Abstract
Background
HIV infection and its treatment, specifically protease inhibitor (PI) therapy, have been associated with an increased risk for cardiovascular disease. Heart rate recovery (HRR) following peak exercise is predictive of future cardiovascular events and mortality in the general population. Nothing is known regarding HRR in individuals infected with HIV on highly active antiretroviral therapy (HAART).
Subjects and methods
HIV-positive subjects on HAART that included a PI (HIV 1 PI, n = 19), HIV-positive subjects on HAART that did not include a PI (HIV 1 noPI, n = 19) and HIV-seronegative age, gender and body mass index (BMI) matched controls (Cntl, n = 15) underwent a graded maximal exercise test on a cycle ergometer to volitional exhaustion. A continuous electrocardiogram was recorded and HRR was monitored every 30 s for 2 min post exercise.
Results
HRR at 1.5 and 2 min was significantly delayed in HIV-positive subjects both on and not on PI-based HAART compared with controls (P<0.01).
Conclusion
HRR is impaired in HIV-positive individuals on HAART, whether or not the HAART includes a PI, compared with age, gender, BMI, and activity level matched HIV-seronegative controls. Abnormal HRR may reflect cardio-autonomic dysfunction and may be an independent risk factor for future cardiac events in HIV-positive individuals that receive HAART.
Keywords: cardiovascular, exercise, HAART, heart rate, HIV
Introduction
HIV-1 infection is associated with a greater risk for cardiovascular disease (CVD) [1], left ventricular dysfunction [2] and myocardial infarction [3]. The treatment of HIV-1-infected individuals with highly active antiretroviral therapy (HAART) may lead to metabolic complications including insulin resistance [4], hyperlipidaemia [5] and central fat accumulation [6]; all known risk factors for CVD. Delayed heart rate recovery (HRR) after peak or submaximal exercise is an indication of an abnormal cardio-autonomic response and a predictor of cardiovascular sudden death [7] and mortality in non-HIV-infected patients [8–10]. It has been reported that autonomic nervous system dysfunction occurs in the early [11] and later stages of HIV infection [12]. In addition, prior investigations have demonstrated that HIV protease inhibitor (PI) exposure adversely affects vascular endothelial function and CVD risk factors [13, 14]. However, the effect of PI on HRR in HIV-positive individuals is currently unknown. We hypothesized that HRR following peak exercise would be delayed in HIV-positive individuals receiving PI-based HAART compared with those receiving non-PI-based HAART and compared with healthy HIV-seronegative controls.
Subjects and methods
Subjects
HIV-infected and HIV-seronegative men and women were recruited from the AIDS Clinical Trials Unit and the Infectious Disease Clinics at the Washington University School of Medicine in Saint Louis, MO, USA. Subjects were assigned to one of three groups: (1) HIV-seropositive individuals currently on HAART that included nucleoside reverse transcriptase inhibitors (NRTIs) plus PI (HIV 1 PI; n = 19, two female subjects, 37% African American, mean age standard deviation (SD) 41 ± 9 years), (2) HIV-seropositive individuals on HAART that included NRTIs, but no PI (HIV 1 noPI; n - 19, three female subjects, 32% African American, mean age 43 ± 6 years), and (3) HIV-seronegative controls who were matched for age, gender and activity level (Cntl; n = 15, two female subjects, 25% African American, mean age 40 ± 10 years). The HAART regimen duration was <6 months in all HIV-positive subjects (HIV + PI: mean 59 ± 40 months; median 66 months; HIV + noPI: mean 80 ± 34 months; median 72 months). HAART components in the groups included: HIV + PI: lopinavir/ritonavir (RTV; n = 10), boosted RTV (n + 9), atazanavir (n = 8), tenofovir (TDF) (n = 8), lamivudine (3TC)/zidovudine (ZDV) (n = 2), TDF/emtricitabine (FTC) (n = 8), didanosine (ddI) (n = 4), nevirapine (n = 4), nelfinavir (n = 1), 3TC (n = 4), FTC (n = 1), ZDV (n = 2), abacavir (ABC) (n = 1), saquinavir (n = 2) and enfuvirtide (T-20) (n = 1); HIV 1 noPI: ABC/3TC/ZDV (n = 4), TDF/FTC (n = 3), ddI (n = 3), 3TC (n = 4), efavirenz (n = 8), nevirapine (n = 4), stavudine (d4T) (n = 2), TDF (n = 1), 3TC/ZDV (n = 1) and ABC (n = 1). No subject had a current opportunistic infection or CD4 count <200 cells/μL (median CD4 counts: HIV + PI, 463 cells/μL; HIV 1 noPI, 603 cells/μL; P 0.03). Time infected with HIV tended to be higher in HIV + noPI (mean ± SD: 130 ± 74 months) than in HIV + PI (95 ± 64 months; P=0.10).
