Abstract
Purpose
Electron paramagnetic resonance (EPR) oximetry using variable length multi-probe implantable resonator (IR), was used to investigate the temporal changes in the ischemic and contralateral brain pO2 during stroke in rats.
Material and Methods
The EPR signal to noise ratio (S/N) of the IR with four sensor loops at a depth of up to11 mm were compared with direct implantation of lithium phthalocyanine (LiPc, oximetry probe) deposits in vitro. These IRs were used to follow the temporal changes in pO2 at two sites in each hemisphere during ischemia induced by left middle cerebral artery occlusion (MCAO) in rats breathing 30% O2 or 100% O2.
Results
The S/N ratios of the IRs were significantly greater than the LiPc deposits. A similar pO2 at two sites in each hemisphere prior to the onset of ischemia was observed in rats breathing 30% O2. However, a significant decline in the pO2 of the left cortex and striatum occurred during ischemia but no change in the pO2 of the contralateral brain was observed. A significant increase in the pO2 of only the contralateral non-ischemic brain was observed in the rats breathing 100% O2. No significant difference in the infarct volume was evident between the animals breathing 30% O2 or 100% O2 during ischemia.
Conclusions
EPR oximetry with IRs can repeatedly assess temporal changes in the brain pO2 at four sites simultaneously during stroke. This oximetry approach can be used to test and develop interventions to rescue ischemic tissue by modulating cerebral pO2 during stroke.
Keywords: cerebral ischemia, reperfusion, stroke, EPR oximetry, implantable resonator, pO2
INTRODUCTION
Tissue pO2 is one of the most important factors in various physiological and pathological processes. Therefore, it will be very useful to develop methods for a direct assessment of tissue pO2 with sufficient sensitivity, accuracy and ease (1,2) to enhance our understanding of pathological processes, such as stroke, which is the foremost cause of long-term disability and mortality in the United States.
Several methods have been developed to measure pO2, including the Clark electrode, optical methods based on fluorescence quenching or phosphorescence quenching, MRI, PET and EPR oximetry (3–8). Each of these methods has specific advantages as well as limitations, especially for repeated measurement of cerebral pO2 (5). Clark electrodes cause trauma when inserted into the brain and potentially affect the pO2 measurements (3). Optical techniques have a very limited depth of measurement (< 2 mm) when made non-invasively from the surface. An alternate approach using an insertable fiberoptic probe can provide measurements at greater depths, but cause acute trauma at the time of measurement (6,9). On the other hand, the methods based on hemoglobin do not provide direct measure of tissue pO2. MRI, PET and EPR imaging require repeated injections of contrast agents, tracers or oximetry probes for serial measurement of the oxygen levels in the tissue of interest (4,7,8). EPR imaging with water soluble probes provide either an average pO2 over a large tissue volume (~0.5 cm3) with high temporal resolution using conventional spectroscopy or high spatial resolution (~1 mm3) though imaging (5). These methods have provided very useful information, however, the need for repeated injection of the probes and tissue trauma associated with some methods limits in vivo applications.
We have focused on the development of EPR oximetry using particulate probes, which requires a one-time implantation of the probe by a minimally invasive procedure but rest of the method for pO2 measurement is entirely non-invasive and can be repeated as desired (2,10–12). The basis of EPR oximetry is the paramagnetic nature of molecular oxygen, which affects the EPR line widths of other paramagnetic species, such as LiPc (oximetry probe), in its vicinity by altering their relaxation rates. The magnitude of change in the EPR line width is directly related to the amount of oxygen that is present in the environment of the paramagnetic probes. The useful properties of LiPc for EPR oximetry are their stability and the strong response of their spectra to the presence of oxygen. The physicochemical properties of the LiPc crystals have been described earlier (1,13)
Tissue pO2 measurements using 1.2 GHz EPR spectrometers can be made at depths of less than 10 mm from the surface, which has limited its application to mainly small experimental models. Consequently, we have developed IRs that can be used to assess pO2 at depths greater than 10 mm from the surface (14). We report the application of IRs with four-probes to assess the dynamic changes in the regional oxygen levels at different depths (3 mm and 5 mm) during ischemia by MCAO in rats. We also have investigated the effect of 100% O2 breathing on ischemic and contralateral brain pO2 and its consequence on infarct volume. These results indicate that EPR oximetry with multiple probes IRs can provide crucial information on the temporal changes in the rat brain pO2 during stroke. We anticipate that this oximetry approach can also be used to study the effect of other pathologies, such as traumatic brain injury and intracranial hemorrhage, on cerebral pO2 and develop interventions that can improve oxygen levels to minimize tissue damage.
