fat meals.
Methods
This double-blind, randomized crossover study included serial assessments of resting energy expenditure (REE), fat and carbohydrate oxidation, triglycerides, insulin, glucose, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) before and after two high-fat meals. During two separate 9.5 hour visits, 43 healthy married couples, ages 24 to 61 (mean=38.22), received either a high saturated fat meal or a high oleic sunflower oil meal, both 930 kcal and 60 g fat. The Structured Diagnostic Interview for DSM-IV assessed mood disorder history. Couples discussed a marital disagreement during both visits; behavioral coding of these interactions provided data on hostile marital behaviors.
Results
Men and women who displayed more hostile behaviors and who also had a mood disorder history had significantly lower post-meal REE, higher insulin, and higher peak triglyceride responses than other participants, with nonsignificant effects for fat and carbohydrate oxidation. Participants with a mood disorder history had a steeper rise in postprandial IL-6 and glucose than those without a past history. Higher levels of hostile behaviors were associated with higher post-meal TNF-α. The two meals did not differ on any outcome assessed.
Conclusions
People spend about 18 of every 24 hours in a postprandial state, and dining with one’s partner is a common daily event. Among subjects with a mood disorder history, the cumulative 6.75-hour difference between high and low hostile behaviors translates into 128 kcal, a difference that could add 7.6 pounds/year. Our findings illustrate novel pathways through which chronic marital stress and a mood disorder history synergistically heighten the risk for obesity, metabolic syndrome, and cardiovascular disease.
Keywords: marriage, depression, resting energy expenditure, triglycerides, insulin, inflammation
1. INTRODUCTION
A turbulent marriage can have a substantial impact, including a three-fold heightened risk for serious coronary events and a three-fold increased likelihood of developing metabolic syndrome (Orth-Gomer et al., 2000; Troxel et al., 2005). Troubled marriages also take a toll on mental health (Rehman et al., 2008; Beach, 2014); for example, unhappy marriages are a potent risk factor for major depressive disorder, associated with a 25-fold increase relative to untroubled marriages (Weissman, 1987).
The amplified risk for depression in distressed marriages is important because depression and chronic stress promote obesity (Raikkonen et al., 2007; Luppino et al., 2010), which contributes to a host of medical problems, including cardiovascular disease, diabetes, and metabolic syndrome. Indeed, depressed people have a 58% increased risk of becoming obese (Luppino et al., 2010). In a large prospective study, older depressed adults gained visceral fat over five years, while nondepressed adults lost visceral fat (Vogelzangs et al., 2008). Longitudinal studies have implicated both depression and marital discord in the development of the metabolic syndrome, which has abdominal obesity as its cornerstone (Troxel et al., 2005; Whisman et al., 2010; Pan et al., 2012; Whisman and Uebelacker, 2012).
During stressful times many people turn to calorie-dense high-fat “comfort” food (Tomiyama et al., 2011), while others may eat less (Dallman, 2010). Rodent studies have shown that stressors can alter energy expenditure as well as fat and carbohydrate metabolism (Moles et al., 2006; Laugero, 2008; Dallman, 2010), and these changes could provide another route to obesity—particularly if they occurred when people were turning to comfort foods.
In accord with the rodent data, we recently demonstrated that greater numbers of prior daily stressors were associated with obesity-promoting metabolic responses to high-fat meals the following day, including lower post-meal resting energy expenditure, lower fat oxidation, and higher insulin (Kiecolt-Glaser et al., 2014). Furthermore, women with a history of depression who also had experienced more prior day stressors had a higher peak postprandial triglyceride response than other participants (Kiecolt-Glaser et al., 2014).
The current study extends our prior work in several notable ways. The average age of the 58 participants in our first study was 53, all were women, and two-thirds were breast cancer survivors (Kiecolt-Glaser et al., 2014) and thus the generalizability of our findings was unclear. Furthermore, in our prior study stressors were self-reported whereas the current study included observational data from two marital interactions.
This study provided the opportunity to examine the impact of an acute stressor, marital conflict, on healthy men and women's metabolic responses to high-fat meals. Higher levels of hostile and negative behavior during marital conflict discussions are strongly related to lower marital satisfaction, a consistent finding in the marital literature (Robles et al., 2014). However, our primary focus was on hostile behavior rather than self-reported satisfaction because of the evidence that objectively measured marital behaviors explain more of the variance in physiological outcomes (Kiecolt-Glaser and Newton, 2001). Based on this rationale, we assessed the joint impact of hostile marital behavior and a mood disorder history on post-meal responses.
Metabolic processes that influence weight regulation and fat storage were our central focus. Resting energy expenditure plays a key role in energy balance and weight control, accounting for 65% to 75% of the total daily energy expenditure; lower daily energy expenditure increases risk for weight gain (Lara et al., 2010). Metabolism of macronutrients, primarily fats and carbohydrates, also influences weight regulation (Flatt, 2012), and lower fat oxidation clearly facilitates weight gain over time (Blaak et al., 2006). Higher levels of insulin stimulate food intake and visceral fat accumulation (Dallman, 2010). Triglycerides are the major form of fat storage in the body. Acute stress can transiently increase triglyceride concentrations and slow triglyceride clearance (Stoney et al., 2002). The magnitude and duration of the postprandial triglyceride response is linked with the progression of atherosclerosis (Boquist et al., 1999; Teno et al., 2000; Pollin et al., 2008).
Earlier dietary studies suggested that ingestion of high-fat meals raises systemic inflammatory responses, with high saturated fat meals in particular promoting greater or more sustained inflammatory responses (Nappo et al., 2002). Accordingly we contrasted a meal high in saturated fatty acids with a meal high in monounsaturated fatty acids, and we assayed IL-6 and TNF-α. However, the serum cytokine changes described in early papers have not been replicated in subsequent studies (Poppitt et al., 2008; Herieka and Erridge, 2014), and thus no meal-related cytokine differences were expected in this project.
We hypothesized that both hostile marital behaviors and a mood disorder history would be associated with lower post-meal energy expenditure and fat oxidation. We investigated whether marital behavior and a mood disorder history predicted post-meal triglycerides, insulin, and inflammation, based on evidence suggesting that marital distress, stressors and a depression history can have adverse effects on each of these dimensions (Stoney et al., 2002; Kiecolt-Glaser et al., 2005; Dallman, 2010; Aschbacher et al., 2014; Kiecolt-Glaser et al., 2014). We also examined whether self-reported marital distress mirrored the marital behavior data as would be expected from other literature (Robles et al., 2014).
2. METHODS
2.1 Design and overview
This double-blind, randomized crossover study assessed metabolic responses following high-fat meals. Couples completed an online screening questionnaire and an in-person screening visit. Eligible couples received one high saturated fat meal and one high oleic sunflower oil meal at the beginning of two separate full-day visits to the Clinical Research Center (CRC), a hospital research unit, with the meal order randomized. Participants were required to consume the entire meal, and both members of a couple received the same meal within a single visit. Visits occurred 1-25 weeks apart (mean=4.45, SD=4.76). Although 55% of visits occurred within 3 weeks, some were more widely spaced as a consequence of participants’ work schedules.
