Abstract
There is growing interest in using antibodies as auxiliary proteins to crystallize proteins. Here, we describe a general protocol for the generation of Nanobodies to be used as crystallization chaperones for the structural investigation of diverse conformational states of flexible (membrane) proteins and complexes thereof. Our technology has the competitive advantage over other recombinant crystallization chaperones in that we fully exploit the natural humoral response against native antigens. Accordingly, we provide detailed protocols for the immunization with native proteins and for the selection by phage display of in vivo matured Nanobodies that bind conformational epitopes of functional proteins. Three representative examples illustrate that the outlined procedures are robust, enabling to solve the structures of the most challenging proteins by Nanobody-assisted X-ray crystallography in a time span of 6 to 12 months.
Keywords: Nanobody, Structural Biology, Crystallization chaperones, Protein conformation
INTRODUCTION
Producing diffraction quality crystals remains the major bottleneck in macromolecular X-ray crystallography. Collective efforts of several laboratories have demonstrated that Nanobodies are exquisite chaperones to crystallize complex biological systems such as membrane proteins1-3, transient multiprotein assemblies2,4-6, transient conformational states1, intrinsically disordered proteins7,8 or can be used as structural probes of protein misfolding and fibril formation9,10. Nanobodies (Nbs) are the small (15kDa) and stable single domain fragments harboring the full antigen-binding capacity of the original heavy chain-only antibodies that naturally occur in Camelids11,12. Nanobodies are encoded by single gene fragments, are easily produced in micro-organisms and exhibit a superior stability compared to derivatives of conventional antibodies like Fabs or scFvs. Because of their compact prolate shape, Nanobodies expose a convex paratope and have access to cavities or clefts on the surface of proteins1,13,14 often inaccessible to conventional antibodies. These cryptic epitopes can be readily recognized by the long CDR3 loop of the Nanobody. In our experience, Nanobodies raised in vivo by immunization against and selected on properly folded proteins systematically recognize discontinuous amino acid segments of the native protein conformation (i.e. conformational epitopes), making them ideal tools to selectively stabilize specific conformational states of (membrane) proteins.
For the discovery of Nanobodies as crystallization chaperones approximately 1 mg of functional protein is required. The generation of in vivo matured Nanobodies can therefore be incorporated in the crystallization pipeline even before the purification of the protein has been fully optimized and scaled up. Nanobodies binding conformational epitopes (conformational Nanobodies) can subsequently be used for preparing pure, homogeneous and highly concentrated monodisperse samples that are required for crystallization15. In case no native purified protein is available, genetic and cell based vaccinations combined with cell based selection approaches have been successfully applied in our lab and elsewhere to generate Nanobodies against target proteins in their native conformation16-18.
Comparisons to other approaches
Here we present a general protocol for the generation, selection and purification of recombinant in vivo matured Nanobodies for structural biology1-5,7,9,19-30 that takes 3-4 months. Our Nanobody discovery platform has the competitive advantage over other recombinant crystallization chaperones31-33 that the cloned Nanobody library represents the full collection of the naturally circulating humoral antigen-binding repertoire of heavy chain-only antibodies, contrary to combinatorial libraries of conventional antibody fragments. Because Nanobodies are encoded by single exons, the full antigen-binding capacity of in vivo matured antibodies can be cloned and efficiently screened for high affinity binders, allowing one to fully exploit the humoral response of large mammals against native antigens. To our knowledge, there are no indications that in vivo matured Nanobodies induce non-native conformations. Surely, immature B cells expressing antibodies that have to pay a substantial energetic penalty for distorting the antigen structure will have a lower probability to proliferate and to differentiate into mature antibody secreting B lymphocytes.
Limitations
With nearly 20 years of experience now, we learned that conformational Nanobodies can be identified against any properly folded protein. In those cases where we failed in a first attempt, we successfully performed new immunizations or pannings, paying special attention to the quality of the antigen, instructing us that good protein biochemistry is the key to success. Although Nanobodies are good at binding conformational epitopes on folded proteins with high affinity, they perform poorly at binding peptides or intrinsically unfolded parts of proteins. For linear epitopes, conventional antibodies may be a better alternative.
Applications
The Nanobodies to be used as crystallization chaperones can also be valuable for other applications in structural biology. For example, domain-specific Nanobodies have been used in single-particle electron microscopy (EM) as a marker to track these domains in particle projections34,35. Because many Nanobodies can be functionally expressed as intrabodies in eukaryotic cells, these single domain antibodies can also be used as biosensors to track conformational properties of their targets inside a living cell36-39. Ultimately, Nanobodies that constrain protein targets in unique disease-linked conformations may facilitate the discovery of new therapeutic molecules40.
EXPERIMENTAL DESIGN
General considerations
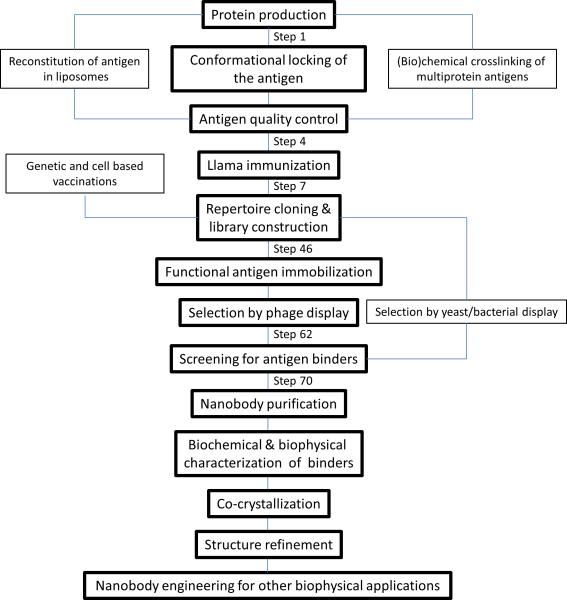
The workflow for generating, isolating and characterizing Nanobodies to be used as crystallization chaperones (Figure 1) is inherently dependent on the nature of the antigen and on the purpose of the structural study. Several steps in the Nanobody discovery process including the preparation of the immunogen, the selection strategy, the screening approach and the functional and biophysical characterization differ when the target is a soluble protein, a membrane protein or a multiprotein complex. To make this protocol broadly applicable for the structural biology community, several modifications to the standard protocol are referred to in the text.
Figure 1.
Workflow for generating conformational Nanobodies for structural biology.
Protein production & antigen quality control
Today, researchers are faced with a bewildering array of methods to produce and purify recombinant proteins41. Although this is not the focus of the paper, supply of properly folded protein is critical for the generation of conformational Nanobodies. We discourage immunization of animals with poorly characterized protein samples. Our standard protocol typically requires 1 mg of the purified protein: 700 μg for immunization and the remaining for all Nanobody selection, identification and characterization efforts. Ideally, a single batch of the purified protein is dispensed into aliquots and stored under conditions that ensure the stability of the protein over time. Often flash freezing in liquid nitrogen and storage at −80°C is favored. We insist on confirming protein quality of one thawed aliquot before the protein is administered as an immunogen. Biochemical or cellular assays that quantitatively assess the functionality of the antigen [e.g. enzymatic/signalling activity, interaction with (radio)ligands or binding of certified conventional antibodies against discontinuous epitopes] can be used to test whether your protein is properly folded. If your protein cannot be tested functionally, we advise performing a rigorous biophysical characterization to confirm its folded state (reviewed in 42). Unless demonstrated not to have any effect on the functionality of the protein, we strongly discourage multiple freeze-thaw cycles in order to minimize protein denaturation. If samples cannot be frozen, consider using freshly prepared protein throughout the immunization and discovery effort.
Conformational locking of the antigens
Nanobodies have been shown to stabilize proteins such as kinases and G protein-coupled receptors in unique biologically relevant conformations33,43,44. To identify such Nanobodies, we advise to immunize animals with proteins constrained in the desired conformation with cofactors, enzyme inhibitors27, orthosteric ligands1, allosteric ligands, or any molecule that conformationally traps the macromolecule in a particular state45. We recommend using ligands that dissociate slowly to maximize the lifetime of the constrained target conformation in the immunized animal. Purified, detergent-solubilized membrane proteins may denature following immunization due to dissociation of detergents. To maintain a native conformation following immunization, reconstitution of the protein into a lipid environment, typically phospholipid vesicles1, or the use of a very tight binding detergent2 may be required. Reconstitution of membrane proteins in lipid vesicles may also reduce ligand dissociation. A ligand trapped in the vesicle can rebind the intravesicular binding site of the protein prolonging the extravesicular exposure of the desired conformational state to the immune system. Alternatively, particular mutations may trap the target in a unique conformational state. Finally, chemical crosslinking between protein domains or different proteins may stabilize epitopes that are unique to the complex2.
Immunogen preparation, camelid immunization & repertoire cloning
Antibodies with a homodimeric heavy chain composition that are devoid of light chains are only found in Tylopoda (camels, dromedaries and llamas) and sharks46. Our protocols can be applied to both llamas, camels, dromaderies and alpacas. All vaccination experiments are executed following the EU animal welfare legislation and after approval of the local ethical committee. It is known that stress can cause immunosuppression. Animals should be manipulated by authorized staff, preferentially an experienced veterinarian. We allow animals to acclimatize to new housing conditions for at least one week before immunization starts.
In llamas, repetitive immunogen administrations generate a robust immune response mediated by both conventional and heavy chain-only antibodies. Building on 10 years of experience, GERBU LQ turns out to be a good immunostimulating adjuvant for conformational Nanobody discovery and is well tolerated by llamas. In general, we inject our animals subcutaneously with cocktails of 1 to 5 antigens mixed with adjuvant. Alternatively, different immunogens can be injected separately at different subcutaneous locations. In case small molecule ligands are used for the conformational locking of an antigen, we always add these compounds in excess to the antigen. In our hands, we successfully reused llamas for different Nanobody discovery projects by respecting a grace period of at least 6 months.
A blood sample of 100 ml from an immunized llama contains sufficient expressing B-cells to clone a diverse set of the affinity-matured Nanobodies with high specificity for their cognate antigen47. Peripheral blood lymphocytes (PBL) should be isolated without delay from the non-coagulated blood for the purification of total RNA and the synthesis of cDNA. From this cDNA, the Nanobody encoding open reading frames can be amplified by PCR and cloned into an appropriate phage display vector.
Primer design
Over the years, several PCR strategies have been developed to amplify the Nanobody gene fragments from lymphocyte cDNA. We prefer to use a two-step nested PCR approach. One pair of primers (CALL001 and CALL002) has been designed for the first PCR using cDNA of B lymphocytes as the template48. The CALL002 primer anneals in a region of the second constant heavy chain domain (CH2) that is conserved among all IgG isotypes of all camelids, whereas the CALL001 primer anneals in a well conserved region of the leader signal sequence of all V elements of family III (by far the most abundant V family in camelids). The primers VHH-Back and VHH-For are used to amplify the Nanobody repertoire via a second nested PCR (See Supplementary Figure 1A). From our experience, the primers described in steps 21 and 24 are adequate to amplify Nanobody encoding genes of family III from dromedary (Camelus dromedaries), camel (Camelus bactrianus), llama (Lama glama) and also alpaca (Vicugna pacos).
Van der Linden and coworkers developed dedicated primers annealing to the hinge of each heavy chain only IgG isotype49 of all camelid species (See Supplementary Figure 1B). Maass et al. designed primers dedicated for alpaca Nanobodies50 (See Supplementary Figure 1C). Kastelic et al. used primers that amplify mixtures of the VHH (variable fragment of heavy chain antibody) and the VH (variable fragment of classical antibody) from llama51 (See Supplementary Figure 1D).
Selection by phage display
Many excellent reviews on selection methodologies to enrich for target specific antibodies against native epitopes have been published52. Phage display is certainly the most robust technique but yeast display53,54 or bacterial display55 can also be used to select Nanobodies from immune libraries. For conformational binders, it is essential to perform the in vitro selection (panning) experiments under conditions (buffer composition including appropriate detergent, pH, temperature, cofactors) that produce the desired conformation of the protein during phage incubation. To reduce the background of phage expressing non-specific Nanobodies, the target protein should be highly pure (> 95%) and homogeneous in conformation.
