Abstract
The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) regulates a battery of antioxidant, detoxification, and cell stress genes. It is activated by oxidative stress and a number of exogenous compounds, one of which is tert-butylhydroquinone (tBHQ), a widely used food preservative. Nrf2 modulates immune responses in numerous rodent models of inflammation, but its effects on human immune cells are not well characterized. The purpose of these studies was to evaluate the effects of the Nrf2 activator tBHQ on early events of T cell activation in primary human cells. Treatment with tBHQ induced mRNA expression of the Nrf2 target genes HMOX-1, GCLC, and NQO1, and also increased NRF2 mRNA expression, albeit to a lesser extent than the other target genes. tBHQ decreased production of the cytokines IL-2 and IFN-γ at both the protein and mRNA levels after stimulation with anti-CD3/anti-CD28 in human peripheral blood mononuclear cells and to an even greater extent in isolated CD4 T cells. Likewise, tBHQ decreased induction of CD25 and CD69 in peripheral blood mononuclear cells and this decrease was even more marked in isolated CD4 T cells. In addition, tBHQ inhibited induction of NFκB DNA binding in anti-CD3/anti-CD28-activated PBMCs. Collectively, these data suggest that tBHQ inhibits activation of primary human CD4 T cells, which correlates with activation of Nrf2 and inhibition of NFκB DNA binding. Although these studies suggest the food additive tBHQ negatively impacts T cell activation, further studies will be needed to fully elucidate the effect of tBHQ on human immune response.
Keywords: Nrf2, CD4 T Cells, tBHQ, human PBMC
1. Introduction
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that acts as a sensor for oxidative stress. Under basal conditions, Nrf2 is tethered in the cytosol to its repressor protein, Kelch-like ECH-associated protein 1 (Keap1), which facilitates the ubiquitination and subsequent proteasomal degradation of Nrf2 [1]. After stimulation by reactive oxygen species or electrophilic stimuli, Nrf2 ubiquitination is disrupted so that Nrf2 translocates to the nucleus. Upon heterodimerizing with small Maf proteins or other binding partners, Nrf2 binds to antioxidant response elements to regulate the transcription of a number of detoxification, antioxidant, and cell stress-related genes [2, 3]. One activator of Nrf2 is tert-butylhydroquinone (tBHQ), a commonly used food additive found in a number of processed foods [4–6].
Nrf2 activation has been shown to have anti-inflammatory effects, and conversely, Nrf2 deletion has been shown to have pro-inflammatory effects [7–9]. Nrf2-null mice develop a lupus-like autoimmune disease, and have increased sensitivity to inflammation and infection in models such as sepsis and lung injury [10–14]. Recently, our laboratory demonstrated that Nrf2 modulates T cell responses in primary mouse CD4 T cells and Jurkat T cells [15, 16]. Collectively, this indicates that Nrf2 modulates immune responses in a variety of different models and cell types. However, the role of Nrf2 in the activation of primary human T cells remains unclear.
T cells are a critical part of the adaptive immune response. Helper (CD4) T cells direct the immune response to different pathogens; thus T cell activation is critical for an effective adaptive immune response. T cell activation is characterized by a number of early events which have important downstream effects, including production of early cytokines, such as IL-2 and IFNγ, and upregulation of CD25 and CD69. IL-2 acts in an autocrine/paracrine fashion to help drive the proliferation and clonal expansion of naïve T cells, among other functions [17]. IFN-γ is the signature cytokine produced by Th1 cells and is important in driving cell-mediated immunity [18]. CD25 is the high affinity subunit of the IL-2 receptor, whereas CD69 is a C-type lectin protein. Both CD25 and CD69 are considered cell surface markers of T cell activation [19]. Several transcription factors have been shown to be important in T cell activation, including NFAT, NFκB, and AP-1 [17, 20]. Our previous studies demonstrated that the Nrf2 activator tBHQ inhibits IL-2 secretion, CD25 expression, and NFκB induction in activated Jurkat cells [16]. The purpose of the present studies was to determine the effects of the Nrf2 activator tBHQ on the early events following T cell activation in primary human cells.
2. Methods
2.1 Peripheral Blood Mononuclear Cell (PBMC) and CD4 T Cell Isolation
Whole human blood was purchased from Innovative Research (Novi, MI). PBMCs were isolated using Lymphocyte Separation Medium following the manufacturer’s protocol (MP Biomedicals, Santa Ana, CA). CD4 T cells were isolated from PBMCs by positive selection using commercially available magnetic bead separation (Miltenyi Biotec, Auburn, CA). Cell treatments are described in figure legends. T cells were activated with purified hamster anti-human CD3ε (clone UCHT1, 1.5 µg/ml), purified hamster anti-human CD28 (clone CD28.2, 1.5 µg/ml), and an F(ab′)2 fragment specific for anti-Syrian hamster IgG that was used to cross-link CD3 and CD28. Anti-CD3 and anti-CD28 were purchased from Affymetrix/E-Bioscience (San Diego, CA), and the F(ab′)2 cross-linker from Jackson ImmunoResearch Laboratories (West Grove, PA).
