Abstract
Oral biofilm (dental plaque) is formed by the initial adhesion of “pioneer species” to salivary proteins that form the dental pellicle on the tooth surface. One such pioneer species, Streptococcus gordonii, is known to bind salivary amylase through specific amylase-binding proteins such as amylase-binding protein A (AbpA). Recent studies have demonstrated that once bound, salivary amylase appears to modulate gene expression in S. gordonii. However, it is not known if this amylase-induced gene expression leads to secretion of proteins that play a role in plaque biofilm formation. In this study we examined the differences in secreted proteomes between S. gordonii KS1 (wild type) and AbpA-deficient (ΔAbpA) strains. We also examined the differentially precipitated secretome proteins following incubation with salivary amylase. The culture supernatants from KS1 and ΔAbpA were analyzed by nano-LC/MS/MS to characterize the whole secreted proteomes of the KS1 and ΔAbpA. A total of 107 proteins were identified in the KS1 and ΔAbpA secretomes of which 72 proteins were predicted to have an N-terminal signal peptide for secretion. Five proteins were differentially expressed between the KS1 and ΔAbpA secretomes; AbpA and sortase B were expressed exclusively by KS1, whereas Gdh, AdcA and GroEL were expressed only by ΔAbpA. Incubation of culture supernatants from KS1 and ΔAbpA with amylase (50 μg/ml) at room temperature for 2 h resulted in the differential precipitation of secretome proteins. Hypothetical protein (SGO_0483), cation-transporting ATPase YfgQ (Aha1), isocitrate dehydrogenase (Icd), sortase A (SrtA), beta-N-acetylhexosaminidase (SGO_0405), peptide chain release factor 1(PrfA) and cardiolipin synthase (SGO_2037) were precipitated by amylase from the KS1 culture supernatant. Among the identified secreted proteins and amylase-precipitated proteins, transcriptional regulator LytR (SGO_0535) and cation-transporting ATPase YfgQ (Aha1) are potential signaling proteins.
Keywords: Amylase-binding proteins, Proteomic analysis, Secretome, AbpA, AbpB, GtfG
Introduction
Streptococcus gordonii belongs to the viridans group streptococci, which includes the mitis, salivarius and anginosus groups. These streptococci are essential for establishment of oral biofilms (dental plaque) and are associated with a healthy oral environment [1-3]. S. gordonii is one of several bacteria known to be pioneer colonizers of newly erupted teeth and cleaned tooth surfaces [4]. The formation of dental plaque initiates with the interaction between early colonizing oral bacteria and salivary components [5]. Such interactions influence the binding of other oral plaque bacteria to tooth surfaces, the aggregation of bacteria to prevent adhesion to the oral surfaces, as well as the effect of antimicrobials on the bacteria within the dental plaque [5].
Amylase is one of the most abundant proteins present in saliva. It is an enzyme that is involved in the digestion of starch to glucose, maltose and maltodextrins, which act as nutritional substrates for oral plaque bacteria. Besides its enzymatic activity, amylase adsorbs to enamel surfaces and also interacts with the pioneer colonizers [6,7]. Amylase is known to bind to the surface of S. gordonii, Streptococcus mitis, Streptococcus cristatus, Streptococcus parasanguinis, and Streptococcus salivarius and several other streptococci, which are collectively termed as amylase-binding streptococci (ABS) [8]. The binding and interaction of amylase with S. gordonii has been investigated most extensively.
S. gordonii produces two amylase-binding proteins (ABPs)-AbpA and AbpB [9,10]. AbpA (20 kDa) is an extracellular, cell wall-associated protein that is expressed at maximal levels during mid-log phase of bacterial growth [9]. AbpB is an 82-kDa extracellular dipeptidyl-peptidase that co-precipitates with AbpA and GtfG from culture supernatants when incubated with salivary amylase [11-13] and binds amylase in the amylase-ligand overlay assay [14]. It is clear though that AbpA is the primary receptor for amylase binding to S. gordonii since inactivation of AbpA essentially eliminates amylase binding to the cell surface [15,16]. However, the exact function of AbpA with regards to binding amylase is not clear. Recent studies have shown that amylase binding can induce gene expression in S. gordonii [16]. Since the binding of amylase to S. gordonii is AbpA dependent, the hypothetical question arises whether AbpA could act as a signaling receptor for salivary amylase-induced gene expression. This led us to investigate the secretome proteins and the amylase precipitated proteins to identify candidate proteins that interact with AbpA.
Although AbpA and AbpB of S. gordonii Challis are the predominant and best studied ABPs, SDS-PAGE analysis of amylase-precipitated proteins indicates the presence of other as yet uncharacterized proteins that appear to interact with amylase [12,17]. The secretome, which is the set of proteins secreted to the cell surface and extracellular space, has not been previously described for S. gordonii. Proteins of the secretome may contain signaling proteins that interact with ABPs to influence biofilm formation or interact with salivary components to induce bacterial cell signaling. In addition, comparison of differentially secreted proteins between wild type KS1 and AbpA-deficient (ΔAbpA) strains may also provide insight into the role of AbpA in S. gordonii. In this study, we performed 1-D electrophoresis followed by nano-liquid chromatography/mass spectrometry/mass spectrometry (nano-LC/MS/MS) analysis to identify the secretome proteins and the amylase-precipitated proteins of S. gordonii.
Materials and Methods
Preparation of secretome proteins
S. gordonii Challis CH1 (wild type), KS1 (kanamycin-resistant wild type), ΔAbpA (KS1ΩabpA) and ΔAbpB (KS1ΩabpB) strains were grown on tryptic soy agar supplemented with 0.5% yeast extract (TSBY) agar plates with appropriate antibiotic selection for 2-3 days at 37°C in a candle jar, as previously described [18]. For secretome preparation, isolated bacterial colonies were inoculated into 10 ml of TSBY broth and cultured overnight (12-14 h) at 37°C in a candle jar. After centrifugation at 5,000×g, the supernatant was collected and filtered using a 0.22-μm microfilter (Corning Inc., Corning, NY) to remove residual bacterial cells. To precipitate proteins, the filtered supernatant was transferred to a clean glass bottle and mixed well with an equal volume of 25% (w/v) trichloroacetic acid (TCA)/acetone. The samples were incubated at −20°C for at least 48 h and then centrifuged at 8,000×g for 10 min at 4°C. After removing the supernatant, the pellets were washed twice with 10 ml of ice-cold acetone. Upon completion of the final centrifugation, the pellets were left in a fume hood for 1-2 h to allow residual acetone to evaporate. The pellets were solubilized in 1% SDS and boiled for 10 min to denature the proteins. Protein in the samples was quantified using the BioRad DC Protein assay (Hercules, CA). Samples containing 15 μg of protein were prepared in 4× loading buffer (LDS buffer, Invitrogen, Carlsbad, CA), loaded onto a 10% SDS-PAGE gel (NuPage gels, Invitrogen, Carlsbad, CA), and resolved by electrophoresis. Gels were stained using the SilverQuest silver staining kit (Invitrogen, Carlsbad, CA). For MS/MS analysis, the samples (15 μg protein/lane) were loaded onto a 10% SDS-PAGE gel and subjected to gel electrophoresis briefly for 5-10 min until the samples completely entered the resolving gel. Electrophoresis was stopped and the gel was stained with Coomassie blue. The single stained band in each lane containing the whole “secreted proteome” was cut from the gel and sent for nano-LC/MS/MS analysis (Fred Hutchinson Cancer Research Center, Seattle, WA). This experiment was repeated from two independent biological samples.
