Abstract
Purpose
We describe a non-invasive PET imaging method that monitors early therapeutic efficacy of BAY 87-2243, a novel small molecule inhibitor of mitochondrial complex I as a function of hypoxia-inducible factor-1α (HIF-1α) activity
Experimental Design
Four PET tracers (18F-FDG, 18F-Fpp(RGD)2, 18F-FLT and 18F-FAZA) were assessed for uptake into tumor xenografts of drug-responsive (H460, PC3) or drug-resistant (786-0) carcinoma cells. Mice were treated with BAY 87-2243 or vehicle. At each point, RNA from treated and vehicle H460 tumor xenografts (n=3 each) was isolated and analyzed for target genes.
Results
Significant changes in uptake of 18F-FAZA, 18F-FLT and 18F-Fpp(RGD)2 (p<0.01) occurred with BAY 87-2243 treatment with 18F-FAZA being the most prominent. 18F-FDG uptake was unaffected. 18F-FAZA tumor uptake declined by 55–70% (1.21±0.10 %ID/g to 0.35±0.1 %ID/g, n=6, vehicle vs. treatment) in both H460 (p<0.001) and PC3 (p<0.05) xenografts 1–3 days post-drug. 18F-FAZA uptake in 786-0 xenografts was unaffected. Decline occurred before significant differences in tumor volume, thus that suggesting 18F-FAZA decrease reflected early changes in tumor metabolism. BAY 87-2243 reduced expression of hypoxia-regulated genes CA IX, ANGPTL4 and EGLN-3 by 99 %, 93% and 83%, respectively (p<0.001 for all), which corresponds with reduced 18F-FAZA uptake upon drug treatment. Heterogeneous expression of genes associated with glucose metabolism, vessel density and proliferation was observed.
Conclusions
Our studies suggest suitability of 18F-FAZA-PET as an early pharmacodynamic monitor on the efficacy of anti-cancer agents that target the mitochondrial complex I and intra-tumor oxygen levels (e.g. BAY 87-2243).
Introduction
Studies into the gene and protein expression profile on cancers(1) have resulted in novel strategies to monitor anti-cancer therapies by radiotracers(2, 3). Such approaches are important since clinical investigations show that expanding tumors lead to heterogeneous patterns of tissue hypoxia(4–6). This has the initial consequence of impeding growth but over time, there will be selection for tumor subpopulations that grow even more aggressively and invasively under hypoxia(7). Clinical investigations carried out over the last two decades have demonstrated that the prevalence of hypoxic tissue areas [i.e., O2 tensions (pO2 values) ≤2.5 mmHg or %O2 of 0.3] is characteristic of locally advanced solid tumors and is a relevant factor in the tumor’s patho-physiome(7). In addition, hypoxic tumors are also known to be especially resistant to radiotherapy(8). Thus, it is evident that hypoxia influences multiple, critical steps in the process of tumorigenesis, often leading to a more malignant and metastatic stage. It is also evident that additional monitoring tools, such as molecular imaging, are required to adjust therapies such that they are more appropriate to the cancer profiles of the individual patient. In order to achieve this, it is vital to understand how to abrogate such a response. Ultimately, one also needs reagents that could potentially resolve tumor hypoxia.
Recently, the experimental drug BAY 87-2243 has been identified that acts by impacting the hypoxia-induced activity of the HIF-1α pathway, principally by inhibiting the activity of mitochondrial complex I(9). A chemically related drug candidate, BAY 84-7296, sensitizes tumors to radiotherapy through the same mechanism, that is, by resolving cancer hypoxia via inhibition of mitochondrial complex I(10). HIF-1α is a transcription factor that plays a key role in initiating and maintaining eukaryotic responses to hypoxic and free radical stress(7, 8, 11, 12). In cell culture and in preclinical tumor xenograft models (with fully functional HIF-1α activity and wild type VHL), BAY 87-2243 has been reported to inhibit growth(9). To image the processes underlying such inhibition, we employed PET (Positron Emission Tomography) technology to monitor the efficacy of the drug, BAY 87-2243, on growing xenografts of human lung cancer (H460) or human prostate cancer (PC3). As a non-responding, negative control, we also tested the drug on xenografts of human renal cell carcinoma (786-0) that possess constitutive HIF2 activity that was initiated by loss of VHL expression(13).
To determine the feasibility of image monitoring on a drug, we selected several 18F-labelled PET tracers that followed specific processes related to carcinogenesis and cancer response to hypoxia. These processes (and their corresponding tracers) include: tumor growth (18F-FLT), tumor energy metabolism (18F-FDG), tumor angiogenesis (18F-FP(PRGD)2) and tumor hypoxia (18F-FAZA). We compared the utility of these tracers to monitor the therapeutic potency of BAY 87-2243. We conclude that 18F-FAZA is the best tracer to monitor therapeutic response from the drug, BAY 87-2243.
Materials and Methods
Pharmaceuticals
Unless otherwise specified all reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1-Cyclopropyl-4-{4-[(5-methyl-3-{3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl}-1H-pyrazol-1-yl)methyl]pyridin-2-yl}piperazine or BAY 87-2243 (chemical structure disclosed in (9) and shown in Supplemental Figure 4) was prepared as a suspension in a solution of 20% Solutol and 10% Ethanol in PBS. Vehicle solution was 20% Solutol/10% Ethanol in PBS.
Cell Culture of H460, PC3 and 786-0
The human cancer cell lines used in this study were: H460 lung carcinoma cells, PC3 prostate cancer cells and 786-0 renal cell carcinomas. All lines were purchased from the American Type Tissue Culture Collection (ATCC, Manassas, VA) and pre-tested for the presence of endotoxins and mycoplasma. No other authentication was done. H460 lung carcinoma cells were grown in RPMI-1640 (high glucose), 2mM GlutaMax, and 1mM Na Pyruvate, 1.5 g/L Na bicarbonate (Gibco BRL/Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS) and 1% penicillin(100 U/mL)/streptomycin (100 mg/mL). PC3 prostate cancer cells were propagated in F12/Kaighn’s medium with 2mM GlutaMax, 1.5 g/L Na bicarbonate(Gibco BRL/Invitrogen, Carlsbad, CA), 10% FBS with 1% penicillin(100 U/mL)/streptomycin (100 mg/mL). 786-0 renal cell carcinoma cultures were grown in RPMI 1640 medium, 10% FBS and 1% penicillin(100 U/mL)/streptomycin (100 mg/mL).