2.2 Cytokine analysis
Concentrations of IL-2 or IFN-γ were quantified in cell supernatants using commercially available human IL-2 or IFN-γ ELISA kits following the manufacturer’s protocol (Biolegend, San Diego CA). Absorbance was quantified on a Bio-Tek µQuant microplate reader (Highland Park, VT).
2.3 mRNA quantification
Total RNA was isolated using TRIzol (Ambion, Life Technologies, Grand Island, NY) extraction according to the manufacturer’s protocol. RNA was reverse transcribed to cDNA after which quantitative-real time PCR was performed using Sybr green analysis (Applied Biosystems, Life Technologies). Fluorescence was detected by a Life Technologies/Applied Biosystems Sequence Detection System 7500, and relative transcript levels were quantified using the ΔΔCT method, comparing the target genes to ribosomal protein L13a. Primer sequences were acquired from qPrimerDepot (http://primerdepot.nci.nih.gov/) and are as follows: RPL13A forward primer, 5’GTTGATGCCTTCACAGCGTA-3’ and reverse primer, 5’-AGATGGCGGAGGTGCAG-3’; IL-2 forward primer, 5’-GCACTTCCTCCAGAGGTTTG-3’ and reverse primer 5’-TCACCAGGATGCTCACATTT-3’; IFN-γ forward primer 5’-TCAGCCATCACTTGGATGAG-3’ and reverse primer 5’-CGAGATGACTTCGAAAAGCTG-3’; CD69 forward primer, 5’-ACAGGAACTTGGAAGGACCC-3’ and reverse primer, 5’-AGAACAGCTCTTTGCATCCG-3’; CD25 forward primer, 5’-TAGGCCATGGCTTTGAATGT-3’ and reverse primer, 5’-ATACCTGCTGATGTGGGGAC-3’; NRF2 forward primer, 5’-TCTTGCCTCCAAAGTATGTCAA-3’ and NRF2 reverse primer, 5’-CACGGTCCACAGCTCATC-3’; NQO1 forward primer, 5’-TCCTTTCTTCAAAGCCG-3’ and NQO1 reverse primer, 5’-GGACTGCACCAGAGCCAT-3’; HMOX-1 forward primer, 5’-GGCTTCCCTCTGGGAGTCT-3’ and HMOX-1 reverse primer, 5’-AGCTGCTGACCCATGACAC-3’; GCLC forward primer, 5’-CTTTCTCCCCAGACAGGACC-3’ and GCLC reverse primer 5’-CAAGGACGTTCTCAAGTGGG-3’ All primers were synthesized by Integrated DNA Technologies (Coralville, IA).
2.4 Flow Cytometry
PBMCs or isolated CD4 cells were labeled with CD4-FITC (Affymetrix/E-Bioscience), CD69-PE/Cy7 (Biolegend), and CD25-APC (Affymetrix/E-Bioscience) for 30 min in the presence of an FcR blocking reagent (Miltenyi Biotec, Auburn, CA) and then washed. Fluorescence was detected by a C6 BD Accuri flow cytometer (BD Accuri, San Jose, CA). Fluorescence was quantified using CFlow software (BD Accuri).
2.5 ELISA-based DNA binding assay
PBMCs were treated with tBHQ (1 µM) or vehicle, then activated with anti-CD3/anti-CD28 30 min later. Three hours after activation, nuclear protein was extracted from 1x107 cells using a commercially available kit (Active Motif, Carlsbad, CA). After extraction, nuclear protein was quantified via the Bradford assay (BioRad). NFκB DNA binding was quantified from 10 µg of nuclear protein, by a commercially available ELISA-based DNA binding assay (Active Motif). Assays were performed per manufacturer’s protocol.
