Abstract
Background
There is no effective pharmacotherapy for the acute respiratory distress syndrome (ARDS), and mortality remains high. Preclinical studies support the efficacy of mesenchymal stem (stromal) cells (MSCs) in the treatment of lung injury. The aim of this phase one clinical trial was to test the safety of a single dose of allogeneic bone marrow-derived MSCs in patients with moderate-to-severe ARDS.
Methods
The STem cells for ARDS Treatment (START) trial was a multi-center, open-label, dose-escalation phase one clinical trial of a single dose of intravenous MSCs in patients with moderate-to-severe ARDS. The trial is registered with clinicaltrials.gov number [NCT01775774]. The first three patients were treated with low dose MSCs (1million cells/kg predicted body weight (PBW)); the next three patients received intermediate dose MSCs (5 million cells/kg PBW); and the final three patients received high dose MSCs (10 million cells/kg PBW). Primary outcomes included the incidence of pre-specified infusion associated events and serious adverse events. Secondary outcomes included standard respiratory and systemic endpoints, 28- and 60-day mortality, and measurement of biologic markers of inflammation and endothelial and epithelial injury. The trial completed enrollment in January 2014.
Findings
There were no pre-specified infusion associated events or treatment-related adverse events in any of the nine patients in this trial. Serious adverse events (SAEs) were subsequently observed in three patients during in the weeks following the infusion: two patients expired >seven days after the MSC infusion, and one patient was discovered to have multiple embolic infarcts of the spleen, kidneys, and brain that were age-indeterminate but thought to have occurred prior the MSC infusion based on MRI results. None of these SAEs were thought to be MSC-related.
Interpretation
A single intravenous infusion of allogeneic, bone marrow-derived human MSCs was well tolerated in 9 patients with moderate to severe ARDS. Based on this phase one experience, we have proceeded to phase two testing of MSCs for moderate to severe ARDS.
Funding
The trial was funded by the National Heart, Lung and Blood Institute (NHLBI U01HL10871301).
Keywords: Pulmonary edema, acute lung injury, mesenchymal stemcells, phase one trial, acute respiratory failure
Background
Despite advances in our understanding of the pathogenesis of the acute respiratory distress syndrome (ARDS), no pharmacologic agent has reduced mortality in ARDS.1 Treatment remains primarily supportive, with lung-protective ventilation and a fluid conservative strategy, as well as early neuromuscular blockade and prone positioning in more severe cases.2-7 Mortality of ARDS has declined modestly with improved ventilator and fluid management but remains high (between 20-40% in clinical studies).1,8
Therapy with allogeneic bone marrow-derived human mesenchymal stem (stromal) cells (MSCs) is attractive as a potential new treatment for ARDS for several reasons. MSCs are multi-potent cells with low immunogenicity that secrete multiple paracrine factors including endothelial and epithelial growth factors, anti-inflammatory cytokines, and antimicrobial peptides.9-20 They are also capable of transferring mitochondria to injured epithelial cells.21 These characteristics are directly relevant to the principal abnormalities that underlie lung injury in patients with ARDS.1
Pre-clinical studies in small animal (mouse and rat) and large animal (sheep) experiments, as well as in an ex vivo perfused human lung model, demonstrated potential efficacy and safety of MSC administration for the treatment of ARDS.9,10,12,13,15,22-24 Zheng et al. recently published the results of a single-center trial testing a single dose of 1 million cells/kg adipose-derived human MSCs in 12 patients with moderate to severe ARDS and reported infusion-related adverse events.25 In addition, MSCs have been tested in over 2000 human patients for a variety of conditions, with no apparent major adverse effects.19 Based on these studies, we conducted a phase one dose escalation trial of bone marrow-derived human MSCs for the treatment of moderate to severe ARDS. This report summarizes the results of that trial.
Methods
Trial design
The STemcells for ARDS Treatment (START) trial was a multi-center, open-label phase one clinical trial to test the safety of a single dose of intravenous MSCs in patients with moderate-to-severe ARDS (clinicaltrials.gov identifier NCT01775774). The purpose was to determine the maximum tolerated MSC dose up to a dose of 10 million cells/kg PBW using three cohorts of three patients each, with a primary focus on safety. The nine patient dose-escalation protocol was selected based on several discussions with and approval by the US Food and Drug Administration (FDA). The protocol included a provision that the Data Safety Monitoring Board (DSMB), the FDA, or the study sponsor could decide to enroll more patients at any dose level if there were any pre-specified infusion-associated adverse events or serious adverse events related to the MSCs.
The first three patients were assigned to receive low dose MSCs (1 million cells/kg predicted body weight (PBW)); the next three patients were assigned to receive intermediate dose MSCs (5 million cells/kg PBW); and the final three patients were assigned to receive high dose MSCs (10 million cells/kg PBW). The dose of 10 million cells/kg PBW was selected as the final target dose of MSCs based on preclinical experiments in a large animal model of ARDS, which showed maximal efficacy as well as favorable safety with this dose.23 Data from the first patient of each cohort and each complete cohort were reviewed for safety prior to proceeding with enrollment of the next patient or escalation of the dose. The protocol was approved by the U.S. Food and Drug Administration and by the institutional review boards of the three participating hospitals.
