Abstract
Background & Aims
Crohn’s disease and ulcerative colitis are associated with increased risk of colorectal cancer (CRC). Surveillance colonoscopy is recommended at 2−3 year intervals beginning 8 years after diagnosis of inflammatory bowel disease (IBD). However, there have been no reports of whether colonoscopy examination reduces the risk for CRC in patients with IBD.
Methods
In a retrospective study, we analyzed data from 6823 patients with IBD (2764 with a recent colonoscopy, 4059 without a recent colonoscopy) seen and followed for at least 3 years at 2 tertiary referral hospitals in Boston. The primary outcome was diagnosis of CRC. We examined the proportion of patients undergoing a colonoscopy within 36 months before a diagnosis of CRC or at the end of the follow-up period, excluding colonoscopies performed within 6 months before a diagnosis of CRC, to avoid inclusion of prevalent cancers. Multivariate logistic regression was performed, adjusting for plausible confounders.
Results
One hundred fifty-four patients developed CRC. The incidence of CRC among patients without a recent colonoscopy (2.7%) was significantly higher than among patients with a recent colonoscopy (1.6%) (odds ratio [OR], 0.56; 95% confidence interval, 0.39−0.80). This difference persisted in multivariate analysis (OR, 0.65; 95% CI, 0.45−0.93) and was robust when adjusted for a range of assumptions in sensitivity analyses. Among patients with CRC, a colonoscopy within 6−36 months before diagnosis was associated with reduced mortality (OR, 0.34; 95% CI 0.12−0.95).
Conclusions
Recent colonoscopy (within 36 months) is associated with a reduced incidence of CRC in patients with IBD, and lower mortality in those diagnosed with CRC
Keywords: Crohn’s disease, ulcerative colitis, pregnancy, delivery, caesarean section, colon cancer, screening, risk factor, early detection
INTRODUCTION
Patients with inflammatory bowel diseases (IBD; Crohn’s disease (CD), ulcerative colitis (UC)) are at an elevated risk of colorectal cancer (CRC)1–7. Early estimates of magnitude of this risk placed it at 18% at 30 years after diagnosis in UC3. Consequently, professional society guidelines recommend surveillance colonoscopies begin 8–10 years after diagnosis, repeating every 2–3 years thereon in individuals with UC or colonic CD7–11. Recent literature has been conflicting on whether there have been secular changes in risk of CRC in IBD patients. The majority of emerging studies from diverse population-based cohorts suggest that there has been a reduction in risk of CRC in IBD4, 5, 12, 13. Several reasons have been proposed to explain this temporal reduction including more wide spread use of maintenance treatment, higher frequency of colectomy, and a greater uptake of surveillance colonoscopy in clinical practice allowing for the detection of dysplastic lesions prior to the development of CRC5.
In the general population, screening colonoscopy has been associated with a reduced incidence and mortality from colorectal cancer14–20. Individuals with a recent negative colonoscopy have a substantial reduction in risk of subsequent significant lesions on follow-up periods even extending to 10 years after the initial exam14, 19, 20. In contrast, data on whether a similar ‘protective’ effect exists in those with IBD has not been examined. Indeed this is an important clinical question to examine as the molecular pathogenesis of colitis-associated-cancer is distinct from that of sporadic carcinoma21, 22. Consequently, the benefits of surveillance colonoscopies cannot be extrapolated from the general population to patients with IBD. Additional factors influence any potential estimates of surveillance in patients with IBD. The temporal decline in CRC risk in patients with IBD may reduce potential benefits of frequent surveillance examinations. Furthermore, controversy also exists regarding the optimal method of performing surveillance exams and the added yield of random biopsies. Given the considerable cost of surveillance exams every 2–3 years in individuals who are often diagnosed with IBD at a young age, it is important to examine if colonoscopies alter the risk of or outcomes after CRC in this patient population.
Consequently, using a large validated cohort of patients with IBD, we examined whether recent colonoscopy alters risk of CRC in patients with IBD, and if the outcomes after CRC diagnosis are different in those who had a recent colonoscopic exam compared to those without.
METHODS
Study Population
The data source for our study was an electronic medical record IBD cohort the development of which has been detailed in previous publications from our group23–27. In brief, we selected all patients with at least one International Classification of Diseases, 9th edition, clinical modification (ICD-9-CM) code for CD or UC. Using a combination of free text concepts identified using natural language processing from clinical notes, endoscopy, pathology, radiology and operative reports, structured codified data (diagnosis and procedure codes) and electronic prescription for medications, we developed and validated an algorithm to accurately define CD or UC in our cohort with a positive predictive value of 97%. Our final population consisted of 5,506 patients with CD and 5,522 patients with UC.
