Abstract
Recent reports of As concentrations in certain food and drinks have garnered public concern and led to a lowering of the US guideline maximum concentration for inorganic As in apple juice and proposed limits for As in rice products. In contrast Se is an essential micro-nutrient that can be limiting when Se-poor soils yield Se-poor food crops. Rare earth element (REE) doubly charged interferences on As and Se can be significant even when initial ICP-MS tuning minimizes doubly charged formation. We analyzed NIST 1547 (peach leaves) and 1515 (apple leaves), which contain high levels of REEs, by quadrupole ICP-MS with (He) collision mode, H2 reaction mode or triple quadrupole ICP-MS (ICP-QQQ) in mass-shift mode (O2 and O2/H2). Analysis by collision cell ICP-MS significantly over-estimated As and Se concentration due to REE doubly charged formation; mathematical correction increased the accuracy of analysis but is prone to error when analyte concentration and sensitivity is low and interferent is high. For Se, H2 reaction mode was effective in suppressing Gd2+ leading to accurate determination of Se in both SRMs without the need for mathematical correction. ICP-QQQ using mass-shift mode for As+ from m/z 75 to AsO+ at m/z 91 and Se+ from m/z 78 to SeO+ at m/z 94 alleviated doubly charged effects and resulted in accurate determination of As and Se in both SRMs without the need for correction equations. Zr and Mo isobars at 91 and 94 were shown to be effectively rejected by the MS/MS capability of the ICP-QQQ.
Introduction
The detection of As in fruit juices and in rice(1) and rice products(2) has generated much public and media interest and there are current efforts aimed at establishing regulations or guidelines for acceptable levels of As in these products, although this remains a contentious topic(3). In 2013 The US FDA proposed a new action level for inorganic As in juice of 10 μg/L(4; 5). A recent WHO document, which records the findings of the eighth meeting of the CODEX Committee on Contaminants in Food proposes a guideline for inorganic As in rice of 0.2 mg/kg(6). The proposed guideline of 0.2 mg/kg for arsenic in rice equates to a digestate concentration of 2 μg/L (assuming a 100X digestion dilution), consequently even small errors in precision and accuracy could result in a food product being classified as above the guideline when in fact it is not Selenium is generally not present at elevated concentrations in most foodstuffs, on the contrary, Se is a micro-nutrient and some populations can be Se-deficient due primarily to Se-poor soils. Hence accurate quantification of Se in food is necessary to assess nutrient status.
In this paper we focus on doubly charged interference from the rare earth elements (REEs) Nd, Sm and Gd, which cause ‘false positives’ at m/z 75 and 78 (7). The REEs have a relatively low 2nd ionization potential ca. 11 – 12 eV and some doubly charged formation is inevitable in an Ar plasma. This is a particular problem for As and Se when the analytes are present at low concentration in the sample compared to the REEs and the effect is exacerbated by the fact that both As and Se are relatively poorly ionized in the plasma, hence, the sensitivity or slope of their calibration is low and false signals at either mass can equate to significant concentration errors. Both Nd and Sm REE have isotopes at 150 amu, where Sm is 7.4% abundant and Nd is 5.6% abundant, while Gd is 20.5% abundant at m/z 156 (Dy is also 0.06% abundant at this mass). Because REEs are immobile in soils, certain soils can be relatively enriched in REEs and consequently food crops grown on these soils may take up higher concentrations of these elements(8; 9). Two NIST standard reference materials, NIST 1515 apple leaves and NIST 1547 peach leaves, that are useful for quality control in food and plant analysis contain low μg kg−1 levels of As and Se in the presence of mg kg−1 levels of REEs. Quantification of As and Se in these two reference materials is confounded by doubly charged interference of the REEs at m/z 75 and 78. While some standard methods recommend monitoring masses at 150 and correcting for doubly charged ions through the use of mathematical equations, it is debatable how widespread this procedure is followed and in any event, correction equations are less accurate than quantification at m/z that is free from any interference. We illustrate the doubly charged effect by analysis of NIST apple leaves and peach leaves and test the effectiveness of interference correction equations, KED (He only) and H2 reaction cell ICPMS and ‘mass shifting’ by use of O2 and O2/H2 as a reaction gas using ICP-QQQ. The ICP-QQQ uses a quadrupole mass filter before (Q1) and after (Q3) the reaction cell. The unit mass resolution of the first quadrupole selectively allows for only a single m/z (eg. mass 78) to enter the collision reaction cell. Thus, except for the analyte and those interferences at the selected mass, no other analyte or polyatomic molecules enter the cell allowing targeted and selected chemical reaction to take place.