Mouse Tumor Xenograft Model
Animal protocols were approved by the Stanford Administrative Panel on Laboratory Animal Care. 14–16 week-old, female nude mice (Charles River Laboratories, Inc.) were inoculated subcutaneously on both scapular shoulder blades with 5×106 human cancer cells per side. The mice were anesthetized with 2% isoflurane in oxygen at 2 L/min during the injections. The tumor xenografts were grown to at least 200 mm3 (3). For H460 cells, a period of 7–10 days post-injection was needed while 786-0 and PC3 cells required 4–6 weeks.
The majority of xenografted mice were used for small animal PET studies. Small animal PET scans were done before vehicle or drug treatment and at defined times after treatment (see Results section). To associate tumor growth kinetics with changes in radiotracer uptake, tumor volume measurements (taken via calipers) were performed at various time points throughout the course of the experiments. At the end of studies, the mice were sacrificed and the tumors excised and frozen in OCT for immunohistochemical analysis. In one set of experiments, the xenografts were used exclusively for real-time PCR studies and selected tumors were isolated at various times pre- and post-drug or vehicle delivery.
Pre-Clinical Experimental Protocols
(a) Time Dependency Experiment
Small animal PET scans were performed 1 day before the oral administration of either vehicle (20% solutol/10% EtOH in PBS) or BAY 87-2243 (9 mg/kg in vehicle solution). Scans were then performed 1, 3, 5 or 7 days after the initiation of the drug or vehicle treatments. Treatments were given via oral gavage daily. Tumor size was also monitored daily starting at Day -1 through the end of studies. Sample size was n=6 tumors in 3 mice. At the end of each study, mice were pre-treated with pimonidazole (9 mg/kg, tail vein injection 1.5h prior to autopsy), sacrificed and tumours frozen in OCT. Samples were then analysed via IHC and RT-PCR to determine molecular response to treatment.
(b) Specificity Experiments
Time dependency of tumor growth and radiotracer uptake in the presence of escalating concentrations of BAY 87-2243 was compared among the treated groups and to the vehicle-control groups. Three separate cohorts of mice (n=6 tumors in three mice per cohort) were given either vehicle-control (20% solutol/10% EtOH in PBS), 3mg/kg BAY 87-2243 (in solutol vehicle) or 9 mg/kg BAY 87-2243. Follow up specificity experiments at 1mg/kg BAY 87-2243 and at 9mg/kg BAY 87-2243 were also undertaken. As a negative control, equivalent preclinical experiments were done on 786-O xenografts.
Radiotracer Production and Purification
Synthesis of 18F-Fpp(RGD)2
All chemicals obtained commercially were of analytical grade (Sigma-Aldrich, St. Louis, MO, USA) and used without further purification. 18F-Fpp(RGD)2 was synthesized as per a standardized protocol(14). Finished reaction products were purified and analyzed via reversed phase HPLC as described in in a previous publication (14). Radiochemical yield was 50 ± 14% (n =10) with specific activity of 18.5×109 Bq 18F/umole FPP(RGD)2. Radiochemical purity was 95%.
Synthesis of 18F-FDG
The synthesis of [18F]FDG was performed according to a previous publication (15). Tail vein injection of tracer was completed on 6-hour fasted athymic nude mice (female, 14–16 weeks). Radiochemical yield was 63 ± 11% (n =10) with specific activity of 5×109 Bq 18F/umole FDG. Radiochemical purity was 95%.
Synthesis of 18F-FLT
The synthesis of [18F]FLT was performed according to a previous publication (3) . Radiochemical yield was 13 ± 1% (n =10) with specific activity of 37×109 Bq 18F/umole FLT. Radiochemical purity was 95%.
Synthesis of 18F-FAZA
18F-FAZA(Supplemental Figure 4) was synthesized as described previously(16–19). Radiochemical yields were approximately 21% at end of the bombardment with specific activity of 9.8×109 Bq 18F/umole. Typically, 7–11 GBq of 18F-FAZA were isolated with a radiochemical purity of generally more than 95%.
Small animal PET scanning
An R4 microPET (Siemens Medical Solutions USA, Inc.) was used for imaging, which has an approximate resolution of 2 mm in each axial direction (20). After a tail vein injection of (3.7±0.3) x 106 Bq of 18F-FP-PRGD2, 18F-FLT, 18F-FDG or 18F-FAZA in 100 μL of PBS, a 3-min prone acquisition scan was performed approximately 60 min after injection (90 minutes for 18F-FAZA). A heating pad, heat lamp, or hot water was used to dilate the tail veins for injections.
Except for those injected with 18F-FDG, the mice were maintained under isoflurane anesthesia during the injection and scanning periods only. In between, the mice were given ad libitum access to food and water. For mice injected with 18F-FDG, the mice were fasted 6-hours before the injections and were kept under anesthesia between the injection and scanning sessions. For all experiments, the mice were kept warm using a heating pad or heat lamp while under anesthesia to maintain a body temperature of around 350C. microPET images were reconstructed with the ordered-subsets expectation-maximization algorithm (OESM)(21) using 16 subsets and 4 iterations. Attenuation correction was not performed.
microPET Image Analysis
Ellipsoidal 3-dimensional regions of interest (ROI) were manually drawn around the edge of the tumor xenograft activity by visual inspection using AMIDE software(22).
Immunohistochemical Analysis
In nude mice bearing pre-established H460 tumor xenografts (80 mm3 in size, n=3 per group), either a single or dual oral treatment was performed with vehicle or BAY 87-2243 (9 mg/kg). One day after, pimonidazole (9 mg/kg, Natural Pharmacia International) was injected via the tail vein. Mice were sacrificed 1.5 hours after the pimonidazole injection. Tumors were excised and processed for pimonidazole staining according to the protocol provided by Natural Pharmacia International(23).
Immunohistochemical assays for CD31 were done on H460 xenografts after one, two, and four treatments of vehicle or BAY 87-2243 (9 mg/kg). Primary antibody was a rat anti-mouse-CD 31 (MEC 13.3; Fa BD Pharmingen), diluted 1:250. Secondary antibody was a biotinylated goat anti-rat antibody (Fa BD Pharmingen), diluted 1:100. All tissues were counterstained with hematoxylin. Calculations of percent hypoxic fraction (PHF) and percent vascular fraction (PVF) was determined by using ImageJ to trace and then to integrate the areas of both positive staining and the total tumor. The hypoxic and vascular fractions were then determined by obtaining the ratio for area of positive staining over total tumor area.
Real-time PCR Analysis
Nude mice bearing pre-established H460 tumor xenografts (grown for 10 days) were treated orally with vehicle or BAY 87-2243 (9 mg/kg qd). RNA from xenograft tumors was isolated from satellite animal groups either before treatment or after 1 and 3 days of treatment with BAY 87-2243 according to published methods(9). Expression levels of the HIF-1α target genes were quantified by RT-PCR pursuant to established protocols(9). Data were normalized to α-actin and were given in arbitrary units as the mean of n=3 animals/treatment group ± standard deviation.