Variables and Outcome
The primary outcome for our study was a diagnosis of CRC determined by diagnosis codes for colon or rectal cancer (ICD-9-CM 153.x – 154.x). In a previous study from our group, we confirmed the high positive predictive value of this diagnostic code in our EMR, consistent with published literature24, 26–28. Our main predictor of interest was undergoing a colonoscopy within 36 months prior to the first date of CRC diagnosis (for cases) or end of follow-up (for non-cases). We selected this interval to be consistent with the recommended intervals from both the American Gastroenterological Association (AGA) and British Society of Gastroenterology (BSG) guidelines9–11. We specifically excluded procedures performed within 6 months prior to CRC diagnosis to exclude procedures leading to the diagnosis of the cancer. Patients who had a colonoscopy within 6 months prior to the CRC diagnosis were still eligible to be counted as having had surveillance colonoscopies if their prior exam was in the 6–36 months prior to CRC diagnosis. We also specifically required at least a 6 month interval between first diagnosis code for IBD and a CRC diagnosis to exclude patients specifically referred to our center for care of prevalent CRC. Among patients who did not have CRC, we required at least 3 years of care in our system, no colectomy during this period, and counted only colonoscopies occurring within 3 years of the date of last visit in our system to ensure a similar time frame of reference as the CRC group. Mortality was determined by linkage to the social security death index with updates every month as described previously24.
Covariates
We extracted information on age at diagnosis of cancer (or end of follow-up), gender, race (white or non-white), duration of follow-up for IBD in our health care system, and a diagnosis of primary sclerosing cholangitis (PSC) established using our previously validated algorithm24. We assessed for any use of immunomodulator (azathioprine, 6-mercaptopurine, or methotrexate) or anti-tumor necrosis factor α (infliximab, adalimumab, certolizumab pegol) therapy, and a history of an IBD-related hospitalization or surgery. Where available, we also calculated median C-reactive protein or erythrocyte sedimentation rate (ESR) levels that we have previously shown to be associated with risk of colorectal cancer in IBD26.
Statistical Analysis
The study was approved by the Institutional Review Board of Partners Healthcare and all statistical analysis was performed using Stata 12.0 (StataCorp, College Station, TX). Categorical variables were expressed as proportions and compared using the chi-square test while continuous variables were summarized using medians and interquartile ranges (IQR) and compared using the t-test. The Mann-Whitney test was used for non-parametric comparisons. Univariate logistic regression was performed to identify factors associated with CRC and variables with a p-value < 0.05 or those that had previously been demonstrated to be associated with CRC were included in a final multivariate model where a p < 0.05 was considered to indicate independent statistical significance.
We performed a number of planned subgroup and sensitivity analysis. We examined the association between colonoscopy and risk of CRC stratifying our cohort by gender, type of IBD, and a diagnosis of PSC. In sensitivity analyses, we increased the interval between first date of IBD diagnosis and CRC to at least 12 months; and also restricted the window for an eligible colonoscopy to be within 12–36 months prior to a diagnosis of CRC or end of follow-up. We also repeated our analysis requiring that our no surveillance arm have at least one prior colonoscopy performed within our health care system, and adjusting for median ESR values. We also adjusted for intensity of healthcare utilization measured as density of number of medical facts, defined as each distinct encounter with the healthcare system including laboratory visits, radiological or surgical procedures, office visits, or inpatient stays. As there is no validated method to ascertain disease location or extent from electronic medical record administrative datasets, we repeated our analysis adjusting for development of penetrating or stricturing small bowel complications in CD as a proxy for those with small bowel involvement. Similarly, as disease extent was not available in UC, we repeated our models adjusting for prescriptions for topical aminosalicylate or corticosteroid therapy as proxy for distal colitis. Finally, to ensure that any inverse association identified is not a reflection of overall healthy behaviors, we examined whether a recent colonoscopy was associated with a diagnosis of non-CRC solid tumors or hematologic malignancies.