Subjects were excluded if they were receiving β-blockers, consumed ≤ 3 alcohol-containing beverages per week, had hepatitis C or B virus infection, reported recreational drug use within the previous 6 months, had a weight change of ± 2% in the previous 3 months, or regularly participated (<2 times per week) in exercise training activities. The HIV + PI group contained one (5%) current smoker and six individuals (32%) with a history of smoking, the HIV 1 noPI group contained four (21%) current smokers and 10 individuals (53%) with a history of smoking, and the Cntl group contained one (7%) current smoker and six individuals (40%) with a history of smoking. The Human Studies Committee at Washington University School of Medicine approved the study and all subjects provided informed consent prior to participation.
Fasting blood chemistry, glucose tolerance and body composition
At the time of enrolment, participants received a physical examination and had a detailed medical history taken, and fasting blood chemistry measurements (i.e. glucose, insulin and lipids/lipoproteins), complete blood cell counts and plasma HIV RNA quantification (Roche Amplicor HIV-1 Monitor®; Roche, Indianapolis, IN, USA) were performed. Serum/plasma analyses were performed as previously described [15]. Metabolic data were collected, as HRR can be related to the metabolic syndrome (MS) and its components [16].
Peak exercise test and HRR measurement
Each subject performed a graded exercise test on an electronically braked cycle ergometer (SensorMedics, Yorba Linda, CA, USA). The work rate on the cycle ergometer started at 20 W and increased by 20 W/min until volitional exhaustion. A 12-lead electrocardiogram (ECG) (Quinton, Bothell, WA, USA) was continuously recorded and the volume of oxygen consumption (VO2; Parvo Medics, Sandy, UT, USA) was continuously measured. Following exhaustion, subjects remained seated on the ergometer and were instructed to remain motionless while their heart rate (HR) was recorded every 30 s for 2 min post exercise. A higher HR at 30-s intervals reflected slower HRR. Exercise tests were considered to be maximal if the peak HR was ≥ 85% of that predicted for age (calculated by subtracting the subject's age from 220) and/or the peak respiratory exchange ratio was ≤ 1.15.
Statistics
Pearson product moment correlations were used to evaluate significant relationships between variables. Student's independent t-tests and one-way analysis of variance (ANOVA) with Tukey HSD post hoc analyses were used to determine inter-group differences. Two-way ANOVA (group × condition) with repeated measures and Tukey HSD were used to determine differences in HR at each minute of recovery. A P ≤ value 0.05 was considered statistically significant. Means ± SD are reported, unless specified otherwise. All statistical analyses were performed using SAS (SAS Institute, Cary, NC, USA).
Results
Insulin levels, high-density lipoprotein (HDL) cholesterol, and total cholesterol were similar among groups, although there was a trend towards lower HDL cholesterol in both HIV-positive groups compared with Cntl (Table 1). Glucose levels and systolic blood pressure were higher, and low-density lipoprotein (LDL) cholesterol was lower in HIV + PI compared with Cntl. Triglycerides were higher in HIV + PI compared with HIV + noPI and Cntl. Resting HR and diastolic blood pressure were higher in both HIV-positive groups compared with Cntl.
Table 1.
