Abstract
Rationale
Clinical literature suggests a link between substance abuse and sleep disturbances. Quetiapine, an atypical antipsychotic has shown efficacy in treating sleep disturbances, with clinical studies showing promise for quetiapine as a treatment for cocaine abuse.
Objective
The goal of this study was to examine the effects of quetiapine on cocaine self-administration and behavioral indices of sleep in monkeys.
Methods
Seven adult male rhesus monkeys, fitted with Actical® activity monitors, were trained to respond under a choice paradigm of food (1.0-g pellets) and cocaine (0.003–0.3 mg/kg per injection) presentation. First, monkeys received acute pretreatment (45 min) with quetiapine (25–75 mg, p.o.) prior to choice sessions; three cocaine doses were studied in combination with quetiapine. Next, the effect of chronic (14–16 days) quetiapine treatment (25–250 mg, p.o., BID) was examined in combination with the lowest preferred cocaine dose (≥ 80% cocaine choice). Behavioral indices of sleep, based on activity measures obtained during lights-out, were recorded throughout the study.
Results
Acute quetiapine decreased cocaine choice in four of the seven monkeys. Chronic quetiapine treatment resulted in initial decreases, but tolerance developed to these effects. Acute doses of quetiapine did not improve sleep efficiency the following night, nor did chronic quetiapine. The first night after discontinuing quetiapine treatment resulted in significant decreases in sleep efficiency and increases in nighttime activity.
Conclusions
These findings do not offer support for the use of quetiapine as a monotherapy for treatment of cocaine abuse nor as an adjunct therapy to treat sleep disturbances associated with stimulant abuse.
Keywords: quetiapine, cocaine, self-administration, actigraphy, sleep, monkeys
INTRODUCTION
Cocaine dependence represents an important area of concern to healthcare professionals and scientists. At present there are no medically approved treatments for cocaine addiction. Clinical literature suggests a link between substance abuse and sleep disturbances (e.g., Puhl et al. 2009; Morgan et al. 2006) although the direct relationship remains to be elucidated (Volkow et al. 2008, 2012). It has been hypothesized that treating an individual’s sleep disturbances could be beneficial in attenuating physiological consequences of cocaine abstinence (Morgan et al. 2008) and therefore improve the duration of abstinence.
Quetiapine is an atypical, second generation, antipsychotic which has approved labeling from the Food and Drug Administration (FDA) for the treatment of several affective disorders including schizophrenia, acute manic or mixed episodes associated with bipolar I disorder and as an adjunctive treatment of major depression (Borison et al. 1996; Malhi and Berk 2002; Emsley and Oosthuizen 2003; Prieto et al. 2010; FDA 2011; Lexicomp Online 2013). It has been proposed that quetiapine exerts its activity primarily through a combination of DA D2/D3 and serotonin (5-HT) 2 (5-HT2) receptor antagonism, although it acts at multiple neurotransmitter systems (Goldstein, 1999; Reeves and Brister 2007; Riedel et al. 2007; Lexicomp Online 2013). In addition to its proposed efficacy in sleep disturbances and other off-label indications, results from clinical studies have shown promise for quetiapine as a treatment for cocaine abuse (Brown et al. 2002, 2003; Sattar et al. 2004; Kennedy et al. 2008).
While it remains possible that quetiapine will be an effective pharmacotherapy for cocaine addition, previous preclinical studies involving monkeys and antipsychotics as potential treatments for cocaine abuse, including studies involving food-cocaine choice, have reported negative outcomes (e.g., Woods et al. 1978; Woolverton and Balster 1979, 1981; Nader et al. 1999; Negus 2003), consistent with clinical findings (e.g. Kampman et al. 2003). However, not all preclinical studies have been negative. Howell et al. (2006) reported that olanzapine could decrease cocaine self-administration in rhesus monkeys at doses that had not noticeable effects on other behaviors. Thus, the purpose of the present study was to examine the effects of acute and chronic quetiapine, administered via the oral route, in monkeys self-administering cocaine in the context of an alternative non-drug reinforcer. In addition, this self-administration paradigm has been shown to significantly affect sleep efficiency in monkeys (Brutcher and Nader 2013); if quetiapine, unlike other antipsychotics, is effective in decreasing cocaine choice, the mechanism may be due to the ability of quetiapine to influence several behavioral indices of sleep in monkeys. Similarities in sleep architecture between rhesus monkeys and humans make them an excellent model for studying human sleep (Daley et al. 2006).
METHODS
Subjects
Seven adult (age 16–18) male rhesus monkeys (Macaca mulatta) with an extensive behavioral history (Hamilton et al. 2010, 2011) including cocaine self-administration under a concurrent cocaine-food choice paradigm (Brutcher and Nader 2013) served as subjects. Monkeys were weighed weekly and healthy body weights were maintained (determined by veterinary staff and somewhat less than free-feeding weights) by food earned during experimental sessions and by supplemental feeding of LabDiet Monkey Chow and fresh fruit no sooner than 30 minutes after the session; water was available ad libitum in the home cage. Each monkey was fitted with an aluminum collar (Model B008, Primate Products, Redwood City, CA) and trained to sit in a standard primate chair (Primate Products). All experimental manipulations were performed in accordance with the 2011 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Institutional Animal Care and Use Committee. Environmental enrichment was provided as outlined in the Animal Care and Use Committee of Wake Forest University Non-Human Primate Environmental Enrichment Plan.
