Abstract
Innate lymphoid cells (ILCs) are lymphocyte-like cells lacking T or B cell receptors that mediate protective and repair functions through cytokine secretion. Among them, type 2 ILC (ILC2) cells are capable of producing type 2 cytokines. We report the existence of an inflammatory (i) ILC2 population responsive to IL-25 that complements IL-33-responsive natural (n) ILC2 cells. iILC2 cells developed into nILC2-like cells in vitro and in vivo, contributing to expulsion of Nippostrongylus brasiliensis. They also acquired IL-17-producing capacity, providing partial protection against Candida albicans. We propose that iILC2 cells are transient ILC progenitors mobilized by inflammation and infection that develop into nILC2-like cells or ILC3-like cells and contribute to immunity to both helminths and fungi.
Keywords: KLRG1, nILC2, iILC2, IL-25, IL-33
INTRODUCTION
Innate lymphoid cells (ILCs) are lymphocyte-like cells that lack specific antigen receptors yet produce effector cytokines matching those of CD4+ T helper (TH) cell subsets. ILCs are classified into three major groups based on their cytokine-producing potential, interferon-γ (IFN-γ)-producing ILC1s, interleukin 13 (IL-13) and IL-5-producing ILC2s and IL-17- or IL-22-producing ILC3s1–3. ILCs provide immunity against infectious agents, participate in inflammatory responses and mediate lymphoid organogenesis and tissue repair, particularly at mucosal barriers. They provide early control of pathogen invasion in a non-antigen-specific manner and are often a critical first-line immune defense.
ILC2s play an important role in type 2 immunity. They were independently described by several groups and designated natural helper (NH) cells, nuocytes or innate helper 2 (Ih2) cells4–6. Although there are some differences in tissue localization and surface marker patterns between these cells, they are considered by many to represent a single cell type, now designated ILC2s based on type 2 cytokine production and associated functions. A separate IL-25-responsive innate cell population was described and termed multipotent progenitors (MPPtype2)7. MPPtype2 cells contribute to helminth immunity but, in contrast to ILC2 cells, can develop into monocytes and macrophages or mast cells and are of myeloid rather than lymphoid lineage8.
ILC2s play a critical role in immune responses to helminth infections, such as Nippostongylus brasiliensis. Dysfunction of ILC2s causes a significant delay in worm expulsion9. ILC2s also participate in allergic inflammation. They mediate influenza-induced airway hyper-reactivity, protease allergen-induced eosinophilic lung inflammation10,11 and have been reported to promote allergic atopic dermatitis in humans12. ILC2 cells also function in tissue remodeling. They contribute to lung tissue repair during virus infection and mediate hepatic fibrosis13,14.
ILC2s act through diverse effector pathways. Through production of IL-13, they induce epithelial goblet cells to secrete mucus and contribute to tissue repair by producing amphiregulin10,13. By secreting IL-5 and IL-9, ILC2s control eosinophil homeostasis and mast cell activation15,16. It has been reported that ILC2s promote B cell production of antibody and activation of CD4+ T cells17–19.
ILC2 development depends on the action of DNA-binding protein inhibitor 2 (Id2) and expression of IL-2 receptor gamma chain (common γ chain; γc), but not on RAG recombinase1,18. GATA-3 is indispensable for ILC2 development and survival19,20. RORα and TCF-1 are also required for ILC2 generation21,22. Additionally, Gfi-1 has been reported to promote development of ILC2 cells and to regulate their cytokine-producing phenotype23.
Two epithelial-derived cytokines, IL-25 and IL-33, have been reported to be crucial for the induction and activation of ILC2s both in humans and in mice24,25. IL-25 is a member of IL-17 family that is associated with TH2-like immune responses. IL-33 is a member of the IL-1 family and its receptor, ST2, is expressed on both TH2 cells and ILC2s, as well as mast cells, basophils and eosinophils26. IL-25 and IL-33 are important in anti-helminth immunity and allergic inflammation. N. brasiliensis infection triggers epithelial cells to release IL-25 and IL-33, which in turn activate ILC2s, causing their expansion and cytokine production27. ILC2 populations with different tissue distribution and cytokine receptor-expression patterns have been reported to have different responsiveness to IL-25 or IL-33. Some ILC2 populations are thought to respond to either IL-25 or IL-33 and some to both. However, the relationship between IL-25-responsive ILC2s and IL-33-responsive ILC2s is still unclear.
Here we report an IL-25-responsive ILC2 cell population that expressed large amounts of KLRG1 and the IL-25 receptor (IL-17RB) but did not express ST2. These cells have a phenotype distinct from both MPPtype2 and conventional ILC2 cells in the lung. They proliferated in response to IL-25 but not to IL-33. They developed into ST2+ ILC2s both in vitro and in vivo. These KLRG1hi cells were elicited early in the course of N. brasiliensis infection, before proliferation of lung-resident ILC2s, and became ILC2-like cells during such infection. KLRG1hi cells also expressed intermediate amounts of RORγt, whereas IL-33-responsive ILC2s did not. KLRG1hi cells have the potential to produce IL-17 and can develop into ILC3-like cells either under “TH17” culture conditions or in response to Candida albicans infection. We propose that the IL-33-responsive ILC2 cells resident at steady state in the lung and fat-associated lymphoid tissues be designated homeostatic or natural ILC2 (nILC2) cells while the KLRG1hi cells that only appear after IL-25 stimulation or infection be designated inflammatory ILC2 (iILC2) cells.
RESULTS
IL-25 induces a lineage-negative KLRG1hi cell population
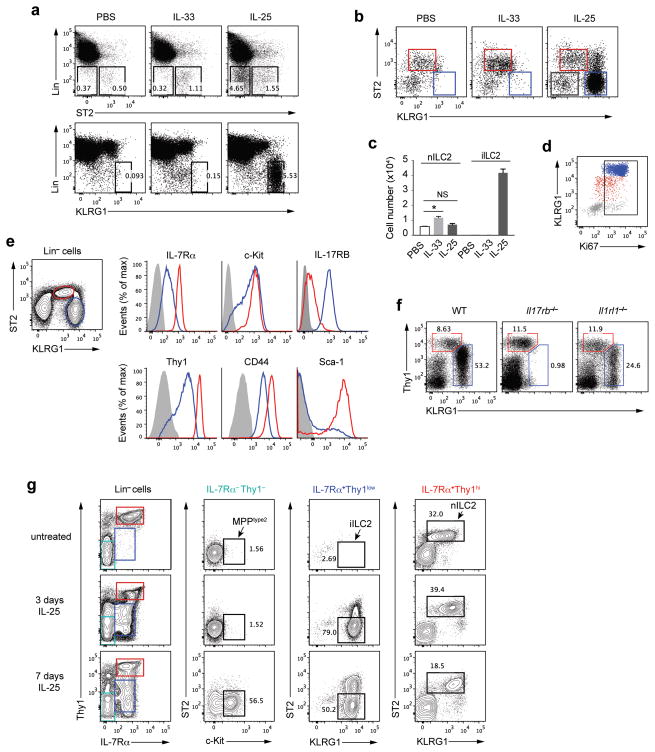
Wild-type mice were treated intraperitoneally (i.p.) with recombinant IL-33 or IL-25 for 3 days. Lung leukocytes were analyzed for ILC surface markers (Fig. 1a). In naïve mice, lung ILC2 cells, characterized as Lin−ST2+, increased 2–3-fold in number in response to IL-33 (Fig. 1a–c). A Lin−ST2− cell population, barely detectable in the lungs of untreated or IL-33-treated mice, appeared after IL-25 treatment (Fig. 1a). This IL-25-induced cell population expressed abundant KLRG1 (Fig. 1a,b). Although KLRG1 is expressed on resident ILC2 cells, its intensity on these cells is substantially less than on the IL-25-responsive population. We designated the Lin−ST2+KLRG1int cells as nILC2s and Lin−ST2−KLRG1hi cells as iILC2s.
