Abstract
Human papillomaviruses (HPVs) cause cancer at multiple anatomic sites in men and women, including cervical, oropharyngeal, anal, vulvar, and vaginal cancers in women and oropharyngeal, anal, and penile cancers in men. In this EUROGIN 2014 roadmap, differences in HPV-related cancer and infection burden by gender and anatomic site are reviewed. The proportion of cancers attributable to HPV varies by anatomic site, with nearly 100% of cervical, 88% of anal, and less than 50% of lower genital tract and oropharyngeal cancers attributable to HPV, depending on world region and prevalence of tobacco use. Often mirroring cancer incidence rates, HPV prevalence and infection natural history varies by gender and anatomic site of infection. Oral HPV infection is rare and significantly differs by gender; yet HPV-related cancer incidence at this site is several-fold higher than at either the anal canal or penile epithelium. HPV seroprevalence is significantly higher among women compared to men, likely explaining the differences in age-specific HPV prevalence and incidence patterns observed by gender. Correspondingly, among heterosexual partners, HPV transmission appears higher from women to men. More research is needed to characterize HPV natural history at each anatomic site where HPV causes cancer in men and women, information that is critical to inform the basic science of HPV natural history and the development of future infection and cancer prevention efforts.
Keywords: human papillomavirus, epidemiology, gender differences, natural history, cancer
Introduction
Over the past several decades, research focused on human papillomaviruses (HPVs) and their relationship to cervical cancer has led to major scientific discoveries, including a clear understanding of the role of HPV as a carcinogen at the cervix, use of HPV testing as part of cervical cancer prevention programs, and the development, testing, licensure, and implementation of two highly efficacious HPV vaccines.1 These discoveries led to notable accomplishments in countries with established HPV vaccination programs, including a significant decline in cervical HPV infection prevalence2, 3 and related pre-cancerous lesions,4–6 as well as a reduction in diagnoses of genital warts.7, 8
More recently, the WHO HPV Monograph,1 published in 2007, recognized for the first time that HPV, particularly HPV 16, is a cause of multiple cancers in men (penile, anal, and a subset of oropharyngeal cancers [OPC]) as well as in women (cervical, vaginal, vulvar, anal, and OPC). Since this publication, there has been a growing interest in understanding the epidemiology of HPV in men and infection at multiple anatomic sites in both men and women. From this more recent and limited body of research, we have observed that there are differences in HPV natural history by gender, as well as differences by anatomic site of infection. Understanding these differences remains essential to the development of efficacious interventions to prevent a multitude of HPV-related cancers afflicting both men and women.
Each year, the EUROGIN roadmap highlights cutting-edge HPV research presented at the EUROGIN Congress. The 2011 roadmap9 focused on HPV-related disease morbidity, as well as HPV and cancer prevention and treatment strategies, whereas the 2012 roadmap10 compared the epidemiology of cervical and HPV-related head and neck cancers. In the EUROGIN 2014 roadmap, we expand upon these publications by reviewing differences in HPV-related cancer and infection burden by gender and anatomic site of infection. The following review summarizes the highlights of the 2013 EUROGIN Congress entitled, “HPV at a Crossroads: 30 Years of Research and Practice” (Florence, Italy; November 3–6, 2013), specifically the session entitled, “EUROGIN 2014 Roadmap: HPV infection in men, genito-anal versus oral, convergences and divergences.”
Ideally, we would report results from large studies focused on both genders. Unfortunately, few studies include both men and women from the same underlying population, and fewer still include sampling at multiple anatomic sites. In the absence of such studies, we review studies that employed similar methodologies from comparable populations and are therefore limited in our ability to directly compare HPV across anatomic sites. For all anatomic sites other than the cervix, there is a paucity of HPV natural history publications. It is important to note that epidemiological estimates among different populations are impacted not only by the true distribution of infection and disease but also by methodological differences among studies, including population sampling strategy, sampling procedures within the anal canal, assay sensitivity, and the number of genotypes detected by a given assay. Many of the limitations of the published literature highlighted here could be overcome by the addition of new HPV natural history studies that include males and females sampled at multiple anatomic sites.
HPV-related cancers
Worldwide, more HPV-related cancers are diagnosed among women compared to men, primarily due to the large burden of cervical cancer. Among men, HPV-related cancers are rare, occurring in ~1–6/100,000 among the general population (e.g., penile, anal, and OPC). However, in some countries, the incidence of OPC appears to be increasing among men. If this trend continues, gender disparities in the HPV-related cancer burden may diminish over time in countries that have implemented effective cervical cancer screening programs.
In 2008, approximately 610,000 of the 12.7 million new cancer cases were attributable to HPV (population attributable fraction [PAF]: 4.8%),11 with 570,000 new cancer cases diagnosed among women (PAF: 9.4%) and 39,000 cases among men (PAF: 0.6%).12 HPV is assumed to be responsible for 100% of cervical cancers, 88% of anal cancers, 70% of vaginal cancers, 50% of penile cancers, and 43% of vulvar cancers,11 with the majority caused by HPV 16 or 18.9 The corresponding percentage for OPC is less well-defined than for the other anatomic sites because of the strong association with tobacco and alcohol use. The PAF of HPV in OPC is estimated to be 26% globally, but HPV prevalence among OPC cases rises to ~50% in North America, Japan, and Australia.11
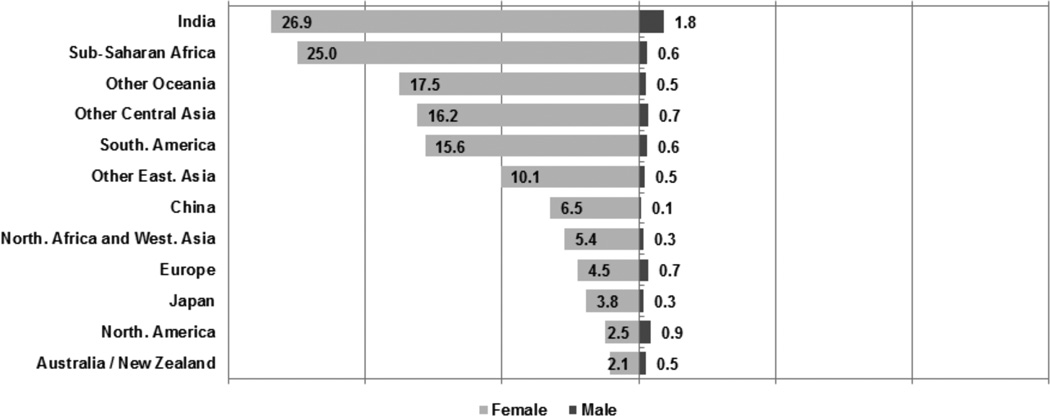
The proportion of total cancer cases attributable to HPV in women and men differs significantly by geographical region and economic development status of the country. Figure 1 shows PAFs of HPV in cancers among women and men globally, across nine geographic regions and three large countries.12 The PAF of HPV in women ranged from ≤2.5% in North America and Australia to approximately 25% in sub-Saharan Africa and India.12 In men, the PAF was highest in India (1.8%) and <1.0% in all other countries or regions.12 When comparing women and men, women have a higher incidence rate of HPV-attributable cancers than do men, and this gender difference is greatest among less-developed countries where a higher burden of cervical cancer is coupled with a lower proportion of OPCs attributable to HPV.11, 12
Figure 1.