Statistical Analysis
Statistical analysis was performed either with Excel 2011 (Microsoft) or with Graph Pad Prism version 6.00 (Graph Pad Software, San Diego, CA). Paired t tests were used when paired data from the same mouse were compared. Unpaired t tests were used when 2 groups of data were compared. When multivariate analyses for groups of more than 3 were needed, ANOVA techniques were employed. Correlation coefficients were calculated when 2 groups of nominal variables were compared. A significance value of P<0.05 was used. Data are reported as mean±SD.
Results
BAY 87-2243 impacts growth of H460 and PC3 but not 786-0
Tumor xenografts of H460 (Figure 1A), PC3 (Figure 1B) and 786-0 (Figure 1C) were established as test models. For both H460 and PC3, the treatment with BAY 87-2243 significantly abrogated tumor growth (P<0.001 vehicle vs treatment, n=6 tumors per condition) when compared to the vehicle controls (Figures 1A and 1B). BAY 87-2243 did not affect the kinetics of tumor growth for 786-0 xenografts (Figure 1C, n=6 tumors per condition) when compared to vehicle controls. In no cases did oral administration of BAY 87-2243 affect the total weight of any animal model that was studied (Supplemental Figure 1).
Figure 1.
Pharmacodynamic (PD) effects of vehicle (●) and BAY 87-2243 (9mg/kg; ■) on tumor growth kinetics in the murine xenograft models of (A) H460 human lung carcinomas (B) PC3 human prostate cancers and (C) 786-O human renal cell carcinomas. Vehicle and drug administered vial daily, oral gavages. N=6 tumor over three mice per cohort. MicroPET readings (shown as
 ) usually done on days -1, 1, and 3 of treatment. Xenograft tumors were grown to a volume size of 200 mm3 before initiation of treatment. * - denotes P<0.001, vehicle vs. treatment.
) usually done on days -1, 1, and 3 of treatment. Xenograft tumors were grown to a volume size of 200 mm3 before initiation of treatment. * - denotes P<0.001, vehicle vs. treatment.
BAY 87-2243 reduces 18F-FAZA uptake and growth in H460 lung carcinoma xenografts in a dose dependent manner
To examine the concordance between tracer uptake and drug efficacy, we examined uptake of [18F]-FDG, [18F]-FPP(RGD)2, [18F]-FLT, 18F-FAZA at time points before, 1 day after and 3 days after vehicle or drug-treatment. In H460 xenografts, reduction of tracer uptake by BAY 87-2243 was most pronounced for 18F-FAZA (Figure 2A–B). One day post-drug treatment, 18F-FAZA uptake declined by 50% compared to pre-scan controls (1.1±0.10 %ID/g to 0.55±0.1 %ID/g, n=6, p<0.001 vehicle vs. treatment). By Day 3, a significant difference (P<0.01 vehicle vs. treatment, n=6 tumors in 3 mice per treatment) in tumor volume between vehicle and treated groups became apparent and this occurred with significantly (P<001) abrogated 18F-FAZA uptake in the tumors from the treated cohorts. Thus, changes in 18F-FAZA uptake occurred before significant changes in tumor size.
Figure 2.
PD effects of vehicle (●) and BAY 87-2243 (9mg/kg, ■) on reduction of 18F-FAZA uptake in the H460 tumor xenograft model in both single dose and dose-dependent manners. (A) Single voxel plane coronal microPET scans (tracer is 18F-FAZA) of nude mice containing H460 tumor xenografts. Scans performed on mice before treatment (left-most column) and 1 (middle column) and 3-days (right-most column) after treatment for either vehicle (upper panel) or 9 mg/kg BAY 87-2243 (lower panel). (B) Quantitative assessments of 18F-FAZA uptake of corresponding tumor xenografts. Uptake expressed in terms of %IDmean/g. Vehicle cohorts represented by solid bars while hatched, open bars represent uptake in tumors from drug-treated animals. (C) Significant (p<0.001 vehicle vs. treatment) percent loss in %IDmean/g values, 1 day after start of drug treatment. (D) Relative loss in uptake occurred before significant percent decline in tumor volume (p<0.001). N=6 tumor over three mice per cohort. * or + − denotes P<0.001, vehicle vs. treatment. Tumors indicated by white arrows. (E) Tumor growth kinetics of H460 xenografts for given oral gavages of vehicle or 3 and 15 mg/kg of BAY 87-2243. (F) Quantitative assessments of 18F-FAZA uptake of corresponding tumor xenografts. Uptake expressed in terms of %IDmean/g. N=6 tumor over three mice per cohort. * or + − denotes P<0.001, vehicle vs. treatment. Tumors indicated by white arrows.
To determine if BAY 87-2243 reduces both tumor growth and 18F-FAZA uptake in a dose dependent manner, murine models of H460 tumor xenografts were given escalating doses of the drug (3 and 15 mg/kg, respectively). At both drug concentrations, tumor sizes started to be reduced 24–48 hours after initial drug treatment. However, the degree of tumor shrinkage was positively related to dosage of BAY 87-2243, namely, the percent reduction of tumor size was greater for input of 15 mg/kg/day BAY 87-2243 than for a dosage of 3 mg/kg/day (n=6 tumor per group, Figure 2E) however the differences were not significant. In a second set of experiments where the effects of dosage 1 and 9 mg/kg BAY 87-2243 were examined, the dose dependent effect on tumor shrinkage with drug treatment was more pronounced and significant(p<0.001, ANOVA vehicle vs treatment group, n=6 tumors per group, Supplemental Figure 7B). Such findings were consistent with separate investigations by Ellinghaus et al. (9). When 18F-FAZA uptake was measured for those same cohorts, we observed that reduction of uptake changed in parallel with increasing BAY 87-2243 dosage (p<0.001 ANOVA for vehicle vs. different treatment concentrations, n=6 tumor per group, Figure 2F and Supplemental Figure 7A). The data from Figures 2E-2F and Supplemental 7A-7B as well as the findings of Ellinghaus et al. (9), all suggest that BAY 87-2243 can act as an anti-tumorigenic agent (for H460 xenografts) in a dose-dependent manner. In addition, the metric of %IDmean/g 18F-FAZA uptake can serve as an early indicator of dosage efficacy of BAY 87-2243 even before significant changes in tumor volume.
