Abstract
Background and Aims
The immediate post-ischemic period is marked by elevated intracellular calcium levels which can lead to irreversible myocyte injury. Del Nido cardioplegia was developed for use in the pediatric population to address the inability of immature myocardium to tolerate high levels of intracellular calcium following cardiac surgery. Our aim in this study is to determine if this solution can be used safely and effectively in an adult, re-operative population.
Methods
All patients undergoing isolated re-operative aortic valve replacement at our institution from 2010 to 2012 were retrospectively reviewed. Demographics, co-morbidities, operative variables, post-operative complications, and patient outcomes were collected. Patients were divided into 2 groups based on cardioplegia strategy used: Whole blood cardioplegia (WB, n=61) and del Nido cardioplegia (DN, n=52).
Results
Mean age in the study was 73.4 ± 14.3 years and 86 patients were male (76.1%). Eighty-four patients had undergone prior CABG (74.3%). Patients in the DN group required significantly lower total volume of cardioplegia (1147.6 ± 447.2 mL DN vs. 1985.4 ± 691.1 mL WB, p<0.001) and retrograde cardioplegia dose (279.3 ± 445.1 mL DN vs. 1341.2 ± 690.8 mL WB, p<0.001). There were no differences in cross-clamp time, bypass time, post-operative complication rate, or patient outcomes between groups.
Conclusions
Del Nido cardioplegia use in an adult, re-operative aortic valve population offers equivalent post-operative outcomes when compared with whole blood cardioplegia. In addition, use of del Nido solution requires lower total and retrograde cardioplegia volumes in order to achieve adequate myocardial protection.
Keywords: Valve repair/replacement, perfusion
INTRODUCTION
The hallmarks of effective myocardial protection are to render the myocardium electromechanically quiescent while simultaneously decreasing cellular metabolic demands in order to prevent intracellular acidosis and reperfusion injury (1,2). Although the mechanisms of ischemia-reperfusion injury are still debated, it is believed that intracellular calcium concentrations are increased in post-ischemic myocardium in part due to an increased intracellular sodium concentration which develops during the ischemic period, forcing the Na+/Ca2+ exchanger to function in the “reverse” direction, thereby increasing calcium influx (3–5). High levels of intracellular calcium are known to cause myocyte hypercontracture, which irreversibly injures cytoskeletal components leading to cell death (3). Initially developed for the pediatric population, del Nido cardioplegic solution (DN) addresses the concern that immature cardiomyocytes are particularly susceptible to reperfusion injury given their inability to tolerate high levels of intracellular calcium in the post-ischemic period (6). Specifically, lidocaine, a sodium channel blocker, and magnesium, a calcium competitor, are added to a calcium-free solution, and mixed with blood. This creates a solution that serves to limit the influx of sodium, producing a depolarized arrest, and also limits calcium influx post-reperfusion following a single dose administration. This technique has been used successfully in the pediatric population since its development in the early 1990s (7,8).
More recently, it has been shown in animal models as well as isolated myocyte models that senescent myocardium is also relatively intolerant of the post-ischemic increase in intracellular calcium following cardioplegic arrest which has led surgeons to consider the use of DN in the adult population (9–11). Since 2011, we have used this solution exclusively in adult cardiac surgical cases, including reoperations. In the current study, we report the use and safety of DN in adults undergoing re-operative aortic valve surgery and document its effect on surgical outcomes.
METHODS
Patient Selection
Consecutive patients undergoing re-sternotomy for isolated aortic valve replacement for aortic stenosis (AS) or aortic insufficiency (AI) were retrospectively studied from 2010–2012, and divided into two cohorts based on the type of cardioplegia administered during surgery: 1) whole blood cardioplegia (WB, n=61) used exclusively in patients from January 2010 until mid-2011, and 2) del Nido cardioplegia solution (DN, n=51) used exclusively from mid-2011 to December 2012. All patients met standard indications for surgery for AS or AI. Patients who were in cardiogenic shock, were undergoing a concomitant cardiac surgical procedure, or had active endocarditis were excluded from this analysis.
