Abstract
Although diarrhea is the predominant bowel dysfunction in as many as one-third of patients with irritable bowel syndrome (IBS), it is unclear whether there is a specific disorder of intestinal fluid or electrolyte secretion in IBS. Diarrhea is generally considered secondary to accelerated colonic transit in patients with IBS. Although a primary secretory diathesis has not been well documented in patients with IBS with diarrhea (IBS-D), several mechanisms that could potentially contribute to intestinal secretion have been reported. Some of these mechanisms also influence motor and secretory dysfunctions that contribute to the pathophysiology of IBS-D. We review the evidence supporting secretion in IBS-D caused by peptides and amines produced by enteroendocrine cells or submucosal neurons, enterocyte secretory processes, and intraluminal factors (bile acids and short-chain fatty acids). Understanding these mechanisms and developing clinical methods for their identification could improve management of patients with IBS-D.
Keywords: pathogenesis, intestine, SCFA, absorption
Introduction
Diarrhea (D) is the predominant bowel dysfunction of up to one-third of patients with irritable bowel syndrome (IBS) and it appears to be dominant, particularly in patients with post-infectious IBS (PI-IBS).1 To date, there is no published evidence that there is a specific disorder of intestinal fluid or electrolyte secretion in IBS;2 thus, the diarrhea in IBS is generally considered secondary to accelerated colonic transit and the reduced volume of the proximal colon. The 24-hour stool weight was significantly correlated with the rate at which radiolabeled solid residue emptied from the ascending and transverse colons, and there was also an inverse relationship between emptying rates of those colonic regions and the maxima1 volume of the proximal colon.3 Reduced volume may result from the increased rectal or colonic tone postprandially and in response to lipids, described in IBS-D.4,5 Although a primary secretory diathesis has not been well documented in IBS-D, several mechanisms that could potentially contribute to intestinal secretion have been reported, and some of these mechanisms also influence motor and secretory dysfunctions that contribute to the pathophysiology of IBS-D.
Peptides and Amines Produced by Enteroendocrine Cells or Submucosal Neurons
Several peptides and amines such as serotonin, as well as granins, are released from enteroendocrine cells by luminal factors in the diet, by metabolites produced following intraluminal digestion of nutrients [into short chain fatty acids (SCFAs)], and by endogenous chemicals such as bile acids.6,7
Table 1 summarizes the literature on the effects of enteroendocrine peptides and amines that may induce intestinal secretion or inhibit absorption of fluids and electrolytes, and the table summarizes information reported in IBS that supports the hypothesis of a role of intestinal secretion in IBS.
Table 1.
Examples of Altered Functions of Peripheral Hormones, Amines and Peptides in IBS
| Mechanisms | Pathophysiology | Release, distribution, action | Biological and clinical correlates | References |
|---|---|---|---|---|
| Granins | Chromogranins (Cg) or secretogranins (Sg) in secretory granules mobilize release of peptide hormones from EC cells | Release of (e.g.) 5-HT, PYY, somatostatin (SS) from secretory granules of EC cells into lumen or interstitial fluid | IBS-D or -A higher fecal Cg A, SG II and III and duodenal CgA cell density; changes not specific for IBS; higher CgA and SG associated with faster colonic transit and weakly with symptoms | Montero-Hadjadje 2009; Ohman 2012; El-Salhy 2012, |
| Serotonin | Derived primarily from EC cells and neurons: mediates intrinsic reflexes (stimulates motility, secretion and vasodilation); activates extrinsic afferents that mediate extrinsic reflexes, sensation | Release from EC cells with re-uptake by the serotonin transporter (SERT) in epithelial cells or platelets; circulating 5-HT represents 5-HT that does not undergo re-uptake | Plasma post-prandial 5-HT elevated in IBS-D, and PI-IBS; reduced in IBS-C; Platelet SERT uptake is disrupted in IBS-D; Mucosal 5-HT elevated in IBS-C and in PI-IBS; Mucosal SERT mRNA expression and immune-reactivity decreased in some reports | Donowitz and Binder 1975; Spiller 2000; Mawe, Hofmann 2013; Houghton 2003, and 2007; Atkinson 2006; Bellini 2003; Franke 2010; Foley 2011; El-Salhy 2013 |