Materials and methods
All samples were acid digested using a MARS6 (CEM, Mathews, NC) closed vessel digestion at 200 °C with a 20 minute ramp and 20 minute hold time. Triplicate samples of NIST (US National Institute of Standards and Technology, Gaithersburg, MD) SRMs 1547 (peach leaves), 1515 (apple leaves) were digested using 0.25g sample 2.5 mls 9:1 HNO3:HCl and diluted to a final digested weight of 25g. NIST 1547 and 1515 contain low μg kg concentrations of As and Se (Table 1) and high concentrations of REEs. NIST 1547 certification gives reference (non-certified) values for Nd, Sm and Gd concentrations at 7, 1, and 1 mg kg−1 and NIST 1515 has reference values for Nd, Sm and Gd concentrations at 17, 3, and 3 mg kg−1, respectively. Samples were run at Dartmouth College by collision cell ICP-MS (Agilent 7700x) using He as the collision gas at 4.5 ml/min and H2 as reaction gas at 6 ml/min and at Agilent Technologies (Wilmington, Delaware) by ICP-QQQ (8800) using O2 or O2/H2 as reaction gas at 3.5 ml/min with AsO+ detection at m/z 91 and SeO+ detection at m/z 94 and 96. Both instrument set-ups used manufacturer-recommended operating conditions and utilized a concentric nebulizer and Peltier cooled double pass spray chamber, internal standard was added online. The Dartmouth system is operated with 5% butanol in the internal standard mixture to equalize the organic plasma load between samples and standards and was calibrated using both external calibration and standard additions; the standard additions data gave more accurate data for both SRMs and is reported here. Both instruments were calibrated using NIST- traceable standards and traceable 2nd-source calibration checks. The measured values were considered not significantly different than the certified values if their 95% confidence interval overlapped with the 95 % confidence range reported on the NIST certificate.
Table 1.
Analysis of As and Se (m/z 78) in NIST 1547 and 1515 in KED and reaction modes uncorrected and corrected for doubly charged interference and by ICP-QQQ. All concentrations are in mg/kg, and are averages of 3 replicate sample digests expressed as mean ± 95% confidence interval.
| Arsenic (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| KED-He | reaction-H2 | ICP-QQQ reaction-O2/H2 | ||||
| SRM | certified | uncorrected | corrected | uncorrected | corrected | uncorrected |
| NIST 1547 | 0.060 ± 0.018 | 0.170 ± 0.016 | 0.068 ± 0.003 | 0.113 ± 0.004 | 0.079 ± 0.004* | 0.065 ± 0.002 |
| NIST 1515 | 0.038 ± 0.007 | 0.250 ± 0.016 | 0.026 ± 0.021 | 0.126 ± 0.005 | 0.047 ± 0.004* | 0.032 ± 0.002 |
| NIST 1547 spiked at 1 mg l−1 Zr and Mo (n=1) | 0.063 | |||||
| Selenium (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| KED-He | reaction-H2 | ICP-QQQ reaction-O2/H2 | ||||
| SRM | certified | uncorrected | corrected | uncorrected | corrected | uncorrected |
| NIST 1547 | 0.120 ± 0.009 | 0.394 ± 0.04 | 0.113 ± 0.02* | 0.119 ± 0.009* | 0.119 ± 0.009* | 0.127± 0.006* |
| NIST 1515 | 0.050 ± 0.009 | 0.808 ± 0.04 | 0.013 ± 0.04* | 0.050 ± 0.003* | 0.050 ± 0.003* | 0.047± 0.006* |
| NIST 1547 spiked at 1 mg l−1 Zr and Mo (n=1) | 0.13 | |||||
Means marked have 95% confidence intervals that overlap with the certified range.
