Abstract
Purpose
To determine the safety and toxicities of sequential MMC + BCG in patients with non-muscle invasive bladder cancer (NMIBC) and explore evidence for potentiation of BCG activity by MMC.
Experimental Design
A 3+3 phase I dose-escalation trial of six weekly treatments was conducted in patients with NMIBC. MMC (10, 20 or 40 mg) was instilled intravesically for 30 minutes, followed by a 10-minute washout with gentle saline irrigation and then instillation of BCG (half or full strength) for 2 hours. Urine cytokines were monitored and compared to levels in a control cohort receiving BCG only. Murine experiments were carried out as described previously.
Results
Twelve patients completed therapy including 3 patients receiving full doses. The regimen was well tolerated with no treatment-related dose limiting toxicities. Urinary frequency and urgency, and fatigue were common. Eleven (91.7%) patients were free of disease at a mean (range) follow-up of 21.4 (8.4-27.0) months. Median post-treatment urine concentrations of IL-2, IL-8, IL-10 and TNF-α increased over the 6-week treatment period. A greater increase in post-treatment urinary IL-8 during the 6-week period was observed in patients receiving MMC + BCG compared to patients receiving BCG monotherapy. In mice, intravesical MMC + BCG skewed tumor-associated macrophages (TAMs) towards a beneficial M1 phenotype.
Conclusions
Instillation of sequential MMC + BCG is safe and tolerable up to 40 mg MMC plus full strength BCG. This approach could provide improved antitumor activity over BCG monotherapy by augmenting beneficial M1 TAMs.
Keywords: bladder, cancer, BCG, and mitomycin
Introduction
Due to the high rate of disease relapse following tumor removal for patients with high-grade Ta-T1 bladder cancer, current guidelines recommend adjuvant administration of intravesical bacillus Calmette-Guérin (BCG, preferred) or mitomycin C (MMC) following complete tumor resection (1, 2). For patients with carcinoma-in-situ (CIS), intravesical BCG is recommended for tumor eradication and prophylaxis (1, 2). Intravesical BCG provides a significant improvement in recurrence-free survival and consistently affords an improvement over intravesical chemotherapy. Unfortunately, up to 70% of patients with high-risk NMIBC will eventually recur despite intravesical BCG and some recurrent tumors will progress to higher stage (3, 4).
Combining intravesical agents with different antitumor mechanisms could improve antitumor efficacy and prevent the emergence of drug-resistant tumors (5). Whereas MMC exerts antineoplastic activity through cross-linking DNA, alkylation, and DNA strand breakage through generation of free radicals (6), precise effector mechanisms mediating the antitumor activity of BCG remain unknown. However, it is generally accepted that intravesical BCG instillation induces non-specific local immunity, which facilitates recruitment of activated T cells important for anti-tumor efficacy (7-9). The established clinical efficacy of these two agents and their disparate antitumor effects prompted several trials testing alternate and sequential delivery of BCG and MMC(5).
In preclinical models, combining BCG plus MMC inhibits growth of bladder cancer more effectively than either individual agent (10, 11). Thus, in addition to the direct antineoplastic activity of MMC, MMC given immediately prior to BCG instillation could also improve BCG activity by promoting BCG uptake into local cells and activation of anti-tumor immune cells.
Clinical evidence for an improved effect of sequential MMC and BCG is supported by results from a recent phase III trial demonstrating that MMC given one day before BCG was more effective than BCG monotherapy (12). Administration of MMC on the same day as BCG is more practical than treating on two separate visits and could boost BCG anti-tumor activity. Nevertheless, there are substantial safety and toxicity concerns for this sequential approach. Urothelial cell disruption mediated through MMC followed by BCG-induced bladder inflammation could result in a breach of the protective urothelium resulting in increased local irritative symptoms or permitting systemic exposure to high levels of MMC and/or BCG (11). The primary objective of our study was to determine the safety and identify toxicities of sequentially combining MMC with BCG in the same treatment setting. We also assessed tumor-infiltrating immune cell populations during sequential therapy.
Methods
Eligibility Criteria
This was a non-randomized, open-label, phase I dose-escalation single center study. All patients had high-grade NMIBC. Clinical stages included CIS, Ta, and T1. All papillary tumors were resected completely prior to enrollment. Patients with T1 disease underwent a restaging transurethral resection of bladder tumor prior to enrollment. Patients receiving prior BCG were eligible only if they were unable or unwilling to undergo radical cystectomy. Additional eligibility criteria included adequate marrow function defined as more than 1,500 blood granulocytes/mm3 and more than 150,000 platelets/mm3, age >18 years and ability to provide informed consent. Immunosuppressed patients (e.g., HIV, chronic steroid use) were excluded. The local Institutional Review Board approved the trial. No study exemptions were granted.
Treatment
Therapy was administered 2-4 weeks following the most recent bladder tumor removal, and was given weekly for 6 weeks. Patients were instructed to refrain from drinking fluids starting 16 hours before and during treatment and to take oral sodium bicarbonate (1.3 g) the night before, the morning of, and 30 minutes prior to therapy. MMC (Accord Healthcare Inc., Durham, NC) was reconstituted and diluted in sterile water. Patients were treated with MMC at 10 mg, 20 mg or 40 mg for 30 minutes. Freeze-dried TICE® BCG preparations (equivalent to approximately 50 mg wet weight) were diluted in 50 ml of sterile saline. Full strength corresponds to 1-8 × 108 colony forming units of BCG. The bladder was completely emptied with a urethral catheter prior to drug instillation; MMC was instilled into the bladder through the urethral catheter, held for 30 minutes and then drained. The bladder was gently irrigated with 60 ml of sterile saline for 10 minutes to facilitate washout of MMC. BCG was then administered with a dwell time of 2 hours. Maintenance therapy with BCG monotherapy was given at 3 months, 6 months, and then every 6 months for a total of 7 maintenance cycles.