Aspecific adsorption onto the solid surface of an ELISA plate is still the most common way to immobilize targets for selection by phage display but this method can result in (partial) denaturation of the protein56. Though the use of streptavidin-coated magnetic beads is a valid alternative, we perform most panning experiments in 96 well plates allowing multiple parallel selection conditions. Our preferred method is to capture biotinylated or tagged target protein on a solid phase coated with neutravidin or a tag-specific antibody, respectively. Alternative strategies to present target proteins during selection are summarized in Table 1. The structural integrity and homogeneity of the presented target is the most decisive factor for selecting Nanobodies with the desired properties. If possible, we try different capturing/immobilization methods, vary the antigen concentration, use different detergents or ligands to keep the target in the desired conformation, try different washing & incubation buffers, or use different elution methods in parallel pannings using different selection wells on the same 96 well plate. In many cases, magnetic beads can be used as a valid alternative to perform selections in solution.
TABLE 1.
Target presentation methods for the identification of Nanobodies with demonstrated crystallization chaperone activity.
| Target selection format | Negative control |
|---|---|
| Biotinylated target protein captured onto a neutravidin coated well27 | Neutravidin coated well |
| Membrane protein that is packed into a (biotinylated) nanodisc and captured onto a neutravidin or antibody coated well2 (see anticipated results) | Irrelevant membrane protein reconstituted in (biotinylated) nanodiscs and captured onto a neutravidin or antibody coated well |
| Protein captured onto an immobilized monoclonal antibody that is specific for your protein or a protein tag (His-tag, Strep-Tag, GST, ...) | Well that is coated with the monoclonal antibody |
| Solid phase immobilized protein by aspecific adsorption6,70 | Empty well |
| Solid phase immobilized membrane protein reconstituted in lipid vesicles1 (see anticipated results) | Well coated with lipid vesicles harboring an irrelevant membrane protein |
| Solid phase immobilized membrane protein that is packed into virus like particles71 | Well coated with an irrelevant virus like particle |
Depending on the magnitude of the heavy chain-only antibody mediated humoral response in the llama, typically one to two rounds of panning are sufficient to enrich for target specific Nanobodies. Rather than to select for target specificity alone, we prefer early on to implement conditions that allow the identification of Nanobodies with the desired functional or biophysical properties: high affinity, stabilization of a unique protein conformation, modulation of receptor function, binding to a particular target domain/epitope, interference with (orthosteric) ligand binding, stabilization of a protein complex amongst others. In case no purified protein with demonstrated functionality is available, Nanobody panning methodologies using target expressing cells (or derivatives) have been reported and are skills implemented in our lab57.
Screening & functional/biophysical characterization of conformational Nanobodies
Following panning, we routinely pick 96 individual clones from distinct selection outputs and express the Nanobodies in the E. coli periplasm in 96-well plates. In the last 10 years, we rarely found Nanobodies that bind linear epitopes. Therefore, we discourage the use of western blot or any other technique that tends to unfold the target. In parallel to the assessment of antigen specificity, assays should be implemented for the identification of Nanobodies with the desired functional or biophysical properties. In case the target is a membrane protein, we advise to additionally assess Nanobody binding to target-overexpressing cells via fluorescence activated flow cytometry.
Our display vectors permit the inducible periplasmic expression of Nanobodies as soluble C-terminally His6 tagged proteins in E. coli strain WK6. Milligram quantities of > 95% pure Nanobody can routinely be obtained by IMAC from the periplasmic extract of a one liter bacterial culture48. As it is not known upfront which Nanobodies will behave as the best crystallization chaperone, it is critical to identify a large panel of sequence diverse Nanobodies (see Anticipated Results). Typically, following a protein based immunization and selection discovery, between 3 and 30 Nanobody families are identified. A Nanobody family is defined as a group of Nanobodies with a high similarity in their CDR3 sequence (identical length and > 80% sequence identity). Nanobodies from the same family derive from the same B-cell lineage and bind to the same epitope on the target.
Nanobodies as crystallization chaperones
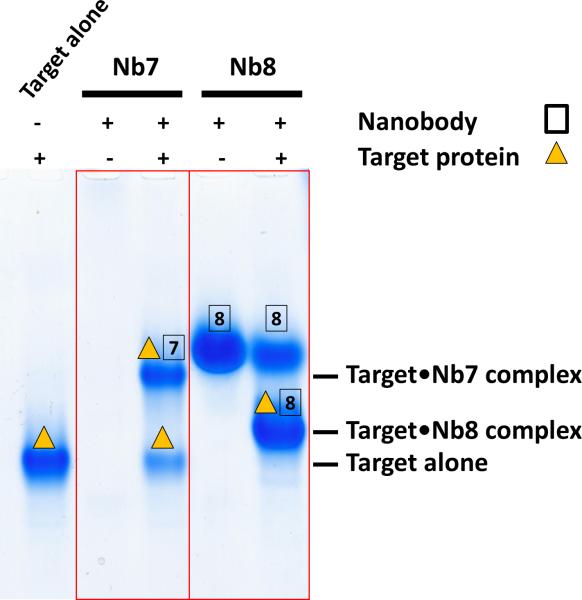
Electrophoretic mobility shift assays on native gels provide a quick and easy strategy to verify whether purified Nanobodies form a homogenous complex with the target protein. Figure 2 shows how mobility shift assays have been used to characterize Nanobodies that bind an editosome protein of T. brucei24. Co-crystallization experiments can be successful just by mixing Nanobody and target protein in a 1.2/1 molar ratio. Alternatively, the complex can be further purified by size exclusion chromatography after co-incubation. Because Nanobodies are resistant to additives, extremes of pH and temperature and proteases58, Nanobodies are ideally suited for screening a broad range of crystallization conditions with variations in pH, ionic strength, temperature, protein concentration, salts, ligands or additives, and type of precipitant. Conditions that can be screened are limited by the stability of the target protein rather than by the stability of the crystallization chaperones. More important is that Nanobodies are extremely soluble proteins (≥ 40 mg ml-1), maximizing the chance that they crystallize in complex with their co-crystallization target, rather than to yield Nanobody-only crystals. Because they are efficiently produced in the periplasm of E.coli, SeMet-labeled Nanobodies may ultimately be used for phasing using single-anomalous dispersion technique (SAD) without the need for introducing SeMet into the target protein28. Alternatively, phase information can be obtained from a molecular replacement solution of the Nanobody in the complex.
Figure 2.
Native gel analysis of Nanobodies interacting with the OB fold of the A1 protein of the editosome of the sleeping sickness parasite Trypanosoma brucei 24. Nanobodies Nb7 and Nb8 were generated using this protocol. The target protein was incubated with Nb7 or Nb8 for 30 min at 4 °C in 20 mM Tris (pH 7.5), 1 mM DTT and 300 mM NaCl. Complex formation of target and Nanobody was analyzed on a 4-15% native gel at 110 V for 1 h and stained by Coomassie Blue. The positions of the target protein alone (lane 1), the target•Nb7 complex (lane 3), the Nanobody Nb8 alone (lane 4), and the target•Nb8 complex (lane 5) are indicated with symbols above the bands. Due to its high isoelectric point, Nb7 is too positively charged to run into the native gel (lane 2).
Considerations about the lab facilities
We advise dedicating two separate labs for Nanobody discovery. One phage-free lab is used for Nanobody repertoire amplification and cloning. We use dedicated reagents for library construction. The other lab is used for all phage work including the amplification of phage and pannings. To reduce phage-contamination we use filter tips and clean bench, equipment and glassware with 1% sodium hypochlorite after each experiment. Use disposables whenever possible and discard in 1% sodium hypochlorite to inactivate remaining phage particles.
MATERIALS
REAGENTS
Acetic acid, glacial (Merck, cat. no. 1.00058.2500) ! CAUTION Glacial acetic acid is corrosive. Avoid exposure to inhalation, skin and eyes.
AEBSF protease inhibitor (Carl Roth, cat. no. 2931.3)
Agarose MP (Roche, cat. no. 11 388 991 001)
AP-conjugate substrate: 4-Nitrophenyl phosphate disodium salt hexahydrate (DNPP, Sigma, cat. no. 71768)
CaCl2.2H2O (Merck, cat. no. 2382)
Chloroform (Merck, cat. no. 1.02445.0250)
Citric acid.H2O (Sigma, cat. no. C1909)
E. coli strains: ▲ CRITICAL Use an amber codon suppression (supE) strain like TG1 (electrocompetent cells ≥ 4 × 1010 cfu μ−1; Lucigen cat. no. 60502-1) for cloning and preparation of phage libraries, and a non-suppressor strain (su−) like WK659 for the expression of Nanobodies
EDTA (Sigma-Aldrich, cat. no. E5134)
ELISA blocking reagent: skimmed milk powder (any commercial provider, e.g. Nestle, Marvell)
Ethanol (Fischer Chemical, cat. no. E/0650DF/15)
Gerbu adjuvant LQ 3000 (GERBU Biotechnik, cat. no. 30000025)
Glycerol (VWR, cat. no. 24387.292)
Guanidine-HSCN (ICN Biomedicals, cat. no. 820991)
HRP-conjugate substrate: Azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS, Sigma-Aldrich, cat. no. A1888)
IPTG dioxane free (Thermo Scientific, cat. no. R0392 )for the induction of Nanobody expression
Isoamylalcohol (Acros Organics, cat. no. 41273-5000)
KAPA Taq DNA polymerase and 10 × Taq buffer A (KAPA Biosystems, cat. no. BK1002) and dNTP mix (Thermo Scientific, cat. no. R0192) for PCR reactions
KCl (VWR, cat. no. 26764.298)
KH2PO4 (Carl Roth, cat. no. 3904.1)
K2HPO4 (Carl Roth, cat. no. P749.2)
M13 helper phage: kanamycin resistant VSCM13 (Stratagene, cat. no. 200251)
MgCl2 (Sigma-Aldrich, cat. no. M8266)
MgSO4.7H2O (Merck, cat. no. 5889)
NaCl (Carl Roth, cat. no. 3957.1)
Na2HPO4 .H2O (Merck, cat. no. 1.06586.1000)
NaH2PO4 .H2O(Carl Roth, cat. no. K300.1)
NH4Cl (Sigma, cat. no. A9434)
NeutrAvidin™ biotin binding protein (Thermo Scientific, cat. no. 31000)
PEG6000 (Sigma-Aldrich, cat. no. 81260)
Phage display vectors: pMES4 (genbank GQ907248) and pMESy4 (genbank KF415192) are pHEN460 derivatives allowing the display and production of soluble His6-tagged (pMES4) or His6-tagged and EPEA-tagged61 (i.e. CaptureSelect™ C-tag) Nanobodies (pMESy4), respectively. See Supplementary Figures 2 and 3 for maps. Nanobodies are under the transcriptional control of the lacZ promoter that is repressed by glucose and induced by IPTG
RNaseOUT recombinant RNase inhibitor (Life Technologies, cat. no. 10777019)
Sodium acetate.3H2O (Merck, cat. no. 1.06267.1000)
Sodium hypochlorite (any supplier of commercial bleach) ! CAUTION Avoid exposure to inhalation, skin and eyes.