After fasting for 12 hours, couples arrived at the CRC and a catheter was inserted in their arm. Following the 25-minute baseline metabolic measurement, couples had 20 minutes to eat their entire meal; all participants were able to consume the full meal within 20 minutes. To maximize the postprandial metabolic impact, the marital problem discussion was introduced two hours post-meal, when triglycerides were expected to begin peaking. Couples remained in the CRC for ~7 hours after meal completion without further food, only water.
Glucose and insulin were sampled before the meal, and post-meal at 45 minutes, 1.5 hours, 2 hours, 2.5 hours, and then hourly (Lairon et al., 2007). Triglycerides were assessed before the meal and then hourly thereafter (Lairon et al., 2007). Serum interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) were assessed before the meal and then every 2 hours post- meal. Metabolic data were obtained during a 25-minute resting baseline and then 20 minutes out of every hour thereafter (Kiecolt-Glaser et al., 2014).
2.2 Participants
Using print and web-based announcements, we recruited 43 healthy couples, ages 24-61, who had been married at least 3 years. Individuals were ineligible if they or their partner had any notable chronic health problems. Other exclusions included smoking, alcohol/drug abuse, an HbA1C > 6.5, anemia, and any prescription medications except birth control pills (N=5) and levothyroxine (N=3). Our exercise criteria were a maximum of 2 hours of vigorous activity per week for BMI < 25 and 5 hours per week for BMI ≥ 25. We prioritized recruitment of unhappy couples as well as heavier sedentary individuals to maximize the likelihood of stress-related metabolic responses. When potential participants applied online, they completed the 16-item version of the Couples Satisfaction Index; the full version was given at the end of the in-person screening (Funk and Rogge, 2007). Happier and thinner couples were overrepresented among applicants, consistent with the evidence that recruitment of unhappy couples is a challenge for marital research in general. Accordingly, in terms of both inclusion and scheduling we prioritized unhappy and overweight participants to represent the full range of interest. We spent considerable time and effort to recruit people who were healthy but overweight; a total of 350 interested individuals were excluded because either they or their spouse did not meet our stringent health criteria. Table 1 lists additional sample characteristics. The institutional review board approved this study, and each participant provided written informed consent before participation.
Table 1.
Participant Characteristics
| Men (N= 43) | Women (n=43) | Overall Sample (n=86) |
|
|---|---|---|---|
| Age, years | 39.25 (9.17) | 37.19 (7.00) | 38.22 (8.18) |
| BMI, kg/m2 | 31.96 (5.06) | 32.17 (6.58) | 32.07 (5.83) |
| Waist, cm | 106.71 (14.72) | 99.14 (13.63) | 102.93 (14.61) |
| Trunk fat, g | 19502.14 (7761.57) |
19375.02 (7382.91) |
19438.58 (7530.19) |
| Activity, hours per week | 3.52 (5.09) | 1.86 (2.00) | 2.70 (3.95) |
| Systolic blood pressure, mmHg | 127.12 (12.18) | 111.67 (12.30) | 119.40 (14.44) |
| Diastolic blood pressure, mmHg | 76.00 (7.21) | 67.72 (8.22) | 71.85 (8.74) |
| Total Cholesterol, mg/dL | 171.95 (27.31) | 170.84 (32.46) | 171.40 (29.82) |
| HDL, mg/dL | 41.81 (7.76) | 46.30 (9.44) | 44.06 (8.88) |
| LDL, mg/dL | 104.90 (24.34) | 101.69 (27.92) | 103.29 (26.09) |
| Fasting triglyceride, mg/dL | 126.30 (57.69) | 114.72 (54.24) | 120.51 (55.97) |
| Fasting glucose, mg/dL | 94.69 (6.05) | 94.48 (7.68) | 94.58 (6.87) |
| Years married | 11.49 (6.64) | ||
| Race | |||
| White | 35 | 35 | 70 |
| Black | 8 | 8 | 16 |
| Education | |||
| Graduate degree | 17 | 20 | 37 |
| College graduate | 13 | 8 | 21 |
| Partial college | 6 | 10 | 16 |
| High school graduate | 5 | 5 | 10 |
| ≤ 11 years high school | 2 | 0 | 2 |
Data shown are mean (SD) or N.
2.3 Standardized pre-study activity and meals
On the day before each of the two study visits, couples received three standardized meals from the CRC’s kitchen to reduce the variability associated with recent food intake. Equations from the Dietary Reference Intakes were used to determine total kcal requirements for each participant based on sex, age, height, weight, and physical activity (2002). Macronutrient targets were 54.9 ± 2.68% carbohydrate, 27.6 ±2.13% fat, and 17.6 ± 0.95% protein. Participants’ last meals, eaten no later than 7:30 PM the night before admission, were light and low in fat (Lairon et al., 2007). Compliance was good: participants consumed 91.18 ± 8.62% of these meals.
2.4 Research meals
Both research meals included eggs, turkey sausage, biscuits, and gravy for a total of 930 kcals, with 60 grams fat, 59 grams carbohydrate, and 36 grams protein (percent of total kcals = 60, 25, 15, respectively). However, following prior research (Poppitt et al., 2008), the saturated:unsaturated fatty acid ratio varied between the meals; the high saturated fat meal contained 16.84 g palmitic and 13.5 g oleic (ratio=1.93), compared to 8.64 g palmitic and 31.21 g oleic for the high oleic sunflower oil meal (ratio=0.67).
As noted earlier, some studies had suggested that high saturated fat meals promoted inflammation (Manning et al., 2008). For example, palmitic acid, the saturated fat chosen for our meal, can stimulate production of IL-6 and TNF-α by adipocytes and adipose tissue macrophages (Ajuwon and Spurlock, 2005; Suganami et al., 2005). Thus, when we designed the study, we were interested in the possibility that the saturated fat meal might interact with stress and depression to fuel inflammation.
2.5 Metabolic data: Resting energy expenditure (REE)
Long-term weight maintenance or energy balance requires that caloric intake equals calories burned. Resting energy expenditure plays a key role in energy balance and weight control, accounting for 65% to 75% of the total daily energy expenditure (Lara et al., 2010). Lower daily energy expenditure increases risk for weight gain and obesity. Weight change prediction models that incorporate metabolic adaptation were used to project the impact of the differences in energy expenditure (Hall et al., 2012).
Metabolic data were obtained using indirect calorimeters (Ultima CPX and CCM Express, MedGraphics, St. Paul, MN). Inspired and expired airflow of oxygen and carbon dioxide were measured after the couples had rested for 30 minutes in a thermoneutral room in adjacent beds. During the 25-minute resting metabolic rate (RMR) measurement, participants reclined at a 30- degree angle and remained still but awake, following standard procedures (Reeves et al., 2004; Kiecolt-Glaser et al., 2014). The same procedure was followed for all subsequent measurements.
