Abstract
The interplay between effector and regulatory T (Treg) cells is crucial for adaptive immunity, but how Treg control diverse effector responses is elusive. We found that the phosphatase PTEN links Treg stability to repression of TH1 and TFH (follicular helper) responses. Depletion of PTEN in Treg resulted in excessive TFH and germinal center responses and spontaneous inflammatory disease. These defects are considerably blocked by deletion of Interferon-γ, indicating coordinated control of TH1 and TFH responses. Mechanistically, PTEN maintains Treg stability and metabolic balance between glycolysis and mitochondrial fitness. Moreover, PTEN deficiency upregulates mTORC2-Akt activity, and loss of this activity restores PTEN-deficient Treg function. Our studies establish a PTEN-mTORC2 axis that maintains Treg stability and coordinates Treg-mediated control of effector responses.
Introduction
The interplay between immune regulatory mechanisms and effector T cell responses is a crucial determinant of adaptive immunity. Among multiple regulatory systems, thymus-derived regulatory T (Treg) cells marked by the transcription factor Foxp3 play a central role in maintaining self-tolerance and preventing autoimmune disease. Recent genetic studies highlight that Treg cells employ distinct transcriptional programs to control effector TH1, TH2 and TH17 responses1, 2. Specifically, Treg cells expressing the transcription factors T-bet, IRF4 and STAT3 orchestrate the control of effector TH1, TH2 and TH17 responses respectively3-5. Owing to their potent suppressive activity and functional diversity, the stability of Treg cells is actively maintained by Foxp3-dependent and independent mechanisms6, 7. However, under certain inflammatory conditions, Treg cells could lose Foxp3 expression and lineage stability and acquire effector functions8-11. Studies also demonstrate the heterogeneity of Treg cells distinguished by the expression of CD25, CCR7 and other molecules12-14. Defining the mechanisms involved in the functional diversification and lineage stability of Treg cells is crucial to understanding immune system regulation.
Follicular helper T (TFH) cells are a subset of CD4+T cells specialized in providing help to B cells for the formation of germinal center (GC) reactions and the development of humoral immunity15. Excessive TFH responses, however, lead to the development of autoimmune diseases including systemic lupus erythematosus (SLE)15, 16. TFH cells are characterized by the preferential expression of the chemokine receptor CXCR5, the co-stimulatory molecule ICOS, the inhibitory molecule PD-1, and the cytokine IL-21. Differentiation of TFH cells requires the interaction with antigen-presenting cells including dendritic cells and B cells, and is further programmed by lineage-specific transcription factors including Bcl6 and Ascl215, 17. Further more, TFH responses are restrained by a specific Treg cell subset, follicular regulatory T cells (TFR), in a Bcl6-dependent manner. TFR cells share phenotypic features with both thymus-derived Treg and TFH cells, as evidenced by the concomitant expression of Foxp3, CTLA4, GITR, Bcl6, ICOS, PD-1 and CXCR5, but are functionally distinct from these conventional populations18, 19. The molecular pathways that orchestrate the generation and function of TFR cells and the interplay with other effector cells have remained unclear.
Emerging studies reveal a central role of mechanistic target of rapamycin (mTOR), a signaling pathway that integrates immune and metabolic cues, in T cell-mediated immune responses20, 21. mTOR signaling is comprised of two distinct complexes: mTOR complex 1 (mTORC1) and 2 (mTORC2), which have unique contributions to effector T cell responses22-24, and functional fitness of Treg cells25. Because of the potent effects of mTOR signaling on T cell responses, multiple mechanisms are evolved to actively suppress mTOR signaling20. For instance, loss of the tumor suppressor Tsc1 aberrantly upregulates mTORC1 activity and disrupts T cell quiescence, homeostasis and functions26. T cell-specific deletion of PTEN, an upstream inhibitor of PI3K-Akt signaling, leads to the development of leukemia and autoimmunity27, 28. As a pluripotent molecule, PTEN antagonizes PI3K activity and thus inhibits both mTORC1 and mTORC2 activities20; PTEN also possesses nuclear functions independent of PI3K-Akt activity29. Although PTEN has been implicated in Treg cells from mice and humans30-32,Treg cells deficient in PTEN show largely normal suppressive activity in vitro33. Therefore, the functional impacts and molecular pathways of PTEN in Treg-mediated immune homeostasis and function remain to be established.
To investigate the in vivo functions and mechanisms of PTEN in Treg cells, we have developed a mouse model to delete PTEN selectively in Treg cells. Treg-specific loss of PTEN is sufficient to induce a systemic lupus-like autoimmune and lymphoproliferative disease. This is associated with excessive TFH and GC B cell responses, as well as exuberant interferon-γ (IFN-γ) production and TH1 reactions. Deletion of IFN-γ considerably rectifies TFH and autoimmune responses, indicating a crucial role of PTEN in Treg cells at coordinately controlling TH1 and TFH responses. Mechanistically, PTEN deficiency results in the loss of Treg functional stability and dysregulated transcriptional and metabolic programs, including the balance between glycolytic activity and mitochondrial fitness. Further, PTEN deletion mainly upregulates mTORC2, not mTORC1 activity, in Treg cells. Depletion of Rictor-mTORC2 activity is sufficient to restore the functional abnormalities observed in PTEN-deficient Treg cells. Finally, we present evidence that PTEN is haploinsufficient in Treg cells. Our studies establish a crucial role of the PTEN-mTORC2 axis in mediating Treg cell stability and homeostasis of the immune system, and highlight that their stability is actively maintained to coordinately regulate the magnitude of TH1 and TFH responses.
Results
Treg deletion of PTEN precipitates an inflammatory disease
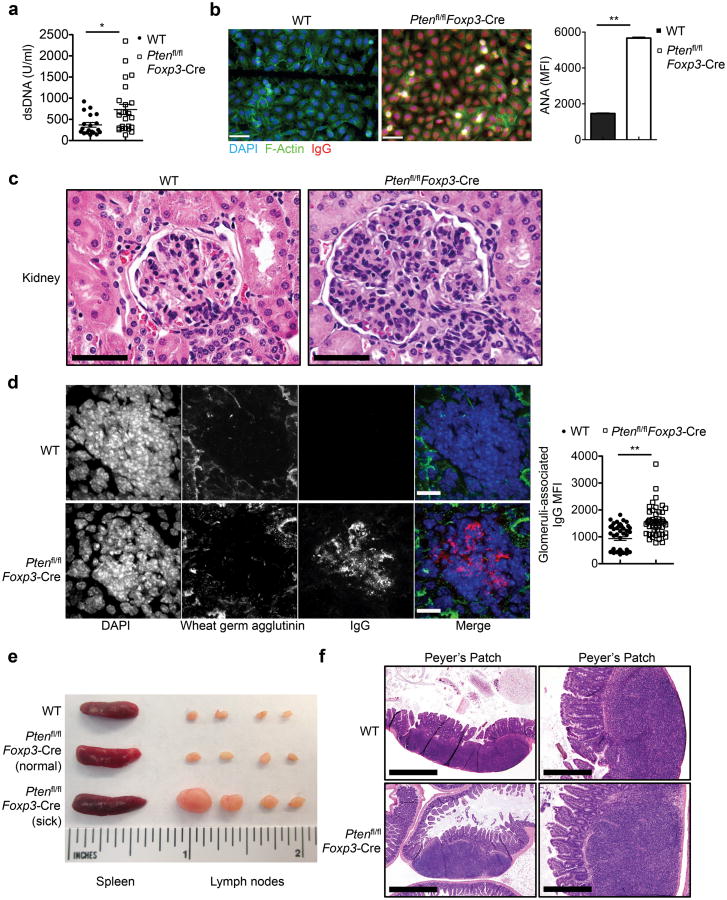
To investigate the requirement of PTEN in the homeostasis and functions of Foxp3+Treg cells, a central regulator of immune tolerance, we crossed mice with loxP-flanked Pten alleles (Ptenfl/fl) with Foxp3YFP-Cre (Foxp3-Cre) mice34 to delete the PTEN conditional alleles specifically in Treg cells but not naive T cells (hereafter Ptenfl/flFoxp3-Cre) (Supplementary Fig. 1a). To examine whether this alters self-tolerance, we measured the amounts of anti-dsDNA and anti-nuclear antigen (ANA) autoantibodies in the serum by ELISA and binding to fixed Hep-2 slides, respectively. As compared with Foxp3-Cre mice (designated WT), Ptenfl/flFoxp3-Cre mice contained significantly increased titers of circulating anti-dsDNA and anti-ANA antibodies (Fig. 1a,b), indicative of autoimmune reactions. This was associated with considerably elevated titers of serum IgG2a, IgG2c and IgG2b isotypes in Ptenfl/flFoxp3-Cre mice. In contrast, IgG1 and IgG3 titers were reduced, while IgM and IgA titers were comparable (Supplementary Fig. 1b-d). As systemic autoimmunity is frequently associated with renal pathology, we examined the structural integrity of the kidney. The size and cellularity of the glomeruli were greatly increased in the kidney from Ptenfl/flFoxp3-Cre mice, indicative of glomerulonephritis (Fig. 1c). Moreover, glomeruli of the Ptenfl/flFoxp3-Cre kidney contained prominent IgG deposits (Fig. 1d). Therefore, loss of PTEN in Treg cells results in dysregulation of serum autoantibodies and antibody isotypes and development of renal pathology, indicating a breakdown of immune tolerance in Ptenfl/flFoxp3-Cre mice.