In-gel trypsin digestion
The gel pieces were destained with 25 mM ammonium bicarbonate in 50% acetonitrile, and subsequently dehydrated using acetonitrile. The proteins were digested overnight with 5 ng/μL trypsin (Promega Corporation) in 50 mM ammonium bicarbonate at 37°C. The peptides were extracted from the gel using 0.1% (v/v) trifluoroacetic acid (TFA) in water after 30 min incubation followed by acetonitrile. The pooled extracts were dried in a SpeedVac and cleaned using ZipTip™ C18 (Millipore Corporation) before MS analysis.
Nano-LC-MS/MS analysis
LC-MS/MS analysis was performed using a LTQ Orbitrap XL mass spectrometer (Thermo Scientific). The LC system configured in a vented format consisted of a fused-silica nanospray needle packed in-house with Magic C18 AQ 100A reverse-phase media (Michrom Bioresources Inc, 25 cm) and a trap (2 cm) containing Magic C18 AQ 200A reverse-phase media. The inner diameters of the analytical and trap columns were 75 μm and 100 μm, respectively. The peptide samples were loaded onto the column and chromatographic separation was performed using a two-mobile-phase solvent system consisting of 0.1% formic acid in water (A) and 0.1% acetic acid in acetonitrile (B) over 60 min from 5% B to 40% B. The mass spectrometer operated in a data-dependent MS/MS mode over the m/z range of 400-1800. For each cycle, the five most abundant ions from each MS scan were selected for MS/MS analysis using 35% normalized collision energy. Selected ions were dynamically excluded for 45 seconds.
Data analysis
Raw MS/MS data were submitted to the Computational Proteomics Analysis System (CPAS), a web-based system built on the LabKey Server v11.2 and searched using the X!Tandem search engine against S. gordonii Challis protein database from UniProt (http://www.uniprot.org/). The search output files were analyzed and validated by ProteinProphet. Peptide hits were filtered with PeptideProphet error rate <0.05, and proteins with probability scores of >0.9 were accepted.
Amylase precipitation of proteins from culture supernatant
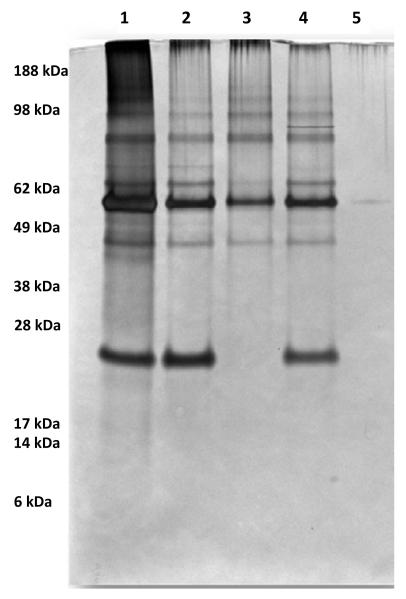
S. gordonii KS1 and ΔAbpA were grown on TSBY agar with appropriate antibiotic selection for 2-3 days at 37°C in a candle jar. Isolated bacterial colonies were inoculated into 10 ml of TSBY broth and cultured overnight (12-14 h) at 37°C in a candle jar. After centrifugation, the culture supernatant was collected and filtered using a 0.22-μm microfilter. Proteins were precipitated with salivary amylase as described previously [16]. Briefly, purified human salivary amylase (50 μg/ml), was added to the filtered supernatant in a sterile tube and incubated statically at room temperature for 2 h followed by centrifugation at 5,000×g for 10 min. After carefully removing the supernatant, the pellet containing the precipitated proteins was solubilized in 1% SDS and boiled for 10 min to denature the proteins. The protein content of the samples was quantified using the BioRad DC Protein assay. Samples of equal protein content (15 μg) were prepared for electrophoresis in 4X loading buffer, loaded onto 10% SDS PAGE gels (NuPage gels, Invitrogen), and subjected to electrophoresis. The gels were silver stained to visualize protein separation. For MS/MS analysis, a similar gel was stained with Coomassie blue and four bands were cut above and one band below the amylase band from both the KS1 and AbpA samples, as shown in Figure 3. Bands were sent for nano-LC/MS/MS analysis (Applied Biomics, Inc, Hayward, CA). As a control, concentrated TSBY was sent to determine potential growth medium derived proteins in the samples. This experiment was repeated from two independent biological samples.
Figure 3. Sample preparation for MS/MS analysis of amylase-precipitated proteins.
Coomassie-stained gels were cut in the pattern depicted above to get 5 bands/slices per strain. Amylase and AbpA bands were avoided as they are well known from past experiments. The gel bands/slices were placed in microcentrifuge tubes containing 70 μl of double distilled water and sent for nano-LC/MS/MS analysis.
Reduction/alkylation and trypsin digestion
DTT was added to a final concentration of 10 mM in 50 mM ammonium bicarbonate and incubated with the gel band at 60°C for 30 min, followed by cooling down to room temperature. Iodoacetamide was then added to a final concentration of 50 mM and incubated in the dark for 30 min at room temperature. The gel band was washed a few times and then digested in-gel with modified porcine trypsin protease (Promega).
NanoLC-MS/MS
NanoLC was carried out using a Dionex Ultimate 3000 (Milford, MA). Tryptic peptides were loaded into a μ-Precolumn Cartridge and separated on an acetonitrile gradient (ranging from 5% to 60%) on a Nano LC column. Fractions were collected at 20-second intervals followed by Mass Spectrometry analysis on AB SCIEX TOF/TOF™ 5800 System (AB SCIEX). Mass spectra were acquired in reflectron positive ion mode. TOF/TOF tandem MS fragmentation spectra were acquired for each ion, averaging 4000 laser shots per fragmentation spectrum (excluding trypsin autolytic peptides and other known background ions).
Data analysis
Both of the resulting peptide mass and the associated fragmentation spectra were submitted to GPS Explorer workstation equipped with MASCOT search engine (Matrix Science, London, UK) to search the database of National Center for Biotechnology Information non-redundant (NCBInr). Searches were performed without constraining protein molecular weight or isoelectric point, with variable carbamidomethylation of cysteine and oxidation of methionine residues, and with one missed cleavage also allowed in the search parameters.
