Abstract
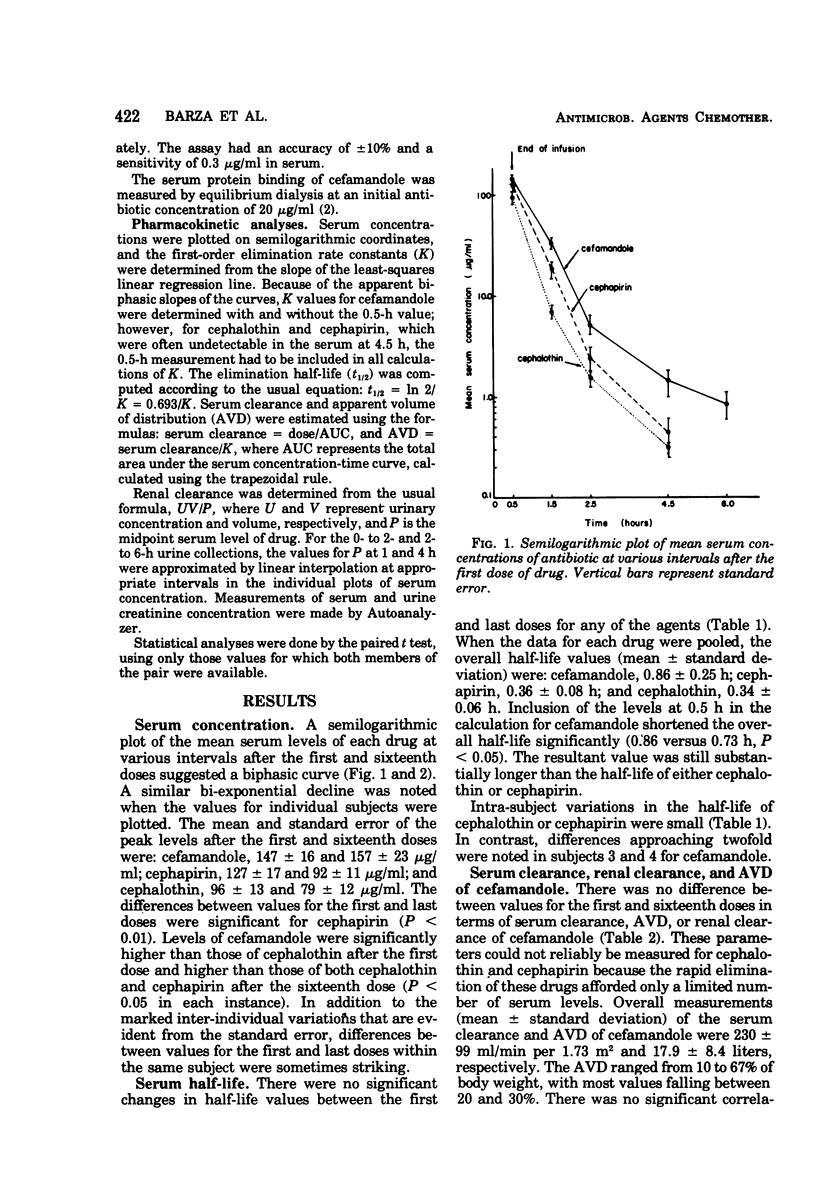
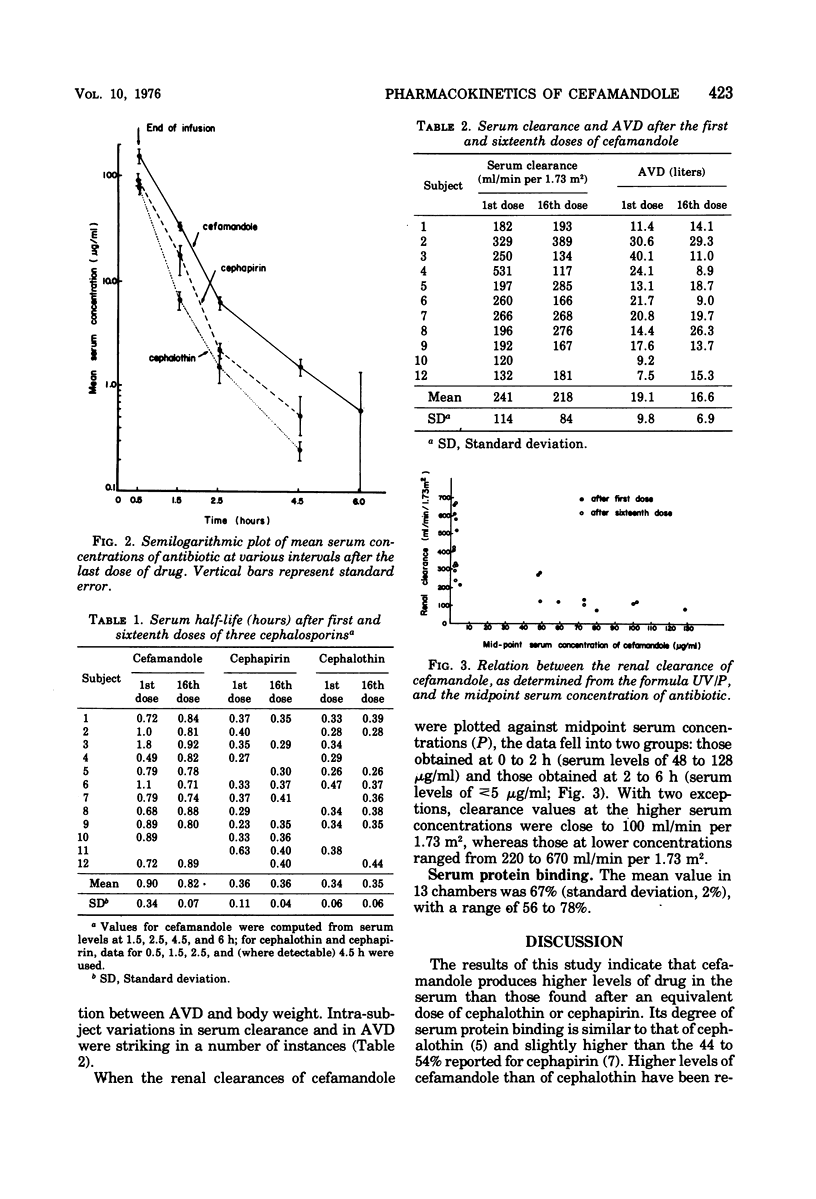
Cefamandole nafate, cephapirin, and cephalothin were administered intravenously in crossover fashion to 12 volunteers, in dosages of 2 g every 6 h for 16 doses. Mean peak levels of cefamandole were approximately 50% higher than those of the other agents. The serum concentration curves appeared to decline bi-exponentially, suggesting that a two-compartment model was most applicable for pharmacokinetic analysis; accordingly, the t½ of cefamandole was significantly longer when the serum peak was omitted from the analysis (0.86 versus 0.73 h, P < 0.05). The half-lives of cephalothin and cephapirin, 0.34 and 0.36 h, respectively, were probably underestimates reflecting the inclusion of distribution-phase values in the calculation. Repeated dosing had no effect on the peak serum levels, half-life, serum clearance, or apparent volume of distribution with one exception: peak serum levels of cephapirin were significantly lower after the sixteenth than after the first dose. Marked variations within a given subject were noted in the half-life and apparent volume of distribution of cefamandole in several instances. Renal clearances of cefamandole exhibited saturation kinetics similar to those of penicillin G.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Meyers B. R., Hirschman S. Z. Cephapirin: pharmacology in normal human volunteers. J Clin Pharmacol New Drugs. 1972 Feb-Mar;12(2):84–88. doi: 10.1002/j.1552-4604.1972.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Barza M., Samuelson T., Weinstein L. Penetration of antibiotics into fibrin loci in vivo. II. Comparison of nine antibiotics: effect of dose and degree of protein binding. J Infect Dis. 1974 Jan;129(1):66–72. doi: 10.1093/infdis/129.1.66. [DOI] [PubMed] [Google Scholar]