Figure 1. Ptenfl/flFoxp3-Cremice develop age-related autoimmune and lymphoproliferative disease.
(a) Quantification of dsDNA-specific IgG in the serum of Pten+/+Foxp3-Cre (WT) and Ptenfl/flFoxp3-Cre mice (2-6 months old). (b) Representative images (scale 60 μm) and quantification of fluorescent intensity (right) of serum ANA IgG autoantibodies detected with fixed Hep-2 slides. (c) Histology images of kidney glomeruli sections stained with H&E (magnification: ×60; scale 50 μm). (d) Immune fluorescence images of kidney glomeruli sections showing IgG deposits (scale 40 μm). (e) Images of spleen and peripheral lymph nodes from WT (upper, ∼5 months old), Ptenfl/flFoxp3-Cre mice prior to the development of lymphoproliferative disease (middle, ∼2.5 months old), and Ptenfl/flFoxp3-Cre mice with lymphoproliferative disease (lower, ∼5 months old). (f) H&E staining of Peyer's patches in the intestine of WT and Ptenfl/flFoxp3-Cre mice (magnification: left, ×4; scale 1mm and right, ×20; scale 200 μm). Data are representative of at least two independent experiments (a-f). *P < 0.05 and **P < 0.001. Data are mean ± s.e.m.
In addition, Ptenfl/flFoxp3-Cre mice spontaneously developed lymphoid hyperplasia, which became prominent when they reached 4-5 months. Specifically, peripheral lymph nodes in Ptenfl/flFoxp3-Cre mice, especially those around the cervical regions, were markedly enlarged. These aged mice also contained a slight increase of the spleen size (Fig. 1e). Moreover, the number and size of lymphoid follicles in Peyer's patches were increased Ptenfl/flFoxp3-Cre mice (Fig. 1f). The development of lymphoadenopathy and Peyer's patch abnormality in Ptenfl/flFoxp3-Cremice indicated an ongoing lymphoproliferative disease.
Altered immune homeostasis upon Treg-specific loss of PTEN
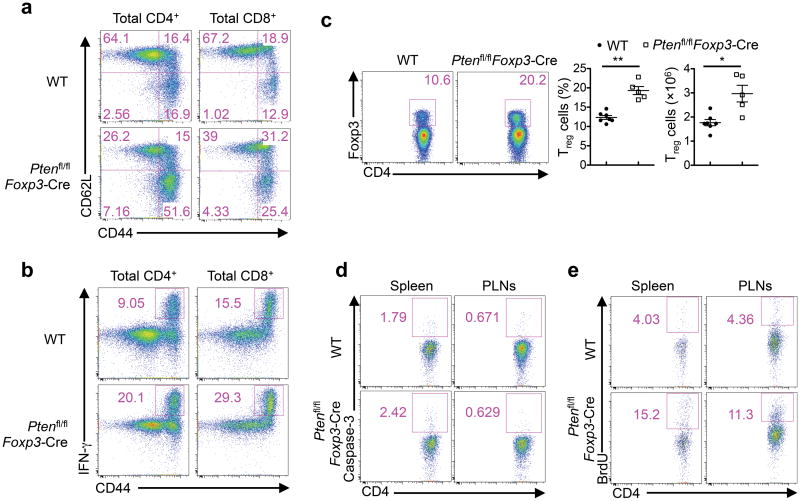
The development of the autoimmune and lymphoproliferative disease prompted us to examine whether homeostasis of the immune system was altered in Ptenfl/flFoxp3-Cre mice. In our following analyses, we used mice at a young age, prior to the development of the lymphoproliferative disease. The numbers of CD4+, CD8+, B cells, dendritic cells, and neutrophils were largely comparable between WT and Ptenfl/flFoxp3-Cre mice (Supplementary Fig. 2a-c). However, Ptenfl/flFoxp3-Cre mice contained increased numbers of activated CD62LloCD44hi effector/memory T cells in the CD4+ and CD8+ compartments (Fig. 2a). Moreover, CD44hi cells from these mice expressed elevated levels of IFN-γ (Fig. 2b). Similarly, expression of CXCR3, a signature chemokine receptor for TH1 cells, was also increased (Supplementary Fig. 2d). In contrast, IL-17 and IL-4 production was largely normal (Supplementary Fig. 2e). Thus, T cells from Ptenfl/flFoxp3-Cre mice were spontaneously activated in vivo, with a propensity to differentiate into the TH1 phenotype.
Figure 2. Increased T cell activation and altered immune homeostasis in Ptenfl/flFoxp3-Cre mice.
(a) Expression of CD62L and CD44 on WT and Ptenfl/flFoxp3-Cre splenic T cells. Numbers in quadrants indicate percent cells in each. (b) Expression of IFN-γ in CD4+ and CD8+ T cells of WT and Ptenfl/flFoxp3-Cre mice. (c) Flow cytometry analysis of Treg cells in WT and Ptenfl/flFoxp3-Cre splenic CD4+ T cells. Below, the frequency and numbers of Treg cells. (d,e) Caspase-3 activity (d) and BrdU staining at 16 h after injection of BrdU (e) in Treg cells from the spleen and peripheral lymph nodes (MLNs) of WT and Ptenfl/flFoxp3-Cre mice. Data are representative of at least ten independent experiments (a-c) and two independent experiments (d,e). *P < 0.05 and **P < 0.001. Data are mean ± s.e.m.
Despite severe autoimmune diseases, Ptenfl/flFoxp3-Cre mice had increased percentage and numbers of Foxp3+Treg cells in the spleen and lymph nodes (Fig. 2c, Supplementary Fig. 2f). To explore the underlying basis for the increased Treg cellularity, we used caspase-3 staining and BrdU incorporation assays to measure Treg apoptosis and proliferation, respectively. Treg cells from WT and Ptenfl/flFoxp3-Cre mice had comparable caspase-3 staining (Fig. 2d), but Ptenfl/flFoxp3-Cre Treg cells had more BrdU incorporation than WT cells (Fig. 2e), indicative of an elevated rate of proliferation. Therefore, PTEN deficiency causes an increased Treg cellularity and proliferation.
Uncontrolled TFH and GC B cells in Ptenfl/flFoxp3-Cre mice
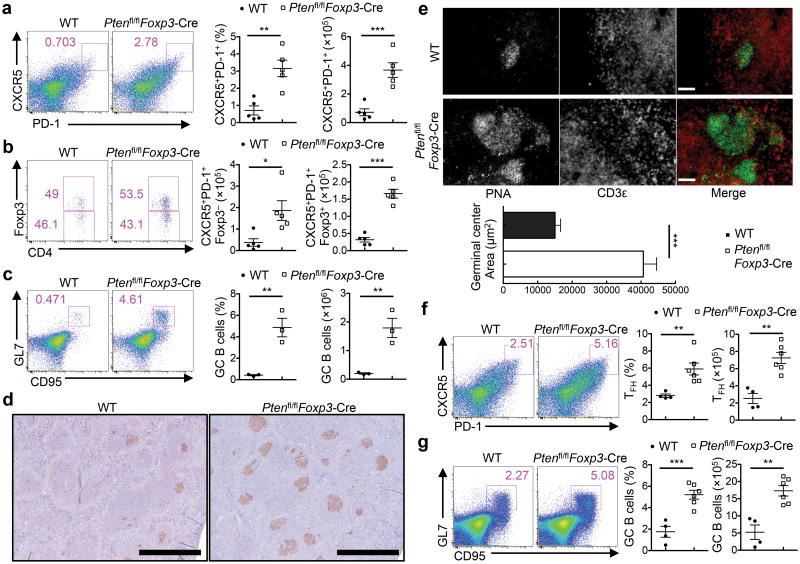
SLE, the prototypical systemic autoimmune disease, is characterized by the heterogeneity of underlying T cell responses. Aside from the roles of the conventional effector (e.g. TH1 and TH17) and regulatory responses, recent studies demonstrate a crucial role of TFH cells in the overproduction of pathogenic autoantibodies and tissue damage in SLE15, 16. The development of an SLE-like symptom in Ptenfl/flFoxp3-Cre mice prompted us to examine whether the TFH response was altered in these mice. Under steady state, only a small percentage of splenic CD4+T cells was stained positive for the TFH signature molecules CXCR5 and PD-1 in WT mice, but the CD4+CXCR5+PD-1+ population was greatly expanded in the spleen and mesenteric lymph nodes (MLNs) of Ptenfl/flFoxp3-Cre mice (Fig. 3a, Supplementary Fig. 3a). Similar changes were noticed for CXCR5+ cells expressing the TFH-specific co-stimulatory molecule ICOS and transcription factor Bcl6 (Supplementary Fig. 3b,c). The CD4+CXCR5+PD-1+ cells can be further divided into immunostimulatory TFH and immunoregulatory TFR cells, as indicated by the absence or presence of Foxp3 expression, respectively18, 19. Both subsets were increased in Ptenfl/flFoxp3-Cre mice (Fig. 3b). Consistent with the increase of CXCR5+ PD-1+ cells, the spleen and MLNs of Ptenfl/flFoxp3-Cre mice contained 3 fold more GC B cells, denoted by the expression of GC signature markers GL7 and CD95 (Fig. 3c, Supplementary Fig. 3d), even though total B cell numbers were largely unaltered (Supplementary Fig. 2b). Moreover, immunohistochemistry showed that the spleen and MLNs of Ptenfl/flFoxp3-Cre mice contained considerably more and larger Peanut Agglutinin (PNA)-positive GCs than did their WT counterparts (Fig. 3d,e). These results revealed spontaneous TFH and GC formation in Ptenfl/flFoxp3-Cremice.