RESULTS
Study Population
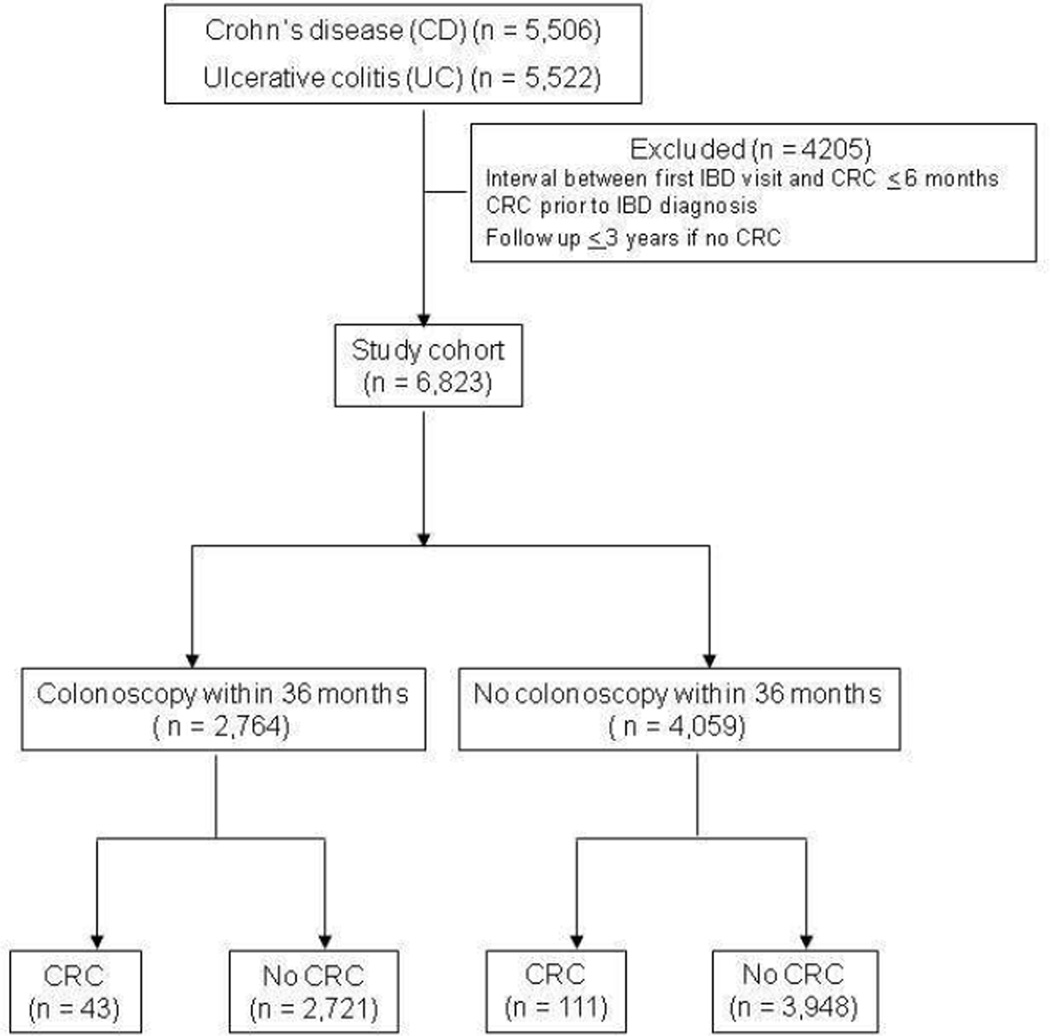
A total of 6,823 patients were included in the study among whom 154 developed CRC during the follow-up (Figure 1). A total of 2,764 patients had an eligible colonoscopy within the 3 years window while the remaining 4,059 did not have a recent colonoscopy. Table 1 compares the characteristics of the two groups of patients. Patients with a recent colonoscopy were more likely to be younger, had slightly longer duration of follow-up, less likely to be women or have a diagnosis of UC. There was no difference in racial distribution between the two groups. As expected, those with a recent colonoscopy were more likely to have severe disease characterized by need for immunomodulator or anti-TNF biologic therapy. A total of 43 patients in the colonoscopy group developed CRC during follow up (1.6%) compared to 111 patients in the no colonoscopy group (2.7%, p=0.001) (Table 1).
Figure 1.
Flowchart depicting flow of patients within the study
Table 1.