Sucrose (Sigma-Aldrich, cat. no. S7903)
SuperScript II Reverse Transcriptase (Life technologies, cat. no. 18064-014)
T4 DNA ligase (1U μl−1) (Thermo Scientific, cat. no. EL0011)
Trisma base (Sigma, cat. no. T6066)
Tri-sodium citrate.2H2O (Sigma-Aldrich, cat. no. W302600)
Trypsin (Sigma-Aldrich, cat. no. T1426) for the elution of phage
Ultrapure™ Phenol:Water (3.75:1, v/v) (Life technologies, cat. no. 15594-047)
Growth media and agar plates
Bactotrypton (Duchefa, cat. no. T1332)
Glucose (Sigma-Aldrich, cat. no. G8270)
LB broth high salt (Duchefa, cat. no. L1704)
Micro agar (Duchefa, cat. no. M1002)
Thiamine hydrochloride (Sigma, cat. no. T1270)
Yeast extract (Duchefa, cat. no. Y1333)
Restriction enzymes
Eco91I (Thermo Scientific, cat. no. ER0391)
NcoI (Thermo Scientific, cat. no. ER0571)
PstI (Thermo Scientific, cat. no. ER0611)
DNA purification kits
PureYield™ Plasmid Miniprep System (Promega, cat. no. A1222)
QIAquick Gel Extraction Kit (Qiagen, cat. no. 28706)
QIAquick PCR purification kit (Qiagen, cat. no. 28106)
Biotinylation kits
EZ-Link Sulfo-NHS-LC-Biotinylation kit (Thermo Scientific, cat. no. 21435)
Biotin Quantitation Kit (Thermo Scientific, cat. no. 28005)
Antibodies
Capture select Biotin anti-C-tag conjugate (Life Technologies, cat. no. 7103252100)
Goat anti-Llama antibody HRP conjugated (Bethyl Laboraties, cat. no. A160)
Goat anti-mouse IgG Alkaline Phosphatase conjugated (Sigma, cat. no. A3562)
Mouse anti-His antibody (Abd Serotec, cat. no. MCA1396)
Antibiotics
Ampicillin sodium salt (Carl Roth, cat. no. K029.2)
Kanamycin monosulfate (Duchefa, cat. no. K0126)
Detergents
Lauroyl sarcosine (Affymetrix, cat. no. 21653)
Tween20 (Sigma-Aldrich, cat. no. P2287 or P1379)
Primers
CALL001: 5’-GTCCTGGCTGCTCTTCTACAAGG-3’
CALL002: 5’-GGTACGTGCTGTTGAACTGTTCC-3’
VHH-Back: 5’-GATGTGCAGCTGCAGGAGTCTGGRGGAGG-3’ (PstI)
VHH-For: 5’-CTAGTGCGGCCGCTGGAGACGGTGACCTGGGT-3’ (Eco91I)
MP57: 5’-TTATGCTTCCGGCTCGTATG-3’
GIII: 5’-CCACAGACAGCCCTCATAG-3’ These custom synthesized primers (Sigma-Aldrich) are stored at −20°C for years as stock solutions at 100 μM in 10 mM Tris-HCl buffer pH 8.5
dN6 Random primers: 5’-NNNNNN-3’ are custom synthesized and sored at −20° for years as a stock solution in 10 mM Tris-HCl buffer pH 8.5 at 2.5 μg/μl
EQUIPMENT & DISPOSABLES
245 × 245 × 25mm square dishes (Thermo Scientific, cat. no. 240835)
96-deepwell plates (Thermo Scientific, cat. no. AB-0932)
96-well conical bottom plates (Thermo Scientific, cat. no. 249944)
96-well U-bottom tissue culture plates with lid (BD, cat. no. 353077)
Benchtop centrifuge for Falcon tubes and 96-well plates, swing out rotor (Eppendorf, cat. no. 5810R)
Blood collection tubes: Venosafe® hematology (Terumo, cat. No. VF-109SDK) and serum gel (Terumo, cat. No. VF-108SAS) tubes
Cell scraper (Costar, cat. no 3011)
Centrifuge for 1.5 and 2 ml tubes (Eppendorf, cat. no. 5430R)
Electroporator: E.coli pulser (Bio-rad, cat. no.1652103)
Falcon conical centrifuge tubes (15 ml, 50 ml) (Fisher Scientific, cat. no. 14-959-49B, 14-432-22)
Gene pulser electroporation cuvette 0.1 cm gap (Bio-rad, cat. no. 165-2089)
Glass beads Ø (2.5-3.5 mm): (VWR, cat. no.2010087)
Heidolph Titramax 1000 vibrating platform (Heidolph-instruments, cat. no. 544-12200-00)
Incubators at 4 °C, 16 °C and 37 °C
Incubators with orbital shaking platform at 37 °C, 170 -200 rpm
Maxisorp 96-well Immuno plates (Nunc, cat. no. 439454)
Multichannel pipettes: Finnpipette F2 8 Channels 30-300 μl (Thermo Scientific, cat. no. 4662030), Finnpipette F2 8 Channels 5-50 μl (Thermo Scientific, cat. no. 4662010)
Nunc Microplate Sealing Tape 236366 (Thermo Scientific, cat. no. 10170671)
PCR tube strips (8-tube strips, Greiner bio-one, cat. no.673210; cap strips, Greiner bio-one, cat. no.373270)
RNase free microtubes (Eppendorf, cat no. 0030 121.589)
Shake flask triple baffled (1 liter, 250 ml) (Sigma-Aldrich, cat. no. F0769, F0644)
Spectrophotometer & ELISA plate reader
Sterile gas permeable adhesive seal (Thermo Scientific, cat. no. AB-0718)
Sterilization filters: 0.22 μM and 0.45 μM (Sarstedt , cat. no.83-1826-001, cat. no.83-1826)
Uni-SepMAXI+ lymphocyte separation tubes (Gentaur, cat. No. NVU16)
REAGENT SETUP
20% glucose Dissolve 20 g glucose in 100 ml ddH2O upon heating. Autoclave. Can be stored at room temperature (RT = 22°C) for months.
20% PEG6000/2.5M NaCl solution Dissolve 200 g of PEG6000 and 146.1 g NaCl in 1 liter ddH2O. Autoclave. Can be stored for months at RT.
▲ CRITICAL Dissolving PEG and NaCl requires stirring for several hours.
2 × TY medium Dissolve 16 g bactotrypton, 5 g NaCl and 10 g yeast extract in 1 liter ddH2O. Autoclave. Can be stored 6 months at 4°C or 1 month at RT.
ABTS reagent Dissolve 0.11 g ABTS in 500 ml 50 mM Na citrate buffer pH 4.0. Filter-sterilize the solution through a 0.2 μm filter and keep at 4 °C. Just before use add 20 μl H2O2 per 10 ml solution. Always prepare fresh.
AEBSF stock solution (4 mg ml−1) Dissolve 4mg AEBSF in 1 ml of PBS. Aliquots can be stored several months at −20 °C.
! CAUTION toxic serine protease inhibitor. Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation.
Ampicillin stock solution (100 mg ml−1) Dissolve 1 g in 10 ml ddH2O and sterilize through a 0.22 μM filter. Aliquots can be stored at −20 °C for at least one year.
Blocking solution Dissolve 1 g skimmed milk powder in 50 ml of target appropriate buffer (vortex for 1 min). Remove non-soluble components by centrifugation at > 3,000g at RT for 10 min. Prepare fresh.
Chloroform/isoamylalcohol solution Mix 24 volumes of chloroform with 1 volume isoamylalcohol and store for months at 4°C in a tightly closed recipient.
Coating buffer Dissolve 8.413 g NaHCO3 in 1 liter ddH2O and adjust pH to 8.2 with 1 M HCl. Can be stored for months at 4°C.
DNPP reagent Dissolve 12.1 g Tris, 1.02 g MgCl2 and 5.84 g NaCl in 1 liter ddH2O, adjust pH with 1 M HCl to pH 9.5 and autoclave. Buffer can be stored for several months at 4 °C. DNPP should be added freshly before use at a final concentration of 2 mg ml−1.
IPTG stock solution (1M) Dissolve 1.19 g in 5 ml ddH2O and sterilize through a 0.22 μM filter. Aliquots can be stored at −20 °C for at least one year.
LB medium Dissolve 25 g LB broth mix in 1 liter ddH2O. Autoclave. Can be stored up to 6 months at 4°C or for 1 month at RT.
10 × M9 minimal salts Dissolve 60 g Na2HPO4.H2O, 30 g KH2PO4, 5 g NaCl and 10 g NH4Cl in 1 liter ddH2O. Adjust to pH 7.4 before autoclaving. Can be stored for months at RT.
Kanamycin stock solution (25 mg ml−1) Dissolve 0.25 g in 10 ml in ddH2O and sterilize through 0.22 μM filter. Aliquots can be stored at −20 °C for at least one year.
LB Amp glucose plates Dissolve 25 g LB broth high salt and 15 g agar in 900 ml ddH2O. Autoclave and cool down to 50 °C. Supplement with 100 ml 20% glucose and 1 ml 100 mg ml−1 Ampicillin (0.20 μM filter sterilized) and pour plates. Plates can be stored for 1 month at 4°C.
M9 Minimal medium plates Mix 20 ml of sterile 10 × M9 minimal salts with 180 ml of hand warm 2% agar solution. Add the following sterile stock solutions: 200 μl of 0.1 M CaCl2, 400 μl of 1 M MgSO4.7H2O, 2 ml of 20% glucose, 1 ml of 1 mg ml−1 thiamine hydrochloride. Can be stored for 1 month at 4°C.
Neutravidin stock solution (1 mg ml−1) Dissolve 10 mg of NeutrAvidin™ biotin binding protein in 10 ml PBS. Aliquots can be stored at −20 °C for months
PBL lysis buffer Dissolve 23.6 g of guanidine-HSCN, 0.37 g of citric acid and 0.25 g of lauroyl sarcosine in 50 ml RNase-free water and adjust to pH 7.0. Aliquots can be stored at −20 °C for months without notable loss of activity.
PBS buffer (10 × stock) Dissolve 2.4 g KH2PO4, 14.1 g Na2HPO4.H2O, 2 g KCl and 80 g NaCl in 1 liter ddH2O (pH 6.8). This buffer will give a pH of 7.4 when diluted to 1 × PBS. Autoclave. Can be stored for months at RT.
PBST Add 100 ml 10 × PBS and 500 μl Tween20 to 900 ml ddH2O. Can be stored for months at RT.
Sodiumacetate (2 M) at pH 4.0 Dissolve 27.2 g Sodiumacetate.3H2O in 100 ml ddH2O and calibrate pH to pH 4.0. Can be stored for months at RT.
TB medium Dissolve 12 g bactotrypton, 2.3 g KH2PO4, 12.5 g K2HPO4, 24 g yeast extract and 2.5 ml glycerol in 1 liter ddH2O. Autoclave and store up to 6 months at 4°C or 1 month at RT.
TES buffer Dissolve 24.22 g Tris, 0.19 g EDTA and 171.15g sucrose in 1 liter ddH2O. Adjust to pH 8.0 with HCl. Can be stored for months at 4°C.
TES/4 buffer Dilute 250 ml TES buffer in 750 ml ddH2O. Can be stored for months at 4°C.
Tris-borate for electrophoresis (TBE) Dissolve 54 g Tris base and 27.5 g boric acid in 800 ml ddH2O. Add 20 ml 0.5 M EDTA (pH 8.0) and add ddH2O to 1 liter. Store at RT in glass bottles for months. Dilute 5 times with ddH2O for agarose gel electrophoresis.
Trypsin stock solution (1mg ml−1) Dissolve 1 g of trypsin in 100 ml PBS. Store aliquots at −20 °C for months.
PROCEDURE
Protein storage and analysis ● TIMING 1 day
Dispense ~1 mg protein in 7 aliquots of 100 μg (for llama immunization) and the remaining amount of protein in 10-20 μg aliquots. Use PCR tubes and Flash freeze aliquots by submerging the tubes in liquid nitrogen62 and transfer for long term storage to −80 °C.
Dispense 10 × 5 ml aliquots of the protein storage buffer (storage buffer composition has to be optimized for each target protein, see ref. 42) and store under appropriate conditions. For each component of the storage buffer, consult the manufacturer's product sheets.
Thaw one protein aliquot rapidly in your hand62 and confirm that the protein is properly folded using at least one quantitative functional or biophysical assay42.
! CAUTION Repeated freeze-thawing may negatively affect protein quality.
Llama immunization ● TIMING 6 weeks
-
4
We routinely immunize llamas with mixtures of 5 different target proteins. Always thaw protein aliquots (from Step 1) rapidly in your hand62 and keep on ice. For the first immunization mix 200 μg of each protein, for the following immunizations mix 100 μg of each protein in a buffer with a composition that does not disrupt the integrity of any of the proteins in the mix. Keep the total volume between 300 μL and 1 ml. If proteins cannot be mixed, separate immunogens can be prepared to be injected separately at different subcutaneous locations near draining lymph nodes. Table 2 gives a non-exhaustive overview of alternative materials that can be used for immunization. ! CAUTION All vaccination experiments should be executed following the applicable animal welfare legislation and must be approved by the local ethical committee. Make sure not to inject additives like sodium azide or (therapeutic) compounds that are toxic to the animals.
-
5
Prior to the first immunogen administration, collect a 4 ml blood sample in a Venosafe serum gel tube and allow to clot for 2 hrs at RT. Centrifuge for 5 min at RT and 3,000g and recover supernatant as the pre-immune serum. Store at −20 °C for measuring the serum conversion (see Step 11).