Characteristics of the study population
| Variable | Controls (n = 11) | HIV + noPI (n = 19) | HIV + PI (n = 19) |
|---|---|---|---|
| BMI | 26 ± 3 | 26 ± 5 | 29 ± 6 |
| Glucose (mg/dL) | 87 ± 5 | 91 ± 8 | 94 ± 5$ |
| Insulin (μU/mL) | 5 ± 2 | 12 ± 14 | 11 ± 6 |
| TG (mg/dL) | 105 ± 57 | 135 ± 62 | 292 ± 286** |
| HDL (mg/dL) | 57 ± 19 | 46 ± 18 | 43 ± 11 |
| Total cholesterol (mg/dL) | 193 ± 36 | 169 ± 35 | 182 ± 33 |
| LDL cholesterol (mg/dL) | 116 ± 19 | 96 ± 26 | 94 ± 27* |
| Resting HR (bpm) | 64 ± 8 | 75 ± 12$$ | 73 ± 12 |
| Peak HR (bpm) | 175 ± 10 | 162 ± 15* | 161 ± 15* |
| Peak VO2 (mL/kg/min) | 33.1 ± 9.7 | 28.8 ± 8.6 | 26.0 ± 5.0 |
| SBP (mmHg) | 111 ± 7 | 122 ± 13 | 129 ± 15* |
| DBP (mmHg) | 69 ± 8 | 81 ± 11* | 79 ± 10* |
P < 0.05 vs. control
P < 0.06 vs. control
P < 0.05 vs. HIV + noPI and control
P < 0.01 vs. control.
bpm, beats per minute; BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HIV + noPI, HIV-positive individuals on highly active antiretroviral therapy (HAART) but no protease inhibitor (PI); HIV + PI, HIV-seropositive individuals on HAART that included a PI; HR, heart rate; LDL, low-density lipoprotein; SBP, systolic blood pressure; TG, triglycerides; VO2, volume of oxygen consumption.
Exercise capacity and HRR
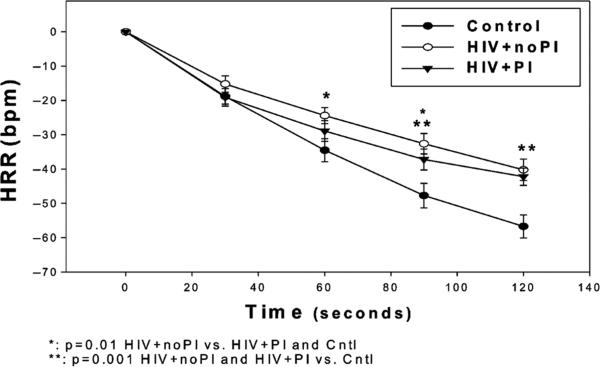
All subjects achieved exercise criteria for peak volume of oxygen consumption (VO2peak) [17] (data not shown). Peak exercise HR was lower in both HIV-positive groups compared with Cntl. VO2peak was similar among groups but there was a trend towards lower VO2peak in HIV-positive subjects combined compared with Cntl. At 1 and 1.5 min post exercise, HRR was slower in HIV + noPI than in HIV + PI and Cntl. At 1.5 and 2 min post-exercise, HR was higher and HRR slower in both HIV + PI and HIV + noPI groups compared with Cntl. The peak HR achieved during exercise tended to weakly correlate with HRR at + min post exercise (r = 0.26, P < 0.06) and was only moderately correlated with HRR at 2 min post exercise (r = 0.50, P<0.001) (Fig. 1). These findings suggest that the lower peak HR achieved during exercise did not fully account for the slower HRR in HIV-positive subjects. VO2peak tended to be lower in both HIV-positive groups and was weakly correlated with HRR at 2 min post exercise (r = 0.36, P = 0.01). Therefore, the trend in lower exercise capacity in HIV-positive subjects appeared to only play a small role in the differences in HRR. There were no significant correlations between 1 and 2 min HRR and CD4 count, or duration of HIV infection or HAART.
Fig. 1.