Characteristics of study population, stratifying by colonoscopy within 3 years
| Characteristic | Colonoscopy (n = 2,764) % |
No colonoscopy (n = 4,059) % |
p-value |
|---|---|---|---|
| Median age (in years) (IQR) | 47 (32 – 61) | 49 (35 – 63) | < 0.0001 |
| Median age at first IBD diagnosis code (IQR) (in years) | 37 (24 – 51) | 39 (27 – 54) | < 0.0001 |
| Median follow-up duration after first IBD code (IQR) (in years) | 8 (5 – 12) | 8 (5 – 11) | 0.06‖ |
| Sex | < 0.0001 | ||
| Female | 52 | 57 | |
| Male | 48 | 43 | |
| Race | 0.94 | ||
| White | 87 | 87 | |
| Non-white | 13 | 13 | |
| Type of IBD | < 0.0001 | ||
| Crohn’s disease | 51 | 46 | |
| Ulcerative colitis | 49 | 54 | |
| Primary sclerosing cholangitis | 3 | 2 | 0.28 |
| Ever immunomodulator use‡ | 40 | 21 | < 0.0001 |
| Ever biologic use‡ | 21 | 9 | < 0.0001 |
| Median C-reactive protein (IQR) (mg/dl)† | 3.7 (1.4 – 10.4) | 4.8 (1.6 – 16.7) | 0.001 |
| Colorectal cancer | 1.6 | 2.7 | 0.001 |
IQR – interquartile range; IBD – inflammatory bowel diseases;
CRP available for 1492 patients without recent colonoscopy and 1747 patients with recent colonoscopy
Immunomodulators comprise azathioprine, 6-mercaptopurine (6-MP), methotrexate) while biologics include infliximab, adalimumab, and certolizumab pegol
Using the Mann-Whitney test for non-parametric comparisons
As expected, patients with CRC were likely to be older, more likely to be male and have a diagnosis of UC or PSC (Supplemental Table 1). The mortality in patients without CRC was 5% compared to 29% in those with a diagnosis of CRC (p < 0.001). Table 2 compares the characteristics of patients with CRC, stratified by whether they had a history of colonoscopy within 6–36 months prior to diagnosis. There was no significant difference in age between the two groups, though patients with CRC and a recent colonoscopy were younger and less likely to be white, but had a longer duration of follow-up.
Table 2.
Characteristics of study population, stratifying by diagnosis of colorectal cancer
| Characteristic | CRC with recent colonoscopy (n = 43) % |
CRC without recent colonoscopy (n = 111) % |
p-value |
|---|---|---|---|
| Median age (in years) (IQR) | 57 (44 – 63) | 58 (46 – 69) | 0.11 |
| Median age at first IBD diagnosis code (IQR) (in years) | 49 (34 – 55) | 53 (40 – 64) | 0.03 |
| Duration of follow-up after first IBD code [Median (IQR)] (in years) | 6 (3 – 10) | 5 (2 – 8) | 0.06‖ |
| Sex | 0.68 | ||
| Female | 39 | 43 | |
| Male | 61 | 57 | |
| Race | 0.004 | ||
| White | 74 | 92 | |
| Non-white | 26 | 8 | |
| Type of IBD | 0.95 | ||
| Crohn’s disease | 45 | 46 | |
| Ulcerative colitis | 54 | 54 | |
| Primary sclerosing cholangitis | 9 | 7 | 0.66 |
| Ever immunomodulator use‡ | 33 | 17 | 0.04 |
| Ever biologic use‡ | 14 | 8 | 0.27 |
| Died | 14 | 34 | 0.012 |
IQR – interquartile range; IBD – inflammatory bowel diseases;
Immunomodulators comprise azathioprine, 6-mercaptopurine (6-MP), methotrexate) while biologics include infliximab, adalimumab, and certolizumab pegol
Using the Mann-Whitney test for non-parametric comparisons
Colorectal cancer Incidence
In the full cohort, having a recent colonoscopy within 3 years was associated with a reduced likelihood of CRC (Odds ratio (OR) 0.56, 95% confidence interval (CI) 0.39 – 0.80) (Table 3). This difference persisted on multivariate analysis adjusting for age, gender, duration of disease, type of IBD, and co-existing PSC (OR 0.65, 95% CI 0.45–0.93). Adjusting for intensity of healthcare utilization (OR 0.61, 95% CI .42 – 0.88) or use of immunomodulator or anti-TNF therapy (OR 0.62, 95% CI 0.43 – 0.89) did not alter our estimates. In the subset of patients who had available ESR measurements prior to cancer diagnosis or end of follow-up, adjusting for quartile of median ESR elevation also did not alter the inverse association between recent colonoscopy and CRC diagnosis.
Table 3.
Risk of colorectal cancer (CRC) associated with a recent colonoscopy (within 36 months) in patients with inflammatory bowel diseases
| Model | Odds ratio(OR) |
95% confidence interval (CI) |
|---|---|---|
| Unadjusted model | 0.56 | 0.39 – 0.80 |
| Fully adjusted model‖ | 0.65 | 0.45 – 0.93 |
| Sensitivity analysis | ||
| Full model + intensity of healthcare utilization‡ | 0.61 | 0.42 – 0.88 |
| Full model + immunomodulator or anti-TNF therapy use | 0.62 | 0.43 – 0.89 |
| Full model + median ESR level# | 0.54 | 0.34 – 0.86 |
| Full model (excluding colonoscopy within 12 months prior to diagnosis) | 0.66 | 0.46 – 0.94 |
ESR – erythrocyte sedimentation rate (ESR) in mm/hr
Fully adjusted model included age, gender, duration of disease, diagnosis of primary sclerosing cholangitis, type of IBD and duration of follow-up
Intensity of healthcare utilization was assessed using density of facts defined as number of distinct encounters with the medical system per year of follow-up
Information of ESR levels were available in 85 patients with colorectal cancer and 4,414 non-CRC controls. Only ESR values prior to diagnosis of colorectal cancer were included.