Bioinformatics
A bioinformatics approach was used to further characterize the proteins for molecular weight, putative functions and domains. To determine molecular weight, FASTA amino acid sequences were obtained from the S. gordonii Challis database at National Center for Biotechnology Information (NCBI) and submitted to http://web.expasy.org/compute_pi/. For prediction of N-terminal signal peptides for secretion, the FASTA amino acid sequences were submitted to http://www.predisi.de/home.html. To determine functional domains, the FASTA amino acid sequences were submitted to the protein BLAST at NCBI.
Results and Discussion
Whole secretome analysis
The secretome of CHI, KS1, ΔAbpA and ΔAbpB, comprising both secreted and cell-associated proteins shed during pellet preparation, were compared. To extend the results of our previous work [11,12], bacteria were grown in nutrient-rich TSBY medium to stationary phase (12-14 h). The use of rich medium avoids the secretion of proteins induced under the stress of nutrient limitation in defined medium. AbpA is secreted throughout the growth phase, whereas AbpB is expressed from late exponential to stationary phase [9], thus examination of the secretome of cells in stationary phase enabled the study of both known ABPs of S. gordonii, as well as other potentially associated secreted proteins.
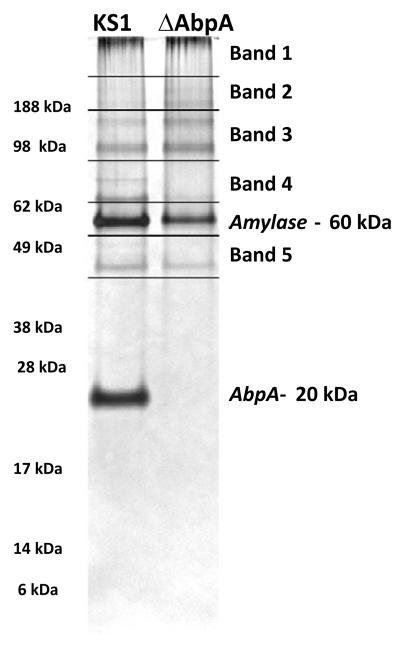
A silver stained SDS-PAGE gel of TCA-precipitated whole secretome samples revealed only minor differences in protein band patterns between CH1 (wild type), KS1 (kanamycin-resistant wild type) and ΔAbpA strains. Although these differences appeared to be minor from the stained protein gels, the presence of a large number of closely spaced bands makes their differentiation difficult. Hence, a mass spectrometric (nano-LC/MS/MS) analysis of the whole secretome samples was performed to identify proteins within each sample. The protein sample was treated with trypsin to generate multiple peptides. Mass spectrometry (MS) allows for differential identification of peptides present in the samples. Differential expression between wild-type and mutant strains is based on presence or absence of the protein-associated peptides in the secretome preparations. There were no differences evident between the CH1 and KS1 secretomes (data not shown) supporting the observations by SDS-PAGE analysis. The TSBY media contains yeast extract, which may contribute to the peptide sequences in the MS analysis. For this reason an MS analysis of TCA precipitate of the TSBY media was performed and no yeast proteins were found (data not shown). Concurrently, in any of the MS analyses for secreted proteomes yeast proteins were not found, however, human keratin was found as a contaminant.
The whole secretomes of KS1 and ΔAbpA were compared. A total of 107 proteins were identified (Tables 1 and 2). This is the first study to identify whole secretome proteins from S. gordonii using nano-LC/MS/MS analysis. The secretome fractions include several classically secreted proteins with N-terminal signal peptides, secreted proteins with enzymatic domains and non-canonical secretory proteins, which mostly include cytosolic proteins. Several hypothetical proteins of unknown functions and uncharacterized domains were also identified.
Table 1.
Whole secretome proteins identified in common from KS1 and ΔAbpA.
| Protein/Gene Name | Gene Index | Predicted Function/Domain | Mol. Wt. (kDa) |
Pep. Id. |
Aa. Seq. Cov. (%) |
|---|---|---|---|---|---|
| General stress protein gsp-781* | SGO_2107 | Unknown | 41 | 211 | 63 |
| Glucosyltransferase G gtfG* | SGO_0497 | Glycosylhydrolase | 178 | 175 | 62.8 |
| Serine protease challisin sgc* | SGO_0566 | Serine protease | 165 | 131 | 52.2 |
| Serine protease degP* | SGO_2150 | Serine protease | 42 | 102 | 43.6 |
| Surface-associated protein cshA* | SGO_0854 | Unknown | 264 | 85 | 40.1 |
| Streptococcal surface protein A sspA* | SGO_0210 | Unknown | 172 | 75 | 38.3 |
| Surface antigen SCP-like domain | SGO_1110 | Unknown | 82 | 72 | 46.2 |
| Membrane protein | SGO_0060 | Membrane protein | 109 | 66 | 41.8 |
| 5′-nucleotidase family protein* | SGO_1247 | N-terminal metallophosphatase domain | 78 | 64 | 55.6 |
| Streptococcal surface protein B sspB* | SGO_0211 | Unknown | 164 | 58 | 38.7 |
| LPXTG cell wall surface protein* | SGO_0707 | Cell wall surface protein | 180 | 52 | 54.6 |
| LPXTG cell wall surface protein* | SGO_1651 | Cell wall surface protein | 84 | 50 | 37.9 |
| Surface-associated protein cshB* | SGO_1148 | Unknown | 244 | 46 | 32.9 |
| Penicillin-binding protein 1B pbp1B | SGO_1928 | Transpeptidase domain | 92 | 34 | 43.6 |
| Exo-beta-D-fructosidase* | SGO_0385 | Glycosyl hydrolases family 32 | 156 | 32 | 29.9 |
| LPXTG cell wall surface protein* | SGO_0890 | Collagen binding domain | 69 | 32 | 22.7 |
| Zinc metalloproteinase B zmpB* | SGO_0408 | IgA1-specific Metallo-endopeptidase N-terminal region |
218 | 28 | 18.2 |
| Putative transcriptional regulator lytR* ∞ | SGO_0535 | Membrane bound transcriptional regulator | 44 | 28 | 44.2 |
| Lipoprotein* | SGO_1082 | Lipoprotein | 37 | 28 | 70.2 |
| LPXTG cell wall surface protein* | SGO_1487 | Cna protein B-type domain | 191 | 28 | 50.7 |
| Putative N-acetylmuramidase* | SGO_2013 | Glycosyl hydrolases family 25 | 127 | 28 | 33.4 |
| Lipoprotein* | SGO_0094 | Unknown | 42 | 26 | 43.1 |
| Putative uncharacterized protein | SGO_1066 | Unknown | 49 | 26 | 53.9 |