Proportion of total cancer cases attributable to HPV (population-attributable fractions [%]) in women and men by geographical region/country (modified from refs 11–12).
Among HPV-attributable cancers occurring in both genders, the number of incident anal cancer cases due to HPV in 2008 was estimated to be higher in women (13,000) than men (11,000), with the proportion attributable to HPV similar among both genders.11 In contrast, the number of HPV-attributable OPCs was much greater in men (17,000) than women (4,400).11 However, these estimates were based on the assumption that the contribution of HPV to OPC etiology was similar in both genders. Case-control studies13 have suggested that the proportion of OPCs attributable to HPV is inversely correlated with population-level smoking prevalence, which varies by geographical region and gender.
Genital HPV infection
Data from studies conducted in North and Latin America indicate that genital HPV prevalence is higher in men14 than in women,15, 16 and age does not appear to influence genital HPV prevalence in men14 but is strongly negatively associated with cervical HPV prevalence in women.15 The proportion of high-risk (HR) and low-risk (LR) HPV infections in women15, 16 appears equivalent (HR: 14–15%; LR: 18%); however, in men, the prevalence of LR HPV (39%) is substantially higher than HR HPV (30%).14 Only a few studies have evaluated genital HPV prevalence by anatomic site. In studies among men, HPV prevalence is highest at the penile shaft17 and lowest at the urethra.17, 18 Among women, HPV prevalence is highest at the cervix and vagina and appears lower at the vulvar epithelium, likely due to the unique vulnerability of the cervical transformation zone to infection.18
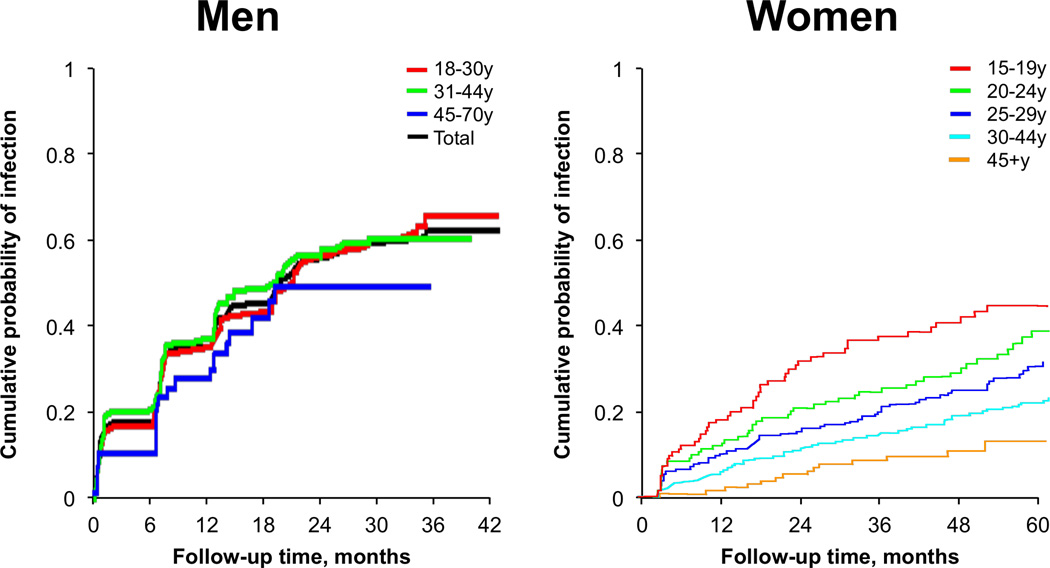
Given the gender differences in HPV prevalence and observed age patterns, it stands to reason that rates of HPV acquisition, as well as rates of clearance, may differ by gender. In the few studies that have evaluated HPV natural history by age among men19 and women,20, 21 we note that the rate of acquiring a new genital HPV infection decreases with age in women20, 21 but does not vary by age in men19 (Figure 2A). However, once HPV is acquired, the median duration of an HPV infection appears comparable between men19 and women22 (Figure 2B), with genital HPV 16 infections typically having a longer duration than most other HR HPV types in both men19 and women.21
Figure 2.
(A) Genital HPV incidence among men and women by age (B) Duration of genital HPV infections among men and women by age (modified from refs. 19, 21, 22).
Several important questions arise from the above observations. Why is the rate of genital HPV acquisition constant over the lifespan of men but not women? Given that HPV is common at multiple anatomic sites within the genital region of men and women, why does cancer incidence differ considerably across genders and anatomic sites (e.g., ~35/100,000 for cervical cancer [unscreened population] versus ~1/100,000 for anal, vulvar, and penile cancers)? How does the local epithelial environment, such as transformation zones of the cervix and anal canal vs. keratinized skin, interact with HPV to determine immune response and rate of progression to cancer?.
Anal HPV infection
Anal HPV has been studied more frequently in men than women, although anal cancer incidence is slightly higher among women than men.23 Studies among men typically focus on HIV-positive individuals or men having sex with men (MSM), populations with high anal cancer incidence (5–131/100,000).24, 25 Among studies of anal HPV prevalence in HIV-negative individuals, twice as many report data among men17, 26–36 than among women.37–42 Anal HPV studies among transgendered persons are rare,43 and we are aware of no studies among women who have sex with women.
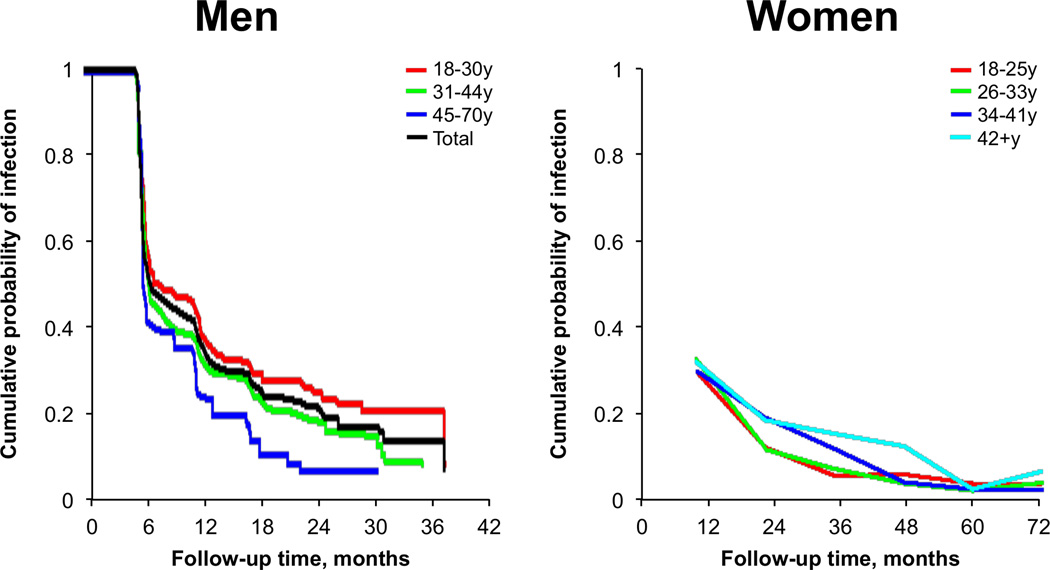
Anal HPV prevalence differs by gender44 and male sexual orientation (Figure 3).26, 27, 32, 34, 35, 37, 39, 42, 45–53 Comparisons of anal HPV incidence and clearance rates are more limited, given the paucity of prospective studies. Unlike anal cancer, anal HPV infection is common among women and men, including heterosexual men.32, 37, 54 A study conducted among 1,378 women ≥18 years recruited from healthcare facilities in Hawaii estimated an anal HPV prevalence of 27%.39 Studies among women that compare cervical and anal HPV infection have shown comparable prevalence estimates at both anatomic sites.37, 39 Recently, a study of 2,107 women in the Costa Rica Vaccine Trial observed a cervical HPV prevalence of 37% and an anal HPV prevalence of 32%.37 As has been shown in most studies of genital HPV, a decline in anal HPV prevalence with increasing age has been observed in women38, 39 but not in men having sex with women (MSW).55 Anal HPV prevalence is typically higher among women with HPV-related cervical disease and women at increased risk for HIV.40, 56–60
Figure 3.