2.5 Statistical analysis
All data were analyzed using SigmaPlot 12.3 (Systat, Chicago, IL). Data were analyzed by one-way ANOVA followed by a Dunnett’s two-tailed post-hoc test. Data are expressed as mean ± standard error. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1 The food additive tBHQ induces expression of the Nrf2 target genes HMOX-1, NQO1, and GCLC in human peripheral blood mononuclear cells (PBMCs)
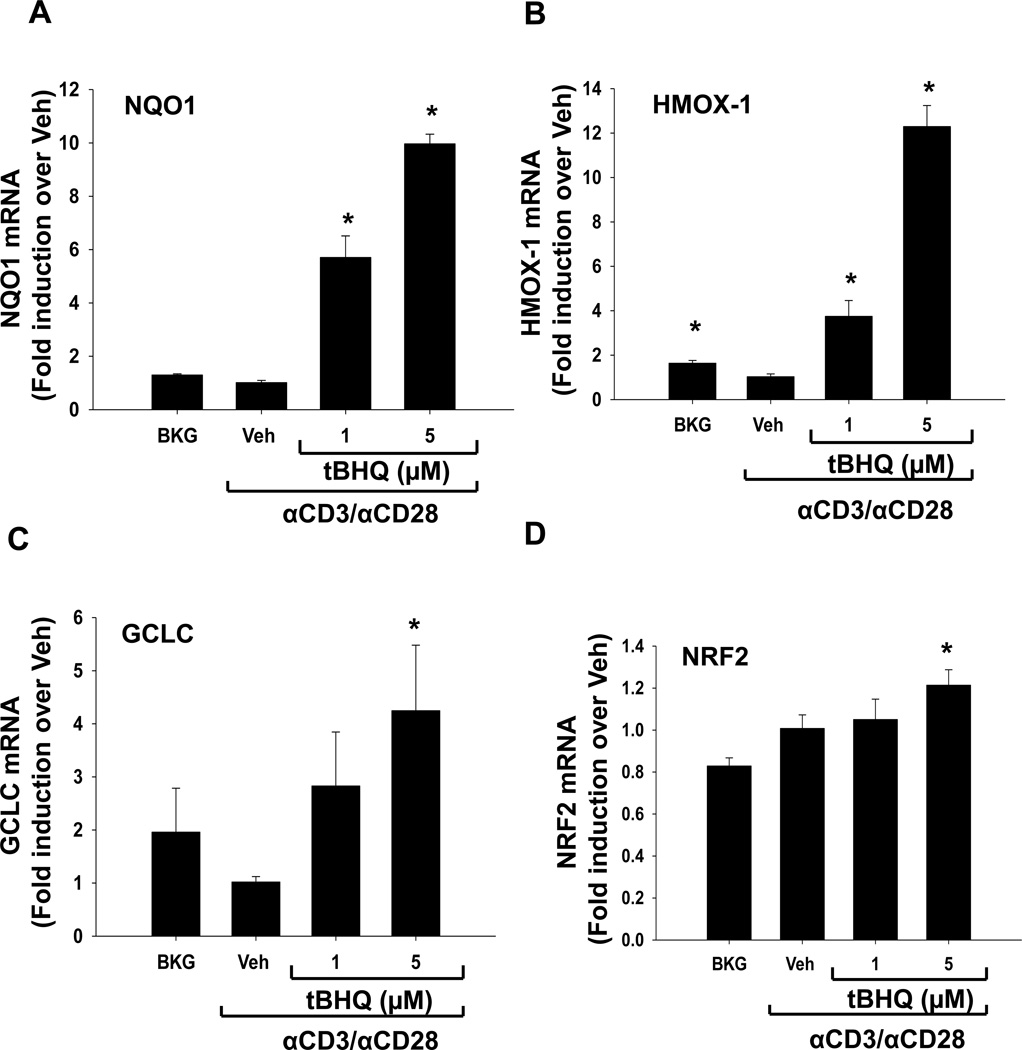
tBHQ is a known activator of Nrf2, and accordingly we investigated the ability of our tBHQ treatment (0.1–5µM) to induce expression of Nrf2 target genes. Consistent with other cell types, tBHQ treatment increased expression of the Nrf2 target genes, heme oxygenase 1 (HMOX-1), NAD(P)H quinone oxidoreductase 1 (NQO1), and glutamate-cysteine ligase, catalytic subunit (GCLC), in human peripheral blood mononuclear cells (Fig. 1) [15, 16]. In addition, tBHQ caused a modest induction of NRF2 mRNA expression itself, which has also been observed previously in primary mouse T cells and suggests that NRF2 upregulates itself. Taken together, these data suggest that the concentrations of tBHQ used in this study activate Nrf2 in primary human PBMCs.
Figure 1. tBHQ treatment induces expression of the Nrf2 target genes NQO1, GCLC, and HMOX as well as NRF2 expression in PBMCs.
PBMCs were either left untreated (Bkg), or pretreated with vehicle (Veh, 0.01% ethanol) or tBHQ (1–5µM) and then activated 30 min later with anti-CD3/anti-CD28. Total RNA was isolated after 6h and mRNA expressions of (A) NQO1, (B) GCLC, (C) HMOX, and (D) NRF2 were quantified by quantitative RT-PCR. * indicates p<0.05 compared to the Veh group.