MATERIALS AND METHODS
Animal and experimental groups
The Dartmouth College Animal Care and Use Program approved the animal protocol. Male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA), each weighing 250–300 g were randomly divided into two experimental groups: (i) Control group (30% O2, N=8); (ii) Hyperoxia group (100% O2, N=6). All animals had EPR probes placed 5–7 days prior to MCAO. The animals were subjected to a 2 h of ischemia followed by reperfusion and sacrifice 22 h later.
Transient middle cerebral artery occlusion (MCAO)
Animals were anesthetized with 2–2.5% isoflurane in 30% O2 for all surgical procedures. During surgery, the body temperature was monitored by a rectal probe and the body temperature was maintained at 37 ± 0.5 °C with the help of a warm water pad. The left middle cerebral artery (MCA) was occluded for 2h with subsequent reperfusion according to the method reported by Longa et al. (15) with few modifications (11,12). Briefly, the left common carotid artery (CCA), internal carotid artery (ICA) and external carotid artery (ECA) were exposed though a midline incision of the neck. A silicone rubber-coated monofilament nylon suture (tip diameter 0.26 mm) was inserted into the ECA through a stump, and the CCA was kept open and intact. The suture was advanced into the ICA 18–20 mm beyond the carotid bifurcation for occlusion to induce ischemia. The neck incision was closed with a silk suture and the rats were kept anesthetized. The incision was re-opened 2h later and the intraluminal suture was carefully withdrawn to allow reperfusion. The CCA and ICA were inspected to ensure the return of pulsation indicative of reperfusion. The incision was again closed and the animals were allowed to recover from anesthesia. The rats then were kept for 22 h with free access to food and water.
All rats were sacrificed after 22 h of reperfusion and the brains were carefully removed and sectioned into 2 mm thick coronal sections, starting 2 mm from the frontal pole. The slices were placed in a 2% 2′,3′,5′-triphenyl-2H-tetrazolium chloride (TTC) solution at room temperature to stain viable brain, which demonstrates mitochondrial dehydrogenase activity (16). The sections were photographed and the volume of the infarct was determined by an investigator blinded to the groups.
Paramagnetic material and implantable resonators (IRs)
The oxygen sensitive LiPc crystals were synthesized in our laboratory (13). The IRs were constructed with a copper wire of diameter 0.15 mm and had two sets of loops, a larger loop (coupling loop) on one end and four small loops (sensor loops (SLs)) on the other end of the transmission line (T), Figure 1. The coupling loop has a diameter of 10 mm and is used to couple inductively to the surface loop resonator of the L-band EPR spectrometer. The sensor loops (inner diameter of 0.2 – 0.3 mm) were loaded with LiPc crystals (50 – 80 μg) and the entire IR was coated by gas permeable Teflon AF2400 (14,17,18). The SLs were implanted in the site of interest, and the coupling loop was placed on the skull below the skin for coupling with the surface loop resonator of the EPR spectrometer (Figure 1a and 1b). The length of the transmission lines (i.e. depth of tissue probed) was 3 mm for SL1, and SL4 and 5 mm for SL2 and SL3 from the surface. The SL1 and SL2 were located in the normal cortex and striatum of the contralateral hemisphere whereas the SL 3 and SL4 were located in the striatum (peripheral area of the infarct) and cortex (core of the infarct) during MCAO, respectively. The IRs were calibrated before implantation into the brain (Figure 1c) and the EPR spectra reflect the average pO2 on the surface of each SL ~ 0.11–0.15 mm2. The diameter and the number of sensor loops, the length of the transmissions lines and the distance between sensor loops can be adjusted based on the experimental requirements.
Figure 1.