Because this was among the first trials to test MSCs in patients with ARDS, the primary objectives were to test the safety and tolerability of the MSC infusion and determine a safe dose of MSCs for our planned phase two study. The secondary objectives were to measure standard respiratory and systemic organ endpoints.
The coordinating center for the trial was at the University of California, San Francisco (UCSF). Eligible study subjects were enrolled at UCSF's Moffitt-Long Hospital, Stanford University, and the Massachusetts General Hospital (MGH).
Source and preparation of MSCs
The allogeneic, bone marrow-derived human MSCs were prepared from bone marrow obtained from a healthy male donor (age 18-45), with support from the NHLBI Production Assistance for Cellular Therapies (PACT) program (David McKenna, MD, Principal Investigator). The mononuclear cell fraction of the bone marrow was enriched and tested for nucleated cells, differential, viability, flow cytometry, and sterility prior to seeding for culture. At 70% confluence, MSCs were lifted and passaged at a low density into a cell factory. At 70-80% confluence of the MSCs, the product was washed, harvested, resuspended, and cryopreserved. Karyotyping/G-banding was normal.
The cryopreserved MSCs were shipped frozen to the clinical sites in a validated liquid nitrogen dry shipper with continuous temperature monitoring device. Upon receipt, the cellular product was inspected and stored in a controlled, continuously monitored liquid nitrogen storage tank. Prior to administration, the MSCs were thawed, washed to remove dimethyl sulfoxide, and resuspended in Plasmalyte-A by the local cell therapy laboratory. The total volume of the MSC infusion was 100mL regardless of dose. The percent viability of the infused MSCs was determined by trypan blue exclusion after the MSCs had been thawed and prepared for infusion. The viability ranged from 50-63% (mean 56%).
Selection of trial subjects
Patients were enrolled in the intensive care units at UCSF, Stanford University, and MGH between July 8, 2013 and January 13, 2014. The inclusion criteria were moderate to severe ARDS as defined by (1) the acute onset of the need for positive pressure ventilation by an endotracheal or tracheal tube, (2) a PaO2/FiO2 ratio < 200 mmHg with at least 8 cmH2O positive end-expiratory airway pressure (PEEP), and (3) bilateral infiltrates consistent with non-cardiogenic pulmonary edema on frontal chest radiograph. The PEEP threshold was set at 8 cmH2O instead of 5 cmH2O to decrease the chance that a patient's hypoxemia was due in significant measure to atelectasis, and narrow the patient population to those with moderate to severe ARDS who would be most likely to benefit from the therapy.
To avoid enrolling patients with late ARDS, the study design excluded patients in whom > 96 hours had passed since meeting the Berlin definition for ARDS.8 Additionally, the MSC infusion had to be initiated within 120 hours of meeting the Berlin definition for ARDS. If the PaO2/FiO2 ratio improved to >300 mmHg on a PEEP of at least 8 cmH2O after enrollment but before infusion, the subject was considered no longer eligible to receive MSCs. Patients were also excluded if they had an active malignancy requiring treatment within the last two years, major trauma in the preceding five days, severe chronic respiratory disease requiring home oxygen or with a baseline PaCO2>50, moderate to severe liver failure (Childs Pugh score > 12), recent deep vein thrombosis or pulmonary embolism, World Health Organization class three or four pulmonary hypertension, or if they were moribund or there was not a commitment to full supportive measures other than cardiopulmonary resuscitation. The full inclusion and exclusion criteria are presented in Table 1.
Table 1. START Inclusion and Exclusion Criteria.
| inclusion criteria |
|---|
|
| Exclusion criteria |
|
Study procedures
Informed consent was obtained after discussion with the patient or an appropriate surrogate. Following informed consent, the cell therapy laboratory was alerted to the enrollment, and a two-hour period of bedside observation of hemodynamic and respiratory parameters was initiated to ensure that the patient was stable prior to the MSC infusion. The baseline stability criteria are listed in Table 2.
Table 2. Baseline Stability Criteria.
| In the supine position, patients must sustain the following for 2 hours prior to MSC infusion: |
|---|
|
After two hours of stability, the infusion was initiated using a standard blood filter tubing set. The cells were infused via gravity over approximately 60-80 minutes, with the infusion rate controlled by the investigator based on droplet count. The physician investigator remained at the bedside for duration of the infusion and for six hours after the infusion was initiated, observing for any signs of an adverse reaction. All patients were ventilated according to the modified ARDS Network lower tidal volume protocol.3 Data collection and on-study measurements are described in detail by Liu et al in a previous publication.26
Safety endpoints
The primary endpoints were (1) the incidence of pre-specified infusion-associated events occurring during the six hour interval beginning with the start of the MSC infusion and (2) serious adverse events (SAEs) unexpected in ARDS patients. Due to concern that infusion of MSCs could lead to transient obstruction of the pulmonary microcirculation with subsequent hemodynamic or respiratory compromise, all patients were monitored closely for any changes in respiratory or cardiovascular parameters by at least one study physician at the bedside during the one-hour infusion and for six full hours following the start of the infusion. Pre-specified infusion associated events are listed in Table 3. The incidence and nature of all serious adverse events were reviewed and independently adjudicated by the DSMB to determine whether they were believed to be related to MSC administration, with special focus on events that would be unexpected in a critically ill patient with ARDS. In addition, serum creatinine, total bilirubin and alanine aminotransferase (ALT) were measured on days three, seven and 14 (after administration of the MSCs) for safety monitoring if subjects were still hospitalized.
