Abstract
This study examines whether prenatal nicotine exposure sensitizes the developing brain to subsequent developmental neurotoxicity evoked by chlorpyrifos, a commonly-used insecticide. We gave nicotine to pregnant rats throughout gestation at a dose (3 mg/kg/day) producing plasma levels typical of smokers; offspring were then given chlorpyrifos on postnatal days 1–4, at a dose (1 mg/kg) that produces minimally-detectable inhibition of brain cholinesterase activity. We evaluated indices for acetylcholine (ACh) synaptic function throughout adolescence, young adulthood and later adulthood, in brain regions possessing the majority of ACh projections and cell bodies; we measured nicotinic ACh receptor binding, hemicholinium-3 binding to the presynaptic choline transporter and choline acetyltransferase activity, all known targets for the adverse developmental effects of nicotine and chlorpyrifos given individually. By itself nicotine elicited overall upregulation of the ACh markers, albeit with selective differences by sex, region and age. Likewise, chlorpyrifos alone had highly sex-selective effects. Importantly, all the effects showed temporal progression between adolescence and adulthood, pointing to ongoing synaptic changes rather than just persistence after an initial injury. Prenatal nicotine administration altered the responses to chlorpyrifos in a consistent pattern for all three markers, lowering values relative to those of the individual treatments or to those expected from simple additive effects of nicotine and chlorpyrifos. The combination produced global interference with emergence of the ACh phenotype, an effect not seen with nicotine or chlorpyrifos alone. Given that human exposures to nicotine and chlorpyrifos are widespread, our results point to the creation of a subpopulation with heightened vulnerability.
Keywords: Acetylcholine, Brain development, Chlorpyrifos, Nicotine, Organophosphate pesticides
INTRODUCTION
Protection of the human population from environmental toxicants requires consideration of subpopulations that may be especially vulnerable. This is particularly important for developmental neurotoxicity, given the widespread use of thousands of neurotoxic chemicals and their likely contribution to the increased incidence of neurodevelopmental disorders (Grandjean and Landrigan, 2006). In the case of the organophosphate pesticides, which represent a large proportion of total worldwide insecticide use (Casida and Quistad, 2004), we already know that there are polymorphisms of PON1, the enzyme that breaks down these neurotoxicants, clearly defining a sensitive subpopulation (Povey, 2010). In recent studies, we have explored how non-genetic factors, particularly an individual’s “chemical history,” might similarly create differential susceptibility, namely, whether prenatal exposures to neuroactive drugs or chemicals sensitize an individual to neurotoxicants encountered later in life. In particular, we showed how drug therapies commonly used in preterm labor enhance the subsequent vulnerability of the developing brain to chlorpyrifos, a commonly-used organophosphate pesticide (Aldridge et al., 2005; Levin et al., 2014; Meyer et al., 2005; Rhodes et al., 2004; Slotkin et al., 2013, 2014a). However, by far the most widely-encountered prenatal drug exposure is nicotine. Maternal cigarette smoking during pregnancy is the single most identifiable and preventable cause of neonatal morbidity and mortality (DiFranza and Lew, 1995), and the nicotine contained in tobacco smoke produces abnormalities of brain development leading to neurobehavioral deficits such as attention deficit/hyperactivity disorder, conduct disorder and affective disorders (Ernst et al., 2001; Herrmann et al., 2008; Slotkin, 2008). Besides active maternal smoking, significant nicotine exposures occur through second-hand smoke or through the use of nicotine replacement products in smoking cessation, or more recently, through the use of “vaping” devices for inhaling nicotine. Nicotine’s direct actions on nicotinic acetylcholine receptors (nAChRs), preempt the timing and intensity of the natural trophic signals ordinarily controlled by acetylcholine (ACh), leading to abnormalities of neuronal cell replication, differentiation and synaptic connectivity (Dwyer et al., 2008; Hohmann and Berger-Sweeney, 1998; Lauder and Schambra, 1999; Slotkin, 2004, 2008). Likewise, chlorpyrifos and other organophosphates target ACh systems as one of their primary modes of action, involving not only inhibition of cholinesterase (the mechanism for systemic toxicity), but also mechanisms that compromise ACh receptors and cell signaling at exposures below the threshold for cholinesterase inhibition (Slotkin, 2004, 2005).
The convergence of nicotine and chlorpyrifos on ACh pathways makes this a likely combination for enhanced vulnerability. At the same time, however, nicotine possesses neuroprotective properties that can attenuate the actions of other neurotoxicants (Belluardo et al., 2000; Ferrea and Winterer, 2009; Kawamata and Shimohama, 2011). Using in vitro models, we found that nicotine can protect neuronotypic cells from the antimitotic actions and oxidative stress induced by chlorpyrifos, while at the same time nicotine itself had adverse effects on neural cell replication and differentiation (Abreu-Villaça et al., 2005; Qiao et al., 2003b, 2005; Slotkin et al., 2007b, 2014b); the balance between protection and damage was highly dependent on the specific developmental stage of the cells (Slotkin et al., 2007b). It is thus difficult to predict whether prior exposure to nicotine might worsen or improve the outcome of subsequent developmental exposure to chlorpyrifos.