Variables collected included age, gender, body surface area (BSA), type of prior operation, pre-operative co-morbidities [hyperlipidemia (HLD), prior myocardial infarct (MI), hypertension (HTN), stroke, chronic obstructive pulmonary disease (COPD), chronic kidney disease with baseline creatinine > 2.0 mg/dL (CKD), atrial fibrillation (AFib), congestive heart failure by New York Heart Association functional classification], need for inotropes/vasopressors upon ICU admission, days on ventilator, post-operative complications [transfusion, infection, acute renal injury (ARI), stroke, transient ischemic attach (TIA), need for permanent pacemaker (PPM), any arrhythmia, need for reintubation], pre and post-operative echocardiographic findings, length of stay, discharge status, and bypass/cardioplegia details (bypass time, cross-clamp time, lowest bypass temperature, antegrade/retrograde/total cardioplegia volume, continuous retrograde cardioplegia usage). This study was approved by the Institutional Review Board of Columbia University in November 2013 and need for informed consent was waived.
Cardioplegia Administration
In cases with significant AI or prior coronary bypass grafting with patent grafts, cardioplegia was delivered in a retrograde and antegrade fashion in both treatment cohorts. In the presence of a patent internal mammary artery graft, individual surgeon preference determined whether the graft was dissected and clamped or continuous retrograde cardioplegia was delivered.
Standard cardioplegia utilized a whole blood cardioplegia (WB) solution (See Table 1 for a comparison of cardioplegia solution composition) given at 4°C with a 4:1 blood mixture administered in antegrade and retrograde fashion as described above for a total volume of approximately one liter. Cardioplegia was re-dosed at 20-minute intervals and a final retrograde warm dose was given prior to reperfusion. For cases using DN, cardioplegia was delivered at 4°C with a 1:4 blood mixture given antegrade in the absence of significant AI. If cross-clamp time was less than 90 minutes, a single one-liter dose was given. In cases with cross-clamp times significantly greater than 90 minutes, re-dosing was performed with 500 ml of solution. No warm dose was given prior to reperfusion. All cardioplegia volumes used in the text and tables are the total volume delivered to the patient, including both the crystalloid component and blood component.
Table 1.
Composition of Whole Blood cardioplegia vs. del Nido Cardioplegia
| Whole Blood Cardioplegia | del Nido Cardioplegia | |
|---|---|---|
| Carrier | D5W | Plasma-lyte A |
| Blood:Crystalloid ratio | 4:1 | 1:4 |
| KCl | 80 mEq | 26 mEq |
| NaHCO3 | 30 mEq | 13 mEq |
| Mannitol | 12.5 g | 3.3 g |
| Lidocaine | 0 | 130 mg |
| Magnesium | 0 | 2 g |
Note: all components below are added to the crystalloid component of the respective cardioplegia and do not reflect the concentration after mixing with blood
Statistical Analysis
Data was analyzed using SPSS version 21 (IBM corp., Armonk, NY). Continuous variables are reported as mean ± standard deviation and compared using independent samples t-tests. Categorical variables are reported as frequency and percentage of total group and compared using Pearson’s chi-squared test or Fisher’s Exact test where applicable. Multivariable logistic regression analysis was performed to determine predictors of post-operative low cardiac output requiring inotropes upon ICU admission and reported as odds ratio (OR) and 95% confidence interval (CI). A propensity matched subgroup analysis was performed using the Greedy 5 to 1 digit matching algorithm in order to control for differences in pre-operative characteristics between groups. Matching was done in 1:1 fashion and matched 46 patients in each group. Patients receiving continuous retrograde cardioplegia were not included in cardioplegia volume analysis given their requirement for significantly higher volumes of cardioplegia during the ischemic period. All p-values ≤ 0.05 are considered significant.