-
6
Immunize the animals 6 times with the freshly prepared immunogen (from Step 4) at weekly intervals. For the 1st, 2nd, 3rd and 5th immunizations, gently mix the immunogen with an equal volume of the GERBU adjuvant to make an emulsion and inject subcutaneously (max. 2 ml) in the neck base of the llama near the bow lymph node. To eliminate the risk of the adjuvant denaturating your protein of interest and destroying its conformational epitopes, we advise not to mix the immunogen with the adjuvant for the 4th and the 6th immunizations. Rather, inject the native immunogen subcutaneously and separately inject an equal volume of GERBU at 5 cm distance of the immunogen to locally boost the immune system.
TABLE 2.
Immunogen formats that have been applied for the generation of conformational Nanobodies.
| Detergent solubilized membrane proteins can be mixed with other soluble proteins if these resist the detergent26. |
| Reconstituted membrane proteins: membrane proteins tend to be more stable when they are reconstituted in lipid vesicles. We successfully immunized llamas with GPCRs, reconstituted in DOPC:LipidA (10:1) vesicles1 as exemplified in the anticipated results. |
| Protein complexes: in case (transient) protein complexes are immunized, we advise to chemically crosslink the complex. In general, we use a commercial mixture of different amine reactive crosslinkers that have been developed for mass spectroscopic detection of protein-protein complexes72. |
| Intact cells or membrane preparations: membrane preparations functionally expressing the target protein are described17,43. |
| Genetic immunization: llamas have been immunized successfully with a DNA-prime proteinboost strategy16,17. |
Blood sampling and lymphocyte preparation ● TIMING 1 day
-
7
Three to five days after the last immunization, collect 100 ml blood from the jugular vein in Venosafe hematology EDTA-coated blood collection tubes and invert twice to inhibit coagulation. Transfer blood samples to the lab and process immediately, following Step 8. ▲ CRITICAL STEP Coagulated blood will impair the extraction of total RNA from the lymphocytes.
-
8
Equally distribute the non-diluted blood into 4 separate Uni-SepMAXI+ tubes and isolate the peripheral blood lymphocytes (PBLs) according to the manufacturer's instructions. Collect PBLs at the interface between the plasma and polysucrose-sodium metrizoate layers and transfer to 4 separate 50 ml Falcon tubes. Recover the plasma (top layer) as the post-immune IgG containing sample, which will be used to measure the serum conversion in Step 11. Plasma samples can be stored at −20 °C for years without notable loss of activity.
-
9
Dilute the PBLs at least 10 fold in PBS and centrifuge for 20 min at 800g and 4 °C.
-
10
Carefully discard the supernatant. Resuspend each cell pellet in at least 5 ml PBS, collect into one 50 ml Falcon tube and divide cells equally over two 50 ml Falcon tubes. Centrifuge as in Step 9, discard the supernatant and place the tubes upside down for few minutes on paper towel. Each pellet contains approx. 5 × 108 PBLs. Preferably, continue immediately with the extraction of total RNA from fresh PBLs (Step 13) without freezing. RNA can also be extracted from pellets that have been stored at −80 °C for periods shorter than 6 months.
Analysis of the serum conversion ● TIMING 2 days
-
11
Use ELISA to compare the titer of the pre-immune serum (from Step 5) and the post-immune plasma (from Step 8) to measure the serum conversion induced by each antigen (as described in Box 1). Make 4 fold serial dilutions of the pre- and post-immune samples starting from 1/200 dilutions in PBS containing 0.1% milk. Use goat anti-llama-HRP conjugate and ABTS reagent to develop the ELISA. Except for immunizations with intrinsically unfolded proteins or peptides, we have always seen that conventional and heavy chain-only antibodies both contribute to the immunogen specific serum conversion.
? TROUBLESHOOTING
Isolation of total RNA ● TIMING 1 day
! CAUTION For all manipulations of RNA, wear gloves and use RNase-free materials and reagents.
-
13
Add 4 ml of PBL lysis65 buffer to approx. 5 × 108 PBLs (from Step 10), lyse the cells and shear the genomic DNA by repeatedly forcing the solution through 19G and 23G needles (6 times each). Transfer the transparent solution to a 15 ml Falcon tube.
-
14
Add 400 μl of 2 M Na-acetate pH 4.0, 4 ml phenol (water saturated) and 2 ml of a 24/1 chloroform/isoamylalcohol solution65. Mix well without vortexing and incubate for 10 min on ice. Centrifuge for 10 min at 3,200g and 4 °C in a swing out rotor.
-
15
Carefully transfer the upper RNA containing water phase to a fresh 15 ml Falcon tube and avoid carrying over interphase material. Add 4 ml phenol (water saturated) and 2 ml Chloroform/Isoamylalcohol (24/1) solution. Mix well and incubate for 10 min on ice. Centrifuge for 10 min at 3,200g and 4 °C in a swing out rotor.
-
16
Transfer 3 ml of the upper phase to a fresh 15 ml Falcon tube and divide over 6 RNase-free microcentrifuge tubes. Add 500 μl of ice cold 100% ethanol to each tube, mix manually and store at −80 °C for at least 30 min (or overnight) to precipitate the nucleic acids.
-
17
Centrifuge for 20 min at 20,000g and 4 °C. Next discard the supernatants and air dry the pellets for 10 min at RT. Dissolve each pellet in 20 μl of RNAse-free water. Incubate for 5 min at RT. If pellets do not dissolve completely, incubate RNA pellets for 10 min at 65 °C. Collect all 6 samples in a single RNase-free microcentrifuge tube.
-
18
Quantify RNA by measuring the optical density at 260 nm. Typically, 100-250 μg of RNA is obtained from 50 ml blood.
■ PAUSE POINT For long time storage at −80 °C, the RNA must be precipitated by mixing 0.05 volumes of 2 M Na Acetate pH 4.0 and 2.5 volumes of 100% ethanol. Precipitated RNA can be stored at −80 °C for one year without detectable degradation.
cDNA synthesis ● TIMING 4 hours
-
19
To prepare for cDNA synthesis, dilute 50 μg of total RNA in 50 μl RNAse-free water in a PCR tube. Add 2.5 μg of dN6 random primers, incubate for 7 min at 65 °C and allow the primers to anneal for 5 min on ice. Precipitate the remaining RNA (as described in Step 18) for later use.
-
20
To synthesize cDNA, add the following components to the PCR tube: 20 μl of 5x SSII Reverse transcriptase buffer, 10 μL of 100 mM DTT, 2 μl of dNTPs (25 mM), 2 μl of RNase out, 2.5 μl of SSII Reverse transcriptase and 12.5 μl of RNAse free water. Incubate for 10 min at 25 °C, 2 h at 42 °C and 15 min at 72 °C.
■ PAUSE POINT cDNA can be stored at −80°C for years without losing the diversity of the antibody repertoire.
Construction of the immune library ● TIMING 5 days
-
21To amplify the variable domains of all immunoglobulin heavy chains (VHs and VHHs) from the cDNA (See Supplementary Figure 1A) with two gene-specific primers CALL001 and CALL002 (See primers design), combine the following components in a PCR tube:
Volume Component Final concentration 1.0 μl cDNA 5.0 μl 10× Kapa Taq buffer A 1× 2.0 μl CALL001 primer (10μM) 0.4 μM 2.0 μl CALL002 primer (10μM) 0.4 μM 1.0 μl dNTPs (10mM each) 200 μM 38.75 μl water 0.25 μl Kapa Taq DNA polymerase (5U/μl) 0,025 U μl−1
Amplify DNA in a thermocycler using the following amplification cycles:
| Cycle | Temperature | Time |
|---|---|---|
| 1 | 95 °C | 3 min |
| 2 to 31 | 94 °C | 1 min |
| 57 °C | 1 min | |
| 72 °C | 45 s + 0.02 min cycle−1 | |
| 32 | 72 °C | 7 min |
▲ CRITICAL STEP We advise to run several PCRs in parallel, starting with 0, 0.5, 1, 2 and 4 μl of cDNA template.
-
22
Analyze the PCR products by electrophoresis on a 1% (w/v) agarose gel. Two DNA fragments should be amplified: fragments of approximately 700 bp representing the heavy chain-only antibody repertoire and fragments of 1,000 bp corresponding to the heavy chain of the conventional antibodies (See Supplementary Figure 1A). Choose that condition where an intense 700 bp band is well separated from the background and repeat this PCR in 8 tubes.
? TROUBLESHOOTING
-
23
To separate and gel-purify the 700 bp PCR fragment from the 1,000 bp fragment, apply the PCR products to 8 lines on a 1% (w/v) TBE agarose gel. Run the gel at 5.5 V cm−1 for 35 min. Cut the 700 bp PCR products from the gel and purify the fragment cut from each lane separately using the QIAquick gel extraction kit following the manufacturer's instructions. Quantify DNA from each purification by measuring the OD260nm.
■ PAUSE POINT The gel-extracted and purified PCR products can be stored at –20 °C for weeks without losing the diversity of the antibody repertoire.
-
24Reamplify the Nanobody encoding genes with nested primers VHH-Back and VHH-For, annealing at framework 1 and framework 4 respectively (See Supplementary Figure 1A) by combining the following components in 8 PCR tubes:
Volume Component Final concentration 5.0 μl Gel-purified PCR product (from Step 23) 0.4 ng μl−1 5.0 μl 10× Kapa Taq buffer A 1× 2.0 μl VHH-Back primer (10μM) 0.4 μM 2.0 μl VHH-For primer (10μM) 0.4 μM 1.0 μl dNTPs (10mM each) 200 μM 34.75 μl water 0.25 μl Kapa Taq DNA polymerase (5U μl−1) 0,025 U μl−1
Amplify the DNA in a thermocycler using the following amplification cycles:
| Cycle | Temperature | Time |
|---|---|---|
| 1 | 94°C | 5 min |
| 2 to 16 | 94°C | 30 s |
| 55°C | 30 s | |
| 72°C | 30 s | |
| 17 | 72°C | 7 min |
-
25
Clean up each PCR reaction with the Qiagen PCR purification kit, according to the manufacturer's instructions and pool all PCR products in one microcentrifuge tube.
-
26
Follow the restriction enzyme manufacturer's instructions to digest 10 μg of the phage display vector pMES4 or pMESy4 with PstI, Eco91I and XbaI in a 250 μl reaction mix. The additional XbaI digestion reduces self-ligation of the vector. In parallel digest 3 μg of the amplified PCR product (from Step 25) with PstI and Eco91I in a 100 μl reaction. Purify the vector and the PCR product using the QIAquick PCR purification kit, according to the manufacturer's instructions.
■ PAUSE POINT Store digested phage display vector and PCR product at −20 °C. Can be stored for minimally six months without loss of cloning efficiency.
-
27
Carry out test ligations to determine the amount of vector and insert needed to generate a library of at least 107 individual transformants by ligating 100ng of the triple digested vector with 30 ng of the PstI/Eco91I digested PCR product (1 to 3 molar ratio) and by ligating 100 ng of the triple digested vector alone for 2 hrs at RT in a 10 μl reaction using 0.5 units of the ligase.
-
28
Purify these ligation mixtures with the QIAquick PCR purification kit, according to the manufacturer's instructions, and transform 5 μl of each into 25 μl electrocompetent TG1 E. coli cells by electroporation according to the manufacturer's instructions.
-
29
Make ten-fold serial dilutions of the recovered transformed cells in LB. Plate 100μL of the 1/10,000, 1/1,000 and 1/100 dilutions on 90 mm LB agar plates containing 100 μg ml−1 ampicillin and 2% glucose and grow ON at 37 °C.
-
30
Count the colonies on each plate. Multiply the number of colonies with the corresponding dilution factor to calculate the electroporation efficiency. Pick 20 separate colonies and resuspend each colony in 50μL ddH2O for colony PCR with primers MP57 & GIII (as described in Step 68). Analyze the PCR products (Step 69) to confirm that the majority (>75%) of the transformants has an insert of the proper size of a Nanobody.
▲ CRITICAL STEP If the electroporation efficiency per 100 ng vector in the vector-insert ligation mix is lower than 5 × 105 or less than 75% of the transformants have an Nb insert, we advise to repeat Steps 26 to 30.
? TROUBLESHOOTING
-
31
Ligate sufficient digested vector and insert prepared in Step 26 to generate a library of at least 107 individual transformants. We typically ligate 15 to 25 times the amount of the test ligation ON at 16 °C.
-
32
Purify the ligation mixture using the QIAquick PCR purification kit on a single column, wash twice with PE solution and elute with 30 μl EB buffer.