| Histamine | Derived primarily from mast cells | Locally released histamine stimulates ileal fluid secretion via H1 receptors, and sensation (possibly via H1 receptors) | Mucosal extracts from IBS activate submucosal and sensory neurons; possible therapeutic benefit with H1 antagonists ketotifen and ebastin in IBS | Linaker 1981 Coelho 1998 Mobarakeh 2000 Barbara 2007 Buhner 2009, Klooker 2010 van Wanrooij 2013 |
| Peptide YY | Derived primarily in enteroendocrine cells | Intraluminal PYY induces small bowel and colon fluid/electrolyte absorption | Rectal biopsy PYY elevated during acute Campylobacter enteritis; normal in PI-IBS by 12 weeks; lower PYY in colonic mucosa in IBS | Playford 1990; Bilchik 1993, and 1994; Liu 1997; Spiller 2000; El-Salhy 2013 |
| NeuropeptideY | Derived from enteric neurons | NPY Y2 receptor agonists reduce intestinal fluid secretion (mice) | NPY levels in both plasma and the sigmoid lower in IBS patients than controls | Zhang et al 2008; Moriya 2010; |
| Somatostatin (SS) | Derived primarily from enteroendocrine cells and neurons: | SS inhibits NHE1 (basolateral in enterocytes), involved in HCO3− secretion | Expression of SS in serum and colonic or rectal mucosa of IBS higher compared with controls; SS in mucosa in IBS-C greater than in IBS-D | Han 2013; Zachos 2005; El-Salhy 2013 |
| Vasoactive Intestinal Peptide (VIP) | Derived mainly from gut secretomotor neurons | VIP modulates secretion and vasodilatation | Sigmoid mucosa and plasma VIP higher in IBS than controls; Rectosigmoid expression of VIP increased on RNA-Seq | Zhang 2008; Han 2013; Camilleri 2014 |
| Purines | P1 and P2 ubiquitous receptors activated by adenosine and extra-cellular nucleotides e.g. ATP | P1A2B receptor regulates colonic Cl− and water secretion; P2Y activates K+, Cl−, HCO3− secretion; inhibits Na+ absorption | Rectosigmoid expression of P2RY4 increased on RNA-Seq | Camilleri 2014 |
EC=enteroendocrine cells; CHO=carbohydrates; RNA-Seq=mRNA sequencing
The prototype mediator of intestinal secretion is the amine, serotonin [5-hydroxytryptamine (5-HT)]; therefore this will be discussed in greater detail than the other mediators. Serotonin is synthesized primarily in the gastrointestinal tract, stored in the mucosal enterochromaffin cells,8 and released in response to mechanical and chemical stimulation. It mediates intrinsic reflexes (e.g., stimulation of propulsive and segmentation motility, epithelial secretion and vasodilation) and activates extrinsic vagal and spinal afferents.9–11
Circulating 5-HT is derived primarily from the gut and represents the 5-HT that does not undergo re-uptake by the serotonin transporter (SERT) in the cells of the epithelial lining. Circulating postprandial 5-HT levels are increased in platelet-depleted plasma (PDP) in IBS-D12,13 and PI-IBS,14 and are reduced14 or unchanged in IBS-C.12 Elevated postprandial 5-HT in PDP in IBS-D and PI-IBS, but not IBS-C12–14, might reflect differences in platelet uptake of 5-HT by SERT, which is disrupted in IBS-D.15–17 The depletion of platelet SERT in IBS-D may reflect primary deficiency in SERT expression in gastrointestinal mucosa, which has been observed in adults and children with IBS.18,19 This is supported by platelet 5-HT levels which are reduced in IBS-D16 and are ~2-fold higher in IBS-C patients compared to healthy controls,12 which, together with decreased postprandial release of 5-HT in IBS-C, suggests increased SERT reuptake activity.14. Alternatively, release of 5-HT to physiological stimuli appears impaired in IBS-C.12
In a study combining IBS-C and PI-IBS patients, there was a negative correlation between plasma 5-HT and colonic transit time;14 although not specifically studied, the transit time may reflect effects of 5-HT on both motor and secretory functions, given the well-established effects of 5-HT on intestinal secretion and colonic transit, as observed in the carcinoid syndrome.20,21
Rectal or colonic mucosal 5-HT levels were increased in PI-IBS22 and in IBS-C and functional constipation,23–26 though no statistically different intensity of serotonin immunoreactivity was observed in any IBS group.27 The increased mucosal content in IBS-C may reflect mucosal storage without release into the lumen or plasma, potentially explaining the lack of fluid secretion, more solid consistency of bowel movements, and low postprandial plasma 5-HT in IBS-C.