Results and Discussion
Both kinetic energy discrimination (KED, collision cell technology) and reactive gases have been used to reduce polyatomic interferences in ICP-MS but their effect, if any, on doubly charged formation is less often reported. The effect of collision (He) and reaction (H2) gases on polyatomic interferents, analyte signal and doubly charged formation at m/z 75 and 78 are shown in figure 1. Both He and H2 effectively reduce polyatomic interferences; however analyte intensity is also reduced as cell gas flow rate is increased. Helium gas flow rate has no effect on reducing doubly charged interference from Sm or Nd at m/z 75 (Figure 1A) or from Gd at m/z 78 (Figure 1C), in fact apparent doubly charged formation increases as He gas flow increases, presumably because KED reduces the singly-charged ion intensity relatively more than the doubly charged ion, which may be due to the difference is size between these two differently charged ions. The optimum He flow for reducing polyatomics and maintaining high signal to noise is 4.5 ml/min. Hydrogen is very effective as a reaction gas in reducing Ar-based interferences, with optimal signal to noise ratio obtained at flow rates ca. 3- 4 ml/min (Figure 1D), however, doubly charged formation for Gd (Figure 1D) and Nd and Sm (Figure 1B) remain high at this gas flow rate. Increasing the H2 flow rate to 5.5 – 6 ml/min effectively removes Gd doubly charged interference while still maintain good signal to noise ratio for Se. Similarly Nd and Sm doubly charged formation is dramatically reduced at this higher flow rate to 0.03 and 0.01 % respectively, at the expense, however, of severe reduction in As intensity and signal to noise ratio.
Figure 1.
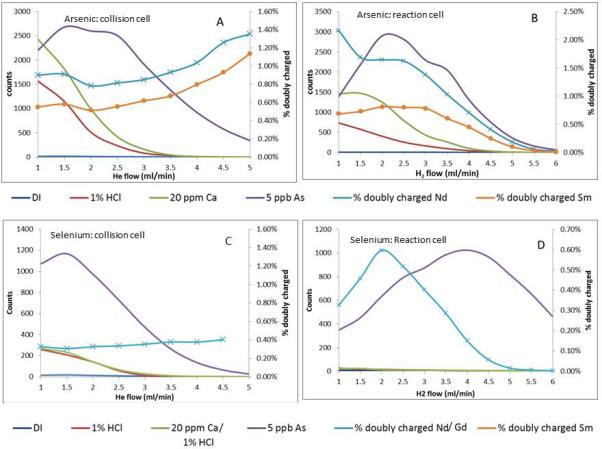
Analyte, polyatomic and doubly charged instrument response at m/z 75 (A, B) and m/z 78 (C,D) as a function of collision (A,C) or reaction (B.D) gas flow rate in a single quadrupole ICP-MS.
Using the method development criteria determined above, NIST SRMs 1515 and 1647 were determined in both He and H2 gas modes with KED. Doubly charged ion formation was 1.1%, 0.9% and 0.25% for Nd, Sm and Gd in He-mode and 0.06%, 0.036% and 0.006% for H2 mode. The trend of decreasing doubly charged formation with increasing REE atomic weight reflects the increase in 2nd ionization potential across the period. Hydrogen is clearly effective in reducing doubly charged interferences in the cell. Approximate instrument detection limits derived from the calibration statistics and used here for comparative purposes were 0.01 μg/L and 0.06 μg/L for As and Se respectively in KED (He) mode and 0.022 μg/L and 0.03 μg/L in H2 mode. The instrument sensitivity for each analyte in each mode, as indicated by the calibration slope, was 1.52 and 0.076 for As in He and H2 mode respectively and 0.15 and 0.25 for Se in He and H2 mode respectively.