2.6 Macronutrient oxidation rates
In addition to resting energy expenditure, metabolism of macronutrients, primarily fats and carbohydrates, also influences weight regulation (Flatt, 2012), and lower fat oxidation rate clearly facilitates weight gain over time (Blaak et al., 2006). Accordingly, fat and carbohydrate oxidation (g/min) were calculated from VO2 and VCO2 using the Weir formulas (Weir, 1949). Adjustments for the protein respiratory quotient were based on estimations of urinary urea nitrogen using the protein consumption from the prior day’s standardized meals (Simonson and DeFronzo, 1990).
2.7 Body Composition
Body composition was assessed by dual x-ray absorptiometry (DXA) (Kennedy et al., 2009). DXA data provided a way to assess both lean body mass, which explains 70-80% of the variance in resting metabolic rate (Nelson et al., 1992), and trunk fat, which contributes to adverse metabolic meal-related responses and obesity-related disease risk (Kennedy et al., 2009).
2.8 Interview data
The mood disorder modules of the Structured Clinical Interview-Nonpatient Version for DSM-IV, nonpatient version (SCID-NP) provided data on lifetime prevalence (First et al., 1996). Interviews were administered by trained clinical psychology graduate students or staff. Consensus meetings reviewed the recorded interviews to obtain diagnoses. SCID data demonstrated that 16 people met criteria for a past mood disorder (MDD=13, and 1 each for depression NOS, bipolar, and dysthymia). Average time since diagnosis was 7.95 years (SD=6.27). Two currently met criteria (1 MDD, 1 dysthymia).
Three 24-hour dietary recalls, administered via telephone, used the USDA Multiple Pass Approach method to assess food intake (Moshfegh et al., 2008). Data, averaged across the interviews, included one weekend day.
2.9 Questionnaires
The 32-item Couples Satisfaction Index (CSI) assessed marital satisfaction (Funk and Rogge, 2007). Developed using item response theory, the CSI can discriminate well between satisfied and dissatisfied couples with greater precision than the most commonly used marital scales (Funk and Rogge, 2007).
During each CRC admission couples completed the widely-used Center for Epidemiological Studies Depression Scale (CES-D) to assess depressive symptoms (Radloff, 1977). Studies have shown acceptable test-retest reliability and excellent construct validity.
The Positive and Negative Affect Schedule (PANAS) provided affect change data during each admission (Watson et al., 1988). The two scales are largely uncorrelated, and show good convergent and discriminant validity when related to state mood scales and other variables (Watson et al., 1988).
2.10 Marital problem discussion
Hostile marital behavior predicts couples’ physiological changes more reliably than self-reports (Kiecolt-Glaser and Newton, 2001). To obtain behavioral data, the experimenter first conducted a 10-20 minute interview to identify the best discussion topics (Kiecolt-Glaser and Newton, 2001; Kiecolt-Glaser et al., 2005), based on each spouse’s Relationship Problem Inventory ratings (Knox, 1971). Couples were then asked to discuss and try to resolve one or more marital issues that the interviewer judged to be the most conflict-producing, e.g., money, communication, or in-laws. The research team remained out of sight while videotaping the subsequent 20-minute problem discussion.
Marital interaction tapes were coded using the Rapid Marital Interaction Coding System (RMICS) which discriminates well between distressed and nondistressed couples (Heyman, 2004). Distressed marriages are characterized by negative affect, conflictual communication, and poor listening skills (Kiecolt-Glaser and Newton, 2001; Kiecolt-Glaser et al., 2005; Robles et al., 2014). Accordingly, the composite index summed four RMICS codes: psychological abuse (e.g., disgust, contempt, belligerence, as well as nonverbal behaviors like glowering), distress- maintaining attributions (e.g., “You're only being nice so I'll have sex with you tonight” or “You were being mean on purpose”), hostility (e.g., criticism, hostile voice tone, or rolling the eyes dramatically) and withdrawal (behaviors that suggest pulling back from the interaction or not listening).
Holley and Gilford’s G was used to quantify inter-rater agreement for the RMICS hostility composite (Holley and Guilford, 1964; Xu and Lorber, 2014). Interrater agreement was high, with a G index of 0.88.
2.11 Assays
IL-6 and TNF-α were multiplexed and measured using an electrochemilluminescence method with Meso Scale Discovery kits, and read using the Meso Scale Discovery Sector Imager 2400. Each subject’s stored samples were assayed for all the cytokine markers in one run, thus using the same controls for all time points (Kiecolt-Glaser et al., 2012). Sensitivity for these serum cytokines was 0.3 pg/ml. The intra-assay coefficient of variation for IL-6 was 3.42%, and the inter-assay coefficient of variation was 8.425%; corresponding values for TNF-α were 2.59% and 8.14%. Use of a catheter can boost IL-6’s normal diurnal rise across the day (Gudmundsson et al., 1997; Vgontzas et al., 2004).
Circulating triglycerides rise within an hour of a high fat meal and can remain elevated for 5 to 8 hours (Lairon et al., 2007). Triglycerides were assayed by the hospital’s clinical laboratory using the standard procedures employed for patient samples.
Higher levels of insulin are lipogenic, enhancing fat storage (Dallman, 2010). The link between stress and increased abdominal obesity may be linked to the responsiveness of abdominal fat tissue to insulin and cortisol (Dallman, 2010). Insulin serum samples were analyzed using chemilluminescence methodology on the Immulite 1000 (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Sensitivity for this assay is 2μIU/ml. Intra-assay coefficient of variation is 5.7% and inter-assay coefficient variation is 6.7%.
Glucose serum samples were analyzed using the Dimension Xpand Clinical Chemistry System (Siemens Medical Diagnostics, Decatur, Ga.). The analytical sensitivity is 1 mg/dL. The intra-assay variability is 0.43%.
2.12 Statistical methods
The primary analytic approach utilized linear mixed models, which allowed explicit modeling of the within-subject correlations both across visits and within a visit, as well as accounting for the clustering of spouses. Specifically, we included random subject-specific meal effects that were allowed to be correlated and a couple-specific random intercept. Cytokine models also included a random effect for assay plate. Triglyceride, insulin, glucose, IL-6, and TNF-α data were natural-log (ln) transformed to better approximate normality of residuals. Exploratory data analyses revealed that the post-meal trajectories of energy expenditure, carbohydrate and fat oxidation, insulin, glucose, IL-6, and TNF-α were approximately linear. Accordingly, we included hours since the meal as a linear fixed effect for these models. The post-meal triglyceride data increased for the first 3-4 hours followed by a decline until the end of the measurement period. Thus, the triglyceride model included a quadratic effect of hours post-meal. There was no hours effect for negative affect as only a single measurement (immediately post-conflict) was used.