Figure 3. The aberrant induction of TFH cell and GC responses in Ptenfl/flFoxp3-Cremice.
(a) Flow cytometry analysis of CXCR5+PD-1+ cells (gated on CD4+TCRβ+ cells) in the spleen of WT and Ptenfl/flFoxp3-Cremice. Right, the frequency and numbers of TFH cells. (b) Analysis of conventional TFH (CD4+CXCR5+PD-1+Foxp3-YFP−) and TFR cells (CD4+CXCR5+PD-1+Foxp3-YFP+) in the spleen of WT and Ptenfl/flFoxp3-Cremice. (c) Analysis of GL7+CD95+ GC B cells (gated on CD19+ B cells) in the spleen of WT and Ptenfl/flFoxp3-Cremice. Right, the frequency and numbers of GC B cells. (d) PNA staining of spleen sections of WT and Ptenfl/flFoxp3-Cremice (magnification, ×4; scale 1mm). (e) Immune fluorescence of MAN sections of WT and Ptenfl/flFoxp3-Cremice for the staining of CD3 (red) and PNA (green) (scale 60 μm). Bottom, quantification of germinal center area. (f,g) Analysis of TFH (f) and GC B cells (g) in the spleen of mice immunized with SRBCs 7 days previously. Data are representative of at least ten independent experiments (a-c) and two independent experiments (d-g). *P < 0.05, **P < 0.01 and ***P < 0.001. Data are mean ± s.e.m.
We next determined whether PTEN deficiency in Treg cells affects TFH and GC responses after TFH-inducing immunization. After immunization with sheep red blood cells (SRBCs), a strong protein antigen, the formation of TFH cells and GC B cells was greatly enhanced in Ptenfl/flFoxp3-Cre mice compared with WT mice (Fig. 3f,g). We observed a similar finding after challenging WT and Ptenfl/flFoxp3-Cre mice with a T cell-dependent antigen, NP-OVA precipitated in alum together with LPS (Supplementary Fig. 3e,f). We conclude that deletion of PTEN in Treg cells results in enhanced TFH and GC reactions both under steady state and upon immunization.
Coordination of TH1 and TFH responses by Treg signaling
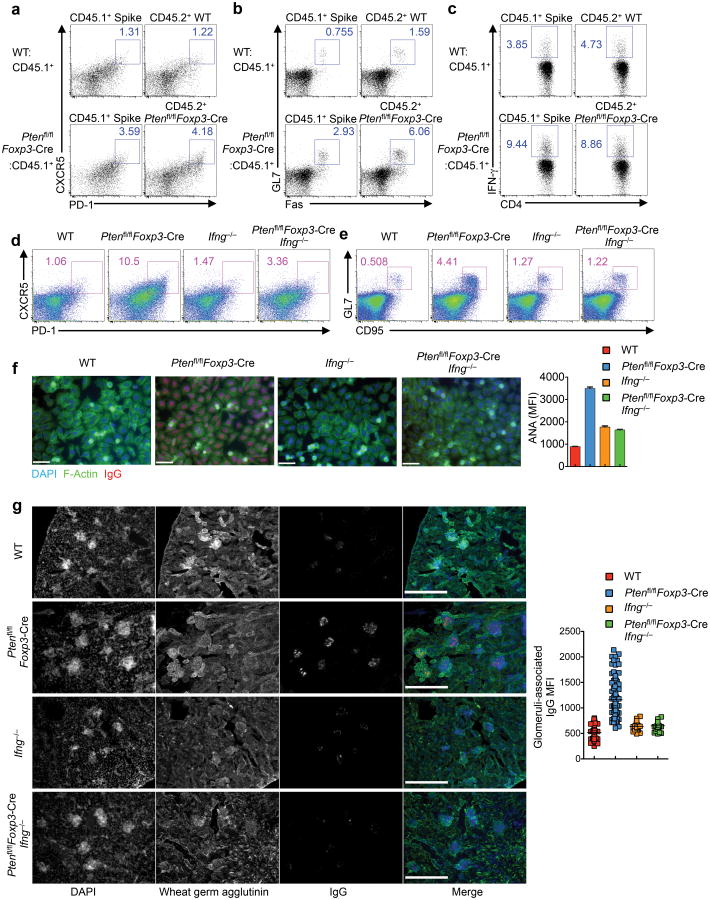
We next investigated whether the increased TFH response in Ptenfl/flFoxp3-Cre mice was a cell-autonomous defect. To this end, we generated mixed bone marrow (BM) chimeras by reconstituting alymphoid Rag1−/− mice with a 1:1 mixture of Ptenfl/flFoxp3-Cre CD45.2+ (donor) and CD45.1+ (spike) BM cells (Ptenfl/flFoxp3-Cre:CD45.1+), and as a control, a mixture of WT and CD45.1 cells (WT:CD45.1+). The frequency of TFH cells was considerably increased in both the donor and spike-derived populations in the Ptenfl/flFoxp3-Cre:CD45.1+ chimeras, as compared with the frequency of TFH cells in the WT:CD45.1+ chimeras (Fig. 4a). Additionally, Ptenfl/flFoxp3-Cre:CD45.1+ chimeras had augmented GC B cells (Fig. 4b). Thus, PTEN deficiency in Treg cells results in a dominantly acting effect on the TFH and GC responses.
Figure 4. Analysis of bone marrow-derived chimeras and Ptenfl/flFoxp3-Cre Ifng−/− mice reveals an important contribution of IFN-γ overproduction to dysregulated TFH responses in Ptenfl/flFoxp3-Cre mice.
(a-c) Sublethally irradiated Rag1−/− mice were reconstituted with a 1:1 mix of CD45.1+ BM and either CD45.2+ WT or Ptenfl/flFoxp3-Cre BM cells. Following reconstitution, the mixed chimeras were analyzed for TFH (a), GC B cells (b), and intracellular staining of IFN-γ in CD4+ T cells (c). (d,e) Analysis of TFH (d) and GC B cells (e) in the spleen of WT, Ptenfl/flFoxp3-Cre, Ifng−/− and Ptenfl/flFoxp3-Cre Ifng−/− mice. (f) Representative images and quantification of fluorescent intensity (right) of ANA IgG autoantibodies detected with Hep-2 slides in the serum from WT, Ptenfl/flFoxp3-Cre, Ifng−/− and Ptenfl/flFoxp3-Cre Ifng−/− mice (scale 60 μm). (g) Representative images of immune fluorescence imaging of kidney sections showing IgG deposits (scale 300 μm), and quantitative analysis (right). Data are representative of three independent experiments (a-e) and one experiment (f,g; n=3 WT, 6 Ptenfl/flFoxp3-Cre, 2 Ifng−/− and 3 Ptenfl/flFoxp3-Cre Ifng−/− mice). Data are mean ± s.e.m.