| Conserved domain protein* | SGO_0021 | Unknown | 20 | 25 | 60.4 |
| Putative uncharacterized protein* | SGO_2136 | Unknown | 48 | 25 | 47.2 |
| Putative uncharacterized protein | SGO_0319 | Unknown | 32 | 23 | 26.1 |
| LysM domain protein* | SGO_0138 | Involved in binding peptidoglycan | 40 | 21 | 17.7 |
| Oligopeptide-binding protein* | SGO_1716 | ABC-type oligopeptide transport system | 73 | 20 | 35.7 |
| LPXTG cell wall surface protein* | SGO_0388 | Zinc carboxypeptidase | 120 | 19 | 22.3 |
| LPXTG cell wall surface protein* | SGO_0430 | Unknown | 96 | 19 | 35.3 |
| Serine/threonine protein kinase | SGO_0600 | Serine/threonine protein kinase | 67 | 18 | 24.6 |
| Maltose/maltodextrin-binding protein* | SGO_0104 | Maltose binding domain | 45 | 17 | 44.2 |
| Oligopeptide-binding lipoprotein hppH* | SGO_1715 | ABC-type oligopeptide transport system | 72 | 17 | 32.2 |
| Protein with prophage function domain | SGO_0067 | Prophage function domain | 52 | 16 | 35 |
| Glyceraldehyde-3-phosphate dehydrogenase gap |
SGO_0207 | Glycolysis | 36 | 15 | 41.1 |
| Transport protein | SGO_0767 | TroA like transporter domain | 32 | 14 | 47.8 |
| LPXTG cell wall surface protein* | SGO_1650 | Collagen binding domain | 77 | 14 | 18.7 |
| Pneumococcal histidine triad A protein | SGO_1313 | Unknown | 124 | 13 | 13 |
| Autolysin, N-acetylmuramidase-like protein | SGO_1138 | Unknown | 26 | 12 | 40.2 |
| Sulfatase* | SGO_1377 | Sulfatase | 83 | 12 | 26.3 |
| LPXTG cell wall surface protein | SGO_2005 | Unknown | 397 | 12 | 4.9 |
| LPXTG cell wall surface protein* | SGO_0107 | Collagen binding domain | 115 | 11 | 10.9 |
| Amylase-binding protein B abpB* | SGO_0162 | Amylase-binding protein | 73 | 11 | 25.5 |
| Lipoprotein* | SGO_0181 | Unknown | 36 | 11 | 36.7 |
| Cell wall binding protein* | SGO_0477 | Glucan-binding domain | 36 | 11 | 42.4 |
| Peptidoglycan N-acetylglucosaminedeacetylase A pgdA* |
SGO_0948 | Peptidoglycan N-acetylglucosaminedeacetylase |
54 | 11 | 21.1 |
| Penicillin-binding protein 2B pbp2B | SGO_1449 | Transpeptidase domain | 75 | 11 | 22.8 |
| Oligopeptide-binding lipoprotein hppG* | SGO_1713 | ABC-type oligopeptide transport | 74 | 11 | 28.5 |
| Lipoprotein* | SGO_0068 | Unknown | 20 | 10 | 42.4 |
| Lipoprotein hlpA* | SGO_0458 | ABC-type metal ion transport system | 32 | 9 | 29.6 |
| Cell wall binding protein* | SGO_0478 | Glucan-binding domain | 37 | 9 | 38 |
| Substrate-binding protein msmE* | SGO_1305 | Sugar transport | 49 | 9 | 35.9 |
| Branched-chain amino acid ABC transporter* | SGO_1630 | Amino acid-binding protein | 41 | 9 | 25.3 |
| Conserved domain protein* | SGO_0018 | Transglycosylase-like domain | 21 | 8 | 25.5 |
| Elongation factor Tu tuf | SGO_0761 | Protein translation | 44 | 7 | 23.4 |
| Enolase eno | SGO_1426 | Glycolysis | 47 | 7 | 17.1 |
| Cell wall binding protein* | SGO_0845 | Glucan-binding domain | 34 | 7 | 12.8 |
| Cell shape-determining protein mreC* | SGO_2108 | Rod shape-determining protein | 31 | 7 | 29.6 |
| Penicillin-binding protein 1A pbp1a* | SGO_0586 | Penicillin binding protein transpeptidase domain |
79 | 6 | 11.7 |
| Putative uncharacterized protein | SGO_0591 | Unknown | 50 | 6 | 10.5 |
| SCP-like extracellular protein* | SGO_0847 | SCP-like extracellular protein domain | 56 | 6 | 14.9 |
| Putative uncharacterized protein | SGO_0332 | Unknown | 53 | 5 | 15.6 |
| Amino acid ABC transporter* | SGO_0982 | Amino acid-binding protein domain | 31 | 5 | 18.7 |
| Foldase protein prsA* | SGO_1572 | PPIase domain | 34 | 4 | 18.8 |
| Penicillin-binding protein 2× pbp2×* | SGO_0575 | Penicillin binding protein transpeptidase domain | 83 | 4 | 5.7 |
| Efflux transporter, RND family, MFP subunit family* |
SGO_0750 | RND family efflux transporter | 42 | 4 | 18.3 |
| Lipoprotein* | SGO_1165 | Unknown | 33 | 4 | 21.6 |
| Sortase A srtA* | SGO_1230 | Cell wall anchoring of LPXTG proteins (Nobbs, 2007) |
28 | 4 | 8.8 |
| LPXTG cell wall surface protein* | SGO_1415 | X-prolyldipeptidylaminopeptidase | 117 | 4 | 7.6 |
| Peptidyl-prolylcis-trans isomerase* | SGO_1463 | Peptidyl-prolylcis-trans isomerase | 29 | 4 | 12.7 |
| Metal ABC transporter substrate-binding lipoprotein* |
SGO_1802 | Metal ABC transporter | 35 | 4 | 22.6 |
| Elongation factor G fusA | SGO_0206 | Protein translation | 77 | 3 | 10.2 |
| Chaperone protein dnaK | SGO_0402 | Protein folding | 65 | 3 | 4.9 |
| L-lactate dehydrogenase ldh | SGO_1232 | L-lactate dehydrogenase | 35 | 3 | 9.1 |
| Putative lipoprotein* | SGO_0004 | Unknown | 20 | 3 | 17.4 |
| Possible cell wall protein* | SGO_0846 | SCP-like extracellular protein domain n | 76 | 3 | 5.2 |
| Glutamine ABC transporter permease and substrate binding protein* |
SGO_1037 | ABC transporter | 53 | 3 | 6.4 |
| Thioredoxin family protein | SGO_1171 | Thioredoxin | 18 | 3 | 31.2 |
| D-Alanyl-D-Alanine carboxypeptidase* | SGO_1585 | D-alanyl-D-alanine carboxypeptidase | 51 | 3 | 6.7 |
| 5′-nucleotidase, lipoprotein* | SGO_1860 | Haloaciddehalogenase-like hydrolases | 32 | 3 | 11.2 |
| Putative uncharacterized protein | SGO_1870 | Unknown | 65 | 3 | 5.1 |
| Putative uncharacterized protein* | SGO_1932 | Unknown | 22 | 3 | 24.6 |
| Lipoprotein* | SGO_2038 | Unknown | 17 | 3 | 21.4 |
| Lipoprotein* | SGO_0227 | Unknown | 23 | 3 | 8.1 |
| Arginine–tRNA ligase argS | SGO_2058 | Arginine–tRNA ligase | 63 | 3 | 6.9 |
| Putative peptidoglycan hydrolase and general stress protein* |
SGO_0212 | Putative peptidoglycan hydrolase | 23 | 3 | 12 |
