Abstract
Studies have examined age-related changes in personality traits from adolescence through young adulthood, finding that aspects of negative emotionality decrease while conscientiousness increases over time. Varied mechanisms may underlie these transitions, including puberty-driven hormonal changes. Here, healthy adolescents completed the Multidimensional Personality Questionnaire-Brief Form and self-report measures of pubertal status at baseline and after two years. Independent of age, pubertal status impacted primary trait scales of the MPQ-BF Constraint factor in a sex-specific manner. Females decreased in Constraint, and particularly Control, while males increased in Constraint, and particularly Harm Avoidance, with advancing puberty. Longitudinal analyses validated these findings for Control. Findings are discussed relative to males’ versus females’ achievement of optimal levels of behavioral control in adolescence.
Keywords: Puberty, Constraint, Control, Sex differences
1. Introduction
Adolescence is fraught with physical and psychological changes, including trait level changes in major domains of personality. Individual difference factors may affect the patterning of change over time.
Age is one potential characteristic of interest. The age-defined transition from late adolescence to young adulthood is robustly characterized by increases in social maturity and behavioral stability, reflected via self-report indices of decreased negative emotionality and increases in control or conscientiousness (Blonigen, Carlson, Hicks, Krueger, & Iacono, 2008; Caspi, Roberts, & Shiner, 2005; Donnellan, Conger, & Burzette, 2007; Roberts, Walton, & Viechtbauer, 2006). Social Investment Theory (Lodi-Smith & Roberts, 2007) suggests that age-related transitions toward more mature behavior reflect individuals’ engagement with adult social roles through relationships, volunteerism, and employment. Intriguingly, sex differences impact these patterns. Neuroticism increases in adolescent girls but not boys, depending on age (Francis, 1993; McCrae et al., 2002). Using the Multidimensional Personality Questionnaire (MPQ: Tellegen, 1982), Roberts, Caspi, and Moffitt (2001), Donnellan et al. (2007) found that males decreased more substantially on Aggression and Alienation between late adolescence and early adulthood than did females. Both found that females increased more markedly in Control and Harm Avoidance than males, as did Blonigen et al. (2008).
Whether similar changes in these traits characterize earlier adolescent development has not been well studied. This transition is important to examine given that peers become increasingly salient to early adolescents’ well-being and expressions of behavioral control (Steinberg, 2008) and that adolescent problem behaviors are predictive of later adjustment (Krueger, 1999).
Ryan (2009) compared younger to older adolescents and found age-related differences in MPQ Constraint and Positive Emotionality (PEM) (lower levels in the younger cohort), as well as Agentic PEM (Social Potency and Achievement), and primary trait scales associated with negative emotionality (NEM: higher levels of Aggression and Alienation in younger adolescents). Males were relatively low in Constraint and its primary subscales, Harm Avoidance and Traditionalism. There was an age-by-sex effect for Stress Reaction, the primary trait scale that most strongly reflects NEM, and for NEM itself. Young females had the highest, while older males had the lowest, Stress Reaction scores.
Thus, regardless of whether one considers the transition from late adolescence to young adulthood or the transition from early adolescence to late adolescence, males and females differ most consistently on primary traits reflecting NEM and Constraint with evidence of sex-specific patterns of maturation. These differences are intriguing given that young girls are vulnerable to internalizing pathology, perhaps due to high levels of negative emotion, while immature boys may be more vulnerable to transient externalizing problems related to insufficient behavioral control and high aggression levels (Roberts, Jackson, Burger, & Trautwein, 2009).
There are several potential explanations for these sexually dimorphic developmental trajectories. Males and females may differ in the rate at which they embrace adult social roles due to environmental influences on socialization. A popular view is that males are more immature than females and for a longer period of time in the adult transition. Alternatively, biological factors might impact males’ and females’ rates of personality development but in distinct ways. Salient biological factors could include different rates of brain development between the sexes (Lenroot & Giedd, 2010), particularly with respect to cortical regions that mediate behavioral and emotional regulation (DeBellis et al., 2001) or hormonal fluctuations related to pubertal development that impact sex differences in brain morphology (Ahmed et al., 2008). If biological factors play a role, then they might serve to propel males and females, in sex-specific ways, toward behavioral maturity and receptivity to the assumption of adult social roles. The goal of this study is to examine whether pubertal status, independent of age, may account for transitions in negative emotionality and facets of behavioral control from early to late adolescence and whether males and females vary in the nature of these transitions.