BAY 87-2243 reduces 18F-FAZA uptake and growth in human PC3 prostate cancer xenografts
To determine if the initial 18F-FAZA findings from H460 could be extended to other human cancer models, we performed similar studies in mice bearing human PC3 xenografts. When time dependent 18F-FAZA uptake studies were performed in the presence of vehicle vs. 9mg/kg/day BAY 87-2243 oral gavage, 18F-FAZA uptake was significantly reduced by nearly 70% in the drug-treated cohorts (1.2±0.10 %IDmean/g to 0.50±0.1 %IDmean/g, n=6 tumors per group, p<0.001 vehicle vs. treatment, Figure 3A–B). For PC3 xenografts, a significant reduction (p<0.001) in 18F-FAZA uptake occurred as early as one day after initiation of drug treatment, before any significant differences in tumor volume appeared between vehicle and drug-treated animals (Figure 3C–D). Differences in 18F-FAZA uptake persisted to day 3 post-initiation of drug treatment, at which point there occurs a significant abrogation of tumor growth with BAY 87-2243 oral gavage. In a similar finding to that with the H460 cohorts, the BAY 87-2243 reagent reduced both 18F-FAZA and tumor growth for PC3 xenografts in a dose-dependent manner (Supplemental data Figure 2).
Figure 3.
Pharmacodynamic (PD) effects of BAY 87-2243 on the reduction of 18F-[FAZA] uptake in the PC3 prostate carcinoma xenograft model: (A) Single voxel plane coronal uPET scans (tracer is 18F-FAZA) of nude mice containing H460 tumor xenografts. Scans performed on mice before treatment (left-most column) and 1 (middle column) and 3-days (right-most column) after treatment for either vehicle (upper panel) or for 9 mg/kg BAY 87-2243 (lower panel). (B) Quantitative assessments of 18F-FAZA uptake of corresponding tumor xenografts. Uptake expressed in terms of %IDmean/g. Vehicle cohorts represented by black bars while patterned bars represent uptake in tumors from drug-treated animals. (C) Significant (p<0.001 vehicle vs. treatment) percent loss in %IDmean/g values, 1 day after start of drug treatment. (D) Relative loss in uptake occurred before significant percent decline in tumor volume (p<0.001). N=6 tumor over three mice per cohort. * or + − denotes P<0.001, vehicle vs. treatment. Tumors indicated by white arrows.
Drug treatment reduces neither 18F-FAZA uptake nor growth in BAY 87-2243-resistant human 786-0 renal cell carcinoma xenografts
BAY 87-2243 did not inhibit the growth of 786-0 human renal cell carcinoma xenografts (Figure 1C). When 18F-FAZA small animal PET was performed on these animals, the scans revealed no significant difference in 18F-FAZA uptake between vehicle treated mice and those treated with BAY 87-2243 (oral gavage, 9mg/kg, Figure 1C, Figure 4).
Figure 4.
Pharmacodynamic (PD) effects of BAY 87-2243 on 18F-FAZA uptake in the 786-0 renal cell carcinoma xenograft model.
(A) Single voxel plane coronal uPET scans (tracer is 18F-FAZA) of nude mice containing 786-O tumor xenografts. Scans performed on mice before treatment (leftmost column) and 1 (middle column) and 3-days (right-most column) after treatment for either vehicle (upper panel) or for 9 mg/kg BAY 87-2243 (lower panel). (B) Quantitative assessments of 18F-FAZA uptake of corresponding tumor xenografts. Uptake expressed in terms of %IDmean/g. Vehicle cohorts represented by solid, black bars while open, spotted bars represent uptake in tumors from drug-treated animals. Tumors indicated by white arrows.
Lack of effect by BAY 87-2243 on 18F-FDG uptake in murine models of H460 and PC3 xenografts
When BAY 87-2243 was administered to nude mice carrying H460 tumor xenografts, no change in 18F-FDG uptake was observed (Figure 5A) between vehicle- and BAY 87-2243 treated mice at 3 days post-treatment (scans for 1 day post-treatment was not done). For mice carrying the human-derived PC3 xenografts, no significant difference in 18F-FDG was observed between vehicle- and BAY 87-2243-treated mice at 1 day and 3 days post-treatment (Figure 5B).
Figure 5.
Summary of the effects of BAY 87-2243 on the uptake of 18F-FDG, 18F-FLT and 18F-FPP(RGD)2 into H460 and PC3 tumor xenografts. BAY 87-2243 does not reduce the uptake of 18F-FDG in the (A) H460 human lung carcinoma and the (B) PC3 human prostate cancer xenograft models. Nude mice bearing pre-established H460 tumors (279 ± 54 mm3 in size) were injected with 18F-FDG (4.1 ± 0.2 MBq) via the tail vein before and 1 or 3 days after initial oral BAY 87-2243 treatment (9 mg/kg). PET imaging was performed 1 hour after the tracer injection. Quantitative tumor uptake of 18F-FDG was determined based on the PET scans. Data expressed as %IDmean/g and are given as the mean of n=6 tumors/treatment group ± SEM
BAY 87-2243 induces a modest reduction in uptake of 18F-FLT in the (C) H460 and the (D) PC-3 prostate carcinoma xenograft models. Nude mice bearing pre-established PC-3 tumors (177 ± 43 mm3 in size) were injected with 18F-FLT (4.1 ± 0.1 MBq) before and 1 and 4 days after initial BAY 87-2243 treatment (1 mg/kg and 9 mg/kg, respectively). PET imaging was performed 1 hour after the tracer injection. Quantitative tumor uptake of 18F-FLT was determined based on the microPET scans and expressed as %IDmean/g. Data are given as the mean of n=6 tumors/treatment group ± SEM.