The potential role of serotonin in the induction of loose bowel movements in IBS-D is supported by the therapeutic effects of selective serotonergic receptor type 3 (5-HT3) antagonists (ondansetron, alosetron, ramosetron). These agents are effective in the overall relief of IBS-D and, particularly, in the normalization of bowel function and reduction of urgency in these patients.28,29
Chromogranins (Cg) and secretogranins (Sg) are present in secretory vesicles of nervous, endocrine, and immune cells, and CgA appears to be involved in intestinal secretion by inducing formation of secretory granules and release of other peptide hormones such as from enteroendocrine cells.30 IBS patients with faster colonic transit have higher levels of fecal CgA, SgII, and SgIII, but lower levels of CgB relative to healthy controls,31 and increased duodenal CgA cell density.27, 32 Overall, the data suggest that granins packaging neuropeptides indirectly stimulate colonic secretion or motility.33,34
Other biogenic peptides that may influence intestinal secretion or absorption are detailed in Table 1. These include somatostatin,35 PYY,36–40 and NPY, all of which increase fluid absorption, and their tissue expression is generally reduced in patients with IBS-D.11,41,42 The mechanism of increased absorption by somatostatin is mediated in part by stimulation of apical membrane Na/H exchange (NHE3 transporter).43 In contrast, IBS-D is associated with increased mucosal expression of VIP and purinergic receptors,44 which are associated with intestinal secretion, and histamine, derived predominantly from mast cells, is generally associated with intestinal secretion.45–51
Intraluminal Factors: Bile Acids and Short Chain Fatty Acids
Bile acids (BA) stimulate colonic motility,52 transit,53 and secretion, primarily through electrogenic chloride secretion (apical chloride channels) and through increase in colonic mucosal permeability54,55 or activation of colonocyte apical Cl−/OH− exchange.56 An additional mechanism mediating effects of intraluminal BAs is the BA receptor, GPBAR1 (or TGR5),57 which is expressed in enteric neurons, enteroendocrine cells,58 and primary spinal afferent and spinal neurons involved in sensory transduction.59 GPBAR1 mediates the prokinetic actions of intestinal BAs, is required for normal defecation in mice, and mediates colonic fluid secretion.60
A systematic review documented bile acid malabsorption (BAM) in a sizeable proportion of patients with chronic functional diarrhea, or IBS-D. BAM was typically identified by the 75SeHCAT retention test which uses a synthetic 75selenium homotaurocholic acid, a BA that is resistant to bacterial degradation, does not undergo passive absorption, and is actively absorbed in the terminal ileum to enter the enterohepatic circulation or excreted into stool, unaltered by its passage through the colon. Normal retention is >15% at 7 days, and moderate and severe malabsorption are respectively defined by retention <10% and<5% respectively; the level of isotope retention predicts response to BA sequestrants. Five studies (429 patients) indicated that 10% (CI: 7–13) of patients had severe BAM (75SeHCAT 7 day retention <5%); and 17 studies (1073 patients) indicated that 32% (CI: 29–35) of patients had moderate BAM (75SeHCAT retention <10%).61 Patients with increased fecal BA excretion (>2337mM/48h, the 90th percentile in healthy controls) have increased small bowel permeability, borderline faster colonic transit and higher CDCA proportion in stool and fecal fat excretion compared to IBS-D patients without increased fecal BA excretion.62
The human small intestine also secretes fluid in response to perfusion with conjugated di-α hydroxyl BAs.63,64 In patients with IBS-D, there is evidence that the small intestinal mucosa is more sensitive to secretory effects of BAs compared to mucosa from healthy controls.65 Variation in GPBAR1 genotype (rs11554825, which is in strong linkage disequilibrium with mutations that alter expression and function of the receptor)66 was significantly associated with colonic transit at 48 hours,67 a surrogate of the intestinal secretory effects of BAs; these genotype data are consistent with a secretory diathesis in patients with IBS-D and could potentially explain the increased secretory response of ileal mucosa to infused BAs.65