All-cause Mortality
Among patients with a diagnosis of CRC, those who had a colonoscopy within 6–36 months prior to diagnosis had a lower mortality (14%) compared to those without a recent colonoscopy (34%) (p=0.012). Adjusting for age, gender, co-morbidity and disease factors, a recent colonoscopy within 6–36 months prior to diagnosis was independently associated with a reduced all-cause mortality (OR 0.34, 95% CI 0.12 – 0.95).
Sensitivity Analysis
We performed a number of sensitivity analyses. Restricting the window for an eligible colonoscopy to between 12–36 months prior to CRC diagnosis did not affect our estimates (OR 0.66, 95% CI 0.46 – 0.94). The association was of similar magnitude for CD (OR 0.68) and UC (OR 0.64), and for both men and women. The association remained significant excluding those with PSC. Adjusting for small bowel stricturing or penetrating complications as a proxy for small bowel involvement in CD did not change our estimate (OR 0.48; 95% CI 0.28 – 0.81). Our estimates were similarly unchanged adjusting for prescriptions of topical aminosalicylates or corticosteroids in those with UC. The inverse association with colonoscopy was specific to CRC and not significant for solid tumors excluding colon cancer (OR 0.97, 95% CI 0.86 – 1.10) or hematologic malignancies (OR 1.25, 95% CI 0.93 – 1.67) suggesting that the association is unlikely to be explained by overall healthy behaviors. To ensure completeness of capture, restricting the analysis to patients who had a primary care doctor within our system resulted in similar estimates. To account for the fact that patients in the no colonoscopy arm may have undergone this procedure elsewhere, we additionally required our non-CRC arm to have at least one colonoscopy within our electronic medical record within the time frame. Our findings remained unchanged by these sensitivity assumptions.
DISCUSSION
It is well established in the general population that colonoscopy reduces risk of and mortality from CRC through removal of adenomas that have cancerous potential and detection of prevalent cancers at an early stage14–20. Information regarding the potential benefit of such colonoscopies in patients with IBD is lacking but is particularly pertinent as the traditional adenoma-carcinoma sequence on which the above premise is based does not apply to colitis associated cancers21, 22. Using a large multi-institutional IBD cohort of nearly 7,000 patients, we demonstrate that IBD patients who underwent a colonoscopy within the past 3 years had a significantly lower risk of CRC.
Recognizing the higher risk of CRC in patients with IBD, guidelines from the AGA, BSG, and the European Crohn’s and Colitis Organization (ECCO) recommend surveillance colonoscopies every 2–3 years once individuals with UC attain a disease duration of 8–10 years7, 8, 11. Recognizing that a similar risk of CRC exists in colonic CD, such patients are recommended to adhere to similar surveillance protocols as those with UC29, 30. However, the potential benefit in terms of reduction in CRC risk or mortality in this population has not been examined previously, yet this is an important question for patients subject themselves to invasive colonoscopies every 2–3 years, and indeed sometimes annually in long-standing disease. Furthermore, the utilization of societal resources by repeated surveillance exams is considerable. Thus establishment of the benefit of surveillance colonoscopy is an important research goal. Reassuringly, we found in our study that individuals with either UC or CD who had a colonoscopy within the past 3 years had a reduction in risk of CRC by nearly half compared to those patients without such recent exam. Additionally and importantly, IBD patients with CRC who had a recent colonoscopy had lower mortality than those with CRC diagnosed without a recent colonoscopy.