Figure 1.
IL-25 induces a Lin−ST2−KLRG1hi cell population distinct from nILC2 or MPPtype2. (a) Wild-type C57BL/6 (B6) mice were treated i.p. with PBS, IL-33 or IL-25 (200ng per mouse per day for each cytokine) daily for 3 days. Leukocytes in the lung were isolated and analyzed by flow cytometry for ST2, KLRG1 and “lineage” expression. Lineage (Lin) includes the antibodies to CD3ε, CD5, CD19, B220, TCRγδ, NK1.1, CD11b, CD11c, Gr-1, FcεR1 and TER119. (b) KLRG1 and ST2 expression on Lin− cells in the lungs of the mice treated as in a. Red gate, nILC2; blue gate, iILC2. (c) Cell numbers of nILC2s or iILC2s in the lungs of the mice treated as in a. (d) Ki67 expression on lung leukocytes from IL-25-treated mice as in the right of b. Red (nILC2) dots were gated on Lin−ST2+KLRG1int cells, blue (iILC2) dots were gated on Lin−ST2−KLRG1hi cells and gray dots were gated on Lin−ST2−KLRG1− cells. (e) Expression of ILC2 markers on Lin− cells from the lung of IL-25-treated mice. Red line, nILC2; Blue line, iILC2; gray shadow, negative control (e.g., Lin+IL-7Rα− cells were gated as negative control for IL-7Rα expression). (f) Wild-type (WT), Il17rb−/− or Il1rl1−/− mice were treated with IL-25 for 3 days and lung leukocytes were analyzed by flow cytometry for lineage, Thy1 and KLRG1 expression. Cells were gated on Lin−. (g) Wild-type B6 mice were untreated or treated with IL-25 for 3 or 7 days. Lung leukocytes were analyzed by flow cytometry for ILC markers expression. MPPtype2 cells were gated on Lin−Thy1−IL-7Rα−ST2−c-Kit+, iILC2s were gated on Lin−Thy1lowIL-7Rα+ST2−KLRG1hi, and nILC2s were gated on Lin−Thy1hiIL-7Rα+ST2+KLRG1int. c, mean ± s.e.m.; NS, not significant; *P≤0.05 (unpaired two-tailed t test). Data are representative of three independent experiments (a–e) or representative of two independent experiments (f,g). a,b,c, n=3 mice for each group in each experiment; d,e, n=2 mice in each experiment; f,g, n=2 mice for each group in each experiment.
Lungs of naïve mice contain 4–5 × 103 nILC2 cells. IL-33 treatment increased that to ~104 while IL-25 caused a statistically insignificant increase in lung nILC2s. By contrast, iILC2s, undetectable in the lungs of untreated or IL-33-treated mice, were present at more than 4 × 104 per mouse in lungs of IL-25-treated mice (Fig. 1c). iILC2s were all Ki67 positive (Fig. 1d), indicating they had proliferated very rapidly in the IL-25-treated animals. iILC2s were also detected in spleen, mesenteric lymph nodes (MLNs), and liver, with few in bone marrow (Supplementary Fig. 1).
Phenotypically iILC2s were c-Kit+CD44+ and expressed less IL-7Rα and Thy1 than nILC2s (Fig. 1e). Most iILC2s lacked Sca-1, which was uniformly expressed on nILC2s. Importantly, iILC2s were IL-17RBhi, whereas nILC2s expressed much less IL-17RB. Thus, iILC2s were ST2−IL-17RB+ and responded to IL-25 but not to IL-33, whereas nILC2s were ST2+ and mainly responded to IL-33. IL-25 treatment did not elicit iILC2s in Il17rb−/− but did in Il1rl1−/− mice that lack ST2 (Fig. 1f).
Previous studies showed that 7-day IL-25-treatment elicits MPPtype2 cells, a myeloid cell population, in MLNs. MPPtype2 cells are characterized as Lin−IL-7Rα−Thy1−ST2−c-Kit+. To address whether MPPtype2 cells also appear in the lung and to clarify the relationship among iILC2, nILC2 and MPPtype2 cells, we examined untreated mice and mice treated with IL-25 daily for 3 days or 7 days for the presence of these three populations among lung leukocytes (Fig. 1g). In untreated mice, only nILC2s (Lin−IL-7Rα+Thy1hiST2+KLRG1int) were observed. 3-day administration of IL-25 induced large numbers of iILC2s (Lin−IL-7Rα+Thy1lowST2−KLRG1hi) but still no MPPtype2 cells. In 7-day treated mice, while iILC2s were still present, we observed the induction of MPPtype2 cells (Lin−IL-7Rα−Thy1−ST2−c-Kit+) (Fig. 1g). Thus, IL-25 induces at least two cell populations in the lung, lymphoid iILC2 cells and myeloid MPPtype2 cells, depending on the duration of treatment.
iILC2 development depends on γc and IL-7Rα
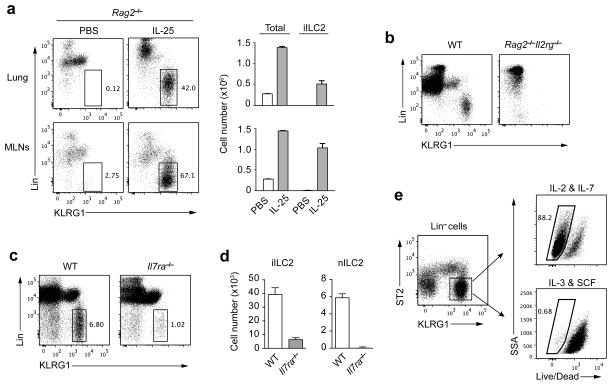
We tested whether iILC2 cells exist in Rag2−/− mice or Rag2−/−Il2rg−/− mice. RAG2-deficient mice treated with IL-25 gave rise to iILC2s in the lung and MLNs (Fig. 2a). Many more iILC2s were elicited by IL-25 in Rag2−/− mice than in wild-type mice. In the lung, ~5 × 105 iILC2s appeared in Rag2−/− mice treated with IL-25 (Fig. 2a), compared to fewer than 5 × 104 in wild-type mice (Fig. 1c). MLNs are much smaller in RAG2-deficient mice than in wild-type mice; however, MLNs of Rag2−/− mice became enlarged after IL-25 treatment. 67% of the total MLN cells in these mice were iILC2 cells (Fig. 2a), with numbers in excess of 1 × 106. By contrast, iILC2s were totally absent in Rag2−/−Il2rg−/− mice treated with IL-25 (Fig. 2b), implying that iILC2s are γc dependent.
Figure 2.
iILC2 development depends on γc chain and IL-7Rα. (a) Left, Rag2−/− mice were treated with PBS or IL-25 for 3 days and leukocytes from lungs or MLNs were analyzed by flow cytometry for lineage and KLRG1 expression. iILC2s were gated as Lin−KLRG1hi. Right, cell numbers of total lymphocytes and iILC2s in the lungs (upper) or MLNs (lower). (b) Lung leukocytes from IL-25-treated wild-type or Rag2−/−Il2rg−/− mice were analyzed by flow cytometry for lineage and KLRG1 expression. (c) Wild-type and Il7ra−/− mice were treated and analyzed as in b. (d) Cell numbers of iILC2s and nILC2s in c. (e) Lin−ST2− KLRG1hi cells were purified by cell sorting from the lungs of IL-25-treated wild-type mice and were cultured either in IL-2 plus IL-7 or in IL-3 plus SCF (10ng/ml for each cytokine). 3 days later, cell viability was determined by Dead/Live staining. Live cells were gated as Dead/Live−. a,d, mean ± s.e.m.. Data are representative of more than five independent experiments (a), three independent experiments (b–d) or two independent experiments (e). a–d, n=3 mice for each group in each experiment; e, n=2 cell culture wells for each condition in each experiment.