To evaluate the impact of marital distress and mood disorder history on post-meal responses, we included a three-way interaction of couples’ hostile behavior by mood disorder history by post-meal hours, along with all lower-order interaction terms. Non-significant interactions (p>0.05) were removed in constructing the final model for each outcome. Marital behavior, as measured by the composite hostile behavior scores, was highly correlated across visits (Spearman r=0.77, p<0.0001) and within couples (Spearman r=0.81, p<0.0001), and thus the dyad’s hostile behavior sum was averaged across visits for use as a predictor in our analyses.
All models controlled for the baseline (pre-meal) measurement of the corresponding outcome by including it as a fixed effect. The interaction of meal type (high oleic sunflower oil vs. high saturated fat) by hours post-meal was included in all models to properly account for the study design (regardless of significance), though it was not of primary interest. All models controlled for age, trunk fat, physical activity, and sex to guard against potential confounding (all measured at the screening visit except trunk fat, which was measured at the first visit). The model for energy expenditure additionally controlled for lean body mass, and the models for carbohydrate oxidation and fat oxidation also controlled for HOMA insulin resistance. To facilitate interpretation of significant interactions involving marital distress, a continuous predictor, we estimated and present post-meal slopes for people with a hostile composite of 21.5 (75th percentile, “higher hostile behavior”) and for people with a hostile composite of 5.5 points (25th percentile, “lower hostile behavior”). The cumulative effect of marital hostility and history of mood disorder on energy expenditure was estimated by calculating the area under the post-meal trajectories (area under the curve with respect to ground) predicted by the mixed model, from the first post-meal time point (15 minutes) to the last (7 hours). The Kenward-Roger degrees of freedom adjustment was used to control type I error (Kenward and Roger, 1997). All analyses were conducted in SAS version 9.3 (Cary, NC).
In the analyses there were five subjects who only contributed data for one visit: one couple who did not have a second visit and three subjects who were sick at one visit. One subject had to be excluded from all analyses (both visits) due to missing physical activity data, a controlling variable in all models. There was sporadic missingness in outcomes measured across the day, but the analysis method (mixed models) allowed subjects with these occasionally missing measurements to be included in analyses.
3. RESULTS
Complete model results are available in the online Supplement, eTables 1-3, for all of the primary analyses, including estimates of within-couple correlations for all outcomes. The Supplement also includes ancillary analyses addressing self-reported marital quality, sleep, typical diet, and blinding. The two meals did not differ on any of the outcomes we assessed, consistent with prior work (Kiecolt-Glaser et al., 2014).
3.1 Primary analyses
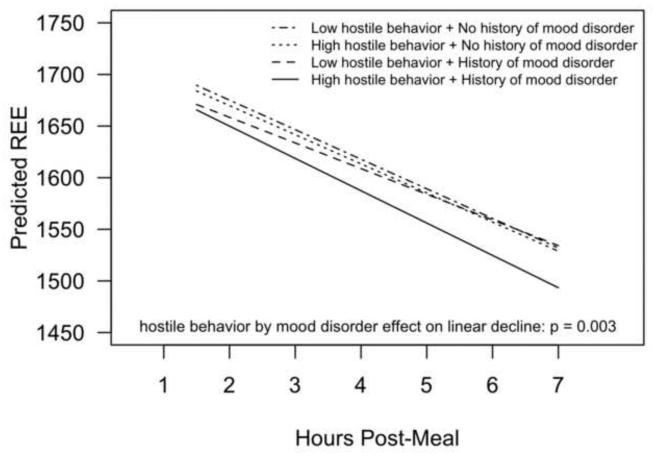
There was a significant three-way hostile behavior by mood disorder history by hours post-meal interaction (p=0.003) predicting postprandial energy expenditure. Participants with a mood disorder history who also had more hostile marital interactions had the steepest postprandial decline in energy expenditure (Figure 1). For participants with a past mood disorder and higher hostile behavior, the estimated post-meal energy expenditure slope was -31.3 (95% CI: −39.5 to −23.1) kcal/hour, compared to −24.8 (95% CI: −34.2 to −15.5) kcal/hour for participants with a past mood disorder and lower hostile behavior (p=0.001). In contrast, among participants without a past mood disorder, there was no effect of hostile behavior on post-meal energy expenditure; the post-meal slope of energy expenditure was −28.6 (95% CI: −33.3 to −24.0) kcal/hour for lower hostile behavior and −28.2(95% CI: −32.1 to −24.3) for higher hostile behavior (p=0.70). Neither hostile behavior nor mood disorder history significantly predicted post meal carbohydrate oxidation (p=0.30, p=0.99, respectively) or fat oxidation (p=0.29, p=0.80, respectively).
Figure 1.
Estimated postprandial decline in resting energy expenditure as a function of couples' hostile behavior and mood disorder history. Results are from a linear mixed model controlling for pre-meal REE, age, lean body mass, trunk fat, physical activity, gender, and meal type.
The three-way interaction of hostile behavior by mood disorder history by hours post-meal predicting postprandial insulin was significant (p=0.0006). For participants with a past mood disorder, insulin levels were higher at the first post-meal measurement (12% higher geometric mean) for higher hostile behavior compared to lower hostile behavior (p=0.02); their average insulin levels did not drop to the level of lower hostile participants until two hours post-meal. However, for participants without a past mood disorder, there was no effect of hostile behavior on postprandial insulin (p=0.79).
The mood disorder by hours post-meal interaction was significant in predicting postprandial glucose (p=0.001). Participants with a mood disorder history had higher glucose at the first post-meal measurement (4.5% higher geometric mean); their average glucose levels did not drop to the level of the participants without a mood disorder history until three hours post-meal. There was not a significant effect of hostile behavior on glucose (p=0.81).
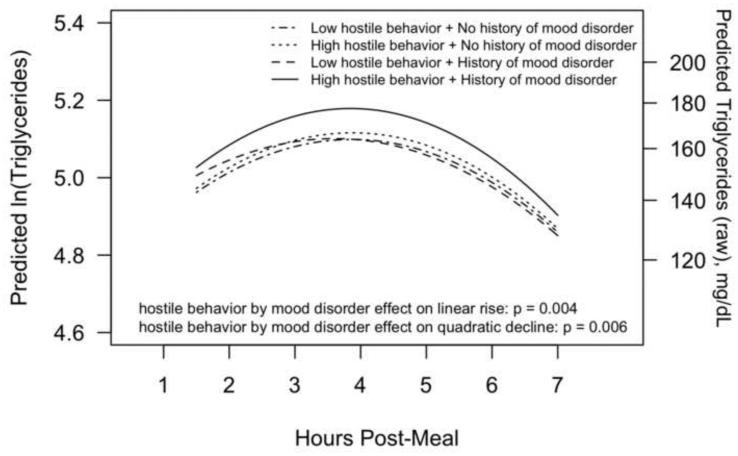
The three-way hostile behavior by mood disorder history by hours post-meal (p=0.004) and hostile behavior by mood disorder history by hours post-meal squared (p=0.006) interactions predicting postprandial triglycerides were significant. Participants who had a mood disorder history and who had more hostile marital interactions had a steeper immediate rise in triglycerides post-meal followed by a steeper decline post-peak compared to other participants (Figure 2).