We therefore explored whether such defect was associated with dysregulated cytokine production. The TH1 cytokines such as IFN-γ have been implicated in potentiating TFH responses35, although opposing evidence also exists36, 37, suggesting a context-dependent effect. In the Ptenfl/flFoxp3-Cre:CD45.1+ mixed chimeras, IFN-γ production from CD4+ and CD8+T cells was enhanced irrespective of the source of donor cells (Fig. 4c, Supplementary Fig. 4a). To determine the functional effects of the augmented IFN-γ production, we crossed Ptenfl/flFoxp3-Cre mice with Ifng−/− mice to generate Ptenfl/flFoxp3-Cre Ifng−/− double knockout mice. Deletion of IFN-γ did not exert strong effects on the production of IL-17 and IL-4 from conventional CD4+T cells (Supplementary Fig. 4b). However, IFN-γ deficiency substantially blocked the elevated frequencies of TFH and GC B cells (Fig. 4d,e) and the increased formation of GCs in Ptenfl/flFoxp3-Cre mice (Supplementary Fig. 4c,d). Moreover, the increased production of serum ANA antibody and the deposition of IgG in the kidney glomeruli of Ptenfl/flFoxp3-Cre mice were essentially rectified in Ptenfl/flFoxp3-Cre Ifng−/− mice (Fig. 4f,g). Thus, the increased production of IFN-γ in Ptenfl/flFoxp3-Cre mice largely accounts for the exacerbated TFH, GC, and autoimmune responses, thereby highlighting the crucial role of PTEN signaling in Treg cells to coordinate TH1 and TFH reactions.
PTEN is crucial in maintaining the stability of Treg cells
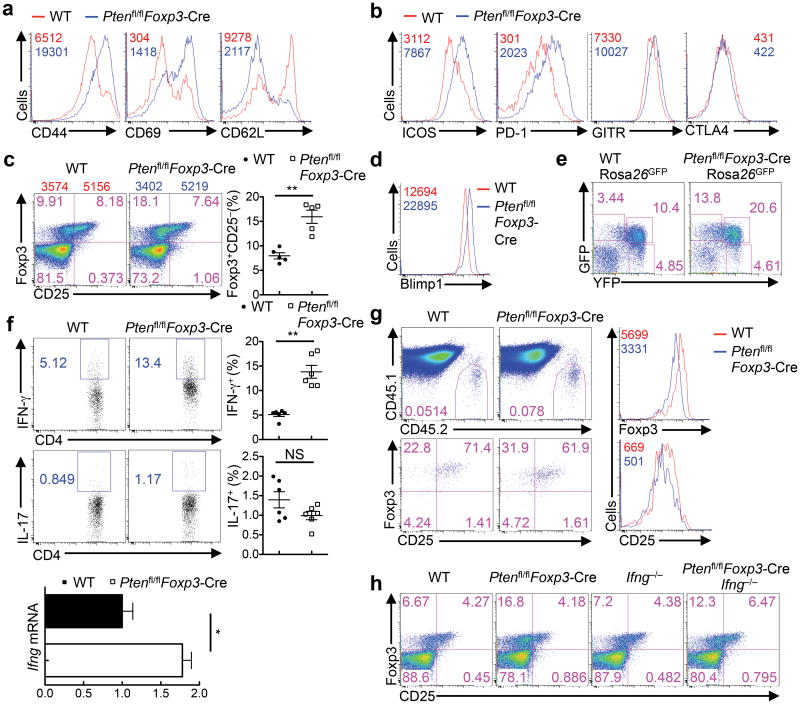
Despite the crucial role of IFN-γ overproduction in disrupting immune homeostasis in Ptenfl/flFoxp3-Cre mice, deletion of IFN-γ did not affect the phenotype of increased Treg cellularity in these mice (Supplementary Fig. 5a). We therefore explored the direct effects of PTEN deficiency on the homeostasis and functionality of Treg cells. As compared with WT counterparts, Treg cells from Ptenfl/flFoxp3-Cre mice showed higher expression of CD44 and CD69 but lower levels of CD62L, indicating an elevated level of activation (Fig. 5a). We next examined Treg-selective effector molecules. Ptenfl/flFoxp3-CreTreg cells showed increased levels of ICOS and PD-1, and to a lesser extent, GITR, whereas the expression of CTLA4 was largely normal (Fig. 5b). In sharp contrast to the elevated expression of activation markers and effector molecules, expression of CD25, a signature molecule of Treg and activated T cells, was markedly downregulated in Ptenfl/flFoxp3-Cre Treg cells, corresponding to the expansion of the Foxp3+CD25− population (Fig. 5c). As compared with Foxp3+CD25+ cells, the Foxp3+CD25− population contained lower levels of Foxp3 expression, as reported previously12. Consistent with this observation, Blimp1, a transcription factor implicated in CD25 downregulation in CD8+T cells38, was upregulated in Ptenfl/flFoxp3-Cre Treg cells (Fig. 5d). These results indicate that PTEN deficiency in Treg cells results in dysregulated expression of multiple Treg activation and phenotypic molecules.
Figure 5. PTEN deficiency impairs Treg stability.
(a,b) Expression of CD44, CD69, CD62L (a) and ICOS, PD-1, GITR and CTLA4 (b) in Treg cells from the spleen of WT and Ptenfl/flFoxp3-Cre mice. Mean fluorescent intensity (MUFI) is presented in the plots. (c) Expression of CD25 and Foxp3 (gated on CD4+TCRβ+ cells) in the spleen of WT and Ptenfl/flFoxp3-Cremice; the numbers above the graphs indicate the mean fluorescent intensity (MUFI) of Foxp3 in CD25− and CD25+ subsets. Right, quantification of Foxp3+CD25− cells. (d) Expression of Blimp1 in the splenic Treg cells of WT and Ptenfl/flFoxp3-Cre mice. (e) Foxp3-YFP and GFP expression in CD4+ T cells from Pten+/+Foxp3-Cre Rosa26GFPand Ptenfl/flFoxp3-Cre Rosa26GFPmice. (f) Intracellular staining of IFN-γ and IL-17 (right, quantification of IFN-γ+ and IL-17+ producing cells in Treg cells), and RNA analysis of IFN-γ in Treg cells of WT and Ptenfl/flFoxp3-Cre mice (IL-17 RNA was undetectable). (g) WT and Ptenfl/flFoxp3-Cre Treg (CD45.2+) were transferred into CD45.1+ recipients, followed by analysis of donor cell percentages (upper) and Foxp3 and CD25 expression (lower). Right, direct overlays of donor-derived WT and Ptenfl/flFoxp3-Cre Treg cells for Foxp3 (upper right) and CD25 levels (lower right). (h) Flow cytometry analysis of Foxp3-YFP+CD25− cells in WT, Ptenfl/flFoxp3-Cre, Ifng−/− and Ptenfl/flFoxp3-Cre Ifng−/− splenic Treg cells. Data are representative of at least three independent experiments (a-f, h) and two independent experiments (g). NS, not significant; *P < 0.05 and **P < 0.001. Data are mean ± s.e.m.
The lineage stability of Treg cells is a matter of considerable interest and debate6, 8-11, 13, but the signaling mechanisms involved are largely unexplored. To determine whether PTEN deficiency affects the stability of Treg cells, we crossed Ptenfl/flFoxp3-Cre mice with a Cre recombination reporter allele with the ubiquitously expressed Rosa26 locus containing a loxP site–flanked STOP cassette followed by the gene encoding green fluorescent protein (GFP)25. In this lineage tracing system, Foxp3Cre-mediated excision of the floxed STOP cassette results in constitutive, heritable expression of GFP, even for those “ex-Treg” cells that have lost Foxp3 expression (the GFP+YFP-Foxp3− population). Ptenfl/flFoxp3-Cre mice contained a striking accumulation of GFP+YFP-Foxp3− population (Fig. 5e), indicating the preferential loss of Foxp3 expression upon PTEN deletion. Additionally, Treg cells from Ptenfl/flFoxp3-Cre mice had increased expression of IFN-γ, whereas IL-17 expression was largely unaltered (Fig. 5f). Moreover, Ptenfl/flFoxp3-Cre Treg cells upregulated signature molecules characteristic of TH1 and TFH cells, including CXCR3 and T-bet, and CXCR5 and Bcl6, respectively, whereas expression of IRF4 and p-STAT3, which are required for Treg-mediated suppression of TH2 and TH17 cells4, 5, remained unchanged (Supplementary Fig. 5b). Consistent with these observations, Ptenfl/flFoxp3-Cre Treg cells contained an expanded T-bet+CXCR3+ population that is important for the regulation of type 1 inflammation (Supplementary Fig. 5c)3. Because the development of this Treg subset is dependent upon IFN-γ signaling3, we examined whether excessive IFN-γ production in Ptenfl/flFoxp3-Cre mice was involved in the dysregulation of T-bet and CXCR3. IFN-γ deficiency had only a partial rescue effect on the expansion of T-bet+CXCR3+ population in PTEN-deficient Treg cells (Supplementary Fig. 5c), indicating that the augmented expression of T-bet and CXCR3 upon PTEN deletion is largely cell intrinsic, not simply secondary to the overproduction of IFN-γ. Finally, to directly test the role of PTEN in maintaining Treg stability, we sorted Foxp3+CD25+ cells from WT and Ptenfl/flFoxp3-Cre mice and transferred them into congenic mice (CD45.1+). The expression of Foxp3 and CD25 was downregulated in PTEN-deficient Treg cells in various organs examined, as compared with WT cells (Fig. 5g, Supplementary Fig. 5d). These complementary approaches indicate that PTEN deficiency results in a loss of Treg stability.
