Abstract
Externalizing behaviors (encompassing antisocial, impulsive, and substance use behaviors) are pervasive and impairing across a multitude of settings and developmental contexts. These behaviors, though often investigated separately, are highly comorbid. Prenatal tobacco exposure in interaction with various genetic influences has predicted later externalizing behavior, and recent evidence supports investigating sex differences in these patterns. In the current study, we extend this work by (a) examining two functional genetic markers in the dopamine system: the transporter gene (DAT1) and the dopamine receptor D4 gene (DRD4) in interaction with prenatal tobacco exposure to predict a latent composite of externalizing behavior and (b) testing whether these patterns differ by sex of youth in a community sample of adolescents (n=176). The relatively small sample is partially offset by high quality, multi-method prospective measurement. We assessed prenatal tobacco exposure using prospective repeated cotinine-corrected reports and externalizing behaviors were assessed utilizing multiple measures across three waves. The interaction between DAT1 (but not DRD4) and prenatal tobacco exposure was statistically significant in boys, and patterns appeared to differ by sex. Risk for externalizing behaviors for exposed boys increased linearly as a function of the 10r DAT1 allele. For exposed girls, there was a trend such that DAT1heterozygotes had a marginally higher risk than homozygotes. This pattern was not explained by passive gene-environment correlation. Elucidating sex-specific pathways through which early adverse exposures and genetic susceptibilities contribute to externalizing behavior can inform early targeted prevention efforts for those children at highest risk.
Keywords: Prenatal tobacco exposure, DAT1, DRD4, gene × environment, externalizing, developmental psychopathology
1. Introduction
Prenatal tobacco exposure (PTE) can cause developmental disruptions of catecholaminergic systems in the fetal brain, particularly the dopamine system, which lead to long-term behavioral problems (Dwyer et al., 2009; Ernst et al., 2001; Navarro et al., 1988). The chemicals in cigarette smoke directly influence fetal development and PTE constricts uteroplacental vessels and creates a hypoxia that results in later hyper-innervation in key systems leading to behavioral disruption (Fried, 1995; Ikemoto, 2007; Navarro et al., 1988; Schultz, 2007; Toro et al., 2008). Experiments with animal models have allowed for separation of potential confounds such as socioeconomic status (SES) from the effects of PTE and furthered biological study via postmortem examinations. In humans, the long-term consequences of PTE and the potential interaction with specific genes are less well-defined, in part due to the challenges of measuring PTE in context with other relevant genetic and environmental influences on behavior.
In the current study, we focus on two dopamine system genes. DAT1 functions to clear dopamine from synapses and has a variable number tandem repeat (VNTR) in the 3’ region with common 9 and 10 repeat variants (9r, 10r). DRD4 contains a 48 base pair VNTR (common repeats: 4r and 7r) on the third exon (Schmidt et al., 2001). These two VNTRs show genetic links to externalizing behaviors (Cook et al., 1995; Gizer et al., 2009; Swanson et al., 2000; Turic et al., 2010). DAT1 interacts with PTE to predict higher rates of attention deficit hyperactive disorder (ADHD) in male adolescents (Becker et al., 2008) and oppositional defiant disorder (ODD) in preschool-aged PTE children (Kahn et al., 2003). DAT1 and DRD4 increase susceptibility to severe ADHD in PTE children (Neuman et al., 2007). One shortcoming of the aforementioned studies is that PTE is often measured retrospectively via maternal report. Wiebe and colleagues (2009) identified an interaction between the dopamine receptor D2 and biologically-measured PTE to predict infant novelty responses. This detailed level of measurement is rare. Utilizing high-quality prospective “best estimate” measures of exposure, we previously demonstrated that monoamine oxidase A (MAOA) genotypes modify exposure pathways differently by child sex: low activity MAOA increased risk in PTE boys whereas high activity MAOA increased risk in PTE girls (Wakschlag et al., 2010).
The existing G × E literature commonly uses either an additive (zero, one or two DAT1 10r or DRD4 7r) or dominant coding system (presence/absence of the 10r/7r) for these genes, which presupposes that more of a certain variant confers risk or protection. An interesting alternative is the heterozygote advantage/disadvantage (heterosis) model, which been demonstrated across animal and human studies for a variety of genes (for a review see Comings & MacMurray, 2000). Previous studies have shown a potential heterozygote advantage/disadvantage in relation to dopamine system functioning. Lee and colleagues (2007) demonstrated that DAT1 9r/10r heterozygotes displayed the most disruptive behavior in an early childhood sample; though their sample was not conducive to testing sex differences. DAT1 homozygous genotypes, conversely, relate to increased inattention to left-side stimuli (a marker for ADHD; Bellgrove et al., 2007), indicating that further research is needed comparing heterosis models to additive models.
In the current study, we expand our examination of sex differences in gene × exposure interaction by focusing on dopamine system genes and testing both additive genetic and heterosis models. We also take a spectrum approach to externalizing behavior, which enhances linkage to mechanisms, has ecological validity, and yields a highly heritable component of behavior (Farmer et al., 2009; Krueger et al., 2002; 2007). We have found that this approach captures phenotypic patterns well (McGrath et al., 2012). While our sample is fairly small relative to many G × E studies, this limitation is partially offset by the high quality measurement of exposure (prospective, repeated measure and includes biologic measures) and of externalizing behavior (multi-faceted, repeated-measures) and rigorous control for gene-environment correlation (rge).