Figure 2.
Estimated postprandial change in triglycerides as a function of couples' hostile behavior and mood disorder history. Results are from a mixed model controlling for pre-meal triglycerides, age, gender, trunk fat, physical activity and meal type. The left vertical axis is on a natural-log transformed scale (as analyzed) and the right vertical axis shows the original (raw) scale.
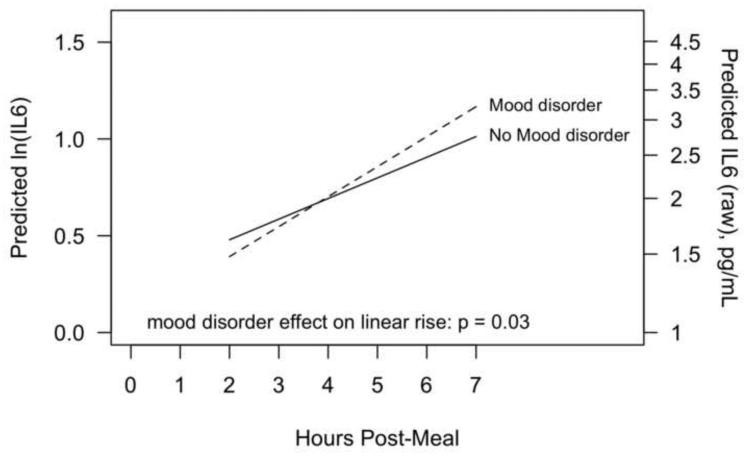
IL-6 increased post-meal (p<0.0001), and there was a significant effect of a past mood disorder on the rate of increase (p=0.03), shown in Figure 3. For participants with a mood disorder history, IL-6 increased at a rate of 0.16 (95% CI: 0.12 to 0.19) ln-pg/mL/hour, compared to only 0.11 (95% CI: 0.09 to 0.13) ln-pg/mL/hour for participants without a mood disorder history. Hostile behavior was not related to post-meal IL-6 (p=0.62).
Figure 3.
Estimated postprandial increase in IL-6 as a function of history of mood disorder. Results are from a linear mixed model controlling for pre-meal IL-6, age, lean body mass, trunk fat, physical activity, gender, and meal type. The left vertical axis is on a natural-log transformed scale (as analyzed) and the right vertical axis shows the original (raw) scale.
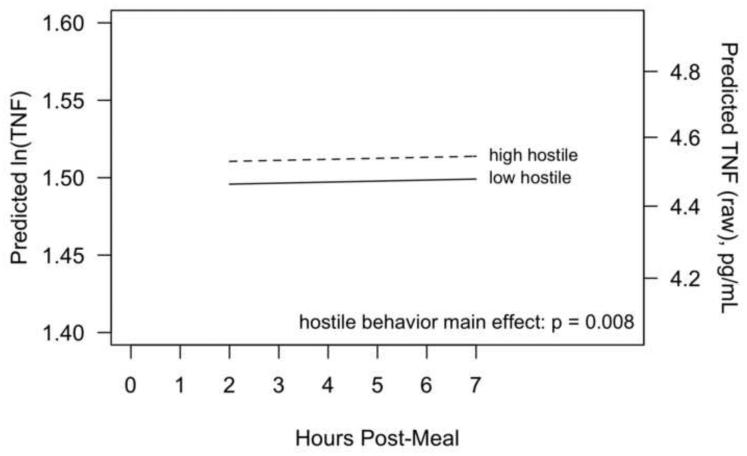
TNF-α did not change over time post-meal (p=0.75), but there was a significant effect of couple hostile behavior on post-meal TNF-α levels, with more negative behavior associated with higher TNF-α (p=0.008), shown in Figure 4. For example, the average ln-TNF-α for participants with more hostile behaviors was 0.015 (95% CI: 0.004 to 0.03) ln-pg/mL higher than participants with fewer hostile behaviors, corresponding to a 1.5% higher geometric mean. There was not a significant effect of mood disorder history on TNF-α (p=0.80). Both cytokine models controlled for trunk fat; results were similar when this was removed from the models.
Figure 4.
Estimated postprandial TNF levels as a function of couple hostile behavior. Results are from a mixed model controlling for pre-meal TNF, age, gender, trunk fat, physical activity and meal type. The left vertical axis is on a natural-log transformed scale (as analysed) and the right vertical axis shows the original (raw) scale.
Both couple hostile behavior (p=0.01) and history of a past mood disorder (p=0.02) significantly were related to higher negative affect immediately post-conflict.
3.2 Ancillary analyses
We evaluated the impact of self-reported marital satisfaction on post-meal responses. These pre-planned secondary analyses were conducted using the dyad’s average CSI scores in place of the couple hostile behavior composite in the mixed models, after controlling on the same covariates. Results from these analyses are displayed in Supplement eTables 1-3. Overall, marital satisfaction showed similar significant associations and patterns with postprandial REE, triglycerides, and insulin, with decreasing marital satisfaction leading to a steeper decline in energy expenditure, higher initial levels of insulin, as well as a steeper immediate rise in triglycerides post-meal followed by a steeper decline post-peak among subjects who had a history of mood disorder (eFigure 1). In addition, the three-way marital satisfaction by mood disorder history by hours post-meal interaction was significant in predicting glucose (eFigure 1). Participants with a mood disorder history who also reported lower marital satisfaction had the highest starting value in post-meal glucose and steepest decline compared to other participants.
Current depressive symptoms, measured by CES-D, were added to all models reported in the primary analyses, replacing mood disorder history. CES-D was not a significant predictor of any outcome except post-meal glucose change and post-conflict negative mood change, where more depressive symptoms were associated with a steeper decline in postprandial glucose (p=0.05) and more negative mood immediately post conflict (p<0.0001).
4. DISCUSSION
Men and women who had more hostile marital interactions and who also had a mood disorder history had lower post-meal energy expenditure, and this disparity was clinically meaningful: the cumulative 6.75-hour total translates into 128 kcal, a difference that could add 7.6 pounds/year for women and 7.7 for men, based on weight change prediction models that incorporate metabolic adaptation (Hall et al., 2012). In addition to energy expenditure, higher levels of hostile behaviors among those who had a mood disorder history were also associated with higher post-meal insulin compared with other participants, with nonsignificant effects for fat and carbohydrate oxidation. Higher insulin levels stimulate food intake and visceral fat accumulation (Dallman, 2010), and thus would act in tandem with lower energy expenditure to promote obesity.