Short Chain Fatty Acids
In healthy volunteers, 2 to 20% of dietary starch escapes absorption in the small bowel,68 providing substrate for the generation of short-chain (<6 carbon) fatty acids (SCFAs) by colonic bacteria and increased delivery of water to the colon.69 SCFAs stimulate intraluminal colonic release of 5-hydroxytryptamine (5-HT)70 from enteroendocrine cells in rats.71 The SCFA, propionate, induced transepithelial ion and fluid secretion in guinea pig distal colon mucosal preparations in vitro and increased the expression of receptor FFA2, which co-localizes with chromogranin A in enteroendocrine cells.72 However, the overall effect of SCFAs is generally to enhance absorption of fluids and electrolytes, effects that are mediated through a common mechanism, that is, the SCFA induced increase in expression of NHE3,73 although the magnitude of effect may differ among the SCFAs. Moreover, the fecal SCFA profile of patients with IBS-D is characterized by lower concentrations of total SCFA, acetate, and propionate, and a higher concentration and percentage of n-butyrate, which is pro-absorptive. Fecal flora from these patients produced less SCFA in an in vitro fermentation system in response to incubations with various carbohydrates and fibers.74 Overall, since the SCFA composition in stool of IBS-D patients has lower amounts of the pro-secretory propionate and higher amounts of the pro-absorptive butyrate, these data suggest that the SCFAs in stool would be associated with less fluid secretion in patients with IBS-D.
Enterocyte Secretory Processes
There are two lines of evidence that support the existence of enterocyte secretory mechanisms in IBS or functional diarrhea. Guanylate cyclase C (GUCY2C) is a transmembrane receptor whose extracellular domain is activated by ligands that may be endogenous (e.g. guanylin and uroguanylin) or exogenous (e.g. E. coli enterotoxin).
A dominant inheritance gain of function variation in GUCY2C gene was reported in a Norwegian family75 with a rare form of familial diarrhea (FD), characterized by onset of symptoms in infancy, chronic, relatively mild diarrhea, diagnosed as IBS-D. Subsequently, it was demonstrated that this dominantly inherited, fully penetrant disease was due to a heterozygous base substitution, c.2519G→T, in exon 22 of chromosome 12, GUCY2C. This functional mutation encodes for the guanylate cyclase-C (GC-C) receptor which induces enterocyte secretion. Conversely, an autosomal-recessive phenotype of meconium ileus (associated with inadequate intestinal fluid and electrolyte secretion) was observed in two unrelated consanguineous Bedouin kindreds, caused by different homozygous loss of function mutations (c.1160A>G; c.2270dupA insertion) in GUCY2C.76
In order to determine whether this mutation might be overlooked in patients with IBS-D outside Norway or this specific family, we explored the association of GUCY2C (c.2519G→T) mutation in 406 patients with IBS and 227 healthy controls from the upper Midwest U.S.A. None of our IBS patients or controls carried the c.2519G→T mutation in GUCY2C, and these results were confirmed by sequencing in 5 randomly selected DNA samples. We concluded that this c.2519G→T mutation in GUCY2C is unlikely to be responsible for IBS in patients from the Midwest U.S.A.; however, the identification of the genetic mutation in association with chronic functional diarrhea or IBS-D in the Norwegian family is consistent with the concept that variations in the functional expression of such receptors involved in enterocyte chloride secretion may result in chronic diarrhea that is mistakenly attributed to IBS-D.
