Abstract
Background
Several clinical variables, such as tumor stage and age, are well-established factors associated with long-term survival following surgical resection of lung cancer. Our aim was to examine the impact of other clinical and demographic variables, controlling for known predictors of long-term survival, in order to investigate how outcomes varied according to important non-clinical factors.
Study Design
The National Cancer Data Base, jointly supported by the Commission on Cancer of the American College of Surgeons and the American Cancer Society, was utilized to identify patients undergoing pulmonary resection for lung cancer and perform a retrospective cohort study. The cohort consisted of patients diagnosed with NSCLC from 2003–2006 who underwent resection, as overall survival data are available only for patients diagnosed through 2006. A Cox proportional hazards survival model was used to examine factors associated with risk of mortality.
Results
A total of 92,929 patients were identified as diagnosed during the study period and undergoing surgical resection for lung cancer. On multivariable analysis, several socioeconomic factors such as lack of insurance, lower income, less education, and treatment at community centers vs. academic/research programs predicted worse overall survival after controlling for disease characteristics known to be predictors of worse survival, such as tumor stage, histology, age, and extent of resection.
Conclusions
Diminished long-term survival after pulmonary resection was associated with a number of socioeconomic factors. To date, this represents the largest database analysis of long-term mortality in patients undergoing surgical resection for lung cancer. The disparities in survival outcomes reported here require further, detailed investigation.
Introduction
Long term survival after surgical treatment for non-small cell lung cancer (NSCLC) has been previously associated with several clinical variables. Well-established factors include tumor stage, lymph node (LN) metastases, distant metastases, histology, tumor grade, sex, and age (1–5). Beyond tumor and patient characteristics, treatment parameters such as completeness of surgical resection, lobar vs. sublobar resection, number of LNs sampled and the use of adjuvant and neoadjuvant chemoradiotherapy, in appropriate clinical settings, have all been associated with survival (5–13). Non-clinical demographic variables such as race, income, and insurance status have been analyzed in a variety of other cancers, such as breast, colon, and gastric cancers, and have been shown to have significant impact on survival (14–16).
Limited knowledge exists in regards to how survival following surgical treatment of lung cancer varies according to important non-clinical factors. In order to continue improving and standardizing quality of care and survival in NSCLC patients, it will be important to identify and address whether similar disparities exist in the treatment of lung cancer patients. Further, few large nationwide retrospective database analyses have been published examining risk factors for survival after resection for NSCLC. Our aim was to examine the impact of clinical and demographic variables that have had limited examination on long-term survival after surgical resection for NSCLC, controlling for known predictors, using the National Cancer Data Base.
Methods
We performed a retrospective cohort study using the National Cancer Data Base (NCDB) to assess risk factors for overall mortality after pulmonary resection for NSCLC only. The NCDB is a joint endeavor of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society that includes registry-level clinical and demographic detail on patients treated at approximately 1,500 CoC-approved hospitals across the country beginning in 1989. Patients diagnosed between 2003–2006 who underwent resection were included as Charlson comorbidity indexes are available only after 2003 and long-term survival data were not yet available for cases diagnosed after 2006. Institutional review board (IRB) approval was waived by the Emory University IRB as the NCDB files are de-identified in regards to both patients and facilities.
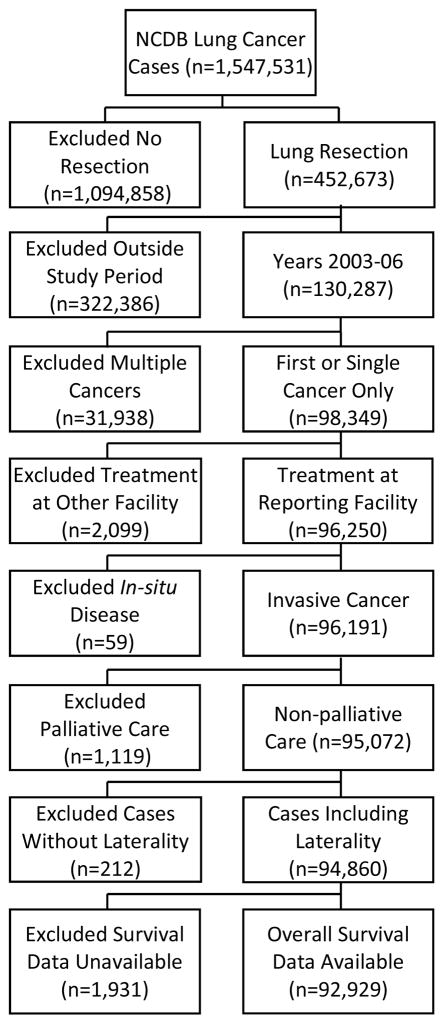
Cases were identified using the NSCLC Participant Use Data file (PUF) from the NCDB. Among all NSCLC cancer patients diagnosed from 2003 through 2006 who then underwent resection in the dataset, the following exclusions were made: cases where the diagnosis was at the reporting facility and all treatment or a decision not to treat was done elsewhere as data would be incomplete, cases with cancer in-situ, patients receiving palliative care, cases where laterality was unknown, and cases without survival information. Only cases with one lifetime cancer or cases where the reported tumor was the first of multiple cancer diagnoses were included in order to avoid confounding with a prior cancer treatment or diagnosis. The patient selection algorithm is shown in Figure 1. For further comparison with resected patients, descriptive statistics were gathered for unresected patients including those treated with palliative intent.
Figure 1.
Patient selection algorithm.
