Abstract
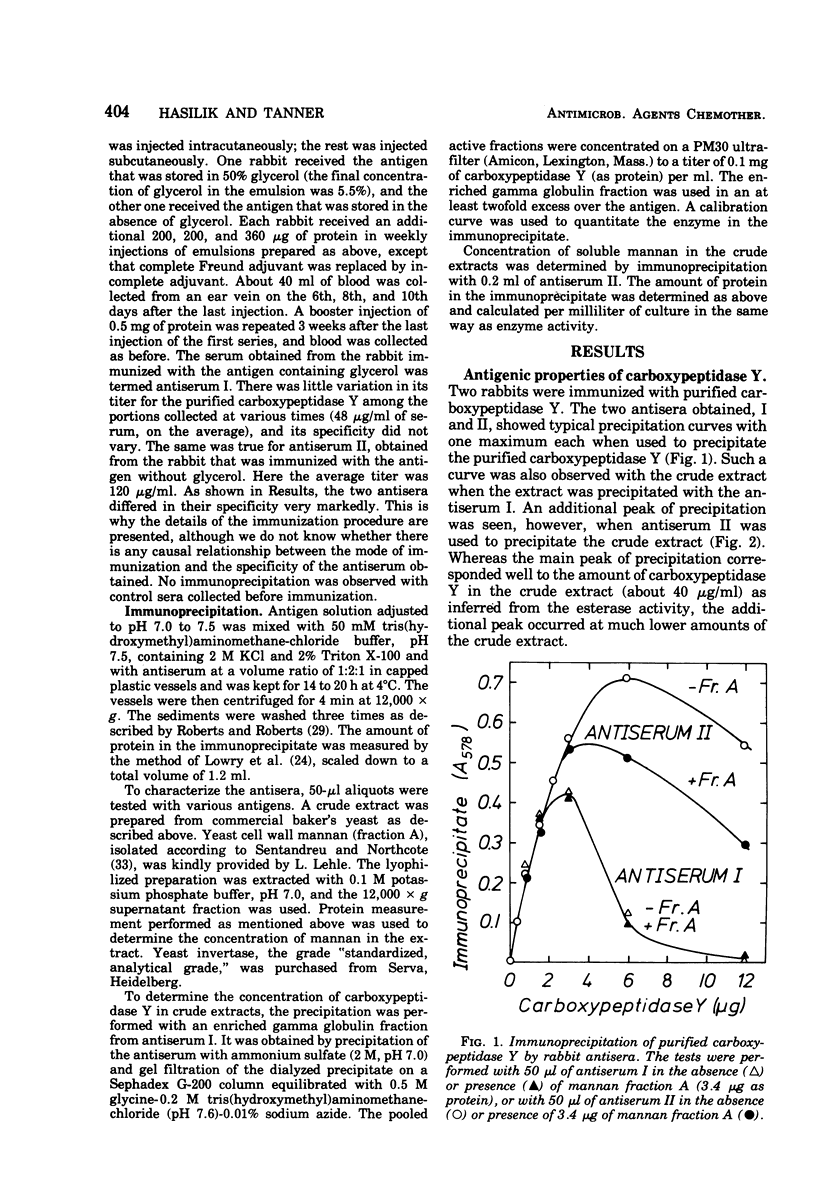
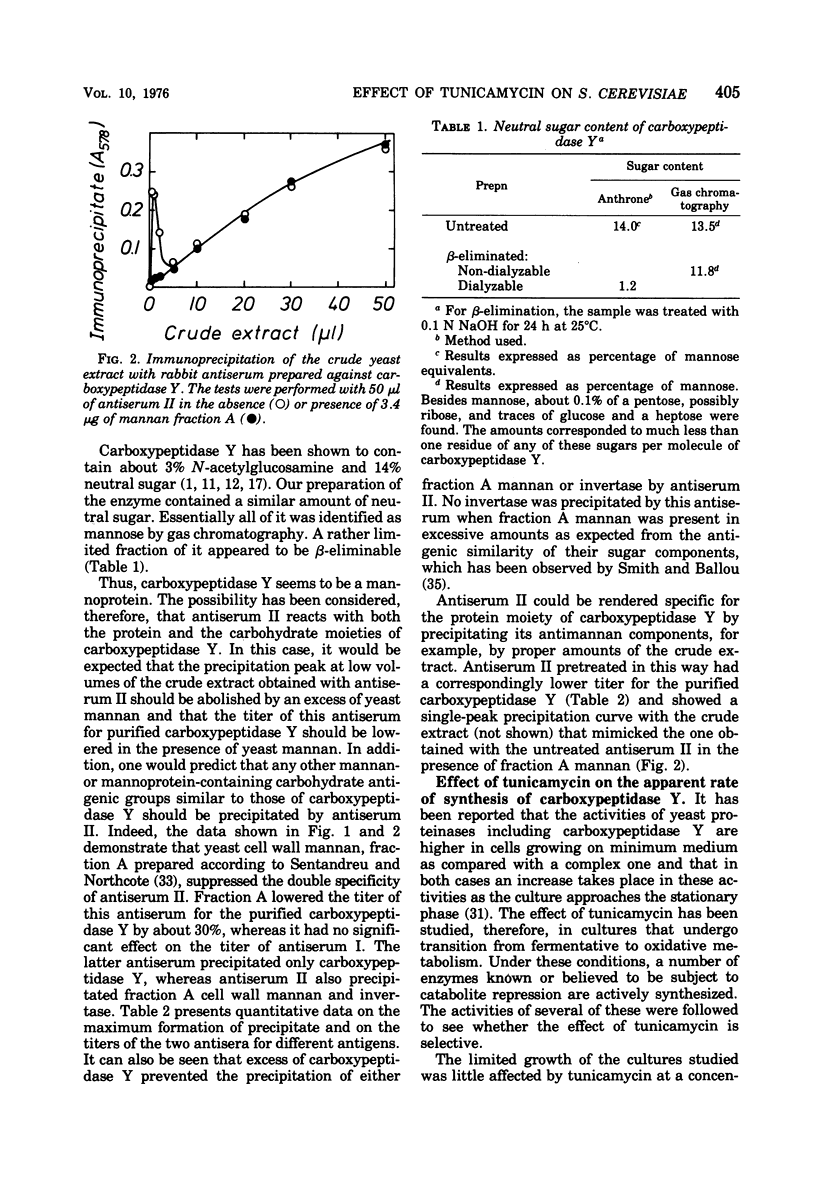
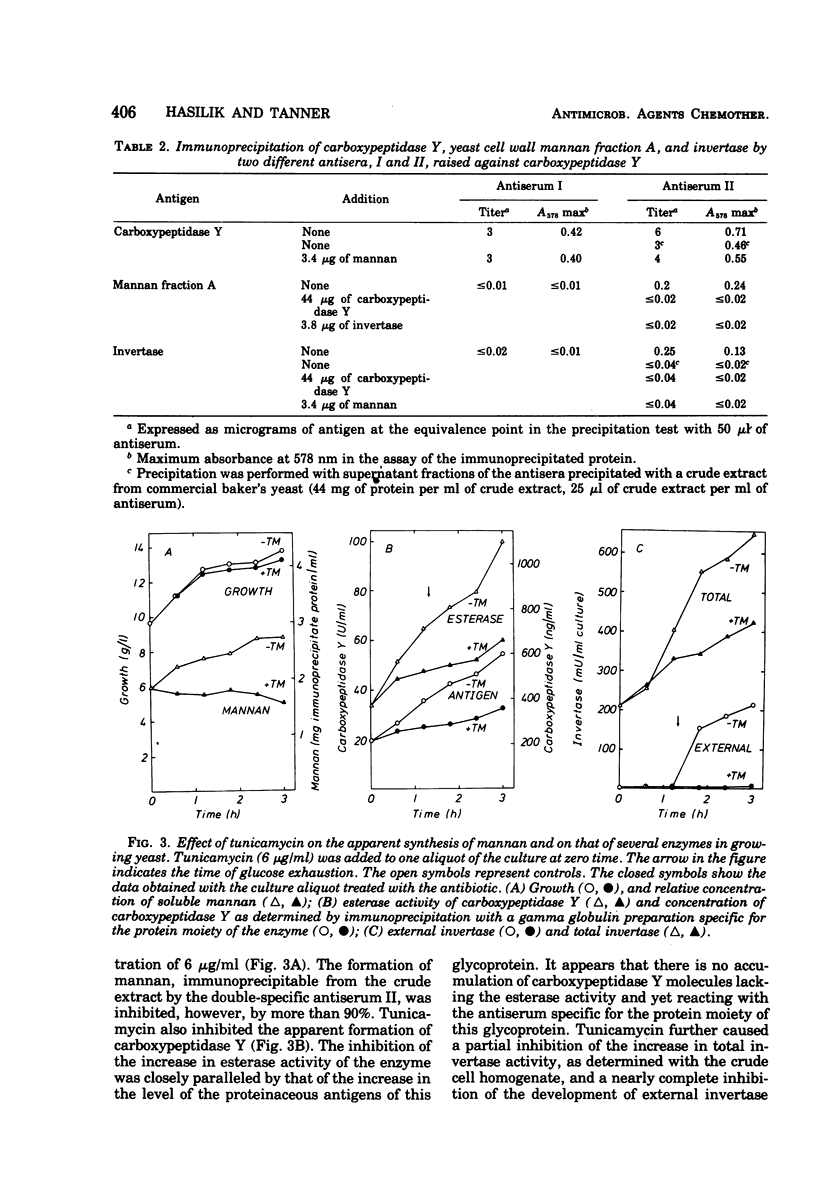
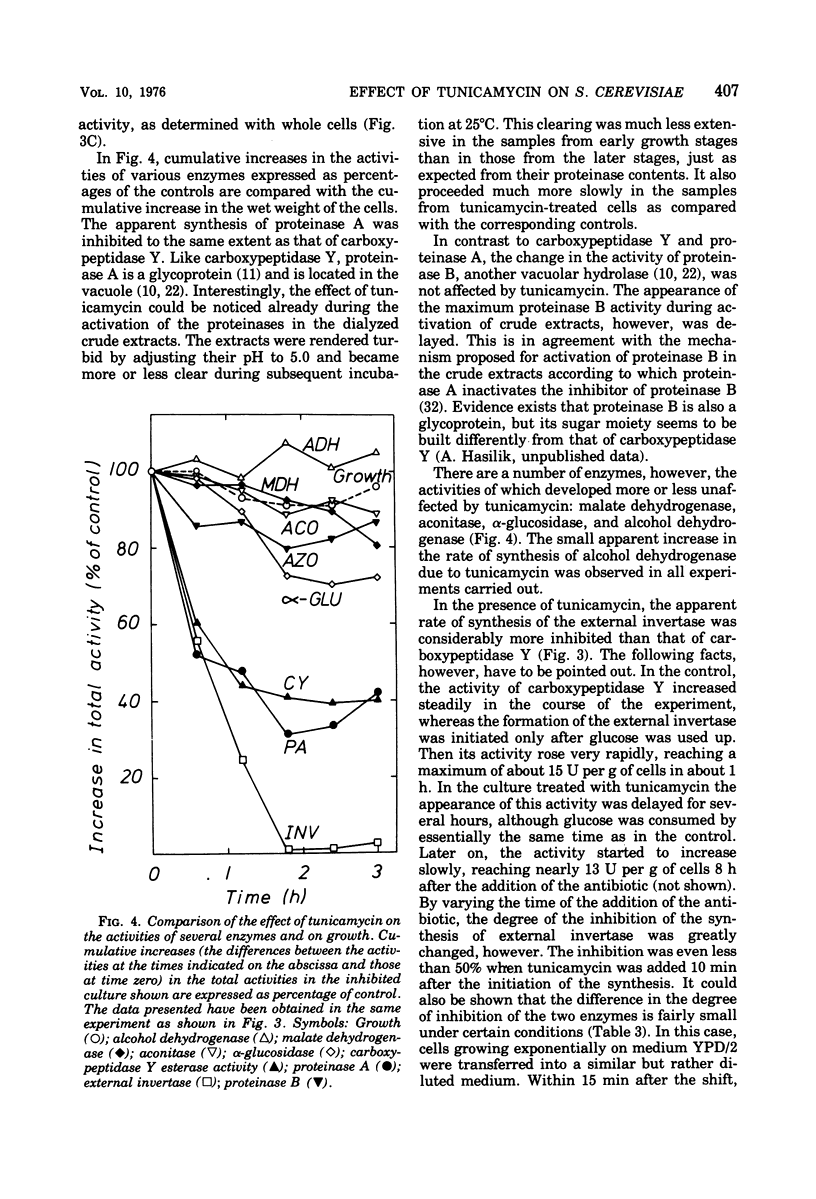
Carboxypeptidase Y from Saccharomyces cerevisiae contains 14% mannose, the only neutral sugar present. An antiserum can be raised in rabbits which reacts with both the protein and the sugar moieties of the enzyme. This antiserum also precipitates yeast invertase and yeast cell wall mannan. Thus carboxypeptidase Y, which is known to be localized in yeast vacuoles, is very probably a mannoprotein. Tunicamycin inhibits the apparent formation of carboxypeptidase Y to a similar extent as that of the externally localized mannoprotein, invertase. No accumulation of an inactive nonglycosylated or partly glycosylated carboxypeptidase Y occurs as determined by the immunoprecipitation technique. Tunicamycin also inhibits the apparent formation of proteinase A, whereas it does not affect the increase in the activities of a number of other enzymes. It is suggested that in the synthesis of glycoproteins there exists a regulatory link between the synthesis of their polypeptide chains and the reactions involved in their glycosylation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babczinski P., Tanner W. Involvement of dolicholmonophosphate in the formation of specific mannosyl-linkages in yeast glycoproteins. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1119–1124. doi: 10.1016/0006-291x(73)90808-5. [DOI] [PubMed] [Google Scholar]