| Putative uncharacterized protein pXO1 | SGO_0059 | Unknown | 11 | 2 | 35.8 |
| Putative uncharacterized protein | SGO_0080 | Unknown | 62 | 2 | 5.3 |
| Putative acyltransferase* | SGO_0112 | Putative acyltransferase | 68 | 2 | 3.5 |
| Putative uncharacterized protein* | SGO_0329 | Unknown | 69 | 2 | 5.4 |
| Aminodeoxychorismatelyase-like protein | SGO_0518 | Unknown | 60 | 2 | 3.3 |
| Pyruvate kinase pyk | SGO_1339 | Glycolysis | 55 | 2 | 7.4 |
| ABC transporter, substrate-binding protein* | SGO_1763 | ABC transporter | 54 | 2 | 7.6 |
| LPXTG cell wall surface protein, glycosyl hydrolase* |
SGO_0208 | Glycosyl hydrolase family 85 | 174 | 2 | 1.6 |
| Lipoprotein* | SGO_1931 | Unknown | 19 | 2 | 9.4 |
| ABC transporter, substrate-binding protein* | SGO_0457 | ABC transporter | 32 | 2 | 6.7 |
| Putative uncharacterized protein | SGO_0832 | Unknown | 13 | 2 | 26.3 |
| Penicillin-binding protein 2A pbp2A | SGO_2010 | Penicillin binding protein transpeptidase domain |
81 | 2 | 2.6 |
| Penicillin-binding protein 3 pbp3* | SGO_1717 | Penicillin-binding protein domain | 46 | 2 | 5.6 |
| Elongation factor Ts tsf | SGO_2000 | Protein translation | 37 | 2 | 7.2 |
| Putative uncharacterized protein | SGO_1756 | Unknown | 19 | 2 | 16.1 |
| Zinc proteinase* | SGO_2009 | Zinc metalloprotease | 34 | 2 | 7.9 |
Mol. Wt. kDa – Molecular weight in kDa (http://web.expasy.org/compute_pi/)
Pep. Id. – No. of peptides identified in MS/MS analysis
Aa. Seq. Cov. – % of the total aminoacid sequence covered by identified peptides
Proteins with N-terminal signal for secretion (http://www.predisi.de/home.html)
Table 2.
Differentially secreted proteins in KS1 and ΔAbpA strains.
| Protein/Gene Name | Gene Index | Predicted Function/Domain | Mol. Wt. kDa |
Pep.
Id. |
Aa. Seq.
Cov. (%) |
KS1 | ΔAbpA |
|---|---|---|---|---|---|---|---|
| Amylase-binding protein abpA* | SGO_2105 | Amylase-binding protein | 20 | 12 | 53.8 | + | − |
| Sortase B srtB* | SGO_2104 | Cell surface protein anchoring | 32 | 2 | 8.4 | + | − |
| Glutamate dehydrogenase gdh | SGO_0276 | Glutamate dehydrogenase | 49 | 3 | 8.7 | − | + |
| Metal binding permease adcA* | SGO_1936 | Metal transport | 56 | 2 | 7.2 | − | + |
| 60 kDa chaperonin groEL | SGO_1885 | Chaperone protein | 57 | 2 | 9.3 | − | + |
Mol. Wt. kDa–Molecular weight in kDa (http://web.expasy.org/compute_pi/)
Pep. Id.–No. of peptides identified in MS/MS analysis
Aa. Seq. Cov.–% of the total aminoacid sequence covered by identified peptides
Proteins with N-terminal signal for secretion (http://www.predisi.de/home.html)
It is notable that the majority of 107 proteins contained an N-terminal signal peptide. In fact, 72 proteins (67%) were predicted to have an N-terminal signal peptide for secretion typical for classically secreted proteins. The other 35 proteins (33%), which did not contain an N-terminal signal peptide, were likely of cytosolic origin or may be considered as non-classical secreted proteins (Table 1). Among the classically secreted proteins predicted to be cell wall surface associated, 11 proteins contained a consensus C-terminal motif Leu-Pro-X-Thr-Gly (LPXTG) cell wall anchor motif and 9 proteins lacked this motif.
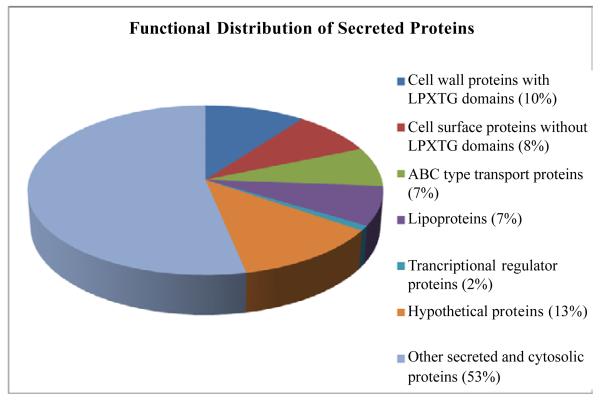
The majority of proteins that have been identified in the secretome of S. gordonii represent proteins observed in the stationary phase of other gram-positive bacteria. Even in rich medium during the stationary phase of growth nutrient limitation and decrease in pH can trigger various stress responses [19]. In Streptococci pyogenes, transition to early stationary phase was associated with acidification of the growth medium to approximately pH 5.5 and a depletion of glucose [20]. Stationary phase cytoplasmic proteins of S. pyogenes grown in Todd-Hewitt broth showed an abundance of enzymes involved in glycolysis and pyruvate metabolism, as well as stress-responsive proteins [21]. An increase in enzymes in central metabolism is thought to enhance the ability to scavenge carbohydrates [22]. Thus, proteins associated with energy production, carbohydrate transport, amino acid transport, lipid transport, nucleotide transport, cell wall biogenesis, protein turnover, and posttranslational modification are expected to be more abundant. In this study, during the stationary phase of growth, we found a significant number of secreted proteins, lipoproteins, ABC type transporter proteins and proteins related to cell wall biogenesis as part of the secretome. The overall functional distribution of the secretome proteins is listed in Figure 1.
Figure 1.
Functional distribution of proteins in whole secretome of Streptococcus gordonii.
Consistent with the silver stained gel, a total of 102 proteins were found in common to both the KS1 and ΔAbpA secretomes (Table 1). Only five proteins were found to be consistently differentially expressed based on the analyses of secretome samples from two independent experiments (Table 2). AbpA and sortase B were identified in the KS1 secretome fraction by LC-MS/MS analysis, whereas they were absent in the ΔAbpA strain. AbpB and GtfG were present in both strains indicating that their secretion was not affected by inactivation of abpA. Glutamate dehydrogenase (SGO_0276), metal-binding (Mn) permease lipoprotein (SGO_1936), and the 60-kDa chaperonin (groEL) (SGO_1885) were identified in the ΔAbpA secretome while they were absent in the KS1 strain (Table 2).