-
33
Perform 5 separate electroporations each using 6 μl of the purified ligation and 25 μl TG1 cells. To each cuvette, add 1 ml of pre-warmed recovery medium (37 °C) and recover all cells in one 50 ml Falcon tube and incubate cells for 1 hr at 37 °C and 170 rpm. Add LB to reach a total volume of 8 ml.
-
34
Use 100 μl of the electroporated cells to make serial dilutions (see Step 29) and calculate the effective library size (see Step 30). Analyze the final insert ratio according to Step 30.
-
35
Divide the remaining suspension over four 245 mm square LB-agar dishes containing 100 μg ml−1 ampicillin and 2% glucose and grow ON at 37 °C.
-
36
When the library size is ≥ 107, recover the library; wet the 245 mm square LB-agar dishes with 4 ml of LB, scrape with a sterile cell scraper and collect all cells in a 50 ml Falcon tube with a sterile pipet. Rinse the plates once more, each with 2 ml LB medium and mix the collected cell suspension with sterile glycerol (20% v/v final concentration). Measure the OD600nm of the suspension, make 10 to 20 aliquots of 150 μl and store at −80 °C. The rest of the suspension can be stored in larger tubes at −80 °C.
■ PAUSE POINT These glycerol stocks of the immune library can be stored at −80 °C for several years without notable loss of repertoire diversity. Immune libraries can be used as the starting point to select Nanobodies each time new reagents or new scientific insights become available.
Rescue and amplification of phage from immune libraries ● TIMING 2 days
-
37
Day 1: Before each round of panning, rescue and amplify Nanobody-displaying phage particles by adding helper phage following the standard rescue protocol: firstly, inoculate 6 OD600nm units of the immune library into a baffled 250 ml Erlenmeyer containing 60 ml of 2 × TY medium supplemented with 100 μg ml−1 ampicillin and 2% glucose. For a typical library size of 108 clones, this inoculum represents 48 copies of each library clone (assuming 1 OD600nm unit of E. coli cells corresponds to 8 × 108 cells ml−1) and theoretically 480 copies of the maximum Nanobody diversity present in a 100 ml blood sample (estimated to be maximally 107). Grow at 37 °C and 200 rpm until cells reach logarithmic phase (corresponding to an OD600nm of 0.5) required for F-pilus production.
▲ CRITICAL STEP Note that Phage display libraries can only be rescued from pMES4 or pMESy4 in an amber codon suppressor strain such as TG1 that contains the F’ conjugative plasmid.
-
38
For phage rescue: transfer 10 ml of the log grown cells to a sterile 50 ml Falcon tube. Superinfect with 4 × 1010 pfu VSCM13 (10 times excess helper phage versus TG1 cells). Mix gently and incubate 30 min at 37 °C without shaking allowing phage to infect the cells.
! CAUTION As TG1 cells are easily contaminated with phage, always use filtertips when working with phage to avoid unintended TG1 infections and discard all tubes containing phage in 1% sodium hypochlorite to inactivate remaining phage particles.
-
39
Centrifuge the infected cells at 2,800g for 10 min, RT and discard supernatant carefully to remove traces of glucose. Resuspend the cell pellet in 50 ml 2 × TY supplemented with 100 μg ml−1 ampicillin and 25 μg ml−1 kanamycin into a baffled 250 ml erlenmeyer. Incubate ON at 37 °C and 200 rpm for the amplification of Nanobody-displaying phage.
-
40
Day 2: Transfer the ON culture to a 50 ml Falcon tube and centrifuge 15 min at 3,200g and 4 °C in a swing out centrifuge to pellet the cells.
-
41
Transfer 40 ml of the supernatant to a new 50 ml Falcon tube and add 10 ml of 20% PEG6000 / 2.5 M NaCl solution. Mix well by inverting the Falcon tube 5 times and keep on ice for at least 30 min to precipitate phage resulting in a homogeneous cloudy suspension.
-
42
Pellet phage particles by centrifugation for 10 min at 3,200g and 4 °C. Discard supernatant and carefully remove remaining liquid by placing the tube upside down on tissue paper.
-
43
Resuspend the precipitated phage particles in 1 ml ice-cold PBS and transfer to a microcentrifuge tube. Centrifuge for 1 min at 20,000g and 4 °C in a microcentrifuge to pellet residual bacteria.
-
44
Transfer the supernatant to a new microcentrifuge tube without disturbing the bacterial pellet and re-precipitate phage by adding 250 μl ice-cold 20% PEG6000/2.5 M NaCl solution. Invert the tube 5-10 times until a homogeneous white suspension appears and keep 10 min on ice.
-
45
Centrifuge for 15 min at 20,000g and 4 °C in an microcentrifuge, remove the supernatant and resuspend the phage pellet in 1 ml ice-cold PBS. Centrifuge for 1 min at 20,000g and 4 °C in a microcentrifuge to completely remove bacterial contaminants. Transfer supernatant to a new tube to recover phage solution.
■ PAUSE POINT Phage is ready to be used for panning and can be stored up to a month at 4 °C without loss of phage infectivity. For longer term storage, add glycerol (20% v/v final concentration) and transfer to −80 °C. Before use, remove glycerol from solution by precipitating phage as described in Steps 44-45.
Antigen presentation & phage selection by panning ● TIMING 3 days per round of selection
-
46
Day 1: Preparative steps. Inoculate a fresh colony of TG1 cells grown on minimal medium into 15 ml LB in a 100 ml culture flask and incubate at 37 °C and 170 rpm. Grow until cells are in the exponential phase (OD600nm = 0.5 – 0.6) and keep sterile on ice.
-
47
To estimate the number of infective recombinant phage, titrate the rescued phage (from Step 45) by preparing serial ten-fold dilutions in a low binding 96-well round bottom culture plate. Typically, prepare up to 1010 fold dilutions by sequentially mixing 10 μl of phage into 90 μl of PBS. Transfer 10 μl of each dilution to a well with 90 μl of TG1 cells (from Step 46). Use multichannel pipettes when possible. Incubate for 15 min at 37 °C without shaking to infect TG1 cells. Carefully pipet 5 μl drops of the infected TG1 cells (one drop per phage dilution) on solid selective medium (LB + 100 μg ml−1 ampicillin + 2% glucose), air-dry drops and grow ON at 37 °C.
-
48
As TG1 cells are easily contaminated with phage, also pipet a 5 μL drop of the non-infected TG1 culture (from Step 46) on solid selective medium as a negative control.
-
49
Coat non-adjacent wells of a Maxisorp 96-well plate with 100 μl of a 2 μg ml−1 neutravidin solution in PBS and seal the plate with Microplate Sealing Tape. Incubate ON at 4 °C. Sealed plates can be stored for two weeks at 4 °C without loss of capturing capacity.
-
50
Day 2: Enriching for target specific recombinant phage. Estimate the phage titer by counting the colonies from the highest dilutions, prepared in Step 47, that have grown ON onto the selective medium.
▲ CRITICAL STEP If the titer of the phage library is lower than 1012 ml−1 colony forming units, we advise to repeat library rescue (Steps 37 to 48).
? TROUBLESHOOTING
-
51
Make sure the log grown TG1 culture (from Step 48) was not infected with phage by visually inspecting the ON incubated solid medium plate. No TG1 colonies should be grown on selective medium.
-
52
Dilute the TG1 culture (from Step 46) 100-fold into 50 ml LB and incubate at 37 °C and 170 rpm. Grow until cells are in the exponential phase (OD600nm = 0.5 – 0.6) and keep on ice until used in Steps 59 and 60.
-
53
Wash the neutravidin-coated wells (from Step 49) three times with 250 μl of PBST, add 250 μl blocking solution and incubate for two hours on a vibrating platform (700 rpm) at RT.
-
54
For each selection well and each control well, pre-incubate 1011 phage (from Step 45) with 10 μl blocking solution in 100 μl of the appropriate selection buffer for 30 min at RT in one microcentrifuge tube by head-over-head rotation. In our hands, the presence of tracer amounts of biotin in milk powder does not affect antigen capturing.
-
55
Biotinylate the target protein for panning using the Thermo Scientific EZ-Link Sulfo-NHS-LCBiotinylation kit, following the manufacturer's instructions.
▲ CRITICAL STEP Although it is beyond the scope of this protocol, it is important to quantify the biotin/target ratio (ideally one) with the Biotin Quantitation Kit and to demonstrate that biotinylation does not interfere with the quality of the target42.
-
56
To immobilize the biotinylated target protein for panning, remove the blocking solution of the neutravidin coated wells (from Step 53) and wash five times with 250 μl of the appropriate selection buffer. For each selection condition, add saturating amounts of the biotinylated target protein (from Step 55; typically 100 nM) in 90 μl of the appropriate selection buffer supplemented with 10 μL blocking solution to a separate selection well. For each selection condition, also fill a neutravidin-coated control well with the corresponding selection buffer only supplemented with blocking solution to act as a negative control. Incubate for 15 min on a vibrating platform (700 rpm) at RT. Wash five times with 250 μl of the appropriate selection buffer.
-
57
For each selection condition, dispense 100 μl of the pre-incubated phage (from Step 54) into both a selection well and a control well and incubate for 2 hrs on a vibrating platform (700 rpm). Carefully remove non-bound phage particles and wash each well 15 times by adding 250 μl of the selection buffer. After each wash step, remove excess liquid by tapping the emptied plates once on a paper towel, avoiding cross contamination of phage to neighboring wells. After the final wash step, carefully remove excess liquid via pipetting.
-
58
To elute phage bound to the wells, add 100 μl of 0.25 mg ml−1 trypsin solution to each selection well and the corresponding control well and incubate for 30 min at RT on a vibrating platform (700 rpm). This step will dissociate phage bound to the immobilized antigen by proteolysis without affecting phage infectivity. Transfer eluted phage from each selection well and the corresponding control well to separate microcentrifuge tubes pre-filled with 5 μl of a 4 mg ml−1 AEBSF solution to inhibit protease activity.
-
59
To allow recovery of the phage that eluted from each well, infect 3 ml of exponentially grown TG1 cells (from Step 52) in a 50 ml Falcon tube with 50 μL of the eluted phage from Step 58 and incubate for 30 min at 37 °C without shaking. Add 7 ml of LB and supplement to 100 μg ml−1 ampicillin and 2% glucose and grow ON at 37 °C and 170 rpm. Store 1 ml aliquots at −80 °C as a glycerol stock (20% v/v) for later use. Glycerol stocks of these selected sub-libraries can be stored for multiple years without detectable loss of Nanobody repertoire diversity.
■ PAUSE POINT Phage can be rescued from this sub-library at any time (see Step 61).
-
60
To estimate the number of infective recombinant phage that eluted from each well, titrate the remaining eluted phage (from Step 58) according to Steps 47 and 50. For a first selection round, typically phage is diluted from 100 up to 105 fold. If the number of phage that eluted from the selection well is at least 100-fold higher than the phage eluted from an appropriate negative control well, we advise to screen individual clones of this selection round for the desired properties (Step 62). When the enrichment factor is below 100, we recommend to rescue this sub-library and proceed with another selection round (see Step 61).
▲ CRITICAL STEP Although one round of selection is often sufficient to identify binders, nonspecific binding limits the target specific phage enrichment that can be achieved per selection round. Therefore consecutive rounds of selection may be needed to select the binders with the desired properties from the immune library. Consider adapting the panning conditions to select Nanobodies with higher affinity or to bias the selection to an epitope of interest57. Take into account that subsequent rounds of selection will reduce diversity.
? TROUBLESHOOTING
-
61
Day 3: Rescue of the eluted phage for consecutive rounds of panning. To rescue and amplify phage for a consecutive round of panning from the ON grown infected TG1 culture or the frozen glycerol stock (from Step 59) , inoculate 500 μl into a baffled 250 ml Erlenmeyer containing 50 ml of 2 × TY supplemented with 100 μg ml−1 ampicillin and 2% glucose. Grow at 37 °C and 200 rpm until cells reach exponential phase. Repeat steps 38 to 60.
Screening for antigen binders ● TIMING 4 days
-
62
To isolate individual clones from an enriched sub-library, prepare 10-fold serial dilutions of the ON grown phage infected TG1 cells (Step 59) in sterile LB. Homogeneously spread 100 μl of the serial dilutions over individual culture plates containing selective medium (LB agar + 100 μg ml−1 ampicillin + 2% glucose) using sterile glass beads and incubate ON at 37 °C. 100 μl of a 105 dilution typically results in approximately 100 single colonies per plate.