- Bai Y., Hay Ashi R. A possible role for a single cysteine residue in carboxypeptidase Y. FEBS Lett. 1975 Aug 1;56(1):43–45. doi: 10.1016/0014-5793(75)80107-4. [DOI] [PubMed] [Google Scholar]
- Bettinger G. E., Young F. E. Tunicamycin, an inhibitor of Bacillus peptidoglycan synthesis: a new site of inhibition. Biochem Biophys Res Commun. 1975 Nov 3;67(1):16–21. doi: 10.1016/0006-291x(75)90276-4. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Physicochemical modifications of lysosomal hydrolases during intracellular transport. Biochem J. 1973 Feb;132(2):267–282. doi: 10.1042/bj1320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Müller H., Holzer H. Compartmentation of the tryptophan-synthase-proteolyzing system in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 1;48(1):117–117. doi: 10.1111/j.1432-1033.1974.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Holzer H., Betz H., Ebner E. Intracellular proteinases in microorganisms. Curr Top Cell Regul. 1975;9:103–156. doi: 10.1016/b978-0-12-152809-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Hsu A. F., Baynes J. W., Heath E. C. The role of a dolichol-oligosaccharide as an intermediate in glycoprotein biosynthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2391–2395. doi: 10.1073/pnas.71.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Isolation and partial characterization of an acid carboxypeptidase from yeast. Biochemistry. 1974 Sep 10;13(19):3871–3877. doi: 10.1021/bi00716a008. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. Movement of extracellular enzymes across cell membranes. Symp Soc Exp Biol. 1974;(28):351–374. [PubMed] [Google Scholar]
- Lehle L., Tanner W. Formation of lipid-bound oligosaccharides in yeast. Biochim Biophys Acta. 1975 Aug 13;399(2):364–374. doi: 10.1016/0304-4165(75)90265-2. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J. Lipid linked sugars in glycoprotein synthesis. Science. 1975 Jun 6;188(4192):986–991. doi: 10.1126/science.167438. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- Lerch K., Fischer E. H. Amino acid sequence of two functional sites in yeast glycogen phosphorylase. Biochemistry. 1975 May 6;14(9):2009–2014. doi: 10.1021/bi00680a031. [DOI] [PubMed] [Google Scholar]
- Manney T. R. Regulation of factors that influence the in vitro stability of tryptophan synthetase from yeast. J Bacteriol. 1968 Aug;96(2):403–408. doi: 10.1128/jb.96.2.403-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H., Betz H., Holzer H. Compartmentation of inhibitors of proteinases A and B and carboxypeptidase Y in yeast. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1051–1057. doi: 10.1016/0006-291x(74)90419-7. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Microheterogeneity of the inner core region of yeast manno-protein. Biochem Biophys Res Commun. 1975 Sep 16;66(2):870–879. doi: 10.1016/0006-291x(75)90590-2. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Proteolytic activities in yeast. Biochim Biophys Acta. 1975 Mar 28;384(1):203–214. doi: 10.1016/0005-2744(75)90109-6. [DOI] [PubMed] [Google Scholar]
- Saheki T., Matsuda Y., Holzer H. Urification and characterization of macromolecular inhibitors of proteinase A from yeast. Eur J Biochem. 1974 Sep 1;47(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C. B., Babczinski P., Lehle L., Tanner W. The role of dolicholmonophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):35–41. doi: 10.1111/j.1432-1033.1974.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Ballou C. E. Immunochemical characterization of the mannan component of the external invertase (beta-fructofuranosidase) of Saccharomyces cerevisiae. Biochemistry. 1974 Jan 15;13(2):355–361. doi: 10.1021/bi00699a021. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Wedgwood J. F., Warren C. D., Jeanloz R. W., Strominger J. L. Enzymatic utilization of P1-di-N-acetylchitobiosyl P2-dolichyl pyrophosphate and its chemical synthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5022–5026. doi: 10.1073/pnas.71.12.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]