AbpA and sortase B (SrtB) were differentially expressed among the secretome proteins in KS1 and ΔAbpA. The absence of AbpA protein in the secretome of ΔAbpA confirms its inactivation. SrtB, a transpeptidase that anchors proteins to the cell wall peptidoglycan through a NXZTN sorting motif [23], was also absent in the secretome of ΔAbpA. The srtB gene locus is downstream of abpA in the S. gordonii Challis (CH1) genome. The absence of SrtB in the secretome of ΔAbpA suggests co-transcription of abpA and srtB. This is also supported by recent transcriptome analysis, which showed that srtB was significantly downregulated in ΔAbpA grown to mid-log phase in TSBY (unpublished data). Considering that abpA and srtB may be transcribed as a polycistronic message, the insertion of the tetracycline resistance gene tet(M), which contains a weak terminator, into abpA may have caused a polar effect on the downstream srtB gene.
Other differentially secreted proteins, including glutamate dehydrogenase, metal-binding (Mn) permease lipoprotein AdcA (SGO_1936), and chaperonin (GroEL) were identified in ΔAbpA but were absent in KS1. These proteins are non-classical secreted proteins, as they lack the N-terminal signal peptide targeting a protein for secretion. Each of these proteins has been shown to play a role in resistance to stress in various organisms. Glutamate dehydrogenase is a housekeeping enzyme in bacteria that functions in nitrogen metabolism as part of the urea cycle by converting glutamate to oxoglutarate. It is usually found in the cytoplasm and the cell membrane. It has been shown that glutamate dehydrogenase functions in providing resistance to stress in yeast cells [24]. In Clostridium difficile, extracellular glutamate dehydrogenase was found to confer resistance to hydrogen peroxide [25]. Metal-binding (Mn) permease lipoproteins may act as surface ligands for host cell matrix proteins [26]. Also, metal-binding (Mn) permease lipoprotein/AdcA belongs to the TroA superfamily of metal binding lipoproteins and may be involved in the sequestration of metal ions like manganese. A TroA knockout in Streptococcus suis resulted in manganese deprivation and reduced virulence in a mouse model [27]. Susceptibility of the TroA mutant to hydrogen peroxide indicated that TroA could be playing a role in oxidative stress [27]. The role of AdcA in S. gordonii remains to be determined.
GroEL is a 60-kDa chaperonin protein that is involved in protein folding in bacterial secretion systems [28]. GroEL is highly conserved among bacterial species and is also used for differentiating various streptococci [29]. Interestingly, it is also a protein that is upregulated along with DnaK under general stress response [30]. GroEL functions to allow unfolded proteins produced by stress to fold and prevent aggregation with other proteins [31]. It is frequently found in secretomes of other bacteria under various stress conditions such as increased oxygen tension and acidic pH [32-34]. It is possible that AbpA may have functions independent of amylase binding that may elicit changes in response to stress.
Amylase-precipitated proteins
Previous studies in our lab identified S. gordonii proteins that interacted with salivary amylase. AbpA (20 kDa), AbpB (82 kDa) and glucosyltransferase G (GtfG; 174 kDa) were the predominant proteins precipitated by amylase from culture supernatants of wild-type strains based on SYRO Red staining of 12% SDS-PAGE gels and MALDI-TOF analysis of eluted proteins [11,12]. In the current study, an additional 5-6 protein bands were revealed by silver stain in the amylase-precipitated protein samples (Figure 2). Silver staining is very sensitive and aids in visualizing proteins even at picogram levels. To identify these proteins, bands were sliced from a duplicate Coomassie-stained gel (Figure 3), as described above, and subjected to nano-LC/MS/MS-analysis. Based on the MS analysis, several proteins were identified in each of the sliced gel bands.
Figure 2. Silver stained 10% SDS-PAGE gel showing amylase-precipitated proteins in various strains of S. gordonii strains.
Lanes: 1-CH1 (WT), 2-KS1 (kanamycin resistant WT), 3-ΔAbpA, 4-ΔAbpB and 5-amylase (control).
The amylase-precipitated proteins identified in KS1 and ΔAbpA are listed in Table 3. AbpA was excluded from the analysis, as no bands were sliced below 40 kDa. Interestingly AbpB and GtfG were not detected in the amylase precipitates of KS1 contrary to previous studies [11,12]. This could be due to several reasons. Growth of cultures to late stationary phase could have degraded some AbpB and GtfG resulting in very low quantities to be precipitated by amylase. Also, the abundant presence of several other amylase-binding proteins could have out-competed the binding of AbpB and GtfG. This is evident by the fact that both AbpB and GtfG were identified by mass spectrometric analysis of TCA-precipitated whole secretome protein fractions. The method of detection can also have a major influence on what is detected. The method of detection in our previous analysis is significantly different from the methods used in this study [11,12]. Previously, after amylase precipitation, the whole protein pellet was solubilized in water and run on a 12% SDS PAGE gel and stained with SYPRO Red. AbpA and GtfG were then identified by N-terminal sequencing of blots. In this study, the resulting pellet following amylase precipitation was solubilized in 1% SDS and boiled for 10 mins. Proteins were quantified after adding SDS containing buffer with a detergent compatible protein assay. Only 10-15 μg protein per lane was loaded on 10% SDS-PAGE gels and silver staining was performed for visualization. Duplicate gels were Coomassie stained and gel slices were submitted for MS analysis. Furthermore, as previously demonstrated, it is not necessary that all of the identified amylase-precipitated proteins bind directly to amylase. GtfG was absent from amylase-precipitated culture supernatants from the AbpA-deficient strain, suggesting that AbpA interacted with and precipitated GtfG [12]. Likewise, the primary proteins like AbpA and AbpB could be bound to amylase and the remaining proteins could be bound to these primary proteins forming a complex of amylase precipitated proteins. Considering that a large number of proteins are potentially part of this amylase-precipitated complex, it is to be determined as to what their exact role would be.
Table 3.
MS/MS analysis of amylase-precipitated proteins from KS1 and ΔAbpA. Underlined proteins are differentially precipitated.