Heart rate recovery following peak exercise. Beats per minute; HIV + protease inhibitor (PI), HIV-positive subjects on highly active antiretroviral therapy (HAART) that included a PI; HIV + noPI, HIV-positive subjects on HAART that did not include a PI.
Because of the known relationship between delayed HRR and metabolic complications in non-HIV-infected individuals [16], we reclassified the HIV-positive subjects into those with (HIV + MS, n = 22) and those without (HIV + noMS, n = 16) the MS as defined by the Adult Treatment Panel (ATP-III) criteria [18]. HRR was significantly slower in HIV + MS at 1 min than in Cntl. However, HRR in both HIV-positive groups with and without MS was significantly slower than in Cntl at 1.5 and 2 min (data not shown). HRR at 2 min was associated with fasting Homeostasis Model Assessment (HOMA) (r = 0.31, P<0.03) but not other metabolic variables. Lastly, because of the known relationship between smoking and delayed HRR [19], HRR was compared between current or past smokers (n = 16) and those who had never smoked (n = 22). No between-group differences were noted.
Discussion
The findings indicate that HIV-positive subjects, regardless of whether they are receiving PI-based HAART or HAART without PI, have impaired HRR following exercise compared with seronegative controls. To our knowledge, this is the first study to report impaired HRR in HIV-positive individuals and supports the notion that cardio-autonomic dysfunction is another component of the CVD risk burden in this population.
HRR following peak exercise is primarily regulated by cardio-vagal (parasympathetic) reactivation, while the sympathetic nervous system predominately regulates HR during exercise [20]. Therefore, a delayed HRR following peak exercise may indicate a sympatho-vagal imbalance [20]. There is a strong relationship between vagal tone and cardiac risk [21] and HRR has been shown to be a predictor of sudden death [7] and cardiovascular and all cause mortality in non-HIV-infected populations [9, 22]. It has been previously reported that abnormalities in HR variability occur at rest in asymptomatic HIV-positive subjects [11, 23]. The findings of the present study suggest that individuals with well-controlled HIV infection on HAART have evidence of early autonomic dysfunction and may be at greater risk for CVD.
Both HIV-positive groups had higher baseline HR and lower peak HR response compared with normal controls, but this finding does not completely account for the delayed HRR in these individuals. The HIV-positive subjects attained 91% of their age-predicted peak HR, whereas normal controls reached 97% of their age-predicted peak HR. An attenuated HR response to exercise (i.e. chrono-tropic incompetence) has been reported to predict CVD events and mortality, despite adjustment for CVD risk factors and fitness levels, in non-HIV-infected individuals [24]. The lower than predicted peak HR during exercise in HIV-positive individuals on HAART may therefore be another indication of cardio-autonomic dysfunction.
Limitations
This study was not powered to address the effects of the type or dose of PI or antiretroviral medication on HRR. This was a cross-sectional study with a relatively small sample size, and the results may not imply a cause and effect relationship. The effects of nutritional factors were not assessed. This study was not powered to examine effects of the MS on HRR, which have been reported elsewhere [16]; however, our data suggest that metabolic complications, specifically impaired glucose metabolism, may play a small role in impaired HRR in HIV-infected individuals. Further research into the role of metabolic complications in abnormal cardio-autonomic function is warranted. Lastly, current or past tobacco use did not predict slower HRR, but the sample size was small, and this should be investigated further.
Conclusions
HRR is impaired in HIV-positive individuals on HAART, regardless of whether the regimen includes a PI, compared with age, gender, body mass index, and activity level matched HIV-seronegative controls. Abnormal HRR may reflect cardio-autonomic dysfunction and may be an independent risk factor for future cardiac events in HIV-positive individuals who receive HAART.
Acknowledgements
We thank the participants for their altruism and patience, the nursing staff of the General Clinical Research Center for their help in performing these studies, and the nursing staff of the AIDS Clinical Trials Unit for identifying potential subjects. We also thank Sam Smith, Sharon Heuerman and Joann Reagan for their technical assistance. This study was supported by the National Institutes of Health grants F32 DK066977-01 (WTC), DK49393 (KEY), DK54163 (KEY), DK59531 (KEY), RR19508 (DNR), AG00078 (DNR), DK63683 (DNR), RR-00036 (General Clinical Research Center), DK020579 (Diabetes Research and Training Center), DK-56341 (Clinical Nutrition Research Unit) and AI25903 (AIDS Clinical Trials Unit).