All ILCs, other than classical IL-7Rα−NK1.1+ NK cells, require IL-7Rα for their development. Treating Il7ra−/− mice with IL-25 led to substantially fewer iILC2s than did treatment of wild-type mice (Fig. 2c,d), indicating IL-7Rα is critical for the IL-25 induction of iILC2s. Interestingly, while no nILC2s were detected in Il7ra−/− mice, iILC2s were not totally abolished in IL-25-treated Il7ra−/− mice (Fig. 2d), suggesting that iILC2s and nILC2s might arise through somewhat different developmental pathways or that more than one cell population may be present in the iILC2 “gate”.
We cultured the sorted iILC2s (Lin−ST2−KLRG1hi) from IL-25-treated wild-type mice in IL-2 plus IL-7 or, alternatively, in IL-3 plus stem cell factor (SCF). iILC2s survived and proliferated in IL-2 plus IL-7 but died in IL-3 plus SCF (Fig. 2e). This pattern of responsiveness differs from that reported for MPPtype2 cells, further indicating that iILC2 cells are distinct from MPPtype2 cells.
iILC2 cells produce type 2 cytokines
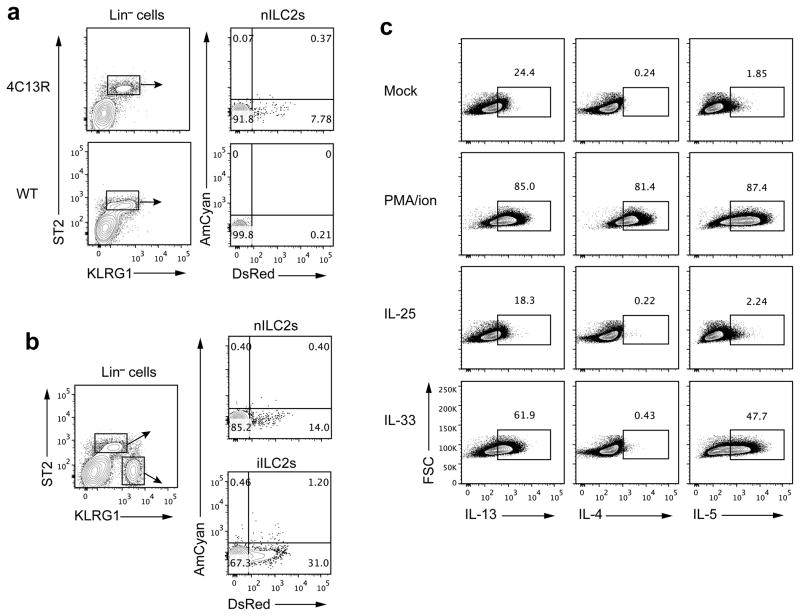
To enumerate type 2 cytokine-producing cells, we generated BAC-transgenic IL-4-AmCyan/IL-I3-DsRed dual reporter mice (4C13R) in which IL-4 production is reported by the expression of AmCyan and IL-13 by destabilized DsRed (Supplementary Fig. 2a). These mice allow detection of IL-4- or IL-13-producing cells without in vitro stimulation. In naïve 4C13R mice, ~2–9% of lung nILC2s produced IL-13 but few if any make IL-4 (Fig. 3a and Supplementary Fig. 2b). After IL-25 administration, the frequency of IL-13-producing nILC2s rose to ~14% but no IL-4-production was observed. Among iILC2s from IL-25-treated mice, ~31% were DsRed+, indicating they were producing IL-13. A few of these cells (~2%) were AmCyan+ (Fig. 3b). Thus, iILC2s share with nILC2 cells the capacity to make type 2 cytokines.
Figure 3.
iILC2 cells produce type 2 cytokines. (a) Lung leukocytes of naive 4C13R or non-transgenic B6 mice were isolated and analyzed by flow cytometry for lineage, KLRG1, ST2, AmCyan (IL-4) and DsRed (IL-13) expression. nILC2s were gated on Lin−ST2+KLRG1int. (b) 4C13R mice were treated i.p. with IL-25 for 3 days, and lung leukocytes were analyzed as in a. nILC2s were gated on Lin−ST2+KLRG1int and iILC2s were gated on Lin−ST2−KLRG1hi. (c) iILC2s, sorted from IL-25-treated wild-type B6 mice, were cultured with IL-2, IL-7 and IL-25 (10ng/ml for each cytokine) for 3 days and then unstimulated (Mock) or stimulated with PMA plus ionomycin, IL-25 (50ng/ml) or IL-33 (50ng/ml) for 6 hours. IL-13, IL-4 and IL-5 production was measured by intracellular cytokine staining followed by flow cytometry. Data are representative of three independent experiments (a–c) (n=2~5 mice for each group in each experiment of a; n=2 mice for each experiment of b; n=3 cell culture wells for each stimulus in each experiment of c).
To further address the cytokine-producing capacity of iILC2s, we purified them from IL-25-treated wild-type mice, cultured them for 3 days in IL-2, IL-7 and IL-25 and then stimulated the cells with IL-25, IL-33 or PMA plus ionomycin (Fig. 3c). Consistent with the results from 4C13R mice, some iILC2s produced IL-13 even without simulation. PMA plus ionomycin induced the great majority of iILC2s to produce IL-4, IL-13 and IL-5. Surprisingly, cultured iILC2s failed to respond to IL-25 but did produce IL-13 and IL-5 in response to IL-33; no IL-4 was produced in response to IL-33 (Fig. 3c). Since iILC2s were sorted as ST2−IL-17RB+ cells, these results suggest that iILC2s might have changed their expression pattern of ST2 and IL-17RB when cultured in vitro. These results emphasize that ILC2 cells, while producing only IL-13 and IL-5 in response to cytokines are competent to produce IL-4 when stimulated with PMA plus ionomycin.
iILC2 cells develop into ST2+ nILC2-like cells
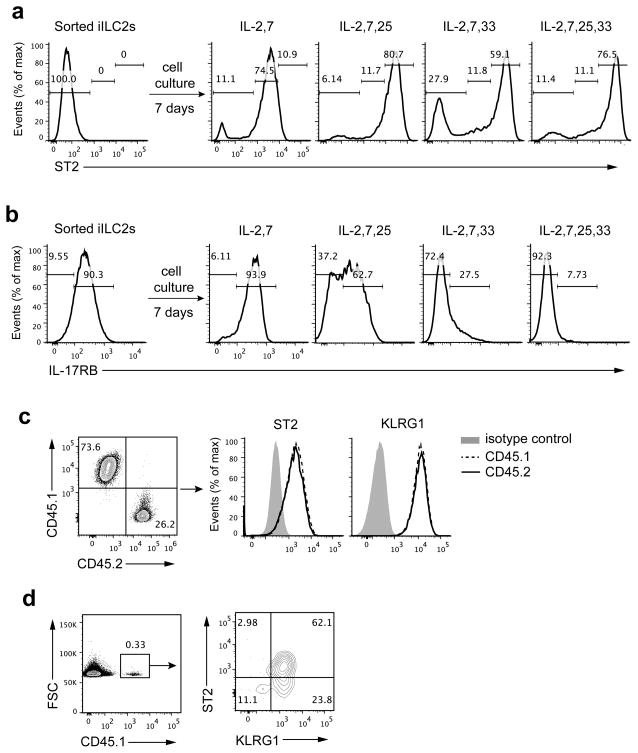
The changed responsiveness of iILC2s to IL-33 and IL-25 suggested they might have upregulated ST2 expression and downregulated IL-17RB expression during culture. We sorted iILC2s from IL-25-treated donors, cultured them in different cytokine combinations and measured ST2 and IL-17RB expression on day 7. The starting cell population was ST2−IL-17RB+ (Fig. 4a,b). 7-day culture in IL-2 and IL-7 resulted in the great majority becoming ST2+. Addition of IL-25 or IL-33 or both to the culture led to even higher expression of ST2 among those cells (Fig. 4a). Culture in IL-2 and IL-7 did not change expression of IL-17RB on day 7; addition of IL-33 or IL-33 plus IL-25 extinguished IL-17RB expression on cultured iILC2s, whereas addition of IL-25 only led to a portion of cells having retained IL-17RB expression (Fig. 4b). iILC2s grew and proliferated in each of the culture conditions although yields were better when either or both IL-25 and IL-33 were present (data not shown). Mesenteric nILC2s have been reported to respond to IL-2 plus IL-25 with upregulation of IL-17RB expression4. However, nILC2-like cells derived from iILC2s by culture in IL-7 and IL-33, which had downregulated IL-17RB, failed to re-express IL-17RB in response to IL-2 and IL-25 (Supplementary Fig. 3).