The results for As and Se determination of NIST 1515 and 1547 by collision cell ICP-MS are shown in Table 1. Accurate analysis of As in either He or H2 mode for either SRM material was not obtained if doubly charged ions were not accounted for The uncorrected As value of 0.25 mg/kg for NIST 1515 is above the proposed 0.2 mg/kg guideline for inorganic As in rice, illustrating that doubly charged interferences can cause large false positives that could potentially lead to unnecessary further analysis for inorganic As content. Uncorrected As values in reaction mode are less than for He mode but still overestimate the certified values by 1.9 and 3.3 times for NIST 1547 and 1515, respectively as residual doubly charged formation and low sensitivity for As at this H2 flow rate lead to significant positive bias. Mathematical correction of doubly charged species for As resulted in a value of 0.068 mg/kg for NIST 1547, within the confidence interval of the certified value. Corrected values for NIST 1515 were also not significantly different than the certified range. However, this was largely due to the high RSD of the measured replicate values, which illustrates a problem of correction equations when the signal is mostly interferent as opposed to analyte. Corrected values for As in H2-reaction mode were not significantly different that the certified values but were at the positive extreme of each SRM confidence interval with recoveries of 132% and 124% for NIST 1547 and 1515, respectively.
Low Se sensitivity in He mode and high Gd in both SRMs (3 mg/kg and 1 mg/kg for NIST 1515 and 1547 respectively) caused large over-estimates of the true Se values when uncorrected. Using correction equations for NIST 1547 gives a value of 0.113 mg/kg which is within the certified confidence range. The corrected value for Se in He mode in NIST 1515 is 0.013 mg/kg and had an unacceptably high CV (150%), again demonstrating the problems of correction equations when the signal is essentially 95% doubly charged interference. In H2 reaction mode the high sensitivity for Se and removal of doubly charged interferences at m/z 78 resulted in accurate values for both SRMs that were not significantly difference than the certified values. Detection limits for Se in H2 mode are better than for He mode and doubly charged formation is reduced to 0.006% such that accurate and precise Se data are obtained. This effect has also been reported in a recent study where it was shown that H2 can eliminate double charge interference for Se in the presence of Gd in blood of patients receiving MRI Gd-based contrasting agents(10).
The pitfalls of mathematical corrections and the general poorer results for As in either He or H2 modes described above led us to investigate other reaction gases. Reaction gas ICP-MS methods(11) have been used successfully for As and Se with gases such as NH3 (14), CH4 (7; 12-15)), and O2 (13; 16) although these studies mostly focused on polyatomic interference removal rather than doubly charged interferences. Reaction with O2 mass shifts As and Se analytes to m/z 91 and 94 (or 96) where background is generally lower and the new m/z less prone to interferences, provided isobars from Zr and Mo at the mass shifted m/z are filtered away from the reaction cell and polyatomic interferences at the new m/z are not created in the cell. We ran both NIST SRM digests by reaction cell ICP-QQQ using either O2 or O2/H2 as the reaction gas. The O2/H2 mixture was included because previous work has shown that the extent of the Se reaction with O2 is enhanced in the presence of H2. The H2 gas reduces Ar-dimer interferences facilitating an increased reaction rate between Se and O2. Table 2 gives the instrument response at each of the mass shifted analyte m/z for the calibration blank and 50 and 100 μg/L solutions of Nd/Dy and Gd/Sm. The slope of the calibration curve and the detection limit, estimated from the standard deviation of 3 independent blank measurements during the run are also shown in Table 2. There is no systematic increase in background signal resulting from 50 or 100 ppb solutions of REEs at the mass shifted analyte masses in either gas mode. In this mass shifted mode it is possible to measure Se 80 isotope which being the most abundant isotope should give lower detection limits. However, Se 78 has slightly lower background signals at the mass shifted m/z and gives slightly lower detection limits of 0.002 μg/l compared to 0.003 μg/l 80SeO, however also note that these are 10 times lower than detection limits for Se by H2-reaction mode by conventional reaction cell on a single quadrupole ICP-MS. For both 78Se and 80Se the calibration slope increases two fold in O2/H2 mode compared to O2 reaction mode indicating increased SeO formation; and for 80Se the background is also significantly lower. Clearly, for Se, there is advantage in using the mixed gas rather than just O2. There is no effect of reaction gas on sensitivity or detection limit for As so either approach is suitable and gives essentially the same results. Detection limits for As are ca. 0.001 μg/l, 10 times lower than for collision cell ICP-MS.