Surgery
Each monkey was prepared with a chronic indwelling venous catheter into a major vein (femoral, internal or external jugular) and subcutaneous vascular port (Access Technologies, Skokie, IL) using aseptic surgical procedures. Anesthesia was induced with Dexmedetomidine (0.04 mg/kg, i.m.) and ketamine (5 mg/kg, i.m.) and maintained with ketamine (5 mg/kg) as needed. Vital signs were monitored for the duration of the surgery. Briefly, a catheter was inserted into a peripheral vein to the level of the vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was then attached to the vascular access port and placed in a pocket formed by blunt dissection. Anesthesia was reversed using atipamezole (0.2 mg/kg, i.m.). Prior to each self-administration session, the back of the animal was cleaned with betadine and 95% EtOH, and the port was connected to the infusion pump located outside the chamber via a 20-gauge Huber Point Needle (Access Technologies). Prior to the start of the daily experiment, the pump was operated for approximately 3 s to fill the port and catheter with the concentration of cocaine (or saline) available during the session. Each port and catheter was filled with heparinized saline solution (100 U/ml) after every experimental session to prolong catheter patency.
Apparatus
The apparatus for operant responding consisted of a ventilated, sound-attenuating chamber (1.5×0.74×0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. Two photo-optic switches (5 cm wide) were located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each switch and a food receptacle between the switches. Illumination of the white lights above each switch served as discriminative stimuli for each reinforcer (see below); illumination of the red lights occurred during reinforcer presentation. The food receptacle was connected with tygon tubing to a pellet dispenser (Gerbrands Corp., Arlington, MA) located on the top of the chamber for delivery of 1.0-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ). An infusion pump (Cole-Palmer, Inc., Chicago, IL) was located on the top of the chamber.
Experiment 1. Effects of quetiapine on cocaine self-administration
For these studies, food reinforcement (three 1.0-gram banana-flavored pellets) was contingent upon completing the response requirement on one switch, while cocaine (0.003–0.3 mg/kg per injection) was contingent on responding on the other manipulandum. For both reinforcers, a fixed-ratio (FR) 30 schedule of reinforcement was used; switching between manipulanda reset the FR value to 30. There was a 30-sec time-out (TO) after each reinforcer presentation Each session began with a forced trial for each reinforcer. For this, the white discriminative stimulus was illuminated above one switch (randomly determined at the start of each session) and 30 responses resulted in the presentation of either 3 food pellets or a 10-sec infusion of the dose of cocaine available that session. There was a 30-sec TO followed by illumination of the other white discriminative stimulus for completing the FR 30 on that switch. Monkeys always completed the two forced trials prior to the start of each experimental session. Also, following five consecutive same-reinforcer choices there was a forced trial on the opposing switch; this forced trial did not count towards the 30 maximum choice trials. Sessions ended after 30 total reinforcers or 60 min. The same cocaine dose or saline remained constant for at least 5 consecutive sessions and until choice was deemed stable (within 20% for 3 consecutive sessions without trends). Following completion of the entire cocaine dose-response curve, the lowest preferred dose (defined as the lowest cocaine dose that engendered ≥ 80% reinforcers being received on the cocaine-associated switch), based on 3-day means of stable performance, was identified for each monkey.
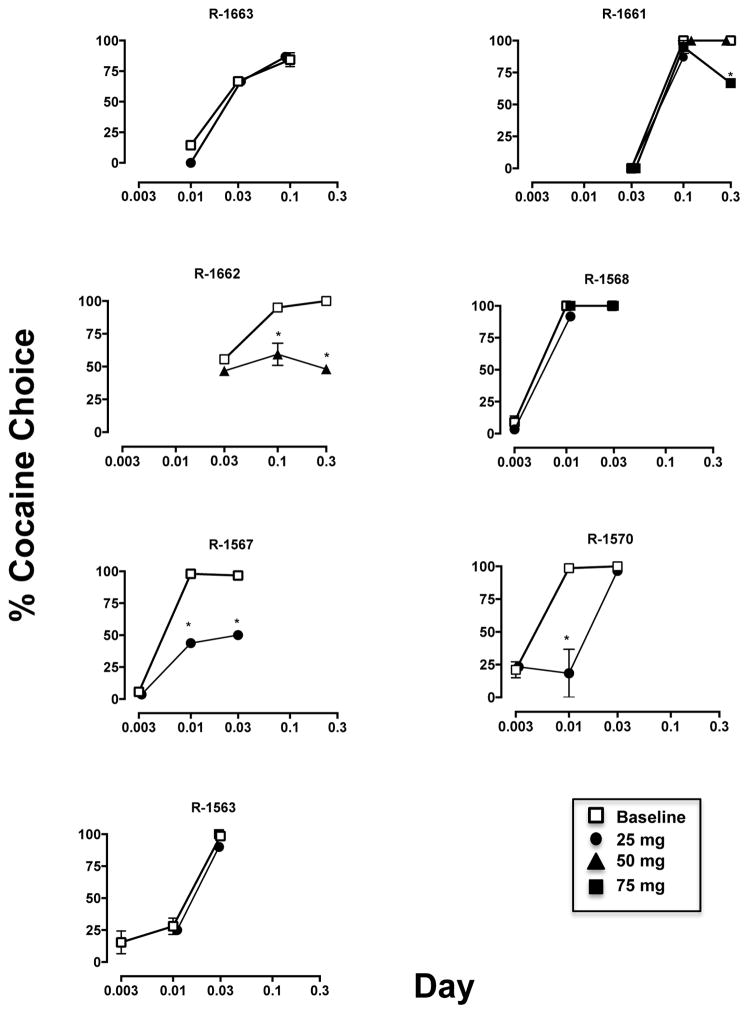
After completion of the cocaine dose-response curve, the effects of acute pretreatment with quetiapine (25–75 mg, P.O. in a food treat), given 45 minutes prior to the session, was examined in each monkey. The first dose tested in each monkey was 25 mg quetiapine. For two monkeys, this dose resulted in significant effects on cocaine choice, so no other doses were tested acutely. For R-1663, 25 mg quetiapine had no noticeable effect, but 50 mg quetiapine resulted in the monkey falling asleep in the primate chair prior to the session, so only 25 mg data are shown (Table 1). For all other monkeys, increasing doses of quetiapine were tested (25, 50 and 75 mg) until there were effects on cocaine choice or observable effects on the monkey. The effects of acute quetiapine administration were examined with three cocaine doses in each monkey; quetiapine was typically given once a week and if cocaine choice was affected, that quetiapine dose was retested.