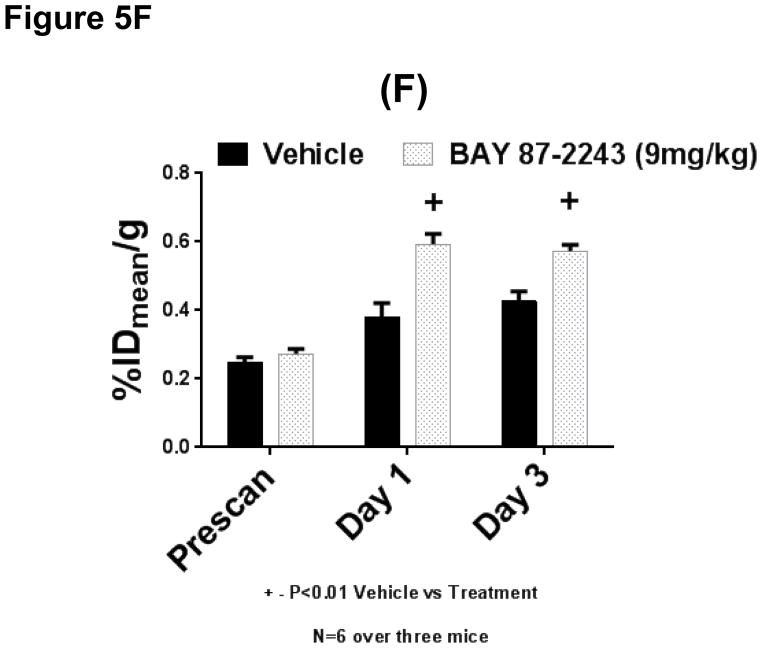
Modest induction of 18F-FPP(RGD)2 uptake, by BAY-87-2243, on H460 tumor xenograft models. (E) Single voxel plane coronal uPET scans (tracer is 18F-FPP(RGD)2) of nude mice containing H460 tumor xenografts. Scans performed on mice before treatment (left-most column) and 1 (middle column) and 3-days (right-most column) after treatment for either vehicle (upper panel) or for 9 mg/kg BAY 87-2243 (lower panel). (F) Quantitative assessments of 18F-FPP(RGD)2 uptake of corresponding tumor xenografts. Uptake expressed in terms of %IDmean/g. Vehicle cohorts represented by solid, black bars while open, spotted bars represent uptake in tumors from drug-treated animals. N=6 tumor over three mice per cohort. * or + − denotes P<0.01, vehicle vs. treatment. Tumors indicated by white arrows.
BAY 87-2243 reduces 18F-FLT uptake in both H460 human lung cancer and human PC3 prostate cancer xenografts
When the effect of BAY 87-2243 on 18F-FLT uptake was examined, by Day 1 post-treatment, there was a 25% reduction in %IDmean/g in treated H460 xenografts compared to vehicles (4.3±0.3 vs. 3.0±0.3 vehicle vs. treatment, p<0.01, n=6 tumors per group, Figure 5C) and there was a reduction by 26% of 18F-FLT uptake for PC3 xenografts (3.9±0.2 vs. 2.9±0.2 vehicle vs. treatment, p<0.01, n=6 tumors per group, Figure 5D). The capacity of BAY 87-2243 to reduce 18F-FLT uptake became more apparent by Day 5 when it was observed that drug-treated H460 xenografts had %IDmean/g values that were reduced by 28% compared to vehicle-controls (4.1±0.3 vs. 3.0±0.3 vehicle vs. treatment, p<0.01, n=6 tumors per group, Figure 5A) while in PC3 xenografts, the equivalent study yielded a reduction of 43% for drug-treated animals compared to vehicle-treated animals (4.0±0.2 vs. 2.4±0.2 vehicle vs. treatment, p<0.01, n=6 tumors per group, Figure 5B). Neither drug nor vehicle treatment significantly affected mouse weight during the time course of this study (data not shown).
Induction of 18F-FPP(RGD)2 uptake by BAY 87-2243 on H460 tumor xenograft models
When the effect of BAY 87-2243 on 18F-FPP(RGD)2 uptake was examined (Figure 5E), the drug increased %IDmean/g over vehicle-treated groups by 55% at 1 day after start of treatment (0.38±0.04 vs. 0.59±0.03, vehicle vs. drug, P<0.01, Figure 5F) but only by 33% at 3 days after initiation of treatment (0.43±0.03 vs. 0.57±0.02, vehicle vs. drug, P<0.01, Figure 5F). Such increases are beyond the boundaries of statistical reliability (corresponding to a coefficient of variation of ~11%) that was initially established by Chang et al. (24).
BAY 87-2243 (QD x 1) reduces pimonidazole and CD31 immunostaining in the H460 tumor xenograft model but with different kinetics
In nude mice bearing pre-established H460 tumor xenografts (80 mm3 in size), a single oral treatment was performed with vehicle or BAY 87-2243 (9 mg/kg). Pimonidazole staining (Figure 6A, arrows) occurred inhomogenously throughout the tumor and tended to describe irregular, circular patterns. Such patterns were consistent with previous observations (25, 26)of solid tumors. Treatment with 9mg/kg BAY 87-2243 resulted in significant reduction in pimonidazole staining (Figure 6B), a result that is consistent with the reduction in [18F]-FAZA uptake in BAY 87-2243 treated H460 xenografts.
Figure 6.
Pharmacodynamic effects on the effects of BAY87-2243 (qd x 1) on the reduction of pimonidazole immunostaining in the H460 lung carcinoma xenograft model. In nude mice, bearing pre-established H460 tumor xenografts (80 mm2 in size), a single oral treatment was performed with vehicle or BAY 87-2243 (9 mg/kg). One day after the treatment, hypoxyprobe (60 mg/kg, Natural Pharmacia International) was injected via the tail vein. Mice were sacrificed 1.5 hours after the hypoxyprobe injection. Tumors were excised and processed for pimonidazole staining according to the protocol provided by Natural Pharmacia International. Histological samples were processed for both (A) vehicle and (B) drug-treated H460 xenografts. BAY 87-2243 also promoted a decline in CD31 staining with respect to vehicle (C, D) but the kinetics is slower; in order to see a decrease, a dosage of qd x 4 was required. Kinetics of the loss of (E) percent hypoxia fraction (%PHF) and (F) percent hypoxia fraction (%PVF) was also calculated.
BAY 87-2243 suppresses HIF-1α target gene expression in the H460 lung carcinoma xenograft model at qd × 1 and qd × 3 dosing. Nude mice bearing pre-established H460 tumor xenografts (grown for 10 days) were treated orally with vehicle or BAY 87-2243 (9 mg/kg qd). RNA from xenograft tumors was isolated from satellite animal groups either before treatment or after 1 and 3 days of treatment with BAY 87-2243. Expression levels of the HIF target genes (G) carbonic anhydrase 9 (CA9), (H) angiopoietin-like 4 (ANGPTL4), and (I) HIF-prolyl hydroxylase-3 (EGLN-3) and (J) the non-HIF target gene HIF-prolyl hydroxylase-2 (EGLN-2; serving as a negative control) was quantified by RT-PCR. Data were normalized to β-actin and are given in arbitrary units as the mean of n=3 animals/treatment group ± standard deviation
The percent hypoxic fraction (PHF) of H460 xenografts at 80 mm3 declines from 28.1±4.5% to 2.6±1.2% (Figure 6E, p<0.001, vehicle vs. treatment, n=3) with one treatment of BAY 87-2243 (9mg/kg). Two to three administrations of BAY 87-2243 are required to induce a significant decrease in percent vascular fraction(PVH) (i.e. from 17.8±3% in vehicle-treated mice to 10.2±3.7% after two treatments to 4.5±1.5% after four BAY 87-2243 treatments; Figure 6C–F, p<0.005, n=3).