Mean prevalence of select anal HPV groups and HPV-16 among HIV-negative men and women in studies with sample sizes ≥ 100 persons. (modified from refs. 26, 27, 32, 34, 35, 37, 39, 42, 45–53).
Among men, anal HPV prevalence varies widely by sexual practice. Studies of anal HPV among MSW observe a prevalence of approximately 12%,54 nearly half that observed among women. In contrast, studies among MSM demonstrate a higher prevalence, with ≥50% of HIV-negative MSM having any type of anal HPV infection, depending on the population sample.24, 27, 28, 54 A 2012 meta-analysis estimated the prevalence of HPV 16 infection to be 12.5% at the anal canal among HIV-negative MSM.24 In general, anal HPV prevalence among MSM is twice that among women, while anal HPV prevalence among women is twice that among MSW. A large study of urban MSM in the United States (US) at high risk for HIV observed a stable anal HPV prevalence across age groups;27 however, a smaller study that enrolled MSM regardless of their risk for HIV observed a declining anal HPV prevalence with age.54 The co-occurrence of genital HPV and anal HPV is common among MSW which, as with women, presents the possibility that sexual and/or non-sexual behaviors commonly transfer the virus between the male genitals and anal canal.32, 55, 61
There are few prospective studies of anal HPV infection among women or men.44 The 12-month cumulative incidence of anal HPV in 431 clinic-based women in Hawaii was approximately 24% for HR HPV types and 14% for LR HPV types.62 Women aged ≥45 years had a 57% lower risk of acquisition compared to women under age 25;62 however, persistence of anal HPV did not differ by age.63 One study64 estimated a several-fold lower anal HPV incidence among MSW than that reported among women, while incidence of anal HPV among HIV-negative MSM was high, with approximately 40% of MSM acquiring an anal HPV genotype annually. Although the data are limited, two studies,64, 65 approximately 75% of MSM had persistent HPV 16 infection for at least six months. Conversely, one of these studies observed that, of 21 MSW with anal HPV 16 infection at baseline, none retained the same HPV genotype at the six-month follow up visit.64 As HPV 16 and 18 are responsible for the majority of anal cancers,23 these HPV 16 persistence data among MSW and MSM help to explain the striking difference in anal cancer incidence between the two groups. Adequately powered prospective studies are needed to fill gaps in knowledge relative to gender differences in anal HPV natural history.
Oral HPV infection
Oral HPV natural history appears to vary significantly by gender. In a recently completed population-based study conducted in the US,66 the age-specific pattern of oral HPV prevalence was similar among men and women, with peak prevalence observed among individuals aged 30–34 years and again among those aged 60–64 years. However, the absolute prevalence of oral HPV (any genotype) was considerably higher in men compared to women ([10.1%; 95% CI: 8.3–12.3%] vs. 3.6% [95% CI: 2.6–5.0%], p <0.001; respectively). In this nationally representative sample, factors independently associated with oral HPV prevalence beyond male gender included older age, higher number of lifetime sexual partners, and current cigarette use (>10 cigarettes per day). In most studies conducted among healthy individuals,67 oral HPV 16 prevalence is low, typically between 0.5% and 1%, and is consistently found to be substantially lower than what is typically observed at the anogenital region for both men14 and women.68
Given the rarity of oral HPV infection, large sample sizes are needed to evaluate oral HPV natural history and to precisely estimate rates of acquisition and clearance. To date, there have been only seven studies that report incidence and persistence of oral HPV,69–75 and few included both men and women.69, 73, 74 To date, the largest study73 to include both genders focused on 1,000 young adults from a university setting. Oral gargle specimens were collected at baseline and three months later. Overall, oral HPV prevalence was rare and higher in men than women (3.2 vs. 1.7%, respectively), as has been consistently shown. For both genders combined, the crude oral HPV incidence rate was 5.7 per 1,000 person-months. Gender was not identified as a significant risk factor for oral HPV acquisition in this study.
The largest prospective study conducted to date to evaluate oral HPV natural history is the HPV Infection in Men (HIM) Study,72 a multinational cohort of >4,000 men aged 18–70 years.14, 19, 76 In the oral sub-cohort (N=1,626 men), 4.4% (95% CI: 3.5–5.6) of men acquired a new oral HPV infection of any type, 1.7% (95% CI: 1.2–2.5) acquired a new oral HR HPV infection, and 0.6% (95% CI: 0.3–1.1) acquired a new oral HPV 16 infection. In a follow-up report of these men,77 oral HPV 16 infections tended to persist beyond one year, and persistence increased significantly with age, potentially explaining the higher prevalence of oral HPV observed at older ages.
The rate of oral HPV incidence in men is an order of magnitude lower than that of genital HPV infection (Table 1).19, 72 Similar findings for oral HPV in women are unavailable, as there have not been sufficiently large natural history studies conducted to date. It is currently unknown why oral HPV prevalence is lower in women compared to men, though several hypotheses exist: (1) men have more sexual partners, thus, more opportunity for HPV exposure; (2) transmission of infection is more efficient when performing oral sex on infected female genitals (i.e., a mucosal surface) compared to transmission when performing oral sex on the keratinized epithelium of a penis; and (3) women, who have some level of systemic immunity from cervical HPV infection,78 may be protected against oral HPV infection, whereas no such protection has been observed in men.79
Table 1.
HPV natural history among men enrolled in the HPV Infection in Men (HIM) Study, by anatomic site of infection.
| Prevalence | Incidence rate per 1,000 person-months |
Median time to clearance (months) |
|
|---|---|---|---|
| Genital HPV14, 19 | 50.4% | 38.4 | 7.5 |
| Anal HPV*,32, 64 | 12.0% | 8.1 | -- |
| Oral HPV72, 76 | 4.0% | 5.6 | 6.9 |
Heterosexual men
HPV serology
As presented above, HPV infections can occur at multiple anatomic sites; once infected, most individuals are able to naturally clear the infection through an immune response. Nine to 24 months following initial HPV infection, a proportion of individuals develop detectable antibodies to the specific HPV type.80, 81 There are several valid laboratory methods that quantify IgG antibodies to type-specific HPV virus-like particles,82 and determining seropositivity is dependent on the assay used and the comparison population. Therefore, comparing results between serological assays is difficult, due to the use of different cutoff values and lack of an international reference population.82
Consistently, women demonstrate a higher HPV 16 seroprevalence than men, regardless of the population studied (Figure 4),83–91 despite a higher genital and oral HPV DNA prevalence observed in men. HPV 6 and 18 seroprevalence are also significantly higher among women compared to men, regardless of the geographic region or risk level of the populations.83–88, 92 However, HPV 11 seroprevalence does not appear to differ by gender in population-based studies published to date.83, 86, 87, 93
Figure 4.