At pubertal onset, gonadal steroid production increases. Females experience dramatic increases in estradiol, particularly between ages 9 and 14. Testosterone increases in males from ages 12 to 14 (Buchanan, Eccles, & Becker, 1992). These hormonal surges have been linked to conduct problems in boys (Rowe, Maughan, Worthman, Costello, & Angold, 2004). Findings in females are inconsistent, likely due to failures to control for menstrual status, but large sample studies indicate that puberty is associated with increased rates of internalizing, particularly anxiety and eating disorders, in girls (Sanborn & Hyaward, 2003), which would be consistent with the notion that females experience some level of over-control or overly exuberant levels of self-monitoring in early puberty. Moreover, pubertal females with high levels of negative affect are at risk for later depression and anxiety (Susman, Dorn, & Chrousos, 1991). As hormone levels stabilize with the resolution of puberty, adolescents of both sexes may experience increases in behavioral stability.
Few studies have empirically considered pubertal development as a mechanism of adolescents’ personality trait change. Canals, Vigil-Colet, Chico, and Marti-Henneberg (2005) assessed 9–10 year-olds over five years using Eysenck’s Personality Questionnaire-Junior Version and reported no influence of pubertal development on adolescent personality change. Age-by-sex effects on neuroticism were found with boys’ scores linearly decreasing until age 15 and girls’ scores decreasing until age 12 before increasing thereafter. Markey, Markey, and Tinsley (2003) longitudinally assessed early adolescent females using a five-factor inventory and found only one pubertal interaction: girls who reached puberty at early ages and were high on Openness to Experience were more likely to take health-related risks. No main effects of pubertal status on personality trait scores were found.
In the current study, self-reported personality traits were measured in adolescents using the Multidimensional Personality Questionnaire-Brief Form (MPQ-BF: Patrick, Curtain, & Tellegen, 2002) and cross-sectionally examined to assess the influence of pubertal status on trait levels. It was hypothesized that after controlling for age, markers of Constraint would be higher and markers of NEM would be lower in late versus early puberty, similar to age-related effects reported in studies of older adolescents. Participants were re-tested after two years. Longitudinal analyses were designed to determine if observed pubertal effects on personality traits were evident within-individuals as pubertal development advanced.
2. Methods
2.1. Participants
Participants, 48 males and 41 females, ages 11–17 (Table 1), were recruited through a database maintained by the University’s Institute of Child Development. When children are born in the metro area, families are sent postcards inviting their research participation. Respondents comprise a volunteer database. Advertisements within the University community were also utilized.
Table 1.
Sample.
| Early-to-mid puberty | Late puberty | |||||
|---|---|---|---|---|---|---|
| N | Age | IQ | N | Age | IQ | |
| Total sample | 34 | 13.36 (1.52) | 116.89 (10.89) | 55 | 15.96 (1.61) | 113.29 (9.41) |
| Males | 27 | 13.45 (1.61) | 117.22 (10.21) | 21 | 16.47 (1.38) | 113.81 (10.77) |
| Females | 7 | 13.10 (1.24) | 115.29 (15.29) | 34 | 15.65 (1.68) | 112.97 (8.62) |
Note: Values represent means and standard deviations. Overall, pubertal groups differed in age, F(1, 87) = 56.01, p < .000, but not IQ, F(1,87) = 2.56, p > .05. Similar patterns held for each sex. Early-to-mid pubertal males and females were similar in age and IQ. In late puberty, males were marginally older, F(1,53) = 3.46, p = .07.
2.2. Screening
Phone interviews were conducted to ascertain eligibility followed by in-person screenings, including semi-structured diagnostic interviews (Kaufman et al., 1997). Participants completed the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) and health questionnaires. Exclusions included medical, psychological, or learning disorders, mental retardation, and psychoactive medicine use. The dataset is part of a larger neuroimaging study. All participants were right-handed and without imaging contraindications. Participants completed a testing session, including the self-report questionnaires described herein. Procedures were approved by the University’s Institutional Review Board.