3.2 The Nrf2 activator tBHQ inhibits production of IL-2 in human PBMCs and isolated CD4 T cells activated with anti-CD3/anti-CD28
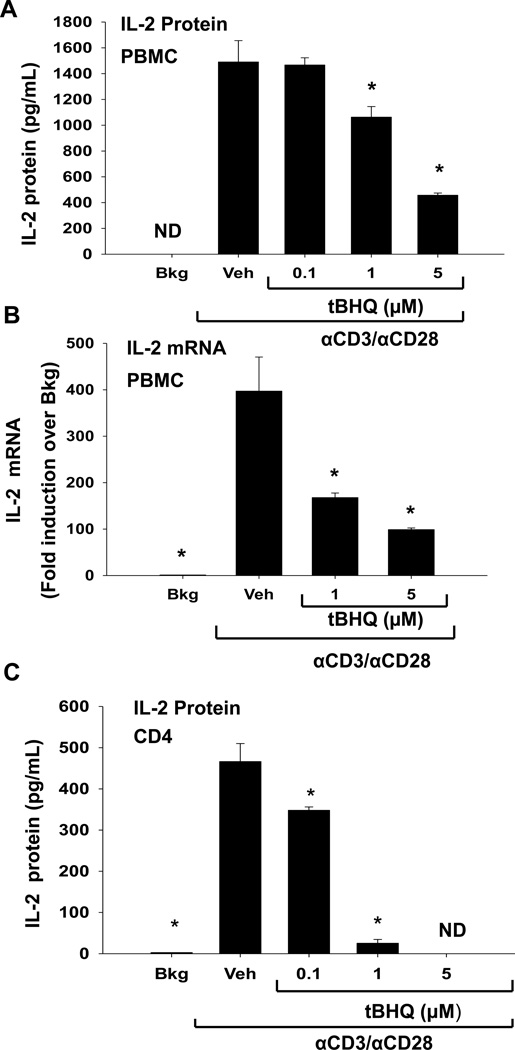
Our previous studies showed the Nrf2 activator tBHQ inhibited IL-2 production in activated Jurkat T cells, but the effect of tBHQ on primary human T cells has not been fully characterized [16]. Therefore, we determined the effect of tBHQ treatment on IL-2 production by PBMCs activated with anti-CD3/anti-CD28, a T cell specific activator. IL-2 protein and mRNA levels were significantly decreased by tBHQ in PBMCs activated with anti-CD3/anti-CD28 (Fig. 2A, B). Interestingly, tBHQ caused a more marked inhibition of IL-2 production in isolated CD4 T cells activated with anti-CD3/anti-CD28, suggesting that isolated T cells are more sensitive to tBHQ than T cells in the mixed PBMC population (Fig. 2C). However, tBHQ did not affect cell viability at these concentrations (data not shown). These studies demonstrate that tBHQ inhibits IL-2 secretion in primary human PBMCs and CD4 T cells, consistent with previous studies in Jurkat cells.
Figure 2. tBHQ treatment inhibits production of the early cytokine IL-2.
(A-B) PBMCs or (C) CD4 cells were either left untreated (Bkg), or pretreated with vehicle (Veh, 0.01% ethanol) or tBHQ (0.1–5µM) and then activated 30 min later with anti-CD3/anti-CD28. (A, C) Supernatants were collected after 24h and analyzed for IL-2 by ELISA. (B) Total RNA was isolated from PBMCs after 6h and mRNA expression of IL-2 was quantified by quantitative RTPCR. * indicates p<0.05 compared to the Veh group, ND indicates samples below the limit of detection.
3.3 The Nrf2 activator tBHQ inhibits production of IFNγ in human PBMCs and isolated CD4 T cells activated with anti-CD3/anti-CD28
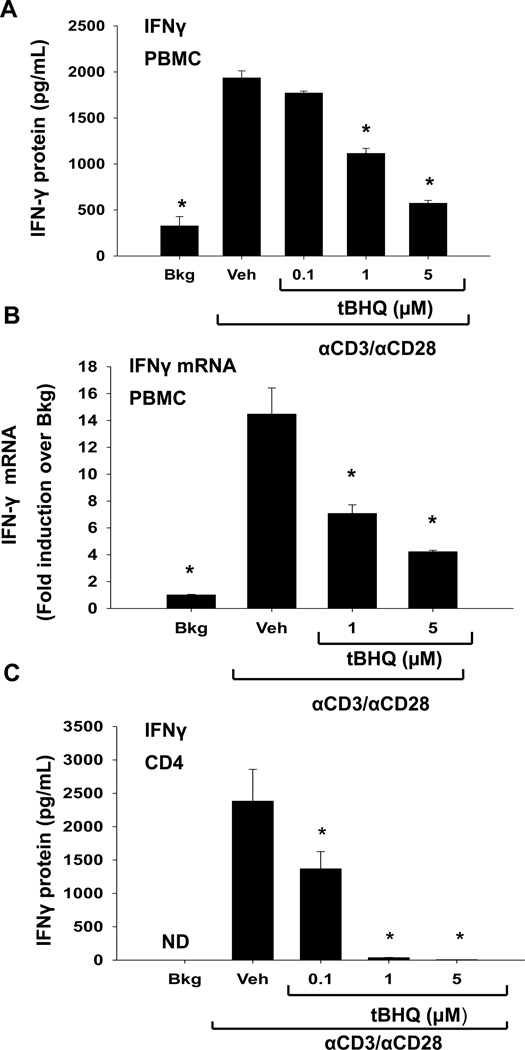
The inhibition of IL-2 production with tBHQ treatment prompted us to investigate the effect of tBHQ on production of IFNγ, which, like IL-2, is rapidly induced in T cells in response to stimulation with anti-CD3/anti-CD28. Similar to its effect on IL-2, tBHQ significantly inhibited IFNγ protein and mRNA production in activated PBMCs (Fig. 3A, B). Likewise, tBHQ inhibited IFNγ production to an even greater extent in isolated CD4 T cells as compared to T cells in the mixed PBMC population (Fig. 3 C). Collectively, these studies demonstrate that tBHQ inhibits IL-2 and IFNγ secretion in primary human PBMCs and isolated CD4 T cells.
Figure 3. tBHQ treatment inhibits production of the cytokine IFNγ.