Study Design and Adverse Event Monitoring
Three patients were assigned to one of four dosing levels using a standard 3+3 phase I dose escalation. The selection of 3 patients per dose cohort was calculated based on described methodology (13) under the assumption that the probability of a true risk of excessive toxicity for the combined treatment was in the range of 10% to 50%. Dose escalation took place among subsequent patients and not within each patient. The first dose level was 10 mg MMC in 20 ml sterile water and half-strength BCG. The second dose level was 10 mg MMC and full strength BCG. The third and fourth dose levels were 20 mg MMC in 20 ml sterile water and 40 mg of MMC in 40 ml sterile water, respectively, followed by full strength BCG. The volume of water was increased for the 40 mg dosage to improve MMC solubility. Three patients were treated at each dose level, or until unacceptable toxicity was observed. If a dose-limiting toxicity (DLT) was experienced in one of three patients, an additional three patients were accrued at that dose level. If at any dose level three or more of six patients experienced DLT, the dose level below that one would be defined as the MTD (maximum tolerated dose). Patients were followed for 2 weeks after completing induction treatment before subsequent patients could be entered at the next higher dose level.
Vital signs were measured shortly before and after administration of the combined agents. Patient-reported side effect questionnaires were administered prior to each weekly instillation to ascertain the previous instillations effects. Physical examinations and assessments of toxicity were performed before and after each administration of chemotherapy. A complete blood count was obtained prior to study enrollment and at weeks 2, 4 and 6. A urinalysis was performed prior to administration of therapy each week. If the urinalysis was abnormal, a urine culture was automatically obtained. Two weeks after completing treatment, patients were contacted to inquire about tolerability and any potential adverse events. Routine surveillance was conducted every 3 months for the first 2 years following biopsy, then every 6 months for 2 years, then annually. Biopsies were performed when clinically indicated per the standard of care (e.g., obvious tumor or erythematous lesion). Response assessment relied on cystoscopic evaluation and biopsies when performed.
Adverse events were graded according to the National Cancer Institute common toxicity criteria version 4.0. Bacterial cystitis was defined as the occurrence of culture-proven cystitis (not BCG-related). Irritative bladder symptoms with negative urine culture were classified as non-infectious cystitis (BCG-related cystitis). Treatment related grade 3 or 4 systemic toxicities or grade 4 local bladder toxicities defined a DLT. No evidence of disease (NED) was defined as absence of visual tumor seen on cystoscopy and cytology.
Urinary Cytokine Measurement
Urine was collected from each patient immediately prior to therapy instillation and 4-6 hours following therapy (first voided urine specimen following evacuation of BCG) on weeks 1, 4 and 6. Urine was also collected on a prospective control population of patients receiving BCG monotherapy (n=5). Samples were filter-centrifuged with a 0.45 μm membrane pore size filter (Corning® Costar® Spin-X® Centrifuge Tube Filter, Corning, NY) for 10 minutes at 800 revolutions per minute to remove debris. Supernatants were stored at -80° C until assayed for cytokines using customized MilliplexTM kits (Millipore, St. Charles, MO) according to the manufacturer's instructions. Data were collected and analyzed using Luminex software (Luminex Corporation, Austin, TX). Samples were run in duplicate, analyzed and standard curves were generated using Bio-Plex Manager v5.0 software (Bio-Rad Laboratories, Hercules, CA). Testing for normality of data distribution was performed using the Shapiro-Wilk normality test. Statistical analysis was performed with Prism 6 (GraphPad Software, Inc., La Jolla, CA) and Stata 10.0 (StataCorp LP, College Station, TX). Data were analyzed by a one-way analysis of variance (ANOVA). When the variance was not equal, a non-parametric test for trend was performed across the groups.
Murine studies
Orthotopic MB49 tumors were established in syngeneic C57BL/6 female mice aged 6-12 months (Jackson Laboratory) as described (8, 14). Briefly, poly-L-lysine (0.1 mg/ml) (Sigma-Aldrich) was instilled into mouse bladders for 20 minutes prior to instillation of 1 × 105 MB49 cells in 50 ml PBS for 1 hour MMC (0.25 mg in 50 μl sterile water) was instilled into the bladder for 20 minutes. BCG (3 × 106 CFUs in 50 μl sterile water) was instilled for 1 hour. In mice receiving MMC + BCG, a 10 minute washout was performed with sterile water after MMC and before BCG. Mouse bladders were surgically harvested under sterile conditions 6 hours after the 4th weekly instillation, minced with 5 ml of collagenase (Sigma-Aldrich, St. Louis, MO) in RPMI medium (2 mg/ml) and incubated at 37° C for 1 hour. Bladder tissue was then repeatedly pipetted through a 1 ml tip, filtered through a 100 μm cell strainer (BD Falcon) and then reconstituted in 14 ml of RPMI. Our Institutional Animal Care and Use Committee approved all animal studies.
Flow cytometry
We isolated and stained cells as previously described (15), using LSR II and FACSAriaII flow cytometers and FACSDiva software (BD Biosciences, San Jose, CA). Antibodies for flow cytometry: antibodies against CD3 (clone 17A2) and CD11b (clone M1/70) were purchased from eBioscience (San Diego, CA); antibodies against CD45 (clone 30-F11) were purchased from BD Bioscience (San Jose, CA); antibodies against CD11c (clone N418) were purchased from Biolegend (San Diego, CA); antibodies against Gr-1 (clone RB6-8C5) were purchased from Biolegend (San Diego, CA); and antibodies against CD8a (clone53-6.7) were purchased from BD Bioscience (San Jose, CA). Tumor-associated macrophages (TAMs) in bladder tissue were identified as CD45+CD11b+Gr-1- cells.