2.3. Self-report measures
The MPQ-BF was used to quantitate mean levels of three broad factors, PEM, NEM, and Constraint, and 11 primary trait scales. PEM is represented by the primary trait scales of Well-Being, Social Potency, Achievement, and Social Closeness. NEM is represented by Stress Reaction, Alienation, and Aggression. Constraint is represented by Control, Harm Avoidance, and Traditionalism. PEM, NEM, and Constraint account for approximately 50.3% of the variance among the primary trait scales (Patrick et al., 2002). Absorption is an independent scale reflecting the tendency to be captivated by sensory and aesthetic experiences. The MPQ-BF contains 155 largely true/false questions. Because existing T-scores for the MPQ were derived from adults; raw scores were used in subsequent analyses (Ryan, 2009).
The Pubertal Development Scale (PDS) assessed pubertal status (Petersen, Crockett, Richards, & Boxer, 1988). The PDS was developed for contexts in which ratings of pictures (Tanner, 1962) are considered inappropriate (Coleman & Coleman, 2002). Both sexes answer questions about growth in height, body hair, and acne. Males answer questions about voice changes and facial hair. Females are asked about breast development and menstrual status. Responses permit categorization of participants into one of five pubertal stages. Stage 1 represents pre-puberty; Stage 5 represents completed puberty. PDS scores correlate well with physician exams (Coleman & Coleman, 2002; Schmitz et al., 2004). Despite the manner in which pubertal status is calculated, most researchers do not analyze pubertal development in a continuous fashion. Few individuals at Stage 1 are fully pre-pubertal, and raters find it difficult to distinguish between late stages (Brooks-Gunn & Warren, 1985; Shirtcliff, Dahl, & Pollak, 2009). Additionally, because it is difficult in non-epidemiological studies to yield samples where each pubertal stage is equivalently represented within each sex, groups may be combined, comparing those in early puberty with those in late puberty (Holm et al., 2009; Quevado, Benning, Gunnar, & Dahl, 2009; Saxton et al., 2010). In the current study, we examined both a five-stage pubertal status variable for our primary analyses and also incorporated a dichotomous approach (findings described in the Supplement). For the dichotomization, participants with a calculated puberty Stage between 1 and 3 were placed in an early-to-mid puberty group; those characterized as Stage 4 or 5 were placed in a late puberty group. This division allows a comparison of those actively experiencing pubertal development with those for whom development is largely complete.
2.4. Longitudinal assessment
Two years later, 80 of 89 participants were re-assessed. Attrition was largely due to relocation among older participants.
3. Results
3.1. Statistical approach
The MPQ-BF was examined for internal consistency. Cronbach’s alphas (Supplement, Table 1) approximate those generated by the normative sample (Patrick et al., 2002), generally exceeding those reported in adolescent studies (Roberts et al., 2001). Descriptive statistics for the full sample are presented in Supplemental Table 2. Trait scores were evaluated for associations with age and sex and examined between pubertal groups using SPSS version 19’s GLM ANCOVA procedure with a full factorial model. A statistical threshold of p < 0.05 was adopted throughout.
3.2. Age, IQ, and sex effects
Increased age was associated with increased Achievement, r(87) = 0.27, p = .01 and with decreased Alienation, r(87) = −0.37, p < .001, Stress Reaction, r(87) = −0.21, p = .05, NEM, r(87) = −0.33, p < .01, and Traditionalism, r(87) = −0.31, p < .01. Decreases in Alienation, Stress Reaction, Traditionalism, and NEM were evident in males (r’s = −0.36 to −0.50) but not females; increased Achievement was evident in females, r(39) = 0.41, p < .01.
IQ was negatively associated with Social Closeness, r(39) = −0.39, p = .01, and Alienation, r(39) = −0.32, p = .04, but only in females.
Sex differences in mean levels of each trait were evaluated, controlling for age and IQ (Supplemental Table 3). Males exhibited higher Aggression and marginally higher Alienation scores. Females exhibited significantly higher PEM, Social Potency and Achievement, and marginally higher Constraint scores.