We also found higher peak triglyceride responses in the presence of higher hostility and a mood disorder history. Larger post-meal triglyceride responses are reliably associated with enhanced cardiovascular risk, in part because they promote intima-media thickening (IMT) of carotid arteries, one surrogate atherosclerosis marker (Nordestgaard et al., 2007). Postprandial triglycerides are potent risk predictors for myocardial infarction, ischemic heart disease, stroke, and cardiovascular death, as highlighted in the compelling data from large prospective studies (Boquist et al., 1999; Teno et al., 2000; Bansal et al., 2007; Nordestgaard et al., 2007; Pastromas et al., 2008; Freiberg et al., 2009; Lindman et al., 2010; Madec et al., 2011; Jackson et al., 2012). Indeed, some cardiovascular researchers have suggested that a “triglyceride tolerance test” using a standardized meal, analogous to a glucose tolerance test, may help identify individuals whose metabolic state predisposes them to higher cardiovascular risk (Bansal et al., 2007; Ridker, 2008).
Other research has shown an association between greater IMT and more frequent negative daily marital interactions (Joseph et al., 2014); similarly, a history of depression was linked to carotid atherosclerosis in middle-aged women (Jones et al., 2003). Our triglyceride data demonstrate how marital distress and a mood disorder history increase cardiovascular risk by altering lipid metabolism.
Both marital distress and depression appear to have a synergistic impact on the development of metabolic syndrome, an important risk factor for both cardiovascular disease and diabetes (Troxel et al., 2005; Whisman et al., 2010; Pan et al., 2012; Whisman and Uebelacker, 2012). This study demonstrates mechanistic pathways through which marital discord and a mood disorder history fuel visceral obesity as well as aberrant glucose and lipid metabolism, central metabolic syndrome symptoms.
Primate models provide further complementary evidence and demonstrate clear health implications for the metabolic changes we observed. In monkeys fed a high-fat, Western-type diet, chronic social stress appears to exacerbate coronary artery atherosclerosis by increasing visceral obesity (Shively et al., 2009). Thus, chronic social stressors can impact coronary heart disease in part by increasing visceral fat, which in turn promotes coronary artery atherosclerosis.
Participants with a mood disorder history had a steeper rise in postprandial IL-6 and glucose than those without a past history. Higher levels of hostile behaviors were associated with higher post-meal TNF-α. Elevated proinflammatory cytokines are associated with depression and marital discord as well as increased risk for many age-related diseases including cardiovascular disease, diabetes, cancer, and frailty and functional decline (Kiecolt-Glaser et al., 2003; Kiecolt-Glaser et al., 2005; Uchino et al., 2013).
This study was originally designed to contrast two different kinds of high-fat meals because some earlier studies suggested that high saturated fat meals may fuel postprandial inflammatory responses (Poppitt et al., 2008; Herieka and Erridge, 2014), and epidemiological studies have linked adherence to a Mediterranean diet with lower IL-6 (Dai et al., 2008; Milaneschi et al., 2011). However, we did not find reliable differences between the two meals in this study or our prior study (Kiecolt-Glaser et al., 2014). Randomized controlled feeding trials that regulated diet over weeks or months have demonstrated that diets rich in monounsaturated fatty acids have a more favorable impact on lipid profiles than high saturated fat diets, consistent with the broader health benefits attributed to Mediterranean-style diets (Allman-Farinelli et al., 2005; Bergouignan et al., 2009). However, a recent review of meal challenge studies—responses to single meals-found that plasma inflammatory markers did not change reliably following high fat meals; in contrast, proinflammatory leukocyte surface markers, mRNA and proteins were elevated in most studies (Herieka and Erridge, 2014). The variability in customary background diets may make it difficult to demonstrate metabolic differences following a single high saturated fat meal compared to a single high oleic sunflower oil meal.
Neither hostile behavior nor a mood disorder history was related to baseline/fasting resting metabolic rate, insulin, glucose, or triglycerides; the typical fasting assessments would not have illuminated the problematic relationships, which only emerged in response to the high-fat meals. Meal challenge paradigms can provide important insights into the ways that stress and depression stimulate adverse metabolic changes.
We cannot determine whether the negative effects of a mood disorder history reflect the scars of past depression or underlying interpersonal deficits that could exacerbate marital difficulties, one limitation (Herr et al., 2007). In addition, it would have been helpful to have more participants with a depression history. In other studies, both currently and formerly depressed men and women had poorer family functioning than those who had no depression history, even years after their depression had remitted (Herr et al., 2007). Furthermore, people with a history of depression experience more major and minor stressors than those without a similar history, and past depression can also boost emotional reactivity to stressors, including relationship stressors (Hammen, 1991; Husky et al., 2009). A history of depression may function as a marker for a high-risk phenotype, while marital conflict is a stressful context that provokes a differential dynamic response. Accordingly, a mood disorder history could act synergistically with marital stress through multiple pathways.
Dining with one’s partner is a common daily event, and calorie-dense high-fat comfort food can be particularly enticing for some individuals during stressful times (Tomiyama et al., 2011). Both of our meals had 930 kcal and 60 g fat as they were designed to mimic common fast food options. For example, a Burger King Double Whopper with cheese has 990 kcal and 64 g fat, while a Big Mac cheeseburger and medium French fries contain 930 kcals and 58 g fat. Thus the metabolic changes observed likely occur with high frequency in response to common meal choices.
Although many people may overeat and gain weight during stress, many eat less and some even lose weight (Dallman, 2010). However, even if people do not gain weight, our data suggest that the effects of hostile marital interactions and a depression history are far from trivial-particularly when considered in the context of the other postprandial metabolic data we reported: higher insulin and a heightened postprandial triglyceride response, which would promote central adiposity and simultaneously heighten the risk of cardiovascular disease. Indeed, one noteworthy paper showed that higher levels of depressive symptoms predicted increased visceral obesity over a five-year period independent of changes in body mass index (Vogelzangs et al., 2008). Thus, even if our participants did not gain weight, the insulin and triglyceride differences we observed provide supportive mechanistic evidence for the depression-related changes in visceral obesity reported in prior research (Vogelzangs et al., 2008).
As described in the introduction, our prior study using this same meal challenge paradigm demonstrated that greater numbers of recent daily stressors were associated with similar obesity-promoting metabolic responses to high-fat meals the following day (Kiecolt-Glaser et al., 2014). These parallel findings highlight both the reliability of the stress- and depression-related effects as well as the importance of further investigations in this arena.
The marital relationship is typically people’s most significant adult relationship and thus a troubled marriage is uniquely stressful, providing regular acute stressors (disagreements) that heighten chronic relationship stress. Distressed families experience roughly twice as many tensions per day as nondistressed families (Margolin et al., 1996). Moreover, distressed couples are more likely to have continuing conflicts that recur in well-established patterns at the same time on subsequent days – and meals provide prime opportunities for these ongoing disagreements (Margolin et al., 1996).
We scheduled the couples’ problem discussion post-meal to maximize its metabolic impact. However, self-reported marital satisfaction (CSI) data parallel the analyses with hostile behavior, and show that the effects observed are not just the result of atypical marital disagreements during study visits, but reflect longer-term, chronic marital distress. The CSI findings are in accord with extensive work demonstrating the stability and external validity of marital conflict coding as a strong predictor of marital satisfaction and stability (Kiecolt-Glaser and Newton, 2001; Heyman, 2004; Robles et al., 2014).