| Bands | KS1 | ΔAbpA |
|---|---|---|
| Band 1 | 50S ribosomal protein L5 Acetyltransferase Hypothetical protein SGO_0483 (Non- secretory) |
50S ribosomal protein L5 |
| Band 2 | 50S ribosomal protein L5 Acetyltransferase Argininosuccinate synthase Cation-transporting ATPase yfgQ (T3 secretion) |
50S ribosomal protein L5 |
| Band 3 | 50S ribosomal protein L5 Isopropylmalateisomerase large subunit Isocitrate dehydrogenase Sortase A-LPXTG cell wall surface protein Beta-N-acetylhexosaminidase (Secreted protein) |
Isopropylmalateisomerase large subunit 50S ribosomal protein L5 |
| Band 4 | 50S ribosomal protein L5 Argininosuccinate synthase Peptide chain release factor 1 |
50S ribosomal protein L5 Acetyltransferase |
| Band 5 | 50S ribosomal protein L5 General stress protein GSP-781 Cardiolipin synthase (Non-secretory) Hypothetical protein SGO_0483 (Non- secretory) |
50S ribosomal protein L5 General stress protein GSP-781 |
Some of the other amylase-precipitated proteins were expressed in the KS1 strain, but absent in the ΔAbpA strain (Table 3). No proteins unique to ΔAbpA were identified. The classically secreted proteins differentially expressed in KS1 included: cation-transporting ATPase YfgQ (T3 secretion) (SGO_1458), sortase A (SGO_1230), and beta-N-acetylhexosaminidase (SGO_0405). The non-classical secreted proteins identified in KS1 included: hypothetical protein (SGO_0483), isocitrate dehydrogenase (SGO_1611), and cardiolipin synthase (SGO_2037). YfgQ is a putative protein with a conserved cation-transporting ATPase domain whose exact function is not known. Sortase A is an enzyme that is involved in cross-linking proteins to the cell wall in gram-positive bacteria. Sortase A recognizes the LPXTG motif present at the N-terminal of cell wall proteins and anchors them to the cell wall [35]. Beta-N-acetylhexosaminidases of glycosyl hydrolase family 20 (GH20) catalyze the removal of beta-1,4-linked N-acetyl-D-hexosamine residues from the non-reducing ends of N-acetyl-beta-D-hexosaminides including N-acetylglucosides and N-acetylgalactosides. These enzymes are broadly distributed in microorganisms, plants and animals, and play roles in various key physiological and pathological processes [36].
The non-classical secreted proteins-hypothetical protein (SGO_0483), isocitrate dehydrogenase (SGO_1611), and cardiolipin synthase (SGO_2037) were identified in the KS1 strain and were absent in the ΔAbpA strain. Isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to alpha-ketoglutarate and can use either NAD(+) or NADP(+) as a cofactor. Recent studies demonstrate that the NADP(+)-dependent isocitrate dehydrogenase, as a source of electrons for cellular antioxidants, is important for protection against oxidative damage [37]. Cardiolipin synthase has an important role in modulating the physical properties of membranes in response to environmental changes. It promotes formation of membrane subdomains that can serve to localize and regulate assembly of protein complexes, which is critical for cell division and membrane transport [38].
Most of the non-classical proteins present in either the whole secretome or those precipitated by amylase usually function in the cytoplasm. While it cannot be ruled out that some of these non-classical secreted proteins may have originated from cell lysis, they may be secreted by other mechanisms such as membrane vesicles or specific membrane microdomains [39-41]. The redox protein thioredoxin, which lacks an N-terminal signal peptide, was found here in the whole secretomes of both KS1 and ΔAbpA, as well as in the secretomes of mammals, plants, and other bacteria [42-44]. Other proteins that include glycolytic pathway enzymes like enolase, membrane associated proteins and chaperone proteins can elicit strong immunogenicity and take part in virulence and pathogenesis of disease [42]. The role of thioredoxin and other secreted proteins lacking N-terminal signal peptides in the extracellular space may be similar to the cytoplasmic function or have a yet unrecognized function.
Summary and Conclusions
We have identified a set of more than 107 proteins in the secretome of S. gordonii for the first time. Some of these proteins are differentially expressed in the ΔAbpA strain as compared to the KS1 strain (wild type) indicating that AbpA may possibly regulate their expression in stationary phase. Some of these differentially expressed proteins include AdcA and GroEL, which are involved in oxidative stress response. Further studies are required to determine the extracellular role of these proteins. By using mass spectrometric analysis we were able to identify the previously uncharacterized amylase-precipitated proteins. It appears that apart from AbpA, AbpB and GtfG there are several other proteins that could bind to salivary amylase (or AbpA), although it is not clear whether this binding is direct or indirect. Future work will focus on the effect of amylase and starch on protein secretion by S. gordonii.
Submit your next manuscript and get advantages of OMICS Group submissions.
Unique features
User friendly/feasible website-translation of your paper to 50 world’s leading languages
Audio Version of published paper
Digital articles to share and explore
Special features
350 Open Access Journals
30,000 editorial team
21 days rapid review process
Quality and quick editorial, review and publication processing
Indexing at PubMed (partial), Scopus, EBSCO, Index Copernicus and Google Scholar etc
Sharing Option: Social Networking Enabled
Authors, Reviewers and Editors rewarded with online Scientific Credits
Better discount for your subsequent articles
Acknowledgments
We would like to thank Yuko Ogata, Ph.D. at the Fred Hutchinson Cancer Research Center for her expert technical assistance in proteomic analysis. This study was funded by NIDCR grant no. DE-022673.
References
- 1.Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 2.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- 4.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–1607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- 5.Scannapieco FA. Saliva-bacterium interactions in oral microbial ecology. Crit Rev Oral Biol Med. 1994;5:203–248. doi: 10.1177/10454411940050030201. [DOI] [PubMed] [Google Scholar]
- 6.Douglas CW. The binding of human salivary alpha-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28:567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- 7.Scannapieco FA, Bergey EJ, Reddy MS, Levine MJ. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989;57:2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilian M, Nyvad B. Ability to bind salivary alpha-amylase discriminates certain viridans group streptococcal species. J Clin Microbiol. 1990;28:2576–2577. doi: 10.1128/jcm.28.11.2576-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwynn JP, Douglas CW. Comparison of amylase-binding proteins in oral streptococci. FEMS Microbiol Lett. 1994;124:373–379. doi: 10.1111/j.1574-6968.1994.tb07311.x. [DOI] [PubMed] [Google Scholar]