The primary outcome measure was overall survival, defined as the number of months between the most definitive surgical procedure on the primary site and the last contact or date of death. Patient demographics, clinical characteristics, and treatment characteristics included as covariates in the analysis are listed in Table 1. Race/ethnicity was defined using five categories: non-Hispanic white; non-Hispanic black; Asian, which included Pacific Islanders and Asian Indians; Hispanic; and all other. Income and education were estimated by matching the patient’s zip code to US Census data. Education was defined as the percentage of adults not graduating high school and income was defined as the median household income. Insurance was categorized as none, private, or government (which included Medicare, Medicaid, and other government-sponsored insurance). The facility type was determined by Commission on Cancer accreditation level and was based on types of services provided, and case volume (17). Community cancer programs treat between 100 and 500 cancer cases per year, comprehensive community cancer programs treat over 500 cancer cases, and academic/research programs (that include National Cancer Institute-designated cancer centers) treat over 500 cancer cases, in addition to providing postgraduate medical education. Analytic cancer stage was reported as pathologic stage when available or clinical stage when pathologic staging data was absent.
Table 1.
Descriptive Statistics
| Variable | Surgical resection, n=92,929, n (%) | No resection* (%) n=234,870 | p Value |
|---|---|---|---|
| Facility type | |||
| Academic/research program | 31,492 (33.9) | 63,241 (26.9) | < 0.001 |
| Comprehensive community cancer program | 52,097 (56.1) | 135,826 (57.8) | |
| Community cancer program/other | 9,340 (10.0) | 35,803 (15.2) | |
| Sex | |||
| Male | 46,653 (50.2) | 132,492 (56.4) | < 0.001 |
| Female | 46,276 (49.8) | 102,378 (43.6) | |
| Race/ethnicity | |||
| White | 72,904 (87.3) | 171,637 (81.5) | < 0.001 |
| Black | 6,513 (7.8) | 26,690 (12.7) | |
| Asian | 1,555 (1.9) | 4,654 (2.2) | |
| Hispanic | 2,147 (2.6) | 6,820 (3.2) | |
| Other | 368 (0.4) | 878 (0.42) | |
| Age, y, mean ± SD | 66.0 ± 10.33 | 67.4 ± 11.5 | < 0.001 |
| Insurance | |||
| Not Insured | 1712 (1.9) | 9960 (4.4) | < 0.001 |
| Govt. Insurance | 55585 (61.1) | 150594 (66.0) | |
| Private Insurance | 33688 (37.0) | 67532 (29.6) | |
| Income | |||
| < $30,000 | 12473 (14.2) | 39527 (17.7) | < 0.001 |
| $30,000 – $34,999 | 17241 (19.6) | 46614 (20.8) | |
| $35,000 – $45,999 | 25324 (28.8) | 64623 (28.9) | |
| $46,000 + | 32995 (37.5) | 73117 (32.7) | |
| Education, % | |||
| >=29 | 14869 (16.9) | 46535 (20.8) | < 0.001 |
| 20–28.9 | 21931 (24.9) | 58512 (26.1) | |
| 14–19.9 | 21778 (24.7) | 54076 (24.2) | |
| < 14 | 29450 (33.5) | 64741 (28.9) | |
| Urban/rural setting | |||
| Metro area | 69948 (80.0) | 178634 (80.2) | 0.179 |
| Urban | 15425 (17.6) | 38797 (17.4) | |
| Rural | 2040 (2.3) | 5356 (2.4) | |
| Charlson/Deyo Score | |||
| 2+ | 10366 (11.2) | 24027 (10.2) | < 0.001 |
| 1 | 31811 (34.2) | 56904 (24.2) | |
| 0 | 50752 (54.6) | 153939 (65.5) | |
| Year of diagnosis | |||
| 2003 | 22671 (24.4) | 58730 (25.0) | < 0.001 |
| 2004 | 22538 (24.3) | 58273 (24.8) | |
| 2005 | 23772 (25.6) | 58789 (25.0) | |
| 2006 | 23948 (25.8) | 59069 (25.2) | |
| Analytic stage | |||
| I | 54350 (62.9) | 17449 (8.3) | < 0.001 |
| II | 14137 (16.4) | 8096 (3.8) | |
| III | 13830 (16.0) | 67527 (32.0) | |
| IV | 4023 (4.7) | 117956 (56.0) | |
| Radiation before surgery | |||
| No | 87756 (95.7) | N/A | |
| Yes | 3953 (4.3) | ||
| Primary tumor site | |||
| Tracheobronchial tree or hilum | 958 (1.0) | 12877 (5.5) | < 0.001 |
| Left upper lobe | 24509 (26.4) | 49063 (20.9) | |
| Left lower lobe | 12768 (13.7) | 23362 (10.0) | |
| Right upper lobe | 30144 (32.4) | 65031 (27.7) | |
| Right middle lobe | 4892 (5.3) | 8851 (3.8) | |
| Right lower lobe | 15443 (16.6) | 29675 (12.6) | |
| Overlapping lesion | 1911 (2.1) | 4172 (1.8) | |
| Not specified | 2304 (2.5) | 41839 (17.8) | |
| Histology | |||
| Unknown | 6984 (7.5) | 73506 (31.3) | < 0.001 |
| Other tumors† | 5663 (6.1) | 4859 (2.1) | |
| Large cell | 4496 (4.8) | 12398 (5.3) | |
| Squamous cell | 26511 (28.5) | 56002 (23.8) | |
| Adenosquamous | 2466 (2.7) | 1556 (0.7) | |
| Adenocarcinoma | 46809 (50.4) | 86540 (36.9) | |
| Grade | |||
| 1 | 10290 (11.1) | 5721 (2.4) | < 0.001 |
| 2 | 34440 (37.1) | 25157 (10.7) | |
| 3 | 34676 (37.3) | 69606 (29.6) | |
| 4 | 2732 (2.9) | 6255 (2.7) | |
| Unknown | 10791 (11.6) | 128131 (54.6) | |
| Tumor size, cm, mean ± SD | 3.42 ± 3.55 | 4.96 ± 5.16 | < 0.001 |
| Surgical procedure | |||
| Pneumonectomy | 7328 (7.9) | N/A | |
| Lobectomy | 69604 (74.9) | ||
| Segmental resection | 2520 (2.7) | ||
| Wedge resection | 13477 (14.5) | ||
| Positive surgical margins | |||
| Yes | 6373 (7.1) | N/A | |
| No | 83958 (92.9) | ||
| Scope of regional lymph node surgery | |||
| No regional lymph node surgery | 9877 (10.7) | N/A | |
| Regional lymph node surgery | 82573 (89.3) | ||
| Regional lymph node positive | |||
| No | 69656 (75.5) | N/A | |
| Yes | 22655 (24.5) | ||
| Mean | 0.63 ± 1.8 | ||
| Regional lymph node examined | |||
| 0–3 | 25676 (30.7) | N/A | |
| 4–6 | 17994 (21.5) | ||
| 7–9 | 14044 (16.8) | ||
| >9 | 25843 (30.9) | ||
| Mean | 7.84 ± 7.43 |
No resection cohort includes patients who received palliative care.