2. Methods
2.1. Participant information
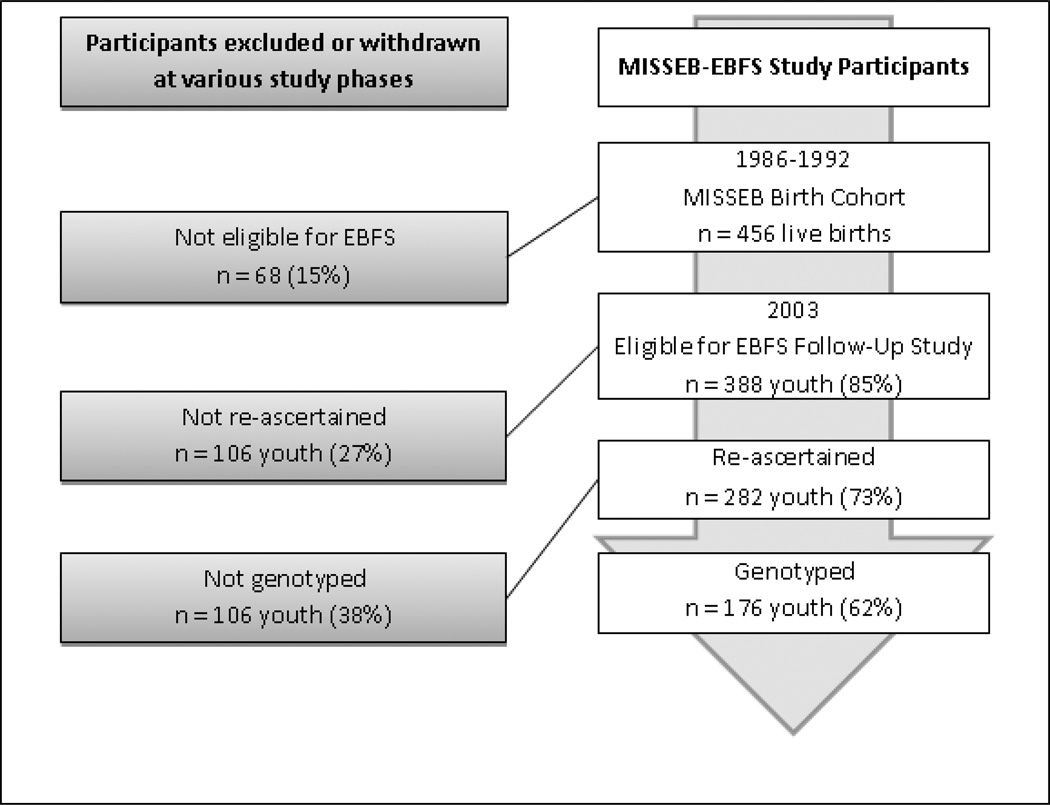
Participants were originally selected as part of a prospective pregnancy cohort of non-Hispanic white women, oversampled for PTE, from a neighborhood clinic in East Boston who participated in the Maternal Infant Smoking Study of East Boston (MISSEB) between 1986 and 1992. Women were initially eligible for the study if they were < 20 weeks pregnant, at least 19 years of age, and attended the targeted East Boston neighborhood health clinic (Hanrahan et al., 1992). An adolescent follow-up (East Boston Family Study; EBFS) was conducted on 282 youth (54% female), ranging in age from 11 to 18 (M = 14.45, SD = 1.74). Hispanic MISSEB participants were excluded because of very low rates of pregnancy smoking, non-White MISSEB participants were excluded from genotyping to avoid participation stratification. Additional EBFS eligibility criteria were delivery of a live infant and participation in a perinatal follow-up wave of MISSEB. Seventy-three percent of eligible youth were ascertained for EBFS and were followed across three annual waves (see Figure 1). Genotyping was conducted on 176 of the non-Hispanic White EBFS sample in Wave 2.
Figure 1.
EBFS study timeline
The EBFS cohort did not differ from the eligible non-participants in terms of maternal smoking status; however, EBFS mothers were more highly educated (75% vs 62% high school completion; χ2 (1) = 6.0, p < .01) and slightly older at time of pregnancy (27 vs 26 years; t(346) = 2.50, p < .01) than mothers who did not participate. The EBFS G × E sub-study did not differ from EBFS eligible non-participants on relevant demographics. Mothers were predominantly working class (mean income $40–50,000 a year).The institutional review board approval at the Beth Israel Deaconess Medical Center in Boston granted study approval. Mothers and youth provided written consent to participate in the study.
2.2. Prenatal exposure
Initial assessment of prenatal exposure took place between 10 to 27 weeks gestation; with 21% of initial assessments occurring in the first trimester. The mean and standard deviation of gestational age at the first pregnancy visit was 15.7 ± 3.6 weeks. Variation is explained by differences in when women sought initial prenatal care. At their first visit, women were asked about current and lifetime smoking status and habits, including the number of cigarettes smoked per day. At each subsequent visit (M = 6 visits, range = 1–12), women were interviewed about current smoking habits. Blood samples were also collected, typically only at the first visit. The majority of the sample (89.9% of women) provided blood samples, most once (59.8%) though some more often (19.1% gave on two visits, 6.3% three visits, 2.5% four visits, .9% five visits, and .1% gave on both six and seven visits). Radioimmunoassay was used to ascertain cotinine levels. Serum cotinine levels from blood were combined with self-reported smoking to form a ‘best-estimate’ measure of exposure (Dukic et al., 2007).