Notably, despite the strong effects of IFN-γ at disrupting immune homeostasis (Fig. 4), deletion of IFN-γ in Ptenfl/flFoxp3-Cre mice did not affect the spontaneous development of the Foxp3+CD25− population (Fig. 5h). Thus, the enhanced IFN-γ expression from Ptenfl/flFoxp3-CreTreg cells is associated with the loss of Treg stability, but is unlikely to be the main driver.
PTEN-dependent transcriptional and metabolic programs
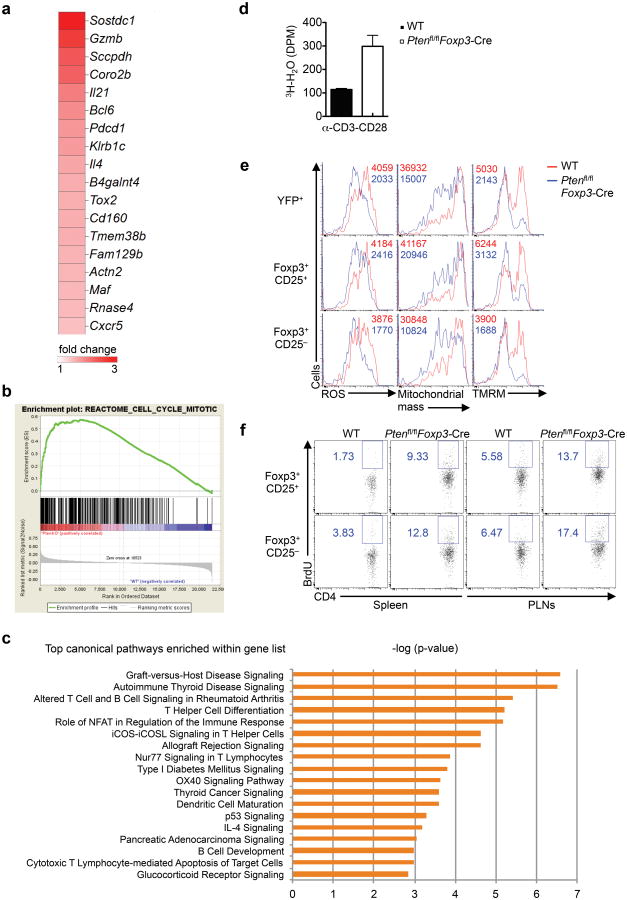
To explore PTEN-dependent molecular mechanisms in Treg cells, we next used microarrays to compare the transcriptional profiles between WT and Ptenfl/flFoxp3-Cre Treg cells. Ptenfl/flFoxp3-Cre Treg cells contained a total of 498 probes (representative of 352 unique genes) with greater than 1.5 fold difference, including 212 upregulated and 286 downregulated probes. Principal component analysis (PCA) showed the clear distinctions between WT and Ptenfl/flFoxp3-Cre samples (Supplementary Fig. 6a). Consistent with the dysregulated stability, loss of PTEN failed to restrain the transcription of genes involved in TFH differentiation, including Gzmb, Il21, Bcl6, Pdcd1, Il4, Maf and Cxcr515, 39 (Fig. 6a). To identify key networks regulated by PTEN, we did gene-set enrichment analysis (GSEA)40 to compare gene expression profiles of WT and PTEN-deficient Treg cells. Remarkably, the top ten of upregulated gene-sets in Ptenfl/flFoxp3-Cre Treg cells were all associated with cell cycle pathways (Fig. 6b, Supplementary Fig. 6b). To examine PTEN-dependent canonical pathways in Treg cells, we next performed the ingenuity pathway analysis (IPA) system by interrogating the differentially expressed genes at the 1.5 fold cut-offs. As shown in Fig. 6c, PTEN deficiency affected multiple canonical pathways implicated in autoimmune diseases, helper T cell differentiation, and immune signaling mediated by transcription factors, co-stimulatory molecules and cytokines. Gene ontology (GO) analysis of these differentially regulated genes also showed that PTEN-deficient Treg cells significantly upregulated groups of genes involved in autoimmune diseases, such as autoimmune thyroid disease and SLE, as well as in cell cycle regulation (data not shown). The transcriptome analysis therefore highlights an important role of PTEN in the regulation of immune response and cell cycle pathways.
Figure 6. PTEN-dependent gene expression and metabolic programs in Treg cells.
(a) Heat maps display expression, relative to the WT mean (over or equivalent to 1.5 fold), of TFH-related genes. (b) GSEA reveals the cell cycle mitotic pathway as one of the most extensively upregulated pathways in Ptenfl/flFoxp3-Cre Treg cells. (c) IPA analysis of canonical pathways controlled by PTEN in Treg cells. (d) Glycolytic activity of Treg cells stimulated with α-CD3-CD28. (e) Analysis of ROS production, mitochondrial mass and mitochondrial membrane potential (tetramethylrhodamine, methyl ester, TMRM) in Treg cells of WT and Ptenfl/flFoxp3-Cremice. (f) Analysis of BrdU incorporation in Treg subsets of WT and Ptenfl/flFoxp3-Cremice. Data are representative of one experiment (a-c; n=3 WT, 3 Ptenfl/flFoxp3-Cre mice), two independent experiments (d,f) and three independent experiments (e).
Cellular metabolic programs, especially glycolysis and mitochondrial oxidative phosphorylation, play an important role in shaping Treg generation and function41, 42, although the molecular mechanisms are unknown. As compared with WT Treg cells, Ptenfl/flFoxp3-Cre Treg cells more strongly upregulated glycolysis after TCR/CD28 stimulation (Fig. 6d). We next examined whether PTEN deficiency affects mitochondrial fitness by measuring multiple parameters associated with mitochondria homeostasis and functions. Ptenfl/flFoxp3-Cre Treg cells showed considerable reduction of reactive oxidative species (ROS), mitochondrial mass, and mitochondrial membrane potential, indicative of impaired mitochondrial fitness (Fig. 6e). Notably, the reduction of these parameters was more prominent in PTEN-deficient Foxp3+CD25− Treg cells than Foxp3+CD25+ cells (Fig. 6e), indicating that impaired mitochondrial function is associated with Treg instability. Moreover, the Foxp3+CD25− Treg subset showed a modestly stronger phenotype of BrdU incorporation than Foxp3+CD25+ cells (Fig. 6f). Altogether, these data establish that PTEN signaling links immune response gene expression, cell cycle progression, and metabolic balance between glycolytic activity and mitochondrial fitness in Treg cells.
PTEN signals via mTORC2 inhibition in Treg cells
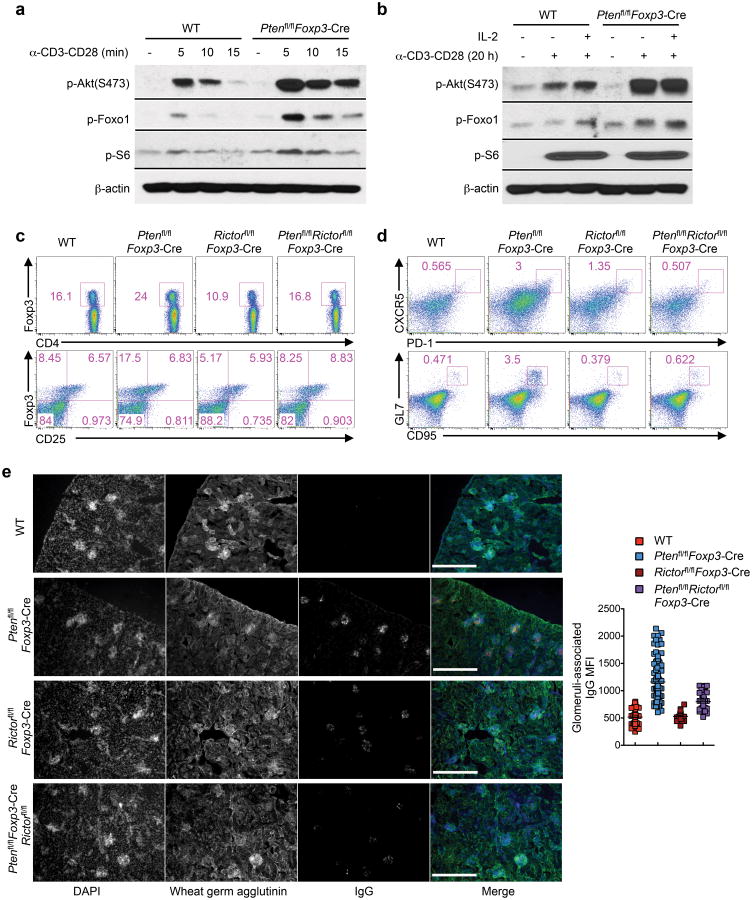
We determined PTEN-dependent biochemical mechanisms in Treg cells by examining mTORC1 and mTORC2 activities, as both are under the control of PTEN in multiple cell types20. In response to short-term anti-CD3-CD28 stimulation (5-15 min), Ptenfl/flFoxp3-Cre Treg cells exhibited slightly elevated phosphorylation of the mTORC1 target S6, as compared with WT Treg cells (Fig. 7a, Supplementary Fig. 7a). In contrast, Ptenfl/flFoxp3-Cre Treg cells exhibited much stronger activation of mTORC2 signaling than WT cells, as indicated by the enhanced phosphorylation of Akt at Ser473 and of the Akt downstream target Foxo1 (Fig. 7a). We next determined the effects of stimulating Treg cells with anti-CD3-CD28 for a longer time (20 h), in the presence or absence of IL-2. Both WT and Ptenfl/flFoxp3-Cre Treg cells responded to these stimuli with robust phosphorylation of S6 and 4E-BP1, indicative of comparable activation of the mTORC1 pathway under these conditions (Fig. 7b, Supplementary Fig. 7b). In contrast, Ptenfl/flFoxp3-Cre Treg cells exhibited much stronger activation of mTORC2 signaling than WT cells (Fig. 7b). Therefore, loss of PTEN results in preferential activation of mTORC2 signaling in Treg cells.