Including, but not restricted to spindle cell carcinoma, mucoepidermoid malignancies, neuroendocrine, and mixed malignant tumors.
LN, lymph node.
Statistical analysis was conducted using SAS Version 9.3. Descriptive statistics for each variable were reported. Kaplan-Meier overall survival curves were generated stratified by clinical and pathologic stage group with the available staging data. Additionally, Kaplan-Meier survival curves were generated stratifying for socioeconomic risk factors for worsened survival in Stage I patients, including treatment facility, insurance, education, and income. Univariate survival analysis for each variable was carried out using the Cox proportional hazards model. A multivariable Cox model was fit. All covariates were entered into the model subject to a backward variable selection method using an alpha=.20 criteria for removal from the model. As a result, urban/rural setting was removed from the model. To assess differences between the surgical resection and non-resection population, ANOVA for numerical covariates and the chi-square test for categorical covariates were conducted.
Results
Sample Descriptive Statistics
A total of 92,929 patients were identified in the NCDB who were diagnosed between 2003 and 2006 and underwent pulmonary resection for non-small cell lung cancer, and met inclusion criteria. The demographics and clinical details of patients at the time of hospital admission for pulmonary resection are summarized in Table 1. These patients were predominantly Caucasians (87%). The most common presentation of lung cancer was stage I (62.9%) tumor located in the right upper (32.4%) or left upper lobe (26.4%). Four percent of patients received neoadjuvant radiation. More than half of the patients had a Charlson/Deyo comorbidity score of zero. The majority of patients underwent lobectomy (74.9%), while 2.7% of patients underwent segmentectomy and 14.5% underwent a wedge/less than one lobe resection. Nearly 90% of patients had some form of lymph node surgery; however, 30.7% of patients had 3 or less lymph nodes examined. The mean number of lymph nodes examined was 7.84, and the mean tumor size was 3.42 cm. Additional demographic information including treatment facility, income, insurance, and education status are as shown. All patients included in the NCDB were treated at Commission on Cancer accredited cancer programs, with 34% treated at academic/research programs including National Cancer Institute-designated cancer centers, 56% at comprehensive community cancer programs, and 10% treated at community or other cancer programs.
Comparison of patients who underwent surgical resection (N=92,929) and those who did not (N=234,870) did show several small, statistically significant differences in regards to baseline demographics (Table 1). The unresected cohort consisted of a higher percentage of men (56.4% vs 50.2%, p < .001), non-Hispanic Black patients (12.7% vs 7.8%, p < .001), patients with government insurance (66.0% vs 61.1%, p < .001), patients from areas with lower income and education, and, surprisingly, lower Charlson comorbidity scores. Additionally, the proportion of unresected patients was higher at community cancer programs (15.2% vs. 10.0%, p < .001). However, further conclusions based on this unadjusted analysis must be tempered by the fact that the proportion of stage IV patients in the unresected cohort was significantly higher (55.9% vs 4.7%, p < .001).
Unadjusted Analysis of Long-Term Survival in Surgically Resected Patients
Median overall survival and Kaplan-Meier survival estimates by both preoperative clinical and postoperative pathologic staging are shown in Supplemental Figures 1 and 2 (online only), respectively. Clinical staging data were available for 38,024 patients and pathologic staging was available for 79,825. Median survival time (MST) for stage I was 74.6 and 80.3 months for clinical and pathologic staging, respectively; 40.3 and 41.3 months for stage II; 31 and 28.9 months for stage III; and 16.3 and 17.2 months for stage IV NSCLC. Five year overall survival rates for clinical and pathologic staging were as follows, 57.2% and 60.5% for stage I, 40.6% and 40.4% for stage II, 34.7% and 31.2% for stage III, and finally 20.3% and 20.1% for stage IV NSCLC.
A univariate analysis of risk factors for long-term mortality after pulmonary resection was performed and is shown in Supplemental Table 1 (online only). Patients with tumors of higher stage, higher grade, positive lymph nodes, positive surgical margins, male gender, need for pneumonectomy vs. wedge resection/less than one lobe, squamous or large cell histology vs. adenocarcinomas, higher comorbidity index, larger tumor size, and older age all had higher hazards of death. Patients of Hispanic (HR 0.90; 95% CI 0.85–0.96) or Asian (HR 0.80; 95% CI 0.74–0.86) descent had improved survival when compared with non-Hispanic white patients.