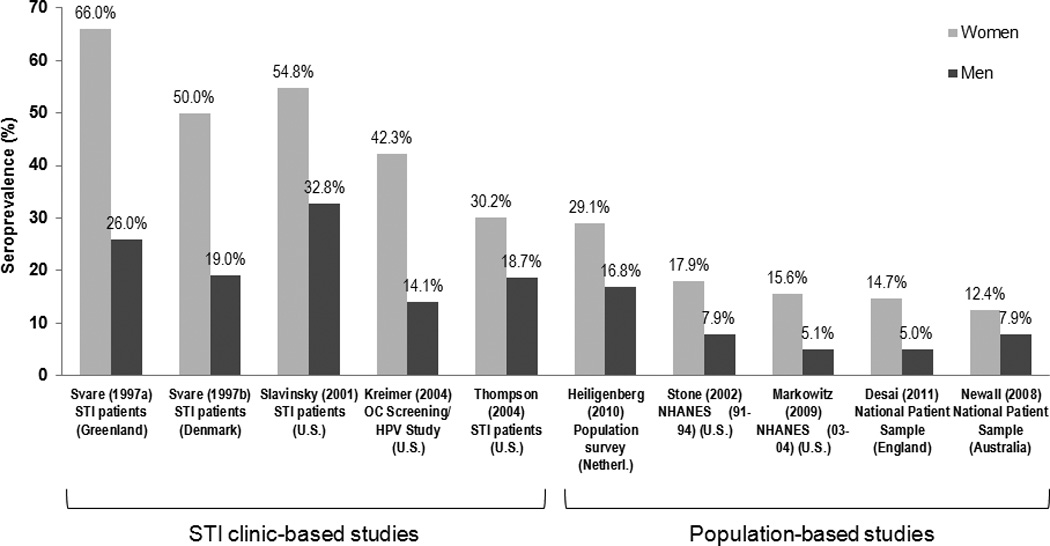
Differences in HPV 16 seroprevalence by gender (modified from refs. 83–91).
Given the differences in HPV seroprevalence by gender, differences in the rates of seroconversion following HPV infection among men and women are of interest. It appears that only a subset of men and women with HPV infection mount a detectable antibody response, with a higher percentage of women seroconverting to HPV 16 and 18 compared to men.81, 94, 95 In one study conducted among young women,94 58–67% developed a detectable HPV 16 antibody within two years following an incident HPV 16 infection, compared to only 7–11% of men.81, 95 Similarly, women were significantly more likely to seroconvert within two years following an incident HPV 18 infection compared to men (54% versus 2%, respectively).81, 96 However, seroconversion following an incident HPV 6 infection was common among women (69%) and men (51%).81, 96
The protection conferred by antibodies generated in response to natural infection against future HPV infections differs between men and women. Three prospective studies of women78, 97, 98 assessed the role of antibodies in protection against future HPV infections. In the Guanacaste cohort,98 no association was observed between serum antibodies above the cutoff level and protection against future HPV infections, while a study in college-aged women97 and the placebo arm of the Costa Rica vaccine trial78 both describe a 50% reduction in risk against future HPV 16 infections among unvaccinated women who had high antibody titers for HPV 16 compared to HPV 16 seronegative women. In the prospective HIM Study,79 HPV 16 seropositivity in unvaccinated men was not associated with reduced risk of future HPV 16 infection, regardless of the level of antibody.
Heterosexual HPV transmission
HPV transmission dynamics are dependent on both viral and host factors, defined by susceptibility (e.g., HPV serostatus among women), contact rate per unit time, transmission probability, and duration of infectiousness.99 In a recent meta-analysis of 30 HPV type-specific concordance studies,100 26% of 2,972 couples were infected with one or more of the same genital HPV types, underscoring the high transmissibility of HPV. Concordance was even higher (63%) when the analysis was restricted to couples that were both genital HPV+. However, the percentage of men with the same HPV type as their HPV+ female sexual partner (36%) was lower than the percentage of women with the same HPV type as their HPV+ male sexual partner (55%). These findings suggest that the epithelial cells of the penile skin are more resistant to HPV infection than the cervical epithelium, the duration of HPV infection is shorter in men than in women, and/or HPV testing is less sensitive in men than in women.99, 100
Five longitudinal heterosexual transmission studies with varying follow-up times and sampling frequencies have been conducted, each of which recruited initially HPV-discordant couples.61, 101–104 In all studies, the incidence of transmission from men to women was lower than the incidence from women to men, supporting the notion that men acquire more transient infections than women (Table 2).19 In one study,105 the per-partnership probability was estimated to be 0.05–0.28 for male-to-female transmission and 0.19–0.81 for female-to-male transmission, highlighting the efficiency of heterosexual HPV transmission. These ranges are understandably correlated with the frequency of HPV testing among the couples, with much higher estimates associated with greater sampling frequency. Another study104 found the highest rates of genital HPV transmission within 24 hours of sexual intercourse, indicating that contamination from the partner may inflate transmission probabilities. Although genital-genital contact appears to account for the majority of heterosexual HPV transmission events, other modes of transmission are possible, including anal-genital, oral-genital, and manual-genital contact, sex toys, and perhaps autoinoculation (i.e., transmission between the genitals, anal canal, oral cavity, or hands of the same individual).
Table 2.
Incidence of genital HPV transmission among heterosexual couples.
| Heterosexual couples, N |
Incidence per 100 person- months (95% CI) |
|||
|---|---|---|---|---|
| Reference | Population | Male-to-female | Female-to-male | |
| 61 | Unites States | 25 | 4.5 (1.5–9.3) | 27.8 (19.0–38.3) |
| 101 | Canada | 179 | 3.5 (2.7–4.5) | 4.0 (3.0–5.5) |
| 102 | South Africa | 486 | 1.2 (0.8–1.7) | 2.8 (2.0–3.9) |
| 104 | United States | 25 | 9.2 (1.1–33.3) | 21.4 (7.8–46.5) |
| 103 | United States | 65 | 0.7 (0.4–1.4) | 1.2 (0.7–2.0) |
HPV appears to be frequently detected at the anal canal and on the hands.61, 104 As such, HPV transmission through non-penetrative sexual contact, such as fingers to genitals or anal canal, has been observed,61, 104, 106 but the majority of HPV+ fingertip specimens likely represent deposition of DNA from genitals rather than true infection. Some anal HPV infections in women may occur as a result of viral shedding of cervical or vaginal HPV infections in vaginal discharge. Indeed, anal and cervical HPV infections occur consecutively,107 suggesting that the vagina and, to a lesser extent, the anal canal, serve as reservoirs for HPV infection at the other anatomical sites. Cross-sectional analyses demonstrating type-specific concordance of HPV infection of the genitals and anal canal among heterosexual men highlight the complexity of determining true sources and targets of viral transmission.54, 55 Understanding heterosexual HPV transmission is further complicated by the possibility of autoinoculation.61 Enhanced understanding of HPV transmission dynamics will assist in promulgating more efficient strategies for the prevention and control of HPV-related cancers.