Figure 7. Dysregulated mTORC2 activity in PTEN-deficient Treg cells responsible for the immune tolerance breakdown.
(a,b) Immunoblots of phosphorylation of Akt (S473), Foxo1 and S6 in resting and short-term stimulated Treg cells (a) and in resting and long-term stimulated Treg cells (b) isolated from WT and Ptenfl/flFoxp3-Cre mice. (c,d) Flow cytometry analysis of WT, Ptenfl/flFoxp3-Cre, Foxp3creRictorfl/fl and Ptenfl/flRictorfl/flFoxp3-Cremice for Foxp3 (upper) and CD25 (lower) expression in CD4+ T cells (c), and proportions of FH cells (upper) and GC B cells (lower) (d). (e) Kidney sections showing IgG deposits (scale 300 μm) and quantification (right; quantitative results of WT and Ptenfl/flFoxp3-Cre mice in Fig. 4g are shown here for comparison). Data are representative of two independent experiments (a,b), three independent experiments (c,d) and one experiment (e; n=2 Foxp3creRictorfl/fl and 3 Ptenfl/flRictorfl/flFoxp3-Cremice). Data are mean ± s.e.m.
To determine the contribution of mTORC2 activity to Ptenfl/flFoxp3-Cre phenotypes, we crossed Ptenfl/flFoxp3-Cre mice with those lacking Rictor that abrogated mTORC2 activity (Ptenfl/flFoxp3-Cre)25 to generate Ptenfl/flRictorfl/flFoxp3-Cre mice. Compared with Ptenfl/flFoxp3-Cre Treg cells with increased cellularity but diminished CD25 expression, Treg cells in Ptenfl/flRictorfl/flFoxp3-Cre mice exhibited normal abundance and CD25 expression (Fig. 7c). Other Treg markers analyzed were also considerably rescued by the deletion of Rictor from Ptenfl/flFoxp3-Cre Treg cells (Supplementary Fig. 7c). Additionally, T cells in Ptenfl/flRictorfl/flFoxp3-Cre mice exhibited largely normal distribution of naïve and effector/memory phenotypes (Supplementary Fig. 7d). Also, the spontaneous development of TFH and TH1 cells and GC B cells observed in Ptenfl/flFoxp3-Cre mice was blocked by Rictor deletion (Fig. 7d, Supplementary Fig. 7e). Moreover, unlike Ptenfl/flFoxp3-Cre mice, Ptenfl/flRictorfl/flFoxp3-Cre mice did not contain IgG deposits in the kidney glomeruli (Fig. 7e), and exhibited normal GC numbers in the spleen and MLNs (Supplementary Fig. 7f,g). Therefore, despite the plethora of pathways identified to mediate PTEN functions in various cells20, PTEN acts in an mTORC2-dependent manner in Treg cells to impinge upon immune homeostasis and tolerance.
PTEN is haploinsufficient in Treg cells
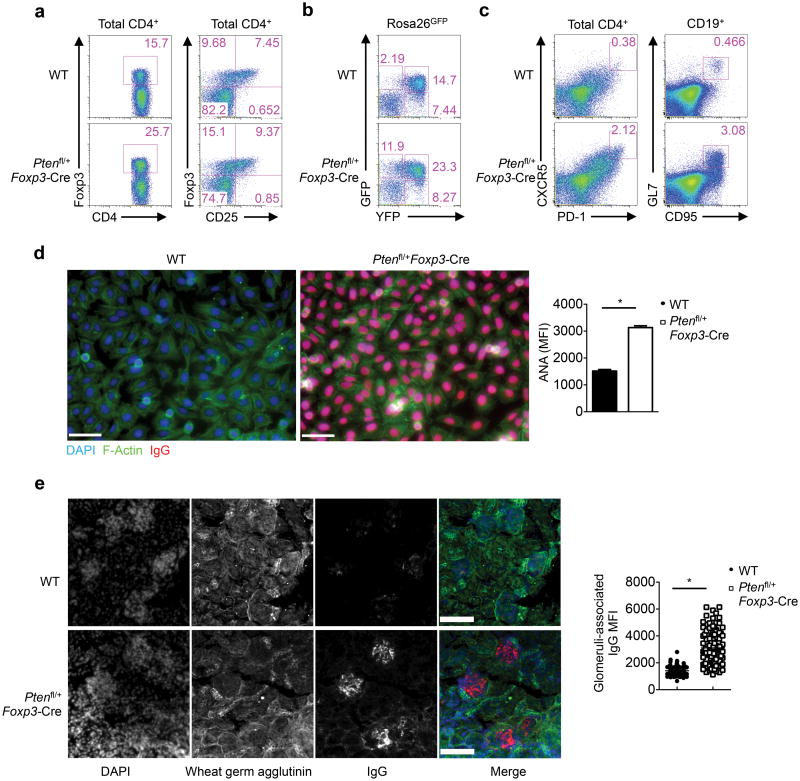
Given the potent effects of PTEN signaling in Treg cells, we asked whether partial loss of PTEN function in Treg cells is physiologically relevant, by analyzing Ptenfl/+Foxp3-Cre mice. As expected, Ptenfl/+Foxp3-Cre Treg cells, but not other T cell subsets, had partial reduction of PTEN expression at both the mRNA and protein levels (Supplementary Fig. 8a,b). Remarkably, heterozygous loss of PTEN resulted in the expansion of Treg cells, associated with considerable downregulation of CD25 expression (Fig. 8a). Ptenfl/+Foxp3-Cre mice also contained a striking accumulation of GFP+YFP-Foxp3− population in the lineage tracing system (Fig. 8b). Moreover, these mice contained increased CXCR5+PD-1+ TFH cells and GL7+CD95+ GC B cells (Fig. 8c), and more abundance of GCs in the spleen (Supplementary Fig. 8c). These findings prompted us to examine whether heterozygous loss of PTEN in Treg cells caused autoimmunity and lymphoid hyperplasia as observed in Ptenfl/flFoxp3-Cre mice. Ptenfl/+Foxp3-Cre mice displayed significantly elevated titers of circulating anti-ANA antibodies (Fig. 8d) and augmented IgG deposits in the kidney glomeruli (Fig. 8e), indicative of systemic autoimmunity. Furthermore, Ptenfl/+Foxp3-Cre mice spontaneously developed lymphoadenopathy (Supplementary Fig. 8d). These results demonstrate that PTEN is haploin sufficient in Treg cells.
Figure 8. Heterozygous loss of PTEN in Treg cells is sufficient to disrupt immune homeostasis.
(a) Flow cytometry analysis of Foxp3 and CD25 expression in WT and Ptenfl/+Foxp3-Cresplenic CD4+ T cells. (b) Foxp3-YFP and GFP expression in CD4+ T cells from Pten+/+Foxp3-CreRosa26GFPand Ptenfl/+Foxp3-Cre Rosa26GFPmice. (c) Analysis of CXCR5+PD-1+ cells (gated on CD4+TCRβ+ cells) and GL7+CD95+ GC B cells (gated on CD19+ B cells) in the spleen of WT and Ptenfl/+Foxp3-Cre mice. (d) Representative images (scale 60 μm) and quantification of fluorescent intensity (right) of serum ANA IgG autoantibodies detected with fixed Hep-2 slides. (e) Immune fluorescence images of kidney glomeruli sections showing IgG deposits (scale 100 μm). Data are representative of at least two independent experiments (a-e). *P < 0.0001. Data are mean ± s.e.m.