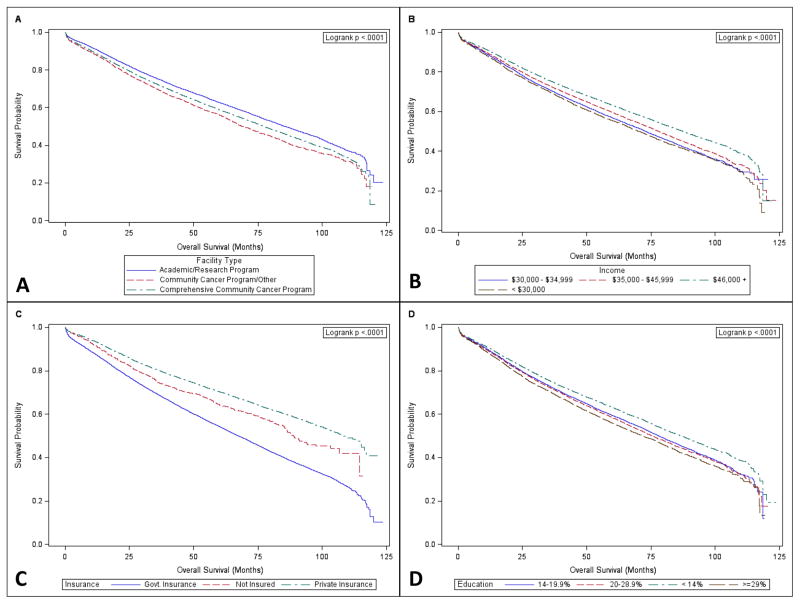
Treatment at an academic/research program (HR 0.79; 95% CI 0.77–0.82) or a comprehensive community cancer program (HR 0.86; 95% CI 0.84–0.89) was associated with better long-term survival, compared with a community or other cancer program. Additionally, patients with government (HR 1.55; 95% CI 1.52–1.58) and no insurance (HR 1.31; 95% CI 1.23–1.41) had worse survival when compared to those with private insurance. Patients living in lower income areas and in areas with lower education rates had worse survival. Risk reduced with a higher number of lymph nodes examined. Kaplan-Meier curves for stage I patients stratified by non-clinical factors having the largest impact on survival, including treatment facility, income, insurance, and education, are shown in Figure 2. These analyses confirmed improved survival with treatment at academic/research programs and comprehensive community cancer programs, with higher incomes, with higher levels of education, and particularly in patients with private insurance.
Figure 2.
Differences in survival for stage I patients after surgical resection for NSCLC by (A) treatment facility type, (B) income, (C) insurance, and (D) education.
Multivariable Analysis of Long-Term Survival
The multivariable survival analysis is shown in Table 2. Risk factors for worse survival are similar to those identified in the univariate models. Community cancer programs compared to comprehensive community programs and academic/research programs, male gender, older age, government or no insurance compared to private insurance, and patients living in lower income areas or areas with lower education rates were all associated with worse survival, when controlling for other well established clinical/pathologic risk factors such as tumor stage, grade, histology, need for pneumonectomy, and lymph node metastases. Our analysis confirmed improved survival after lobectomy (HR 0.86; 95% CI 0.83–0.89) compared with wedge resection when controlling for other risk factors, but not for segmentectomy (0.95, 95% CI 0.89–1.02). Lack of regional LN surgery (HR 1.20; 95% CI 1.15–1.25) and the presence of LN metastases (HR 1.39; 95% CI 1.34–1.45) were both associated with worse survival. A positive surgical margin was associated with a hazard ratio of 1.54 (95% CI 1.48–1.6). Additionally, survival improved with examination of a higher number of LNs. Finally, no difference was noted between non-Hispanic white and black patients, while the survival benefit of Hispanic ethnicity or Asian race persisted on multivariable analysis.
Table 2.
Multivariable Analysis of Overall Survival after Surgical Therapy for Lung Cancer
| Overall survival, mo | ||||
|---|---|---|---|---|
| Covariate | Hazard Ratio | 95% CI | HR, p value | Type 3, p value |
| Facility type | ||||
| Academic/research program | 0. 90 | 0.86 – 0.93 | < 0.001 | < 0.001 |
| Comprehensive community cancer program | 0.93 | 0.90 – 0.96 | < 0.001 | |
| Community cancer program/other | - | - | - | |
| Sex | ||||
| Male | 1.31 | 1.28 – 1.34 | < 0.001 | < 0.001 |
| Female | - | - | - | |
| Race/ethnicity | ||||
| Other | 0.95 | 0.80 – 1.13 | 0.564 | < 0.001 |
| Hispanic | 0.89 | 0.83 – 0.95 | 0.001 | |
| Asian | 0.76 | 0.70 – 0.83 | < 0.001 | |
| Black | 1.01 | 0.97 – 1.05 | 0.635 | |
| White | - | - | - | |
| Age, per year | 1.03 | 1.03 – 1.03 | < 0.001 | < 0.001 |
| Insurance | ||||
| Not insured | 1.23 | 1.13 – 1.34 | <. 001 | < 0.001 |
| Government insurance | 1.15 | 1.11 – 1.18 | <. 001 | |
| Private insurance | - | - | - | |
| Income | ||||
| < $30,000 | 1.08 | 1.03 – 1.13 | < 0.001 | < 0.001 |
| $30,000 – $34,999 | 1.07 | 1.03 – 1.11 | < 0.001 | |
| $35,000 – $45,999 | 1.05 | 1.02 – 1.09 | < 0.001 | |
| $46,000 + | - | - | - | |
| Education, % | ||||
| >=29 | 1.11 | 1.06 – 1.16 | < 0.001 | < 0.001 |
| 20–28.9 | 1.06 | 1.02 – 1.09 | 0 .002 | |
| 14–19.9 | 1.05 | 1.02 – 1.08 | 0 .004 | |
| < 14 | - | - | - | |
| Charlson/Deyo score | ||||
| 2+ | 1.36 | 1.32 – 1.41 | < 0.001 | < 0.001 |
| 1 | 1.13 | 1.10 – 1.15 | < 0.001 | |
| 0 | - | - | - | |
| Analytic stage | ||||
| IV | 2.54 | 2.41–2.67 | < 0.001 | < 0.001 |
| III | 1.66 | 1.60–1.73 | < 0.001 | |
| II | 1.30 | 1.25–1.36 | < 0.001 | |
| I | - | - | - | |
| Radiation before surgery | ||||
| Yes | 1.30 | 1.23 – 1.38 | < 0.001 | < 0.001 |
| No | - | - | - | |
| Primary tumor site | ||||
| Tracheobronchial tree or hilum | 0.88 | 0.78–1.00 | 0.043 | < 0.001 |
| Left upper lobe | 1.02 | 0.99–1.05 | 0.237 | |
| Left lower lobe | 1.09 | 1.05–1.13 | < 0.001 | |
| Right middle lobe | 0.98 | 0.93–1.03 | 0.410 | |