Results
Patient Characteristics
Twelve patients (7 males, 5 females) were treated with sequential MMC + BCG (Table 1). The median age was 67 (range 52-77). Five patients had CIS including one patient with pure CIS. Seven patients had T1 including one patient with pure T1. Prior intravesical therapy is designated by the number of cycles. Five patients had received prior BCG therapy including one patient who received prior BCG and prior induction MMC (Table 1). Three patients had received a prior 6-week cycle and either a 3-week maintenance cycle or repeat 6-week cycle of BCG (designated as BCG × 2). One patient had received prior radiation therapy to the bladder for T1 disease at an outside facility. This patient exhibited gross, but clinically insignificant hematuria and had no other urinary symptoms prior to starting therapy.
Table 1. Patient characteristics and response by stage and level.
| Pt. No. | Disease Stage | Prior Therapy | MMC | BCG | Bladder Status | Follow-up (mo) |
|---|---|---|---|---|---|---|
| 1 | HG Ta with LVI | None | 10 mg in 20 ml | Half | NED | 27.0 |
| 2 | T1 | None | 10 mg in 20 ml | Half | NED | 28.0 |
| 3 | T1, nested variant | BCG | 10 mg in 20 ml | Half | NED | 24.8 |
| 4 | HG Ta and T1 | BCG × 2 | 10 mg in 20 ml | Full | NED | 22.8 |
| 5 | CIS | None | 10 mg in 20 ml | Full | NED | 22.6 |
| 6 | CIS, HG Ta, and T1 | None | 10 mg in 20 ml | Full | NED | 20.7 |
| 7 | LG and HG Ta | BCG × 2 | 20 mg in 20 ml | Full | HG Ta | 24.5 |
| 8 | HG and LG Ta | BCG | 20 mg in 20 ml | Full | NED | 18.7 |
| 9 | CIS and T1 | XRT | 20 mg in 20 ml | Full | NED | 21.2 |
| 10 | HG Ta | None | 40 mg in 40 ml | Full | NED | 18.2 |
| 11 | T1, CIS | None | 40 mg in 40 ml | Full | NED | 19.3 |
| 12 | T1, CIS | BCG × 2 and MMC | 40 mg in 40 ml | Full | NED | 8.4 |
BCG, bacillus Calmette-Guérin. CIS, carcinoma in-situ. HG, high grade. LG, low grade. LVI, lymphovascular invasion. MMC, Mitomycin C. NED, no evidence of disease. XRT, γ-irradiation therapy. Mo, months.
Safety and Toxicities
No patients experienced treatment related DLTs and no severe treatment-related adverse events were observed. Neither neutropenia nor thrombocytopenia was observed. Grade 1 adverse events were common with 11 (92%) patients reporting mild cystitis (urinary frequency, dysuria, or urgency) at some time during therapy. No significant trend in cystitis symptoms was observed for cumulative exposure of combined therapy (Table 2). Grade 2 adverse events included hematuria requiring temporary discontinuation of treatment, placement of urethral catheter and irrigation of bladder. One patient developed a drug-resistant urinary tract infection requiring intravenous antibiotics (grade 3). This infection was not attributed to the intervention and therefore did not trigger a cohort expansion per the study protocol. No other grade 3 or higher adverse events were observed.
Table 2. Number of patients experiencing adverse events by dose group.
| Dose | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| 10 mg MMC/Half BCG (n=3) | 10 mg MMC/Full BCG (n=3) | 20 mg MMC/Full BCG (n=3) | 40 mg MMC/Full BCG (n=3) | |||||||||
| Adverse Event Grade* | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Chills | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 |
| Fatigue | 3 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 |
| Fever | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Hematuria | 2 | 0 | 0 | 2 | 1 | 0 | 3 | 0 | 0 | 2 | 1 | 0 |
| Non-infectious cystitis | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 0 | 0 | 1 | 2 | 0 |
| Bacterial cystitis | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Grading according to CTCAE v4.0.
Therapy was postponed due to either gross hematuria or evidence of urinary tract infection. This included one patient with a drug-resistant urinary tract infection that had multiple delays and therapy was spread out over the course of 12 weeks. The most common patient-reported symptoms include urinary frequency, urinary urgency, and weakness or fatigue (supplemental Table). Weakness or fatigue was reported in 50% after weeks 2 and 3 of therapy and 67% of patients reported weakness or fatigue after week 6 of therapy (supplemental Table). Subjective fever or chills was reported in 33% of patients following week 6 of therapy. The maximum temperature elevation during therapy was 38.8° C with no patients experiencing grade 2 temperature elevation (39-40° C).
Urinary Cytokines
Prior to drug instillation, low to absent levels of urinary cytokines were detected in all patients at weeks 1, 4 and 6 (data not shown). Urinary IL-12 was undetectable in pre- and post-treatment urine samples (data not shown). Evidence for enhanced broad immune activation following repeated doses of therapy was observed as the median urinary cytokines increased for several cytokines over the 6-week treatment course (Table 3).
Table 3. Median post-treatment urinary cytokines by week of therapy.