While to our knowledge no prior studies have specifically addressed reduction in the risk of CRC with surveillance colonoscopy in IBD, a few have examined the association between surveillance and mortality. In cohort of 41 patients who developed CRC in the context of UC, cancer was detected at an earlier stage in the group undergoing surveillance, and resulted in better 5-year survival31. Karlen et al. similarly showed improved survival in patients undergoing colonoscopy32. However, conflicting data also exists with other authors suggesting no mortality benefit to surveillance colonoscopy in patients with IBD33. Intriguing epidemiologic trends support this plausibility. Several recent studies have demonstrated a temporal decline in examined temporal trends in incidence of CRC across diverse IBD cohorts5, 12, 34 though data has not always been consistent4. Researchers have speculated on reasons for the temporal decrease in risk of CRC. Inflammation is felt to be an important determinant of CRC in IBD2, 21, 22. Greater use of maintenance treatment for IBD may result in more prolonged periods of remission resulting in reduced risk of CRC. However, more regular use of surveillance colonoscopies could also explain this temporal reduction in risk of CRC though not all countries that have demonstrated this reduction in CRC risk have reported high rates of adherence to surveillance exams. In the Northern California study, despite no change in CRC risk, there was a significant increase in the utilization of colonoscopy between 1998 and 2010.
There are a few potential explanations for the reduced CRC incidence associated with surveillance colonoscopies in IBD. First, such colonoscopies may allow endoscopic removal of polypoid or non-polypoid dysplastic lesions, thereby reducing risk of CRC. Unresectable lesions may trigger a referral for colectomy prior to development of CRC,thereby improving outcomes. Second, it may detect invisible dysplasia that can also either result in referral to colectomy or be followed up more intensively leading to early diagnosis of high grade dysplasia or cancer. Third, one may speculate that IBD patients who do not undergo colonoscopy are likely different in GI-related behavior when compared to those who undergo such procedures, and may have less frequent follow-up, suboptimal titration of their medical treatments, and poor adherence to recommended medical regimens. We do not believe this to explain our findings for a few reasons. First, there is inconsistent data suggesting a chemopreventive effect to any of the current IBD medications. Thus, adherence to existing medical therapies is unlikely to directly explain differences in CRC risk through a pharmacologic effect. One may hypothesize that the colonic inflammation is better controlled in patients undergoing frequent colonoscopies to assess for mucosal healing, and earlier up-titration of medications. Indeed such an approach has been recently demonstrated to be feasible and improve outcomes. However, this hypothesis only further supports the potential importance of colonoscopy in reducing CRC risk in IBD patients and suggests an alternate mechanism for this inverse association in addition to identification of dysplastic lesions.
Several studies have examined adherence to surveillance colonoscopies in patients with IBD35. As many of these studies focused on those with long-standing disease, our rates of recent colonoscopy are not immediately comparable. In the most directly comparable study from Kaiser Northern California, during the 2007–2010 period, 28% of CD and 24% of UC patients underwent a colonoscopy4. In comparison, in our study, 40% of those in the no CRC group compared to 28% of those in the CRC group had a history of recent colonoscopy within a 3 year time frame prior to diagnosis or end of follow-up. In a study from the CESAME cohort in France, among patients with long-standing CD or UC, 54% had a surveillance colonoscopy during the 41-month study period36, also comparable with our shorter window of 36 months. In contrast, other referral center studies have shown surveillance rates of up to 80% in a single Canadian center37. The reasons for non-adherence to surveillance protocols are multi-factorial including demographic, patient factors related to perception of risk, logistics, as well as provider factors35.
The reduction in mortality in the CRC group who had a recent colonoscopy is consistent with data in the general population where there is emerging data that colonoscopy is associated with a reduction in long-term CRC incidence and mortality14–20. While we did not have information specifically on cause of death or stage of CRC in our study, our findings demonstrate that IBD-CRC patients who had a recent colonoscopy had significantly reduced overall mortality on follow-up even after adjusting for age, gender, race, and non-cancer co-morbidity. Thus, one can speculate that in the IBD cohort, recent colonoscopy could plausibly be associated with a diagnosis of early stage prevalent cancer resulting in better overall outcomes. Studies of interval cancers in the general population support a similar hypothesis38.
We performed a number of sensitivity analyses to examine the robustness of our findings. The inverse association with between recent colonoscopy and CRC risk was robust on adjustment for medication use suggesting that the association with colonoscopy identified in our study was not due to patients undergoing colonoscopy receiving more appropriate escalation in their medical treatment. That the inverse association remained significant after adjusting for median ESR or CRP values suggest that inflammation or treatment thereof is unlikely to be a significant confounder. Furthermore, the higher rates of immunosuppressive treatment in those undergoing colonoscopy suggest that this group may indeed be at a higher risk of CRC by virtue of their inflammatory burden. The inverse association with screening colonoscopy and CRC incidence and mortality is also unlikely to be explained by differential healthcare seeking behaviors between the two groups or healthy life style choices. There was no effect on our estimates on adjusting for intensity of healthcare utilization, and indeed the association with colonoscopy was restricted to CRC and not for other solid organ tumors or hematologic cancers, supporting the specificity of our findings.