Table 1.
Doses of quetiapine for both acute and chronic treatment studies.
| Monkey | Acute QTP Dose (mg) | Chronic QTP Dose (mg) |
|---|---|---|
| R-1563 | 25, 75 | 250 |
| R-1567 | 25 | 25 |
| R-1568 | 75 | 200 |
| R-1570 | 25 | 100 |
| R-1661 | 25–75 | 100 |
| R-1662 | 25, 50 | 100 |
| R-1663 | 25, 50 | 100 |
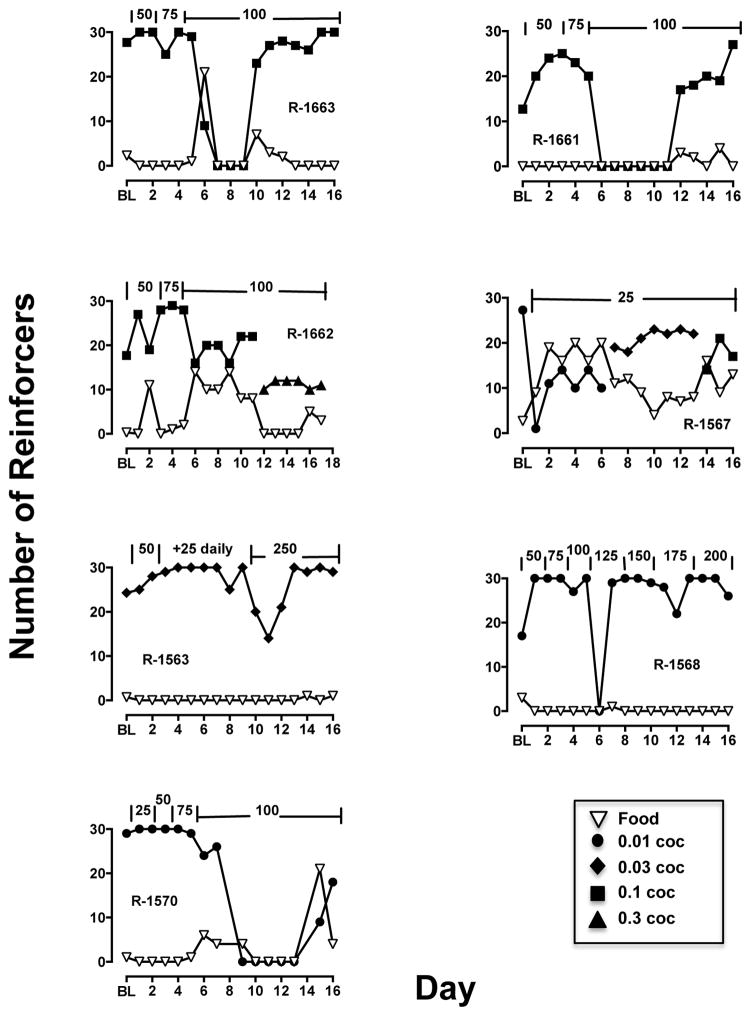
Following these experiments, the effects of twice-daily quetiapine treatment was examined in each monkey using the lowest preferred dose of cocaine. Monkeys self-administered their lowest preferred cocaine dose until responding was deemed stable prior to beginning the chronic treatment. Chronic oral administration of quetiapine (25–250 mg, BID) began with an evening dosing (at approximately 1500) and the AM dose occurred 45 min prior the session; the initial dose tested was the highest dose administered acutely. Cocaine choice sessions and twice daily quetiapine treatment continued daily (Mon–Sun). If the quetiapine dose did not affect choice responding for 3 consecutive sessions, the dose was raised by 25 mg. The entire chronic treatment lasted 14–16 days. Chronic quetiapine treatment was terminated (withdrawal, WD in the figures) by omitting the PM treatment, so the choice data will have one additional quetiapine treatment point than the sleep data, with that point represented as WD in the figure. Unfortunately, choice studies were not conducted the next morning after terminating quetiapine treatment.
Experiment 2. Effects of quetiapine on behavioral indices of sleep
In order to quantify nighttime activity, each monkey was fitted with an Actical® (Phillips Respironics, Bend, OR) activity monitor, an omnidirectional accelerometer that measures the subject’s physical activity, secured to the collar. Actigraphy measures have been used to assess sleep-wake patterns in rhesus macaques (Barrett et al. 2009) and in humans (Ancoli-Israel et al. 2003; Kushida et al. 2001; Sadeh et al. 1995; Sadeh and Acebo 2002) and are considered a valid index of human sleep (Sadeh et al. 1995; Kushida et al. 2001). Further, recent review indicated pharmacological and non-pharmacological changes in sleep measures can be detected using actigraphy (Sadeh 2011). Of note, activity measures (or lack thereof) can only be inferred as sleep since it is possible that the monkey is awake but not moving. Activity was recorded in 30-sec epoch lengths (2880 epochs/day) and the period of activity during the lights-out cycle was quantified. For quantification of sleep measures data were downloaded and analyzed using Actiware Sleep 3.4 (Mini-Matter Co. Inc., Bend, OR) software. Sleep efficiency, defined as total sleep time as a percentage of the period of time with the lights out (600 min) was examined for both acute and chronic quetiapine treatment, as was total nighttime activity (see Brutcher and Nader 2013 for more details).