| Median (IQR) urinary cytokine concentration (pg/ml) | ||||
|---|---|---|---|---|
| Cytokine | Week 1 | Week 4 | Week 6 | P-value* |
| IL-2 | 0.0 (0-0.5) | 25.7 (7.6-73.1) | 40.3 (15.5 – 147.2) | 0.006 |
| IL-4 | 0.16 (0-1.7) | 0.62 (0.08-6.1) | 3.88 (1.87-20.18) | 0.18 |
| IL-8 | 171.7 (54.1-1061) | 681.7 (413.3-1724) | 3212 (958.2-4787) | 0.007 |
| IL-10 | 2.3 (1.6-3.1) | 29.5 (2.3-654.2) | 121.7 (9.8-510.4) | 0.023 |
| IL-17 | 3.5 (2.7-8.0) | 66.0 (16.4-411.1) | 623.9 (41.4-942.6) | 0.085 |
| IFN-γ | 0.0 (0.0-4.3) | 17.0 (0.7-42.8) | 107.9 (1.0-239.6) | 0.072 |
| TNF-α | 2.3 (1.3-44.8) | 569.6 (21.6-2445) | 2149 (42.1-2449) | 0.024 |
P-value by non-parametric test for trend. IFN, interferon. IL, interleukin. TNF, tumor necrosis factor. IQR, interquartile range
Urinary IL-2 and IL-8 following BCG administration have been associated with response to BCG (16). We measured urinary IL-2 and IL-8 in patients receiving sequential MMC + BCG and in a control population of patients receiving BCG monotherapy (Figure 1). In patients treated with BCG monotherapy, median urinary IL-2 at weeks 1, 4, and 6 were 25.1 pg/ml (IQR 23.4-41.4 pg/ml), 48.6 pg/ml (IQR 25.5-58.9 pg/ml), and 40.7 pg/ml (IQR 27.8-50.4 pg/ml), respectively. In patients treated with BCG monotherapy, median urinary IL-8 at 1, 4, and 6 weeks were 162.6 pg/ml (IQR 44.1-1463 pg/ml), 1130 pg/ml (IQR 179.9-8,144 pg/ml), and 1001 pg/ml (IQR 596.2-1730 pg/ml), respectively. Two patients experienced a peak of IL-8 at 4 weeks, including 1 patient receiving 10 mg MMC and1/2 dose BCG and 1 patient receiving 40 mg MMC and full dose BCG (Figure 1). However, 9 (75%) patients experienced the highest IL-8 measurement at the 6th week dose, which is consistent with previous observations in BCG monotherapy (17).
Figure 1. Urinary IL-2 and IL-8 in patients treated with sequential MMC + BCG or BCG monotherapy.
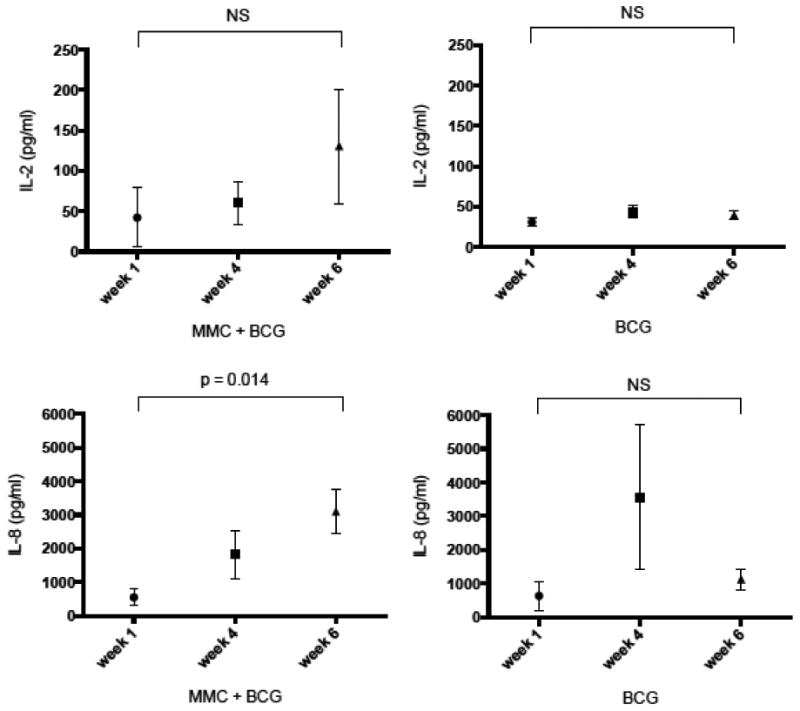
Urine cytokines were measured at second void following intravesical administration. A. Median (IQR) urinary IL-2 in patients treated with sequential MMC + BCG. B. Median (IQR) urinary IL-2 in patients treated with BCG monotherapy. C. Median (IQR) urinary IL-8 in patients treated with sequential MMC + BCG. D. Median (IQR) urinary IL-8 in patients treated with BCG monotherapy.
Sequential MMC + BCG polarizes TAMs towards an M1 phenotype in tumor-bearing mice
Because urinary IL-8 was consistently elevated in patients treated with repeated doses of sequential MMC + BCG, we hypothesized that MMC could facilitate IL-8 production by recruiting macrophages to the bladder during BCG therapy. The response to sequential intravesical MMC + BCG versus control treatment in the urinary bladder was evaluated in bladder tumor-bearing C57BL/6 mice. The percentage of bladder infiltrating CD11b+Gr-1- TAMs among tumor-infiltrating lymphocytes (CD45+ cells) was significantly increased following sequential instillation of MMC + BCG compared to MMC alone or BCG alone (Figure 2, p <0.05 versus either single agent). However, the total number of TAMs was not significantly different between groups. Notably, no significant increase in bladder-infiltrating CD3+ T cells or CD8+ T cells was observed for sequential MMC + BCG instillation (Figure 2).
Figure 2. Sequential MMC + BCG increases percentage of TAMs among tumor-infiltrating leukocytes.
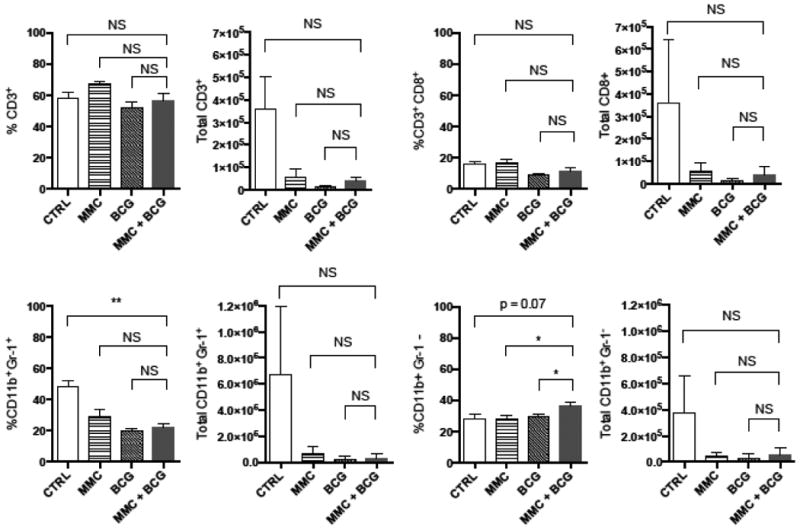
MB49 tumors were implanted on mouse bladders and then treated with PBS, MMC, BCG, or sequential MMC + BCG instillation into bladders of female C57BL/6 mice (n=4 per group). Bladders were harvested and digested at 6 hours following the 4th treatment. Percentage of cells represents gating on live singlet CD45+ bladder cells. TAMs were identified as CD11b+Gr-1- cells. *P < 0.05; **P < 0.005, using a two-tailed, unpaired t-test. NS, not significant.