Conclusions
HPV causes cancer in both men and women. The HPV-related cancer burden remains higher in women than men, even in countries that have effective cervical cancer screening programs. Emerging data indicate that HPV infection appears to vary significantly by gender as well as across anatomic sites. This variation in HPV prevalence may explain the differences in cancer incidence rates observed by gender in some cases (e.g., OPC and oral HPV infection are both higher in men than in women); however, in other cases, cancer rates are remarkably similar among men and women (e.g., anal cancer), despite large differences in HPV prevalence. In addition, within a population, emerging data demonstrate that HPV prevalence varies dramatically by anatomic site evaluated, indicating that for the same sexual exposure, susceptibility to infection varies by epithelia. Adding to the complexity of the viral-host interaction are differences in the adaptive immune response to natural HPV infection observed between men and women.
In summary: (1) HPV infection patterns differ by anatomic site (higher prevalence in the genitals versus oral region); (2) HPV infection and clearance rates differ by gender; (3) transmission rates differ by gender, with higher female-to-male compared to male-to-female transmission; and (4) immune response to HPV differs by anatomic site of infection and is stronger and more protective against re-infection in women than men. Altogether, these observations lead us to conclude that more research is needed to fully characterize HPV natural history at each of the anatomic sites where HPV causes cancer in men and women, which is critical to inform the basic science of HPV natural history and the development of future infection and cancer prevention efforts.
Acknowledgments
The authors thank the EUROGIN 2013 conference organizers, Mr. Jerome Vignat for unpublished data on HPV-attributable fractions, and Dr. Beibei Lu for providing the serology figure.
A.R.G. is the recipient of a current investigator-initiated grant (IISP39582) from Merck and is on the Speaker’s Bureau of Merck. A.R.K. is the Co-Principal Investigator on the long-term follow-up of the Costa Rica Vaccine Trial, sponsored by the National Cancer Institute with vaccine donated by GlaxoSmithKline Biologicals under a clinical trials agreement. J.M. has participated in Merck Steering Committees and on the Advisory Board of Sanofi Pasteur MSD, Gen-Probe, and Roche Diagnostics.
Funding sources: This work was supported, in part, by the National Cancer Institute at the National Institutes of Health (Cancer Prevention Fellowship R25T CA147832 to SLS) and the American Cancer Society (Postdoctoral Fellowship PF-13-222-01 – MPC to CMPC).
Abbreviations
- HPV
Human papillomavirus
- OPC
oropharyngeal cancer
- PAF
population attributable fraction
- M/F
male/female
- HPV+
HPV-positive
- MSM
men who have sex with men
- MSW
men who have sex with women
- qHPV
quadrivalent HPV
Footnotes
Novelty and impact: This is the first manuscript to review differences in HPV natural history and disease by gender and epithelial site of infection, information needed to inform prevention of HPV-related disease.
Conflicts of interest: No other authors reported conflicts of interest.
References
- 1.Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 2.Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 3.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, Bateson D, McNamee K, Garefalakis M, Garland SM. Fall in human papillomavirus prevalence following a national vaccination program. Journal of Infectious Diseases. 2012;206:1645–1651. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 4.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia--nationwide follow-up of young Danish women. Journal of the National Cancer Institute. 2014;106:djt460. doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 5.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–2092. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 6.Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, Niccolai LM, Schafer S, Unger ER, Markowitz LE. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;31:109–113. doi: 10.1016/j.vaccine.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 7.Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Guy RJ. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ (Clinical research ed) 2013;346:f2032. doi: 10.1136/bmj.f2032. [DOI] [PubMed] [Google Scholar]
- 8.Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–2010. American journal of public health. 2012;102:833–835. doi: 10.2105/AJPH.2011.300465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbyn M, de Sanjose S, Saraiya M, Sideri M, Palefsky J, Lacey C, Gillison M, Bruni L, Ronco G, Wentzensen N, Brotherton J, Qiao YL, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. International Journal of Cancer. 2012;131:1969–1982. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillison ML, Castellsague X, Chaturvedi A, Goodman MT, Snijders P, Tommasino M, Arbyn M, Franceschi S. Eurogin Roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. International Journal of Cancer. 2014;134:497–507. doi: 10.1002/ijc.28201. [DOI] [PubMed] [Google Scholar]
- 11.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi S, Vignat J, de Martel C. Comparison of HPV-related disease in men and women EUROGIN International Congress Florence, Italy. 2013 [Google Scholar]
- 13.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncology. 2013 doi: 10.1016/j.oraloncology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, Papenfuss MR, Abrahamsen M, Jolles E, Nielson CM, Baggio ML, Silva R, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–2043. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 16.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, Alfaro M, Sherman ME, Wacholder S, Chen S, Rodriguez AC, Burk RD. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 17.Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, Markowitz LE, Smith D, Harris RB. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. Journal of Infectious Diseases. 2007;196:1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzon L, Militello V, Pagni S, Franchin E, Dal Bello F, Mengoli C, Palu G. Distribution of human papillomavirus types in the anogenital tract of females and males. J Med Virol. 2010;82:1424–1430. doi: 10.1002/jmv.21733. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, Smith D. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Wacholder S, Tarone R, Burk RD. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 21.Muñoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Munoz A. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. Journal of Infectious Diseases. 2004;190:2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. Journal of the National Cancer Institute. 2010;102:315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2010;46:S20–S26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, Hillman RJ, Petoumenos K, Roberts J, Tabrizi SN, Templeton DJ, Grulich AE. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, Kirk GD, D'Souza G, Bosch RJ, Brooks JT, Napravnik S, Hessol NA, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Arguelles ME, Melon S, Junquera ML, Boga JA, Villa L, Perez-Castro S, de Ona M. Human papillomavirus infection in a male population attending a sexually transmitted infection service. PLoS One. 2013;8:e54375. doi: 10.1371/journal.pone.0054375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin-Hong PV, Vittinghoff E, Cranston RD, Buchbinder S, Cohen D, Colfax G, Da Costa M, Darragh T, Hess E, Judson F, Koblin B, Madison M, et al. Age-Specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE study. Journal of Infectious Diseases. 2004;190:2070–2076. doi: 10.1086/425906. [DOI] [PubMed] [Google Scholar]