The same principle applies to congenital Na+ diarrheas (rare autosomal recessive disorders characterized by polyhydramnios, hyponatremia, metabolic acidosis, and diarrhea with high sodium content) associated with genetic variations in the SLC9/NHE gene family. The latter is a plasma membrane and organellar family of Na+/H+ exchangers (particularly NHE3 and NHE8) which constitute the major way Na+ is absorbed in the kidney and GI tract.77
Congenital chloridorrhea is an autosomal recessive disorder involving the gene, solute carrier family 26 member 3 (SLC26A3), which encodes a transmembrane protein that functions as an apical epithelial Cl−/HCO3− exchanger in the colon. The defect in this transporter hinders the absorption of chloride and the secretion of bicarbonate. SLC26A3 is coupled to a Na+/H+ exchanger (NHE2 and/or NHE3) that leads to intestinal loss of sodium, chloride, and fluid due to a chloride-rich diarrhea.78 These congenital diarrheas usually present in infancy; however, they may also cause chronic diarrhea,79 and the patients may rarely present with chronic diarrhea later in life and may receive a diagnosis of IBS-D.80
A second line of evidence comes from recent observations based on expression studies of jejunal or colonic mucosa in patients with IBS. Several groups have reported alterations in expression of tight junction proteins in the jejunal81 or rectosigmoid mucosa of patients with IBS-D; in some studies, these changes were associated with increased mucosal permeability.82,83 However, these alterations do not necessarily prove an intestinal secretory mechanism in IBS-D.
On the other hand, increased mucosal expression of genes demonstrated on RNA sequencing for ion channels suggests more directly that the mucosa of patients with IBS-D may have intrinsic secretory properties.44 Thus, there are two enterocyte secretory mechanisms that are overexpressed in rectosigmoid mucosa of patients with IBS-D: GUC2AB (guanylate cyclase 2B receptor associated with enterocyte chloride channel activation in response to uroguanylin); and PDZD3 (a protein that associates with guanylate cyclase C and regulates cGMP production following receptor stimulation and chloride secretion).84
Conclusion
Although a primary secretory diathesis has not been well documented in IBS-D, several mechanisms that could potentially contribute to intestinal secretion have been reported. Understanding these mechanisms and developing clinical methods for their identification have the potential to enhance management of patients with IBS-D.
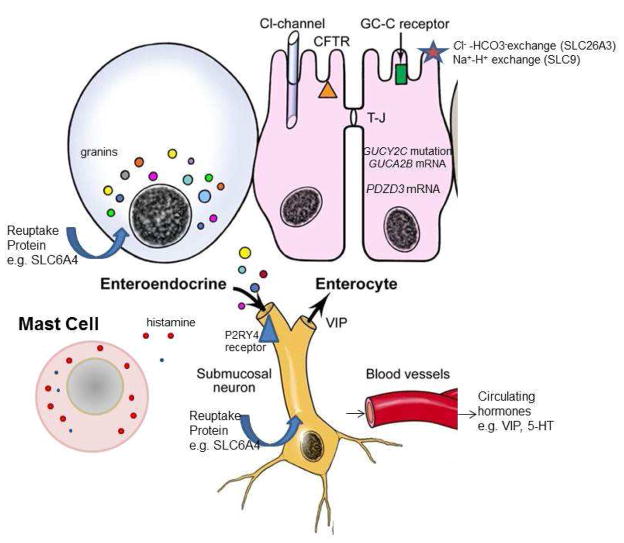
Figure 1.