RESULTS
Patient Demographics
Patient comorbidities, surgical indications, and pre-operative ejection fraction (EF) are presented in Table 2. There were 113 total patients studied, 61 in the WB group and 52 in the DN group. Overall mean age was 73.4 ± 14.3 years (range 24–95 years). Eighty-six patients (76.1%) were male and 84 patients (74.3%) had undergone prior CABG. Indications for surgery included AS (83.2%) and AI (16.8%). There were no significant differences between clinical characteristics across groups.
Table 2.
Baseline Demographics
| Total | WB | DN | P-value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Total (n) | 113 | 61 | 52 | ||
| Age (years, mean ± SD) | 73.4 ± 14.3 | 72.4 ± 16.4 | 74.7 ± 11.5 | 0.397 | |
| Male (%) | 86 (76.1) | 47 (77.0) | 39 (75.0) | 0.799 | |
| BSA (m2, mean ± SD) | 1.92 ± 0.24 | 1.94 ± 0.26 | 1.90 ± 0.23 | 0.387 | |
| STS Score (mean ± SD) | 4.6 ± 3.2 | 4.7 ± 3.8 | 4.4 ± 2.3 | 0.573 | |
| Comorbidities | |||||
| Diabetes mellitus, n (%) | 29 (25.7) | 16 (26.2) | 13 (25.0) | 0.881 | |
| Hypertension, n (%) | 101 (89.4) | 53 (86.9) | 48 (92.3) | 0.351 | |
| Hyperlipidemia, n (%) | 79 (69.9) | 43 (70.5) | 36 (69.2) | 0.884 | |
| COPD, n (%) | 20 (17.7) | 10 (16.4) | 10 (19.2) | 0.694 | |
| Prior stroke, n (%) | 14 (12.4) | 8 (13.1) | 6 (11.5) | 0.800 | |
| Previous MI, n (%) | 23 (20.4) | 11 (18.0) | 12 (23.1) | 0.507 | |
| CKD (Cr > 2), n (%) | 19 (16.8) | 10 (16.4) | 9 (17.3) | 0.897 | |
| Prior CABG, n (%) | 84 (74.3) | 46 (75.4) | 38 (73.1) | 0.777 | |
| Surgical Indications | |||||
| Aortic stenosis, n (%) | 94 (83.2) | 47 (77.0) | 47 (90.4) | 0.059 | |
| Aortic insufficiency, n (%) | 19 (16.8) | 14 (23.0) | 5 (9.6) | 0.059 | |
| EF (%, mean ± SD) | 47.9 ± 12.3 | 46.8 ± 12.5 | 48.2 ± 12.2 | 0.558 | |
| NYHA (grade, mean ± SD) | 2.5 ± 0.9 | 2.6 ± 0.9 | 2.4 ± 1.0 | 0.294 | |
Definitions: BSA=body surface area, CABG=coronary artery bypass grafting, CKD=chronic kidney disease, COPD=chronic obstructive pulmonary disease, EF=ejection fraction, MI=myocardial infarction, NYHA=New York Heart Association, STS=Society of Thoracic Surgery
Intra-operative variables
Operative variables are presented in Table 3. Although the volume of antegrade cardioplegia was higher in the DN group vs. WB group (p=0.001), the volumes of retrograde and total cardioplegia were markedly higher in the WB group (p<0.001 for both comparisons). The lowest temperature on bypass was also significantly lower for WB compared to DN (31.7 ± 1.4 °C WB vs. 33.0 ± 1.1 °C DN, p<0.001), however, it is impossible to determine if this was due to higher total cardioplegia volume in the WB group or variability in cooling preferences amongst surgeons‥ There was no significant difference in cross-clamp time or total bypass time between groups. Continuous retrograde cardioplegia was used in 8 patients in WB and 6 patients in DN. Although not routinely recorded, we did not observe a noticeable difference in the time to restoration of cardiac rhythm, number of cardioversions needed, or time to weaning from bypass between groups.