Figure 4.
iILC2 cells develop into ST2+ nILC2-like cells both in vitro and in vivo. (a,b) iILC2s sorted from IL-25-treated Rag2−/− mice were cultured in different cytokine combinations as indicated. ST2 and IL-17RB expression were measured on day 7 by flow cytometry. (c) iILC2s, sorted from IL-25-treated CD45.1 Rag1−/− mice, and nILC2s, sorted from naive CD45.2 Rag1−/− mice, were mixed at a ratio of 2.7:1 iILC2s to nILC2s and cultured with IL-2, IL-7 and IL-25. The ratio of CD45.1: CD45.2 and expression of ST2 and KLRG1 were measured on day 7 by flow cytometry. (d) iILC2s, sorted from IL-25-treated CD45.1 Rag1−/− mice, were intravenously (i.v.) transferred (5 ×105 cells per mouse) into Rag2−/−Il2rg−/− mice. 8 days later, transferred cells in the lung of recipient mice were analyzed for KLRG1 and ST2 expression by flow cytometry. Data are representative of two independent experiments (a–d) (n=3 cell culture wells for each condition in each experiment of a, b; n=2 repeats in each experiment of c; n=2 recipient mice in each experiment of d).
To exclude the possibility that the purified iILC2s were contaminated with nILC2s, which might have overgrown to become the predominant population during the 7-day culture, we performed a co-culture experiment. CD45.1 iILC2s and CD45.2 nILC2s were each sort purified and mixed at a ratio of 2.7:1 iILC2s to nILC2s. After 7-day culture in IL-2, IL-7, IL-25 and IL-33, the ratio of CD45.1:CD45.2 cells remained essentially the same (2.8:1) but all the cells were ST2+KLRG1int (Fig. 4c), indicating that the two cell populations expanded to the same extent and establishing that iILC2s converted to cells with a nILC2 phenotype.
While the majority of KLRG1hi cells were ST2− at day 3 after in vivo IL-25 treatment, there were a small proportion of these cells that were ST2+ or ST2low. By day 7 of in vivo IL-25 treatment, ST2− and ST2+ cells were present in an equal proportion among the KLRG1hi population (Fig. 1g). This result suggests that iILC2s gave rise to nILC2-phenotype cells in vivo. To address this possibility, we transferred sorted iILC2s into Rag2−/−Il2rg−/− mice. 8 days later, more than 60% of transferred cells found in the lung had become ST2+ nILC2-like cells (Fig. 4d). We propose that IL-25-induced iILC2s are transient progenitors of nILC2s or of cells similar to nILC2s.
iILC2 cells regulate anti-helminth immunity
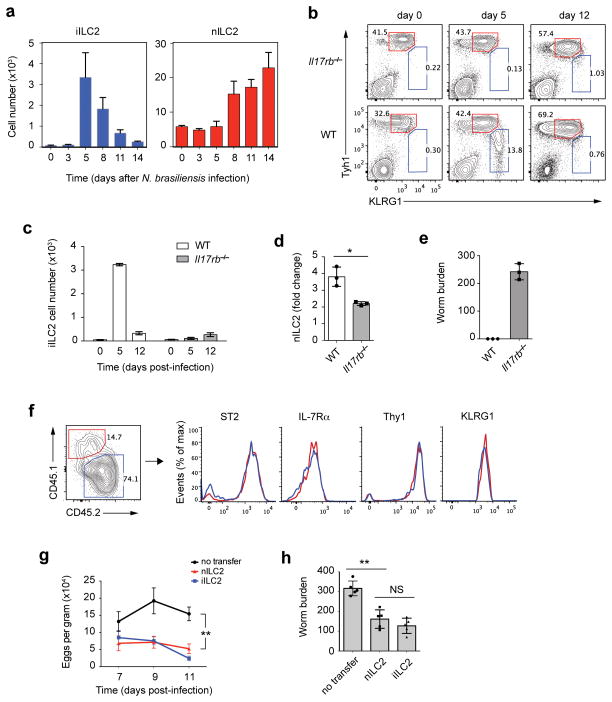
We tested whether helminth infection elicits iILC2 cells. Wild-type mice (Fig. 5a), or 4C13R reporter mice (Supplementary Fig. 4a), were infected with N. brasiliensis and lung ILCs were analyzed at various times. iILC2s first appeared on day 5 post infection. At that time, nILC2s had not yet proliferated. Thereafter, the numbers of iILC2s decreased while nILC2s increased. On day 14, most iILC2s had disappeared but nILC2s had increased 3~4 fold compared to uninfected mice (Fig. 5a). N. brasiliensis-activated nILC2s produced IL-13 and some produced IL-4, but iILC2s were better cytokine producers (Supplementary Fig. 4a).
Figure 5.
iILC2 cells regulate anti-helminth immunity. (a) Wild-type B6 mice were infected with N. brasiliensis for various days and leukocytes in the lungs were counted and analyzed by flow cytometry. nILC2s were gated as Lin− Thy1hi KLRG1int and iILC2s were gated as Lin−Thy1low KLRG1hi. (b) Wild-type or Il17rb−/− mice were infected with N. brasiliensis for various days and leukocytes in the lungs were analyzed as described in a. (c) Absolute cell numbers of iILC2s in b. (d) nILC2 expansion on day 12 relative to day 0 in b. (e) Worm burden in the intestine on day 12. (f) 2.5 ×104 nILC2s (sorted from naive CD45.1 Rag1−/−) and 20 ×104 iILC2s (sorted from IL-25-treated CD45.2 Rag1−/− mice) were mixed and i.v. transferred into Rag2−/−Il2rg−/− mice, which were infected with N. brasiliensis on the same day. 14 days later, transferred cells in the lung were analyzed for the ratio of CD45.1: CD45.2 and for ILC2 surface markers by flow cytometry. (g) 2 ×105 sorted nILC2s or iILC2s were i.v. transferred into Rag2−/−Il2rg−/− mice that were infected with N. brasiliensis. Worm eggs in the feces were counted on day 7, day 9 and day 11 after infection. (h) Worm burden in intestine of the mice in g were counted on day 14 post infection. a,c,d,e,g,h, mean ± s.e.m.; NS, not significant; *P≤0.05, **P≤0.01 (unpaired two-tailed t test). Data are representative of two independent experiments (a,f,g,h) or represent one experiment (b–e) (n=3 mice for each group in each experiment of a; n=3 mice in b–e; n=2 recipient mice in each experiment of f; n=5 mice for each group in each experiment of g,h).