The critically important cells involved in intestinal secretory processes are the enteroendocrine cells and enterocytes in the epithelium, as well as mast cells and submucosal neurons in the lamina propria and submucosa. From granin vesicles in the enteroendocrine cells, a number of peptides or amines are released to induce intestinal secretion of chloride ions, typically by activation of submucosal neurones or the enterocytes. Similarly, release of proteases and histamine from mast cells induces intestinal secretion. Ion transport channels such as SLC26A3 and SLC9 are important in absorption of sodium and chloride ions and may be mutated to result in congenital diarrhea that may be identified later in life. Rare familial mutations in the gene for the guanylate cyclase C receptor may result in familial diarrhea that is attributed to chronic functional diarrhea. In patients with IBS-diarrhea, increased expression of factors involved in intestinal secretion (e.g., PDZD3, GUCA2B, VIP) or reduced expression of pro-absorptive peptides (e.g., somatostatin) have been reported and are the subject of ongoing research.
Acknowledgments
The author thanks Mrs. Cindy Stanislav for secretarial assistance.
Funding support: Dr. Camilleri is supported by grant R01-DK92179 from National Institutes of Health.
Footnotes
Conflicts of interest: None
Authors’ contributions: M. Camilleri: Conceptualization and writing of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 2.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassallo M, Camilleri M, Phillips SF, et al. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–108. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 4.Steens J, Van Der Schaar PJ, Penning C, et al. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:241–247. doi: 10.1046/j.1365-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 5.Simrén M, Abrahamsson H, Björnsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20–27. doi: 10.1136/gut.48.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd M, Modlin IM, Gustafsson BI, et al. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol. 2008;295:G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 7.Peregrin AT, Ahlman H, Jodal M, et al. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol. 1999;127:887–894. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol. 2001;17:99–103. doi: 10.1097/00001574-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman JM, Tyler K, Maceachern SJ, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson W, Lockhart S, Whorwell PJ, et al. Altered 5 hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Houghton LA, Atkinson W, Whitaker RP, et al. Increased platelet depleted plasma 5 hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 15.Bellini M, Rappelli L, Blandizzi C, et al. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–2711. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 16.Franke L, Schmidtmann M, Riedl A, et al. Serotonin transporter activity and serotonin concentration in platelets of patients with irritable bowel syndrome: effect of gender. J Gastroenterol. 2010;45:389–398. doi: 10.1007/s00535-009-0167-y. [DOI] [PubMed] [Google Scholar]
- 17.Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–1443. doi: 10.1053/j.gastro.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Faure C, Patey N, Gauthier C, et al. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donowitz M, Binder HJ. Jejunal fluid and electrolyte secretion in carcinoid syndrome. Am J Dig Dis. 1975;20:1115–1122. doi: 10.1007/BF01070754. [DOI] [PubMed] [Google Scholar]
- 21.von der Ohe M, Camilleri M, Kvols LK, et al. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 22.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lincoln J, Crowe R, Kamm MA, et al. Serotonin and 5-hydroxyindoleacetic acid are increased in the sigmoid colon in severe idiopathic constipation. Gastroenterology. 1990;98:1219–1225. doi: 10.1016/0016-5085(90)90336-y. [DOI] [PubMed] [Google Scholar]
- 24.Miwa J, Echizen H, Matsueda K, et al. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 25.Zhao RH, Baig MK, Thaler KJ, et al. Reduced expression of serotonin receptor(s) in the left colon of patients with colonic inertia. Dis Colon Rectum. 2003;46:81–86. doi: 10.1007/s10350-004-6500-x. [DOI] [PubMed] [Google Scholar]