BAY 87-2243 suppresses HIF-1α target gene expression in the H460 tumor xenograft model at both 1 day and 3 days post-drug delivery
Analysis of HIF-1α target gene expression by RT-PCR revealed that BAY 87-2243 reduced expression of the hypoxia-regulated genes CA IX, ANGPTL4 and EGLN-3 by 99 %, 93 % and 83% respectively (Figure 6G–I, p<0.001 for three), which corresponds to reduced 18F-FAZA uptake upon drug treatment. EGLN-2, a gene not controlled by HIF-1α, was unaffected (Figure 6J). Reduced 18F-FLT uptake was not accompanied by reduced expression of TK1 (Supplemental Figure 5). Expression levels of genes associated with glucose metabolism did not fully match unchanged 18F-FDG uptake: GLUT-1 expression and GLUT-3 expression were reduced while HK-2 decreased by 75 % (Supplemental Figure 6, p<0.01). We detected low integrin beta-3 (ITGB3) expression in untreated xenografts but were unable to detect transcripts after BAY 87-2243 treatment (Supplemental Figure 5).
Discussion
BAY 87-2243 is effective in inhibiting the growth of human lung and prostate cancer xenografts (H460 and PC3) in nude mice whereas the human renal cell carcinoma 786-0 was shown to be resistant to BAY 87-2243 treatment. Of the four tracers studied, BAY 87-2243 affected most dramatically the uptake of 18F-FAZA (before significant changes in tumor volume). Thus, from a pragmatic standpoint, 18F-FAZA appears to be the most promising of these tracers for clinical translation. The decrease in 18F-FAZA uptake with BAY 87-2243 challenge suggests that the drug exerts its action through a process of reoxygenation. Indeed, tumor reoxygenation by BAY 87-2243 via inhibition of mitochondrial complex I has been reported by previous investigators(9).
The dramatic change in 18F-FAZA uptake does not preclude the potential success of other radiotracers that was studied to date. The known capability of BAY 87-2243 and related compounds to resolve tumor hypoxia(9, 10) would inevitably have downstream impact on the tumor microenvironment, on tumor proliferation and on glucose metabolism(27-30). However, while BAY 87-2243 consistently down-regulated hypoxia-associated gene expression in parallel with a diminution of 18F-FAZA tracer uptake, we did not observe such consistent changes in the expression of gene targets associated with angiogenesis, glucose metabolism or proliferation. In turn, the changes in uptake of the corresponding tracers were not as consistent as we had originally surmised. These findings illustrate the complex manner in which changes in the hypoxic status of a tumor affects downstream biological processes. The mutational status of a tumor (Kras, GLUT1 etc.) most likely will affect a cancer cell’s response to hypoxia as well(30). To tease out these complex interactions (and to assign appropriate tracers to track such processes) will involve several careful studies in the future.
[18F]-FAZA belongs to a class of tracers known as nitroimidazoles. A number of nitroimidzole-based tracers (e.g. misonidazole [MISO] or fluoromisonidazole[FMISO]) have seen clinical PET utilization(18, 31, 32). 18F-labelled fluoroazomycin arabinoside(Supplemental Figure 4) or [18F]-FAZA may represent some advance upon pre-existing nitroimidazole tracers because it is more readily diffusible across cell membrane yet more readily alkylating at neutral pH(31). Consequently, this may yield greater signal to noise (i.e., hypoxic tissue-to-normoxic tissue activity concentration) ratios than other nitroimidazoles such as fluoromisonidazole (FMISO), intravenous iodoacetate (IAA) or misonidazole (MISO) (16, 18, 33). Nevertheless, to establish translatability of 18F-FAZA, further studies on additional types of human hypoxic tumors are needed.
With increasing hypoxia, superoxide anions accumulate. Nitroimidzoles readily accept electrons from these highly reactive species and through a multistep process driven by hypoxia-activated nitroreductase (e.g. xanthine oxidase, lipoxygenases and NADPH oxidases) the hydroxylamine moiety of nitroimidazoles becomes reduced to a relatively inactive amine derivative(34). However, intermediates along this pathway are known to be highly alkylating and thus, readily form covalent bonds with resident macromolecules(31, 35). The molecular construct is then trapped within the cell. Thus the level of trapping is reflective of the amount of nitroreductase activity in the cell and therefore of the extent of hypoxia in tissue (31).
Changes in tumor oxygenation (i.e. pO2) are presumably related to changes in tumor vascularity and accessibility of blood-borne tracers to tumor(36). Thus, any abrogations in 18F-FAZA uptake as seen with BAY 87-2243 treatment may be a reflection of changes in accessibility by the nitroimidazole-like tracer to the cancer’s vasculature (and consequently, accessibility to the cancer tissue itself). If tracer accessibility would be truly compromised due to disruption of vascular normality by BAY 87-2243 treatment, then one would expect a general decline of all tracers employed in our studies. In fact, we observe tracer uptake being either increased ([18F]-FPP(RGD)2), decreased ([18F]-FLT) or unchanged([18F]-FDG) following administration of BAY 87-2243. Thus, the data in aggregate would suggest that changes in 18F-FAZA uptake tracks biochemical and genomic changes induced by hypoxic- (or hypoxia-like) stress occurring with BAY 87-2243 administration rather than a physiological alteration of blood vessel patency that results in reduced accessibility of tracer to tumor.
In assessing for the presence of pimonidazole immunostaining (an established marker for hypoxia), histological sections show a marked decline within one day after BAY 87-2243 treatment (Figure 6A–B). The reliability of such histological observations has been confirmed by follow-up molecular expression studies of HIF-1α-associated genes (carbonic anhydrase 9 (CA9), angiopoietin-like 4 (ANGPTL4), and HIF-prolyl hydroxylase-3 (EGLN-3) (see Figure 6)), which demonstrated that BAY 87-2243 significantly inhibits the expression of the corresponding genes. The subsequent loss in CD31 staining in H460 xenografts would suggest that the process of reoxygenation eventually diminishes vessel density within treated H460 xenografts. However, such an interpretation should be taken with caution as no such result after hypoxia resolution occurred with squamous cell carcinoma xenografts(10).