a: Axial view of rat skull with trephination positions of the sensor loops. b: Schematic of rat brain showing the location of the infarct in the brain (shaded area) and the location of the sensor loops in the ischemic core (sensor loop 4, SL4), peripheral area (sensor loop 3, SL3) and contralateral striatum (sensor loop 2, SL2), and cortex (sensor loop 1, SL1). AP: anterior-posterior; ML; medial-lateral; DV: dorsal-ventral. c: Calibration plot of a 4-SL IR. The response of each SL to different concentrations of perfused oxygen and regression coefficients (R2).
Multi-site EPR oximetry with IRs
For implantation of IRs, the rats were anesthetized (2–2.5% isoflurane in 30% O2) and a small incision (1.5–2.0 cm) was made on the skin and four burr holes were drilled by using a 23 gauge needle on the skull at predefined co-ordinates (2.5 and 4 mm left and 2.5 and 4 mm right of the midline and 1 mm posterior to the bregma). Four probes of IRs were gently inserted into the brain tissue though these burr holes by using a stereotaxic frame. The holes were cleaned and sealed with bone wax, and the skin sutured.
The oximetry measurements were performed on a 1.2 GHz EPR spectrometer equipped with a surface loop resonator specially designed for in vivo experiments (19,20). The rats were placed between the poles and the head positioned in the center of the EPR magnet. The surface loop resonator was gently placed over the head region with the coupling loop of IRs and adjusted to obtain the best tuning of the spectrometer. A magnetic field gradient of 3.0 G/cm was used to separate the EPR spectra from each SL (21). The peak-to-peak line widths of the EPR spectra were used to determine pO2 by using appropriate calibration of the IRs used in the study.
Experimental protocol
In vitro phantom experiments
The in vitro experiments were carried out in 2.0% agarose gel (phantom) to compare the EPR line width (LW) and S/N of the IRs with different lengths of the transmission lines: (i) mm and 11 mm (IR-T6 & 11), and (ii) 3 mm and 5 mm (IR-T3 & 5) (Figure 2a & 2b). Four aggregates of LiPc crystals (approximately 100 μg each) were directly implanted using a 23 Ga needle/plunger into the gel at the same depth (Figure 2c). Three to four samples for each experiment were used to compare the LW and S/N of IRs with LiPc deposits. The EPR settings for in vitro experiments was: the incident microwave power: 0.08–0.8 mW for IRs, 0.8–80 mW for LiPc deposits; gradient: 2.8 – 70 mT/m for IRs and LiPc deposits; modulation frequency 24 kHz; magnetic field center 425 G; scan time 10 sec, scan range 3 – 30 G, modulation amplitude not exceeding one third of the peak-to-peak line width.
Figure 2.
Schematic of phantoms (2.0% agarose gel) used for in vitro experiments with an IR fabricated with (a) 6 mm and 11 mm transmission lines, IR-T6 & 11, (b) 3 mm and 5 mm transmission lines, IR-T3 & 5 and (c) LiPc deposits at depths of 3mm and 5 mm. Typical EPR spectra recorded from (d) IR-T6 & 11, (e) IR-T3 & 5 and (f) LiPc deposits.
In vivo experiments
Five to seven days after the implantation of IRs with transmission lines of 3 mm and 5 mm (T 3 & 5 mm), the animals were anesthetized (1.5% isoflurane with 30% oxygen) and a pre-treatment (baseline) pO2 measurements was made for 30 min at the four sites. Thereafter, MCAO was performed and pO2 measurements were repeated for 30 min during which all the animals continued to breathe 30% oxygen. After the confirmation of a significant decrease in pO2 (ischemia), the breathing gas was switched to 100% O2 for 60 min and then switched back to 30% O2 in the last 30 min of MCAO. The animals of the control group continued to breathe 30% O2 during MCAO. The typical EPR spectra collected during the baseline, ischemia and reperfusion are shown in Figure 3. During the experiments, the body temperature of the rats was maintained at 37 ± 0.5°C using a heated water blanket and warm air forced though the core of the magnet. The heart rate (H) and blood hemoglobin oxygen saturation (SpO2) were continuously measured by a Pulse Oximeter (Nonin 8600V Pulse Oximeter, Phoenix, Arizona) throughout the experiments. The EPR settings for in vivo experiments were: the incident microwave power: 0.08–0.8 mW, gradient: 70–168 mT/m and other parameters described for in vitro experiments.