- 26.Costedio MM, Coates MD, Brooks EM, et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am J Gastroenterol. 2010;105:1173–1180. doi: 10.1038/ajg.2009.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri M. Pharmacological agents currently in clinical trials for disorders of neurogastroenterology. J Clin Invest. 2013;123:4111–4120. doi: 10.1172/JCI70837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2013 Dec 12; doi: 10.1136/gutjnl-2013-305989. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montero-Hadjadje M, Elias S, Chevalier L, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Öhman L, Stridsberg M, Isaksson S, et al. Altered levels of fecal chromogranins and secretogranins in IBS: relevance for pathophysiology and symptoms? Am J Gastroenterol. 2012;107:440–447. doi: 10.1038/ajg.2011.458. [DOI] [PubMed] [Google Scholar]
- 32.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacangelo AL, Eiden LE. Chromogranin A: current status as a precursor for bioactive peptides and a granulogenic/sorting factor in the regulated secretory pathway. Regul Pept. 1995;58:65–88. doi: 10.1016/0167-0115(95)00069-n. [DOI] [PubMed] [Google Scholar]
- 34.Camilleri M. Editorial: fecal granins in IBS: cause or indicator of intestinal or colonic irritation? Am J Gastroenterol. 2012;107:448–450. doi: 10.1038/ajg.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubel KA. Intestinal nerves and ion transport: stimuli, reflexes, and responses. Am J Physiol. 1985;248:G261–G271. doi: 10.1152/ajpgi.1985.248.3.G261. [DOI] [PubMed] [Google Scholar]
- 36.Playford RJ, Domin J, Beacham J, et al. Preliminary report: role of peptide YY in defense against diarrhoea. Lancet. 1990;335:1555–1557. doi: 10.1016/0140-6736(90)91378-n. [DOI] [PubMed] [Google Scholar]
- 37.Bilchik AJ, Hines OJ, Adrian TE, et al. Peptide YY is a physiological regulator of water and electrolyte absorption in the canine small bowel in vivo. Gastroenterology. 1993;105:1441–1448. doi: 10.1016/0016-5085(93)90149-7. [DOI] [PubMed] [Google Scholar]
- 38.Bilchik AJ, Hines OJ, Zinner MJ, et al. Peptide YY augments postprandial small intestinal absorption in the conscious dog. Am J Surg. 1994;167:570–574. doi: 10.1016/0002-9610(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 39.Liu CD, Newton TR, Zinner MJ, et al. Intraluminal peptide YY induces colonic absorption in vivo. Dis Colon Rectum. 1997;40:478–482. doi: 10.1007/BF02258396. [DOI] [PubMed] [Google Scholar]
- 40.Moriya R, Shirakura T, Hirose H, et al. NPY Y2 receptor agonist PYY(3-36) inhibits diarrhea by reducing intestinal fluid secretion and slowing colonic transit in mice. Peptides. 2010;31:671–675. doi: 10.1016/j.peptides.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Han B. Correlation between gastrointestinal hormones and anxiety-depressive states in irritable bowel syndrome. Exp Ther Med. 2013;6:715–720. doi: 10.3892/etm.2013.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Yan Y, Shi R, et al. Correlation of gut hormones with irritable bowel syndrome. Digestion. 2008;78:72–76. doi: 10.1159/000165352. [DOI] [PubMed] [Google Scholar]
- 43.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 44.Camilleri M, Carlson P, et al. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1089–G1098. doi: 10.1152/ajpgi.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linaker BD, McKay JS, Higgs NB, et al. Mechanisms of histamine stimulated secretion in rabbit ileal mucosa. Gut. 1981;22:964–970. doi: 10.1136/gut.22.11.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coelho AM, Fioramonti J, Bueno L. Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig Dis Sci. 1998;43:727–737. doi: 10.1023/a:1018853728251. [DOI] [PubMed] [Google Scholar]
- 47.Mobarakeh JI, Sakurada S, Katsuyama S, et al. Role of histamine H(1) receptor in pain perception: a study of the receptor gene knockout mice. Eur J Pharmacol. 2000;391:81–89. doi: 10.1016/s0014-2999(00)00060-1. [DOI] [PubMed] [Google Scholar]
- 48.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 49.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 51.van Wanrooij S, Wouters MM, Van Oudenhove L, et al. Effect of the H1-receptor antagonist ebastin on visceral perception and clinical symptoms in IBS. Gastroenterology. 2013;144(Suppl 1):S160. [Google Scholar]