- 28.Dona MG, Palamara G, Di Carlo A, Latini A, Vocaturo A, Benevolo M, Pimpinelli F, Giglio A, Moretto D, Impara G, Giuliani M. Prevalence, genotype diversity and determinants of anal HPV infection in HIV-uninfected men having sex with men. Journal of Clinical Virology. 2012;54:185–189. doi: 10.1016/j.jcv.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Zhou F, Li X, Yang Y, Ruan Y, Jin Q. Anal HPV infection in HIV-positive men who have sex with men from China. PLoS One. 2010;5:e15256. doi: 10.1371/journal.pone.0015256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Qian HZ, Sun J, Gao L, Yin L, Li X, Xiao D, Li D, Sun X, Ruan Y, Milam DF, Pan SW, et al. Anal human papillomavirus infection among HIV-infected and uninfected men who have sex with men in Beijing, China. Journal of acquired immune deficiency syndromes (1999) 2013;64:103–114. doi: 10.1097/QAI.0b013e31829b6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiviat NB, Critchlow CW, Holmes KK, Kuypers J, Sayer J, Dunphy C, Surawicz C, Kirby P, Wood R, Daling JR. Association of anal dysplasia and human papillomavirus with immunosuppression and HIV infection among homosexual men. AIDS. 1993;7:43–49. doi: 10.1097/00002030-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Nyitray A, Nielson CM, Harris RB, Flores R, Abrahamsen M, Dunne EF, Giuliano AR. Prevalence of and risk factors for anal human papillomavirus infection in heterosexual men. Journal of Infectious Diseases. 2008;197:1676–1684. doi: 10.1086/588145. [DOI] [PubMed] [Google Scholar]
- 33.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, Marshall JB, Radley D, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 34.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. Journal of Infectious Diseases. 1998;177:361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 35.Phanuphak N, Teeratakulpisarn N, Pankam T, Kerr SJ, Barisri J, Deesua A, Rodbamrung P, Hongchookiat P, Chomchey N, Phanuphak P, Sohn AH, Ananworanich J, et al. Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. Journal of acquired immune deficiency syndromes (1999) 2013;63:472–479. doi: 10.1097/QAI.0b013e3182918a5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vajdic CM, van Leeuwen MT, Jin F, Prestage G, Medley G, Hillman RJ, Stevens MP, Botes LP, Zablotska I, Tabrizi SN, Grulich AE. Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men. Sexually Transmitted Infections. 2009;85:330–335. doi: 10.1136/sti.2008.034744. [DOI] [PubMed] [Google Scholar]
- 37.Castro FA, Quint W, Gonzalez P, Katki HA, Herrero R, van Doorn LJ, Schiffman M, Struijk L, Rodriguez AC, DelVecchio C, Lowy DR, Porras C, et al. Prevalence of and risk factors for anal human papillomavirus infection among young healthy women in Costa Rica. Journal of Infectious Diseases. 2012;206:1103–1110. doi: 10.1093/infdis/jis458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez BY, Ka'opua LS, Scanlan L, Ching JA, Kamemoto LE, Thompson PJ, Zhu X, Shvetsov YB, Tofaeono J, Williams VT. Cervical and anal human papillomavirus infection in adult women in American Samoa. Asia-Pacific journal of public health / Asia-Pacific Academic Consortium for Public Health. 2013;25:19–31. doi: 10.1177/1010539511410867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez BY, McDuffie K, Zhu X, Wilkens LR, Killeen J, Kessel B, Wakabayashi MT, Bertram CC, Easa D, Ning L, Boyd J, Sunoo C, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:2550–2556. doi: 10.1158/1055-9965.EPI-05-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessol NA, Holly EA, Efird JT, Minkoff H, Schowalter K, Darragh TM, Burk RD, Strickler HD, Greenblatt RM, Palefsky JM. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23:59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierangeli A, Scagnolari C, Selvaggi C, Cannella F, Riva E, Impagnatiello A, Bernardi G, Ciardi A, Moschella CM, Antonelli G, Indinnimeo M. High detection rate of human papillomavirus in anal brushings from women attending a proctology clinic. The Journal of infection. 2012;65:255–261. doi: 10.1016/j.jinf.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Schlecht NF, Burk RD, Nucci-Sack A, Shankar V, Peake K, Lorde-Rollins E, Porter R, Linares LO, Rojas M, Strickler HD, Diaz A. Cervical, anal and oral HPV in an adolescent inner-city health clinic providing free vaccinations. PLoS One. 2012;7:e37419. doi: 10.1371/journal.pone.0037419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.dos Ramos Farias MS, Picconi MA, Garcia MN, Gonzalez JV, Basiletti J, Pando Mde L, Avila MM. Human papilloma virus genotype diversity of anal infection among trans (male to female transvestites, transsexuals or transgender) sex workers in Argentina. Journal of Clinical Virology. 2011;51:96–99. doi: 10.1016/j.jcv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Nyitray AG. Comparing and contrasting the natural history of anal HPV among women and men EUROGIN International Congress Florence, Italy. 2013 [Google Scholar]
- 45.Hessol NA, Holly EA, Efird JT, Minkoff H, Weber KM, Darragh TM, Burk RD, Strickler HD, Greenblatt RM, Palefsky JM. Concomitant anal and cervical human papillomavirusV infections and intraepithelial neoplasia in HIV-infected and uninfected women. AIDS. 2013;27:1743–1751. doi: 10.1097/QAD.0b013e3283601b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xi LF, Critchlow CW, Wheeler CM, Koutsky LA, Galloway DA, Kuypers J, Hughes JP, Hawes SE, Surawicz C, Goldbaum G, Holmes KK, Kiviat NB. Risk of anal carcinoma in situ in relation to human papillomavirus type 16 variants. Cancer Res. 1998;58:3839–3844. [PubMed] [Google Scholar]
- 47.Nyitray AG, Carvalho da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, Papenfuss M, Villa LL, Lazcano-Ponce E, Giuliano AR. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. The Journal of infectious diseases. 2011;203:49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Critchlow CW, Hawes SE, Kuypers JM, Goldbaum GM, Holmes KK, Surawicz CM, Kiviat NB. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Pierangeli A, Scagnolari C, Degener AM, Bucci M, Ciardi A, Riva E, Indinnimeo M, Mancini G, D'Ettorre G, Vullo V, Antonelli G. Type-specific human papillomavirus-DNA load in anal infection in HIV-positive men. AIDS. 2008;22:1929–1935. doi: 10.1097/QAD.0b013e32830fbd7a. [DOI] [PubMed] [Google Scholar]