Figure 3.
The typical EPR spectra from an implanted multiple SL-IR (IR-T3 & 5) for the simultaneous measurement of cerebral pO2 in both hemispheres of a rat brain recorded at 0 min (baseline), 30, 60, 90 min of ischemia (I), 30 min of reperfusion (R), and 22 h of reperfusion (R/22 h). The narrowing of the line width (intense EPR lines) corresponds to a decrease in local pO2 when compared with the baseline. The numbers shown are the pO2 values in mmHg acquired from each SL using multi-site EPR oximetry.
Statistical analysis
A paired T test was used to compare the pO2 values at four sites (SL) within the same group. The paired comparison reduces the effects of animal-to-animal heterogeneity and eliminates differences in the baseline pO2. The comparisons between groups including line width and S/N ratio, the pO2 values and infarct volume were made using a student’s T test for unpaired samples. The tests were two-sided and a change with a p-value <0.05 was considered statistically significant. All data are expressed as mean ± SE, n is the number of in vitro samples and N is the number of rats in each group.
RESULTS
In vitro calibration of IR in gas phase
The line widths of the EPR spectrum acquired from each sensor loop of the IR-T3 & 5 were a linear function of pO2, Figure 1c. Some differences in the response of the sensor loops at high oxygen concentrations were observed. Therefore, each sensor loop was calibrated individually and the line width was converted to pO2 using the corresponding calibration. Similar procedures were followed with the calibration of IR-T6 & 11.
In vitro EPR measurements in phantom (2.0% agarose gel)
The line widths of each sensor loop of IR-T6 & 11 and IR-T3 & 5 implanted in phantom are shown in Figure 4a. No significant differences in the line widths of the sensor loops were observed. The line width of the sensor loops were pooled together and compared with the line width obtained without gradient, Figure 4b. The differences in the LW of the IR-T6 & 11 and IR-T3 & 5 with or without gradient were 4.1±2% and 2.1±1.5%, respectively. The LW of the IRs recorded without gradient were similar to that of the LiPc deposits, however, the S/N ratio of the LiPc deposits were significantly smaller (p<0.01, Figure 4c, 4d). We were not able to separate the EPR signals from the four LiPc deposits even at higher gradients and microwave power.
Figure 4.
Line width (LW) and signal to noise (S/N) ratio of IR-T6 & 11, IR-T3 & 5 and LiPc deposits in phantoms (2.0% agarose gel). a, the LW acquired from each SL of IR-T6 & 11 (grey column) and IR-T3 & 5 (black column); b, the LW of the four SLs with gradient applied (solid column) and the LW obtained without applied gradient (upward diagonal) from the IR-T6 & 11and IR-T3 & 5; c & d, LW and S/N of the IR-T6 & 11, IR-T3 & 5 and LiPc deposits without applied gradient. Mean ± SE, n = 3 – 5. **p<0.01, compared with IR-T6 & 11 and IR-T3 & 5, respectively.
Measurement of cerebral pO2 during MCAO and the effect of 100% O2
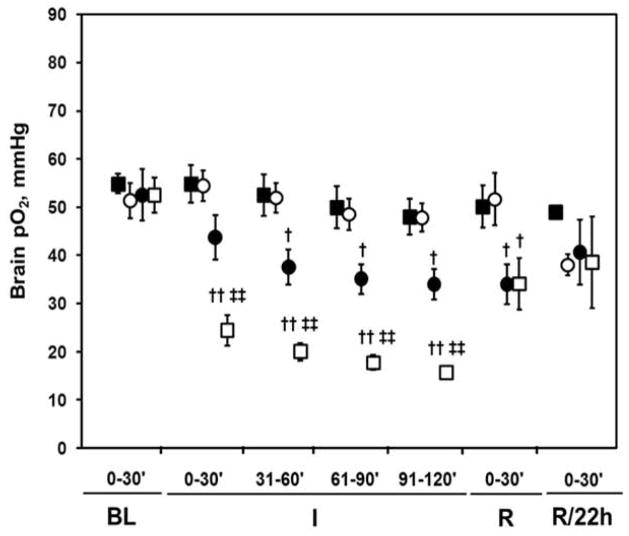
In the control group, no significant difference in the baseline (pre-ischemic) pO2 between all the sites was observed, Figure 5. The mean pO2 decreased significantly in the cortex (ischemic core, SL4) during the first 30 min of MCAO (p<0.01), while the pO2 of the contralateral hemisphere (SL2 and SL1) remained at the baseline level. The extent of decline in the pO2 of the core and striatum was 41±4% and 84±5% of the baseline in the first 30 min of MCAO, respectively (Table 1). There were significant differences in the extent of decline in the pO2 between the ischemic core and peripheral area during MCAO (p<0.01). The mean pO2 of the core and peripheral area did not return to baseline during the first 30 min of reperfusion (p < 0.05). However, the mean brain pO2 of all the four sites returned to baseline at 22 h of reperfusion (Figure 5, Table 1).