-
63
We typically screen 96 to 192 individual clones per target in total and pick at least 24 candidates per enriched selection condition. To prepare master plates, inoculate single colonies into wells of a 96-well round bottom culture plate filled with 100μl 2 × TY supplemented with 100 μg ml−1 ampicillin, 2% glucose and 10% glycerol per well. Inoculate one well with a TG1 transformant expressing an irrelevant Nanobody as the negative control and assign one well without inoculum to monitor possible well to well contaminations. Grow ON at 37 °C without shaking.
■ PAUSE POINT We store these master plates containing glycerol stocks of E. coli expressing monoclonal Nanobodies at −80 °C. Individual clones can be recovered from master plates that have been stored for years.
-
64
To prepare E. coli periplasmic extracts containing Nanobody, inoculate 10 μl of an ON grown master plate in a 96-deepwell plate containing 1 ml 2 × TY + 100 μg ml−1 ampicillin + 0.1% glucose per well. Cover the plates with a sterile gas permeable adhesive seal and grow 3 hrs at 37 °C and 200 rpm until cells are in the exponential phase. Induce Nanobody expression by adding 100 μl of 10 mM IPTG in 2 × TY per well and shake for 4-6 hrs at 37 °C and 200 rpm. Pellet cells at 3,200g for 10 min. Discard supernatant in a single movement by turning the plate upside down followed by a simple sudden vertical move downwards. Place the plate upside down on a paper towel and freeze the cell pellets at −20 °C for 30 min.
■ PAUSE POINT Frozen cell pellets can be stored at −20 °C for months without loss of Nanobody activity.
-
65
Thaw the deep well plate containing cell pellets for 15 min at RT. To release the Nanobodies from the periplasm, add 100 μl PBS to each well. Incubate for 30 min at RT on a vibrating platform (700 rpm). Pellet cell debris by centrifuging for 10min at 3,200g and 4 °C. Carefully recover 90 μl of the supernatant without disturbing the cell pellet and transfer to a new 96-well V-bottom plate. The recovered solution typically contains up to 500 nM of Nanobody.
-
66
As a first step, we screen for target specificity via ELISA according to the protocol described in Box 1. We routinely screen 5-fold diluted periplasmic extracts. Detect Nanobody-binding by incubating with a His-tag specific secondary antibody and an anti-mouse-AP conjugate according to the manufacturer's instructions. Use the DNPP reagent to develop the ELISA.
! CAUTION If the target also contains a His-tag, use appropriate secondary antibody and conjugate.
? TROUBLESHOOTING
-
67
Optionally, the same periplasmic extracts can be used in other biochemical, cellular or biophysical screens to further characterize the functional properties of the Nanobodies. For membrane anchored targets, we suggest to also screen via flow cytometry to assess Nanobody specificity to the target in its native membrane environment17.
Sequence determination of positive clones ● TIMING 2-3 day
-
68Thaw the master plate (Step 63) and resuspend 5μl of the cells expressing Nanobody with the desired properties in 45 μl sterile water. Use 5 μl of this cell suspension as a template with vector specific primers MP57 and GIII in the following PCR:
Volume Component Final concentration 5μl Template (cell suspension) 2,5μl 10× Kapa Taq buffer A 1× 0,5μl Forward primer MP57 (10 μM stock) 0.2 μM 0,5μl Reverse primer GIII (10 μM stock) 0.2 μM 0,5μl dNTPs (10 mM stock each) 200 μM 0,2μl Kapa Taq DNA polymerase (5U μl−1 stock) 0.02 U μl−1 15,8μl ddH2O
Amplify the DNA using the following PCR conditions:
| Cycle | Temperature | Time |
|---|---|---|
| 1 | 95 °C | 2 min |
| 2 to 28 | 94 °C | 30 s |
| 55 °C | 30 s | |
| 72 °C | 40 s | |
| 29 | 72 °C | 7 min |
-
69
Analyze the PCR products via gel electrophoresis on a 1% agarose gel. Clones expressing a genuine Nanobody will generate a fragment of approximately 700bp. Determine the DNA sequence using MP57 or GIII as sequencing primers. Group Nanobodies with a high similarity in their CDR3 sequence (identical length and > 80% sequence identity) into sequence families. Nanobodies from the same family derive from the same B-cell lineage and bind to the same epitope on the target.
Expression and purification of Nanobodies ● TIMING 5 days
! CAUTION To obtain the highest yields, we advise to express soluble Nanobody in the original display vector in the periplasm of E. coli strain WK6.
-
70
Inoculate a single colony derived from individual wells of the master plate (Step 63) in 5 ml LB supplemented with 100 μg ml−1 ampicillin and 2% glucose and grow ON at 37°C. Prepare plasmid from the TG1 cells using the PureYield™ Plasmid Miniprep System. Transform the purified plasmid into E. coli WK6. Inoculate a single colony into a 50 ml sterile Falcon tube containing 10 ml LB supplemented with 100 μg ml−1 ampicillin, 2% glucose and 1 mM MgCl2. Grow pre-culture ON at 37 °C and 170 rpm.
-
71
Inoculate 330 ml TB supplemented with 100 μg ml−1 ampicillin, 0.1% glucose and 1 mM MgCl2 in a 1 liter baffled flask with 3 ml of the preculture. Shake at 37 °C and 170 rpm until OD600nm = 0.7. Induce Nanobody expression with 1 mM of IPTG (final concentration) and grow ON at 170 rpm and 28 °C. Alternatively induce Nanobody expression for 4 hrs at 170 rpm and 37 °C.
-
72
Harvest bacteria by centrifuging 15 min at 9,000g at RT. Carefully resuspend the cell pellet of 1 liter culture in 15 ml ice cold TES and incubate for at least 1 hr on ice on an orbital shaking platform. Add 30 ml of TES/4 to the resuspended pellet and shake for 45 min on ice on an orbital shaking platform. Centrifuge 30 min at 10,000g at 4°C and recover the supernatant as the periplasmic extract.
-
73
Purify the His-tagged Nanobodies from the periplasmic extract using IMAC according to the manufacturer's instructions. This expression and purification protocol routinely yields 1 to 10 mg per liter culture with an estimated purity ≥ 95%. For crystallography grade Nanobodies, a subsequent polishing step via size exclusion chromatography is often required.
? TROUBLESHOOTING
TROUBLESHOOTING
Troubleshooting advice can be found in Table 3.
TABLE 3.
Troubleshooting table
| Step | Problem | Possible reasons | Solution |
|---|---|---|---|
| 11 | No serum conversion | Biotinylation denaturates target | Use a lower molar excess of biotin reagent for target biotinylation and assess folding (Step 55) Assess serum conversion with non biotinylated target |
| Low target immunogenicity | Immunize other animals and use an alternative immunogen format (Table 2). Because camelids are outbred, the magnitude of the serum conversion between animals may differ |
||
| 22 | No Nanobody repertoire amplified | RNA degraded | Collect new blood sample and extract PBLs (Steps 7-10), make new buffers, prepare fresh RNA (Steps 13-18) and verify integrity of RNA via agarose gel electrophoresis |
| 30 | Library size < 107 or insert percentage < 75% | Inefficient digestion by PstI-Eco91I | Evaluate integrity and concentration of digested vector and Nanobody repertoire before ligation via gel electrophoresis. |
| Low transfection efficiency | Assess transformation efficiency of TG1 with supercoiled plasmid. Optimize the vector to insert ratio. Use fresh T4 DNA ligase. |
||
| 50 | Phage titer below 1012 ml−1 | Repression of lacZ promoter | Do not add glucose during overnight phage amplification (Step 39) |
| Low helper phage infectivity | Prepare new helper phage and measure infectivity (Step 38) | ||
| 60 | Enrichment < 10 after two panning rounds | Aspecific binding of phage | Use alternative blocking agent (eg BSA, Steps 53-56). |
| Protein prep not homogeneous | Use a better purified protein sample for panning (Step 55) | ||
| Biotinylation denaturates target protein | Biotinylate target with lower molar excess of biotin reagent and assess folding (Step 55) Repeat panning using an alternative target presentation method (Table 1) | ||
| Immune titer mediated by conventional IgGs | The presence of target specific Nanobodies in the each phage (sub)library can be addressed by performing an ELISA (Box 1) using phage from Step 45 as the sample in step 3 of Box 1. Use an M13 specific antibody conjugate for detection. |
||
| 66 | No target specific Nanobodies identified | Low Nanobody expression in TG1 periplasm | Perform Western Blot to confirm that Nanobodies are expressed in step 65. |
| Quality of the immobilized target | Confirm the quality of the immobilized target with an existing (conformational) antibody. Repeat ELISA using an alternative target presentation method (Table 1). |
||
| 73 | Poor Nanobody yield | Low expression | Use WK6 for expression. Some Nanobodies have the intrinsic property to express poorly in E. coli. In this case we advise to try and express another Nanobody from the same family. |
● TIMING
Steps 1-3, protein storage and analysis: 1 day
Steps 4-6, immunization: 6 weeks
Steps 7-10, blood sampling and lymphocyte preparation: 1 day
Steps 11-12, analysis of the serum conversion: 2 days
Steps 13-18, isolation of total RNA: 1 day
Steps 19-20, cDNA synthesis: 4 hours
Steps 21-36, construction of the immune library: 5 days
Step 37-45, rescue and amplification of phage from immune libraries: 2 days
Step 46-61, antigen presentation & selection by panning: 3 days per round of panning
Step 62-67, screening for antigen binders, 4 days
Steps 68-69, sequence determination, 2-3 days
Steps 70-73, expression and purification of Nanobodies, 5 days
ANTICIPATED RESULTS
Three representative examples of Nanobody discovery programs with distinct levels of complexity are presented below. Each program led to the identification of conformational Nanobodies that were instrumental to growing diffracting crystals that allowed high-resolution structures to be determined.
Nanobody assisted X-ray crystallography of BACE2
Inhibition of the aspartic protease BACE2 has recently been shown to lead to improved control of glucose homeostasis and to increased insulin levels in insulin-resistant mice66. BACE2 may therefore be of high importance in drug discovery as a target for the expansion of functional pancreatic cell mass in diabetes. We generated a series of BACE2 specific Nanobodies for Nanobody-assisted crystallography to create a toolbox of well-diffracting BACE2 crystals grown from solutions with different pH and with different packing interactions and different active-site conformations to repeatedly and rapidly obtain co-crystal structures with previously intractable inhibitor series27.
A BACE2 specific humoral response was induced in two llamas. One llama was immunized with recombinant BACE2 and the other was injected with inhibitor saturated BACE2 according to Steps 4-6. Both llamas were simultaneously immunized with two other target proteins. As the protein storage buffers of the three immunogens were not compatible, all targets were injected at separate locations. A display library was constructed in pMESy4 using pooled RNA extracted from blood samples collected 4 and 8 days after the final antigen boost according to Steps 7-36.
Panning was performed on neutravidin captured biotinylated BACE2 in presence of excess inhibitor in appropriate selection buffer (20 mM Bis-Tris propane pH 7.0, 0.15 M NaCl, 10% glycerol, 0.3% CHAPS). Biotinylated BACE2 was prepared using EZ-Link NHS-Chromogenic-Biotin according to the instructions of the manufacturer. Following two rounds of panning, 129 out of 138 Nanobodies scored positive in an ELISA using neutravidin captured biotinylated BACE2 in presence of excess inhibitor. After sequence analysis 30 unique BACE2 specific Nanobodies were identified belonging to 9 sequence families. Between 1 and 13 sequence variants were identified per family.
Six Nanobodies, all belonging to different sequence families with an affinity between 1 and 100 nM and covering 3 distinct epitopes (as determined by surface plasmon resonance) were selected for cocrystallisation. XA4813 yielded six different crystal forms diffracting up to 1.5 Å resolution for both wild-type and mutant BACE227. The Nanobody co-crystals allowed determining high resolution structures of BACE2 inhibitor complexes that could not be obtained with other BACE2 crystals. A high-resolution structure of BACE2 in complex with 2 Nanobodies (XA4813 and XA4815) was also obtained.