We readily acknowledge several limitations to our study. First, while our cohort was considerably larger than most prior studies examining epidemiology of CRC in patients with IBD, it was comprised of patients seeking care at two tertiary referral centers and affiliated centers in the Greater Boston area. As such, this may represent a cohort with more severe disease. However, as this is indeed the group that is likely to be at a greater risk of CRC owing to their inflammatory burden, it is a pertinent group to examine this question. Second, our cohort was not restricted to those with disease duration of longer than 8 years, and while we adjusted for duration of follow-up after the first diagnosis code of IBD, we were not able to accurately define duration since diangosis. However, there was no difference in the median disease duration between the groups based on receiving a colonoscopy within 3 years, and one would expect disease duration to be non-differentially distributed between the two groups, or favor greater colon cancer risk in those receiving colonoscopy either due to persistent disease activity or longer disease duration. Third, we did not have information on the indication for the colonoscopy, quality of surveillance colonoscopies, and whether the recommended number of biopsies was obtained. It is plausible that the intensity and quality of surveillance exams were higher in this group many of whom were under the care of IBD specialists. Further studies in population-based cohorts are necessary to more accurately define the real-world impact of surveillance practices on CRC incidence and outcomes in IBD. Yet, within the context of our study, this is unlikely to significantly affect our estimates as misclassification of ‘non-surveillance’ colonoscopies as being of the same quality of surveillance exams would bias our effect sizes towards the null, making ours a conservative estimate. As well, bias in selecting high risk patients for colonoscopy would make our findings of lower CRC incidence in this group more robust. Furthermore, recent studies have suggested that most dysplastic lesions, and certainly most cancers in IBD, are visible on white-light exam with a low yield for random biopsies. Consequently, the difference between a ‘diagnostic’ and a ‘surveillance’ colonoscopy would be unlikely to significantly alter our findings. As colon cancer fortunately remains uncommon, large studies are essential to ensure sufficient power to examine the question about the effectiveness of surveillance exams in population-based cohorts. Fourth, as no distinct diagnosis codes exist for dysplasia, we were not able to examine the effect of colonoscopy on the incidence of dysplastic changes in colitis. However, one would expect that a diagnosis of dysplasia would result in more frequent colonoscopies, biasing that group towards having a higher risk of CRC. Further, as there is greater homogeneity in the management of CRC than dysplasia, this allowed us to more accurately examine the effect of recent colonoscopy on CRC outcomes without significant confounding.
In conclusion, using a large IBD cohort, we demonstrate that colonoscopy was associated with a reduction in risk of CRC. Furthermore, individuals with colitis-associated cancer who underwent a recent colonoscopy within 6–36 months prior to CRC diagnosis had significantly reduced mortality compared to those without a recent exam. Continued colonoscopic surveillance in IBD patients at an elevated risk appears to be warranted, as is focus on ensuring the optimal technique and interval for such surveillance exams. Policy should also focus on increasing adoption and adherence to surveillance protocols.
Supplementary Material
Acknowledgments
Sources of Funding: The study was supported by NIH U54-LM008748. A.N.A is supported by funding from the American Gastroenterological Association and from the US National Institutes of Health (K23 DK097142). K.P.L. is supported by NIH K08 AR060257 and the Harold and Duval Bowen Fund. E.W.K is supported by grants from the NIH (K24 AR052403, P60 AR047782, R01 AR049880).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial conflicts of interest: None
Specific author contributions:
An author may list more than one contribution, and more than one author may have contributed to the same element of the work
Study concept and design – Ananthakrishnan,
Data Collection – Ananthakrishnan, Gainer, Cagan, Cai, Churchill, Kohane, Shaw, Liao, Murphy
Analysis – Ananthakrishnan
Preliminary draft of the manuscript – Ananthakrishnan
Approval of final version of the manuscript – Ananthakrishnan, Gainer, Cagan, Cai, Churchill, Kohane, Shaw, Liao, Murphy
REFERENCES