- 52.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 53.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: A pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 55.Gullikson GW, Cline WS, Lorenzsonn V, et al. Effects of anionic surfactants on hamster small intestinal membrane structure and function: relationship to surface activity. Gastroenterology. 1977;73:501–511. [PubMed] [Google Scholar]
- 56.Alrefai WA, Saksena S, Tyagi S, et al. Taurodeoxycholate modulates apical Cl-/OH- exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 57.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 58.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieu T, Jayaweera G, Bunnett NW. Gpba: A G protein-coupled receptor for bile acids and an emerging therapeutic target for disorders of digestion and sensation. Br J Pharmacol. 2013 Sep 24; doi: 10.1111/bph.12426. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wedlake L, A’Hern R, Russell D, et al. Systematic Review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 62.Camilleri M, Busciglio I, Acosta A, et al. Pathophysiology in diarrhea-predominant irritable bowel syndrome: effect of increased bile acid synthesis or excretion. Gastroenterology. (abstract, in press) [Google Scholar]
- 63.Wingate DL, Phillips SF, Hofmann AF. Effect of glycine-conjugated bile acids with and without lecithin on water and glucose absorption in perfused human jejunum. J Clin Invest. 1973;52:1230–1236. doi: 10.1172/JCI107290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broor SL, Slota T, Ammon HV. Cholesterol reduces the effects of dihydroxy bile acids and fatty acids on water and solute transport in the human jejunum. J Clin Invest. 1980;65:920–925. doi: 10.1172/JCI109746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oddsson E, Rask-Madsen J, Krag E. A secretory epithelium of the small intestine with increased sensitivity to bile acids in irritable bowel syndrome associated with diarrhoea. Scand J Gastroenterol. 1978;13:408–416. [PubMed] [Google Scholar]
- 66.Hov JR, Keitel V, Laerdahl JK, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Camilleri M, Shin A, Busciglio I, et al. GPBAR1 genotype predicts bowel function and pathophysiology in diarrhea-predominant irritable bowel syndrome. Gastroenterology. (abstract, in press) [Google Scholar]
- 68.Stephen AM, Haddad AC, Phillips SF. Passage of carbohydrate into the colon. Direct measurements in humans Gastroenterology. 1983;85:589–595. [PubMed] [Google Scholar]
- 69.Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 70.Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 71.Mitsui R, Ono S, Karaki S, et al. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 72.Karaki S, Kuwahara A. Propionate-induced epithelial K(+) and Cl(-)/HCO3(-) secretion and free fatty acid receptor 2 (FFA2, GPR43) expression in the guinea pig distal colon. Pflugers Arch. 2011;461:141–152. doi: 10.1007/s00424-010-0889-y. [DOI] [PubMed] [Google Scholar]
- 73.Musch MW, Bookstein C, Xie Y, et al. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G687–G693. doi: 10.1152/ajpgi.2001.280.4.G687. [DOI] [PubMed] [Google Scholar]
- 74.Treem WR, Ahsan N, Kastoff G, et al. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 75.Fiskerstrand T, Arshad N, Haukanes BI, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 76.Romi H, Cohen I, Landau D, et al. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012;90:893–899. doi: 10.1016/j.ajhg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Aspects Med. 2013;34:236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pieroni KP1, Bass D. Proton pump inhibitor treatment for congenital chloride diarrhea. Dig Dis Sci. 2011;56:673–676. doi: 10.1007/s10620-010-1491-z. [DOI] [PubMed] [Google Scholar]
- 79.Binder HJ. Causes of chronic diarrhea. N Engl J Med. 2006;355:236–239. doi: 10.1056/NEJMp068124. [DOI] [PubMed] [Google Scholar]
- 80.Lok KH, Hung HG, Li KK, et al. Congenital chloride diarrhea: a missed diagnosis in an adult patient. Am J Gastroenterol. 2007;102:1328–1329. doi: 10.1111/j.1572-0241.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 81.Martínez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 82.Martínez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736–746. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1262–G1269. doi: 10.1152/ajpgi.00294.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lamprecht G, Seidler U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol. 2006;291:G766–G777. doi: 10.1152/ajpgi.00135.2006. [DOI] [PubMed] [Google Scholar]