Table 3.
Intra-operative measurements
| Total (n=113) | WB (n=61) | DN (n=52) | P-value | |
|---|---|---|---|---|
| Cross-clamp time (min, mean ± SD) | 64.5 ± 19.7 | 67.2 ± 20.1 | 61.3 ± 18.9 | 0.111 |
| CPB time (min, mean ± SD) | 97.1 ± 30.7 | 100.5 ± 34.3 | 93.1 ± 25.7 | 0.204 |
| Lowest temperature (°C, mean ± SD) | 32.3 ± 1.4 | 31.7 ± 1.4 | 33.0 ± 1.1 | <0.001 |
| Continous retrograde, n (%) | 14 (12.4) | 8 (13.1) | 6 (11.5) | 0.800 |
| ACP dosea (mL, mean ± SD) | 748.3 ± 335.2 | 644.2 ± 366.4 | 868.3 ± 249.2 | 0.001 |
| RCP dosea (mL, mean ± SD) | 847.8 ± 787.6 | 1341.2 ± 690.8 | 279.3 ± 445.1 | <0.001 |
| TCP dosea (mL, mean ± SD) | 1596.1 ± 772.2 | 1985.4 ± 691.1 | 1147.6 ± 447.2 | <0.001 |
continuous retrograde cases were not included in cardioplegia dose analysis
Definitions: ACP=antegrade cardioplegia, CPB=cardiopulmonary bypass, RCP=retrograde cardioplegia, TCP=total cardioplegia
Outcomes
Patient outcomes, post-operative variables, and complications are presented in Table 4 and Figure 1. One patient died in the perioperative period (multisystem organ failure due to sepsis) during the study in the WB group versus none in the DN group. There was a trend towards lower transfusion requirements in the DN group (p=0.07). There was no difference in post-operative EF, need for post-operative inotropes, days on the ventilator, or ICU length of stay. However, hospital length of stay was significantly shorter in the DN group vs. the WB group (7.9 ± 3.4 days vs. 10.1 ± 7.2 days, p=0.035). There were no significant differences in the rates of post-operative complications including infection, ARI, Afib, any arrhythmia, stroke, TIA, or the need for new PPM. No post-operative MIs were observed during the study. In multivariable logistic regression analysis, only post-operative EF was predictive of need for inotropic support upon admission to the ICU (OR 0.94, 95% CI 0.90–0.98, p=0.002).
Table 4.
Postoperative variables
| Total (n=113) | WB (n=61) | DN (n=52) | P-value | |
|---|---|---|---|---|
| Inotropes in ICU, n (%) | 40 (35.4) | 21 (34.4) | 19 (36.5) | 0.815 |
| Ventilation (days, mean ± SD) | 2.0 ± 3.4 | 1.6 ± 2.7 | 2.4 ± 4.0 | 0.191 |
| Re-intubation, n (%) | 3 (2.7) | 3 (4.9) | 0 (0) | 0.248 |
| PRBCs (units, mean ± SD) | 1.8 ± 2.2 | 2.1 ± 2.6 | 1.4 ± 1.7 | 0.070 |
| Post-op EF (%, mean ± SD) | 47.6 ± 10.8 | 46.8 ± 10.9 | 48.5 ± 10.7 | 0.401 |
| ICU stay (days, mean ± SD) | 3.5 ± 5.4 | 4.1 ± 7.0 | 2.8 ± 2.0 | 0.193 |
| Hospitalization (days, mean ± SD) | 9.0 ± 5.9 | 10.1 ± 7.2 | 7.9 ± 3.4 | 0.035 |
| Discharged to rehab, n (%) | 25 (22.3) | 14 (23.3) | 11 (21.1) | 0.855 |
| Mortality, n (%) | 1 (0.9) | 1 (1.6) | 0 (0) | 1.000 |
Definitions: EF=ejection fraction, PRBCs=packed red blood cells
Figure 1.