Table 3. Pre-Specified Infusion Associated Events.
| Any of the following occurring within 6 hours of MSC infusion: |
|---|
|
Secondary endpoints: respiratory and systemic endpoints
Respiratory efficacy endpoints included the lung injury score (LIS), duration of mechanical ventilation, and number of ventilator-free days at day 28. The LIS, a widely used measure of severity of lung injury, is composed of four components: (1) chest radiograph; (2) PaO2/FIO2 ratio; (3) PEEP; and (4) static compliance of respiratory system.27,28 At time points when the patient was not ventilated with positive pressure ventilation, the PEEP was treated as ≤ 5 cm H20, the PaO2/FIO2 ratio was treated as ≥ 300 mmHg, the compliance was not calculated, and the sum of points was divided by three instead of four.
Systemic outcomes included daily sequential organ failure assessment (SOFA) score, duration of vasopressor use (including day of enrollment), number of ICU-free days at day 28, and 28-day and 60-day mortality. The SOFA score incorporates the severity of organ dysfunction and predicts outcomes in critically ill patients.29,30 We calculated the SOFA score daily for study days one through 14, using the worst values for each parameter in the 24-hour period. When a single value required for calculation of the SOFA score was missing, we carried forward the value from the previous measurement.
Biologic Measurements
We also measured biologic markers in plasma collected at baseline, six hours post-infusion, and days one, two, and three. These included markers of inflammation, epithelial injury, and endothelial injury, selected based on the proposed mechanism of action of MSCs in ARDS and on the results of previous preclinical studies. Specifically, we measured inflammatory markers IL-6 and IL-8, a marker of lung epithelial injury (receptor for advanced glycation end products (RAGE)), and a marker of endothelial injury (angiopoeitin-2). Biomarkers were measured by enzyme-linked immunoassays (ELISAs) at baseline, six hours, days one and three (all ELISA kits from R&D systems, Minneapolis, MN). The remaining biomarkers listed in our clinical protocol were not measured in the phase 1 portion of this trial.
Data safety and monitoring
A Data Safety Monitoring Board (DSMB) including critical care physicians and a biostatistician with phase one trial experience was constituted for this trial and was responsible for reviewing data on each cohort of three patients at each dosing level and making recommendations regarding continuing, stopping, or altering the trial. In addition, a designated external medical monitor and scientific review committee (SRC) evaluated the first patient in each dosing cohort after seven full days of observation before enrollment proceeded. At the conclusion of the trial, the DSMB and SRC determined whether or not phase two testing was recommended, and if so, the dose of MSCs that should be administered.
Statistical methods
We report the incidence of all serious adverse events, including death, as well as the incidence of pre-specified infusion-associated events and non-serious adverse events thought to be related to the MSC infusion. Baseline and on-study LIS, SOFA, and APACHE scores among the treatment groups were compared using Analysis of Variance (ANOVA). Systemic clinical outcomes (including duration mechanical ventilation, ventilator-free days, duration of vasopressor and ICU-free days) and biomarker values were compared using Kruskal–Wallis one-way analysis of variance. The software package used for all statistical analyses was STATA version 12.1 (College Station, Texas). Remaining analyses are descriptive.
Role of the funding source
The trial was funded by the National Heart, Lung and Blood Institute (NHLBI U01HL10871301). The sponsors of the trial had no role in study design, nor did they participate in the collection, analysis, or interpretation of the data. The sponsors had no access to the raw data, nor did they have any role in writing this report. The corresponding author (MM) had full access to all of the data and the final responsibility to submit for publication.
Findings
As planned, nine patients were enrolled: three patients received the low dose (1 million cells/kg PBW), three patients received the intermediate dose (5 million cells/kg PBW), and three patients received the high dose (10 million cells/kg PBW) MSCs. Baseline characteristics of each patient are presented in Table 4. Most of the patients (seven of nine) had pneumonia or aspiration as the primary cause of ARDS. While several patients met criteria for severe ARDS when first identified by the study team, all nine had moderate ARDS by PaO2/FiO2 ratio at the official time of enrollment. Clinical variables, including mean age, APACHE III score, PaO2/FiO2 ratio, and lung injury score were similar across the three dosing groups at baseline.