Two distinct phenotypes exist for TAMs: M1 macrophages have anti-tumor properties whereas M2 macrophages exert an immunosuppressive phenotype(18). TAMs were isolated from treated tumor-bearing mouse bladders by cell sorting and characterized according to gene expression and MHC class II surface staining (Figure 3). Interestingly, TAMs isolated from sequential MMC + BCG treated mice exhibited an M1 phenotype characterized by low il10 and arg1 gene expression, high il6 expression by qPCR, and high MHC II surface staining by flow cytometry. In contrast, PBS control treated tumor-bearing TAMs exhibited an M2 phenotype characterized by high il10 and arg1, low il6, and low MHC II. cxcl1 (the mouse IL-8 homologue) was expressed in all TAMs but increased among M2 TAMs in PBS control-treated mice. Expression of cxcl1 among TAMs was increased in sequential MMC + BCG compared to BCG alone but this difference was not significant (p=0.07).
Figure 3. Sequential MMC + BCG polarizes TAMs towards an M1 phenotype.
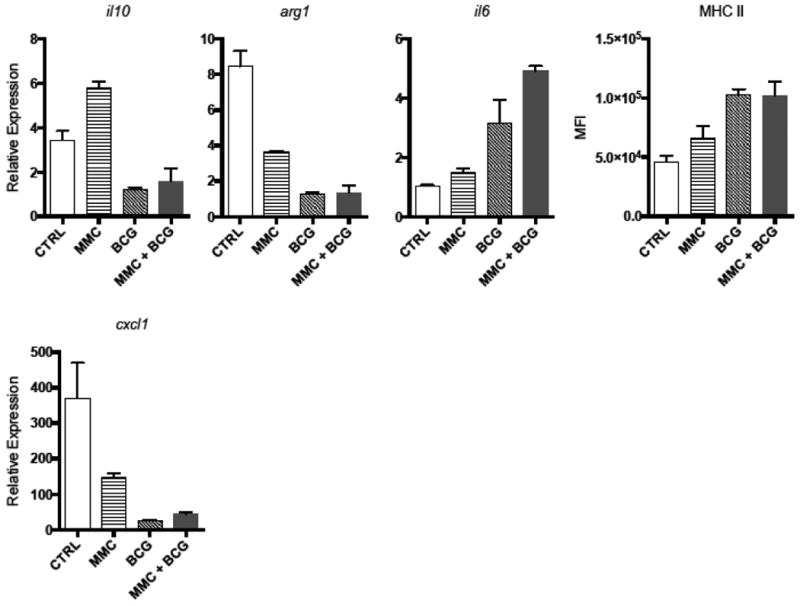
Following treatment under different conditions, bladder cells were stained for flow and sorting. A. Expression analysis by qPCR of il10, arg1, and il6 mRNA and mean fluorescence intensity (MFI) of MHC was performed on CD45+CD11b+Gr-1- TAMs after gating on live singlets (mouse bladders pooled within each treatment group). B. Expression analysis of cxcl1 by qPCR on CD45+CD11b+Gr-1- TAMs.
Clinical Response
All patients were free of obvious papillary tumors in the bladder at week 6 following therapy as demonstrated by cystoscopy. One patient who declined maintenance therapy developed a low-grade non-invasive papillary lesion at 12 months and a subsequent high-grade non-invasive papillary lesion at 18 months after therapy. Eleven (91.7%) patients had no evidence of bladder recurrence with a mean (range) follow-up of 21.4 (8.4-27.0) months for the entire group (Table 1). Four patients with prior intravesical BCG therapy remain free of disease. One patient had a dystrophic calcified lesion at first follow up surveillance cystoscopy. Biopsy of this lesion was negative for malignancy and consistent with post-infectious granulomata.
Discussion
This phase I study tested the safety and toxicities of a novel combined regimen of intravesical MMC followed by intravesical BCG instillation in patients with high-risk NMIBC. This combination treatment was safe with no DLTs observed, including doses up to 40 mg of MMC with full strength BCG. Thus a MTD among the selected doses was not defined. The regimen was associated with a high frequency of mild side effects including urinary frequency, urinary urgency, and weakness or fatigue. Therapy was delayed by at least one week in several patients. However, no serious local or systemic toxicities were encountered. Interestingly, we identified macrophage polarization towards a beneficial M1 phenotype during treatment with BCG and sequential MMC + BCG in a murine model of bladder cancer. These data suggest that combination treatment efficacy could be improved by increasing the numbers of these beneficial cells, a concept requiring additional investigations.
MMC was chosen as the chemotherapeutic agent to combine with BCG due to familiarity and clinical experience with this agent for treating bladder cancer (19-21). In addition, studies carried out by Wientjes and colleagues provide the depth of MMC penetration, the associated drug concentrations, and rate at which the drug concentration decrease following MMC wash-out (22). Based on these findings, we estimated that a washout period of 5-10 minutes would provide sufficient time to eliminate all MMC from the bladder. Our combination strategy was designed to optimize tumor control rates, preserve the immune response to BCG, and assure biocompatibility (i.e., limit possibility of MMC to inhibit BCG growth directly).