N. brasiliensis-induced iILC2s were IL-25 dependent; they were absent in infected Il17rb−/− mice (Fig. 5b,c). The expansion of nILC2s was also significantly impaired in Il17rb−/− mice (Fig. 5d), suggesting that iILC2s make a substantial contribution to nILC2 numbers in N. brasiliensis infection. Intestinal worm burden was substantially greater in Il17rb−/− mice (Fig. 5e), implying that IL-25-dependent iILC2s are important for worm expulsion5.
These results are consistent with the N. brasiliensis-induced iILC2s being a transient progenitor population that develops into nILC2-like cells and participates in the expulsion of helminths. To test this possibility, we transferred CD45.1 nILC2s and CD45.2 iILC2s together into Rag2−/−Il2rg−/− mice and infected the recipient mice with N. brasiliensis. 14 days later, the ratio of CD45.1:CD45.2 cells remained similar to that in the injected cell populations but all the iILC2s had developed into IL-7Rα+Thy1hiST2+KLRG1int nILC2-like cells (Fig. 5f). In addition, transferred iILC2s developed into nILC2-like cells in the absence of nILC2s (Supplementary Fig. 4b). These results imply that iILC2s are indeed transient progenitors of nILC2s during helminth infection.
We asked whether iILC2s could protect mice against N. brasiliensis infection. Rag2−/−Il2rg−/− mice cannot expel worms of this parasite because of absence of T cells and ILCs. Between day 7 and day 11 post N. brasiliensis infection of Rag2−/−Il2rg−/− mice, many eggs in mouse feces were detected (Fig. 5g) and adult worms were observed in the intestine on day 14 (Fig. 5h). Transfer of iILC2s into Rag2−/−Il2rg−/−-mice significantly reduced the number of eggs in the feces and the worm burden in the intestine, comparable to that observed by the transfer of similar numbers nILC2s (Fig. 5g,h), indicating that iILC2s and/or their descendants can limit worm expansion.
iILC2 cells can develop into IL-17 producers
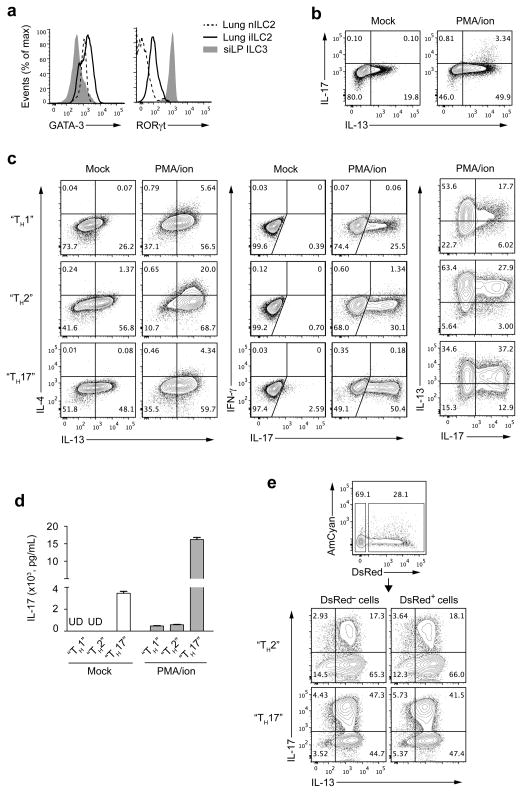
We compared the expression of GATA-3 and RORγt among lung iILC2s, lung nILC2s and small intestine lamina propria (siLP) ILC3s (Fig. 6a). Both ILC2 and ILC3 expressed GATA-3, as recently reported20. As expected, nILC2 cells expressed more GATA-3 than ILC3 cells. iILC2s expressed even more GATA-3 than nILC2s. iILC2s also expressed an intermediate amount of RORγt, less than ILC3 cells, but significantly distinct from nILC2s (Fig. 6a). In keeping with the presence of RORγt, a small proportion of freshly isolated IL-13-producing iILC2s also produced IL-17 upon stimulation with PMA plus ionomycin (Fig. 6b).
Figure 6.
iILC2 cells express RORγt and have the capacity to develop into IL-17 producers. (a) Flow cytometry analysis of GATA-3 and RORγt expression on nILC2s and iILC2s from lung of IL-25-treated wild-type B6 mice and ILC3s (Lin−IL-7Rα+RORγthi) from small intestinal lamina propria (siLP) of naïve mice. (b) Freshly sorted iILC2s from IL-25-treated Rag2−/− mice were stimulated with or without PMA plus ionomycin for 6 hours. IL-13 and IL-17 production was measured with intracellular cytokine staining, followed by flow cytometry. (c) iILC2s sorted from IL-25-treated Rag2−/− mice were cultured in various conditions (described in Methods). 7 days after culture, cells were stimulated with or without PMA plus ionomycin for 6 hours and production of IL-4, IL-13, IL-17 and IFN-γ were determined by intracellular staining. (d) iILC2s were cultured as in c for 7 days and secreted IL-17 protein in supernatants of cultured cells was measured with or without PMA plus ionomycin stimulation, by enzyme-linked immunosorbent assay. UD, undetectable. (e) iILC2s from IL-25-treated 4C13R mice were divided into DsRed+ and DsRed− populations through cell sorting. The purified populations were cultured in either “TH2” conditions or “TH17” conditions for 7 days. IL-13 and IL-17 production was measured by intracellular staining after 6-hour stimulation with PMA plus ionomycin. d, mean ± s.e.m.. Data are representative of three (a–c) or two (d,e) independent experiments (n=2 mice for each group in each experiment of a; n=2 cell culture wells for each condition or stimulus in each experiment of b,c,e; n=3 cell culture wells for each condition in each experiment of d).
Naïve CD4+ T cells can differentiate into diverse TH cell subsets, either in response to distinct pathogenic pressure in vivo or under polarized culture conditions in vitro. We mimicked in vitro conditions for TH differentiation in our cultures of iILC2s, although omitting antigen-presenting cells. More than 50% of iILC2s cultured with IL-4, anti-IFN-γ and anti-IL-12 (TH2 conditions), in addition to the usual iILC2 culture conditions, produced IL-13 and, upon stimulation with PMA plus ionomycin, ~90% of the cells produced IL-13 (Fig. 6c). Among iILC2s cultured with the addition of IL-12 and anti-IL-4 (TH1 conditions) or TGF-β and IL-6 (TH17 conditions), fewer produced IL-13 with or without PMA plus ionomycin. This difference was particularly pronounced for PMA plus ionomycin-induced IL-4 production (Fig. 6c). iILC2s cultured under any conditions failed to produce IFN-γ. Impressively, 50% of iILC2s in “TH17” culture produced IL-17 in response to PMA plus ionomycin while fewer iILC2s cultured under “TH1” or “TH2” conditions could make IL-17 (Fig. 6c). Most IL-17 producers also produced IL-13. Some cells cultured under “TH17” conditions produced IL-17 even without PMA plus ionomycin stimulation, which was confirmed by measuring secreted IL-17 protein in the supernatant of cultured cells (Fig. 6d).
To determine whether the potential of iILC2s to become IL-13–IL-17 double producers was limited to a subset of these cells or was a general property, we sorted DsRed+ and DsRed− iILC2s from IL-25-treated 4C13R mice and cultured them under “TH17” or “TH2” conditions. After culture, both groups were equivalent in their capacity to produce IL-17, although those cultured under “TH17” conditions were superior to those cultured under “TH2” conditions (Fig. 6e). We also used IL-17-RFP reporter mice to perform similar experiments. Both IL-17 producers and non-IL-17 producers, after PMA plus ionomycin stimulation, had the same potential to become IL-13–IL-17 double producers upon culture (Supplementary Fig. 5). This finding was consistent with the homogenous expression of RORγt in freshly isolated iILC2s. Thus, iILC2s express both GATA-3 and RORγt. They produce IL-13 and also can produce IL-17 under certain culture conditions in vitro.