A-B) PBMCs or (C) CD4 cells were either left untreated (Bkg), or pretreated with vehicle (Veh, 0.01% ethanol) or tBHQ (0.1–5µM) and then activated 30 min later with anti-CD3/anti-CD28. (A, C) Supernatants were collected after 24h and analyzed for IFNγ by ELISA. (B) Total RNA was isolated from PBMCs after 6h and mRNA expression of IFN-γ was quantified by quantitative RTPCR. * indicates p<0.05 compared to the Veh group, ND indicates samples below the limit of detection.
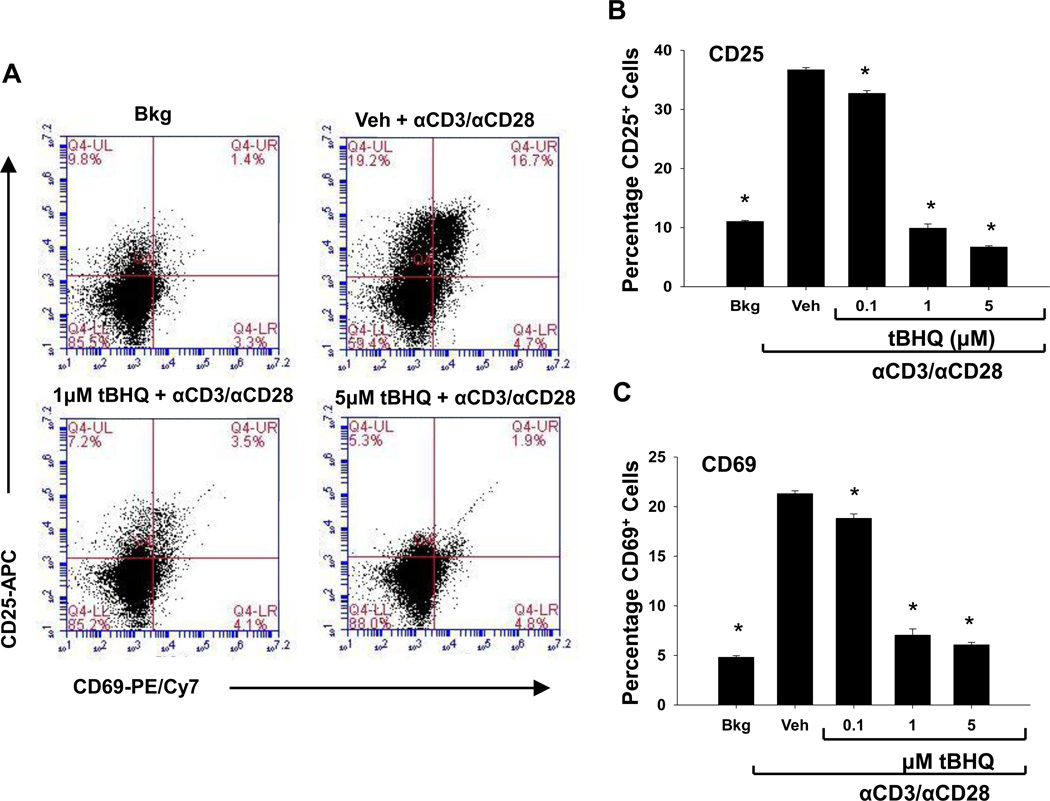
3.4 The food additive tBHQ inhibits the expression of CD25 and CD69 in PBMCs and primary CD4 T cells
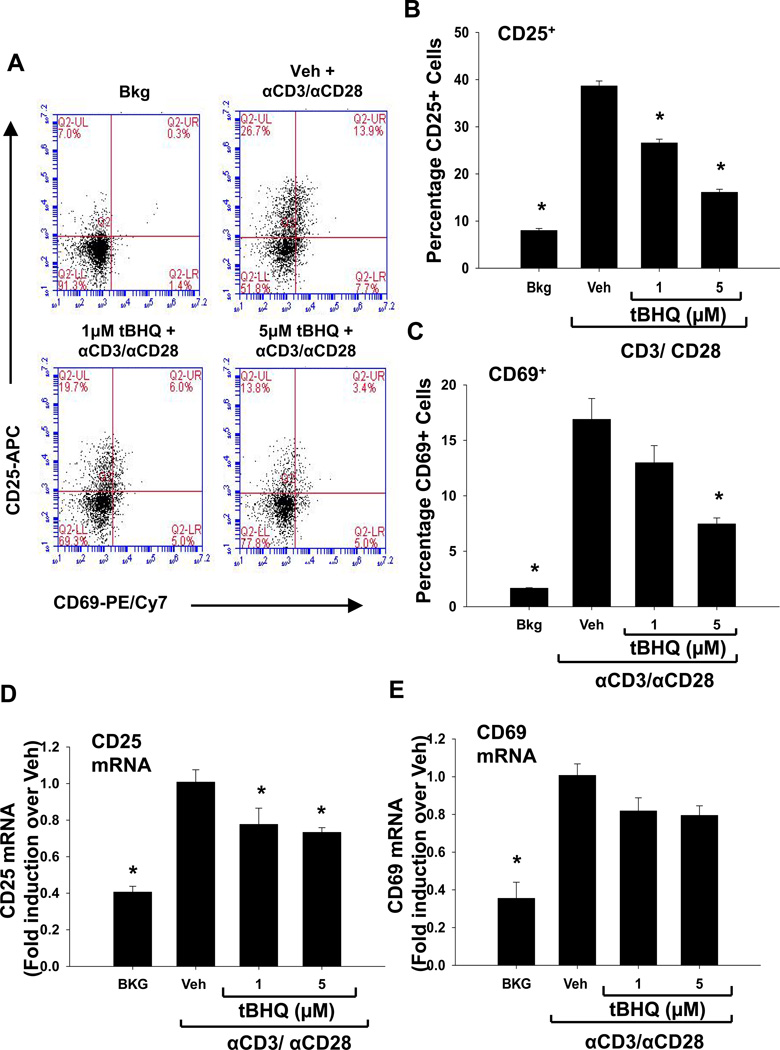
The inhibition of IL-2 and IFN-γ by tBHQ in anti-CD3/anti-CD28-activated T cells prompted us to investigate the effect of tBHQ on other early events following T cell activation. Shortly after T cell activation, the cell surface proteins, CD25 and CD69, are induced, so we next quantified CD25 and CD69 expression. CD25 protein expression was significantly decreased compared to vehicle at 1µM and at 5µM tBHQ in the PBMCs and at 0.1µM, 1µM, and 5µM the CD4 T cells (Fig. 4 A and B and Fig. 5, A and B). Similarly, CD69 protein expression was also decreased by tBHQ in both (Fig. 4 A and C, Fig. 5, A and C). At the mRNA level, CD25 expression was inhibited upon tBHQ treatment in PBMCs (Fig. 4 D). CD69 mRNA expression in the PBMCs showed a trend toward a decrease with tBHQ treatment, but was not statistically significant (Fig. 4 E). Isolated CD4 T cells were more sensitive to the effects of tBHQ on CD25 and CD69 than CD4 T cells cultured in the mixed PBMC population. Overall, these studies indicate that the Nrf2 activator tBHQ inhibits multiple events associated with early T cell activation.
Figure 4. tBHQ treatment inhibits the expression of the early cell surface markers CD25 and CD69 in CD4+ T cells within the PBMC cell population.
PBMCs were either left untreated (Bkg), or pretreated with vehicle (Veh, 0.01% ethanol) or tBHQ (0.1–5µM) and then activated 30 min later with anti-CD3/anti-CD28. Cells were collected after 24h and labeled with fluorochrome-conjugated antibodies against CD25 and CD69. CD4+ T cells were gated prior to analysis of CD25 and CD69 expression. A) Representative dot plots from a PBMC experiment, and quantification of (B) CD25 and (C) CD69 protein expression. Total RNA was isolated from PBMCs 6h after activation, and mRNA expressions of (D) CD25 and (E) CD69 were quantified by q-RT-PCR. * indicates p<0.05 compared to the Veh group.