Data Analysis
The primary dependent variables were the percent cocaine choice (defined as the number of reinforcers earned on the drug switch divided by the total completed choices on both switches, multiplied by 100), the number of food reinforcers and injections earned per component, sleep efficiency and total nighttime activity. For individual monkeys, t-tests were used to analyze the effect of acute quetiapine treatment on cocaine choice. To examine the effect of chronic quetiapine on cocaine choice, a one-way repeated measures ANOVA was used for each monkeys’ lowest preferred dose. For monkey R-1563, the lowest preferred dose (P) changed during the study so his data are shown, but excluded from mean data and from statistical analyses. For all analyses, p<0.05 was considered statistically significant.
Drugs
(−)-Cocaine was supplied by the National Institute on Drug Abuse (Bethesda, MD) and dissolved in sterile 0.9% saline; different cocaine doses were studied by changing the concentration of cocaine delivered over 10 seconds. Quetiapine tablets (25 and 50 mg) and powder were obtained from LKT Laboratories Inc. (St. Paul, MN). Oral doses up to 100 mg were given by placing tablets inside a treat (e.g. banana, Starburst); for higher doses, quetiapine powder was weighed and placed inside the treat. Quetiapine was administered orally 45 min prior to experimental sessions (~ 0700) and during twice daily treatment, the second dose was administered approximately 8 hrs later (~ 1500). An investigator (R.E.B.) observed the monkey consume the treat and if a monkey did not eat the treat or only ate part of the treat, the data were not included.
RESULTS
Experiment 1. Effects of quetiapine on cocaine self-administration
Under baseline conditions, the frequency of cocaine choice increased in a dose-related manner, with individual-subject variability in the sensitivity of cocaine preference over three 1.0-g food pellets (Fig. 1, open symbols). For three of the seven monkeys, the lowest preferred dose was 0.01 mg/kg cocaine, for one monkey it was 0.03 mg/kg and for three monkeys it was 0.1 mg/kg cocaine. Total trials completed (food + cocaine choice trials) did not vary as a function of cocaine dose (see Table 2). Pretreatment with acute doses (Table 1) of quetiapine resulted in significant (p < 0.001) decreases in the percentage of cocaine choice in four out of seven monkeys; in one monkey (R-1663) the lowest quetiapine dose (25 mg) had no effect, but the next higher dose (50 mg) resulted in complete sedation. The ability of quetiapine to reduce cocaine choice did not appear to be dependent on the potency of cocaine (i.e., the lowest preferred dose) (Fig. 1; closed symbols). If quetiapine had an effect, it was most likely at the lowest preferred dose, except in R-1661. For two animals, increasing the cocaine dose did not attenuate the effects of acute quetiapine (R-1662, R-1567). Examination of the average number of reinforcers earned, for cocaine and food, at all three doses tested revealed no significant difference between baseline cocaine self-administration and following acute quetiapine treatment (Table 2).
FIGURE 1.
Effect of acute quetiapine (25–75 mg) treatment (filled symbols) on the percent of baseline cocaine choice (open squares) for individual monkeys. *p < 0.001
Table 2.
Cocaine and Food Choice Trials Under Baseline and Acute Quetiapine Treatment
| − 1/2 log | Preferred | + 1/2 log | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | QTP | BL | QTP | BL | QTP | |||||||
| Monkey | COC | Fd | COC | Fd | COC | Fd | COC | Fd | COC | Fd | COC | Fd |
| R-1663 | 4.3 | 25.7 | 0 | 10 | 20 | 10 | 20 | 10 | 25.3 | 4.7 | 26 | 4 |
| R-1662 | 16.7 | 13.3 | 14 | 16 | 11 | 2 | 14 | 13.5 | 4.2 | 0.2 | 6.5 | 7 |
| R-1567 | 1.7 | 28.3 | 1 | 27 | 29.5 | 0.5 | 11.5 | 14.5 | 28.9 | 1.1 | 15 | 15 |
| R-1563 | 1 | 28.7 | 6 | 19 | 6 | 24 | 5 | 15 | 25.7 | 0.3 | 15 | 0 |
| R-1568 | 2.7 | 27.3 | 1 | 29 | 27 | 0 | 30 | 0 | 30 | 0 | 30 | 0 |
| R-1570 | 6.3 | 23.7 | 7 | 23 | 29.5 | 0.5 | 5.5 | 12.5 | 27 | 0 | 30 | 0 |
| R-1661 | 0 | 30 | 0 | 30 | 12.7 | 0 | 19 | 0 | 5.4 | 0 | 10 | 5 |
| AVG | 4.7 | 25.3 | 4.1 | 22.0 | 19.4 | 5.3 | 15.0 | 9.4 | 20.9 | 0.9 | 18.9 | 4.4 |
| SEM | 2.3 | 2.3 | 2.1 | 3.0 | 3.9 | 3.7 | 3.6 | 2.7 | 4.6 | 0.7 | 3.9 | 2.2 |
Chronic quetiapine treatment was studied when monkeys self-administered their lowest preferred cocaine dose, so at the start of treatment most reinforcers were associated with cocaine (Fig. 2, filled squares). Treatment with quetiapine rarely showed reallocation of responding from the cocaine-associated switch to the food-associated switch; R-1662 and R-1567 are the exceptions. In these two monkeys, chronic quetiapine reduced cocaine choice to approximately 50%. For the remaining five monkeys, quetiapine did not affect choice until doses were administered that decreased overall number of reinforcers per session. In these cases, tolerance developed to the effects on reinforcement frequency and choice returned to >80% cocaine by the end of quetiapine treatment (Fig. 2).