- 50.Donà MG, Palamara G, Di Carlo A, Latini A, Vocaturo A, Benevolo M, Pimpinelli F, Giglio A, Moretto D, Impara G, Giuliani M. Prevalence, genotype diversity and determinants of anal HPV infection in HIV-uninfected men having sex with men. Journal of Clinical Virology. 2012;54:185–189. doi: 10.1016/j.jcv.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Dona MG, Benevolo M, Vocaturo A, Palamara G, Latini A, Giglio A, Moretto D, Rollo F, Impara G, Ensoli F, Pimpinelli F, Di Carlo A, et al. Anal cytological abnormalities and epidemiological correlates among men who have sex with men at risk for HIV-1 infection. BMC Cancer. 2012;12:476. doi: 10.1186/1471-2407-12-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hessol NA, Holly EA, Efird JT, Minkoff H, Schowalter K, Darragh TA, Burk RD, Strickler HD, Greenblatt RM, Palefsky JA. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23:59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez BY, Ka'opua LS, Scanlan L, Ching JA, Kamemoto LE, Thompson PJ, Zhu X, Shvetsov YB, Tofaeono J, Williams VT. Cervical and Anal Human Papillomavirus Infection in Adult Women in American Samoa. Asia Pac J Public Health. 2012 doi: 10.1177/1010539511410867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyitray AG, Carvalho da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, Papenfuss M, Villa LL, Lazcano-Ponce E, Giuliano AR. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. J Infect Dis. 2011;203:49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nyitray AG, Smith D, Villa L, Lazcano-Ponce E, Abrahamsen M, Papenfuss M, Giuliano AR. Prevalence of and risk factors for anal human papillomavirus infection in men who have sex with women: a cross-national study. J Infect Dis. 2010;201:1498–1508. doi: 10.1086/652187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawford R, Grignon AL, Kitson S, Winder DM, Ball SL, Vaughan K, Stanley MA, Sterling JC, Goon PK. High prevalence of HPV in non-cervical sites of women with abnormal cervical cytology. BMC Cancer. 2011;11:473. doi: 10.1186/1471-2407-11-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hillemanns P, Ellerbrock TV, McPhillips S, Dole P, Alperstein S, Johnson D, Sun XW, Chiasson MA, Wright TC., Jr Prevalence of anal human papillomavirus infection and anal cytologic abnormalities in HIV-seropositive women. AIDS. 1996;10:1641–1647. doi: 10.1097/00002030-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Moscicki AB, Durako SJ, Houser J, Ma Y, Murphy DA, Darragh TM, Farhat S, Wilson CM. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS. 2003;17:311–320. doi: 10.1097/00002030-200302140-00004. [DOI] [PubMed] [Google Scholar]
- 59.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. Journal of Infectious Diseases. 2001;183:383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 60.Valari O, Koliopoulos G, Karakitsos P, Valasoulis G, Founta C, Godevenos D, Dova L, Paschopoulos M, Loufopoulos A, Paraskevaidis E. Human papillomavirus DNA and mRNA positivity of the anal canal in women with lower genital tract HPV lesions: predictors and clinical implications. Gynecologic Oncology. 2011;122:505–508. doi: 10.1016/j.ygyno.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 61.Hernandez BY, Wilkens LR, Zhu X, Thompson P, McDuffie K, Shvetsov YB, Kamemoto LE, Killeen J, Ning L, Goodman MT. Transmission of human papillomavirus in heterosexual couples. Emerging infectious diseases. 2008;14:888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Ning L, Killeen J, Kamemoto L, Hernandez BY. Acquisition of anal human papillomavirus (HPV) infection in women: the Hawaii HPV Cohort study. Journal of Infectious Diseases. 2008;197:957–966. doi: 10.1086/529207. [DOI] [PubMed] [Google Scholar]
- 63.Shvetsov YB, Hernandez BY, McDuffie K, Wilkens LR, Zhu X, Ning L, Killeen J, Kamemoto L, Goodman MT. Duration and clearance of anal human papillomavirus (HPV) infection among women: the Hawaii HPV cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48:536–546. doi: 10.1086/596758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nyitray AG, Carvalho da Silva RJ, Baggio ML, Smith D, Abrahamsen M, Papenfuss M, Lin HY, Quiterio M, Salmeron J, Lazcano-Ponce E, Villa LL, Giuliano AR. Six-month incidence, persistence, and factors associated with persistence of anal human papillomavirus in men: the HPV in men study. J Infect Dis. 2011;204:1711–1722. doi: 10.1093/infdis/jir637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chin-Hong PV, Husnik M, Benet DJ, Buchbinder S, Colfax G, Cranston RD, Da Costa M, Judson F, Koblin B, Mayer KH, Herrera R, Palefsky J. High incidence and persistence of anal human papillomavirus infection among HIV-negative sexually active men who have sex with men: The EXPLORE Study 24th International Papillomavirus Conference Beijing, China; 2007. [Google Scholar]
- 66.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Graubard BI, Chaturvedi AK. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreimer AR, Bhatia RK, Messeguer AL, Gonzalez P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sexually Transmitted Diseases. 2010;37:386–391. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 68.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet infectious diseases. 2007;7:453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 69.Beachler DC, D'Souza G, Sugar EA, Xiao W, Gillison ML. Natural history of anal vs oral HPV infection in HIV-infected men and women. Journal of Infectious Diseases. 2013;208:330–339. doi: 10.1093/infdis/jit170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edelstein ZR, Schwartz SM, Hawes S, Hughes JP, Feng Q, Stern ME, O'Reilly S, Lee SK, Fu Xi L, Koutsky LA. Rates and determinants of oral human papillomavirus infection in young men. Sexually Transmitted Diseases. 2012;39:860–867. doi: 10.1097/OLQ.0b013e318269d098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kero K, Rautava J, Syrjanen K, Willberg J, Grenman S, Syrjanen S. Smoking increases oral HPV persistence among men: 7-year follow-up study. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2014;33:123–133. doi: 10.1007/s10096-013-1938-1. [DOI] [PubMed] [Google Scholar]
- 72.Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, Abrahamsen M, Hildesheim A, Villa LL, Salmeron JJ, Lazcano-Ponce E, Giuliano AR. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013;382:877–887. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pickard RK, Xiao W, Broutian TR, He X, Gillison ML. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sexually Transmitted Diseases. 2012;39:559–566. doi: 10.1097/OLQ.0b013e31824f1c65. [DOI] [PubMed] [Google Scholar]
- 74.Rintala M, Grenman S, Puranen M, Syrjanen S. Natural history of oral papillomavirus infections in spouses: a prospective Finnish HPV Family Study. Journal of Clinical Virology. 2006;35:89–94. doi: 10.1016/j.jcv.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 75.Videla S, Darwich L, Canadas MP, Coll J, Pinol M, Garcia-Cuyas F, Molina-Lopez RA, Cobarsi P, Clotet B, Sirera G, Group H-HS. Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sex Transm Dis. 2013;40:3–10. doi: 10.1097/OLQ.0b013e31827e87bd. [DOI] [PubMed] [Google Scholar]
- 76.Kreimer AR, Villa A, Nyitray AG, Abrahamsen M, Papenfuss M, Smith D, Hildesheim A, Villa LL, Lazcano-Ponce E, Giuliano AR. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev. 2011;20:172–182. doi: 10.1158/1055-9965.EPI-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pierce Campbell CM, Kreimer AR, O'Keefe MT, Lin HY, Fulp W, Abrahamsen M, Villa LL, Lazcano-Ponce E, Trotti A, Kish JA, Caudell JJ, Giuliano AR. Oral HPV16 persistence among participants of the HPV Infeciton in Men (HIM) Study EUROGIN International Congress Florence, Italy. 2013 [Google Scholar]
- 78.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, Quint W, van Doorn LJ, Sherman ME, Xhenseval V, Herrero R, Hildesheim A. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. Journal of the National Cancer Institute. 2010;102:1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu B, Viscidi RP, Wu Y, Lee JH, Nyitray AG, Villa LL, Lazcano-Ponce E, da Silva RJ, Baggio ML, Quiterio M, Salmeron J, Smith DC, et al. Prevalent Serum Antibody Is Not a Marker of Immune Protection against Acquisition of Oncogenic HPV16 in Men. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. Journal of Infectious Diseases. 2000;181:1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 81.Lu B, Viscidi RP, Villa LL, Lazcano-Ponce E, Salmeron J, da Silva RJ, Baggio ML, Quiterio M, Smith DC, Abrahamsen M, Papenfuss M, Giuliano AR. Capsid antibody response to genital HPV 6, 11, 16, and 18 infection in healthy men 28th International Papillomavirus Conference San Juan, Puerto Rico; 2012. [Google Scholar]