Nanobodies stabilizing the active β2 adrenoreceptor conformational state
Efforts to obtain an agonist-bound active-state GPCR structure have proven difficult due to the inherent instability of this state in the absence of a G protein. We generated Nanobodies to the human β2 adrenoreceptor (β2AR) that exhibits G protein-like behaviour, and obtained an agonist-bound, active-state crystal structure of the receptor-Nanobody complex1. For this purpose, β2AR truncated after amino acid 365 (β2AR-365) having an amino terminal Flag epitope tag was expressed in Sf9 insects cells and purified by sequential M1 antibody and alprenolol affinity chromatography as described67. To reconstitute the receptor in lipid vesicles, purified β2AR-365 was immobilized on an anti-Flag M1 column and equilibrated with 10 column volumes of a mixture of 5 mg ml−1 DOPC and 0.5 mg ml−1 Lipid A in 1% (w/v) octylglucoside, 100 mM NaCl, 20 mM Hepes pH 7.5, 2 mM CaCl2 and 1 μM of the superagonist BI-167107. The β2AR was then eluted in the same buffer without CaCl2 supplemented with EDTA and Flag peptide. Lipid vesicles were then dialyzed against PBS containing 1 μM agonist at 4 °C to remove detergent. The concentration of the reconstituted protein was adjusted to 5 mg ml−1, aliquoted and stored at −80 °C.
A single llama received six weekly administrations of the reconstituted truncated β2AR according to Steps 4-6. Lymphocytes were isolated from 50 ml of blood of the immunized llama and total RNA was prepared from these cells according to Steps 7-18. The coding sequences of the Nanobody repertoire were amplified by RT-PCR and cloned into the phage display vector pMES4 according to Steps 19-36. β2AR specific phages were enriched by two rounds of in vitro selection on 96 well Maxisorp plates coated with the lipid vesicle reconstituted β2AR-365 receptor according to Steps 37-61. Antigen bound phage was recovered from antigen-coated wells either with thriethylamine pH 11 and neutralized with Tris-HCl pH 7 or by the addition of freshly grown TG1 E.coli cells.
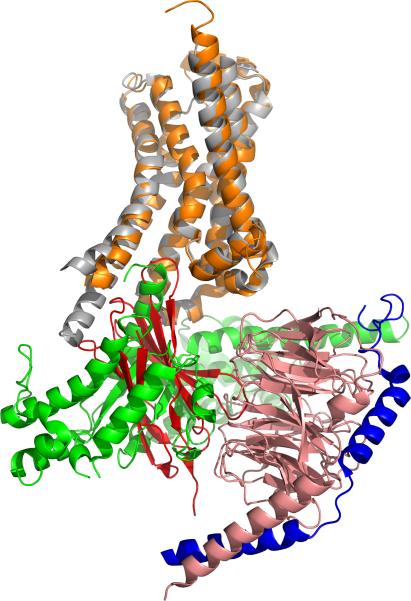
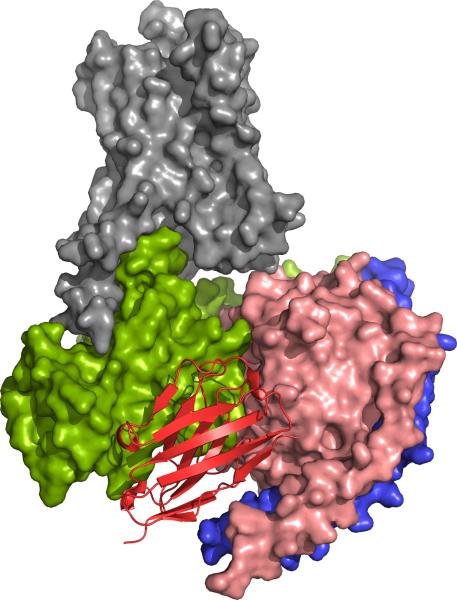
After two rounds of biopanning, 96 individual colonies were randomly picked and the Nanobodies were produced as a soluble His-tagged protein in the periplasm of the TG1 cells. Solid-phase ELISA identified 16 different conformational Nanobodies that recognize native agonist-bound β2AR-365, but not the heat denatured receptor. Seven clones recognized agonist-bound (BI-167107) but not inverse agonist-bound receptor (ICI-118,551). Of these, Nb80 was chosen for co-crystallization experiments because it showed G-protein-like properties upon binding to both wild-type β2AR and β2AR–T4L. Crystals of BI-167107 bound β2AR–T4L in complex with Nb80 were obtained in lipid bicelles and lipidic cubic phase (LCP) at pH8.0 in 39–44% PEG400, 100 mM Tris, 4% DMSO and 1% 1,2,3-heptanetriol. Nb80 binds to the cytoplasmic cavity of the β2AR, with the third complementarity-determining region (CDR3) loop projecting into the core of the receptor (Figure 3).
Figure 3.
Nb80 is a structural mimic of GαS and stabilizes the active-state conformation of β2AR. Nb80 (red) and GαS (green) bind the same intracellular cavity of the agonist bound β2 adrenoreceptor in the β2AR•Nb80 complex1 (PDB3P0G; β2AR in orange, Nb80 in red) and the β2AR•Gs complex2 (PDB3SN6; β2AR in gray, GαS in green, Gβ in salmon and Gγ in blue).
Nanobodies stabilizing the β2 AR-Gs multiprotein complex
The β2 adrenoreceptor activation of Gs, the stimulatory G protein for adenylyl cyclase, has long been a model system for GPCR signaling. We used Nanobody-assisted X-ray crystallography to solve the crystal structure of the active state ternary complex composed of agonist-occupied monomeric β2AR and nucleotide-free Gs heterotrimer. Formation of a stable β2AR-Gs complex from the Gs heterotrimer and BI-167107 bound β2AR-365 in 0.02% MNG-368 was accomplished as described2. MNG-3 (also known as NG310, Anatrace) is a detergent that has high avidity for the β2AR and a very slow dissociation rate compared to other detergents such as dodecyl maltoside. This is important for maintaining the native conformation of the receptor following immunization. From negative stain electron microscopy imaging34, we observed that the α-helical domain of Gαs was flexible and therefore possibly responsible for poor crystal quality. Targeted stabilization of this domain was addressed by immunizing two llamas with 100 μg of the bis(sulfosuccinimidyl)glutarate (BS2G, Pierce) cross-linked BI167107•β2AR•Gs ternary complex followed by 3 bi-weekly shots of 50 μg. After completing the immunization, peripheral blood lymphocytes were isolated from the immunized animals to extract total RNA and prepare cDNA according to Steps 13-20.
For each llama, a separate phage display library was constructed in pMESy4 (Steps 21 to 36). Nanobodies specific for the multiprotein complex were enriched by two rounds of panning on i) the BI167107•β2AR•Gs ternary complex embedded in Apo A-I biotinylated high-density lipoprotein particles69 (rHDL) or ii) on the BS2G crosslinked BI167107•β2AR•Gs ternary complex. For the first panning strategy, biotinylated rHDL particles containing the BI167107•β2AR•Gs ternary complex were immobilized on a neutravidin coated Maxisorp plate at 0.5 μM per well in 20 mM Hepes (pH 8.0), 100 mM NaCl, 1 mM EDTA, 100 μM TCEP, and 100 nM BI167107. For the second panning strategy the BS2G cross-linked BI167107•β2AR•Gs ternary complex was solid phase coated on an ELISA plate at 1 μg per well. To elute complex specific phage, the wells were treated with trypsin according to Step 58. After 2 rounds of selection, 24 colonies were randomly picked from each enriched sub-library and expressed the Nanobodies in the periplasm of E. coli according to Steps 64-65.
Two immunizations and two separate selection strategies lead to the discovery of 8 unique sequence families that are specific for the complex. 6 of these families enriched both on biotinylated rHDL particles containing the BI167107•β2AR•Gs ternary complex and on the solid phase coated BS2G crosslinked BI167107•β2AR•Gs ternary complex. None of nanobodies bound to β2AR-365 receptor alone. To identify Nanobodies providing stabilization to the G protein subunits the agonist•β2AR•Gs ternary complex was analyzed by analytical size exclusion chromatography in the presence and absence of the nonhydrolyzable GTP analog GTPγS. It was found that Nb352 and Nb3734,38 protect the BI167107•β2AR•Gs complex from dissociation by GTPγS.
The T4L–β2AR•Gs•Nb35 complex was used to obtain crystals in LCP (7.7MAG) that diffracted to 2.9Å. In the crystal structure, Nb35 packs at the interface of the Gβ and Gα subunits, with CDR1 interacting primarily with Gβ and a long CDR3 loop interacting with both Gβ and Gα subunits (Figure 4).
Figure 4.
Structure of the β2AR•Gs complex solved by Nanobody-enabled X-ray crystallography. Surface representation of the active state ternary complex composed of agonist-occupied monomeric β2AR (grey) and nucleotide-free Gs heterotrimer. Nb35 (Red, cartoon representation) binds at the interface of the Gα (green) and Gβ (salmon) subunits. Gγ is represented in blue.
Supplementary Material
Box 1 ELISA ● TIMING 2 days.
Unless otherwise mentioned, all incubation steps are performed at RT on a vibrating shaking platform (700 rpm) or any temperature that is required to keep the protein stable.
Add 100 μl of a 2-5 μg ml−1 neutravidin solution in PBS to individual wells of a 96-well Maxisorp plate. Incubate overnight (ON) at 4 °C. Wash the wells three times with 250 μl of PBST. Add 250 μl of blocking solution to all wells and block plate for two hours. Wash 5 times with 250 μl PBST.
Add 100 μl of the biotinylated target protein (diluted to 1 μg ml−1 in the protein storage buffer) per well. Include appropriate negative controls in separate wells (e.g. irrelevant biotinylated protein) and incubate for 30 min. Wash 5 times with 250 μl of the protein storage buffer.
Add 100 μl of the diluted sample in protein storage buffer containing 0.1 % milk to each well and incubate for 1 hr. Wash 5 times with 250 μl of the protein storage buffer. For serum conversion assays use serial dilutions of the pre- and post-immune sera as described in Step 11. For Nanobody screening use a 5 fold dilution of periplasmic extracts as described in Step 50.
If required, add secondary antibody in 100μl protein storage buffer containing 0.1% milk and incubate for 1 hr. Wash 5 times with 250 μl of the protein storage buffer.
Add the conjugate (enzyme-labeled anti-immunoglobulin) in 100 μl of the protein storage buffer containing 0.1% milk and incubate for 1 hr. Wash 5 times with 250 μl of the protein storage buffer.
Develop the reaction by adding 100 μl of the appropriate colorimetric substrate (ABTS or DNPP reagent) and measure absorption at appropriate wavelength.
ACKNOWLEDGMENTS
We thank members and associates of the Steyaert, Muyldermans, Hol & Kobilka labs, past and present, for their assorted contributions over the years to this work. In particular, we acknowledge the contributions of Nele Buys and Young-jun Park. The Steyaert lab was supported by the Fonds Wetenschappelijk Onderzoek-Vlaanderen through research grants G011110N and G049512N, Innoviris Brussels trough Impulse Life Science program BRGEOZ132, the Belgian Federal Science Policy Office through IAP7-40 and by SBO program IWT120026 from the Flemish Agency for Innovation by Science and Technology. Brian Kobilka received support from NIH grants R01NS028471 and R01GM083118 and from the Mathers Foundation. The research in the laboratory of Wim Hol was supported by NIAID and NIGMS of the National Institutes of Health under award numbers AI34501 and GM077418. S.T. received a doctoral fellowship from the Fonds Wetenschappelijk Onderzoek-Vlaanderen. S.G.F.R is supported by the Lundbeck Foundation.