Discussion
Extensive progress has been made on the transcriptional and molecular pathways underlying effector T cell diversity and plasticity. In contrast, how these distinct effector T cell responses are controlled by extrinsic mechanisms is poorly understood. Moreover, despite the emerging evidence for the adoption of TH-specific transcription factors by Treg cells to suppress the corresponding effector responses3-5, we have little understanding about the signaling mechanisms that program these suppressive activities. Here we, together with Huynh et al. (companion manuscript), identify PTEN as a crucial molecular pathway in Treg cells that coordinately regulates TFH and TH1 responses (Supplementary Fig. 8e). In particular, loss of PTEN in Treg cells results in exacerbated TFH and GC responses and disrupted immune tolerance and homeostasis. Ablation of IFN-γ function reveals the hierarchy between Treg-mediated control of TH1 and TFH responses, with the production of the TH1 signature cytokine IFN-γ a prerequisite for the potentiation of TFH responses in Ptenfl/flFoxp3-Cre mice. Further more, the repression of TH1 and TFH responses is associated with the active maintenance of Treg cell stability enforced by PTEN signaling. At the molecular levels, PTEN controls the transcriptional program and metabolic balance in Treg cells, and it mainly signals via inhibition of mTORC2 activity. Our studies therefore establish that the PTEN-mTORC2 axis acts as a central pathway to orchestrate Treg cell stability and restrict TH1 and TFH responses.
The lineage stability of Treg cells remains contentious6, 8-13 but is an issue of utmost importance, as it directly impinges upon Treg-mediated control of health and disease and therapeutic strategies2. Using lineage tracing and adoptive transfer systems, we show that PTEN-deficient Treg cells are more prone to lose Foxp3 expression in vivo. Moreover, PTEN deficiency results in apparently hyper-activated Treg cells, as evidenced by increased cycling, upregulation of activation and phenotypic markers, and aberrant induction of TH1 and TFH signature molecules. Consistent with this, PTEN deletion results in loss of CD25 expression, which is normally downregulated in Treg cells after in vivo activation and proliferation43. While representing only a minor proportion of Foxp3+ Treg cells under steady state, the Foxp3+CD25− population likely makes an important contribution, via conversion or selection, to the generation of ex-Treg cells under inflammatory or lymphopenic environment. Therefore, our studies highlight that the stability of Treg cells under steady state requires active enforcement by PTEN, which functions, at least in part, by preventing overt Treg activation and CD25 downregulation.
Generation of TFH cells requires unique transcriptional programs and is antagonized by TFR cells18, 19. However, TFR cells can also promote antigen-specific high-affinity B cell responses19 and influenza-specific GC reactions44. Extensive crosstalk also exists between the differentiation of TFH cells and other effector lineages15. Here we show that PTEN signaling in Treg cells is crucial in the repression of TFH responses, which is further linked to Treg stability and Treg-mediated control of TH1 responses. Strikingly, loss of PTEN in Treg cells results in spontaneous TFH differentiation and GC formation, and the development of SLE-like autoimmune symptoms. Associated with these immune defects is the dysregulated expression of multiple molecules involved in TFH and TFR responses, including IL-4, IL-21, Bcl6, Blimp1 and Granzyme B, which likely underlies the loss of proper TFR functions in PTEN-deficient Treg cells. Moreover, the uncontrolled TFH responses in Ptenfl/flFoxp3-Cre mice are dependent upon the TH1 signature cytokine IFN-γ. Notably, the relationship between TH1 and TFH cells is complex and remains controversial. For instance, whereas IFN-γ is essential in driving TFH cells in the Roquinsan/san autoimmunity model35, it is dispensable for the differentiation of TFH cells induced by viral infection37. Further, T-bet induction dampens rather than enhances the TFH differentiation program36. Our studies demonstrate that TFH and TH1 responses are coordinately regulated by Treg cells, with the IFN-γ production required for TFH and GC reactions. However, we cannot conclude the direct contribution of the exacerbated TH1 response, independent of TFH cells, to the autoimmune and lymphoproliferative phenotypes.
As one of the most frequently mutated tumor suppressor genes, PTEN acts in murine T cells to prevent development of leukemia and autoimmunity27. More recent studies unveil that these two effects can be dissociated as they are derived from abnormalities in the thymus and periphery, respectively28. PTEN is also implicated in the regulation of effector T cell responses. Deletion of PTEN in activated T cells enhances effector responses but does not lead to autoimmunity or cancer45. miRNAs targeting PTEN expression are also implicated in shaping TFH responses46. We found that deletion of PTEN in Treg cells leads to the exacerbated effector T cell responses and loss of immune tolerance and tissue homeostasis. PTEN-deficient Treg cells dominate over WT cells in mediating TFH and GC responses, which are mediated, at least in part, by dysregulated IFN-γ expression. Moreover, PTEN functions in a haploin sufficient manner in Treg cells. These results together establish PTEN signaling in Treg cells as a unique regulator of immune homeostasis and function.
How does PTEN function in Treg cells at the molecular levels? Our microarray, metabolic and immunological assays reveal the important role of PTEN in linking cell cycle and metabolic machineries and expression of immune effector genes in Treg cells. Given the opposing effects of glycolytic and mitochondrial metabolism on Treg cells41, 42, the disrupted balance between these activities in PTEN-deficient Treg cells is likely an important contributor to the phenotypic alteration, a notion that requires additional investigation. Of note, despite the metabolic functions of PTEN identified in non-immune cells, the underlying mechanisms are highly complex and poorly understood47. Here we establish the PTEN-mTORC2 axis as a central determinant of Treg stability and Treg-mediated control of effector responses, although the involvement of mTORC2-independent pathways cannot be excluded48. Mechanistically, this signaling pathway likely orchestrates both the metabolic and transcriptional programs, such as those mediated by Foxo1 and Blimp17, 38, in impinging upon Treg stability and functions. We note that loss of Treg stability also results from Treg-specific deletion of neuropilin-1 and Foxo17, 32, and future work is required to explore the crosstalk between these molecular pathways in Treg cells.
In summary, our study has unveiled the interplay between the PTEN-mTORC2 signaling axis and the transcriptional and metabolic regulation in Treg cells as a new mechanism for enforcing Treg stability and Treg-mediated repression of TH1 and TFH responses. Given the potent effects of selective PTEN deficiency in Treg cells on immune tolerance and tissue homeostasis, even under steady state conditions or upon a partial loss of function, the identification of this signaling axis provides a new target for therapeutic intervention of systemic autoimmune and lymphoproliferative diseases.
Methods
Mice
Ptenfl/fl, CD45.1+, Rag1−/−, Ifng−/− and Rosa26GFP (a loxP-site-flanked STOP cassette followed by the GFP-encoding sequence was inserted into the Rosa26 locus) mice were purchased from the Jackson Laboratory. Rictor−/− mice have been described previously25. Foxp3YFP-Cre mice were a gift from A. Rudensky34. Ptenfl/flFoxp3-Cre mice were used at 10-12 weeks old unless otherwise noted, with the age and gender-matched WT mice containing the Foxp3cre allele as controls. BM chimeras were generated by transferring 7 × 106 T-cell-depleted BM cells into sub-lethally irradiated (5 Gy) Rag1−/− mice, followed by reconstitution for at least 2.5 months. All mice were kept in a specific pathogen-free facility in the Animal Resource Center at St. Jude Children's Research Hospital. Animal protocols were approved by the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital.
Flow cytometry
For analysis of surface markers, cells were stained in PBS containing 2% (wt/vol) BSA, with anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-TCRβ (H57-597), anti-CD69 (H1.2F3), anti-CD25 (PC61.5), anti-CD44 (1M7), anti-CD62L (MEL-14), anti-CD45.1 (A20), anti-CD45.2 (104), anti-PD-1 (J43), anti-GL7 (GL-7), anti-CD95 (15A7), anti-ICOS (C398.4A), anti-GITR (DTA-1), anti-CD19 (1D3), anti-CXCR3 (CXCR3-173), anti-MHCII (M5/114.15.2), anti-CD11b (M1/70), anti-CD11c (N418), anti-Ly6G (RB6-8C5; all from eBioscience). CXCR5 was stained with biotinylated anti-CXCR5 (clone 2G8) and streptavid in-conjugated PE (both from BD Biosciences). Intracellular Foxp3 (FJK-16s), T-bet (4B10), IRF4 (3E4), IFN-γ (XMG1.2), IL-4 (11B11), IL-17 (17B7; all from eBioscience), CTLA4 (UC10-4B9; BioLegend), p-STAT3 (4/P-STAT3), PTEN (A2B1), Bcl6 (K112-91; all from BD Biosciences) were analyzed by flow cytometry according to the manufacturer's instructions. Blimp1 (3H2-E8) was purchased from Thermo Scientific. For intracellular cytokine staining, T cells were stimulated for 4 h with PMA plus ionomycin in the presence of monensin before being stained according to the manufacturer's instructions (eBioscience). BrdU and caspase-3 staining was performed as per the manufacturer's instruction (BD Biosciences). For staining mitochondria, lymphocytes were incubated for 30 min at 37°C with 10 nM Mito Tracker Deep Red (Life Technologies) or 20 nM TMRM (tetramethylrhodamine, methyl ester; ImmunoChemistry Technologies) after staining surface markers. ROS were measured by incubation with 5 μMMito SOX™ Red (Life Technologies) after staining surface markers. Flow cytometry data were acquired on LSRII or LSR Fortessa (BD Biosciences) and analyzed using Flowjo software (Tree Star).