| Right lower lobe | 1.13 | 1.13–1.17 | < 0.001 | |
| Overlapping lesion | 1.19 | 1.11–1.28 | < 0.001 | |
| Not specified | 1.09 | 1.02–1.18 | 0.028 | |
| Right upper lobe | - | - | - | |
| Histology | ||||
| Unknown histology | 1.04 | 0.99–1.08 | 0.101 | < 0.001 |
| Other tumors† | 0.79 | 0.73–0.86 | < 0.001 | |
| Large cell | 1.20 | 1.13–1.27 | < 0.001 | |
| Squamous cell | 1.05 | 1.02–1.08 | < 0.001 | |
| Adenosquamous | 1.09 | 1.03–1.16 | 0.006 | |
| Adenocarcinoma | - | - | - | |
| Grade | ||||
| Unknown | 1.26 | 1.19–1.33 | < 0.001 | < 0.001 |
| 4 | 1.51 | 1.39–1.64 | < 0.001 | |
| 3 | 1.50 | 1.43–1.56 | < 0.001 | |
| 2 | 1.34 | 1.29–1.40 | < 0.001 | |
| 1 | - | - | - | |
| Tumor size, cm | 1.02 | 1.02–1.02 | <0.001 | < 0.001 |
| Surgical procedure | ||||
| Pneumonectomy | 1.10 | 1.05–1.17 | < 0.001 | < 0.001 |
| Lobectomy | 0.86 | 0.83–0.89 | < 0.001 | |
| Segmental resection, including lingulectomy | 0.95 | 0.89–1.02 | 0.166 | |
| Wedge resection/less than one lobe | - | - | - | |
| Positive surgical margins | ||||
| Yes | 1.54 | 1.48–1.60 | < 0.001 | < 0.001 |
| No | - | - | - | |
| Scope of regional LN surgery | ||||
| No regional LN surgery | 1.20 | 1.15–1.25 | < 0.001 | < 0.001 |
| Regional LN surgery | - | - | - | |
| Regional LN positive | ||||
| Yes | 1.39 | 1.34–1.45 | < 0.001 | < 0.001 |
| No | - | - | - | |
| Regional LN examined | ||||
| >9 | 0.84 | 0.81–0.86 | < 0.001 | < 0.001 |
| 7–9 | 0.88 | 0.85–0.91 | < 0.001 | |
| 4–6 | 0.91 | 0.88–0.94 | < 0.001 | |
| 0–3 | - | - | - | |
Including, but not restricted to spindle cell carcinoma, mucoepidermoid malignancies, neuroendocrine, and mixed malignant tumors.
LN, lymph node.
Treatment Characteristics by Facility Type
Given the identified differences in survival based on socioeconomic factors and treatment facility, we further examined treatment differences based on treatment facility (Table 3). No clinically significant differences were identified in baseline demographics and socioeconomic factors in patients treated at each facility type other than a slightly higher incidence of patients with Stage 1 disease treated at comprehensive community cancer programs (64.1% vs 61.8% at community programs and 61.3% at academic/research programs, p < .001). A small (1%) statistically significant difference was noted in incidence of wedge resections performed at community cancer programs, however again no clinically significant difference was identified. A significant difference was noted, though, in performance of regional LN resection and total number of LN examined, with patients at academic and comprehensive community programs significantly more likely to have both any LN dissection (90.3% and 89.4%, respectively vs. 85.8% at community programs) as well as to have a greater number of LN examined. Despite this, minimal, thought statistically significant, differences were noted in likelihood of having positive regional LNs identified (25.7 vs. 23.8 vs. 24.8% respectively, p < .001).
Table 3.
Treatment Characteristics by Facility Type
| Covariate | Community cancer program, n=9,340 | Comprehensive community cancer program, n=52,097 | Academic/research program, n=31,492 | p Value |
|---|---|---|---|---|
| NCDB analytic stage group | ||||
| 0–1 | 5471 (61.8) | 31029 (64.1) | 17850 (61.3) | < 0.001 |
| II | 1506 (17.0) | 7999 (16.5) | 4632 (15.9) | |
| III | 1425 (16.1) | 7317 (15.1) | 5088 (17.5) | |
| IV | 453 (5.1) | 2042 (4.2) | 1528 (5.3) | |
| Surgical procedure | ||||
| Wedge resection | 1464 (15.7) | 7428 (14.3) | 4585 (14.6) | < 0.001 |
| Segmental resection | 267 (2.9) | 1283 (2.5) | 970 (3.1) | |
| Lobectomy | 6823 (73.1) | 39434 (75.7) | 23347 (74.1) | |
| Pneumonectomy | 786 (8.4) | 3952 (7.6) | 2590 (8.2) | |
| Positive surgical margins | ||||
| No | 8125 (91.0) | 47017 (92.7) | 28816 (93.9) | < 0.001 |
| Yes | 806 (9.0) | 3684 (7.3) | 1883 (6.1) | |
| Scope of regional LN surgery | ||||
| No regional LN surgery | 1315 (14.2) | 5516 (10.6) | 3046 (9.7) | < 0.001 |
| Regional LN surgery | 7934 (85.8) | 46327 (89.4) | 28312 (90.3) | |
| Radiation before surgery | ||||
| No | 8875 (96.5) | 49378 (96.1) | 29503 (94.8) | < 0.001 |
| Yes | 319 (3.5) | 2018 (3.9) | 1616 (5.2) | |
| Regional LN positive | ||||
| No | 6949 (75.2) | 39438 (76.2) | 23269 (74.3) | < 0.001 |
| Yes | 2292 (24.8) | 12317 (23.8) | 8046 (25.7) | |
| Regional LN examined | ||||
| 0–3 | 3256 (39.8) | 15250 (32.8) | 7170 (24.9) | < 0.001 |
| 4–6 | 1768 (21.6) | 10733 (23.1) | 5493 (19.1) | |
| 7–9 | 1250 (15.3) | 8004 (17.2) | 4790 (16.6) | |
| >9 | 1907 (23.3) | 12578 (27.0) | 11358 (39.4) |
LN, lymph node.