We also probed whether nILC2s also have such plasticity. When cultured under “TH1”, “TH2” or “TH17” conditions, only ~2–5% of nILC2 cells produced IL-17 upon PMA plus ionomycin stimulation. This result was independent of culture conditions (Supplementary Fig. 6), implying that nILC2s have less capacity to develop into IL-17 producers than do iILC2s.
iILC2 cells contribute to anti-fungal immunity
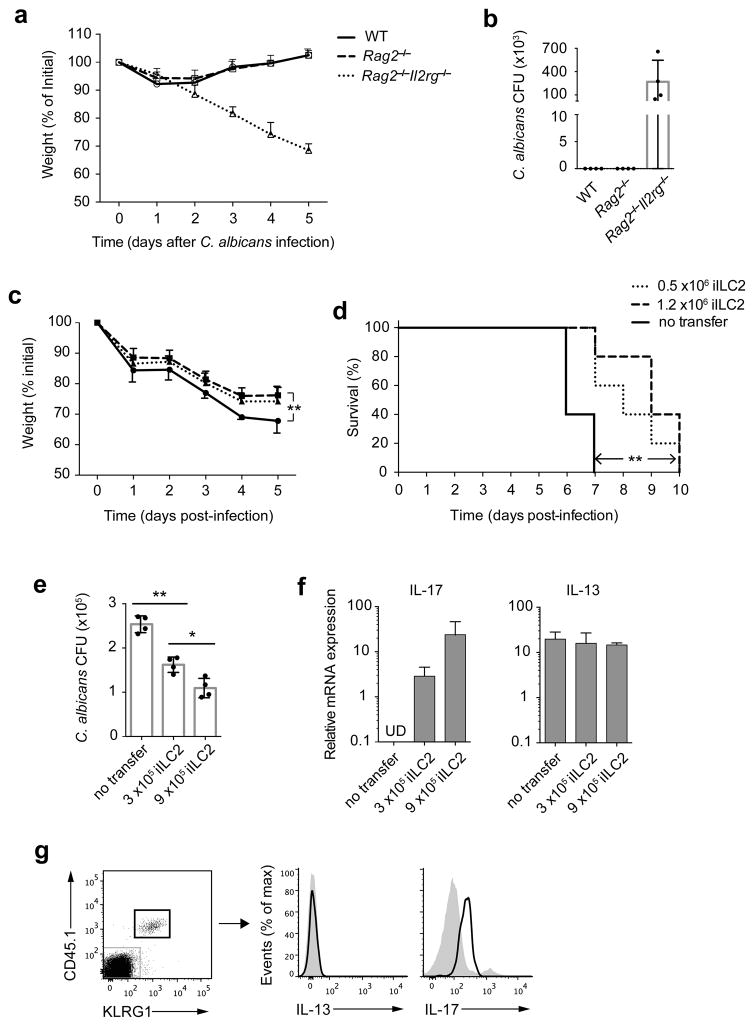
Since iILC2 cells can develop into IL-17 producers in vitro, we asked whether they play a role in controlling IL-17-sensitive pathogens. A previous study showed that IL-17-producing ILC3s were important for protection in C. albicans oral infection28. Wild-type, Rag2−/− and Rag2−/−Il2rg−/− mice were infected with C. albicans sublingually. Both wild-type and Rag2−/− mice lost ~10% body weight by 1–2 days after infection but recovered completely (Fig. 7a). In contrast, Rag2−/−Il2rg−/− mice displayed continued weight loss and all died by day 7 of infection (Fig. 7a,d). On day 6, a large number of C. albicans CFU was detected in the tongue tissue of Rag2−/−Il2rg−/− mice but not in wild-type or Rag2−/− mice (Fig. 7b). ILCs from tongue-draining LNs in infected Rag2−/− mice massively proliferated compared to uninfected mice (Supplementary Fig. 7), indicating that in C. albicans oral infection,γc-dependent cells, presumably ILCs, play a major role in control of infection. In addition, ~35% of the ILCs expressed IL-17RB, of which a portion were also KLRG1+ (Supplementary Fig. 7), suggesting that the IL-25-resposive ILCs may play some role in the innate lymphocyte response to C. albicans infection.
Figure 7.
iILC2 cells contribute to anti-fungal immunity. (a) Wild-type, Rag2−/− and Rag2−/−Il2rg−/− mice were infected sublingually with C. albicans and weighed daily. (b) Fungal burden in entire tongue of the mice as in a on day 5 post infection. (c,d) 0.5 ×106 or 1.2 ×106 iILC2s sorted from IL-25-treated Rag2−/− mice were i.v. transferred into Rag2−/−Il2rg−/− mice. Recipient mice were infected sublingually with C. albicans on the next day. The mice were observed for survival and weighed daily. (e) 3 ×105 or 9 ×105 iILC2s sorted from IL-25-treated CD45.1 Rag1−/− mice were i.v. transferred into Rag2−/−Il2rg−/− mice, followed by C. albicans sublingual infection on next day. On day 5 post infection, two-thirds of the tongue tissue was analyzed for fungal burden. (f) The other one-third tongue tissue in e was analyzed for IL-17 and IL-13 mRNA expression. (g) Lung leukocytes of the mice receiving 9 ×105 iILC2s in e were analyzed for IL-13 and IL-17 production without stimulation, by intracellular cytokine staining. a,c, mean ± s.e.m.; **P≤ 0.01 (paired two-tailed t test). b,e,f, mean ± s.e.m.; *P≤0.05, **P≤0.01 (unpaired two-tailed t test). d, **P≤0.01 (log-rank (Mantel-Cox) test). Data are representative of three (a) or two (b–g) independent experiments (n=3~5 mice for each group in each experiment of a–g).
We transferred 0.5 or 1.2 × 106 iILC2 cells into Rag2−/−Il2rg−/− mice and carried out C. albicans sublingual infection. iILC2s significantly diminished the weight loss of infected mice, with the transfer of 1.2 × 106 iILC2s limiting weight loss by ~1/3 (Fig. 7c). iILC2s also improved survival by 4 days (Fig. 7d). C. albicans CFU in the tongue tissue was reduced in the two groups that received iILC2s, with the group that had received 9 × 105 cells in another experiment showing a lower number of CFU in the tongue (Fig. 7e), confirming that iILC2s can provide partial protection against the pathogen.
There was no detectable IL-17 mRNA in “no transfer” Rag2−/−Il2rg−/− mice; in contrast, IL-17 mRNA was easily detected in the tongues of mice that had received iILC2s, being greater in the tongues of mice that had received a larger number of iILC2s (Fig. 7f). Yet, transfer of iILC2s did not enhance IL-13 expression in the tongue tissue. We also analyzed the cytokine production by transferred iILC2s in the lung of infected mice by flow cytometry. 5 days after transfer into C. albicans-infected mice, iILC2s were producing IL-17 but not IL-13 (Fig. 7g), indicating they became ILC3-like cells. Thus, iILC2 cells display a degree of in vivo plasticity and can provide partial protection against C. albicans.
DISCUSSION
ILCs are a first line of defense in many infectious models. They reside in barrier tissues and respond quickly to infections. Transient activation and expansion in numbers of ILCs occurs upon cytokine or pathogen stimulation. ILC2 cells show cytokine-based activation and expansion in response to IL-25 and/or IL-33. Whether the same cell populations respond to the two different cytokines had not been clarified. In the original report of nuocytes5, the heterogeneous expression of ST2 and IL-17RB could have been due to a mixture of separate cell populations, IL-17RB-expressing ILC2 cells and ST2-expressing ILC2 cells.