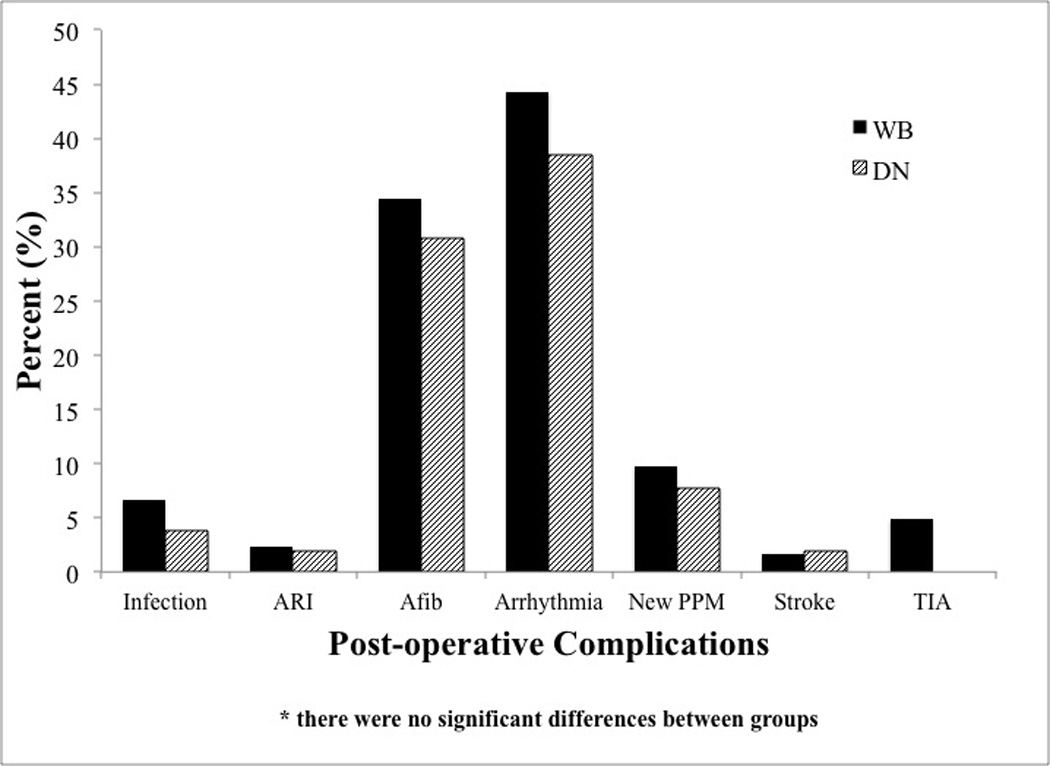
Postoperative complications by cardioplegia group. (Abbreviations: Afib=atrial fibrillation, ARI=acute renal injury, PPM=permanent pacemaker, TIA=transient ischemic attack)
Propensity Analysis
Propensity score matching paired 46 patients in each group for comparison. Mean age for this subgroup was 73.0 ± 14.2 years and there were no significant differences in demographics or co-morbidities between groups. Total and retrograde cardioplegia doses were significantly lower in the DN group when compared to the WB group (total: 1147.6 ± 447.2 mL DN vs. 1952.3 ± 641.2 mL WB (p<0.001), retrograde: 279.3 ± 445.1 mL DN vs. 1285.1 ± 60.1 mL WB (p<0.001). There was no difference in cross-clamp or bypass time between groups, however, the lowest temperature on bypass was slightly higher in the DN group (32.9 ± 1.1 °C DN vs. 31.8 ± 1.1 °C WB, p<0.001). There were no significant differences in transfusion requirements, days on ventilator, post-operative complications, ICU or hospital length of stay, or survival to discharge.