3.3. Pubertal status effects
To examine pubertal status effects, three univariate ANCOVAs were conducted entering each MPQ higher order factor (PEM, NEM, and Constraint) as the dependent variable. The five-level pubertal stage variable and sex were entered as between-subjects factors. Age and IQ were covaried. Main effects and puberty by sex interactions were examined. If significant findings for pubertal status were observed, then the primary trait scales for that factor were also examined. PEM yielded no significant findings. For NEM, there was a significant age effect, F(1,79) = 7.42, p = .008, , consistent with the above correlation. For Constraint, there was a main effect of sex, F(1,79) = 11.10, p = .001, , with higher levels in females as well as a significant pubertal stage by sex interaction, F(2,79) = 7.19, p = .001, . No other main effects or interactions were observed. Of note is that all individuals in pubertal Stages 1–2 are male; Stage 5 is biased toward females.
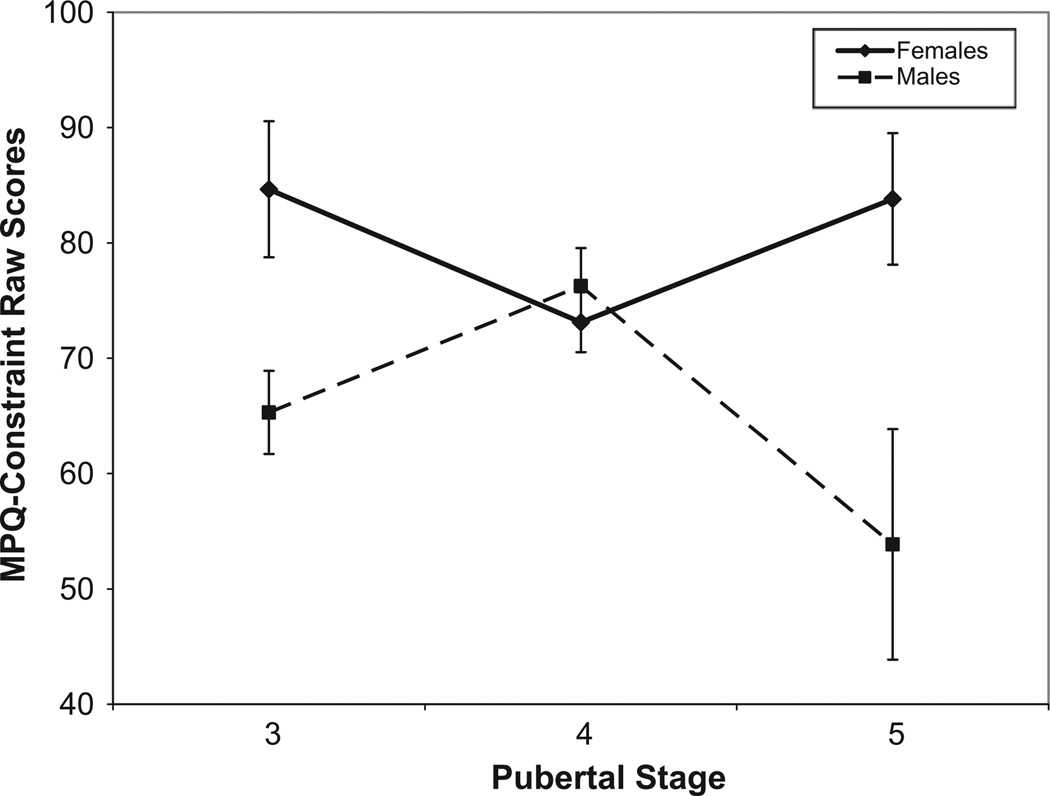
Accordingly, the interaction was investigated in two ways. The first approach was to examine the change in Constraint among pubertal Stages 3, 4 and 5 between the sexes (Fig. 1). In females, pubertal stage predicted Constraint scores (p = .013; ). The quadratic (p = .013) but not the linear (p = .43) contrast was significant. Pubertal status also predicted Constraint scores in males (p = .028, ) again with a significant quadratic (p = .013) rather than linear (p = .631) effect. Males significantly increased in Constraint between Stages 3 and 4 (F(1,31) = 5.32, p = .028) with a non-significant decline from Stages 4 to 5 (p = .073). Females declined from Stages 3 to 4, F(1,30) = 5.07, p = .03. Their subsequent increase between Stages 4 and 5 was not significant (p = .327).
Fig. 1.