Figure 5. tBHQ treatment inhibits the expression of the early cell surface markers CD25 and CD69 in isolated CD4+ T cells.
CD4 T cells were either left untreated (Bkg), or pretreated with vehicle (Veh, 0.01% ethanol) or tBHQ (0.1–5µM) and then activated 30 min later with anti-CD3/anti-CD28. Cells were collected after 24h and labeled with fluorochrome-conjugated antibodies against CD25 and CD69. CD4+ T cells were gated prior to analysis of CD25 and CD69 expression. A) Representative dot plots and quantification of (B) CD25 and (C) CD69 protein expression. * indicates p<0.05 compared to the Veh group.
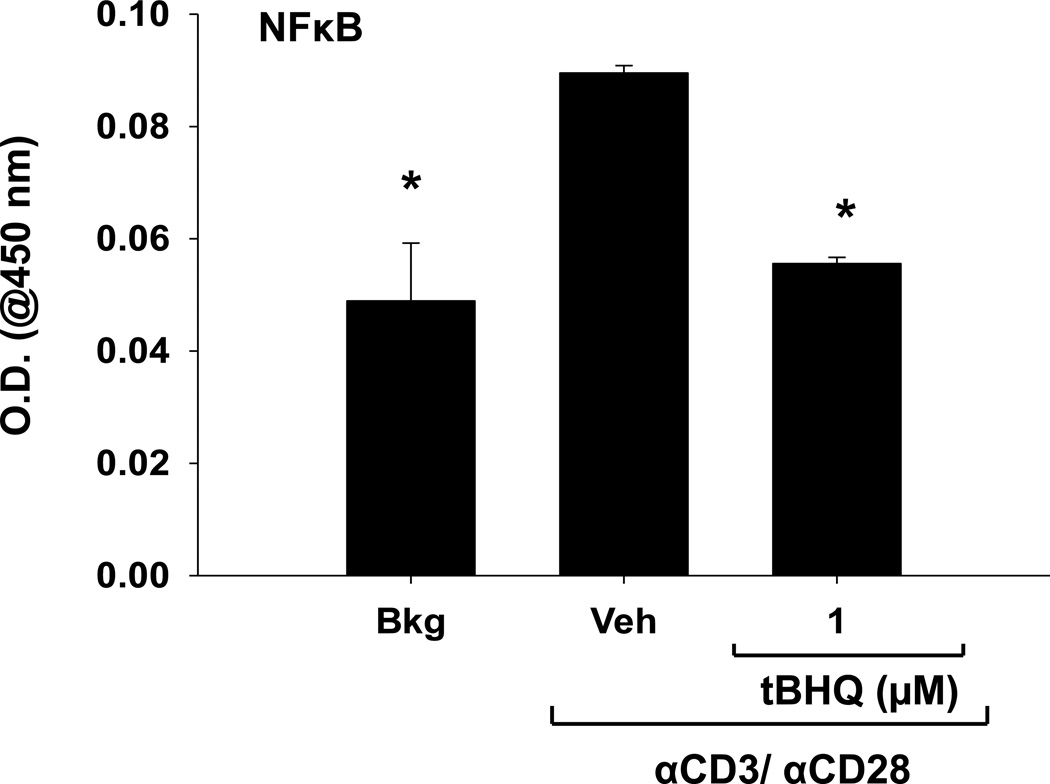
3.5 The Nrf2 activator tBHQ inhibits NFκB DNA binding in PBMCs
Because tBHQ affects multiple genes induced after T cell activation, we next considered transcription factors that regulate such genes. Several transcription factors have been shown to be involved in T cell activation, including NFAT, AP-1, and NFκB, all of which regulate IL-2 expression, among other genes. In addition, previous studies have shown that tBHQ treatment decreases NFκB activation in other cell types [16, 21]. Accordingly, we next investigated the effect of tBHQ treatment on NFκB activation. 3h after activation with anti-CD3/anti-CD28, we observed a decrease in NFκB DNA binding in PBMCs treated with 1µM tBHQ (Fig. 6). Taken together, these studies indicate that tBHQ treatment inhibits multiple events of T cell activation, which correlates with inhibition of NFκB activity.
Figure 6. tBHQ treatment inhibits NFκB DNA binding in PBMCs.
PBMCs were left untreated (Bkg), or pretreated with vehicle (Veh, 0.01% ethanol) or tBHQ (1µM), then activated 30 min later with anti-CD3/anti-CD28. 3h after activation, cells were collected and nuclear protein was isolated. p65 NFκB DNA binding activity was quantified spectrophotometrically by measuring absorbance at 450 nm.
4. Discussion
The current studies are the first to demonstrate that the Nrf2 activator tBHQ inhibits many of the early events associated with T cell activation in primary human T cells. We have shown that tBHQ treatment upregulates the Nrf2 target genes HMOX-1, GCLC, and NQO1, which strongly suggests Nrf2 is activated at the concentrations of tBHQ used in these studies. This is consistent with studies by Morzadec et. al., where treatment of human CD4 T cells with tBHQ induced NQO1 mRNA expression [22]. We also show that tBHQ markedly inhibits production of IFNγ and IL-2 in anti-CD3/anti-CD28-activated PBMCs and to an even greater extent in isolated human CD4 T cells. Similarly, tBHQ significantly suppressed the induction of CD25 and CD69 in CD4 T cells. Finally, tBHQ treatment decreases NFκB DNA binding activity in PBMCs. Collectively, these studies suggest that tBHQ inhibits activation of primary human T cells, as evidenced by decreased cytokine production and decreased expression of CD25 and CD69.