- 82.Scherpenisse M, Schepp RM, Mollers M, Mooij SH, Meijer CJ, Berbers GA, van der Klis FR. Comparison of different assays to assess human papillomavirus (HPV) type 16- and 18-specific antibodies after HPV infection and vaccination. Clinical and vaccine immunology : CVI. 2013;20:1329–1332. doi: 10.1128/CVI.00153-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Desai S, Chapman R, Jit M, Nichols T, Borrow R, Wilding M, Linford C, Lowndes CM, Nardone A, Pebody R, Soldan K. Prevalence of human papillomavirus antibodies in males and females in England. Sexually Transmitted Diseases. 2011;38:622–629. doi: 10.1097/OLQ.0b013e31820bc880. [DOI] [PubMed] [Google Scholar]
- 84.Heiligenberg M, Michael KM, Kramer MA, Pawlita M, Prins M, Coutinho RA, Dukers-Muijrers NH, Waterboer T. Seroprevalence and determinants of eight high-risk human papillomavirus types in homosexual men, heterosexual men, and women: a population-based study in Amsterdam. Sexually Transmitted Diseases. 2010;37:672–680. doi: 10.1097/OLQ.0b013e3181e71069. [DOI] [PubMed] [Google Scholar]
- 85.Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16, -18, and -33 seroprevalence. Sexually Transmitted Diseases. 2004;31:247–256. doi: 10.1097/01.olq.0000118425.49522.2c. [DOI] [PubMed] [Google Scholar]
- 86.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. Journal of Infectious Diseases. 2009;200:1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 87.Newall AT, Brotherton JM, Quinn HE, McIntyre PB, Backhouse J, Gilbert L, Esser MT, Erick J, Bryan J, Formica N, MacIntyre CR. Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46:1647–1655. doi: 10.1086/587895. [DOI] [PubMed] [Google Scholar]
- 88.Slavinsky J, 3rd, Kissinger P, Burger L, Boley A, DiCarlo RP, Hagensee ME. Seroepidemiology of low and high oncogenic risk types of human papillomavirus in a predominantly male cohort of STD clinic patients. International journal of STD & AIDS. 2001;12:516–523. doi: 10.1258/0956462011923615. [DOI] [PubMed] [Google Scholar]
- 89.Stone KM, Karem KL, Sternberg MR, McQuillan GM, Poon AD, Unger ER, Reeves WC. Seroprevalence of human papillomavirus type 16 infection in the United States. Journal of Infectious Diseases. 2002;186:1396–1402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- 90.Svare EI, Kjaer SK, Nonnenmacher B, Worm AM, Moi H, Christensen RB, van den Brule AJ, Walboomers JM, Meijer CJ, Hubbert NL, Lowy DR, Schiller JT. Seroreactivity to human papillomavirus type 16 virus-like particles is lower in high-risk men than in high-risk women. Journal of Infectious Diseases. 1997;176:876–883. doi: 10.1086/516505. [DOI] [PubMed] [Google Scholar]
- 91.Thompson DL, Douglas JM, Jr, Foster M, Hagensee ME, Diguiseppi C, Baron AE, Cameron JE, Spencer TC, Zenilman J, Malotte CK, Bolan G, Kamb ML, et al. Seroepidemiology of infection with human papillomavirus 16, in men and women attending sexually transmitted disease clinics in the United States. Journal of Infectious Diseases. 2004;190:1563–1574. doi: 10.1086/423817. [DOI] [PubMed] [Google Scholar]
- 92.Plitt SS, Sherman SG, Viscidi RP, Strathdee SA, Fuller CM, Taha TE. Human papillomavirus seroprevalence among young male and female drug users. Sexually Transmitted Diseases. 2007;34:676–680. doi: 10.1097/01.olq.0000258309.42765.ac. [DOI] [PubMed] [Google Scholar]
- 93.Hariri S, Dunne EF, Sternberg M, Unger ER, Meadows KS, Karem KL, Markowitz LE. Seroepidemiology of human papillomavirus type 11 in the United States: results from the third National Health And Nutrition Examination Survey, 1991--1994. Sexually Transmitted Diseases. 2008;35:298–303. doi: 10.1097/OLQ.0b013e31815abaef. [DOI] [PubMed] [Google Scholar]
- 94.Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, Kiviat N, Galloway DA. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. Journal of Infectious Diseases. 1996;174:927–936. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 95.Edelstein ZR, Carter JJ, Garg R, Winer RL, Feng Q, Galloway DA, Koutsky LA. Serum antibody response following genital {alpha}9 human papillomavirus infection in young men. Journal of Infectious Diseases. 2011;204:209–216. doi: 10.1093/infdis/jir242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carter JJ, Madeleine MM, Shera K, Schwartz SM, Cushing-Haugen KL, Wipf GC, Porter P, Daling JR, McDougall JK, Galloway DA. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61:1934–1940. [PubMed] [Google Scholar]
- 97.Ho GY, Studentsov Y, Hall CB, Bierman R, Beardsley L, Lempa M, Burk RD. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. Journal of Infectious Diseases. 2002;186:737–742. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]
- 98.Viscidi RP, Schiffman M, Hildesheim A, Herrero R, Castle PE, Bratti MC, Rodriguez AC, Sherman ME, Wang S, Clayman B, Burk RD. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiology, Biomarkers and Prevention. 2004;13:324–327. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 99.Veldhuijzen NJ, Snijders PJ, Reiss P, Meijer CJ, van de Wijgert JH. Factors affecting transmission of mucosal human papillomavirus. The Lancet infectious diseases. 2010;10:862–874. doi: 10.1016/S1473-3099(10)70190-0. [DOI] [PubMed] [Google Scholar]
- 100.Reiter PL, Pendergraft WF, 3rd, Brewer NT. Meta-analysis of human papillomavirus infection concordance. Cancer Epidemiology, Biomarkers and Prevention. 2010;19:2916–2931. doi: 10.1158/1055-9965.EPI-10-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burchell AN, Coutlee F, Tellier PP, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. Journal of Infectious Diseases. 2011;204:1723–1729. doi: 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mbulawa ZZ, Johnson LF, Marais DJ, Coetzee D, Williamson AL. The impact of human immunodeficiency virus on human papillomavirus transmission in heterosexually active couples. The Journal of infection. 2013;67:51–58. doi: 10.1016/j.jinf.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 103.Nyitray AG, Lin HY, Fulp WJ, Chang M, Menezes L, Lu B, Abrahamsen M, Papenfuss M, Gage C, Galindo CM, Giuliano AR. The role of monogamy and duration of heterosexual relationships in human papillomavirus transmission. The Journal of infectious diseases. 2014;209:1007–1015. doi: 10.1093/infdis/jit615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Widdice L, Ma Y, Jonte J, Farhat S, Breland D, Shiboski S, Moscicki AB. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. Journal of Infectious Diseases. 2013;207:1286–1294. doi: 10.1093/infdis/jit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, Kjaer SK, Palefsky J. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30(Suppl 5):F24–F33. doi: 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O'Reilly S, Kiviat NB, Koutsky LA. Detection of genital HPV types in fingertip samples from newly sexually active female university students. Cancer Epidemiology, Biomarkers and Prevention. 2010;19:1682–1685. doi: 10.1158/1055-9965.EPI-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV Cohort Study. Journal of Infectious Diseases. 2010;201:1331–1339. doi: 10.1086/651620. [DOI] [PMC free article] [PubMed] [Google Scholar]