To arrive at this best-estimate, data from the different prenatal visits were combined via hierarchical modeling of cotinine metabolism. Bayesian methods were applied that took into account reported amount smoked, average clearance rates for pregnancy smoking and individual cotinine level. This technique was employed to determine a cotinine-based correction factor by correcting for the individual-level exponential-decay in the blood of each woman, as a function of time since last cigarette and self-reported smoking pattern using methods developed by Dukic et al (2007). This approach corrects for underreporting and nondisclosure. Larger individual deviations from the exponential-decay model indicated less accuracy in maternal report, and scores were adjusted accordingly. The cotinine-calibration method algorithm adjusted maternal self-report, on average, + 2.62 cigarettes per day (SD = 3.25, range = 0.32 – 9.24). This yielded a normally-distributed continuous measurement of PTE (M = 6.88, SD = 8.67). Forty-eight percent of the sample was exposed and the average exposure was over half a pack: M (SD) = 14.29 (7.11) cigarettes per day. Exposure by trimester showed little variability (rs > .90), and so we did not examine trimester-specific exposure influences.
2.3. Externalizing behavior
The ADHD, ODD, conduct disorder (CD), and substance use (nicotine, alcohol, and marijuana) scales from the Diagnostic Interview for Children (C-DISC-IV; Shaffer et al., 2000) were assessed at three separate times using continuous symptom scores from both mother and youth report. The DISC allows primarily for “yes/no” answer choices, with an occasional “sometimes” or “somewhat” response and is designed to identify symptoms occurring within the past four weeks or past 12 months. The DISC was supplemented by the Antisocial Behavior Checklist, a delinquency measure (ASBC; Zucker et al., 1994) ascertained from youth at each assessment time. Reporters used a four point scale (1 = never; 4 = often) to answer questions such as “Have you ever cheated in school?” and “Have you ever sold marijuana or other illegal drugs?” Most scales displayed some extent of right skew, and so raw scales were summed, transformed to their normally-distributed natural log, and used as indicators of externalizing. These transformed indicators improved model fit, but did not change the pattern of results.
2.4. Covariates
Mother and youth age, SES, prenatal alcohol and drug use, maternal and paternal antisocial behavior and harsh parenting were all considered for inclusion as covariates to explain externalizing behavior. See Table 1 for descriptive characteristics of the sample measures and covariates.
Table 1.
Sample Characteristics and Descriptives
| Covariates | Mean (SD) | Range |
|---|---|---|
| Maternal Age | 42.56 (5.89) | 32–59 |
| Youth Age | 15.4 (1.77) | 11–18 |
| Socioeconomic Status | 0.00 (0.68) | −2.00 – 1.28 |
| Harsh Parenting | −0.01 (0.16) | −0.25–0.58 |
| Maternal Antisocial Behavior | 12.87 (10.46) | 0–66 |
| Paternal Antisocial Behavior | 18.32 (1.77) | 0–66 |
| % of youth who are girls | 54% | |
| Prenatal Drug Exposure | 23% | |
| % used illegal substances | ||
| Prenatal Alcohol Exposure: | ||
| % drank, but < 2 drinks/sitting | 32% | |
| % drank, 2 or > drinks/sitting | 25% | |
| Outcomes | Girls Mean (SD) |
Boys Mean (SD) |
| Average Antisocial/CD symptoms (3 waves) | 3.39(2.92) | 4.23(3.60)a |
| Average ODD symptoms (3 waves) | 4.90(2.07) | 4.88(2.39) |
| Average ADHD symptoms (3 waves) | 6.07(3.90) | 6.07(3.65) |
| Average substance use symptoms (3 waves) | 1.70(5.00) | 1.29(3.84) |
Note. All averages are a combination of parent and youth report. Harsh parenting factor score based on maternal self-report and youth report of maternal and paternal parenting.
CD = Conduct Disorder, ODD = Oppositional Defiant Disorder, ADHD = Attention Deficit Hyperactive Disorder;
Boys exhibited marginally more antisocial/CD symptoms than girls: t(178) = −1.82, p < .10.
2.4.1. Socioeconomic status
We examined maternal employment (67% employed outside the home), education (75% completed high school) and total household income (M = $40–50,000). Education level did not correlate significantly with total household income, but all other correlations were significant. Z scores of the three variables were mean composited to form an index of SES.
2.4.2. Other substances
Prenatal alcohol and drug use were controlled for in order to disentangle general substance use during pregnancy from the relations between PTE and child outcomes. Maternal self-report of alcohol and drug use was collected at each prenatal assessment. Alcohol use was assessed by maternal report on the 51-item Michigan Alcoholism Screening Test (MAST; Zung, 1982); scores higher than five indicate alcoholism (19.3% of the sample). Maternal drug use was also self-reported on the 28-item Drug Abuse Screening Test (DAST; Skinner, 1982); scores higher than six indicate drug misuse (17.8% of the sample). Categorical variables were created for both substances, drawing from all available prenatal assessments for alcohol (never drank, drank but never more than two drinks, drank two or more drinks) and drug use (never used vs. used). See Table 1 for frequencies of these categorical variables.