Figure 5.
Dynamic changes of mean cerebral pO2 in the control group in ischemic cortex (□), striatum (●) and contralateral striatum (○) and contralateral cortex (■) before, during and after middle cerebral artery (MCA) occlusion in rats (mean ± SE, N = 8). BL: baseline; I: ischemia; R: reperfusion; R/22 h: reperfusion at 22 h. † p <0.05, ††p <0.01, compared with baseline pO2 in same site; ‡‡p <0.01, compared with site 3 (ischemic striatum, SL3).
Table 1.
percentage changes of baseline cerebral pO2 during 2 h of ischemia, during the first 30 min of reperfusion and after 22 h of reperfusion
| Groups | Sites | BL (mmHg) | I (% of BL) | R (% of BL) | R/22 h (% of BL) | |||
|---|---|---|---|---|---|---|---|---|
| 0–30 (min) | 0–30 | 31–60 | 61–90 | 91–120 | 0–30 (min) | 0–20 (min) | ||
| (min) | ||||||||
| 100% O2 (N=6) | 1 | 51±2.7 | 102±7 | 141±15† | 145±12† | 111±9 | 99±4 | 90±3 |
| 2 | 50±2.3 | 96±3 | 146±13† | 155±9†† | 109±5 | 95±5 | 89±5 | |
| 3 | 50±5.9 | 75±12 | 93±18 | 95±24 | 67±10† | 76±10 | 91±17 | |
| 4 | 48±3.4 | 37±3†† | 47±5††‡ | 41±5†† ‡ | 31±5††‡‡ | 67±11† | 88±10 | |
| Control (N=8) | 1 | 55±2.1 | 100±6 | 96±7 | 91±7 | 87±6 | 92±8 | 96±4 |
| 2 | 51±3.6 | 110±11 | 105±10 | 98±9 | 97±11 | 103±11 | 81±3† | |
| 3 | 53±5.4 | 84±5† | 73±4† | 68±4† | 66±4† | 68±9† | 99±15 | |
| 4 | 53±3.7 | 41±4†† ‡‡ | 36±3††‡‡ | 33±3††‡‡ | 29±3††‡‡ | 66±9† | 98±10 | |
BL: baseline; I: ischemia; R: reperfusion; R/22h: reperfusion at 22 h. Values are mean ± SE.
p <0.05,
p <0.01, compared with baseline pO2 in same site;
p <0.05,
p <0.01, compared with site 3 (ischemic striatum, SL3).
In the 100% O2 group, no significant difference in the mean baseline pO2 between the four sites was observed, Figure 6. The extent of decline in the pO2 in the core and peripheral area during the first 30 min of ischemia was 37±4% (p<0.01) and 75±12% respectively, while the pO2 of the contralateral hemisphere (SL2 and SL1) remained unchanged. Furthermore, there were significant differences between the ischemic cortex and striatum tissue pO2 during 120 min of MCAO (p<0.05), Fig 6. The pO2 of the peripheral area was not significantly different from the baseline during 60 min of breathing 100% O2, which is different from the control animals. However, the pO2 declined significantly when the breathing gas was switched back to 30% during the last 30 min of ischemia (p<0.05, Table 1 and Figure 6). The pO2 of the contralateral sites also increased significantly during breathing of 100% O2 but returned to baseline when the 100% O2 was discontinued. The pO2 of the ischemic core and peripheral area remained significantly lower than the baseline only during the first 30 min of reperfusion.
Figure 6.
