Abstract
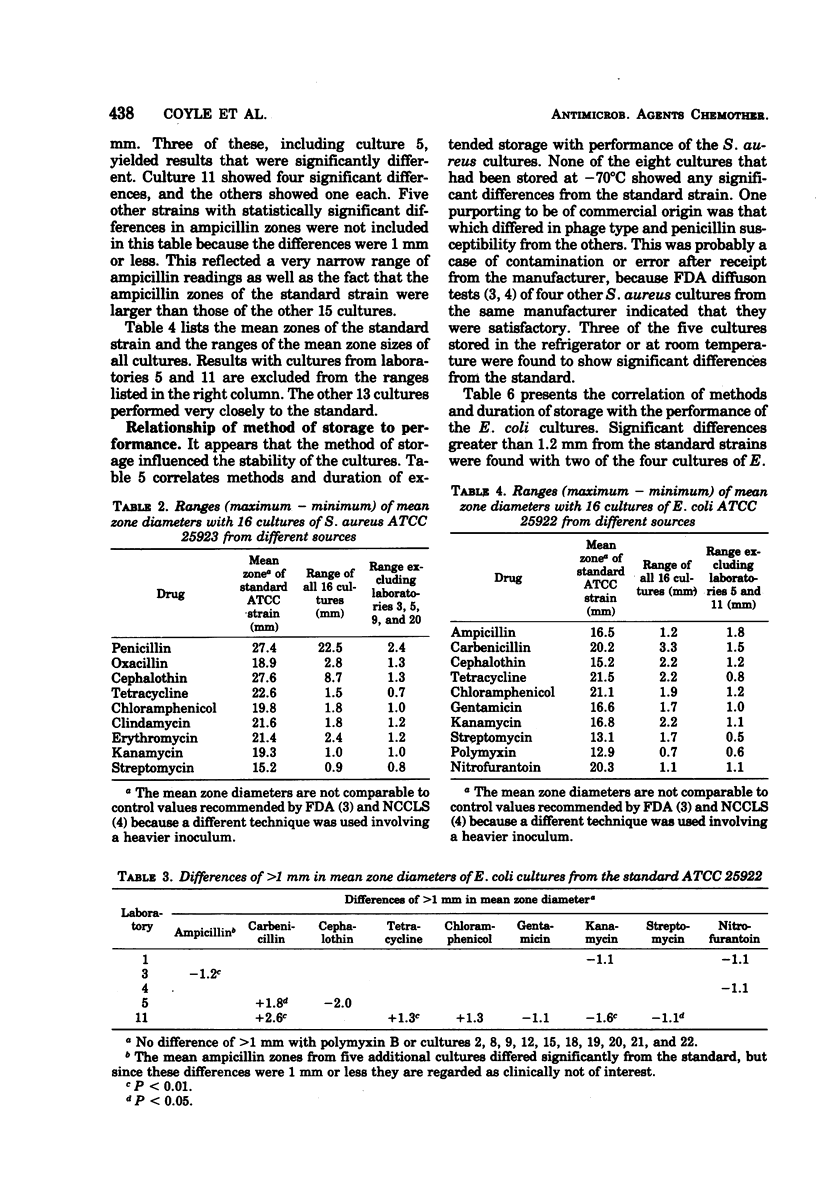
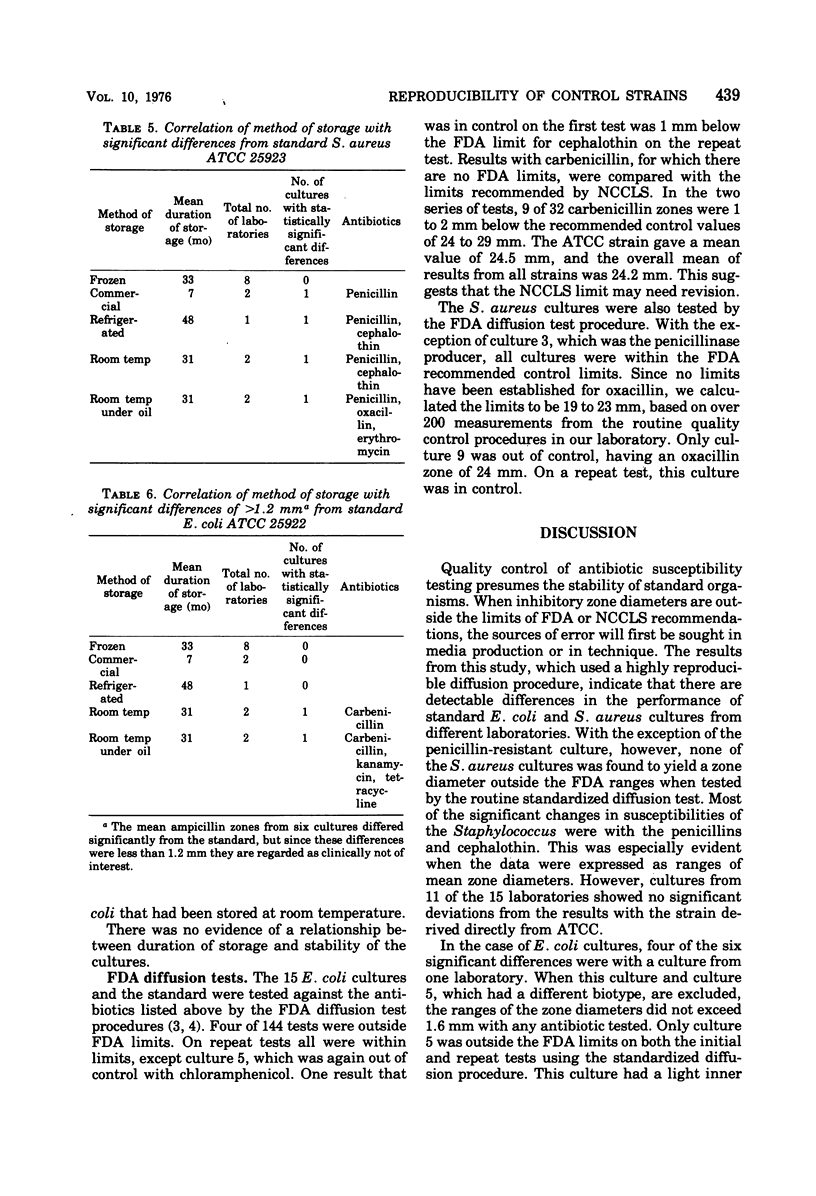
Inter- and intralaboratory reproducibility of susceptibility testing requires stable control strains. The Food and Drug Administration diffusion procedure recommends the Seattle strains of Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) for this purpose. It was of interest to determine the present reproducibility of control cultures maintained in various laboratories over several years. Fifteen cultures each of S. aureus and E. coli were obtained from laboratories in different parts of the country. Their performance was compared with strains directly derived from ATCC. Diffusion susceptibility tests using a modified overlay technique were made with four replicates. Seven of the eight statistically significant differences in responses of the staphylococci were to penicillin, methicillin, or cephalothin. One culture was a penicillinase producer with a zone 15 mm less than the standard strain. Eleven of the 15 cultures showed no significant deviations or differences greater than 2 mm from the results with the strain derived directly from ATCC. All except the penicillinase producer were of identical phage type. Among 150 organism-antibiotic combinations tested with E. coli, all but one reading were within 2 mm of the standard. Four of the six statistically significant differences were in a culture from one laboratory. The stability of the cultures appears to have been influenced by the method of storage. Cultures that were kept frozen during extended storage were remarkably stable. Significant differences were found in cultures from four of five laboratories that maintained cultures in refrigerators or at ambient temperature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- OEDING P., OSTERVOLD B. The stability of Oxford Staphylococcus H and other penicillin reference strains. Acta Pathol Microbiol Scand. 1959;46:149–155. doi: 10.1111/j.1699-0463.1959.tb00327.x. [DOI] [PubMed] [Google Scholar]