Table 4. Baseline Characteristics.
| Cohort/Patient | Age (years) | sex | Apache III | Primary cause of ARDS | Tidal Volume (mL/kg PBW) | Platean Pressure (cm H2O) | PEEP (cm H2O) | PaO2/FiO2 (mmHg) | Lung Injury Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 million cells/kg PBW | |||||||||
| Patient 1 | 29 | Female | 81 | Preeclampsia | 7.0 | 28 | 10 | 173 | 3.25 |
| Patient 2 | 86 | Female | 121 | Pneumonia | 6.6 | 31 | 10 | 101 | 3.25 |
| Patient 3 | 59 | Female | 130 | Aspiration | 6.0 | 25 | 10 | 168 | 2.50 |
| 5 million cells/kg PBW | |||||||||
| Patient 4 | 67 | Female | 133 | Aspiration | 6.3 | 21 | 10 | 105 | 2.75 |
| Patient 5 | 62 | Female | 109 | Pneumonia | 5.6 | 20 | 14 | 111 | 2.50 |
| Patient 6 | 46 | Female | 83 | Aspiration | 6.0 | 19 | 10 | 153 | 3.00 |
| 10 million cells/kg PBW | |||||||||
| Patient 7 | 52 | Male | 121 | Pneumonia | 7.1 | 23 | 10 | 154 | 2.75 |
| Patient 8 | 55 | Female | 127 | Sepsis (Biliary) | 5.9 | 34 | 10 | 194 | 3.50 |
| Patient 9 | 38 | Male | 68 | Pneumonia | 6.0 | Not measured | 8 | 118 | 2.33 |
PBW = predicted body weight; PEEP = positive end expiratory pressure.
Infusion-associated events and serious adverse events
All patients tolerated the MSC infusion well and there were no pre-specified infusion associated adverse events. No patient suffered any immediate complication or respiratory or cardiovascular compromise in the six hours following the MSC infusion, and there were no cardiac arrests or deaths within 24 hours of the MSC infusion. Specifically, there were no significant changes in heart rate, mean arterial pressure, or oxygen saturation in any of the three dosing groups during the infusion or in the immediate post-infusion period (Figure 1). Additionally, safety laboratory values (mean serum creatinine, total bilirubin, and alanine aminotransferase) were not significantly changed for any of the three dosing groups.
Figure 1. Respiratory and Hemodynamic Parameters During and Post-MSC Infusion.
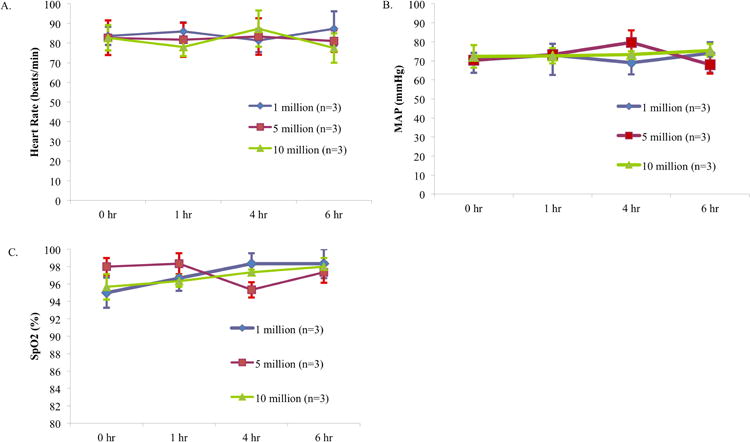
Mean (+/- SD) values for each dosing group for (A) heart rate (beats per minute), (B) mean arterial pressure (mmHg), and (C) arterial oxygen saturation as measured by pulse oximeter (SpO2)(%) at baseline, one, four, and six hours from start of MSC infusion.
There were three patients who subsequently developed serious SAEs in the weeks following the infusion: two patients expired >seven days after the MSC infusion, and one patient was discovered to have multiple embolic infarcts of the spleen, kidneys, and brain that were age-indeterminate but thought to have occurred prior the MSC infusion based on MRI results. This third SAE was determined by investigators to be unexpected in ARDS. All three SAEs were reviewed by the SRC and DSMB, and based on their independent analyses, none were thought to be related to MSC administration. Details of the SAEs are presented in Table 5.
Table 5. Serious Adverse Events.
| Patient | Pre-specified Infusion Associated Events | Other adverse events | Description |
|---|---|---|---|
| 2 | None | Death on day 9 | Patient never recovered from ARDS/sepsis, developed worsening multi-organ failure and shock on study day 6, expired on study day 9. |
| 3 | None | Infarcts of kidneys, spleen, brain | Multiple, likely embolic infarcts of spleen, kidneys, and brain discovered incidentally on study day 0 and study day 1. Believed to have occurred prior to MSC infusion based on MRI results. Extensive work-up for embolic source was negative except for small abnormality of the mitral valve. Culture negative endocarditis was cited as a possible cause of emboli. Patient recovered and was discharged to an acute rehabilitation facility. |
| 5 | None | Respiratory arrest on day 24, death on day 31 | Patient recovered from ARDS and was discharged from ICU. On study day 24 suffered respiratory arrest believed related to aspiration and was transferred back to ICU. Developed worsening multi-organ failure and expired on study day 31. No resuscitation was attempted in keeping with family wishes. |
Mortality
Two patients expired within 60 days of study infusion, for a mortality rate of 22% (2/9). One death occurred in the low dose group on study day nine, and one death occurring in the intermediate dose group on study day 31. Each death was reviewed in detail and neither was believed to be related to study participation. Vital status and study day at discharge for each patient are listed in Table 6.