Figure 2.
Effect of chronic quetiapine (doses annotated across top of each graph in mg) treatment on the number of food (open, inverted triangles) and cocaine (filled symbols) reinforcers earned for individual monkeys.
Experiment 2. Effects of quetiapine on behavioral indices of sleep
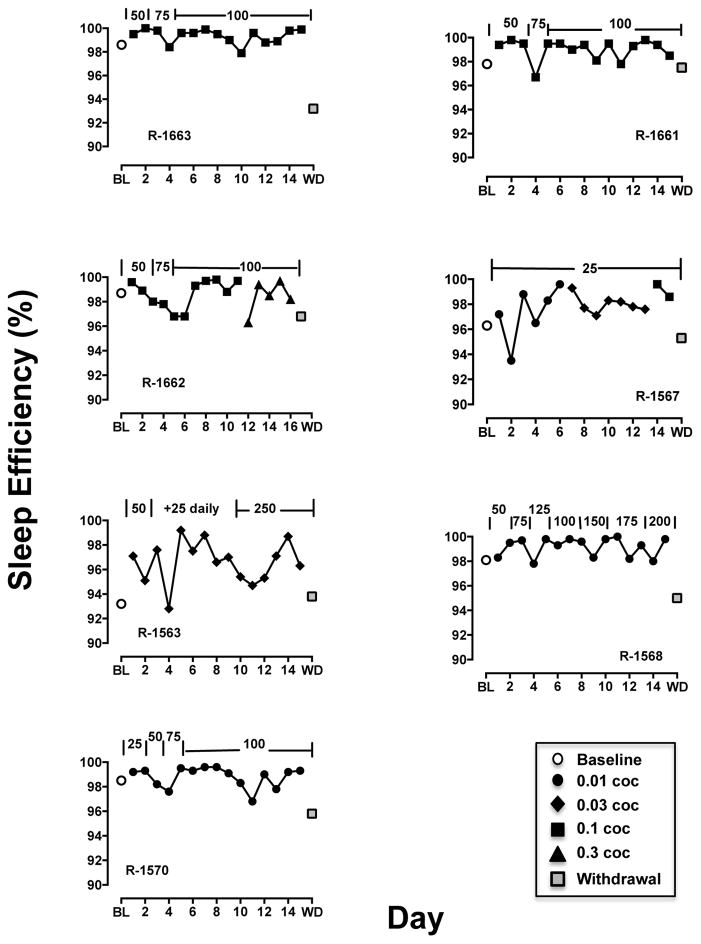
Sleep efficiency was approximately 97% (Table 3) and was not different across three cocaine doses (preferred dose and one-half log-unit above and below that dose). Quetiapine, given as a single acute dose, did not affect the sleep efficiency the evening following cocaine self-administration (Table 3). Chronic twice-daily quetiapine treatment significantly (F(2,10) = 9.25, p < 0.01) affected sleep efficiency (Fig. 3, left panel); post-hoc analyses indicated that following one day of termination from quetiapine treatment, sleep efficiency was significantly (p<0.05) below that observed while on QTP treatment. Examination of individual-subject data (Fig. 4) revealed improvements in sleep efficiency primarily in monkeys with baseline sleep efficiency measures less than 98% and little change in monkeys that were already near maximal in sleep efficiency. One day after quetiapine treatment was terminated, sleep efficiency decreased in all monkeys (Fig. 4, gray squares). There was also a significant effect (F(2,10) = 11.28, p < 0.01) of QTP treatment on nighttime activity (Fig. 3, right panel) and post-hoc tests indicated that activity was significantly greater during WD than under baseline and during QTP treatment (p < 0.05).
Table 3.
Mean sleep efficiency (± SEM) for the preferred (P) dose of cocaine and doses one-half log-unit above (−1/2) and below (+1/2) that dose under baseline (BL) and following acute quetiapine (QTP).
n=7
n=6
FIGURE 3.
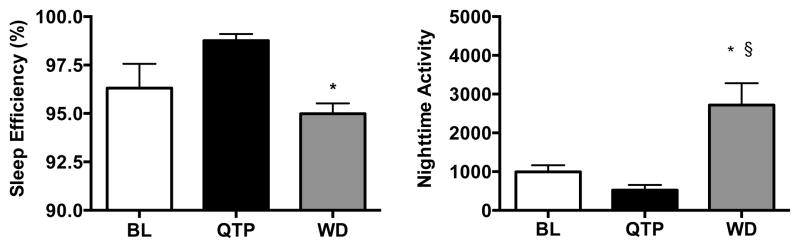
Effect of chronic quetiapine (black bar) and one day of withdrawal (WD) from quetiapine (gray bar) on nighttime sleep efficiency (left panel) and nighttime activity (right panel) following self-administration of the lowest preferred dose of cocaine (white bar). *p < 0.05 compared to QTP, §p < 0.05 compared to BL, n=6
Figure 4.
Effect of chronic quetiapine treatment (doses annotated across top of each graph in mg) on % sleep efficiency under baseline (BL; open circle), following cocaine self-administration sessions (filled symbols) and one day after withdrawal of quetiapine treatment (WD; gray square) for individual monkeys.