Interestingly, the isolated CD4 T cells were more sensitive to tBHQ than the PBMCs, with a greater decrease in IFNγ and IL-2 protein production and a greater reduction in CD25 and CD69 cell surface upregulation at the same concentration of tBHQ. In addition, tBHQ caused a decrease in cytokine production at lower concentrations in isolated CD4 T cells compared to PBMCs. This difference is likely due to the presence of other cells types in the PBMC population which seemingly exert a protective effect. For example, the presence of other immune cell types in the PBMC population likely increases the costimulatory signals received by T cells, which may be protective against the negative effect of tBHQ on T cell activation.
The U.S. Food and Drug Administration (FDA) set a maximum limit for tBHQ of 200 mg/kg in foods, which is lower than the international standard for certain food types [23]. Because tBHQ is most often used to prevent rancidification of oils, this limit is typically applied to cooking oils, but is also applicable to many other types of food as well. Although the acceptable daily intake (ADI) for tBHQ is currently 0.7 mg/kg/day, actual intake is difficult to determine. A variety of different methods have been used to estimate the daily intake of tBHQ in the U.S. and other countries. Estimation by poundage in the U.S. yielded values 80–90% below the ADI. However, estimation based upon model diets yielded much higher values in which the mean intake was roughly half the ADI, whereas intake of individuals in the 90th percentile was estimated to exceed the ADI (0.74 mg/kg/day). Estimates based upon the international standards were considerably higher (1.2 mg/kg/day), as were estimates of daily tBHQ intake in other countries, such as the U.K. and Australia/New Zealand (0.7 – 6.9 mg/kg/day) [4]. Notably, it has been reported that healthy volunteers who consumed 100 – 150 mg of tBHQ had serum concentrations in the high micromolar range [24]. Because many of the estimated daily intakes in the U.S. and other countries approach or even surpass 100 – 150 mg/day of tBHQ, this suggests that the concentrations of tBHQ used in the current studies are relevant and applicable to humans.