Footnotes
AUTHOR CONTRIBUTIONS J.S. developed the concept of Nanobody-assisted crystallography in collaboration with W. G. J. H. and B.K.K.; E.P., T.L., S.T., A.W. and J.S. worked out the protocol. E.P., T.L., S.M., W.G.J.H., B.K.K. and J.S. contributed to the introduction. E.P., T.L., S.G.F.R., A.R., B.K.K. and J.S. performed the experiments described in the anticipated results and all authors participated in discussions on technical and conceptual aspects of the protocol and the editing of the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–80. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–55. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low C, et al. Nanobody mediated crystallization of an archeal mechanosensitive channel. PLoS One. 2013;8:e77984. doi: 10.1371/journal.pone.0077984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranova E, et al. SbsB structure and lattice reconstruction unveil Ca2+ triggered S-layer assembly. Nature. 2012;487:119–22. doi: 10.1038/nature11155. [DOI] [PubMed] [Google Scholar]
- 5.Park YJ, Pardon E, Wu M, Steyaert J, Hol WG. Crystal structure of a heterodimer of editosome interaction proteins in complex with two copies of a cross-reacting nanobody. Nucleic Acids Res. 2012;40:1828–40. doi: 10.1093/nar/gkr867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korotkov KV, et al. Structural and functional studies on the interaction of GspC and GspD in the type II secretion system. PLoS Pathog. 2011;7:e1002228. doi: 10.1371/journal.ppat.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loris R, et al. Crystal structure of the intrinsically flexible addiction antidote MazE. J Biol Chem. 2003;278:28252–7. doi: 10.1074/jbc.M302336200. [DOI] [PubMed] [Google Scholar]
- 8.Abskharon RN, et al. Combining in-situ proteolysis and microseed matrix screening to promote crystallization of PrPc-nanobody complexes. Protein Eng Des Sel. 2011;24:737–41. doi: 10.1093/protein/gzr017. [DOI] [PubMed] [Google Scholar]
- 9.Domanska K, et al. Atomic structure of a nanobody-trapped domain-swapped dimer of an amyloidogenic beta2-microglobulin variant. Proc Natl Acad Sci U S A. 2011;108:1314–9. doi: 10.1073/pnas.1008560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilliams T, et al. Nanobodies Raised against Monomeric alpha-Synuclein Distinguish between Fibrils at Different Maturation Stages. J Mol Biol. 2013;425:2397–411. doi: 10.1016/j.jmb.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Muyldermans S, Cambillau C, Wyns L. Recognition of antigens by single-domain antibody fragments: the superfluous luxury of paired domains. Trends Biochem Sci. 2001;26:230–5. doi: 10.1016/s0968-0004(01)01790-x. [DOI] [PubMed] [Google Scholar]
- 12.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–97. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 13.Lauwereys M, et al. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998;17:3512–20. doi: 10.1093/emboj/17.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Genst E, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci U S A. 2006;103:4586–91. doi: 10.1073/pnas.0505379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newby ZE, et al. A general protocol for the crystallization of membrane proteins for X-ray structural investigation. Nat Protoc. 2009;4:619–37. doi: 10.1038/nprot.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch-Nolte F, et al. Single domain antibodies from llama effectively and specifically block T cell ecto-ADP-ribosyltransferase ART2.2 in vivo. FASEB J. 2007;21:3490–8. doi: 10.1096/fj.07-8661com. [DOI] [PubMed] [Google Scholar]
- 17.Laeremans T, et al. Genetic immunization for producing immunoglobulins against cell-associated antigens such as P2X7, CXCR7 or CXCR4. 2010 WO Patent 2,010,070,145.
- 18.Roovers RC, et al. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies. Cancer Immunol Immunother. 2007;56:303–317. doi: 10.1007/s00262-006-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmyter A, et al. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996;3:803–11. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- 20.Decanniere K, et al. A single-domain antibody fragment in complex with RNase A: non-canonical loop structures and nanomolar affinity using two CDR loops. Structure. 1999;7:361–370. doi: 10.1016/s0969-2126(99)80049-5. [DOI] [PubMed] [Google Scholar]
- 21.Desmyter A, et al. Three camelid VHH domains in complex with porcine pancreatic alpha-amylase. Inhibition and versatility of binding topology. J Biol Chem. 2002;277:23645–50. doi: 10.1074/jbc.M202327200. [DOI] [PubMed] [Google Scholar]
- 22.Dumoulin M, et al. A camelid antibody fragment inhibits the formation of amyloid fibrils by human lysozyme. Nature. 2003;424:783–8. doi: 10.1038/nature01870. [DOI] [PubMed] [Google Scholar]
- 23.Korotkov KV, Pardon E, Steyaert J, Hol WG. Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure. 2009;17:255–65. doi: 10.1016/j.str.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YJ, et al. The structure of the C-terminal domain of the largest editosome interaction protein and its role in promoting RNA binding by RNA-editing ligase L2. Nucleic Acids Res. 2012;40:6966–77. doi: 10.1093/nar/gks369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderhaegen S, et al. Structure of an early native-like intermediate of beta2-microglobulin amyloidogenesis. Protein Sci. 2013;22:1349–57. doi: 10.1002/pro.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward AB, et al. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc Natl Acad Sci U S A. 2013;110:13386–91. doi: 10.1073/pnas.1309275110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banner DW, et al. Mapping the conformational space accessible to BACE2 using surface mutants and cocrystals with Fab fragments, Fynomers and Xaperones. Acta Crystallogr D Biol Crystallogr. 2013;69:1124–37. doi: 10.1107/S0907444913006574. [DOI] [PubMed] [Google Scholar]
- 28.Tereshko V, et al. Toward chaperone-assisted crystallography: protein engineering enhancement of crystal packing and X-ray phasing capabilities of a camelid single-domain antibody (VHH) scaffold. Protein Sci. 2008;17:1175–87. doi: 10.1110/ps.034892.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinelli S, et al. Lactococcal bacteriophage p2 receptor-binding protein structure suggests a common ancestor gene with bacterial and mammalian viruses. Nat Struct Mol Biol. 2006;13:85–9. doi: 10.1038/nsmb1029. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, et al. Structures of a key interaction protein from the Trypanosoma brucei editosome in complex with single domain antibodies. J Struct Biol. 2011;174:124–36. doi: 10.1016/j.jsb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koide S. Engineering of recombinant crystallization chaperones. Curr Opin Struct Biol. 2009;19:449–57. doi: 10.1016/j.sbi.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukowska MA, Grutter MG. New concepts and aids to facilitate crystallization. Curr Opin Struct Biol. 2013;23:409–16. doi: 10.1016/j.sbi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol. 2011;21:567–72. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westfield GH, et al. Structural flexibility of the Gαs α-helical domain in the β2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A. 2011;108:16086–91. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera-Calzada A, et al. Structure of a bacterial type IV secretion core complex at subnanometre resolution. EMBO J. 2013;32:1195–1204. doi: 10.1038/emboj.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vercruysse T, et al. Measuring cooperative Rev protein-protein interactions on Rev responsive RNA by fluorescence resonance energy transfer. RNA Biol. 2011;8:316–24. doi: 10.4161/rna.8.2.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothbauer U, et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods. 2006;3:887–9. doi: 10.1038/nmeth953. [DOI] [PubMed] [Google Scholar]
- 38.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–8. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vercruysse T, Pardon E, Vanstreels E, Steyaert J, Daelemans D. An intrabody based on a llama single-domain antibody targeting the N-terminal alpha-helical multimerization domain of HIV-1 rev prevents viral production. J Biol Chem. 2010;285:21768–80. doi: 10.1074/jbc.M110.112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson AD. Antibody-enabled small-molecule drug discovery. Nat Rev Drug Discov. 2012;11:519–25. doi: 10.1038/nrd3756. [DOI] [PubMed] [Google Scholar]
- 41.Structural Genomics C, et al. Protein production and purification. Nat Methods. 2008;5:135–46. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vedadi M, Arrowsmith CH, Allali-Hassani A, Senisterra G, Wasney GA. Biophysical characterization of recombinant proteins: a key to higher structural genomics success. J Struct Biol. 2010;172:107–19. doi: 10.1016/j.jsb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jahnichen S, et al. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc Natl Acad Sci U S A. 2010;107:20565–70. doi: 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paalanen MM, et al. The development of activating and inhibiting camelid VHH domains against human protein kinase C epsilon. Eur J Pharm Sci. 2011;42:332–9. doi: 10.1016/j.ejps.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Lee GM, Craik CS. Trapping moving targets with small molecules. Science. 2009;324:213–5. doi: 10.1126/science.1169378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flajnik MF, Deschacht N, Muyldermans S. A Case Of Convergence: Why Did a Simple Alternative to Canonical Antibodies Arise in Sharks and Camels? PLoS Biol. 2011;9:e1001120. doi: 10.1371/journal.pbio.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Genst E, Saerens D, Muyldermans S, Conrath K. Antibody repertoire development in camelids. Dev Comp Immunol. 2006;30:187–98. doi: 10.1016/j.dci.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Conrath KE, et al. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob Agents Chemother. 2001;45:2807–12. doi: 10.1128/AAC.45.10.2807-2812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Linden R, et al. Induction of immune responses and molecular cloning of the heavy chain antibody repertoire of Lama glama. J Immunol Methods. 2000;240:185–95. doi: 10.1016/s0022-1759(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 50.Maass DR, Sepulveda J, Pernthaner A, Shoemaker CB. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs). J Immunol Methods. 2007;324:13–25. doi: 10.1016/j.jim.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kastelic D, et al. A single-step procedure of recombinant library construction for the selection of efficiently produced llama VH binders directed against cancer markers. J Immunol Methods. 2009;350:54–62. doi: 10.1016/j.jim.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–16. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 53.Koide A, Koide S. Single Domain Antibodies. Springer; 2012. Affinity Maturation of Single-Domain Antibodies by Yeast Surface Display. pp. 431–443. [DOI] [PubMed] [Google Scholar]
- 54.Ryckaert S, Pardon E, Steyaert J, Callewaert N. Isolation of antigen-binding camelid heavy chain antibody fragments (nanobodies) from an immune library displayed on the surface of Pichia pastoris. J Biotechnol. 2010;145:93–8. doi: 10.1016/j.jbiotec.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Fleetwood F, et al. Surface display of a single-domain antibody library on Gram-positive bacteria. Cell Mol Life Sci. 2013;70:1081–93. doi: 10.1007/s00018-012-1179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nizak C, Moutel S, Goud B, Perez F. Selection and application of recombinant antibodies as sensors of Rab protein conformation. Method enzymol. 2005;403:135–153. doi: 10.1016/S0076-6879(05)03012-0. [DOI] [PubMed] [Google Scholar]
- 57.Verheesen P, Laeremans T. Single Domain Antibodies. Springer; 2012. Selection by Phage Display of Single Domain Antibodies Specific to Antigens in Their Native Conformation. pp. 81–104. [DOI] [PubMed] [Google Scholar]
- 58.Muyldermans S. Single domain camel antibodies: current status. J Biotechnol. 2001;74:277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 59.Zell R, Fritz HJ. DNA mismatch-repair in Escherichia coli counteracting the hydrolytic deamination of 5-methyl-cytosine residues. EMBO J. 1987;6:1809–15. doi: 10.1002/j.1460-2075.1987.tb02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–6. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 61.De Genst EJ, et al. Structure and properties of a complex of alpha-synuclein and a single-domain camelid antibody. J Mol Biol. 2010;402:326–43. doi: 10.1016/j.jmb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Deng J, et al. An improved protocol for rapid freezing of protein samples for long-term storage. Acta Crystallogr D Biol Crystallogr. 2004;60:203–4. doi: 10.1107/s0907444903024491. [DOI] [PubMed] [Google Scholar]
- 63.Hamers-Casterman C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 64.Daley LP, Gagliardo LF, Duffy MS, Smith MC, Appleton JA. Application of monoclonal antibodies in functional and comparative investigations of heavy-chain immunoglobulins in new world camelids. Clin Diagn Lab Immunol. 2005;12:380–6. doi: 10.1128/CDLI.12.3.380-386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–5. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 66.Esterhazy D, et al. Bace2 is a beta cell-enriched protease that regulates pancreatic beta cell function and mass. Cell Metab. 2011;14:365–77. doi: 10.1016/j.cmet.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 67.Kobilka BK. Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal Biochem. 1995;231:269–71. doi: 10.1006/abio.1995.1533. [DOI] [PubMed] [Google Scholar]
- 68.Chae PS, et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods. 2010;7:1003–8. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam AY, Pardon E, Korotkov KV, Hol WG, Steyaert J. Nanobody-aided structure determination of the EpsI:EpsJ pseudopilin heterodimer from Vibrio vulnificus. J Struct Biol. 2009;166:8–15. doi: 10.1016/j.jsb.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez Pajuelo M, Hermans G, Vanlandschoot P. Panning phage libraries with lipoprotein particles expressing the target antigen. 2011 WO Patent 2,011,083,141.
- 72.Bich C, et al. Reactivity and applications of new amine reactive cross-linkers for mass spectrometric detection of protein-protein complexes. Anal Chem. 2010;82:172–9. doi: 10.1021/ac901651r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.