Discussion
Despite improvements in diagnosis, cancer care, and operative care of NSCLC, survival rates remain poor and have shown little improvement. As a result, lung cancer continues to be the leading cause of cancer related death in the United States (18). Several studies have examined clinical risk factors for worse long term survival in early stage and surgically resected disease (1, 2, 5, 6). Our aim was to examine socioeconomic risk factors for worsened survival after controlling for these previously identified clinical risk factors. To this end we utilized the National Cancer Data Base, a nationwide oncology outcomes database which captures 70% of new cancer diagnoses in the United States (19). To date, this represents the largest national retrospective database analysis of surgically resected NSCLC that examines long-term survival following treatment. Analysis of the NCDB confirmed association with worse survival of these previously identified clinical factors, including age, tumor stage, squamous, adenosquamous, or large cell histology, higher grade, lower lobe location, lymph node status, postoperative margin status, surgical procedure, and the need for preoperative radiation (Table 2). In addition, the results of our analysis found that several disparities exist in the treatment of lung cancer. After controlling for these clinical factors, several demographic and socioeconomic characteristics were identified as being associated with worse survival. These included male gender, less education, lower income, government and no insurance, and treatment at non-comprehensive community cancer programs.
Using the NCDB, we analyzed overall survival while controlling for 20 possible confounding variables (Table 1). Overall stage-specific survival by both clinical and pathologic stage, as shown in Supplemental Figures 1 and 2 (online only), was consistent with previously published literature (1). Mean survival time (MST) by pathologic stage reported by the International Association for the Study of Lung Cancer (IASLC) 7th edition staging project were 119 and 81 months for stage Ia and Ib, 49 and 31 months for stage IIa and IIb, 22 and 13 months for stage IIIa and IIIb, and 17 months for stage IV. These results were comparable to survival by pathologic stage in our study (Supplemental Figure 2, online only). By clinical stage, however, MST was better in our study, with IASLC results showing MST of 60 and 43 months for stage Ia and Ib, 34 and 18 months for stage IIa and IIB, 14 and 10 months for stage IIIa and IIIb, and 6 months for stage IV (Supplemental Figure 1, online only). This likely reflects the fact that patients undergoing all treatment modalities, including non-surgical modalities, were included in the IASLC analysis, whereas this study includes only patients undergoing surgical resection. Further, any patients with early clinical stage not undergoing surgical resection in the IASLC analysis were likely medically unfit for surgery and would have other competing causes of death, resulting in lower earlier stage survival by clinical stage.
After controlling for clinical characteristics, the goal of this study was to identify additional key demographic and socioeconomic risk factors for mortality from NSCLC. Previous analyses of national, regional, and institutional databases have confirmed gender, age, and variably race as risk factors for worse survival (4, 20–25). Fu, et al., have previously shown a rising incidence of lung cancer in women, along with better overall survival rates for women at all stages of cancer using the Surveillance, Epidemiology, and End Results (SEER) database (4). Tong, et al., have recently reported lower perioperative mortality (HR 0.56) in women undergoing pulmonary resection in the Society of Thoracic Surgeons (STS) database (20). Our analysis confirmed these results, with male gender associated with a higher hazard of death after controlling for other risk factors. Similarly, increased age, a well-established risk factor for worse survival, was associated with increased risk.
The association between race/ethnicity and survival with lung cancer has been more variable in the literature. Analysis of the SEER database by Bach, et al., has shown 5–10% worse overall survival for black patients with early stage NSCLC (21). However, black patients were significantly less likely to undergo surgical resection. When looking at patients only undergoing surgery, no difference was identified in this analysis. Further analysis of the SEER database looking at all patients with NSCLC found better overall survival in Hispanic patients (HR 0.85) and slightly worse survival in black patients (HR 1.091) (22). Analysis of the California Cancer Registry in stage I patients has shown improved survival in patients of Asian descent, but no difference in survival between all other races after adjusting for socioeconomic status and rates of surgical resection (25). Similarly, in a study of 500 patients, Aldrich, et al., found that while black patients were more likely to be diagnosed at a later stage of disease, no stage-specific survival difference was seen between black and white patients in an underserved population (23). Finally, in a single institutional study of 900 patients, Bryant, et al., reported that stage specific survival was higher in white patients. However, after controlling for socioeconomic status, smoking, and neoadjuvant chemotherapy, this advantage was lost (24). Similar to these previous analyses, our analysis showed no difference in survival between non-Hispanic white and black patients after surgical resection, after controlling for other risk factors, such as income and insurance. Additionally, as only patients who underwent surgical resection were included in our multivariable analysis, this confounding variable was excluded. Interestingly, we did identify a significantly higher proportion of black patients in the unresected group (Table 1), similar to the results of Bach, et al (21). However, as there were a significantly higher proportion of stage IV patients in the unresected group, it is unclear if this difference is secondary to differences at stage of presentation or truly a difference in likelihood of undergoing surgical resection. As a result, this significant confounder would affect any further interpretation of this unadjusted data. Finally, patients of Hispanic ethnicity and Asian race showed improved survival similar to the analyses by Saeed and Ou (22, 25). Overall, these results suggest minimal impact of race and ethnicity on survival after controlling for socioeconomic status and access to care.