CONCLUSIONS
DN solution was developed to address the specific needs of immature myocardium encountered in neonatal and pediatric cardiac surgery, namely the inability of myocytes to tolerate high levels of intracellular calcium that characterize the post-ischemic period and can lead to irreversible myocardial injury. The solution is given as a one-time dose for cross-clamp times less than 90 minutes and no warm dose is given prior to reperfusion. This technique has been used successfully in the pediatric population for almost two decades. Recently, reports have suggested that senescent myocardium may also be intolerant of high intracellular calcium levels in the post-ischemic period (10), which led our group to hypothesize that DN may also be useful in the adult population, both because of its improved calcium handling and its ease of use.
Based on our analysis of patients undergoing re-operative aortic valve replacement, we demonstrate that DN cardioplegia offers equivalent outcomes when compared to WB with regard to short-term post-operative survival, EF, need for hemodynamic support, and complications. In addition, a lower total cardioplegia volume was required. As a result of our findings presented here, all adult cardiac operations at our institution now utilize DN cardioplegia for myocardial protection with continued good result.
Given that only one dose is used in the majority of cases, we initially hypothesized that the lower total volume of DN needed to achieve safe myocardial protection would lead to shorter cross-clamp times as repeat dosing is not necessary. Furthermore, we surmised that a lower total cardioplegia volume would lead to less hemodilution and, therefore, lower post-operative transfusion requirements. Although there was a trend towards lower transfusion requirements in the DN group in the overall analysis, our hypotheses did not reach statistical significance in our data analysis. Despite this fact, DN use promotes an uninterrupted case workflow given the lack of repeat dosing at 20-minute intervals. This technical subtlety, although difficult to quantify, represents a significant advantage, as time limitations become less of a burden.
Optimal dosing and timing of del Nido cardioplegia has not been definitively established for adult patients. Typically, only one dose of cardioplegia is given over the entire ischemic period assuming its duration is limited to approximately 90 minutes or less, although this time cutoff has not been tested experimentally and requires further investigation. Debate exists on how to approach a situation where more than 90 minutes but less than 180 minutes is needed, as giving another full dose of cardioplegia may result in prolonged time to restoration of a cardiac rhythm if the cross-clamp is removed only a few minutes after the second dose is given (8). In our series, only 6 cases in the DN group had a cross-clamp time of greater than 90 minutes (93, 94, 95, 95, 97, and 127 minutes). Redosing was only done in the case with 127 minutes of ischemic time at 60 minutes and only half of a full dose was given. Spontaneous rhythm was restored immediately upon reperfusion.
Approximately 75% of our patient population had undergone previous coronary artery bypass grafting and had patent left internal mammary artery grafts, posing a unique challenge to myocardial protection. Multiple reports exist on how to approach this situation (12–14). At our institution, we employ two main strategies: dissect out the patent graft and clamp versus deliver continuous retrograde cardioplegia in order to protect myocardium served by the patent graft which is left unclamped. In this series, there were 14 cases of continuous retrograde cardioplegia (8 WB, 6 DN) with no difference in post-operative outcomes. This suggests that continuous retrograde cardioplegia using DN is safe for use in re-operations where the patent IMA graft is left unclamped.
The main limitation of this study is its retrospective, descriptive nature conducted at a single institution. Additionally, gross measures of outcome such as post-operative echocardiographic findings, need for inotropic support, and post-operative complication rate were used as a surrogate for adequate myocardial protection on a cellular level. Post-operative cardiac enzymes or recording of post-bypass septal function may have provided more insight into direct cardiac injury. Additional variables that may have been useful include exact time to restoration of cardiac rhythm, number of cardioversions needed, and time to weaning of bypass. Our study is underpowered to adequately detect non-inferiority using need for post-operative inotropic support as our reference variable. Myocardial tissue analysis was not performed to evaluate for acute myocardial injury. We are unable to delineate the appropriate time of re-dosing of DN from the current data, and this issue will need to be further investigated in the future. Finally, no long-term follow-up of myocardial protection, such as changes in regional wall motion or ejection fraction, were performed. Further long-term outcome data will be required to validate our current findings.