Our data dovetail with a recent paper showing that chronic stress was associated with diet-related abdominal fat and insulin resistance in postmenopausal women (Aschbacher et al., 2014), as well as longitudinal studies implicating depression and marital discord in the development of the metabolic syndrome (Gallo et al., 2003; Troxel et al., 2005; Whisman et al., 2010; Pan et al., 2012; Whisman and Uebelacker, 2012). Our current study augments this literature by providing key mechanistic evidence illustrating how high-fat diets promote visceral fat accumulation and insulin resistance in the face of marital distress and a depression history.
Unlike our couples who were only given one meal, most people eat every 4-5 hours, in addition to having snacks that contain fat, and thus many of the adverse metabolic alterations would persist throughout the day (Lairon et al., 2007). Consequently, our results are likely to substantially underestimate actual risk.
Both marital discord and depression have notable physiological repercussions, as documented in the poorer clinical outcomes for conditions ranging from cardiovascular disease to metabolic syndrome to diabetes (Orth-Gomer et al., 2000; Gallo et al., 2003; Jones et al., 2003; Troxel et al., 2005; Joseph et al., 2014; Whisman et al., 2014). This study illustrates novel pathways through which a troubled marriage and a mood disorder history could contribute to each of these high risk conditions. Accordingly, treatments that address marital distress and/or depression could benefit both mental and physical health.
Supplementary Material
Highlights.
During two 9.5 hour visits, 43 healthy married couples received two high-fat meals. Behavioral coding of couples’ problem discussions provided data on hostile behavior. Hostile behavior and past depression heighted post-meal insulin.
Hostile behavior and past depression slowed post-meal energy expenditure (REE). The joint REE behavior/depression difference translates into128 calories per day.
Acknowledgements
The study was supported in part by NIH grants CA154054, CA172296, UL1TRR025755, and CA016058. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We are grateful to Michael Di Gregorio, M.A., for his role as a key organizer and experimenter, and Susan Glaser, PhD, who conducted the interviews to identify the best topics for couples’ conflict discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The_authors_had_no_potential_conflicts_of_interest
The authors had no potential conflicts of interest. This information is also contained in the declaration signed by each author.
References
- Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. National Academy Press; Washington, DC: 2002. [DOI] [PubMed] [Google Scholar]
- Ajuwon KM, Spurlock ME. Palmitate activates the nf-kappab transcription factor and induces IL-6 and tnfalpha expression in 3t3-l1 adipocytes. J. Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- Allman-Farinelli MA, Gomes K, Favaloro EJ, Petocz P. A diet rich in high-oleic-acid sunflower oil favorably alters low-density lipoprotein cholesterol, triglycerides, and factor vii coagulant activity. J. Am. Diet. Assoc. 2005;105:1071–1079. doi: 10.1016/j.jada.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Kornfeld S, Picard M, Puterman E, Havel PJ, Stanhope K, Lustig RH, Epel E. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. doi: 10.1016/j.psyneuen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA-J. Am. Med. Assoc. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- Beach SRH, South, C.R.A.S.C. Interpersonal relationships and health: Social and clinical psychological mechanisms. Oxford University Press; New York, NY, US: 2014. The couple and family discord model of depression: Updates and future directions; pp. 133–155. [Google Scholar]
- Bergouignan A, Momken I, Schoeller DA, Simon C, Blanc S. Metabolic fate of saturated and monounsaturated dietary fats: The Mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog. Lipid Res. 2009;48:128–147. doi: 10.1016/j.plipres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Blaak EE, Hul G, Verdich C, Stich V, Martinez A, Petersen M, Feskens EF, Patel K, Oppert JM, Barbe P, Toubro S, Anderson I, Polak J, Astrup A, Macdonald IA, Langin D, Holst C, Sorensen TI, Saris WH. Fat oxidation before and after a high fat load in the obese insulin-resistant state. J. Clin. Endocrinol. Metab. 2006;91:1462–1469. doi: 10.1210/jc.2005-1598. [DOI] [PubMed] [Google Scholar]
- Boquist S, Ruotolo G, Tang R, Bjorkegren J, Bond MG, de Faire U, Karpe F, Hamsten A. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation. 1999;100:723–728. doi: 10.1161/01.cir.100.7.723. [DOI] [PubMed] [Google Scholar]
- Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. User’s guide for the Structured Clinical Interview for DSM-IV Axis I disorders—research version. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Flatt JP. Misconceptions in body weight regulation: Implications for the obesity pandemic. Crit. Rev. Clin. Lab. Sci. 2012;49:150–165. doi: 10.3109/10408363.2012.712904. [DOI] [PubMed] [Google Scholar]
- Freiberg J, Tybjaerg-Hansen A, Nordestgaard BG. Triglycerides, depression, and risk of ischemic stroke in reply. JAMA-J. Am. Med. Assoc. 2009;301:1339–1339. [Google Scholar]
- Funk JL, Rogge RD. Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. J. Fam. Psychol. 2007;21:572–583. doi: 10.1037/0893-3200.21.4.572. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Troxel WM, Kuller LH, Sutton-Tyrrell K, Edmundowicz D, Matthews KA. Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosom. Med. 2003;65:952–962. doi: 10.1097/01.psy.0000097350.95305.fe. [DOI] [PubMed] [Google Scholar]
- Gudmundsson A, Ershler WB, Goodman B, Lent SJ, Barczi S, Carnes M. Serum concentrations of interleukin-6 are increased when sampled through an indwelling venous catheter. Clin. Chem. 1997;43:2199–2201. [PubMed] [Google Scholar]
- Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. J. Abnorm. Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutri Food Res. 2014;58:136–146. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- Herr NR, Hammen C, Brennan PA. Current and past depression as predictors of family functioning: A comparison of men and women in a community sample. J. Fam. Psychol. 2007;21:694–702. doi: 10.1037/0893-3200.21.4.694. [DOI] [PubMed] [Google Scholar]
- Heyman RE. Rapid Marital Interaction Coding System (RMICS) In: Kerig PK, Baucom DH, editors. Couple observational coding systems. Lawrence Erlbaum Associates; Nahwah, New Jersey: 2004. pp. 67–94. [Google Scholar]
- Holley JW, Guilford JP. A note on the g-index of agreement. Educ. Psych. Meas. 1964;24:749–753. [Google Scholar]
- Husky M, Mazure C, Maciejewski P, Swendsen J. Past depression and gender interact to influence emotional reactivity to daily life stress. Cognit. Ther. Res. 2009;33:264–271. [Google Scholar]
- Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220:22–33. doi: 10.1016/j.atherosclerosis.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Bromberger JT, Sutton-Tyrrell K, Matthews KA. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch. Gen. Psychiatry. 2003;60:153–160. doi: 10.1001/archpsyc.60.2.153. [DOI] [PubMed] [Google Scholar]