Cross-sectional changes in Constraint by sex from pubertal Stages 3 to 5.
The second strategy for the analysis of the pubertal stage by sex interaction was to be more inclusive regarding the study sample, dividing it at the median (pubertal Stage 3.0). Individuals in Stages 1–3 were compared to those in Stages 4–5. Findings are summarized in Supplemental Table 5 and generally confirm that males and females maximally differ in their Constraint scores between pubertal Stage 3 and later stages.
The first-order traits comprising the Constraint factor were similarly examined. There were no significant effects for Traditionalism.
For Harm Avoidance, there were main effects of age, F(1,79) = 7.23, p < .01, , and sex, F(1,79) = 3.89, p = .05, , and a pubertal stage by sex interaction, F(2,79) = 4.65, p = .01, . Males had lower scores than females. In females, there were no main effects of pubertal stage. The effect was significant in males, where the quadratic effect was at a trend level with all five pubertal stages included (p = .068) and significant when the analysis was restricted to Stages 3–5 (p = .018). The increase in Harm Avoidance between Stages 3 and 4 was significant (p = .018). There was no significant change between Stages 4 and 5 (p = .123).
For Control, there were main effects of age, F(1,79) = 4.22, p < .05, , sex, F(1,79) = 7.25, p < .01, , and a pubertal stage by sex interaction, F(2,79) = 4.28, p < .05, . Values were higher in females. The effect of pubertal status was significant only in females and best described by a linear trend (p = .025). Females demonstrated a significant decline in Control between Stages 3 and 4 (p = .020) but remained stable thereafter.
Interestingly, although all aspects of puberty are present to greater degrees in later versus earlier stages, the transition from Stage 3 to later stages is marked by the onset of menarche in this sample’s females (χ2(1) = 41.0, p = .000), which is a notable index of hormonal change.
These overall patterns are confirmed by partial correlations between the full five-level pubertal stage variable as well as the dichotomized (early-mid versus late) variable in relation to MPQ trait scores (Supplemental Table 4). The dichotomized variable best captures the nature of the significant associations within each sex, suggesting a discontinuity in the expression of Constraint-related traits between mid and late puberty.
3.4. Confirmatory longitudinal assessment
Of 80 retested participants, 48 were in pubertal Stages 4 or 5 at Time 1, retaining that status over time. Of 32 individuals in Stages 1–3 at Time 1, 15 remained so at Time 2; 17 transitioned to late puberty. For each group, repeated measures ANCOVAs were conducted, entering scores on the Constraint, Control, and Harm Avoidance scales at Time 1 versus Time 2 to determine puberty-associated change. Sex was evaluated between subjects, covarying age and IQ.
For those that remained in late puberty from Time 1 to Time 2, there were no changes over time in any variable.
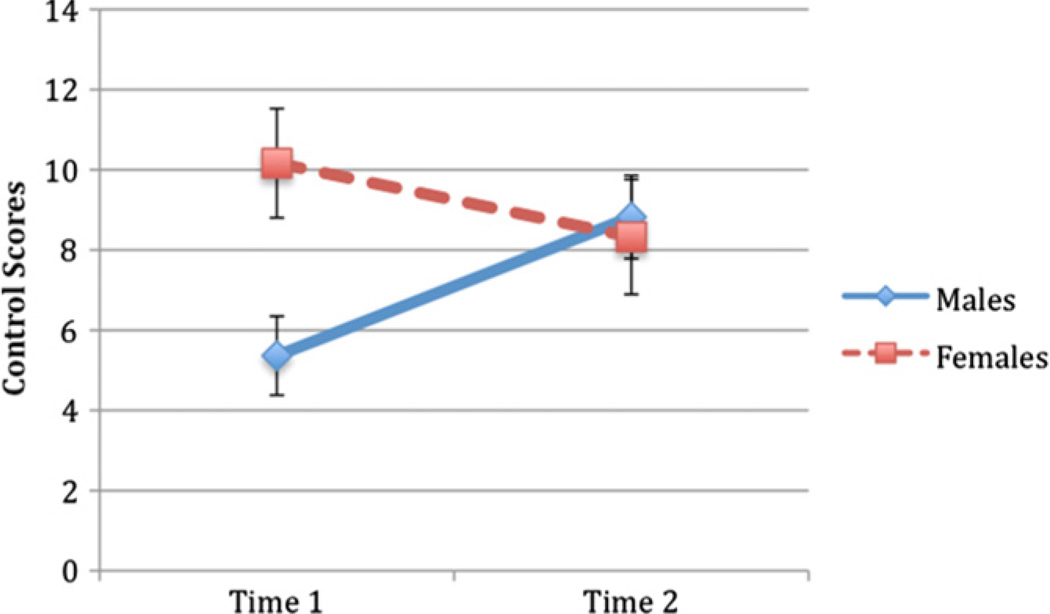
For the group that transitioned from early-mid to late puberty, there was a Time by Sex interaction for Control F(1,13) = 5.77, p < .05, Males and females differed at Time 1 (early-to-mid puberty, with females demonstrating higher scores), F(1,13) = 6.68, p < .05, , but were indistinct at Time 2 (Table 2 and Fig. 2).