References
- 1.Passalaris JD, Sepkowitz KA, Glesby MJ. Coronary artery disease and human immunodeficiency virus infection. Clin Infect Dis. 2000;31:787–797. doi: 10.1086/313995. [DOI] [PubMed] [Google Scholar]
- 2.Longo-Mbenza B, Seghers LV, Vita EK, Tonduangu K, Bayekula M. Assessment of ventricular diastolic function in AIDS patients from Congo: a Doppler echocardiographic study. Heart. 1998;80:184–189. doi: 10.1136/hrt.80.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 4.Yarasheski KE, Tebas P, Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calza L, Manfredi R, Chiodo F. Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:10–14. doi: 10.1093/jac/dkh013. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. Aids. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YJ, Lauer MS, Earnest CP, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–2057. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 9.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 10.Lipinski MJ, Vetrovec GW, Froelicher VF. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am J Cardiol. 2004;93:445–449. doi: 10.1016/j.amjcard.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Correia D, Rodrigues De Resende LA, Molina RJ, et al. Power spectral analysis of heart rate variability in HIV-infected and AIDS patients. Pacing Clin Electrophysiol. 2006;29:53–58. doi: 10.1111/j.1540-8159.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruttimann S, Hilti P, Spinas GA, Dubach UC. High frequency of human immunodeficiency virus-associated autonomic neuropathy and more severe involvement in advanced stages of human immunodeficiency virus disease. Arch Intern Med. 1991;151:2441–2443. doi: 10.1001/archinte.1991.00400120079013. [DOI] [PubMed] [Google Scholar]
- 13.Zhong DS, Lu XH, Conklin BS, et al. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:1560–1566. doi: 10.1161/01.atv.0000034707.40046.02. [DOI] [PubMed] [Google Scholar]
- 14.Fu W, Chai H, Yao Q, Chen C. Effects of HIV protease inhibitor ritonavir on vasomotor function and endothelial nitric oxide synthase expression. J Acquir Immune Defic Syndr. 2005;39:152–158. [PubMed] [Google Scholar]
- 15.Reeds DN, Yarasheski KE, Fontana L, et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab. 2006;290:E47–E53. doi: 10.1152/ajpendo.00236.2005. [DOI] [PubMed] [Google Scholar]
- 16.Spies C, Otte C, Kanaya A, Pipkin SS, Schiller NB, Whooley MA. Association of metabolic syndrome with exercise capacity and heart rate recovery in patients with coronary heart disease in the heart and soul study. Am J Cardiol. 2005;95:1175–1179. doi: 10.1016/j.amjcard.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 6th ed. Lippincott, Williams & Wilkins; Baltimore: 2000. [Google Scholar]
- 18.National Institutes of Health . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) National Institutes of Health; Bethesda, MD: 2001. [Google Scholar]
- 19.Srivastava R, Blackstone EH, Lauer MS. Association of smoking with abnormal exercise heart rate responses and long-term prognosis in a healthy, population-based cohort. Am J Med. 2000;109:20–26. doi: 10.1016/s0002-9343(00)00441-1. [DOI] [PubMed] [Google Scholar]
- 20.Pierpont GL, Stolpman DR, Gornick CC. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton Nerv Syst. 2000;80:169–174. doi: 10.1016/s0165-1838(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 21.Lauer M, Froelicher ES, Williams M, Kligfield P. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771–776. doi: 10.1161/CIRCULATIONAHA.105.166543. [DOI] [PubMed] [Google Scholar]
- 22.Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–838. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 23.Mittal CM, Wig N, Mishra S, Deepak KK. Heart rate variability in human immunodeficiency virus-positive individuals. Int J Cardiol. 2004;94:1–6. doi: 10.1016/j.ijcard.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]