2.4.3. Genetic liabilities
We accounted for rGE, in two ways: (1) we included parental antisocial history and harsh parenting in multivariate models to index genetic liability for externalizing behaviors and (2) we tested whether prenatal smoking status varied by maternal DAT1 or DRD4 genotype as a direct test of whether women with a risk genotype for antisocial behavior were more likely to persist in pregnancy smoking than women without that risk genotype. Mothers reported on their own behavior on the Antisocial Behavior Checklist (ASBC; Zucker et al., 1994). This is a 45-item measure that assesses current delinquent behaviors and retrospective childhood delinquent behaviors. Mothers’ reported on fathers lifetime history of aggressive and delinquent behaviors with questions from the Adult Behavior Checklist (Achenbach, 1997) and the Antisocial Personality Items of the Diagnostic Interview Schedule (DIS-IV; Robins, Cottler, Bucholz & Compton, 1995). These questions were specifically validated for maternal report of fathers’ behavior (Caspi et al., 2001) and scale scores were standardized and composited to form paternal antisocial behavior. Mothers used a four point scale (1 = never; 4 = often) to answer items such as: “Hit your spouse/partner during an argument,” and “Had intercourse with more than one person in a single day.” The total scores were normally distributed (skew < 2, kurtosis < 7). Cronbach’s α exceeded .75 for mothers’ report of both parents antisocial behavior.
Harsh discipline was a mean composite of mother and youth report on their relationship using the 17-item Punitive Discipline subscale of the Hetherington Discipline Scale (Hetherington et al., 1992). Participants were asked to respond on a seven point scale (1 = more than once a day; 7 = 0 times in the last month). Mother and youth report were both normally distributed and significantly correlated, r(147) = .31, p < .01. Sample items included: “Yelled at me about something I did wrong” (youth version) and “how much you yelled at this child about something he/she did wrong,” (mother version). Cronbach’s α exceeded .75 for both scales.
2.5. Youth and maternal DNA extraction, genotyping, and analysis
Saliva was collected in OraGene DNA collection kits (DNA Genotek, Kanata, Ontario, Canada) and extracted using their prepIT·L2P solution for manual extraction. After extraction, DNA was quantitated with a fluorescent Quant-iT PicoGreen dsDNA Assay (Life Technologies, Grand Island, NY, USA) and normalized to a concentration of 10 ng/ul. For DAT1, PCR was carried out in a 10 ul volume containing 30 ng of genomic DNA, 0.5 uM of each primer, 5’-FAM-TGTGGTGTAGGGAACGGCCTGAG-3’ and reverse primer, 5’-(GTTTCTT)CTTCCTGGAGGTCACGGCTCAAGG-3’ (with pigtail sequence within parentheses) 0.05 mM of each dNTP (dATP, dCTP, dGTP, dTTP), 1 × EXT PCR buffer (including 1.5 mM MgCl2), 1µL GC melt, and 0.3 units DyNAzyme EXT polymerase (Thermo Scientific, Waltham, MA, USA). with an initial denaturation step at 96 °C for 12 min followed by 40 cycles of 30 sec at 96 °C, 45 sec at 68 °C, and 3 min at 72 °C. For DRD4 DNA was amplified with forward primer, 5’-FAMGCGACTACGTGGTCTACTCG- 3’ and reverse primer 5’-(GTTTCTT)GGTCTGCGGTGGAGTCTG-3’ (with pigtail sequence within parentheses) in a 10 ul volume containing 10 ng of genomic DNA, 0.2 uM of each primer, 0.05 mM of each dNTP (dATP, dCTP, dGTP, dTTP), 1 × EXT PCR buffer (without MgCl2), 1µL GC melt, 1 mM MgCl2 (1.5mM MgCl2 led to poor amplification of the 7 repeat allele relative to shorter alleles and departure from HWE) and 0.3 units DyNAzyme EXT polymerase using the same cycling parameters as DAT1 except the annealing temperature was 55 °C. Products were separated on a 3730XL Genetic Analyzer (Life Technologies) in the UIC Research Resources Center DNA Services Facility. Alleles were called blind to phenotype data using Genemapper v 3.7.
The DAT1 3’ VNTR was coded for the putative variant of interest (10r). Individuals could have zero, one, or two 10 repeats. The DRD4 VNTR was similarly coded for the 7r variant, with individuals possessing zero, one, or two 7 repeats. Genotype frequencies divided by sex are presented in Table 2. There were no significant differences in genotype frequency by sex (χ2 ps > .05). Both VNTRs were tested for violation of Hardy-Weinberg Equilibrium considering them as both bi- and multi-allelic loci. In the former case we used the exact test developed by Wigginton and colleagues (2005). For the latter, we used an exact test to check for an excess or deficit of homozygous genotypes. The p-values for all tests were greater than 0.05.
Table 2.
Distributions of Genotypes for Girls and Boys
| Girls | Boys | |
|---|---|---|
| Genotype | N (% of girls) | N (% of boys) |
| DAT1 | ||
| 0 10r | 7 (7.6%) | 8 (11.1%) |
| 1 10r | 45 (48.9%) | 33 (45.8%) |
| 2 10r | 40 (43.5%) | 31 (43.1%) |
| DRD4 | ||
| 0 7r | 50 (54.3%) | 55 (69.6%) |
| 1 7r | 37 (40.2%) | 19 (24.1%) |
| 2 7r | 5 (5.4%) | 5 (6.3%) |
Note. VNTRs are coded for major genotype, Hardy Weinberg Equilibrium was tested prior to downcoding.
10r = 10 repeat, 7r = 7 repeat
Sex * genotype differences were not significant (χ2 p’s > .05).