- Bergeron M. G., Brusch J. L., Barza M., Weinstein L. Significant reduction in the incidence of phlebitis with buffered versus unbuffered cephalothin. Antimicrob Agents Chemother. 1976 Apr;9(4):646–648. doi: 10.1128/aac.9.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E. C., Berger S., Barza M., Jacobus N. V., Tally F. P. Activity of cefamandole and other cephalosporins against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1976 May;9(5):852–855. doi: 10.1128/aac.9.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong I. W., Ralph E. D., Engelking E. R., Kirby W. M. Clinical pharmacology of cefamandole as compared with cephalothin. Antimicrob Agents Chemother. 1976 Jan;9(1):65–69. doi: 10.1128/aac.9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottendorf G. H., Price K. E., Van Harken D. R. Comparative plasma bactericidal activity of cephapirin and cefazolin. Curr Ther Res Clin Exp. 1975 Aug;18(2):364–370. [PubMed] [Google Scholar]
- Kirby W. M., De Maine J. B., Serrill W. S. Pharmacokinetics of the cephalosporins in healthy volunteers and uremic patients. Postgrad Med J. 1971 Feb;47(Suppl):41–46. [PubMed] [Google Scholar]
- Meyers B. R., Ribner B., Yancovitz S., Hirschman S. Z. Pharmacological studies with cefamandole in human volunteers. Antimicrob Agents Chemother. 1976 Jan;9(1):140–144. doi: 10.1128/aac.9.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale C. H., Greene D. S., Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975 Dec;64(12):1899–1926. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- PERS M. Penicillin clearance as kidney function test, determinations with and without collection of urine. Scand J Clin Lab Invest. 1954;6(4):341–348. doi: 10.3109/00365515409134873. [DOI] [PubMed] [Google Scholar]
- Shemonsky N. K., Carrizosa J., Levison M. E. In vitro activity and pharmacokinetics in patients of cefamandole, a new cephalsoporin antibiotic. Antimicrob Agents Chemother. 1975 Dec;8(6):679–683. doi: 10.1128/aac.8.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling P. G., Craig W. A., Gordon A. L., Kunin C. M. Pharmacokinetics of cefazolin in normal and uremic subjects. Clin Pharmacol Ther. 1974 Apr;15(4):344–353. doi: 10.1002/cpt1974154344. [DOI] [PubMed] [Google Scholar]