Table 6. Secondary respiratory and systemic results.
| Dosing Cohort | Patient number | Duration of mechanical ventilation (days) | Ventilator-free days (through day 28) | Oxygenation Index (day 3) | Duration of vasopressor use (days) | ICU-free days (through day 28) | Vital status and day of discharge |
|---|---|---|---|---|---|---|---|
| Low Dose | 1 | 5 | 24 | 2.33 | 0 | 24 | Alive, day 8 |
| 2 | 10 | 0 | 13.91 | 10 | 0 | Dead, day 9 | |
| 3 | 11 | 18 | 5.78 | 4 | 14 | Alive, day 22 | |
| Intermediate Dose | 4 | 7 | 22 | 4.63 | 0 | 21 | Alive, day 34 |
| 5 | 31 | 0 | 5.36 | 2 | 0 | Dead, day 31 | |
| 6 | 3 | 27 | * | 3 | 26 | Alive, day 5 | |
| High Dose | 7 | 3 | 26 | 10 | 0 | 22 | Alive, day 7 |
| 8 | 17 | 12 | * | 0 | 9 | Alive, day 25 | |
| 9 | 19 | 20 | 6.39 | 0 | 18 | Alive, day 14 |
Oxygenation index = FiO2 × mean airway pressure/PaO2
Extubated.
Respiratory Outcomes
The mean lung injury score declined (improved) between baseline and day three in all three dosing groups (Figure 2). Numerically, the greatest decrease in LIS was observed in the high dose cohort and the smallest decrease was observed in the low dose cohort (high dose 2·9→1·6 (-45%), intermediate dose 2·8→1·8 (-36%), low dose 3→2·1 (-30%)), though these differences between groups were not statistically significant (p = 0·8720). None of the patients received rescue therapies for refractory hypoxemia (no extracorporeal membrane oxygenation and no inhaled nitric oxide or vasodilators). Two of the nine patients were extubated prior to study day three. One patient was extubated on two different occasions, however required re-intubation within 48 hours both times (primarily due to hepatic encephalopathy), and was never successfully liberated from the ventilator prior to death. Finally, one of the nine patients was never extubated and remained on mechanical ventilation with non-resolving ARDS and worsening multi-organ failure until death on study day nine. The duration of mechanical ventilation, number of ventilator-free days (as of day 28), and oxygenation index for each patient are listed in Table 6.
Figure 2. Lung Injury Score.
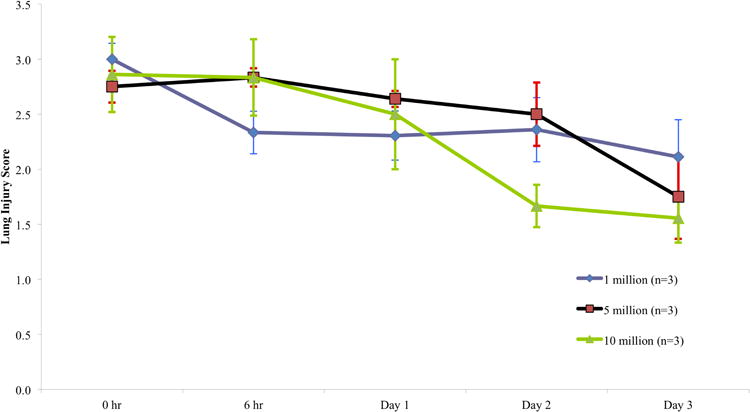
Mean (+/- SD) lung injury score (LIS) for each dosing group at basleline, six hours from start of MSC infusion, and study days one, two, and three. The LIS is calculated from four variables: (1) number of affected quadrants on chest radiograph; (2) severity of hypoxia as measured by PaO2/FIO2 ratio; (3) level of PEEP; and (4) the static compliance of respiratory system.26
Systemic Clinical Outcomes
Mean SOFA score declined in all three dosing groups over the first three days (Figure 3). As with LIS, the greatest numerical decline was observed in the high dose cohort and the smallest decrease was observed in the low dose cohort (high dose 7→3·7 (-48%), intermediate dose 8·7→6·7 (-23%), low dose 8→7·7 (-4%)). The differences among groups were not statistically significant (p = 0 ·7653). Duration of vasopressor administration and number of ICU-free days are listed in Table 6.
Figure 3. Sequential Organ Failure Assessment (SOFA) Score.