Table 2.
ICP-MS response at mass shifted m/z for As and Se in the presence of REEs and sensitivity and estimated detection limits for As and Se at these m/z by ICP-QQQ.
| 75 -> 91 As | 78 -> 94 Se | 80 -> 96 Se | ||||
|---|---|---|---|---|---|---|
| [ O2 ] | [ O2/H2 ] | [ O2 ] | [ O2/H2 ] | [ O2] | [ O2/H2] | |
| Blank (cps) | 6.00 | 36.00 | 0.00 | 2.00 | 27.33 | 6.00 |
| 50 ppb Nd/Dy (cps) | 22.67 | 43.33 | 0.67 | 0.00 | 25.33 | 7.33 |
| 100 ppb Nd/Dy (cps) | 8.00 | 19.33 | 0.00 | 0.00 | 22.67 | 2.67 |
| 50 ppb Gd/Sm (cps) | 17.33 | 44.00 | 2.00 | 3.33 | 20.00 | 6.67 |
| 100 ppb Gd/Sm (cps) | 16.00 | 76.67 | 4.00 | 4.00 | 20.00 | 6.00 |
| slope (cps/ng l−1) | 6.40 | 6.33 | 0.26 | 0.53 | 0.60 | 1.30 |
| estimated detection limit (μg l−1) | 0.0010 | 0.0013 | 0.0090 | 0.0022 | 0.0135 | 0.0032 |
The results for analysis of the NIST 1515 and 1547 using the ICP-QQQ approach are shown in Table 1. For As in NIST 1515 and 1547 the measured values were 0.032 and 0.065 respectively, both well within the respective certified confidence range for the SRM. Similarly, recoveries for Se are excellent, both being within the certified range for NIST 1515 and 1547. Hence doubly charged interference from the high REE levels of these two SRMs is eliminated through this O2/H2 reaction mode approach. Zirconium and Mo have isotopes at m/z 91 and 94 but should be rejected by the first quadrupole filter prior to entering the cell. To test this, an aliquot of NIST 1547 was spiked at 1 mg/L Zr and Mo and the results are also shown in Table 1. The measured values for As and Se are not different than the unspiked samples proving that the MS/MS capability is effective at rejecting isobars of similar mass to the new mass-shifted analyte.
Conclusions
Doubly charged formation is best addressed by reaction cell based approaches as collision cell ICP-MS is not effective at removing doubly charged species and correction equations are prone to error. Either H2 to remove the doubly charged species or O2/H2 to mass shift Se at m/z 94 are effective for Se analysis. For As, using O2 to mass shift to AsO at m/z 91 was the most effective approach. ICP-QQQ also had 10-fold lower detection limits than the single quadrupole ICP-MS, which makes it particularly suited to low level determination of As and Se in complex sample matrices. The extent and concentrations of REEs in food products is not well studied but their occurrence in two NIST reference materials suggests REEs can be expected in some foods depending on soil concentrations during crop production. At a minimum, m/z 150 and 156 should be monitored during any analysis to flag samples where doubly charged formation could be problematic, but reaction cell approaches in general and ICP-QQQ approaches in particular are most appropriate in making accurate low level analyses.
Acknowledgements
Brian Jackson acknowledges the support of NIEHS P42 ES007373, NIEHS P01 ES022832, EPA RD83544201 for the work presented herein.
Contributor Information
Brian Jackson, Trace element Analysis Core Laboratory, Earth Sciences, Dartmouth College, Hanover, NH..
Amir Liba, Agilent Technologies, Wilmington, Delaware..