DISCUSSION
The present study investigated quetiapine, an atypical antipsychotic that has shown efficacy in treating sleep disturbances, as a potential treatment option for cocaine addiction using a monkey model of cocaine abuse. Acute oral administration of quetiapine resulted in decreases in cocaine choice in some, but not all the monkeys. When tested chronically (twice daily oral administration), quetiapine showed positive effects in two of seven monkeys, but tolerance developed to the reductions in cocaine choice in most of the subjects. Neither single acute administration of quetiapine nor chronic treatment improved sleep efficiency the following night, and discontinuation of quetiapine treatment resulted in significant disruptions in sleep efficiency and increases in nighttime activity, perhaps indicative of withdrawal symptoms. These findings do not offer support for the use of quetiapine as a monotherapy for treatment of cocaine abuse nor as an adjunct therapy to treat sleep disturbances associated with stimulant abuse.
To our knowledge, these are the first studies to examine the potential of quetiapine as a treatment option for cocaine addiction in a rhesus monkey model of cocaine abuse, although these results are inconsistent with reports showing positive results of quetiapine for reducing substance abuse (Brown et al. 2003; Sattar et al. 2004; Pinkofsky et al. 2005; Potvin et al. 2006). One explanation for the divergent effects on cocaine use is that many of the studies showing positive results with quetiapine are in the presence of a comorbid psychiatric condition. The lifetime prevalence rates of substance abuse in patients with bipolar disorder are as high as 60% (Regier et al. 1990; Strakowski and DelBello 2000). Decreases in the quantity of substance abuse have been noted following administration of antipsychotic medications to control psychotic symptoms (Volkow et al. 2002). These findings suggest that in the absence of a psychotic disorder quetiapine treatment may lack efficacy in treating cocaine abuse. To support this hypothesis, a recent study which included patients with comorbid bipolar disorder and alcohol dependence, revealed that quetiapine decreased alcohol consumption, craving for alcohol and psychiatric symptom intensity (Martinotti et al. 2008). Interestingly, in that study, quetiapine was used as a monotherapy and not given as adjunctive pharmacological treatment. Other studies report the efficacy of quetiapine as adjunctive therapy. A retrospective chart review examining the potential benefits of quetiapine in substance dependence disorders (alcohol, marijuana, amphetamine and cocaine) revealed a mean decrease in Likert score (a measure of craving) with negative breathalyzer and urine test results (Sattar et al. 2004). Quetiapine was also examined as add-on therapy in a group of outpatients with bipolar disorder and cocaine dependence. It was reported that quetiapine administration significantly decreased drug cravings and the amount of money spent on cocaine, with a trend toward a reduction on days of cocaine use (Brown et al. 2002). However, contrary to the above hypothesis, quetiapine efficacy was also investigated for the treatment of cocaine dependence in individuals who lacked clinical symptoms of affective disorders and the results showed quetiapine significantly decreased craving for cocaine, with a downward trend in the money spent on cocaine (Kennedy et al. 2008). Additional studies using different preclinical models of cocaine abuse may be necessary to fully evaluate the efficacy of this treatment in deceasing cocaine self-administration.
It is possible that quetiapine may be beneficial in treating substance abuse by inhibiting sleep disturbances associated with drug addiction. Quetiapine is commonly used off-label for a variety of conditions, one of which is insomnia (Hartung et al. 2008; Philip et al. 2008; Wine et al. 2009; Dolder and McKinsey 2010), although results from a meta-analysis described quetiapine efficacy as inconclusive (Maher et al. 2011). There are, however, several studies showing positive results of quetiapine for treating insomnia (Robert et al. 2005; Wiegand et al. 2008; Tassniyom et al. 2010; Endicott et al. 2012; Sheehan et al. 2012). Clinical treatment guidelines recommend use of antipsychotics in patients with concomitant psychiatric disorders and insomnia (NIH 2005; Schutte-Rodin et al. 2008). A recent study showed that sedation associated with quetiapine administration was reported in 100% of the participants receiving quetiapine in the absence of psychatric symptoms, which decreased to 75% by the end of week 1 of treatment to 29% at the end of week 6 (Kennedy et al. 2008). In the present study, the high baseline measures of sleep efficiency most likely precluded any significant improvements following quetiapine administration. A study examining the efficacy of mirtazapine (a sleep-promoting agent) in patients with comorbid depression and cocaine dependence showed that mirtazapine was superior to placebo in improving sleep, however it was not more effective than placebo in reducing cocaine use (Afshar et al. 2012). Importantly, the present findings indicated that following discontinuation of chronic quetiapine administration there was evidence of disruptions in sleep efficiency, suggesting development of physical dependence over two weeks of treatment.
The present findings support earlier work (Brutcher and Nader 2013) indicating that variables that affect cocaine-induced changes in sleep do not necessarily affect cocaine self-administration. While the choice paradigm provides an index of reinforcing strength that is sensitive to recent pharmacological and environmental manipulations (Banks and Negus 2012), it may be a less sensitive baseline for studying variables that disrupt sleep compared to simple schedules of reinforcement. For example, using an FR schedule of reinforcement, Andersen et al. (2013) reported significant correlations between methamphetamine intake and several behavioral measures of sleep. In contrast, Brutcher and Nader (2013) found no effect of sleep disruption on cocaine choice and, in the present study, no effect of enhancing sleep efficiency on cocaine choice. Future studies using higher cocaine doses or longer self-administration sessions that result in pronounced disruptions in sleep efficiency may be needed to fully evaluate the use of quetiapine to treat cocaine-related disruptions in sleep in combination with another drug to treat cocaine addiction (Karila et al. 2011; Fox et al. 2012).
Acknowledgments
We would like to thank Tonya Calhoun and Michael Coller for excellent technical assistance and Dr. Paul Czoty for comments on an earlier version of this manuscript. This research was supported by the National Institute on Drug Abuse grants DA025120 and DA012460.