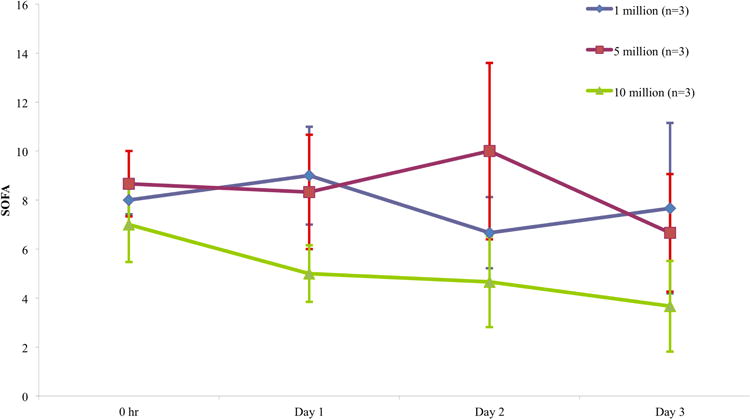
Mean (+/- SD) SOFA score for each dosing group at basleline, six hours from start of MSC infusion, and study days one, two, and three. The SOFA score quantifies the severity of organ dysfunction in six systems (respiratory, coagulation, hepatic, cardiovascular, renal, and neurologic), and predicts outcomes in critically ill patients.28,29
Plasma Biomarker Profiles
Median levels and interquartile ranges of IL-6, IL-8, RAGE, and Ang-2 levels in patient plasma at baseline, six hours, day one, and day three are listed in Table 7. Median levels of all three biomarkers declined between baseline and day three. There were no significant differences in the magnitude of decline among groups for any of the biomarkers (p = 0·3679, 0·3189, 0·3189, and 0·8669, respectively).
Table 7.
Median biomarker concentrations [interquartile range] at baseline, 6 hours, days 1 and 3.
| Marker | Day | 6 hr | Day1 | Day 3 |
|---|---|---|---|---|
| IL-6 (pg/mL) | 762 [419, 1198] | 557 [91, 734] | 317 [150, 736] | 62 [20, 140] |
| IL-8 (pg/mL) | 35 [18, 48.5] | 26 [16, 39] | 29 [19, 55] | 16 [8, 47] |
| ANG-2 (pg/mL) | 7507 [3977, 14950] | 8168 [4415, 13000] | 10900 [4593, 18200] | 6922 [4783, 18700] |
| RAGE (pg/mL) | 2749 [894, 5060] | 2841 [1055, 4333] | 1790 [882, 5080] | 1308 [1268, 2437] |
Interpretation
Intravenous administration of a single dose of bone marrow-derived human MSCs was well tolerated in this phase one trial in nine patients with moderate to severe ARDS, with no evidence of pre-specified infusion associated adverse events, immediate clinical instability, or dose-limiting toxicity at any of the doses tested. Based on external review by the SRC and DSMB, none of the SAEs observed in our trial were related to MSC infusion. Thus, the primary outcomes suggest that all three doses of MSCs are safe in patients with moderate to severe ARDS.
The mortality rate in this cohort was 22%, which is lower than the expected mortality in patients with moderate ARDS according to the Berlin severity stages (32%), and similar to the mortality rate reported by Kangelaris et al. in ARDS patients with a similar baseline LIS (23%).8,27 Thus, the mortality rate in our trial is in keeping with (or lower than) the expected mortality rate in moderate ARDS patients and critically ill patients more generally.
The favorable changes observed in LIS and SOFA score with the high dose of MSCs (10 million cells/kg PBW) compared to both lower doses are consistent with the hypothesis that higher doses of MSCs might provide greater clinical benefit. However, none of these differences were statistically significant, and given the lack of a control group in this phase one trial, we cannot conclude that these differences reflect a true dose response.
Although median levels of IL-6, RAGE, and Ang-2 levels all decreased between baseline and day three, there was no apparent dose effect. Additionally, these markers of inflammation and epithelial/endothelial injury are known to decline over time in patients with ARDS treated with low tidal volumes.31-33 Thus, without a matched control group, we cannot conclude that the observed biomarker changes were related to MSC therapy. The phase 2 iteration of this trial will include a control group to compare the same biomarkers in the MSC-treated and placebo patients. In addition, the phase 2 protocol includes mini bronchoalveolar lavage at 48 hours in order to sample the distal airspaces and permit measurement and comparison of the same biomarkers sampled from the placebo treated control patients versus the MSC-treated patients.
Interestingly, Zheng et al. recently published the results of a single-center, randomized, double-blind, and placebo-controlled trial in which 12 patients with moderate to severe ARDS were randomized in a one:one fashion to receive either a single dose of 1 million cells/kg allogeneic adipose-derived human MSCs or saline placebo.25 In this trial as in ours, there were no infusion toxicities or MSC-related serious adverse events. Secondary outcomes including ventilator-free days and ICU-free days were similar in both groups. While no changes in biomarkers (including surfactant protein D, IL-6, and IL-8) were observed in the placebo group, day five serum levels of surfactant protein D were significantly lower in the MSC group, and there was a non-significant trend towards lower levels of IL-6 as well. The Zheng et al trial had several important limitations: (1) the only dose tested in the 6 patients who received MSCs was the lowest dose tested in our trial,(1 million cells/kg), which is 1/10th of the dose that showed maximal efficacy - and no increased toxicity - in the large animal model we previously described;24 and (2) the MSCs were adipose-derived and re-cultured in the patient's own serum after enrollment, a technique that diverges from the standard within the field. These important differences limit the generalizability of their findings, as well as further comparison to the phase 1 trial that we are currently reporting.