Several transcription factors have been shown to be important in T cell activation, including NFAT, AP-1, and NF-κB, all of which regulate IL-2 as well as other genes that are induced upon T cell activation [17]. An important signaling event in T cell activation is calcium influx, which ultimately leads to NFAT activation [17]. Although our recent studies showed that calcium influx was delayed and decreased by tBHQ in activated Jurkat cells, NFAT activation was not affected by tBHQ. However, these studies demonstrated that tBHQ inhibited NFκB p65 transcriptional activity in activated Jurkat T cells [16]. Previous studies have also shown that Nrf2 inhibits NF-κB signaling in various cell types, making this a likely mechanism for the effects seen in the current studies [11]. Indeed, we see that tBHQ treatment decreases NFκB DNA binding activity in nuclear extracts from PBMCs.
Activation of T cells is an important event for the initiation of a variety of adaptive immune responses. Such responses are critical for host defense against a number of bacterial and viral pathogens as well as fungi and parasites. The current studies are the first to demonstrate that the food preservative tBHQ impairs primary human T cell activation as evidenced by decreased expression of CD25 and CD69 in CD4 T cells and suppressed production of the cytokines IFNγ and IL-2. Further studies will be needed to determine the effect of tBHQ on human host defense, however. Overall, these studies suggest that Nrf2 represents a novel regulatory pathway in primary human T cells.
Highlights.
-
1)
The food additive tBHQ activates the transcription factor Nrf2 in human PBMCs.
-
2)
tBHQ inhibits production of IL-2 and IFN-γ in activated CD4 T cells.
-
3)
The Nrf2 activator tBHQ inhibits induction of CD25 and CD69 in activated CD4 T cells.
-
4)
NFκB DNA binding is suppressed by tBHQ in anti-CD3/anti-CD28-activated PBMCs.
Acknowledgements
This work was supported by the NIH grants ES018885 and T32 ES007255. The authors would like to thank Bob Crawford, Heather Dover, Jenna Bursley, Kelly VanDenBerg, Tyler Maser and the lab of Dr. Bryan Copple for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alexandra E. Turley, Email: turleyal@msu.edu.
Joseph W. Zagorski, Email: zagorsk4@msu.edu.
Cheryl E. Rockwell, Email: rockwelc@msu.edu.
References
- 1.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 3.Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Safety Evaluation of Certain Food Additives. International Programme on Chemical Safety. 1999:42.
- 5.Wang XJ, Hayes JD, Higgins LG, Wolf CR, Dinkova-Kostova AT. Activation of the NRF2 signaling pathway by copper-mediated redox cycling of para- and ortho-hydroquinones. Chem Biol. 2010;17:75–85. doi: 10.1016/j.chembiol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, Abdalrahman A, Lai Y, Janicki JS, Ward KW, Meyer CJ, Wang XL, Tang D, Cui T. Dihydro-CDDO-trifluoroethyl amide suppresses inflammatory responses in macrophages via activation of Nrf2. Biochem Biophys Res Commun. 2014;444:555–561. doi: 10.1016/j.bbrc.2014.01.101. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Khor TO, Saw CL, Lin W, Wu T, Huang Y, Kong AN. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol Pharm. 2010;7:2185–2193. doi: 10.1021/mp100199m. [DOI] [PubMed] [Google Scholar]
- 10.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 11.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rockwell CE, Zhang M, Fields PE, Klaassen CD. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol. 2012;188:1630–1637. doi: 10.4049/jimmunol.1101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zagorski JW, Turley AE, Dover HE, VanDenBerg KR, Compton JR, Rockwell CE. The Nrf2 activator, tBHQ, differentially affects early events following stimulation of Jurkat cells. Toxicol Sci. 2013;136:63–71. doi: 10.1093/toxsci/kft172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 18.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 19.Biselli R, Matricardi PM, D'Amelio R, Fattorossi A. Multiparametric flow cytometric analysis of the kinetics of surface molecule expression after polyclonal activation of human peripheral blood T lymphocytes. Scand J Immunol. 1992;35:439–447. doi: 10.1111/j.1365-3083.1992.tb02879.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Ma Q, Kinneer K, Ye J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64:211–219. doi: 10.1124/mol.64.2.211. [DOI] [PubMed] [Google Scholar]
- 22.Morzadec C, Macoch M, Sparfel L, Kerdine-Romer S, Fardel O, Vernhet L. Nrf2 expression and activity in human T lymphocytes: stimulation by T cell receptor activation and priming by inorganic arsenic and tert-butylhydroquinone. Free Radic Biol Med. 2014;71:133–145. doi: 10.1016/j.freeradbiomed.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 23.USFDA. Title 21 - Food and Drugs. 2013. FDA food and drug regulations 21CFR172.185. [Google Scholar]
- 24.WHO. Toxicological evaluation of some food colours, thickening agents, and certain other substances. WHO Food Additive Series No. 8. 1975