The contribution of BAY 87-243 to maintenance of prolyl hydroxylase activity has been proposed previously(9) based upon the observations that: a) BAY 87-2243 acts principally through inhibition of oxidative phosphorylation via inhibition of the mitochondrial complex I of the respiratory chain and b) HIF-1α stabilization was abrogated by inhibition of the complex I component of the mitochondrial respiratory chain. Helbig et al. (10) showed that a chemically similar drug, BAY-84-7296, resolved tumor hypoxia through the same mechanism, namely, by inhibition of the mitochondrial complex I. With drug challenge, hypoxia and hypoxic ROS activity are decreased and subsequently, there is less degradation of prolyl hydroxylase which leads to destabilization of HIF-1α. One consequence of reoxygenation is the loss of nitroreductase activity(13), thereby leaving less nitroimadazolamine products to be metabolized. As the FAZA tracer is a nitroimadozolamine, this would result in less 18F-FAZA tracer being trapped in tumors. 18F-FAZA uptake is thus an effective monitor of BAY 87-2243 efficacy as it can be argued that tracer uptake is related to the drug’s ability to form mitochondrial ROS and thereby reflect the degree to which HIF-1α activity (and hence response to hypoxia) has been compromised. This mechanism can also explain the lack of change in 18F-FAZA uptake with BAY 87-2243 treatment for 786-0 xenografts as 786-0 cells have high glycolytic activity and are not dependent upon oxidative phosphorylation(37, 38). Phenotypically, 786-0 cells present an oxygenated phenotype (as opposed to a hypoxic one) and thus baseline 18F-FAZA uptake would also be low. Functional VHL-1 complexes in 786-0 cells are lost(39), thus the lack of change in tracer uptake with BAY 87-2243 treatment would be consistent with a drug that targets the functionality of the VHL-1 complex in relation to O2 levels. In Ellinghaus et al. (9), BAY 87-2243 treatment of renal carcinoma RCC4 cells (that also possess a VHL loss-of-function mutation) did not show any effect on the constitutively activated HIF-1α pathway. This further supports our view of BAY 87-2243’s mode of action, that is, no direct effect on HIF-1α or components of the HIF-1α pathway
Taken together, we present here evidence that BAY 87-2243 abrogates hypoxia via complex I inhibition in responsive models such as H460 cells and PC3 cells. Most observations such as reduced FAZA tracer uptake and HIF-1α pathway suppression can be explained by such reoxygenation of the formerly hypoxic tumor areas. Responsive tumors are thus characterized by the occurrence of hypoxic areas, which is not fulfilled with the 786-O model, where 18F-FAZA uptake is unchanged. Furthermore, H460 and PC3 cells harbor functional VHL, which means that HIF-1α pathway is activated upon hypoxia thereby resulting in enhanced glycolysis that can contribute to ATP supply. Upon inhibition of complex I by BAY 87-2243, ATP generation via oxidative phosphorylation is diminished and at the same time reoxygenation abrogates HIF-1α pathway activation along with glycolysis-driven ATP generation. The concurrent reduction of ATP supply by complex I inhibition and diminished glycolysis together with suppression of growth promoting factors generated by an activated HIF-1α pathway may explain, in part, the observed growth inhibition of the H460 and PC3 xenograft models. However, the lack of significant alterations of 18F-FDG uptake upon BAY 87-2243 treatment suggests that the impact on diminished glycolysis/ATP supply remains uncertain. One needs to compare ATP levels on drug treatment between responsive and non-responsive models to resolve these conundrums.
One direction in future studies will involve delineating BAY 87-2243’s impact subsequent to changes in hypoxic status. Promising avenues include: effect of drug on entry of pentose into the Tri-carboxylic acid cycle, on glucose transport, on thymidine salvage, and on integrin induction. Furthermore, the inhibition of mitochondrial complex I (9, 10) by BAY 87-2243 might provide a new approach for sensitization towards localized radiotherapy of formerly hypoxic tumors that have now become reoxygenated (10). Such studies should provide crucial insights on targeting select patient populations and on improving choice and use of radiotracers for clinical monitoring of cancer therapies.
Supplementary Material
Translational Relevance.
The heterogeneous composition of cancers (especially hypoxic ones) results in variability of therapeutic response to (currently rigid) clinical regimens. We describe an innovative approach to individualize treatments by using a mechanism-associated PET imaging tracer (18F-FAZA) to closely monitor the efficacy of a novel anti-cancer drug, BAY 87-2243. By assessing the degree to which the drug affects tracer uptake, one can then judge the effectiveness of the drug and thereby adjust drug dosage at early time points before significant changes in tumor volumes. The level of tracer uptake can also be used to assess staging of the disease as well as evolution of resistance with treatment before significant reduction in the rate of tumor shrinkage. The utility of 18F-FAZA as a monitor of drug action also implies that in the future radiologists will have more PET tracers in their toolbox (other than the non-specific 18F-FDG) to monitor clinical conditions and therapies.
Acknowledgments
We thank the Stanford Small Animal Imaging Facility and the Stanford Radiochemistry Facility for their invaluable technical support
Funding Sources
This project was made possible by funding from Bayer Pharma AG.
Abbreviations
- 18F-FAZA
18F-fluoroazomycin arabinoside tracer
- 18F-FDG
18F-Deoxyglucose tracer
- 18F-FLT
18F-Thymidine tracer
- 18F-Fpp(RGD)2
18F-Pegylated cyclic RGD peptide dimer tracer
- 786-0
Human Renal Cell Carcinoma Cell
- ANGPTL4
Angiopoietin-like 4
- BAY 87-2243
1-Cyclopropyl-4-{4-[(5-methyl-3-{3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl}-1H- pyrazol-1-yl)methyl]pyridin-2-yl}piperazine
- CA9
Carbonic Anhydrase
- H460
Human Lung Cancer Cell Line
- HIF-1α
Hypoxia-Inducible Factor 1-alpha
- %ID/g
% Injected Dose per gram of tissue
- IHC
Immunohistochemistry
- EGLN-2
Prolyl hydroxylase-2
- EGLN-3
HIF-1α prolyl hydroxylase-3
- OCT
Optimal Cutting Temperature Matrix
- PBS
Phosphate-Buffered Saline
- PC3
Human Prostate Cancer Cell Line
- PD
Pharmacodynamic
- PET
Positron Emission Tomography
- RT-PCR
Real-time Polymerase Chain Reaction Technique
- VHL
Von Hippel-Lindau Tumor Suppressor
Footnotes
Potential Conflict of Interests: K.U., P.E., V.G., and D.B. are employees of Bayer-HealthCare AG. E.C., H.L. and S.S.G. have received grant support from Bayer-HealthCare AG. No conflict of interests with S.L. and Z.C.