Footnotes
FINANCIAL DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
R.E.B. and M.A.N. designed the experiments. R.E.B. performed the behavioral studies, and analyzed the data. The manuscript was written by R.E.B. and M.A.N.
References
- Afshar M, Knapp CM, Sarid-Segal O, Devine E, Colaneri LS, Tozier L, Waters ME, Putnam MA, Ciraulo DA. The efficacy of mirtazapine in the treatment of cocaine dependence with comorbid depression. Am J Drug Alcohol Abuse. 2012;38:181–186. doi: 10.3109/00952990.2011.644002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Diaz MP, Murnane KS, Howell LL. Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology. 2013;227:101–107. doi: 10.1007/s00213-012-2943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012:281768. doi: 10.1155/20212/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early adverse rearing experiences alter sleep-wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology. 2009;34:1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison RL, Arvanitis LA, Miller BG. ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. U.S. SEROQUEL Study Group. J Clin Psychopharmacol. 1996;16:158–169. doi: 10.1097/00004714-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Brown ES, Nejtek VA, Perantie DC, Bobadilla L. Quetiapine in bipolar disorder and cocaine dependence. Bipolar Disord. 2002;4:406–411. doi: 10.1034/j.1399-5618.2002.02229.x. [DOI] [PubMed] [Google Scholar]
- Brown ES, Nejtek VA, Perantie DC, Rajan Thomas N, Rush AJ. Cocaine and amphetamine use in patients with psychiatric illness: a randomized trial of typical antipsychotic continuation or discontinuation. J Clin Psychopharmacol. 2003;23:384–388. doi: 10.1097/01.jcp.0000085412.08426.08. [DOI] [PubMed] [Google Scholar]
- Brutcher RE, Nader MA. The relationship between cocaine self-administration and actigraphy-based measures of sleep in adult rhesus monkeys. Psychopharmacology. 2013;229:267–274. doi: 10.1007/s00213-013-3101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs S, Rodenbeck A, Guan Z, Pohlmann K, Jordan W, Meier A, Ruther E. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174:421–429. doi: 10.1007/s00213-003-1759-5. [DOI] [PubMed] [Google Scholar]
- Daley JT, Turner RS, Freeman A, Bliwise DL, Rye DB. Prolonged assessment of sleep and daytime sleepiness in unrestrained Macaca mulatta. Sleep. 2006;29:221–231. [PubMed] [Google Scholar]
- Dolder CR, McKinsey J. Quetiapine for sleep in patients with dementia. Consult Pharm. 2010;25:676–679. doi: 10.4140/TCP.n.2010.676. [DOI] [PubMed] [Google Scholar]
- Emsley R, Oosthuizen P. The new and evolving pharmacotherapy of schizophrenia. Psychiatri Clin North Am. 2003;26:141–63. doi: 10.1016/s0193-953x(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Endicott J, Svedsater H, Locklear JC. Effects of once-daily extended release quetiapine fumarate on patient-reported outcomes in patients with generalized anxiety disorder. Neuropsychiatr Dis Treat. 2012;8:301–311. doi: 10.2147/NDT.S32320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. [Accessed 29 Jan 2013];Seroquel (quetiapine fumarate) medication guide. 2011 http://www.fda.gov/downloads/Drugs/DrugSafety/usm089126.pdf.
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM. Quetiapine fumarate (Seroquel): a new atypical antipsychotic. Drugs Today (Barc) 1999;35:193–210. doi: 10.1358/dot.1999.35.3.533849. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Gage HD, Nader MA. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2010;210:481–488. doi: 10.1007/s00213-010-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Nader MA. Behavioral characterization of adult male and female rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2011;213:799–808. doi: 10.1007/s00213-010-2038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung DM, Wisdom JP, Pollack DA, Hamer AM, Haxby DG, Middleton L, McFarland BH. Patterns of atypical antipsychotic subtherapeutic dosing among Oregon Medicaid patients. J Clin Psychiatry. 2008;69:1540–1547. doi: 10.4088/jcp.v69n1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM, Lindsey KP, Kimmel HL. Olanzapine-induced suppression of cocaine self-administration in rhesus monkeys. Neuropsychopharmacology. 2006;31:585–593. doi: 10.1038/sj.npp.1300828. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Sparkman T, O’Brien CP. A pilot trial of olanzapine for the treatment of cocaine dependence. Drug Alcohol Dep. 2003;70:265–273. doi: 10.1016/s0376-8716(03)00009-7. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–559. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- Karila L, Reynaud M, Aubin HJ, Rolland B, Guardia D, Cottencin O, Benyamina A. Pharmacological treatments for cocaine dependence: is there something new? Curr Pharm Des. 2011;17:1359–1368. doi: 10.2174/138161211796150873. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Wood AE, Saxon AJ, Malte C, Harvey M, Jurik J, Kilzieh N, Lofgreen C, Tapp A. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28:221–224. doi: 10.1097/JCP.0b013e318166f50d. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disoriented patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lexicomp Online. [Accessed 29 Jan 2013];Quetiapine use: labeled indications and mechanism of action. 2013 http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7598.