Imaging and immunohistochemistry
For cryosections, kidneys and MLNs were freshly frozen in OCT embedding medium. Ten μm thick cryosections were fixed with cold acetone for 5 min prior to rehydration in TBS. Non-specific binding was blocked by incubation in TBS containing 2% BSA and 5% normal donkey serum for 30 min prior to incubation with primary antibodies (2 μg/ml) overnight at 4°C. Slides were washed for 15 min in TBS prior to incubation with AF568-conjugated goat anti-mouse antibody (1 μg/ml; Life Technologies), AF568-conjugated donkey anti-goat antibody (1 μg/ml; Life Technologies), Cy5-conjugated donkey anti-goat antibody (1 μg/ml; Jackson ImmunoResearch), or AF488-conjugated streptavid in (1 μg/ml; Life Technologies) for 1 h at room temperature. Slides were washed for 15 min in TBS prior to mounting in Vectashield hard set with DAPI (Vector Laboratories). Fluorescent images were acquired using a Zeiss Axiovert 200M and 20X EC Plan-NeoFluar objective, detected using a Cascade II EMCCD camera (Photometrics) and analyzed using Slidebook software (3i Intelligent Imaging Innovations). Large image composites were acquired with a Nikon Ti-E inverted microscope with 20X CFI Plan Apochromat Lambda objective and iXon DU897 EMCCD camera, using NIS-Elements software. Visualization of ANA antibodies was performed by staining fixed Hep-2 slides (MBL). Specifically, serum samples were applied to the slide and incubated for 2 h at room temperature followed by 15 min washing in TBS. Bound murine antibodies were detected using AF568-conjugated goat anti-mouse antibody (1 μg/ml; Life Technologies) for 1 h at room temperature, while AF488-conjugated phalloidin (Life Technologies) was utilized to visualize f-Actin and nuclei were detected using DAPI. CD3 (sc-1127 (M-20)) antibody was purchased from Santa Cruz, Biotinylated Peanut Agglutinin (PNA) from Vector Laboratories (B-1075), and IgD (558597) from BD Biosciences.
For paraffin sections, spleen, kidney and Peyer's patches were fixed by immersion in 10% (vol/vol) neutral buffered formalin solution. Fixed tissues were embedded in paraffin, sectioned and stained with hematoxylin and eosin, and the clinical signs of autoimmune diseases were analyzed by an experienced pathologist (P. Vogel).
Immunization
For experiments involving antigen-induced TFH and GC B cell response, antigen for immunization was prepared by mixing NP14-OVA (14 molecules of NP linked to OVA; Biosearch Technologies), 10% KAl(SO4)2 dissolved in PBS at a ratio of 1:1, in the presence of LPS (Escherichia coli strain 055:B5; Sigma) and at pH 7. NP-OVA (100 μg) and LPS (10 μg) precipitated in alum was injected intraperitoneally, as described46. Alternatively, a fresh preparation of PBS-washed (1 × 109) SRBCs from Colorado Serum Company (31112) was injected intravenously to induce a robust splenic GC response.
Serum antibodies
Autoantibodies (dsDNA) and immunoglobulin subclasses were measured with kits from Alpha Diagnostic International (5110) and Millipore (MGAMMAG-300K), respectively.
Cell purification and adoptive transfer
Lymphocytes were isolated from lymphoid organs (spleen and peripheral lymph nodes that included inguinal, auxiliary and cervical lymph nodes) and naïve and Treg cells were sorted on a MoFlow (Beckman-Coulter) or Reflection (i-Cyt). For adoptive transfer, CD4+CD25+Foxp3-YFP+ cells from WT and Ptenfl/flFoxp3-Cre mice (CD45.2+) were transferred to the congenically marked (CD45.1+) recipients. Seven days after the transfer, mice were euthanized for the analysis of Foxp3 and CD25 expression.
RNA and immunoblot analysis
Real-time PCR analysis was performed with primers and probe sets from Applied Biosystems, as described49. Immunoblots were performed as described previously50, using the following antibodies: p-S6 (2F9), p-4E-BP1 (236B4), p-Foxo1 (9461), Akt phosphorylated at Ser473 (D9E), PTEN (138D6; all from Cell Signaling Technology), and β-actin (AC-15; Sigma).
Glycolysis assay
Treg cells were stimulated with plate-bound anti-CD3-CD28 for 6 h, and glycolytic flux was measured by detritiation of [3-3H]-glucose, as previously described42.
Gene expression profiling and gene-set enrichment analysis
RNA samples from freshly isolated Treg cells from WT and Ptenfl/flFoxp3-Cremice were analyzed with the Affymetrix HT MG-430 PM Gene Titan peg array, and expression signals were summarized with the robust multi-array average algorithm (Affymetrix Expression Console v1.1). Lists of differentially expressed genes by 1.5 fold or more were analyzed for functional enrichment using the Ingenuity Pathways (www.ingenuity.com). Gene-set enrichment analysis within canonical pathways was performed as described40. The microarray data has been deposited into the GEO series database (GSE63625).
Statistical analysis
P values were calculated with Student's t-test (GraphPad Prism). P values of less than 0.05 were considered significant. All error bars represent the s.e.m.
Supplementary Material
Acknowledgments
The authors acknowledge J. Wei, D. Bastardo Blanco and S. Brown for help with immunological assays, and C. Cloer and B. Rhode animal colony management, A. Rudensky for Foxp3YFP-Cre mice, and St. Jude Immunology FACS core facility for cell sorting. This work was supported by NIH AI105887, AI101407, CA176624 and NS064599, American Cancer Society, and Crohn's & Colitis Foundation of America (to H.C.), and by a postdoctoral fellowship from the Arthritis Foundation (to K.Y.).
Footnotes
Author contributions: S.S. and K.Y. designed and performed cellular, molecular, and biochemical experiments and contributed to writing the manuscript; C.G. performed imaging assays; P.V. performed hist pathology analysis; GUN. performed Bioinformatics analyses; and H.C designed experiments, wrote the manuscript, and provided overall direction.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 3.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhry A, et al. CD4+ Regulatory T Cells Control TH17 Responses in a Stat3-Dependent Manner. Science. 2009 doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang W, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 10.Bailey-Bucktrout SLE, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comatose N, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 12.Comatose N, et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miaow T, et al. Plasticity of Foxp3(+)T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Miguel KS, et al. CCR7 provides localized access to IL-2 and defines home statically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corty S. Follicular helper CD4 T cells (FH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 16.Linter man MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linter man MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell JD, Pol lizzie KN, Headlamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del gaffe GM, et al. The kins mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K, et al. T Cell Exit from Quiescence and Differentiation into Th2 Cells Depend on Raptor-mTORC1-Mediated Metabolic Reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zing H, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suki A, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, et al. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J Cling Invest. 2010;120:2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song MS, et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben singer SJ, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanin-Zhorov A, et al. Scaffold protein Disc large homology 1 is required for T-cell receptor-induced activation of regulatory T-cell function. Proc Natl Acad Sci U S A. 2012;109:1625–1630. doi: 10.1073/pnas.1110120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del gaffe GM, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh PT, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Trigs. J Cling Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Lee SK, et al. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Nakayamada S, et al. Early Th1 cell differentiation is marked by a FH cell-like transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray JP, et al. Transcription factor STAT3 and type I interferon's are co repressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin HM, et al. Epigenetic modifications induced by Blimp-1 Regulate CD8 (+) T cell memory progression during acute virus infection. Immunity. 2013;39:661–675. doi: 10.1016/j.immuni.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, et al. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat Immunol. 2014;15:667–675. doi: 10.1038/ni.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sub romanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michael RD, et al. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chi LS, et al. HIF1a-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Trig cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavin MA, Clarke SR, Negro E, Gall egos A, Rudensky A. Homeostasis and energy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 44.Leon B, Bradley J, Land FE, Randall TD, Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific FH responses by controlling IL-2 availability. Nat Common. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sound DR, et al. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J Immunol. 2012;188:5935–5943. doi: 10.4049/jimmunol.1102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang AG, et al. MicroRNAs of the miR-17 approximately 92 family are critical regulators of T(FH) differentiation. Nat Immunol. 2013;14:849–857. doi: 10.1038/ni.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Cao I, et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell. 2012;149:49–62. doi: 10.1016/j.cell.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei J, Yang K, Chi H. Cutting Edge: Discrete Functions of mTOR Signaling in Invariant NET Cell Development and NKT17 Fate Decision. J Immunol. 2014;193:4297–4301. doi: 10.4049/jimmunol.1402042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.