2.6. Statistical analyses
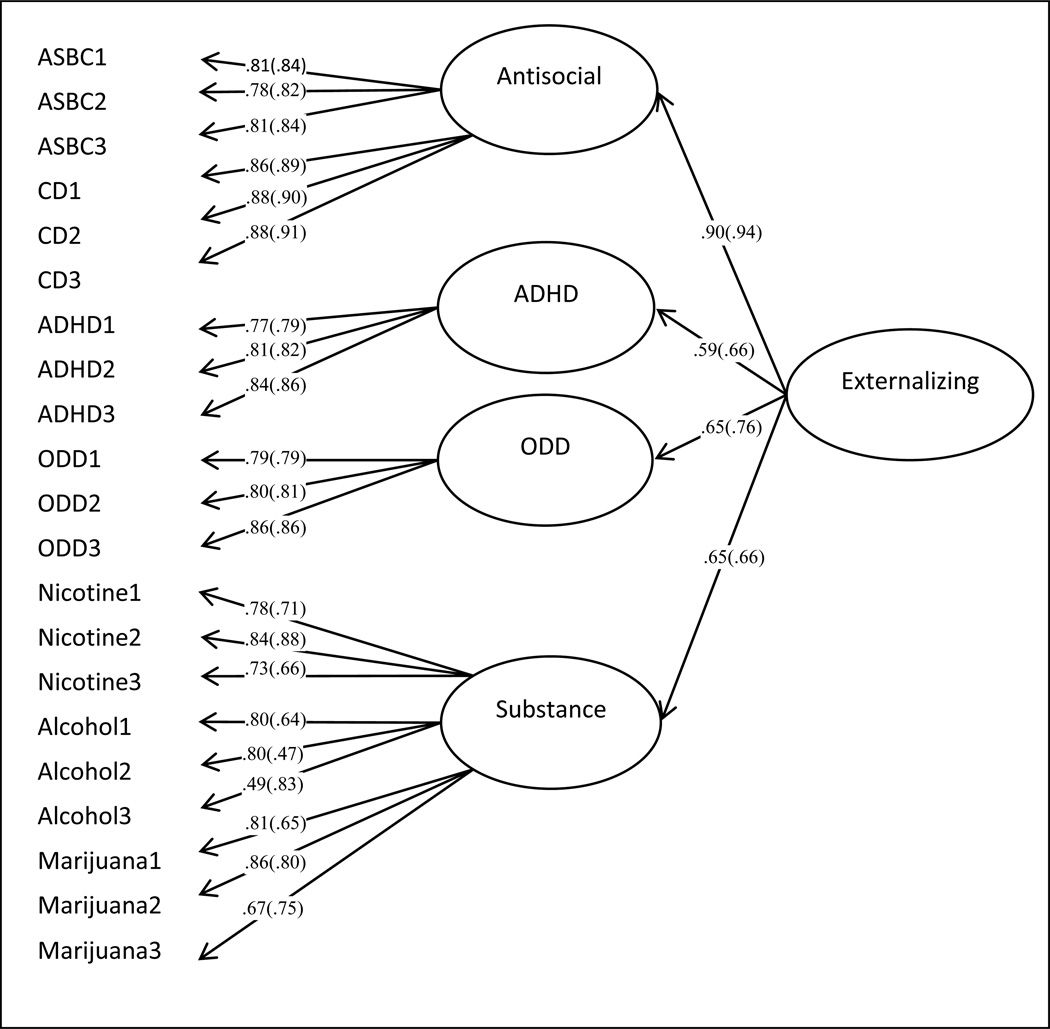
We created an externalizing spectrum latent variable from four lower-order latent variables, each with three time points: antisocial (CD and ASBC scales), ODD, ADHD, and substance use. Backward elimination procedures were undertaken for inclusion of covariates into final models. Multigroup regression analyses (split by sex) with a latent outcome were conducted in Mplus v. 6.12 (Muthén & Muthén, 1998–2011), using maximum likelihood (ML estimator) to handle missing data. Fit indices were acceptable for girls [χ2=148.13(120), p > .01, CFI = .97, RMSEA = .05, SRMR = .06] and boys [χ2=129.69(115), p > .05, CFI = .97, RMSEA = .05, SRMR = .06] separately; although the chi-square was less significant for boys than for girls. Latent indicators were correlated significantly (ranging from .43 to .65) and all loaded significantly onto the externalizing behavior latent. See Figure 2 for factor loadings of the indicators onto the four lower-order latent variables and the higher-order externalizing spectrum latent variable, separate for boys and girls. Factor structure was equivalent across boys and girls; a partially invariant model (loadings constrained to be equal across sex) did not fit significantly worse than separate models. Predictive paths from genes and PTE were allowed to vary freely by sex. Despite expected sex differences in mean levels of externalizing behavior, the raw data revealed few differences in any of the scales by sex. Boys did exhibited marginally more symptoms of conduct disorder, t(178) = −1.82, p < .10, but for all other scales the means did not differ significantly. There were no significant sex differences in prenatal exposure, either via the amount of cigarettes smoked (corrected for cotinine) or categorical PTE status (46% females and 51% males were exposed).
Figure 2.
Initial higher order latent factor model for externalizing spectrum behaviors. Estimates presented separately for: girls (boys). Factor loadings are standardized and all are significant at p < .01. Loadings were constrained to be equal across sexes in the final model. This did not significantly worsen model fit. 1 = Time 1; 2 = Time 2; 3 = Time 3; ASBC = Antisocial Behavior Checklist; CD = conduct disorder; ADHD = attention deficit hyperactive disorder; ODD = oppositional defiant disorder.