ACKNOWLEDGEMENTS
The authors would like to thank Rashmi Advani for her help with data collection. We would also like to thank James Beck and Linda Mongero for their assistance in ensuring a smooth transition to the use of del Nido cardioplegia and for their continued strong leadership of the perfusion department.
Footnotes
DISCLOSURES
none
REFERENCES
- 1.Hearse DJ, Steward DA, Braimbridge MV. Cellular protection during myocardial ischemia: the development and characterization of a procedure for the induction of reversible ischemic arrest. Circulation. 1976;54(2):193–202. doi: 10.1161/01.cir.54.2.193. [DOI] [PubMed] [Google Scholar]
- 2.Hearse DJ. Myocardial protection during ischemia and reperfusion. Mol Cell Biochem. 1998;186(1–2):177–184. [PubMed] [Google Scholar]
- 3.Piper HM, Garcia-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann Thorac Surg. 1999;68(5):1913–1919. doi: 10.1016/s0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- 4.Van Emous JG, Nederhoff MG, Ruigrok TJ, et al. The role of the Na+ channel in the accumulation of intracellular Na+ during myocardial ischemia: consequences for post-ischemic recovery. J Mol Cell Cardiol. 1997;29(1):85–96. doi: 10.1006/jmcc.1996.0254. [DOI] [PubMed] [Google Scholar]
- 5.Choi YH, Cowan DB, Wahlers TC, et al. Calcium sensitisation impairs diastolic relaxation in post-ischemic myocardium: implications for the use of Ca2+ sensitising inotropes after cardiac surgery. Eur J Cardiothoracic Surg. 2010;37(2):376–383. doi: 10.1016/j.ejcts.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra Corpor Technol. 2012;44(3):98–103. [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien JD, Howlett SE, Burton HJ, et al. Pediatric cardioplegia strategy results in enhanced calcium metabolism and lower serum troponin T. Ann Thorac Surg. 2009;87(5):1517–1523. doi: 10.1016/j.athoracsur.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 8.Charette K, Gerrah R, Quaegebeur J, et al. Single dose myocardial protection technique utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2012;27(2):98–103. doi: 10.1177/0267659111424788. [DOI] [PubMed] [Google Scholar]
- 9.Willems L, Zatta A, Holmgren K, et al. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38(2):245–256. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.O’Blenes SB, Friesen CH, Ali A, et al. Protecting the aged heart during cardiac surgery: the potential benefits of del Nido cardioplegia. J Thorac Cardiovasc Surg. 2011;141(3):762–770. doi: 10.1016/j.jtcvs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Govindapillai A, Hua R, Rose R, et al. Protecting the aged heart during cardiac surgery: Use of del Nido cardioplegia provides superior functional recovery in isolated hearts. J Thorac Cardiovasc Surg. 2013;146(4):940–948. doi: 10.1016/j.jtcvs.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Byrne JG, Karavas AN, Filsoufi F, et al. Aortic valve surgery after previous coronary artery bypass grafting with functioning internal mammary artery grafts. Ann Thorac Surg. 2002;73(3):779–784. doi: 10.1016/s0003-4975(01)03456-7. [DOI] [PubMed] [Google Scholar]
- 13.Savitt MA, Singh T, Agrawal S, et al. A simple technique for aortic valve replacement in patients with a patent internal mammary artery bypass graft. Ann Thorac Surg. 2002;74(4):1269–1270. doi: 10.1016/s0003-4975(02)03762-1. [DOI] [PubMed] [Google Scholar]
- 14.Bar-El Y, Kophit A, Cohen O, et al. Continuous retrograde cardioplegia simplifies aortic valve replacement in the presence of a patent internal mammary artery. Ann Thorac Surg. 2003;76(4):1337–1338. doi: 10.1016/s0003-4975(03)00506-x. [DOI] [PubMed] [Google Scholar]