Intravesical MMC could improve the response to intravesical BCG by improving BCG uptake into bladder tissue and subsequent BCG immune activity. Chemotherapy can promote the activation and production of immune effector cells when applied locally prior to antigen stimulation (23-25). Ratliff and coworkers have shown that binding of BCG to the bladder wall is a critical step towards evoking an immune response and producing antitumor activity in the bladder (26-28). Urothelial disruption induced by intravesical instillation of chemotherapy results in increased adherence of BCG to the bladder wall and improves the systemic immune response to BCG (28). Of importance, prior animal studies found that simultaneous instillation of intravesical MMC and intravesical BCG rendered a dose-dependent improvement in survival compared to BCG or MMC monotherapy (11).
Several trials have tested alternating chemotherapy with BCG using various sequential or alternating strategies (reviewed in (5)). There is limited clinical data regarding the simultaneous administration of chemotherapy with BCG due to concerns regarding safety and potential antibacterial activity of chemotherapy (5). However, the activity of BCG combined with chemotherapy is poorly understood. Biocompatibility of MMC and BCG has been investigated. When held in suspension together for 3 hours, MMC did not induce clumping of Mycobacteria but did inhibit the growth of BCG in vitro (29). The influence of this bacterial growth inhibition on the immunostimulatory properties and antitumor activity of BCG is unknown. Mycobacterial cell wall components are important immunostimulants (30). Nevertheless, a washout period was incorporated in our treatment regimen to avoid any potential compromise in the BCG effects as a result of direct antibacterial activity from MMC.
In the only other trial that examined the effect of simultaneous combined therapy, a combined approach of intravesical chemotherapy with BCG was poorly tolerated (31). Intravesical epirubicin 50 mg was given for 2 hours followed by intravesical BCG for 2 hours and all patients experienced moderate to severe cystitis and fever. In fact severe side effects led to discontinuation of therapy in 36% of patients and therapy was delayed in 50% due to side effects (31). The reasons for the improved tolerability observed in our combination compared to that observed with epirubicin and BCG are unknown. Epirubicin is an anthracycline that acts by intercalating DNA strands and triggering DNA cleavage by topoisomerase. MMC is an aziridine-containing agent that crosslinks DNA. We speculate that the increase in side effects observed with epirubicin was related to increased bladder damage with epirubicin and subsequent BCG exposure. This may have been because of longer duration of therapy with epirubicin (2 hours) or more bladder epithelial disruption from epirubicin versus MMC. A difference in side effects attributable to differential effects on BCG-related side effects is also likely given that the tolerability of these chemotherapeutic agents is similar when used as monotherapy (32).
Two-thirds of patients receiving sequential MMC + BCG reported weakness or fatigue following week 6 of therapy. It is unclear, however, to what extent this was related to treatment since 42% also reported these symptoms at week 1 of therapy. Notably, local and systemic side effects were similar to recent reports on patients receiving BCG monotherapy (33). Using prospective data collection, Brausi and colleagues reported fever (8.8%), bacterial cystitis (21%), BCG-related cystitis (33.1%), urinary frequency (23.1%), malaise (15.5%), and systemic side effects (30.4%) after full-dose induction BCG monotherapy (33).
Increased urinary IL-2 and IL-8 after BCG instillation have been reported as positive prognostic factor for responsiveness to BCG therapy making these important cytokines to evaluate (34-36). Urinary cytokine levels increased significantly with repeated instillations of sequential MMC + BCG indicating immune activation with therapy, consistent with other reports in BCG monotherapy (34, 37) and demonstrates that MMC does not appear to prevent BCG-mediated immune activation. Mean urinary IL-2 levels in our sequential MMC + BCG and BCG-only cohorts were similar to results from prior studies (35, 36). In the study by Saint, et al., for example, mean peak urinary IL-2 was 15.1 pg/ml for patients who had recurrence after BCG and 93.3 pg/ml for patients who did not recur after BCG (35).
There are conflicting reports on the prognostic significance of urinary IL-8 in predicting response to BCG (34, 38, 39). We identified an increase in urinary IL-8 during sequential MMC + BCG treatment and we expected to find increased IL-8 by TAMs during combination therapy in our mouse model. Instead, we found decreased CXCL1 (the mouse IL-8 homologue) in M1 TAMs during treatment compared to M2 TAMs from placebo-treated bladders. Other cell types including epithelial and endothelial cells also produce IL-8. Thus the relevance of urinary IL-8 could depend on the cells making IL-8 rather than the quantity, or might not predict efficacy in this combination treatment, a finding requiring additional studies.
Macrophages have been observed in urine and bladder tissue of patients treated with BCG (40, 41) and in vitro studies demonstrate BCG-mediated macrophage cytotoxicity against bladder cancer cells (42, 43). In addition, several urinary cytokines following BCG exposure are produced by macrophages (41, 44, 45). TAMs represent a substantial portion of the cells in tumors and have been associated with adverse prognosis in various human cancers (46). M1 TAMs are generally considered beneficial, activate pro-inflammatory, antitumor activity and antagonize generally detrimental M2 macrophage functions (47, 48). M2 TAMs suppress antitumor immunity and promote tumor growth (46, 49). Infiltration of M2 TAMs in bladder tumors is associated with poor response to BCG immunotherapy (50). However, direct evidence for a role of TAMs in BCG therapy has not been described. Interestingly, we observed clear macrophage M1 polarization during treatment with BCG and sequential MMC + BCG. Additional mechanistic studies should be conducted to elucidate the role of macrophage polarization in BCG-mediated bladder cancer immunotherapy.