Table 2.
Longitudinal assessment from early-to-mid to late puberty.
| Males | Females | |||
|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | |
| Constrainta | 61.37 (4.51) | 71.02 (4.88) | 86.83 (6.36) | 85.46 (6.88) |
| Control | 5.31 (1.05) | 8.71 (1.09) | 10.26 (1.48) | 8.53 (1.54) |
| Harm avoidanceb | 4.28 (1.17) | 4.75 (1.04) | 7.50 (1.65) | 8.97 (1.47) |
Note: Values represent estimated marginal means covarying for age and IQ.
Significant.
Marginally significant main effects of sex.
Fig. 2.
Longitudinal changes in Control from mid to late puberty.
For the group that remained in early-mid puberty from Time 1 to Time 2, 13 of 14 individuals were male, so sex differences could not be modeled. MPQ Constraint, Control, and Traditionalism were unchanged over time. Harm avoidance declined, F(1,10) = 6.75, p = .03, .
4. Discussion
This is one of few studies to separate the influences of age and pubertal development on self-reported personality traits in typically developing adolescents. Consistent with the literature in older adolescents (Blonigen et al., 2008), increasing age was associated with decreasing levels of NEM and several primary trait markers (Stress Reaction and Alienation). This pattern was strongest in males. Females showed age-related increases in Achievement, broadly consistent with observations of increases in industriousness and conscientiousness into young adulthood (Roberts & DelVecchio, 2000). Traditionalism, reflecting one’s tendency to espouse stringent moral values, decreased with age, perhaps reflecting adolescents’ rejection of parental authority as they individuate (Roberts et al., 2009). Thus, age impacts changes in facets of NEM and Constraint with evidence of sex-specific trends in development. Unlike other studies, though, we suggest that pubertal development may motivate distinct facets of personality development in males versus females independent of chronological age.
Cross-sectional analyses indicate that primary traits scales within the broad domain of Constraint change as a function of pubertal development in both sexes, but involving distinctly different first-order traits for males versus females. Mid versus late pubertal males reported significantly lower Constraint scores, an effect driven by change in Harm Avoidance. In females, puberty-related decreases in Constraint were marked by declines in Control. Analyses of individuals who progressed from mid to late puberty over time indicated that males and females maximally differ in Control in early-to-mid puberty, ultimately arriving at equivalent scores. This pattern was supported by the cross-sectional data as well in that there were not significant differences found between Stages 4 and 5.
High levels of Control in early-to-mid pubertal females may indicate rigid levels of self-monitoring. In contrast, males may start at lower-than-ideal levels in early to middle stages of puberty and then increase over time. Low control is related to externalizing tendencies, including impulsivity, substance misuse, and rule-breaking (Krueger, McGue, & Iacono, 2001; Roberts et al., 2009). Therefore, puberty-driven increases and decreases in the primary trait markers Harm Avoidance and Control in males and females, respectively, may reflect the ‘‘maturing out’’ of over-control (females) versus externalizing (males) tendencies. These suggestions are speculative given that the current sample was free of psychopathology. Anecdotally, parents tend to report similar patterns with respect to challenges faced in rearing girls versus boys in the early adolescent period. While boys provoke their parents through displays of under-control, particularly in the presence of peers early in adolescence (Beausay, 2001), parents may become alarmed when their previously well-behaved mid-adolescent daughters pursue more independence in social and sexual contexts (Baumrind, 1991), coincident with declines in Control.