Another relevant recent trial was a phase one dose escalation trial of intratracheal human umbilical cord blood-derived MSCs in nine preterm infants at high risk for bronchopulmonary dysplasia (BPD).34 Again, similar to the results of our trial, the therapy was well tolerated, although in this case there was also a suggestion of benefit in terms of respiratory outcomes. In terms of biomarker response, the authors observed a decline in inflammatory cytokines following MSC therapy, although it is unclear whether this was due to the immunomodulatory effects of the MSCs or merely reflected the natural course of inflammation in the development of BPD.34 Therefore, although the source and dose of MSCs differed among these trials, and conclusions about efficacy and biomarker response are unwarranted, the consistency in the results in terms of tolerability and short-term safety is encouraging.
Finally, Weiss et al. conducted a multi-center, double-blind, placebo controlled randomized trial of four monthly intravenous infusions of 100 million MSCs in 62 patients with moderate-to-severe chronic obstructive pulmonary disease. There were no infusional toxicities, deaths, or serious adverse events deemed related to MSC administration.35 Taken together with our trial, these findings suggest but do not prove that MSC infusions are well-tolerated in patients with either acute or chronic respiratory compromise.
There are some limitations to this small phase one trial. First and foremost, with only nine patients, we cannot generalize our phase one experience, nor draw conclusions about either the efficacy or long-term safety of MSCs for ARDS. Indeed, the absence of any statistically significant differences in secondary outcomes should be interpreted as a reflection of the lack of statistical power in this small study, rather than as confirmation of lack of effect.
The limitations of a small sample size are further amplified by the inherent challenges of conducting clinical trials in critically ill patients, in whom it is often difficult to discern whether medical events are related to underlying critical illness or the experimental therapy being tested. In this trial, the requirement of baseline stability prior to infusion was intended to decrease the noise of critical illness and make it more feasible to identify harmful effects of the MSC infusion.
Finally, although there were no significant differences in baseline LIS, SOFA score, or APACHE III score among the different dosing cohorts, it remains possible that differences in baseline severity of illness confounded the secondary outcomes we observed in terms of change in LIS and SOFA score. For example, none of the patients in the high dose cohort were treated with vasopressors, which could mean the improvement observed in that cohort was due to the absence of shock, rather than to the increased dose of MSCs. Indeed, the optimal dose of MSCs remains unclear; although the high dose of 10 million MSCs/kg showed greater efficancy in the preclinical study of severe lung injury in sheep,23 and the higher dose in this trial was well tolerated, it remains uncertain if that dose was more effective than lower doses in this trial, or if an even higher dose or repeated doses would be tolerated or provide additional benefit.
In conclusion, a single intravenous MSC infusion of up to 10 million cells/kg PBW was well-tolerated in patients with moderate to severe ARDS in this phase one trial. There were no serious adverse events related to MSC administration after six-months of follow-up. This favorable tolerability and short-term safety profile is in keeping with prior research on MSCs for other clinical indications. Based on the recommendations of the DSMB, we are currently conducting a randomized, double-blind placebo-controlled phase two clinical trial of 10 million MSCs/kg PBW in 60 patients with moderate to severe ARDS, with a primary focus on safety and secondary outcomes including respiratory, systemic, and biologic endpoints.
Research in context.
Systematic Review
This trial was planned based on extensive pre-clinical testing of mesenchymal stem (stromal) cells (MSCs) for acute lung injury carried out by our research group, as well as based on a review of published articles identified by searches of Medline, Current Contents, PubMed, and references from relevant articles using the search terms “MSC”, “mesenchymal stem cells”, “mesenchymal stromal cells”, “marrow stromal cells”, “acute respiratory distress syndrome”, “acute lung injury”, and “sepsis”. We also reviewed studies of MSCs in humans for other indications, such as acute myocardial infarction and chronic obstructive pulmonary disease. The many preclinical studies reviewed suggest that MSC therapy holds substantial therapeutic promise for ARDS, and the human trials suggest that MSCs are well-tolerated in various disease states.
Interpretation
The present trial demonstrates that a single intravenous dose of MSCs of up to 10 million cells/kg predicted body weight was well tolerated in 9 patients with moderate-to-severe ARDS. This safety profile is in keeping with the favorable safety record of MSCs in previous trials for other indications, and also the limited number of trials that have tested MSCs for respiratory problems. These findings indicate that it is safe to proceed to phase 2 testing of MSCs for ARDS in a larger cohort of patients, at the highest dose tested. At this time, it is premature to make any conclusions about the long-term safety or efficacy of MSCs for the treatment of ARDS.