3. Results
The externalizing behavior latent was regressed on DAT1 and DRD4 genotype, PTE, and the two resultant interactions (GDAT1 × EPTE; GDRD4 × EPTE) using two separate multigroup regression analyses (one for each interaction model). There was a significant GDAT1 × EPTE interaction for boys (linear) and a marginal interaction for girls (heterosis; see Table 3). The final model predicted 23% of the variance in externalizing behavior for girls and 60% for boys.
Table 3.
Interaction of Prenatal Exposure x Dopamine Genotypes in Predicting a Latent Externalizing Spectrum for Girls and Boys
| Girls | Boys | |||||
|---|---|---|---|---|---|---|
| Linear Effects | Estimate | SE | p | Estimate | SE | p |
| Age | .41 | .07 | .00 | .38 | .08 | .00 |
| PTE | .09 | .09 | .37 | .12 | .11 | .26 |
| DAT1 10r | −.07 | .10 | .45 | .07 | .11 | .53 |
| DRD4 7r | −.01 | .10 | .51 | −.02 | .11 | .88 |
| Heterosis Effects | ||||||
| DAT1 | .10 | .12 | .38 | .15 | .13 | .26 |
| DRD4 | −.02 | .13 | .75 | −.02 | .13 | .88 |
| Interactionsa | ||||||
| PTE*DAT1 | −.23 | .20 | .26 | .50 | .15 | .00 |
| PTE*DRD4 | .03 | .14 | .82 | −.16 | .14 | .58 |
| PTE*heterosis DAT1 | .21 | .12 | .08 | −.09 | .15 | .54 |
| PTE* heterosis DRD4 | .04 | .14 | .75 | .09 | .14 | .54 |
Note. Age, SES, harsh parenting, parent antisocial behavior, maternal prenatal drug and alcohol use, and maternal genotype were considered as covariates. Age was retained in final models; other covariates did not significantly predict latent externalizing or alter the pattern of effects.
Interactions tested in separate models.
There was no main effect of DAT1 on either girls or boys, however; examination of the significant GDAT1 × EPTE for boys yielded a linear simple slope [B(SE) = .50(.15), p < .01] in which the 10r conveys risk to PTE boys (see Figure 3). There was a marginal GDAT1 × EPTE effect such that PTE girls heterozygous for DAT1 appeared to display more externalizing than homozygous girls, however this failed to reach significance (p = .08). This finding is at the trend-level and should be interpreted with caution, but we believe that these differing patterns underscore the importance of considering sex differences and nonlinear effects. The GDRD4 × EPTE interaction was not significant for either boys or girls.
Figure 3.
Externalizing stratified by DAT1 genotype and exposure status for boys and girls.
We then examined whether the most infrequent genotype, zero 10r alleles (boys N = 8; girls N = 7), could be having undue influence on the results. To test this concern, we performed post-hoc analyses using only the more common DAT1 genotypes categories. This post hoc test compared youth with one DAT1 10r allele to those homozygous for the 10r. For girls, the new dichotomous GDAT1 × EPTE interaction marginally related to externalizing behavior [B(SE) =−.26, p < .08]. Exposed girls with two 10R alleles displayed marginally lower externalizing scores than girls with only one 10R allele. The previously significant GDAT1 × EPTE interaction for boys remained significant with the dichotomous DAT1 coding; B(SE) = .36 (.16), p < .05. Boys homogeneous for 10r displayed significantly more externalizing behavior than did boys heterozygous for DAT1 10r.
Finally, to account for possible rGE, we used a 2×3 chi-square test of heterogeneity to examine whether mothers’ prenatal smoking status varied as a function of maternal genotype. Analyses did not support rGE. Maternal smoking status and maternal genotype were uncorrelated (DAT1 χ2 (2) = .09, p = .96 and DRD4 χ2 (2) = 3.16, p = .21). In addition, the GDAT1 × EPTE interaction was robust to control for maternal and paternal antisocial behavior and harsh parenting in multivariate models.
4. Discussion
Recent studies suggest that accounting for sex differences may elucidate the role of exposure × gene interactions in the origins of developmental psychopathology pathways. We use a sample recruited during pregnancy with strong prospective, repeated measures of exposure to examine sex differences in interactions of an early adverse exposure (PTE) and dopamine system genes to predict externalizing behavior. Our findings demonstrate that risk genotypes that modify PTE effects may vary for boys and girls. In particular, we have found (1) a gene × PTE interaction for DAT1 in boys; (2) that DAT1 genetic susceptibility did not increase externalizing behavior risk in the absence of exposure; and (3) that the genetic risk profile for girls and boys appears to vary. Specifically, in PTE youth, DAT1 10r is a risk factor for boys, whereas there is a trend-level indication that having a DAT1 heterozygous genotype may be a risk factor for girls.
The expression of a genotype is dependent upon environmental influences (Crews, 2008). Sex is an aspect of the environment that is relevant throughout prenatal development; with sex differences observed in DNA methylation (the process through which genes are turned ‘on’ or ‘off’), methyltransferases (enzymes that transfer methyl groups), methyl-binding proteins, and corepressor proteins (proteins that inhibit the expression of genes); all important components in the epigenetics or expression of genetic potential (McCarthy et al., 2009). Similarly, PTE has been previously demonstrated to have a significant and lasting effect on the development of brain and behavior by disrupting the development of catecholaminergic systems (including the dopamine system; Dwyer et al., 2009; Ernst et al., 2001; Navarro et al., 1988). Previous research in this area has consistently demonstrated the negative effect of PTE on components of the externalizing spectrum (Becker et al., 2008; Kahn et al., 2003; Wiebe et al., 2009). However, based on previous literature from our group and others demonstrating important sex differences in relation to gene × PTE studies (Becker et al., 2008; Kahn et al., 2003; Wakschlag et al, 2010), we have tested for these differences in relation to DAT1 and in prediction of a latent externalizing pattern. Our findings extend prior work by demonstrating a linear pattern of GDAT1 × EPTE for boys only.