An intriguing issue for further investigation concerns the precise mechanisms through which pubertal development promotes these changes. By definition, a biological mechanism is implicated. From early to late adolescence, the brain’s regulatory circuits become more efficient, allowing the prefrontal cortex to exert increasing levels of executive control in the face of motivational and emotional impulses (Somerville, Jones, & Casey, 2010). Recent studies suggest that development of the capacity for executive control in most situations may asymptote around age 16 (Steinberg, Cauffman, Woolard, Graham, & Banich, 2009), which in this sample corresponds to changes in status from mid to late puberty. Given that the Control scale indexes processes typically ascribed to frontal lobe development (Luciana, Collins, Olson, & Schissel, 2009) and is the facet of conscientiousness most reliably linked to innate temperament as well as to low externalizing (Roberts et al., 2009), these findings are compelling in suggesting that aspects of executive function that rest at the intersection of cognition and personality may not only be grounded in brain development but, more specifically, sex-specific patterns of hormonal regulation.
How is it that pubertal development spurs the maturation of brain circuits involved in behavioral control? Recent work suggests that sex-based hormone-modulated differences, as a function of puberty, structure the rate at which different brain regions develop in male versus female adolescents to establish functional maturity (Ahmed et al., 2008). Given that the ultimate goal is for the individual to possess sufficient capacity for behavioral regulation to function adaptively in adulthood, these changes from early to late puberty may prime older adolescents to be increasingly receptive to engagement with adult roles, consistent with Social Investment Theory (Lodi-Smith & Roberts, 2007). Thus, pubertal surges in gonadal hormones may dysregulate girls’ and boys’ behaviors in distinct ways early in adolescence but may drive sex-specific developmental cascades that structure brain development and the approach towards adult-like levels of behavioral stability by late adolescence.
The validation of cross-sectionally observed findings within subjects assessed repeatedly over time is a strength of our approach. However, a notable limitation concerns this study’s small sample size. Replication of these patterns in larger samples where each stage of puberty is strongly represented within each sex may clarify exactly when, in the course of pubertal development, these changes are pivotal. Another limitation concerns our method for measuring pubertal development given that physicians’ examinations are optimal. Moreover, since hormonal levels were not assessed, the relationship between hormones, brain development, and personality discussed in this paper also remain speculative. Despite these caveats, this approach to analyzing pubertal influences on personality traits, as reflected through a three-factor assessment of broad personality traits, their primary trait markers, and their development, is novel in providing support for puberty-driven hormonal change as a mechanism that underlies the maturation of, and individual differences in, self-regulatory capacities in adolescence.
Supplementary Material
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.paid.2011.08.001.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature Neuroscience. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumrind D. Effective parenting during the early adolescent transition. In: Cowan PA, Heatherington EM, editors. Family transitions. Hillsdale, N.J: Lawrence Erlbaum Associates; 1991. pp. 111–363. [Google Scholar]
- Beausay W. Teenage boys: Surviving and enjoying these extraordinary years. Colorado Springs, Colorado: Waterbrook Press; 2001. [Google Scholar]
- Blonigen DM, Carlson MD, Hicks BM, Krueger RF, Iacono WG. Stability and change in personality traits from late adolescence to early adulthood: A longitudinal twin study. Journal of Personality. 2008;76:239–266. doi: 10.1111/j.1467-6494.2007.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP. Measuring physical status and timing in early adolescence. A developmental perspective. Journal of Youth and Adolescence. 1985;14:163–189. doi: 10.1007/BF02090317. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones? Evidence for activational effects of hormones on moods and behaviors at adolescence. Psychological Bulletin. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Canals J, Vigil-Colet A, Chico E, Marti-Henneberg C. Personality changes during adolescence. The role of gender and pubertal development. Personality and Individual Differences. 2005;39:179–188. [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annual Review of Psychology. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: A review. Journal of Adolescence. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshaven MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, Conger RD, Burzette RG. Personality development from late adolescence to young adulthood: Differential stability, normative maturity, and evidence for the maturity–stability hypothesis. Journal of Personality. 2007;75:237–263. doi: 10.1111/j.1467-6494.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- Francis LJ. The dual nature of the Eysenckian neuroticism scales: A question of sex differences. Personality and Individual Differences. 1993;15:43–59. [Google Scholar]