Acknowledgments
This trial was supported by NHLBI U01 HL10871301, an NIH/NCRR UCSF-CTSI Grant RR024131 (T1 Catalyst Award), and the NIH-supported Production Assistance for Cellular Therapies group (Molecular and Cellular Therapeutics, University of Minnesota), Contract # HHSN268201000008C. The authors also appreciate the support of the intensive care nursing at UCSF, Stanford Medical Center and the Massachusetts General Hospital. We appreciate the work of Jason Abbott who carried out the biologic measurements and Brian Daniel, RRT who assisted with adherence to the ARDS Network lung protective ventilation protocol. The authors also thank the Data Safety Monitoring Board (Gordon Bernard, MD, Chair; Herbert Wiedemann, MD; Kevin Delucchi, PhD; and Scott Emerson, MD, PhD) and the Medical Monitor (Marc Moss, MD).
Footnotes
Contributors: All authors contributed to this manuscript. KDL, HZ, LC, CSC, JWL, AL, DM, BTT, and MAM designed the trial. JGW, LC, MM, XF, KC, RV, AJR, JL, JWK, EKB, AL, DM, BTT, and MAM collected data. JGW, KDL, HZ, XF, AJR, JL, JWK, EKB, DM, BTT, and MAM analyzed data. JGW, KDL, HZ, CSC, JWL, AJR, JL, JWK, EKB, AL, DM, BTT, and MAM interpreted data. JGW, KDL, and MAM provided the plan and wrote the manuscript, while CSC, AJR, JL, AL, DM, and BTT provided editorial overview and modified the report. HZ prepared the figures.
Declaration of Interests: All authors report receiving grant support from the National Heart, Lung, and Blood Institute for their work on this project. In addition, AR reports receiving grant support from the Parker B. Francis Foundation. DM reports additional grants from the National Heart, Lung, and Blood Institute (PACT program). KDL reports receiving grant support from the National Institute of Kideny and Digestive Diseases as well as financial/nonfinancial interests in Astute (adjudicator of clinical events), Abvie (advisory board member), Complexa (scientific advisory board member), Amgen (stockholder), Cytopheryx (Data and Safety Monitoring Board), Chemocentryx (consultant), and Abbot (receives assay reagents). CSC reports grant support from GlaxoSmithKline and consulting work with GlaxoSmithKline and Cerus. BTT reports consulting work with GlaxoSmithKline. MAM reports grant support from the National Institute of Allergy and Infectious Diseases, GlaxoSmithKline, as well as consulting work with GlaxoSmithKline, Cerus, and Roche-Genentec (Chair of Data and Safety Monitoring Board).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer G. Wilson, Email: jenny.wilson@ucsf.edu.
Kathleen D. Liu, Email: kathleen.liu@ucsf.edu.
Hanjing Zhuo, Email: hanjing.zhuo@ucsf.edu.
Lizette Caballero, Email: Lizette.Caballero@ucsfmedctr.org.
Xiaohui Fang, Email: xiaohui.fang@ucsf.edu.
Katherine Cosgrove, Email: kcosgrove@partners.org.
Rosemary Vojnik, Email: rvojnik@stanford.edu.
Carolyn S. Calfee, Email: carolyn.calfee@ucsf.edu.
Jae-Woo Lee, Email: leejw@anesthesia.ucsf.edu.
Angela J. Rogers, Email: angela.rogers@stanford.edu.
Joseph Levitt, Email: joseph.levitt@stanford.edu.
Jeanine Wiener-Kronish, Email: jwiener-kronish@mgh.harvard.edu.
Ednan K. Bajwa, Email: ebajwa@partners.org.
Andrew Leavitt, Email: leavitta@labmed2.ucsf.edu.
David McKenna, Email: mcken020@umn.edu.
B. Taylor Thompson, Email: tthompson@partners.org.
Michael A. Matthay, Email: michael.matthay@ucsf.edu.
References
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silversides JA, Ferguson ND. Clinical review: Acute respiratory distress syndrome-clinical ventilator management and adjunct therapy. Crit Care. 2012;17:225. doi: 10.1186/cc11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 6.Hu SL, He HL, Pan C, et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care. 2014;18:R109. doi: 10.1186/cc13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 8.The ARDS Definition Task Force. Acute Respiratory Distress Syndrome:The Berlin Definition. JAMA. 2012;307:8. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E.coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J RespCrit Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J RespCrit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise Review: Mesenchymal Stem Cells for Acute Lung Injury: Role of Paracrine Soluble Factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respiratory Medicine. 2014 doi: 10.1016/S2213-2600(14)70217-6. Early Online Publication. [DOI] [PubMed] [Google Scholar]
- 21.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curley GF, Hayes M, Ansari B, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 23.McAuley DF, Curley GF, Hamid UI, et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell MolPhysio. 2014;306:L809–815. doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asmussen S, Ito H, Traber DL, et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69:819–825. doi: 10.1136/thoraxjnl-2013-204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng G, Lanfang H, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory Research. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu KD, Wilson JG, Zhuo H, et al. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann Intensive Care. 2014;4:22. doi: 10.1186/s13613-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 28.Kangelaris KN, Calfee CS, May AK, Zhuo H, Matthay MA, Ware LB. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care. 2014;4:4. doi: 10.1186/2110-5820-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 31.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 32.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med. 2012;40:1731. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. JPediatr. 2014;164:966–972. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. CHEST. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]