Our analysis did confirm a significant impact of socioeconomic status, insurance status, and treatment facility on overall survival as evidenced in the multivariable analysis. Patients with private insurance fared best, while lack of insurance and government insurance were associated with significantly worse survival (Figure 2C). Further, lower income and lower education level, as measured by percent of adults not graduating from high school, were associated with statistically significant worse survival (Figure 2B and 2D). Patient’s residing in an area with a median income less than $30,000 as well as those within an area in which >=29% of residents did not graduate from high school showed the worst survival among income and education levels. These results are similar to those of previously published, smaller studies (25, 26). Analysis of the California Cancer Registry found a statistically significant association between worse survival and lower socioeconomic status, as a composite measure of education, income, and occupation (25). Additional analysis of this same registry found that patients with Medicare (OR=0.87), Medicaid (OR=0.45), or no insurance (OR=0.45) were significantly less likely to undergo lobectomy (27). While this may explain some of the impact of insurance on survival, in our analysis insurance status (no insurance vs. insurance) remained an independent predictor for worse survival even after controlling for surgical procedure. While some small statistically significant differences in regards to socioeconomic factors were identified between patients who did and did not undergo resection, no clinically relevant differences were noted. Further, differences that were identified would again be confounded by differences in staging. Additional analysis is therefore needed to determine if these identified socioeconomic risk factors are reflective of treatment bias and other access to care difficulties.
In addition to these socioeconomic risk factors, several clinical risk factors identified in this analysis merit further discussion. Surgical procedure, specifically in regards to sublobar resection vs lobectomy has been the subject of significant study. While results of these multiple studies are varied, particularly in tumors smaller than 2 cm, most papers conclude worse survival after sublobar resection (5–9). Though this study was not specifically designed to compare sublobar resection with lobectomy, our analysis confirmed improved survival after lobectomy when compared with wedge resection after controlling for other confounding variables including tumor size and LN status. Interestingly, no difference was noted in survival between wedge resection and segmentectomy. Further prospective study of this question is still needed and is currently ongoing. Positive regional LN have also been identified as a major risk factor in previous studies. In our study, the presence of positive LN conferred a significantly worse risk of death. Only positive margins, higher tumor grade, and higher stage were associated with poorer survival. Lack of any LN examination also resulted in worse survival. Further, more lymph nodes examined were associated with improved survival, with the best survival associated with > 9 LN examined. This is similar to results from multiple other authors (10–12). Together, these results lend further evidence for the need for systematic sampling of mediastinal nodes, at a minimum, during pulmonary resection for lung cancer.
Finally, treatment at an academic or comprehensive community cancer program was an independent predictor of better overall survival when compared with a non-comprehensive community cancer program (Figure 2A). To further examine the reason for this, we compared patient characteristics at each type of treatment facility. Interestingly, no clinically significant differences in patient demographics and other identified socioeconomic risk factors were seen. However, patients treated at academic/research programs and comprehensive community cancer programs were more likely to undergo regional LN surgery and were also significantly more likely to have > 9 LN examined (Table 3). Implications of this could include patients at academic/research programs being diagnosed with more advanced stage and thereby more likely to receive appropriate adjuvant chemotherapy. Yet despite these findings, the likelihood of having positive nodes identified was only approximately 1% higher at academic institutions, and is presumably reflected in the only 1% higher incidence of stage III disease at academic institutions. Minimal differences were identified in the type of resection performed between treatment facilities, though we did identify a 3% lower incidence of positive surgical margins at academic programs.
Previous studies of the Nationwide Inpatient Sample and SEER-Medicare databases have established improved perioperative and overall survival in patients with NSCLC treated by general thoracic or cardiothoracic surgeons (28,29). Further, it is well established that long-term survival is improved in patients treated by cancer specialists (30, 31). With over 30% of pulmonary resections for NSCLC in the United States performed by general surgeons, this will be an important risk factor to monitor (29). Unfortunately, data regarding surgeon specialty training was unavailable in the NCDB. Improved survival at academic and comprehensive community cancer programs may therefore be related to surgeon training in addition to facility type and likelihood of having adequate numbers of regional LN sampled, as patients treated at academic and high volume community centers would be more likely to be treated by a board certified thoracic surgeon.
Implications of these findings include possible improved long-term survival at high volume centers. There is a trend towards regionalization of complex surgical procedures, such as hepato-pancreato-biliary cancer surgery (32). However, data regarding lung cancer surgery and its relationship with volume remains conflicted, with minimal improvement on in-hospital mortality at high volume centers (33, 34). Interestingly, regionalization of thoracic surgical practice in Ontario, Canada did result in a 5% reduction in 30 day mortality after pneumonectomy, though no change was seen for lobectomy (35). Further, most studies regarding regionalization have focused on in-hospital or 30 day mortality. Limited data exist regarding improved long-term survival as suggested in this study, which would be affected by both surgical and oncologic care.
Limitations of this analysis include its retrospective observational nature, and as a result, its associated selection biases. Additionally, as there was considerable variability in reporting of clinical vs pathologic staging, it was necessary to use analytic stage for the multivariable analysis. Further, given the very large sample size in this analysis, small differences, which may not be clinically relevant, can still be statistically significant, such as the impact of race. Analysis of the impact of comorbidities on survival is also limited in that specific details regarding medical comorbidities are lacking from the NCDB. The Charlson/Deyo comorbidity index, though, is included as an overall marker of a patient’s medical status. Finally, as long-term survival data was only available on patients diagnosed through 2006 in the NCDB, analysis of a more contemporary cohort could not be performed. On the other hand, strengths of this study include long-term longitudinal follow-up for vital status overall and the large size and nationwide distribution of this database, reflecting care given to nearly 70% of cancer patients in the US. As a result, this analysis reflects the general care of NSCLC patients across the United States. Therefore, these results can be more readily generalized to patients undergoing surgical resection for NSCLC, especially as compared to other retrospective datasets that represent smaller or more homogeneous populations. Finally, with 20 variables included in the multivariable analysis, this study represents the most comprehensive retrospective analysis of national NSCLC data focused on long-term survival post-resection.