- Holm SM, Forbes EE, Ryan MD, Phillips ML, Tarr JA, Dahl RE. Reward related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Personality traits in late adolescence predict mental disorder in early adulthood. A prospective-epidemiological study. Journal of Personality. 1999;67:39–65. doi: 10.1111/1467-6494.00047. [DOI] [PubMed] [Google Scholar]
- Krueger RF, McGue M, Iacono WG. The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences. 2001;30:1245–1259. [Google Scholar]
- Lenroot RK, Giedd J. Sex differences in the adolescent brain. Brain and Cognition. 2010;72:46. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi-Smith JL, Roberts BW. Social investment and personality: A meta-analytic analysis of the relationship of personality traits to investment in work, family, religion, and volunteerism. Personality and Social Psychology Review. 2007;11:68–86. doi: 10.1177/1088868306294590. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Olson EA, Schissel AM. Tower of London performance in healthy adolescents: The development of planning skills and associations with self-reported inattention and impulsivity. Developmental Neuropsychology. 2009;34:461–475. doi: 10.1080/87565640902964540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CN, Markey PM, Tinsley BJ. Personality, puberty, and pre-adolescent girls’ risky behaviors: Examining the predictive value of the five-factor model of personality. Journal of Research in Personality. 2003;37:405–419. [Google Scholar]
- McCrae RR, Costa PT, Terracciano A, Parker WD, Mills CJ, De Fruyt F, et al. Personality trait development from age 12 to age 18: Longitudinal, cross-sectional, and cross-cultural analyses. Journal of Personality and Social Psychology. 2002;83:1456–1468. [PubMed] [Google Scholar]
- Patrick CJ, Curtain JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Quevado KM, Benning SD, Gunnar MR, Dahl R. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Development and Psychopathology. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Caspi A, Moffitt T. The kids are alright: Growth and stability in personality development from adolescence to adulthood. Journal of Personality and Social Psychology. 2001;81:670–683. [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Jackson JJ, Burger J, Trautwein U. Conscientiousness and externalizing psychopathology: Overlap, developmental patterns, and etiology of two related constructs. Development and Psychopathology. 2009;21:871–888. doi: 10.1017/S0954579409000479. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132:1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Worthman CM, Costello EJ, Angold A. Testosterone, antisocial behavior, and social dominance in boys: Pubertal development and biosocial interaction. Biological Psychiatry. 2004;55:546–552. doi: 10.1016/j.biopsych.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Ryan R. Age differences in personality: Adolescents and young adults. Personality and Individual Differences. 2009;47:331–335. [Google Scholar]
- Sanborn K, Hayward C. Hormonal changes at puberty and the emergence of gender differences in internalizing disorders. In: Hayward C, editor. Gender differences in puberty. Cambridge: Cambridge University Press; 2003. pp. 29–60. [Google Scholar]
- Saxton TK, Kohoutova D, Roberts SC, Jones BC, DeBruine LM, Havlicek J. Age, puberty, and attractiveness judgments in adolescents. Personality and Individual Differences. 2010;49:857–862. [Google Scholar]
- Schmitz KE, Hovell MF, Nichols JF, Irvin VL, Keating K, Simon GM, et al. A validation study of early adolescents’ pubertal self-assessments. Journal of Early Adolescence. 2004;24:357–384. [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Cauffman E, Woolard J, Graham S, Banich M. Are adolescents less mature than adults? Minors’ access to abortion, the juvenile death penalty, and the alleged APA “flip-flop”. American Psychologist. 2009;64:583–594. doi: 10.1037/a0014763. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Chrousos GP. Negative affect and hormone levels in young adolescents: Concurrent and predictive perspectives. Journal of Youth and Adolescence. 1991;20:167–190. doi: 10.1007/BF01537607. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. Springfield, IL: Charles C. Thomas; 1962. [Google Scholar]
- Tellegen A. Brief manual for the multidimensional personality questionnaire. Minneapolis: University of Minnesota; 1982. Unpublished manuscript. [Google Scholar]
- Wechsler D. Manual for the wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.