- Joseph NT, Kamarck TW, Muldoon MF, Manuck SB. Daily marital interaction quality and carotid artery intima-medial thickness in healthy middle-aged adults. Psychosom. Med. 2014;76:347–354. doi: 10.1097/PSY.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Shea JL, Sun G. Comparison of the classification of obesity by BMI vs. Dual-energy x-ray absorptiometry in the Newfoundland population. Obesity. 2009;17:2094–2099. doi: 10.1038/oby.2009.101. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain. Behav. Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, Belury MA. Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.05.018. DOI: 10.1016/j.biopsych.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T. Marriage and health: His and hers. Psychol. Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. Marriage happiness. Research Press; Champaign, IL: 1971. [Google Scholar]
- Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur. J. Clin. Nutr. 2007;61:1145–1161. doi: 10.1038/sj.ejcn.1602749. [DOI] [PubMed] [Google Scholar]
- Lara J, Taylor MA, Macdonald IA. Energy expenditure in humans: The influence of activity, diet and the sympathetic nervous system, Clinical obesity in adults and children. Third Wiley-Blackwell; 2010. pp. 151–163. [Google Scholar]
- Laugero KD. Filling in the gaps of chronic psychological stress disease models: What's metabolic profiling have to do with it? Endocrinology. 2008;149:2712–2713. doi: 10.1210/en.2008-0328. [DOI] [PubMed] [Google Scholar]
- Lindman AS, Veierod MB, Tverdal A, Pedersen JI, Selmer R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian counties study. Eur. J. Epidemiol. 2010;25:789–798. doi: 10.1007/s10654-010-9501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Madec S, Corretti V, Santini E, Ferrannini E, Solini A. Effect of a fatty meal on inflammatory markers in healthy volunteers with a family history of type 2 diabetes. Br. J. Nutr. 2011;106:364–368. doi: 10.1017/S0007114511000286. [DOI] [PubMed] [Google Scholar]
- Manning PJ, Sutherland WHF, McGrath MA, de Jong SA, Walker RJ, Williams MJA. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity. 2008;16:2046–2052. doi: 10.1038/oby.2008.334. [DOI] [PubMed] [Google Scholar]
- Margolin G, Christensen A, John RS. The continuance and spillover of everyday tensions in distressed and nondistressed families. J. Fam. Psychol. 1996;10:304–321. [Google Scholar]
- Milaneschi Y, Bandinelli S, Penninx BW, Vogelzangs N, Corsi AM, Lauretani F, Kisialiou A, Vazzana R, Terracciano A, Guralnik JM, Ferrucci L. Depressive symptoms and inflammation increase in a prospective study of older adults: A protective effect of a healthy (Mediterranean-style) diet. Mol. Psychiatry. 2011;16:589–590. doi: 10.1038/mp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D'Amato FR. Psychosocial stress affects energy balance in mice: Modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, Staples RC, Cleveland LE. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- Nelson KM, Weinsier RL, Long CL, Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am. J. Clin. Nutr. 1992;56:848–856. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA-J. Am. Med. Assoc. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease - the Stockholm female coronary risk study. JAMA-J. Am. Med. Assoc. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastromas S, Terzi AB, Tousoulis D, Koulouris S. Postprandial lipemia: An under-recognized atherogenic factor in patients with diabetes mellitus. Int. J. Cardiol. 2008;126:3–12. doi: 10.1016/j.ijcard.2007.04.172. [DOI] [PubMed] [Google Scholar]
- Pollin TI, Damcott CM, Shen HQ, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR. A null mutation in human apoc3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, Chan YK, McArdle BH, Cooper GJS. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein to a high-fat dietary load. Nutrition. 2008;24:322–329. doi: 10.1016/j.nut.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psych. Meas. 1977;1:385–401. [Google Scholar]
- Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women - a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- Reeves MM, Davies PSW, Bauer J, Battistutta D. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J. Appl. Physiol. 2004;97:130–134. doi: 10.1152/japplphysiol.01212.2003. [DOI] [PubMed] [Google Scholar]
- Rehman US, Gollan J, Mortimer AR. The marital context of depression: Research, limitations, and new directions. Clin. Psychol. Rev. 2008;28:179–198. doi: 10.1016/j.cpr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Fasting versus nonfasting triglycerides and the prediction of cardiovascular risk: Do we need to revisit the oral triglyceride tolerance test? Clin. Chem. 2008;54:11–13. doi: 10.1373/clinchem.2007.097907. [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychol. Bull. 2014;140:140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity. 2009;17:1513–1520. doi: 10.1038/oby.2009.74. [DOI] [PubMed] [Google Scholar]
- Simonson DC, DeFronzo RA. Indirect calorimetry: Methodological and interpretative problems. Am. J. Physiol. 1990;258:E399–412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- Stoney CM, West SG, Hughes JW, Lentino LM, Finney ML, Falko J, Bausserman L. Acute psychological stress reduces plasma triglyceride clearance. Psychophysiology. 2002;39:80–85. doi: 10.1017/S0048577202010284. [DOI] [PubMed] [Google Scholar]
- Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- Teno S, Uto Y, Nagashima H, Endoh Y, Iwamoto Y, Omori Y, Takizawa T. Association of postprandial hypertriglyceridemia and carotid intima/media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23:1401–1406. doi: 10.2337/diacare.23.9.1401. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36:1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Gallo LC, Kuller LH. Marital quality and occurrence of the metabolic syndrome in women. Arch. Intern. Med. 2005;165:1022–1027. doi: 10.1001/archinte.165.9.1022. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Bosch JA, Smith TW, Carlisle M, Birmingham W, Bowen KS, Light KC, Heaney J, O'Hartaigh B. Relationships and cardiovascular risk: Perceived spousal ambivalence in specific relationship contexts and its links to inflammation. Health Psychol. 2013;32:1067–1075. doi: 10.1037/a0033515. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman ATF, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BWJH. Depressive symptoms and change in abdominal obesity in older persons. Arch. Gen. Psychiatry. 2008;65:1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM. Advances in psychiatric epidemiology: Rates and risks for major depression. Am. J. Public Health. 1987;77:445–451. doi: 10.2105/ajph.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Li A, Sbarra DA, Raison CL. Marital quality and diabetes: Results from the health and retirement study. Health Psychol. 2014;33:832–840. doi: 10.1037/hea0000064. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Uebelacker LA. A longitudinal investigation of marital adjustment as a risk factor for metabolic syndrome. Health Psychol. 2012;31:80–86. doi: 10.1037/a0025671. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Uebelacker LA, Settles TD. Marital distress and the metabolic syndrome: Linking social functioning with physical health. J. Fam. Psychol. 2010;24:367–370. doi: 10.1037/a0019547. [DOI] [PubMed] [Google Scholar]
- Xu S, Lorber MF. Interrater agreement statistics with skewed data: Evaluation of alternatives to Cohen's kappa. J. Consult. Clin. Psychol. 2014 doi: 10.1037/a0037489. DOI: 10.1037/a0037489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.