Here, we present evidence that responsiveness to IL-25 and IL-33 is a property of distinct cell types and propose that two ILC2 developmental pathways exist, a “natural or homeostatic ILC2 (nILC2) pathway” and an “inflammatory ILC2 (iILC2) pathway”.
In naïve mice, ST2+ nILC2s are resident in the lung and other tissues and become cytokine producers and expand moderately in number in response to IL-33 stimulation. These cells initiate IL-13 production quickly after N. brasiliensis infection although they show little expansion in cell number until after day 5. A second population of IL-13-producing ILCs arises in response to IL-25 treatment. These IL-17RB+ cells lack ST2 and express large amounts of KLRG1. We designate them iILC2s. iILC2 cells are undetectable in the lung and in most other peripheral tissues in naïve mice but show massive elicitation in response to IL-25 treatment, which is even greater in Rag2−/− mice. iILC2s appear by day 5 of N. brasiliensis infection, prior to the induction of TH2 cells or to the expansion of nILC2s. Expansion in numbers of nILC2 cells in N. brasiliensis-infected mice is significantly impaired in Il17rb−/− mice, which lack iILC2s. Based on their phenotypic changes in transfer experiments, we conclude that iILC2s become nILC2-like cells during N. brasiliensis infection. Thus, IL-25-induced iILC2s act as transient progenitors for nILC2-like cells. Since the number of nILC2s at barrier surfaces at steady state is low and their proliferation is both slow and limited, iILC2s appear to be an important source of ILC2 cells to combat helminth infection.
Development of iILC2s is dependent on expression of γc chain; iILC2s express IL-7Rα, as do nILC2 cells. iILC2s are induced normally in mice lacking ST2 and nILC2 numbers are normal in mice lacking IL-17RB, implying that development of the two populations is independent of the “alternative” cytokines.
The cytokine receptor expression and responsiveness pattern of iILC2s is dynamic. In vitro, highly purified iILC2s quickly acquire ST2 and IL-33 responsiveness while losing responsiveness to IL-25 and expression of IL-17RB, particularly when cultured in the presence of IL-33. When iILC2s are transferred into untreated mice, they become ST2+ nILC2-like cells in the lung.
We have reported that GATA-3 expression and STAT5 and NF-κB activation are essential for inducing TCR-independent ST2 expression in TH2 cells26. A similar requirement probably exists in ILC2 cells. IL-25-induced iILC2s express very large amounts of GATA-3 as do nILC2 cells; IL-7 activates STAT5 in both cell types; both IL-25 and IL-33 trigger NF-κB activation. Thus, in vitro induction of ST2 expression on iILC2s in response to the STAT5 activators IL-2 and IL-7 and to the addition of either IL-25 or IL-33 would seem quite reasonable.
Important differences between iILC2 and nILC2 cells are that iILC2s express an intermediate level of RORγt whereas nILC2s do not, and nILC2s are identified in lung and fat-associated lymphoid tissues but are hardly found in spleen or liver while iILC2s are present in many sites after IL-25 treatment, including lung, MLNs, spleen, liver and bone marrow.
Effector CD4+ TH cells have been shown to have the capacity to alter their cytokine-producing phenotype in response to infectious challenges or cytokine exposure29. Whether various types of ILCs also have plasticity or flexibility in their cytokine-producing potential is unknown. Generally, ILCs are considered to be terminally differentiated cells. Most are resident in barrier tissues and some constitutively produce cytokines. It has been reported that Gfi-1-deficiency leads ILC2 cells to lose their restricted cytokine-production phenotype; these cells can produce both IL-13 and IL-17 upon stimulation with PMA plus ionomycin, suggesting that ILCs may have plasticity in their cytokine-producing potential. We show here that iILC2s display a degree of plasticity or multipotentiality. In contrast to nILC2 cells, they express both GATA-3 and RORγt and have the capacity to develop into either nILC2-like cells or ILC3-like cells.
We propose that there are two ILC2 developmental pathways, one giving rise to tissue resident nILC2 cells and one to iILC2s. We have clarified the relationship between IL-33-resposive ILC2 and IL-25-resposive ILC2 cells and demonstrated that IL-17RB-expressing iILC2s and their immediate progenitors are precursors of an ST2-expressing ILC2 population during inflammation and infection. We also report that iILC2s display the capacity to be converted into distinct cytokine producers. Although ILCs lack antigen specific receptors, they read and distinguish types of microbial pathogens through epithelial-derived cytokines, to react properly and to orchestrate immune responses.
METHODS
4C13R Transgenic Mice
Transgenic mice expressing AmCyan under Il4 gene regulatory elements and destabilized DsRed (DsRed-DR) under Il13 gene regulatory elements (4C13R) were generated using BAC-recombineering technology with galK-selection. The BAC clone (RP97.23H11) containing the TH2 locus control region and the Il13, Il4 and Kif3a genes was obtained from Children’s Hospital of Oakland Research Institute. The plasmids p-AmCyan1-N1 and p-DsRed-Express-DR were obtained from Clontech. The start codon ATG of the Il4 gene in the BAC was targeted with a galK construct containing homology arms at both the 5′ and 3′ ends of the galK gene. galK was subsequently targeted with an AmCyan. Then, similarly, the ATG of Il13 gene in the “AmCyan-IL-4 BAC” construct was replaced with a DsRed-DR by galK-selection. The final BAC was fully sequenced and then linearized by digestion with AscI. Microinjection of the linear construct into B6 oocytes was followed by transfer into pseudopregnant foster mothers. The pups were screened to identify the mice containing both AmCyan and DsRed-DR by Southern blotting. The correlation of AmCyan and DsRed-DR expression with simultaneous IL-4 and IL-13 expression was shown by culturing CD4+ T cells from the transgenic mice under TH2 conditions for 3 d and then stimulating with PMA plus ionomycin for 4 h, after which cells were stained for IL-4 and IL-13 expression. The in vivo expression of AmCyan and DsRed-DR was also examined in Schistosoma mansoni-infected mice by flow cytometry.
Mice
Wild-type C57BL/6 mice were obtained from Taconic or Jackson Laboratory. B6/SJL (CD45.1 congenic), Rag1−/− (CD45.1 congenic), Rag2−/−, Rag1−/−, and Rag2−/−Il2rg−/− mice were from Taconic. Il7ra−/− mice were from Jackson Laboratory. IL-17F-RFP reporter mice were from C. Dong. Il1rl1−/− mice were from A. McKenzie. Il17rb−/− mice were generated in U. Siebenlist’s lab. All the mice used for experiments were between 6~18 weeks old. For N. brasiliensis or C. ablicans infections, mice were females at 8~10 weeks of age. All animal experiments were performed under the approval of NIAID Animal Care and Use Committee.