Dynamic changes of mean cerebral pO2 in 100% O2 group in the ischemic cortex (□), striatum areas (●) and contralateral striatum (○) and contralateral cortex (■) before, during and after middle cerebral artery (MCA) occlusion in rats (mean ± SE, N = 6). Shaded area indicates the duration of the 100% O2 inhalation. BL: baseline; I: ischemia; R: reperfusion; R/22 h: reperfusion at 22 h. †p <0.05, ††p <0.01, compared with baseline pO2 in same site; ‡p <0.05, ‡‡p <0.01, compared with site 3 (ischemic striatum, SL3).
Measurement of animal physiology and infarct volume
Table 2 shows the arterial oxyhemoglobin saturation (SpO2), heart rate (HR) and core temperature of animals breathing 30 % O2 or 100% O2. SpO2 values significantly increased after 100% O2 as compared to the baseline and control group. On the other hand, a significant increase in the H and body temperature during ischemia as compared to the baseline was observed only in the control group.
Table 2.
Physiological parameters
| Group | Parameters | BL (min) | I (min) | R (min) | R/22h (min) | ||
|---|---|---|---|---|---|---|---|
| 0–30 | 0–30 | 31–90 | 91–120 | 0–30 | 0–20 | ||
| 100% O2 (N=6) | SpO2 (%) | 92±2.1 | 90±2.0 | 97±0.7†‡ | 91±1.3 | 92±1.2 | 91±3.2 |
| HR (beats/min) | 355±7 | 360±20 | 376±17 | 389±19 | 77±15 | 350±11 | |
| Temp. (°C) | 37±0.1 | 36±0.2 | 37±0.3 | 37±0.4 | 37±0.2 | 36±0.3 | |
| Control (N=8) | SpO2 (%) | 93±1.5 | 93±1.8 | 94±1.2 | 94±0.7 | 94±1.7 | 95±1.1 |
| HR (beats/min) | 388±20 | 401±29 | 443±21†‡ | 458±18†† | 412±16 | 383±21 | |
| Temp. (°C) | 36±0.2 | 36±0.4 | 38±0.3 | 38±0.3† | 37±0.2 | 37±0.1 | |
BL: baseline; I: ischemia; R: reperfusion; R/22h: reperfusion at 22 h; SpO2: arterial oxyhemoglobin saturation; HR: heart beat rate; Temp.: core body temperature. Values are mean ± SE.
p<0.05,
p<0.01, compared with baseline;
p<0.05, compared with control group.
120min of MCAO followed by 22h of reperfusion resulted in a significant infarct in the ischemic hemisphere of all surviving rats in both groups. However, no significant difference in the infarct volume with 100% O2 (200.7 ± 38.5 mm3) compared to the control group (251.8 ± 37.1 mm3) was observed. These results are consistent with our previous finding (12).
DISCUSSION
We have developed IRs to overcome the limited depth of tissue pO2 measurement with direct implantation of particulate oximetry probes at L-band EPR (14,17,22). The IRs were constructed from enameled copper wires and can be used to measure pO2 at any depth from the surface. For simultaneous measurement of tissue pO2 at multiple sites, IRs with multiple SLs can be used with magnetic field gradients. Our in vitro experiments demonstrate a significantly greater S/N with IRs compared to direct LiPc implants. Additionally, the line widths of the IRs with different lengths of the transmission lines for pO2 measurement at different depths (IR-T6 & 11 and IR-T3 & 5) had similar line widths in the presence or absence of the magnetic field gradients. These results indicate that the spatial resolution as small as 1.5 mm between the SLs can be easily achieved by applying appropriate magnetic field gradient without any distortion of the EPR signals.