In summary, our analysis of the NCDB, the largest retrospective analysis of NSCLC patients undergoing pulmonary resection, identified several risk factors associated with worse overall survival. After controlling for well-established clinical characteristics known to effect long-term survival, our analysis has identified several socioeconomic disparities, including income, education levels, insurance status, and treatment at lower volume community programs, affecting survival after resection as well. The reason for these findings continues to be unclear, however, as minimal differences were identified in surgical management by treatment facility. Additional patient-specific data is needed to elucidate the underlying cause of these identified disparities. With increasing emphasis on survival with NSCLC and lung cancer screening, it will be important to focus future efforts and interventions towards identifying and treating these patients at higher risk for limited long term survival after surgical resection.
Supplementary Material
Acknowledgments
Support: Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Ramalingam is paid as a consultant for Abbvie, Astra Zeneca, Boehringer Ingelheim, Celgene, Novartis, Genentech, Lilly, Gilead, Biodesix, Aveo, and Ariad.
Abstract presented at the American College of Surgeons 100th Annual Clinical Congress, San Francisco, October 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 2.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110:1532–1541. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 4.Fu JB, Kau TY, Severson RK, Kalemkerian GP. Lung cancer in women: analysis of the national surveillance, epidemiology, and end results database. Chest. 2005;127:768–777. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 5.Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg. 2011;92:1943–1950. doi: 10.1016/j.athoracsur.2011.05.091. [DOI] [PubMed] [Google Scholar]
- 6.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest. 2011;139:491–496. doi: 10.1378/chest.09-2547. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 8.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 9.Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg. 2011;92:1819–1823. doi: 10.1016/j.athoracsur.2011.06.099. [DOI] [PubMed] [Google Scholar]
- 10.Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer. 2009;66:365–371. doi: 10.1016/j.lungcan.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg. 2012;93:1614–1619. doi: 10.1016/j.athoracsur.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol. 2008;3:880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 13.Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest. 2011;139:1124–1129. doi: 10.1378/chest.10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Hsu TW, Chang CM, et al. The effect of individual and neighborhood socioeconomic status on gastric cancer survival. PLoS One. 2014;9:e89655. doi: 10.1371/journal.pone.0089655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hole DJ, McArdle CS. Impact of socioeconomic deprivation on outcome after surgery for colorectal cancer. Br J Surg. 2002;89:586–590. doi: 10.1046/j.1365-2168.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- 17.Surgeons ACo. [Accessed June 18, 2014];Commission on Cancer Categories of Accreditation. Available at: http://www.facs.org/cancer/coc/categories3.html#.
- 18.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 19.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong BC, Kosinski AS, Burfeind WR, Jr, et al. Sex differences in early outcomes after lung cancer resection: Analysis of the Society of Thoracic Surgeons General Thoracic Database. J Thorac Cardiovasc Surg. 2014;148:13–18. doi: 10.1016/j.jtcvs.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 22.Saeed AM, Toonkel R, Glassberg MK, et al. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival: an analysis of the Survival, Epidemiology, and End Results database. Cancer. 2012;118:4495–4501. doi: 10.1002/cncr.26686. [DOI] [PubMed] [Google Scholar]
- 23.Aldrich MC, Grogan EL, Munro HM, et al. Stage-adjusted lung cancer survival does not differ between low-income Blacks and Whites. J Thorac Oncol. 2013;8:1248–1254. doi: 10.1097/JTO.0b013e3182a406f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant AS, Cerfolio RJ. Impact of race on outcomes of patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:711–715. doi: 10.1097/JTO.0b013e31817c60c7. [DOI] [PubMed] [Google Scholar]
- 25.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Low socioeconomic status is a poor prognostic factor for survival in stage I nonsmall cell lung cancer and is independent of surgical treatment, race, and marital status. Cancer. 2008;112:2011–2020. doi: 10.1002/cncr.23397. [DOI] [PubMed] [Google Scholar]
- 26.Caposole MZ, Miller K, Kim JN, et al. Elimination of socioeconomic and racial disparities related to lung cancer: Closing the gap at a high volume community cancer center. Surg Oncol. 2014;23:46–52. doi: 10.1016/j.suronc.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groth SS, Al-Refaie WB, Zhong W, et al. Effect of insurance status on the surgical treatment of early-stage non-small cell lung cancer. Ann Thorac Surg. 2013;95:1221–1226. doi: 10.1016/j.athoracsur.2012.10.079. [DOI] [PubMed] [Google Scholar]
- 28.Schipper PH, Diggs BS, Ungerleider RM, Welke KF. The influence of surgeon specialty on outcomes in general thoracic surgery: a national sample 1996 to 2005. Ann Thorac Surg. 2009;88:1566–1572. doi: 10.1016/j.athoracsur.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Farjah F, Flum DR, Varghese TK, Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg. 2009;87:995–1004. doi: 10.1016/j.athoracsur.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 31.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 32.Colavita PD, Tsirline VB, Belyansky I, et al. Regionalization and outcomes of hepato-pancreato-biliary cancer surgery in USA. J Gastrointest Surg. 2014;18:532–541. doi: 10.1007/s11605-014-2454-z. [DOI] [PubMed] [Google Scholar]
- 33.Otake H, Yasunaga H, Horiguchi H, et al. Impact of hospital volume on chest tube duration, length of stay, and mortality after lobectomy. Ann Thorac Surg. 2011;92:1069–1074. doi: 10.1016/j.athoracsur.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 34.Kozower BD, Stukenborg GJ. The relationship between hospital lung cancer resection volume and patient mortality risk. Ann Surg. 2011;254:1032–1037. doi: 10.1097/SLA.0b013e31821d4bdd. [DOI] [PubMed] [Google Scholar]
- 35.Sundaresan S, McLeod R, Irish J, et al. Early results after regionalization of thoracic surgical practice in a single-payer system. Ann Thorac Surg. 2013;95:472–478. doi: 10.1016/j.athoracsur.2012.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.