The absence of correlation between local or systemic symptoms and drug dose could be due to the limited number of patients in this study. The relatively high proportion of patients experiencing weakness or fatigue is notable. Because BCG monotherapy is also associated with considerable side effects, additional studies with a comparison group will be needed to determine the relative tolerability of this regimen over BCG alone. Another limitation of this study is that serial plasma collections were not performed to evaluate for MMC or BCG systemic absorption, which could have contributed to systemic symptoms. Nevertheless it is notable that we observed no severe toxicities and no DLTs in the high dose group. The effect of BCG uptake and subsequent immune stimulation following MMC exposure is unknown and the antitumor or cancer prevention potential of this combined approach warrants further exploration. This trial provides evidence that intravesical MMC combined with intravesical BCG is safe and potentially tolerable and should prompt additional clinical studies.
Supplementary Material
Statement of Translational Relevance.
This study demonstrates the safety and potential tolerability of a novel approach to treating urothelial bladder carcinoma in which BCG immunotherapy is delivered into the bladder immediately following delivery of intravesical mitomycin. Our findings indicate that intravesical chemotherapy could potentiate the activity of BCG immunotherapy by increasing beneficial M1 tumor-associated macrophages in the bladder. These findings have clear relevance to clinical medicine, as this work will be followed with a clinical trial to test the clinical efficacy of this sequential approach against standard BCG monotherapy.
Acknowledgments
We thank Dr. Guillaume Wientjes for intellectual support for this study.
NIH Funding: This work is supported through the Clinical and Translational Science Award grant (8UL1 TR000149) and the Cancer Therapy and Research Center at the University of Texas Health Science Center San Antonio (P30 CA054174)
Other support: This work is supported through the Owens Foundation, the Holly Beach Public Library Association, San Antonio Medical Foundation, the Daisy M. Skinner Endowment, and the Roger L. and Laura D. Zeller Charitable Foundation Chair in Urologic Cancer.
Footnotes
Disclosures: There are no financial disclosures
IRB approval: HSC20120003H
References
- 1.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 2.Association. AU. Guidelines for the Management of Non-Muscle Invasive Bladder Cancer (Stages Ta, T1, and TIS): 2007 Update. Updated 2007 [cited March 28, 2013]; Available from: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm.
- 3.Lerner SP, Tangen CM, Sucharew H, Wood D, Crawford ED. Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol. 2009;27:155–9. doi: 10.1016/j.urolonc.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 5.Hilton WM, Ercole B, Parekh DJ, Sonpavde G, Ghosh R, Svatek RS. Efficacy of combined intravesical immunotherapy and chemotherapy for non-muscle invasive bladder cancer. Expert Rev Anticancer Ther. 2011;11:949–57. doi: 10.1586/era.11.69. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J, Spanswick VJ, Smyth JF. Re-evaluation of the molecular pharmacology of mitomycin C. European journal of cancer. 1995;31A:1928–33. doi: 10.1016/0959-8049(95)00364-9. [DOI] [PubMed] [Google Scholar]
- 7.Jinesh GG, Chunduru S, Kamat AM. Smac mimetic enables the anticancer action of BCG-stimulated neutrophils through TNF-alpha but not through TRAIL and FasL. J Leukoc Biol. 2012;92:233–44. doi: 10.1189/jlb.1211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biot C, Rentsch CA, Gsponer JR, Birkhauser FD, Jusforgues-Saklani H, Lemaitre F, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med. 2012;4:137ra72. doi: 10.1126/scitranslmed.3003586. [DOI] [PubMed] [Google Scholar]
- 9.Vita F, Siracusano S, Abbate R, Ciciliato S, Borelli V, Soranzo MR, et al. BCG prophylaxis in bladder cancer produces activation of recruited neutrophils. The Canadian journal of urology. 2011;18:5517–23. [PubMed] [Google Scholar]
- 10.Rajala P, Kaasinen E, Rintala E, Jauhiainen K, Nurmi M, Alfthan O, et al. Cytostatic effect of different strains of Bacillus Calmette-Guerin on human bladder cancer cells in vitro alone and in combination with mitomycin C and interferon-alpha. Urological research. 1992;20:215–7. doi: 10.1007/BF00299720. [DOI] [PubMed] [Google Scholar]
- 11.Matsushima M, Horinaga M, Fukuyama R, Yanaihara H, Kikuchi E, Kawachi M, et al. Enhanced antitumor effect of combination intravesical mitomycin C and bacillus Calmette-Guerin therapy in an orthotopic bladder cancer model. Oncol Lett. 2011;2:13–9. doi: 10.3892/ol.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solsona E, Madero R, Chantada V, Fernandez JM, Zabala JA, Portillo JA, et al. Sequential Combination of Mitomycin C Plus Bacillus Calmette-Guerin (BCG) Is More Effective but More Toxic Than BCG Alone in Patients with Non-Muscle-invasive Bladder Cancer in Intermediate- and High-risk Patients: Final Outcome of CUETO 93009, a Randomized Prospective Trial. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Dalbagni G, Russo P, Sheinfeld J, Mazumdar M, Tong W, Rabbani F, et al. Phase I trial of intravesical gemcitabine in bacillus Calmette-Guerin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol. 2002;20:3193–8. doi: 10.1200/JCO.2002.02.066. [DOI] [PubMed] [Google Scholar]
- 14.Loskog A, Ninalga C, Hedlund T, Alimohammadi M, Malmstrom PU, Totterman TH. Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim. 2005;39:384–93. doi: 10.1258/002367705774286475. [DOI] [PubMed] [Google Scholar]