References
- 1.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–93. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 2.Tseng JR, Stuart D, Aardalen K, Kaplan A, Aziz N, Hughes NP, et al. Use of DNA microarray and small animal positron emission tomography in preclinical drug evaluation of RAF265, a novel B-Raf/VEGFR-2 inhibitor. Neoplasia. 2011;13:266–75. doi: 10.1593/neo.101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng JR, Dandekar M, Subbarayan M, Cheng Z, Park JM, Louie S, et al. Reproducibility of 3'-deoxy-3'-18F-fluorothymidine microPET studies in tumor xenografts in mice. J Nucl Med. 2005;46:1851–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L, Greenwood TR, Artemov D, Raman V, Winnard PT, Jr, Heeren RM, et al. Localized hypoxia results in spatially heterogeneous metabolic signatures in breast tumor models. Neoplasia. 2012;14:732–41. doi: 10.1593/neo.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans SM, Hahn SM, Magarelli DP, Koch CJ. Hypoxic heterogeneity in human tumors: EF5 binding, vasculature, necrosis, and proliferation. Am J Clin Oncol. 2001;24:467–72. doi: 10.1097/00000421-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–74. [PubMed] [Google Scholar]
- 7.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer metastasis reviews. 2010;29:285–93. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer metastasis reviews. 2007;26:333–9. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 9.Ellinghaus P, Heisler I, Unterschemmann K, Haerter M, Beck H, Greschat S, et al. BAY 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities and regulates HIF-1a protein expression by inhibition of mitochondrial complex I. Cancer Medicine. 2013;2:611–24. doi: 10.1002/cam4.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helbig L, Koi L, Bruchner K, Gurtner K, Hess-Stumpp H, Unterschemmann K, et al. Hypoxia-inducible factor pathway inhibition resolves tumor hypoxia and improves local tumor control after single-dose irradiation. Int J Radiat Oncol Biol Phys. 2014;88:159–66. doi: 10.1016/j.ijrobp.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Life with Oxygen. Science. 2007;318:62–4. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009;100:1366–73. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin FT, Shen B, Liu S, Berganos RA, Chang E, Mittra E, et al. First experience with clinical-grade ([(1)(8)F]FPP(RGD(2)): an automated multi-step radiosynthesis for clinical PET studies. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2012;14:88–95. doi: 10.1007/s11307-011-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandekar M, Tseng JR, Gambhir SS. Reproducibility of 18F-FDG microPET studies in mouse tumor xenografts. J Nucl Med. 2007;48:602–7. doi: 10.2967/jnumed.106.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souvatzoglou M, Grosu AL, Roper B, Krause BJ, Beck R, Reischl G, et al. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: a pilot study. Eur J Nucl Med Mol Imaging. 2007;34:1566–75. doi: 10.1007/s00259-007-0424-3. [DOI] [PubMed] [Google Scholar]
- 17.Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, et al. Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine. 2005;62:897–901. doi: 10.1016/j.apradiso.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–13. [PubMed] [Google Scholar]
- 19.Picchio M, Beck R, Haubner R, Seidl S, Machulla HJ, Johnson TD, et al. Intratumoral spatial distribution of hypoxia and angiogenesis assessed by 18F-FAZA and 125I-Gluco-RGD autoradiography. J Nucl Med. 2008;49:597–605. doi: 10.2967/jnumed.107.046870. [DOI] [PubMed] [Google Scholar]
- 20.Knoess C, Siegel S, Smith A, Newport D, Richerzhagen N, Winkeler A, et al. Performance evaluation of the microPET R4 PET scanner for rodents. Eur J Nucl Med Mol Imaging. 2003;30:737–47. doi: 10.1007/s00259-002-1052-6. [DOI] [PubMed] [Google Scholar]
- 21.Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–9. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 22.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–7. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 23.Pimonidazole_protocol. http://searchcosmobiocojp/cosmo_search_p/search_gate2/docs/NPI_/HP210020080201pdf.
- 24.Chang E, Liu S, Gowrishankar G, Yaghoubi S, Wedgeworth JP, Chin F, et al. Reproducibility study of [(18)F]FPP(RGD)2 uptake in murine models of human tumor xenografts. Eur J Nucl Med Mol Imaging. 2011;38:722–30. doi: 10.1007/s00259-010-1672-1. [DOI] [PubMed] [Google Scholar]
- 25.Hendriksen EM, Span PN, Schuuring J, Peters JP, Sweep FC, van der Kogel AJ, et al. Angiogenesis, hypoxia and VEGF expression during tumour growth in a human xenograft tumour model. Microvasc Res. 2009;77:96–103. doi: 10.1016/j.mvr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Young RJ, Moller A. Immunohistochemical detection of tumour hypoxia. Methods Mol Biol. 2010;611:151–9. doi: 10.1007/978-1-60327-345-9_12. [DOI] [PubMed] [Google Scholar]
- 27.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 28.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 29.Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014;2:3. doi: 10.1186/2049-3002-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(Suppl 2):129S–48S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 32.Chapman JD. Hypoxic sensitizers--implications for radiation therapy. N Engl J Med. 1979;301:1429–32. doi: 10.1056/NEJM197912273012606. [DOI] [PubMed] [Google Scholar]
- 33.Beck R, Roper B, Carlsen JM, Huisman MC, Lebschi JA, Andratschke N, et al. Pretreatment 18F-FAZA PET predicts success of hypoxia-directed radiochemotherapy using tirapazamine. J Nucl Med. 2007;48:973–80. doi: 10.2967/jnumed.106.038570. [DOI] [PubMed] [Google Scholar]
- 34.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. European journal of nuclear medicine. 1995;22:265–80. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 35.Miwa GT, West SB, Walsh JS, Wolf FJ, Lu AY. Drug residue formation from ronidazole, a 5-nitroimidazole. III. Studies on the mechanism of protein alkylation in vitro. Chem Biol Interact. 1982;41:297–312. doi: 10.1016/0009-2797(82)90107-7. [DOI] [PubMed] [Google Scholar]
- 36.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 37.Biswas S, Troy H, Leek R, Chung YL, Li JL, Raval RR, et al. Effects of HIF-1alpha and HIF2alpha on Growth and Metabolism of Clear-Cell Renal Cell Carcinoma 786–0 Xenografts. J Oncol. 2010;2010:757908. doi: 10.1155/2010/757908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hervouet E, Cizkova A, Demont J, Vojtiskova A, Pecina P, Franssen-van Hal NL, et al. HIF and reactive oxygen species regulate oxidative phosphorylation in cancer. Carcinogenesis. 2008;29:1528–37. doi: 10.1093/carcin/bgn125. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Zhang W, Kondo K, Klco JM, St Martin TB, Dufault MR, et al. Gene expression profiling in a renal cell carcinoma cell line: dissecting VHL and hypoxia-dependent pathways. Mol Cancer Res. 2003;1:453–62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.