The pO2 values at the four sites in the brain were stable over 30 min of baseline measurement in both control and 100% O2 groups. The tissue pO2 decreased significantly in the core (SL 4) and peripheral area (SL 3) but the pO2 of the contralateral hemisphere (SL 2 and SL 1) did not change during MCAO. A significant increase in the pO2 of the contralateral brain during 60 min of breathing 100% O2 was evident, but no such changes were observed in the ischemic region. Additionally, no significant difference in the infarct volume between the control and 100% O2 group was observed. These results are consistent with our previous report in which LiPc implants were used for pO2 measurement in rats subjected to 120 min of MCAO (12). Liu et al have reported the pO2 of the penumbra using LiPc implants in rats breathing different concentration of oxygen (30%, 70%, 95% or 100%) during 90 min of MCAO (23). The rats breathing 95% or 100% oxygen not only had reduced infarct volume, but also maintained the pO2 at the pre-treatment level. Singhal et al. noted that 100% O2 leads to reduced infarct size, measured by serial MRI after 2 h MCAO followed by 1h of reperfusion (24). Pan et al. compared rats breathing mixed oxygen/nitrogen (30%/70%), 100% oxygen, or mixed oxygen/heliox (30%/70%) during filament-induced 120 min of MCAO. A significant decrease in infarct volume (TTC at 24 h) was observed in rats breathing 30%/70% O2 using heliox (4%) compared to 100% O2 (16%) (25). However, Beynon et al. did not find any beneficial effect of 120 min of breathing 100% O2 initiated 60 min after transient filament-induced MCAO for 150 min (26). No significant reduction in the infarct volume or clinical impairment in the 100% O2 group compared to the control group was observed after 90 min of MCAO by Eschenfelder et al.(27).
No significant increase in the pO2 of the ischemic regions was observed during 60 min of breathing 100% O2 in our experiments, which is consistent with no change in the infarct volume compared to the control. The contradicting results discussed above are likely due to a difference in the MCAO model, duration of ischemia, lack of direct measurement of pO2 to verify the changes in oxygen levels, and different durations of breathing100% O2. Additional studies are planned to systematically investigate the effect of these variables on the cerebral pO2, cerebral blood flow and infarct volume.
CONCLUSIONS
These results demonstrate that EPR oximetry using multi-probe IRs potentially can be used to investigate the effect of ischemia-reperfusion on the cerebral pO2 at several sites simultaneously and repeatedly over days. The IRs provide significantly better S/N ratio as compared to direct LiPc implants and can be used to measure pO2 at depths greater than 10 mm from the surface. No significant change in the tissue pO2 of the ischemic region and consequently infarct volume was observed with 60 min of breathing 100% O2 during 120 min of MCAO as compared to the control group. The IRs based EPR oximetry approach will be especially useful to study cerebral tissue pO2 in large experimental models, including primates, to develop and test interventions to increase oxygen levels in the ischemic regions to salvage cerebral tissue in stroke.
Acknowledgments
EPR Center for Viable Systems, Geisel School of Medicine at Dartmouth.
Footnotes
CONFLICT OF INTEREST NOTIFICATION
No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43(7):1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 2.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9(8):1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Hara JA, Khan N, Hou H, Wilmo CM, Demidenko E, Dunn JF, Swartz HM. Comparison of EPR oximetry and Eppendorf polarographic electrode assessments of rat brain PtO2. Physiological measurement. 2004;25(6):1413–1423. doi: 10.1088/0967-3334/25/6/007. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, Alsner J, Sorensen BS, Toustrup K, Jakobsen S, Petersen J, Petersen H, Theil J, Nordsmark M, Overgaard J. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother Oncol. 2012 doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, Cook JA, Golman K, Ardenkjaer-Larsen JH, Subramanian S, Mitchell JB. Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A. 2002;99(4):2216–2221. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. Br J Radiol. 1999;72(859):627–630. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 7.Gaertner FC, Souvatzoglou M, Brix G, Beer AJ. Imaging of hypoxia using PET and MRI. Curr Pharm Biotechnol. 2012;13(4):552–570. doi: 10.2174/138920112799436267. [DOI] [PubMed] [Google Scholar]
- 8.Carlin S, Humm JL. PET of hypoxia: current and future perspectives. J Nucl Med. 2012;53(8):1171–1174. doi: 10.2967/jnumed.111.099770. [DOI] [PubMed] [Google Scholar]