We acknowledge that the small sample size in the current study is a limitation, and further research would benefit by testing these sex differences in larger, more representative samples. This would also be beneficial to testing whether PTE girl DAT1 heterozygotes are at increased risk for externalizing, given our marginal findings. Our genetic data collection was also limited to White, non-Hispanic adolescents to avoid population stratification (base differences in allelic frequencies between subpopulations) which can result in false positive results. Methods that infer genetic ancestry may be utilized in future to enable systematic looks at different ethnic groups (Liu, Zhang, Liu & Arendt, 2013; Price, Zaitlen, Reich & Patterson, 2010). An additional strength of this work is that by controlling for other prenatal influences (alcohol and drugs), we are more confident that our results reflect the true interaction between prenatal tobacco exposure and DAT1. Future studies could include other prenatal teratogens, such as caffeine or stress.
We considered variants in the dopamine system as ones likely to influence externalizing spectrum behaviors. Dopamine is expressed at higher concentrations in the brain than most other neurotransmitters and has a broad receptor distribution, making it an excellent candidate for genetic studies (Baskerville & Douglas, 2010; Bell & Deater-Deckard, 2007). We found that the dopamine transporter, DAT1, significantly interacted with PTE in boys, while the dopamine receptor GDRD4 × EPTE interaction was not significant. The lack of significance with DRD4 could also be a result of low minor allele frequency; there are a small number of youths with the 7r allele, or it could indicate that DRD4 is sensitive to a different environment. Our focus on dopamine system genes reflects the literature illustrating that dopamine is both sensitive to the disruption of PTE and has a strong influence on later behavioral outcomes via the reward-response mechanism (Ikemoto, 2007; Schultz, 2007) by way of activating the mesolimbic pathway (which spans from the ventral tegmental area of the midbrain through the nucleus accumbens into the prefrontal cortex). It is also worth repeating that our models were more successful in explaining variance in externalizing for boys than girls (60% vs. 23%) even though boys and girls had very similar mean levels of externalizing behaviors. The current study’s marginal findings for girls may indicate that a different gene, or set of genes, is more salient for explaining externalizing for girls.
The present study and much of our prior work is limited by considering only a single locus at a time. An interesting alternative for future work has recently been suggested, i.e. a cumulative genetic index, akin to Belsky and Beaver’s (2011) approach wherein DAT1, DRD2, DRD4, 5HTTLPR (serotonin transporter), and MAOA were combined into a single index that was then tested as a moderator of the parenting – self regulation pathway in adolescence. While this is an interesting future direction, the present and prior results from EBFS (Wakschlag, 2010) indicate that a cumulative genetic index may differ greatly by sex. The cumulative genetic approach holds considerable potential, but further research is needed to take sex differences into account in determining which gene variants pose a risk across sexes.
The current study additionally offers a practical application of the externalizing spectrum approach. This approach is gaining traction in the literature and reflects the emphasis in the DSM-5 on spectrum disorders, as well as empirical evidence that this approach is more informative for genetic research (Krueger, 1999; Krueger & Markon, 2006; Krueger et al., 2005). Modeling externalizing behavior as a latent spectrum allows for an investigation of the underlying commonalities that result in a range of related behavioral outcomes. In the current study, we conceptualize the externalizing spectrum as one common latent factor that encompasses risk for antisocial, disinhibitory, and substance use behaviors. Finally, externalizing behavior in the current study was collected at three separate time points. This was done to improve the accuracy and precision of measurement. The data did not allow for a longitudinal growth model in the current study due to high stability in the constructs across the duration of the follow-up. Future longitudinal work with a longer span of time between measurement occasions could examine individual change and growth within the framework of PTE, sex, and dopamine system functioning.
5. Conclusion
These findings offer an initial examination into the potential interrelations between variants in DAT1 and DRD4, sex, and prenatal exposure to cigarettes. PTE has dynamic and lasting influences on the brain (Dwyer et al., 2009) and subsequent behavior. The work of disentangling the ways in which genes and sex interact with prenatal exposure is at its naissance. Failure to consider relevant environments leads to null or contradictory results (Gizer et al.,2009; Propper et al., 2007), and so future studies should carefully investigate sex differences when studying gene-environment interplay.
Highlights.
We examine dopamine gene by prenatal tobacco exposure interactions.
Prenatal tobacco exposure measurement included repeated biological assays.
A multigroup model examines sex differences in gene-environment interactions.
Externalizing in prenatally-exposed boys increased linearly by DAT1 genotype.
Acknowledgements
This work was supported by NIDA Grant DA15223 to Dr. Wakschlag, including support to Dr. Cook. Drs Wakschlag and Cook were also supported by the Walden & Jean Young Shaw and Children’s Brain Research Foundations. We thank Ira Tager, the founder of MISSEB for his support for the EBFS follow-up and the EBFS research staff and participants, who made this work possible.
Abbreviations
- ADHD
attention deficit hyperactive disorder
- CD
conduct disorder
- DAT1
dopamine receptor gene
- DRD4
dopamine receptor D4
- ODD
oppositional defiant disorder
- PTE
prenatal tobacco exposure
- SES
socioeconomic status
- VNTR
variable number tandem repeat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Contributor Information
T. Caitlin O’Brien, Email: tcobrien@northwestern.edu.
Brian S. Mustanski, Email: brian@northwestern.edu.