Antibodies and Reagents
The following fluorochrome-conjugated antibodies were used for flow cytometry. Anti-CD3ε (Clone ID, 145-2C11), anti-CD5 (53-7.3), anti-CD19 (1D3), anti-B220 (RA3-6B2), anti-CD11b (M1/70), anti-CD 11c (N418), anti-NK1.1 (PK136), anti-TCRγδ (eBioGL3), anti-Gr-1 (RB6-8C5), anti-FcεR1 (MAR-1), anti-CD4 (RM4-5), anti-CD8a (53-6.7), anti-CD49b (DX5), anti-TER119 (TER-119), anti-IL-7Rα (A7R34), anti-Thy1.2 (30-H12), anti-CD44 (IM7), anti-c-Kit (2B8), anti-Sca-1 (D7), anti-CD45.1 (A20), anti-CD45.2 (104), anti-IL-13 (eBio13A), anti-IFN-γ (XMG1.2), anti-IL-5 (TRFK5), anti-IL-17A (eBio17B7), anti-RORγt (AFKJS-9) and anti-T-bet (4B10) antibodies were from eBioscience. Anti-KLRG1 (2F1), anti-IL-4 (11B11), anti-Ki67 (B56) and anti-GATA-3 (L50-823) antibodies were from BD Biosciences. Anti-ST2 antibody (DJ8) was from MD Bioproducts. Anti-IL-17RB antibody (752101) was from R&D Systems. Recombinant IL-25 and IL-33 were from R&D Systems. For in vitro cell culture, recombinant IL-2, IL-7, IL-3, SCF, IL-4, IL-12, IL-1β, IL-6, IL-23 and TGF-β were from Peprotech or R&D Systems, and neutralizing antibodies, including anti-IL-4 (11B11), anti-IL-12 (C17.8) and anti-IFN-γ (XMG1.2), were from Harlan Laboratories. LIVE/DEAD fixable dead cell stain kit was from Life Technologies.
Flow Cytometry and Cell Sorting
Cells in PBS solution with 3% FBS were blocked with anti-CD16/CD32 (2.4G2, Harlan Laboratories) and then were incubated with fluorochrome-conjugated antibodies with LIVE/DEAD fixable dead cell stain dye. Staining and washing were performed at 4 °C. Cells were analyzed on an LSRII flow cytometer (BD Biosciences) and data was analyzed with FlowJo software version 10.0.6. For cell sorting, cells were stained and washed in a PBS solution with 10% FBS, but LIVE/DEAD dye was omitted. Cells were purified on FACS Aria cell sorter (BD Biosciences).
Intracellular Staning
For cytokine staining, monensin was added in culture media for last 2 hours during stimulation. After surface staining, cells were fixed with 4% Paraformaldehyde and permeabilized with 0.5% Triton-X100 in PBS solution, and then incubated with fluorochrome-conjugated cytokine antibodies. For GATA-3, RORγt and Ki67 staining, Foxp3/Transcription Factor Staining Buffer Set was used according to the manufacturer’s instruction (eBioscience).
Isolation of Leukocytes from Lung Tissue
Lung tissues were harvested after perfusion and disrupted into small pieces and then were digested with Liberase TM (Roche) plus DNase I (Roche) at 37 °C for 20 min. Tissue pieces were strained into single cells, and the leukocytes were purified by centrifugation using 40% Percoll (GE Healthcare Life Sciences) in PBS solution, followed by ACK solution (Life technologies) treatment.
In vitro “polarization” of iILC2s Cells
iILC2s purified through cell sorting from lungs of IL-25-treated Rag2−/− mice were cultured in various conditions for 7 days. For “TH1” conditions, IL-7 (10ng/ml), IL-2 (10U/ml), IL-12 (10ng/ml) and anti-IL-4 (10μg/ml) antibody were added in culture media; for “TH2” conditions, IL-7 (10ng/ml), IL-2 (10U/ml), IL-33 (10ng/ml), IL-4 (100U/ml), anti-IFN-γ (10μg/ml) and anti-IL-12 (10μg/ml) antibodies were added in culture media; for “TH17” conditions, IL-7 (10ng/ml), IL-2 (10U/ml), IL-1β (10ng/ml), IL-6 (10ng/ml), IL-23 (20ng/ml), TGF-β (5ng/ml), anti-IFNγ (10μg/ml), anti-IL-4 (10μg/ml), and anti-IL-12 (10μg/ml) antibodies were used in culture media. The culture media was refreshed every 2 or 3 days.
N. brasiliensis infection
Mice were injected subcutaneously with 300 third-stage (L3) N. brasiliensis larvae and cell transfer was performed on the same day if required. Mouse feces were collected from individual mouse on day 7 to day 9 post infection and eggs in feces were counted. Worm burden in small intestines were measured on day 14.
C. albicans Oral infection
Cotton swabs were immersed in a solution of 1 × 108 CFU C. albicans (Strain SC5314) for 5 min and then were placed sublingually in the mice for 45 min while mice were anesthetized. Cell transfer was performed the day before if required. Mice were weighed daily and death/survival was observed. For determining fungal CFU, tongues were disrupted in PBS solution and cultured on YPD plates for 2 days.
Quantitative PCR
One third of the mouse tongue was disrupted in TRIZol Reagent and total RNA was purified according to the manufacturer’s protocol (Life Technologies). Reverse transcription was performed by using Oligo(dT)20 primers. TaqMan probes were used to measure the expression of Il13 (Mm00434204_m1) and Il17a (Mm00439618_m1), and mRNA expression level was adjusted based on the expression level of Gapdh (Mm03302249_g1) (Life Technologies).
Enzyme-linked immunosorbent assay (ELISA)
Sorted cells were cultured under various conditions in 96-well plates, with 2 × 103 cells per well. With or without PMA plus ionomycin stimulation, supernatants of cultured cells were collected and diluted. The secreted IL-17 protein concentration was determined by using a mouse IL-17AF (heterodimer) ELISA Ready-Set-Go kit (eBioscience).
Statistical Analysis
Sample or experiment sizes were determined empirically for sufficient statistical power. No statistical tests were used to predetermine the size of experiments. No samples were excluded specifically from analysis, and no randomization or blinding protocol was used. GraphPad Prism 6 software was used for statistical analysis. Survival curves were analyzed according to Kaplan-Meier estimator and the difference between two groups was determined by Log-rank (Mantel-Cox) test. Statistical difference for other experiments was determined by two-tailed t-test. P values of ≤ 0.05 was considered to represent means that were statistically different. Statistical analysis was performed on groups with similar variance. Limited variance was observed within sample groups.
Supplementary Material
Acknowledgments
We thank J. Zhu for critical reading of the manuscript, J. Edwards for her assistance in preparation of sorter purified cells, L. Feigenbaum of the SAIC Laboratory Animal Sciences Program for injection of the recombinant BAC into oocytes, transfer into pseudopregnant females and screening of pups, C. Dong, MD Anderson Cancer Center, Texas, for IL-17F-RFP reporter mice and A. McKenzie, MRC Laboratory of Molecular Biology, Cambridge, UK, for Il1rl1−/− mice. We thank members of Laboratory of Immunology, NIAID, for discussions. This work was supported by the NIH NIAID Division of Intramural Research.
Footnotes
AUTHOR CONTRIBUTIONS
Y.H. and W.E.P. designed and interpreted the experiments and wrote the manuscript. Y.H. did the experiments. L.G. assisted with the experiments and read the manuscript. J.Q. did the C. albicans oral infection and P.R.W. assisted in the design and interpretation of C. albicans experiments. J.F.U. provided N. brasiliensis and helped to design and interpret N. brasiliensis experiments. X.C. generated 4C13R transgenic mice. U.S. provided Il17rb−/− mice. J.H.-L. assisted with cell culture and flow cytometry.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interest.
References
- 1.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 2.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 4.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 5.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saenz SA, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motomura Y, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHedlidze T, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilhelm C, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magri G, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15:354–364. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyler T, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi R, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SH, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spooner CJ, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat Immunol. 2013;14:1229–1236. doi: 10.1038/ni.2743. [DOI] [PubMed] [Google Scholar]
- 24.Hwang YY, McKenzie AN. Innate lymphoid cells in immunity and disease. Adv Exp Med Biol. 2013;785:9–26. doi: 10.1007/978-1-4614-6217-0_2. [DOI] [PubMed] [Google Scholar]
- 25.Mjosberg JM, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 26.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 28.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 29.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.