- 9.O’Hara JA, Hou H, Demidenko E, Springett RJ, Khan N, Swartz HM. Simultaneous measurement of rat brain cortex PtO2 using EPR oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiological measurement. 2005;26(3):203–213. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 10.Hou H, Khan N, O’Hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Demidenko E, Lu S, Steffen RP, Swartz HM. Increased oxygenation of intracranial tumors by efaproxyn (efaproxiral), an allosteric hemoglobin modifier: In vivo EPR oximetry study. International journal of radiation oncology, biology, physics. 2005;61(5):1503–1509. doi: 10.1016/j.ijrobp.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 11.Hou H, Grinberg OY, Grinberg SA, Demidenko E, Swartz HM. Cerebral tissue oxygenation in reversible focal ischemia in rats: multi-site EPR oximetry measurements. Physiological measurement. 2005;26(1):131–141. doi: 10.1088/0967-3334/26/1/012. [DOI] [PubMed] [Google Scholar]
- 12.Hou H, Grinberg O, Williams B, Grinberg S, Yu H, Alvarenga DL, Wallach H, Buckey J, Swartz HM. The effect of oxygen therapy on brain damage and cerebral pO(2) in transient focal cerebral ischemia in the rat. Physiological measurement. 2007;28(8):963–976. doi: 10.1088/0967-3334/28/8/017. [DOI] [PubMed] [Google Scholar]
- 13.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90(12):5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou H, Dong R, Li H, Williams B, Lariviere JP, Hekmatyar SK, Kauppinen RA, Khan N, Swartz H. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: a multisite EPR oximetry with implantable resonators. J Magn Reson. 2012;214(1):22–28. doi: 10.1016/j.jmr.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longa E, Weinstein P, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 16.Bederson J, Pitts L, Germano S, Nishimura M, Davis R, Bartkowski H. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 17.Hou H, Li H, Dong R, Mupparaju S, Khan N, Swartz H. Cerebral oxygenation of the cortex and striatum following normobaric hyperoxia and mild hypoxia in rats by EPR oximetry using multi-probe implantable resonators. Advances in experimental medicine and biology. 2011;701:61–67. doi: 10.1007/978-1-4419-7756-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinguizli M, Jeumont S, Beghein N, He J, Walczak T, Lesniewski PN, Hou H, Grinberg OY, Sucheta A, Swartz HM, Gallez B. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosensors & bioelectronics. 2006;21(7):1015–1022. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Salikhov I, Hirata H, Walczak T, Swartz HM. An improved external loop resonator for in vivo L-band EPR spectroscopy. J Magn Reson. 2003;164(1):54–59. doi: 10.1016/s1090-7807(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 20.Hirata H, Walczak T, Swartz HM. Electronically tunable surface-coil-type resonator for L-band EPR spectroscopy. J Magn Reson. 2000;142(1):159–167. doi: 10.1006/jmre.1999.1927. [DOI] [PubMed] [Google Scholar]
- 21.Smirnov AI, Norby SW, Clarkson RB, Walczak T, Swartz HM. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med. 1993;30(2):213–220. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Hou H, Sucheta A, Williams BB, Lariviere JP, Khan MN, Lesniewski PN, Gallez B, Swartz HM. Implantable Resonators - A Technique for Repeated Measurement of Oxygen at Multiple Deep Sites with In Vivo EPR. Advances in experimental medicine and biology. 2010;662:265–272. doi: 10.1007/978-1-4419-1241-1_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Liu W, Ding W, Miyake W, Rosenberg G, Liu K. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(10):1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- 24.Singhal A, Wang X, Sumii T, Mori T, Lo E. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002;22(7):861–868. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Zhang H, VanDeripe DR, Cruz-Flores S, Panneton WM. Heliox and oxygen reduce infarct volume in a rat model of focal ischemia. Exp Neurol. 2007;205(2):587–590. doi: 10.1016/j.expneurol.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Beynon C, Sun L, Marti HH, Heiland S, Veltkamp R. Delayed hyperbaric oxygenation is more effective than early prolonged normobaric hyperoxia in experimental focal cerebral ischemia. Neurosci Lett. 2007;425(3):141–145. doi: 10.1016/j.neulet.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Eschenfelder CC, Krug R, Yusofi AF, Meyne JK, Herdegen T, Koch A, Zhao Y, Carl UM, Deuschl G. Neuroprotection by oxygen in acute transient focal cerebral ischemia is dose dependent and shows superiority of hyperbaric oxygenation. Cerebrovasc Dis. 2008;25(3):193–201. doi: 10.1159/000113856. [DOI] [PubMed] [Google Scholar]