Andrew Skol, Email: askol@uchicago.edu.
Edwin H. Cook, Jr, Email: ecook@psych.uic.edu.
Lauren S. Wakschlag, Email: lauriew@northwestern.edu.
References
- Achenbach TM. Manual for the Young Adult Self-Report and Young Adult Behavior Checklist. Burlington: University of Vermont Department of Psychiatry; 1997. [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. J Pediatr. 2008;152:263–269. doi: 10.1016/j.jpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Chambers CD, Johnson KA, Daibhis A, Daly M, Hawi Z, et al. Dopaminergic genotype biases spatial attention in healthy children. Mol Psychiatr. 2007;12:786–792. doi: 10.1038/sj.mp.4002022. [DOI] [PubMed] [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. J Child Psychol Psyc. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Taylor A, Smart M, Jackson J, Tagami S, Moffitt T. Can women provide reliable information about their children’s fathers? Cross-informant agreement about men’s lifetime antisocial 16ehavior. J Child Psychol Psyc. 2001;42:915–920. doi: 10.1111/1469-7610.00787. [DOI] [PubMed] [Google Scholar]
- Comings DE, MacMurray JP. Molecular heterosis: A review. Mol Genet Metab. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J of Hum Genet. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front in Neuroedocrinol. 2008;29:344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukic VM, Niessner M, Benowitz N, Hans S, Wakschlag L. Modeling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: A deterministic approach. Nicotine Tob Res. 2007;9:453–465. doi: 10.1080/14622200701239530. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Therapeut. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Psy. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR, Kosty DB, Lewinsohn PM. Refinements in the hierarchical structure of externalizing psychiatric disorders: Patterns of lifetime liability from mid-adolescence through early adulthood. J Abnorm Psychol. 2009;188:699–710. doi: 10.1037/a0017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA. Prenatal exposure to marijuana and tobacco during infancy, early and middle childhood: Effects and an attempt at synthesis. Arch Toxicol Suppl. 1995;17:233–260. doi: 10.1007/978-3-642-79451-3_21. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- Hetherington EM, Clingempeel WG. Coping with marital transitions: A family systems perspective. Monogr Soc Res Child Serial No. 227. 1992;57:2–3. [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens – olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Pine DS, Schoeny ME, Henry DB, Gollan JK, Moy G, et al. Maternal depressive history, teen 5HTTLPR genotype, and the processing of emotional faces: Exploring mechanisms of risk. Behav Res Ther. 2011;49:80–84. doi: 10.1016/j.brat.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Khoury J, Nichols WC, Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. J Pediatr. 2003;143:104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiat. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J of Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Understanding psychopathology: Melding behavior genetics, personality, and quantitative psychology to develop an empirically based model. Curr Dir Psychol Sci. 2006;15:113–117. doi: 10.1111/j.0963-7214.2006.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, personality: An integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional spectrum. J of Abnorm Psychol. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Waldman I, Van Hulle CA, Rathouz P, Pelham WE, et al. Association of dopamine transporter genotype with disruptive behavior disorders in an eight-year longitudinal study of children and adolescents. Am J Med Genet B. 2007;144B:310–317. doi: 10.1002/ajmg.b.30447. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang D, Liu H, Arendt Robust methods for population stratification in genome wide association studies. BMC Bioinformatics. 14:132–144. doi: 10.1186/1471-2105-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LM, Mustanski B, Metzger A, Pine DS, Kistner-Griffin E, Cook E, et al. A latent modeling approach to genotype-phenotype relationships: Maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Arch Women Ment Hlth. 2012;15:269–282. doi: 10.1007/s00737-012-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus v. 6.12 [computer program] Los Angeles, CA: Muthén & Muthén; 1998–2011. [Google Scholar]
- Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: Effects on development of catecholamine systems. J of Pharmacol Exp Ther. 1988;244:940–944. [PubMed] [Google Scholar]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiat. 2007;6:1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Price AL, Zaitlen NA, Reich D, Patterson N. New approaches to population stratification in genome-wide association studies. Nat Rev Genet. 2010;11:459–463. doi: 10.1038/nrg2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper C, Willoughby M, Halpern CT, Carbone MA, Cox M. Parenting quality, DRD4, and the prediction of externalizing and internalizing behaviors in early childhood. Dev Psychobiol. 2007;49:619–632. doi: 10.1002/dev.20249. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH. Association of DRD4 with attention problems in normal childhood development. Psychiat Genet. 2001;11:25–29. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Psy. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, et al. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. PNAS. 2000;97:4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, et al. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacol. 2008;33:1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- Turic D, Swanson J, Sonuga-Barke E. DRD4 and DAT1 in ADHD: Functional neurobiology to pharmacogenetics. Pharmgenomics Pers Med. 2010;3:61–78. doi: 10.2147/pgpm.s6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol Psychiatr. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, Stopp C, Respass J, Stewart P, Jameson TR, et al. Gene-environment interactions across development: Exploring DRD2 genotype and prenatal smoking effects on self-regulation. Dev Psychol. 2009;45:31–44. doi: 10.1037/a0014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A Note on Exact Tests of Hardy-Weinberg Equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R, Noll R, Ham H, Fitzgerald H, Sullivan L. Assessing Antisociality with the Antisocial Behavior Checklist: Reliability and Validity Studies. University of Michigan & Michigan State University; 1994. Unpublished. [Google Scholar]