Jenny Nelson, Agilent Technologies, Wilmington, Delaware..
References
- 1.Zhu Y-G, Williams PN, Meharg AA. Exposure to inorganic arsenic from rice: A global health issue? Environmental Pollution. 2008;154:169–171. doi: 10.1016/j.envpol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL. Arsenic, organic foods, and brown rice syrup. Environ Health Perspect. 2012;120:623–626. doi: 10.1289/ehp.1104619. PMCID:3346791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meharg AA, Raab A. Getting to the bottom of arsenic standards and guidelines. Environmental Science & Technology. 2009;44:4395–4399. doi: 10.1021/es9034304. [DOI] [PubMed] [Google Scholar]
- 4.U.S.FDA Guidance for industry. Arsenic in apple juice: Action level. 2013 [Google Scholar]
- 5.Fda sets limit for arsenic in apple juice. Chemical & Engineering News. 2013;91:21–21. [Anonymous] [Google Scholar]
- 6.COMMISSION CA. Report of the eighth session of the codex committee on contaminants in foods. CL2014/11-CF. Geneva, Switzerland: 2014. [Google Scholar]
- 7.Guo W, Hu S, Wang Y, Zhang L, Hu Z, Zhang J. Trace determination of selenium in biological samples by ch4-ar mixed gas plasma drc-icp-ms. Microchemical Journal. 2013;108:106–112. [Google Scholar]
- 8.Li X, Chen Z, Chen Z, Zhang Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in fujian province, southeast china. Chemosphere. 2013;93:1240–1246. doi: 10.1016/j.chemosphere.2013.06.085. [DOI] [PubMed] [Google Scholar]
- 9.Hu S, Xue J, Lin Y, Yu J-P, Zhou J. Determination of rare earth elements in navel oranges from different geographical regions of china by inductively coupled plasma-mass spectrometry. Analytical Letters. 2014;47:1400–1408. [Google Scholar]
- 10.Harrington CF, Walter A, Nelms S, Taylor A. Removal of the gadolinium interference from the measurement of selenium in human serum by use of collision cell quadrupole inductively coupled plasma mass spectrometry (q-icp-ms). Annals of clinical biochemistry. 2014;51:386–391. doi: 10.1177/0004563213504386. [DOI] [PubMed] [Google Scholar]
- 11.D'Ilio S, Violante N, Majorani C, Petrucci F. Dynamic reaction cell icp-ms for determination of total as, cr, se and v in complex matrices: Still a challenge? A review. Analytica Chimica Acta. 2011;698:6–13. doi: 10.1016/j.aca.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 12.Wei YH, Zhang JY, Zhang DW, Luo LG, Tu TH. Simultaneous determination of se, trace elements and major elements in se-rich rice by dynamic reaction cell inductively coupled plasma mass spectrometry (drc-icp-ms) after microwave digestion. Food Chemistry. 2014;159:507–511. doi: 10.1016/j.foodchem.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Hu S, Li X, Zhao J, Jin S, Liu W, Zhang H. Use of ion-molecule reactions and methanol addition to improve arsenic determination in high chlorine food samples by drc-icp-ms. Talanta. 2011;84:887–894. doi: 10.1016/j.talanta.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Sucharova J. Optimisation of drc icp-ms for determining selenium in plants. Journal of Analytical Atomic Spectrometry. 2011;26:1756–1762. [Google Scholar]
- 15.Wang R-Y, Hsu Y-L, Chang L-F, Jiang S-J. Speciation analysis of arsenic and selenium compounds in environmental and biological samples by ion chromatography-inductively coupled plasma dynamic reaction cell mass spectrometer. Analytica Chimica Acta. 2007;590:239–244. doi: 10.1016/j.aca.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Suh JK, Kang N, Lee JB. Direct determination of arsine in gases by inductively coupled plasma-dynamic reaction cell-mass spectrometry. Talanta. 2009;78:321–325. doi: 10.1016/j.talanta.2008.10.042. [DOI] [PubMed] [Google Scholar]