- 15.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 16.Zuiverloon TC, Nieuweboer AJ, Vekony H, Kirkels WJ, Bangma CH, Zwarthoff EC. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol. 2012;61:128–45. doi: 10.1016/j.eururo.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Sagnak L, Ersoy H, Ozok U, Senturk B, Ercil H, Bahar G, et al. Predictive value of urinary interleukin-8 cutoff point for recurrences after transurethral resection plus induction bacillus Calmette-Guerin treatment in non-muscle-invasive bladder tumors. Clin Genitourin Cancer. 2009;7:E16–23. doi: 10.3816/CGC.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 18.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Frontiers in immunology. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90. doi: 10.1097/01.ju.0000125486.92260.b2. quiz 435. [DOI] [PubMed] [Google Scholar]
- 20.Solsona E, Iborra I, Ricos JV, Monros JL, Casanova J, Dumont R. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999;161:1120–3. [PubMed] [Google Scholar]
- 21.Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–56. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Wientjes MG, Badalament RA, Wang RC, Hassan F, Au JL. Penetration of mitomycin C in human bladder. Cancer Res. 1993;53:3314–20. [PubMed] [Google Scholar]
- 23.Tan BT, Limpens J, Koken M, Valster H, Scheper RJ. Local administration of various cytostatic drugs after subcutaneous immunization enhances delayed-type hypersensitivity reaction to sheep red blood cells in mice. Scand J Immunol. 1986;23:605–9. doi: 10.1111/j.1365-3083.1986.tb01994.x. [DOI] [PubMed] [Google Scholar]
- 24.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 25.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–8. [PubMed] [Google Scholar]
- 26.Hudson MA, Ritchey JK, Catalona WJ, Brown EJ, Ratliff TL. Comparison of the fibronectin-binding ability and antitumor efficacy of various mycobacteria. Cancer Res. 1990;50:3843–7. [PubMed] [Google Scholar]
- 27.Ratliff TL, Palmer JO, McGarr JA, Brown EJ. Intravesical Bacillus Calmette-Guerin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guerin. Cancer Res. 1987;47:1762–6. [PubMed] [Google Scholar]
- 28.Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990;85:62–7. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsogiannis IC, Psaroudaki Z, Perrea D, Paniara O, Melekos MD, Zervas A, et al. In vitro biocompatibility between mitomycin-C (MMC) and bacillus Calmette-Guerin (BCG) Anticancer Res. 2006;26:4569–73. [PubMed] [Google Scholar]
- 30.Chin JL, Kadhim SA, Batislam E, Karlik SJ, Garcia BM, Nickel JC, et al. Mycobacterium cell wall: an alternative to intravesical bacillus Calmette Guerin (BCG) therapy in orthotopic murine bladder cancer. J Urol. 1996;156:1189–93. doi: 10.1016/s0022-5347(01)65748-3. [DOI] [PubMed] [Google Scholar]
- 31.Erol A, Ozgur S, Basar M, Cetin S. Trial with bacillus Calmette-Guerin and epirubicin combination in the prophylaxis of superficial bladder cancer. Urol Int. 1994;52:69–72. doi: 10.1159/000282576. [DOI] [PubMed] [Google Scholar]
- 32.da Silva FC, Ferrito F, Brandao T, Santos A. 4′-epidoxorubicin versus mitomycin C intravesical chemoprophylaxis of superficial bladder cancer. Eur Urol. 1992;21:42–4. doi: 10.1159/000474798. [DOI] [PubMed] [Google Scholar]
- 33.Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65:69–76. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]
- 35.Saint F, Kurth N, Maille P, Vordos D, Hoznek A, Soyeux P, et al. Urinary IL-2 assay for monitoring intravesical bacillus Calmette-Guerin response of superficial bladder cancer during induction course and maintenance therapy. Int J Cancer. 2003;107:434–40. doi: 10.1002/ijc.11352. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe E, Matsuyama H, Matsuda K, Ohmi C, Tei Y, Yoshihiro S, et al. Urinary interleukin-2 may predict clinical outcome of intravesical bacillus Calmette-Guerin immunotherapy for carcinoma in situ of the bladder. Cancer Immunol Immunother. 2003;52:481–6. doi: 10.1007/s00262-003-0384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisiaux A, Thiounn N, Timsit MO, Eladaoui A, Chang HH, Mapes J, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol. 2009;181:1571–80. doi: 10.1016/j.juro.2008.11.124. [DOI] [PubMed] [Google Scholar]
- 38.Thalmann GN, Dewald B, Baggiolini M, Studer UE. Interleukin-8 expression in the urine after bacillus Calmette-Guerin therapy: a potential prognostic factor of tumor recurrence and progression. J Urol. 1997;158:1340–4. [PubMed] [Google Scholar]
- 39.Rabinowitz R, Smith DS, Tiemann DD, Hudson MA. Urinary interleukin-8/creatinine level as a predictor of response to intravesical bacillus Calmette-Guerin therapy in bladder tumor patients. J Urol. 1997;158:1728–31. doi: 10.1016/s0022-5347(01)64111-9. discussion 31-2. [DOI] [PubMed] [Google Scholar]
- 40.Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette-Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–8. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 41.de Boer EC, de Jong WH, van der Meijden AP, Steerenberg PA, Witjes F, Vegt PD, et al. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urological research. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, Yamada H, Evanoff DP, Chen X. Role of Th1-stimulating cytokines in bacillus Calmette-Guerin (BCG)-induced macrophage cytotoxicity against mouse bladder cancer MBT-2 cells. Clin Exp Immunol. 2006;146:181–8. doi: 10.1111/j.1365-2249.2006.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Murine IL-2 secreting recombinant Bacillus Calmette-Guerin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol. 2000;164:526–31. [PubMed] [Google Scholar]
- 44.de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 45.Jackson AM, Alexandroff AB, Kelly RW, Skibinska A, Esuvaranathan K, Prescott S, et al. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. Clin Exp Immunol. 1995;99:369–75. doi: 10.1111/j.1365-2249.1995.tb05560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 47.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. Journal of ovarian research. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nardin A, Abastado JP. Macrophages and cancer. Front Biosci. 2008;13:3494–505. doi: 10.2741/2944. [DOI] [PubMed] [Google Scholar]
- 50.Suriano F, Santini D, Perrone G, Amato M, Vincenzi B, Tonini G, et al. Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J Exp Clin Cancer Res. 2013;32:87. doi: 10.1186/1756-9966-32-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