- 1.Andersen V, Halfvarson J, Vogel U. Colorectal cancer in patients with inflammatory bowel disease: can we predict risk? World J Gastroenterol. 2012;18:4091–4094. doi: 10.3748/wjg.v18.i31.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaden J. Review article: colorectal carcinoma and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):24–30. doi: 10.1111/j.1365-2036.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrinton LJ, Liu L, Levin TR, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–389. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 5.Jess T, Loftus EV, Jr, Velayos FS, et al. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis. 2006;12:669–676. doi: 10.1097/00054725-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Loftus EV., Jr Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35:517–531. doi: 10.1016/j.gtc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Sebastian S, Hernandez V, Myrelid P, et al. Colorectal cancer in inflammatory bowel disease: Results of the 3rd ECCO pathogenesis scientific workshop (I) J Crohns Colitis. 2013 doi: 10.1016/j.crohns.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Farraye FA, Odze RD, Eaden J, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–774. 774 e1–774 e4. doi: 10.1053/j.gastro.2009.12.035. quiz e12-3. [DOI] [PubMed] [Google Scholar]
- 9.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 11.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 12.Jess T, Simonsen J, Jorgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375 e1–381 e1. doi: 10.1053/j.gastro.2012.04.016. quiz e13-4. [DOI] [PubMed] [Google Scholar]
- 13.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology. 2010;138:870–876. doi: 10.1053/j.gastro.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Kahi CJ, Imperiale TF, Juliar BE, et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–775. doi: 10.1016/j.cgh.2008.12.030. quiz 711. [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh AM, Giovannucci EL, Fuchs CS, et al. Screening endoscopy and risk of colorectal cancer in United States men. Cancer Causes Control. 1998;9:455–462. doi: 10.1023/a:1008884021049. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner H, Chang-Claude J, Seiler CM, et al. Long-term risk of colorectal cancer after negative colonoscopy. J Clin Oncol. 2011;29:3761–3767. doi: 10.1200/JCO.2011.35.9307. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Burstein E, Fearon ER. Colitis and cancer: a tale of inflammatory cells and their cytokines. J Clin Invest. 2008;118:464–467. doi: 10.1172/JCI34831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Normalization of Plasma 25-Hydroxy Vitamin D Is Associated with Reduced Risk of Surgery in Crohn's Disease. Inflamm Bowel Dis. 2013 doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Mortality and extraintestinal cancers in patients with primary sclerosing cholangitis and inflammatory bowel disease. J Crohns Colitis. 2014 doi: 10.1016/j.crohns.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Cai T, Savova G, et al. Improving Case Definition of Crohn's Disease and Ulcerative Colitis in Electronic Medical Records Using Natural Language Processing: A Novel Informatics Approach. Inflamm Bowel Dis. 2013;19:1411–1420. doi: 10.1097/MIB.0b013e31828133fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananthakrishnan AN, Cheng SC, Cai T, et al. Serum Inflammatory Markers and Risk of Colorectal Cancer in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ananthakrishnan AN, Cheng SC, Cai T, et al. Association Between Reduced Plasma 25-Hydroxy Vitamin D and Increased Risk of Cancer in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dregan A, Moller H, Murray-Thomas T, et al. Validity of cancer diagnosis in a primary care database compared with linked cancer registrations in England. Population-based cohort study. Cancer Epidemiol. 2012;36:425–429. doi: 10.1016/j.canep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn's colitis: results of a surveillance program spanning 25 years. Clin Gastroenterol Hepatol. 2008;6:993–998. doi: 10.1016/j.cgh.2008.03.019. quiz 953-4. [DOI] [PubMed] [Google Scholar]
- 30.Ekbom A, Helmick C, Zack M, et al. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357–359. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 31.Choi PM, Nugent FW, Schoetz DJ, Jr, et al. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–424. doi: 10.1016/0016-5085(93)90715-o. [DOI] [PubMed] [Google Scholar]
- 32.Karlen P, Kornfeld D, Brostrom O, et al. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–714. doi: 10.1136/gut.42.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch DA, Lobo AJ, Sobala GM, et al. Failure of colonoscopic surveillance in ulcerative colitis. Gut. 1993;34:1075–1080. doi: 10.1136/gut.34.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winther KV, Jess T, Langholz E, et al. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 35.Friedman S, Cheifetz AS, Farraye FA, et al. Factors that affect adherence to surveillance colonoscopy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:534–539. doi: 10.1097/MIB.0b013e3182802a3c. [DOI] [PubMed] [Google Scholar]
- 36.Vienne A, Simon T, Cosnes J, et al. Low prevalence of colonoscopic surveillance of inflammatory bowel disease patients with longstanding extensive colitis: a clinical practice survey nested in the CESAME cohort. Aliment Pharmacol Ther. 2011;34:188–195. doi: 10.1111/j.1365-2036.2011.04711.x. [DOI] [PubMed] [Google Scholar]
- 37.Kottachchi D, Yung D, Marshall JK. Adherence to guidelines for surveillance colonoscopy in patients with ulcerative colitis at a Canadian quaternary care hospital. Can J Gastroenterol. 2009;23:613–617. doi: 10.1155/2009/691850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950–960. doi: 10.1053/j.gastro.2014.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.