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, Wang Z, Timmer M, Sultzer D, Shekelle PG. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. J Am Med Assoc. 2011;306:1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Berk M. Pharmacotherapy of bipolar disorder: the role of atypical antipsychotics and experimental strategies. Hum Psychopharmacol. 2002;17:407–412. doi: 10.1002/hup.437. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Andreoli S, Di Nicola M, Di Giannantonio M, Sarchiapone M, Janiril L. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnoses alcoholics. Hum Psychopharmacol. 2008;23:417–424. doi: 10.1002/hup.944. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep architecture, cocaine and visual learning. Addiction. 2008;103:1344–1352. doi: 10.1111/j.1360-0443.2008.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin AK. Possible intranasal quetiapine misuse. Am Society of Health-System Pharmacists. 2007;64:723–725. doi: 10.2146/ajhp060226. [DOI] [PubMed] [Google Scholar]
- Murphy D, Bailey K, Stone M, Wirshing WC. Addictive potential of quetiapine. Am J Psychiatry. 2008;165:918. doi: 10.1176/appi.ajp.2008.08020277. [DOI] [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1999;147:143–152. doi: 10.1007/s002130051154. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. State-of-the-science conference statement on manifestations and management of chronic insomnia in adults. 2005 doi: 10.1093/sleep/28.9.1049. http://consensus.nih.gov/2005/insomniastatement.htm. [DOI] [PubMed]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Philip NS, Mello K, Carpenter LL, Tyrka AR, Price LH. Patterns of quetiapine use in psychiatric inpatients: an examination of off-label use. Ann Clin Psychiatry. 2008;20:15–20. doi: 10.1080/10401230701866870. [DOI] [PubMed] [Google Scholar]
- Pinkofsky HB, Hahn AM, Campbell FA, Rueda J, Daley DC, Douaihy AB. Reduction of opioid-withdrawal symptoms with quetiapine. J Clin Psychiatry. 2005;66:1285–1288. doi: 10.4088/jcp.v66n1011. [DOI] [PubMed] [Google Scholar]
- Potvin S, Kouassi E, Lipp O, Bouchard RH, Roy MA, Demers MF, Gendron A, Astarita G, Piomelli D, Stip E. Endogenous cannabinoids in patients with schizophrenia and substance abuse disorder during quetiapine therapy. J Psychopharmacol. 2008;22:262–269. doi: 10.1177/0269881107083816. [DOI] [PubMed] [Google Scholar]
- Prieto E, Mico JA, Meana JJ, Majadas S. Neurobiological bases of quetiapine antidepressant effect in the bipolar disorder. Act Esp Psiquiatr. 2010;38:22–32. [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacol Biochem Behav. 2009;94:262–270. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran AV, Al-Subaie A, Abraham G. Quetiapine: novel uses in the treatment of depressive and anxiety disorders. Expert Opin Investig Drugs. 2010;19:1187–1204. doi: 10.1517/13543784.2010.515586. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. Southern Med J. 2007;100:834–836. doi: 10.1097/SMJ.0b013e3180f62d53. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) study. J Am Med Assoc. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Riedel M, Muller N, Strassnig M, Spellmann I, Severus E, Moller HJ. Quetiapine in the treatment of schizophrenia and related disorders. Neuropsychiatr Dis Treat. 2007;3:219–235. doi: 10.2147/nedt.2007.3.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Hamner MB, Kose S, Ulmer JG, Deitsch SE, Lorberbaum JP. Quetiapine improves sleep disturbances in combat veterans with PTSD. J Clin Psychopharmacol. 2005;25:387–388. doi: 10.1097/01.jcp.0000169624.37819.60. [DOI] [PubMed] [Google Scholar]
- Sadeh A. the role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Sattar SP, Bhatia SC, Petty F. Potential benefits of quetiapine in the treatment of substance dependence disorders. Rev Psychiatr Neurosci. 2004;29:452–457. [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysee D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Locklear J, Svedsater H, Datto C. Long-term functioning and sleep quality in patients with major depressive disorder treated with extended-release quetiapine fumarate. Int Clin Psychopharmacol. 2012;27:239–248. doi: 10.1097/YIC.0b013e328356ac78. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP. The co-occurrence of bipolar and substance use disorders. Clin Psychol Rev. 2000;20:191–206. doi: 10.1016/s0272-7358(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Tassniyom K, Paholpak S, Tassniyom S, Kiewyoo J. Quetiapine for primary insomnia: a double blind, randomized controlled trial. J Med Assoc Thai. 2010;93:729–734. [PubMed] [Google Scholar]
- Volkow ND, Ding Y, Fowler J, Wang G. Cocaine addiction: Hypothesis derived from imaging studies with PET. J Addict Dis. 1996;15:55–71. doi: 10.1300/J069v15n04_04. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferre S. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, Ma J, Pradhan K, Tomasi D, Thanos PK, Ferre S, Jayne M. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand MH, Landry F, Bruckner T, Pohl C, Vesely A, Jahn T. Quetiapine in primary insomnia: a pilot study. Psychopharmacology. 2008;196:337–338. doi: 10.1007/s00213-007-0968-8. [DOI] [PubMed] [Google Scholar]
- Wine JN, Sanda C, Caballero J. Effects of quetiapine on sleep in nonpsychiatric and psychiatric conditions. Ann Pharmacother. 2009;43:707–713. doi: 10.1345/aph.1L320. [DOI] [PubMed] [Google Scholar]
- Woods JH, Herring S, Winger G. Chlorpromazine- and haloperidol-induced changes in some behavioral effects of cocaine and amphetamine. In: Deniker P, Radouco-Thomas C, Villeneuve A, editors. Neuro-Psychopharmacology. Pergamon Press; Oxford, NY: 1978. pp. 1485–1502. [Google Scholar]
- Woolverton WL, Balster RL. The effects of lithium on choice between cocaine and food in the rhesus monkey. Commun Psychopharmacol. 1979;3:309–318. [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. Effects of antipsychotic compounds in rhesus monkeys given a choice between cocaine and food. Drug Alcohol Dep. 1981;8:69–78. doi: 10.1016/0376-8716(81)90088-0. [DOI] [PubMed] [Google Scholar]