- 10.Scannapieco FA, Haraszthy GG, Cho MI, Levine MJ. Characterization of an amylase-binding component of Streptococcus gordonii G9B. Infect Immun. 1992;60:4726–4733. doi: 10.1128/iai.60.11.4726-4733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Tanzer JM, Scannapieco FA. Identification and analysis of the amylase-binding protein B (AbpB) and gene (abpB) from Streptococcus gordonii. FEMS Microbiol Lett. 2002;212:151–157. doi: 10.1111/j.1574-6968.2002.tb11259.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri B, Rojek J, Vickerman MM, Tanzer JM, Scannapieco FA. Interaction of salivary alpha-amylase and amylase-binding-protein A (AbpA) of Streptococcus gordonii with glucosyltransferase of S. gordonii and Streptococcus mutans. BMC Microbiol. 2007;7:60. doi: 10.1186/1471-2180-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhuri B, Paju S, Haase EM, Vickerman MM, Tanzer JM, et al. Amylase-binding protein B of Streptococcus gordonii is an extracellular dipeptidyl-peptidase. Infect Immun. 2008;76:4530–4537. doi: 10.1128/IAI.00186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers JD, Haase EM, Brown AE, Douglas CW, Gwynn JP, et al. Identification and analysis of a gene (abpA) encoding a major amylase-binding protein in Streptococcus gordonii. Microbiology. 1998;144:1223–1233. doi: 10.1099/00221287-144-5-1223. [DOI] [PubMed] [Google Scholar]
- 15.Rogers JD, Palmer RJ, Jr., Kolenbrander PE, Scannapieco FA. Role of Streptococcus gordonii amylase-binding protein A in adhesion to hydroxyapatite, starch metabolism, and biofilm formation. Infect Immun. 2001;69:7046–7056. doi: 10.1128/IAI.69.11.7046-7056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikitkova AE, Haase EM, Vickerman MM, Gill SR, Scannapieco FA. Response of fatty acid synthesis genes to the binding of human salivary amylase by Streptococcus gordonii. Appl Environ Microbiol. 2012;78:1865–1875. doi: 10.1128/AEM.07071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikitkova AE, Haase EM, Scannapieco FA. Taking the starch out of oral biofilm formation: molecular basis and functional significance of salivary alpha-amylase binding to oral streptococci. Appl Environ Microbiol. 2013;79:416–423. doi: 10.1128/AEM.02581-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanzer JM, Thompson A, Sharma K, Vickerman MM, Haase EM, et al. Streptococcus mutans out-competes Streptococcus gordonii in vivo. J Dent Res. 2012;91:513–519. doi: 10.1177/0022034512442894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Len AC, Harty DW, Jacques NA. Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiology. 2004;150:1339–1351. doi: 10.1099/mic.0.27008-0. [DOI] [PubMed] [Google Scholar]
- 20.Chaussee MS, Somerville GA, Reitzer L, Musser JM. Rgg coordinates virulence factor synthesis and metabolism in Streptococcus pyogenes. J Bacteriol. 2003;185:6016–6024. doi: 10.1128/JB.185.20.6016-6024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaussee MA, Dmitriev AV, Callegari EA, Chaussee MS. Growth phase-associated changes in the transcriptome and proteome of Streptococcus pyogenes. Arch Microbiol. 2008;189:27–41. doi: 10.1007/s00203-007-0290-1. [DOI] [PubMed] [Google Scholar]
- 22.Harder W, Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- 23.Bierne H, Garandeau C, Pucciarelli MG, Sabet C, Newton S, et al. Sortase B, a new class of sortase in Listeria monocytogenes. J Bacteriol. 2004;186:1972–1982. doi: 10.1128/JB.186.7.1972-1982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YJ, Kim KJ, Kang HY, Kim HR, Maeng PJ. Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells. J Biol Chem. 2012;287:44221–44233. doi: 10.1074/jbc.M112.375360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girinathan BP, Braun SE, Govind R. Clostridium difficile glutamate dehydrogenase is a secreted enzyme that confers resistance to H2O2. Microbiology. 2014;160:47–55. doi: 10.1099/mic.0.071365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cellier M. Bacterial genes controlling manganese accumulation. Wiley-VCH; Weinheim: 2001. [Google Scholar]
- 27.Wichgers Schreur PJ, Rebel JM, Smits MA, van Putten JP, Smith HE. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol. 2011;193:5073–5080. doi: 10.1128/JB.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moparthi SB, Sjölander D, Villebeck L, Jonsson BH, Hammarström P, et al. Transient conformational remodeling of folding proteins by GroES-individually and in concert with GroEL. J Chem Biol. 2013;7:1–15. doi: 10.1007/s12154-013-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng LJ, Hsueh PR, Tsai JC, Chen PW, Hsu JC, et al. groESL sequence determination, phylogenetic analysis, and species differentiation for viridans group streptococci. J Clin Microbiol. 2002;40:3172–3178. doi: 10.1128/JCM.40.9.3172-3178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zotta T, Ricciardi A, Ciocia F, Rossano R, Parente E. Diversity of stress responses in dairy thermophilic streptococci. Int J Food Microbiol. 2008;124:34–42. doi: 10.1016/j.ijfoodmicro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Ellis RJ. Chaperomics: in vivo GroEL function defined. Curr Biol. 2005;15:R661–R663. doi: 10.1016/j.cub.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Chitlaru T, Gat O, Grosfeld H, Inbar I, Gozlan Y, et al. Identification of in vivo-expressed immunogenic proteins by serological proteome analysis of the Bacillus anthracis secretome. Infect Immun. 2007;75:2841–2852. doi: 10.1128/IAI.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pumirat P, Saetun P, Sinchaikul S, Chen ST, Korbsrisate S, et al. Altered secretome of Burkholderia pseudomallei induced by salt stress. Biochim Biophys Acta. 2009;1794:898–904. doi: 10.1016/j.bbapap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Vayssier C, Mayrand D, Grenier D. Detection of stress proteins in Porphyromonas gingivalis and other oral bacteria by western immunoblotting analysis. FEMS Microbiol Lett. 1994;121:303–307. doi: 10.1111/j.1574-6968.1994.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 35.Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slámová K, Bojarová P, Petrásková L, Kren V. β-N-acetylhexosaminidase: what’s in a name..? Biotechnol Adv. 2010;28:682–693. doi: 10.1016/j.biotechadv.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, Park JW. Cellular defense against singlet oxygen-induced oxidative damage by cytosolic NADP+-dependent isocitrate dehydrogenase. Free Radic Res. 2003;37:309–316. doi: 10.1080/1071576021000050429. [DOI] [PubMed] [Google Scholar]
- 38.Davlieva M, Zhang W, Arias CA, Shamoo Y. Biochemical characterization of cardiolipin synthase mutations associated with daptomycin resistance in enterococci. Antimicrob Agents Chemother. 2013;57:289–296. doi: 10.1128/AAC.01743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosch J, Caparon M. A microdomain for protein secretion in Gram-positive bacteria. Science. 2004;304:1513–1515. doi: 10.1126/science.1097404. [DOI] [PubMed] [Google Scholar]
- 40.Sumei L, Klein MI, Heim KP, Fan Y, Bitoun JP, et al. Streptococcus mutans eDNA is up-regulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol. 2014 doi: 10.1128/JB.01493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KJ, Bae SM, Lee MR, Yeon SM, Lee YH, et al. Proteomic analysis of growth phase-dependent proteins of Streptococcus pneumoniae. Proteomics. 2006;6:1274–1282. doi: 10.1002/pmic.200500415. [DOI] [PubMed] [Google Scholar]
- 42.Choi CW, Lee YG, Kwon SO, Kim HY, Lee JC, et al. Analysis of Streptococcus pneumoniae secreted antigens by immuno-proteomic approach. Diagn Microbiol Infect Dis. 2012;72:318–327. doi: 10.1016/j.diagmicrobio.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Maddi A, Bowman SM, Free SJ. Trifluoromethanesulfonic acid-based proteomic analysis of cell wall and secreted proteins of the ascomycetous fungi Neurospora crassa and Candida albicans. Fungal Genet Biol. 2009;46:768–781. doi: 10.1016/j.fgb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Dang Y, Wang X, Lu H, Wang X, et al. Comparative proteomic analyses of Streptococcus suis serotype 2 cell wall-associated proteins. Curr Microbiol. 2011;62:578–588. doi: 10.1007/s00284-010-9747-6. [DOI] [PubMed] [Google Scholar]