Accordingly, in the present work, we examined the impact of fetal nicotine exposure on the subsequent effects of postnatal chlorpyrifos directed toward the development of ACh systems in the rat brain. Nicotine was given throughout gestation via implanted osmotic minipump, at a dose (3 mg/kg/day) that produces nicotine plasma levels similar to those in moderate smokers (Lichtensteiger et al., 1988; Murrin et al., 1987; Trauth et al., 2000); we specifically chose a lower dose than in our previous work simulating heavy smoking (Slotkin, 2004, 2008), so as to produce submaximal changes in order to leave room for additional effects of chlorpyrifos. Chlorpyrifos was then given daily on postnatal days (PN) 1–4 at a dose of 1 mg/kg, a regimen that produces just-detectable inhibition of brain cholinesterase and that disrupts neurobehavioral development, but that is not systemically toxic (Slotkin, 1999, 2004, 2005; Song et al., 1997). This exposure model successfully predicts both the neurobehavioral deficits and abnormalities of brain structure seen in children exposed prenatally to chlorpyrifos (Bouchard et al., 2011; Engel et al., 2011; Rauh et al., 2006, 2011, 2012). Our study thus encompassed four treatment groups: control, nicotine alone, chlorpyrifos alone, and nicotine followed by chlorpyrifos.
We assessed the impact on brain regions comprising the major ACh projections and their corresponding cell bodies, focusing on well-established markers of ACh synaptic function: the concentration of α4β2 nAChRs, binding of hemicholinium-3 (HC3) to the presynaptic high-affinity choline transporter, and activity of choline acetyltransferase (ChAT). The α4β2 nAChR is the most abundant nAChR subtype in the mammalian brain (Flores et al., 1992; Happe et al., 1994; Whiting and Lindstrom, 1987, 1988) and underlies the ability of ACh systems to release other neurotransmitters involved in reward, cognition and mood (Buisson and Bertrand, 2001, 2002; Dani and De Biasi, 2001; Fenster et al., 1999; Quick and Lester, 2002). High-affinity choline transporters and ChAT are both constitutive components of ACh nerve terminals but they differ in their regulatory mechanisms and hence in their functional significance. ChAT is the enzyme that synthesizes ACh, but is not regulated by nerve impulse activity, so that its presence provides an index of the development of ACh projections (Happe and Murrin, 1992; Kreider et al., 2005, 2006; Slotkin, 2004, 2008). In contrast, HC3 binding to the choline transporter is directly responsive to neuronal activity (Klemm and Kuhar, 1979; Simon et al., 1976), so that comparative effects on HC3 binding and ChAT enables the characterization of both the development of innervation and presynaptic impulse activity. Accordingly, in addition to assessing HC3 binding and ChAT activity, we determined the HC3/ChAT ratio as an index of presynaptic activity relative to the number of cholinergic nerve terminals (Abreu-Villaça et al., 2004; Slotkin et al., 1994, 2007a). We also contrasted the specific effects on ACh synaptic development with assessments of systemic toxicity (maternal weight gain, litter characteristics, postnatal body and brain region weights), and determinations of whether prenatal nicotine treatment enhanced the subsequent ability of chlorpyrifos to inhibit cholinesterase.
MATERIALS AND METHODS
Animal treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats were shipped on the second day of gestation by climate-controlled truck (total transit time < 1 h), housed individually and allowed free access to food and water. There were four treatment groups, each comprising 10–12 litters: controls (prenatal vehicle infusion + postnatal vehicle injections), nicotine treatment alone (nicotine infusion + postnatal injections), chlorpyrifos treatment alone (vehicle infusion + chlorpyrifos injections), and those receiving the combined treatment (nicotine infusion + chlorpyrifos injections). On gestational day 4, before implantation of the embryo in the uterine wall, each animal was quickly anesthetized with ether, a small area on the back was shaved, and an incision made to permit s.c. insertion of a Model 2ML2 Alzet minipump, after which the incision was closed with wound clips. The pumps contained nicotine bitartrate dissolved in bacteriostatic water so as to deliver 3 mg/kg/day of nicotine free base, with the dosage determined by the initial body weights of the dams (215 ± 2 g); control pumps contained bacteriostatic water and equivalent concentrations of sodium bitartrate. Because weights increased with gestation, the dose rate fell accordingly to 2 mg/kg/day, but the dose rates remained well within the range that produces nicotine plasma levels similar to those in moderate smokers (Fewell et al., 2001; Trauth et al., 2000). It should be noted that the pump, marketed as a two week infusion device, actually takes approximately 17 days to be exhausted completely (information supplied by the manufacturer) and thus the nicotine infusion terminates on the 21st day of gestation; in earlier work, we confirmed the termination of nicotine delivery coinciding with the calculated values (Trauth et al., 2000). Parturition occurred during gestational day 22, which was also taken as PN0.
After birth, pups were randomized within treatment groups and litter sizes were culled to 10 (5 males and 5 females) to ensure standard nutrition. Control and nicotine-treated litters were then assigned to either the vehicle or chlorpyrifos postnatal treatment groups. Chlorpyrifos was dissolved in dimethylsulfoxide to provide consistent absorption (Whitney et al., 1995) and pups were injected subcutaneously at a dose of 1 mg/kg in a volume of 1 ml/kg once daily on postnatal days 1–4; control pups received equivalent injections of the dimethylsulfoxide vehicle. This regimen has been shown previously to produce developmental neurotoxicity, including robust effects on ACh systems, without eliciting growth retardation or any other signs of systemic toxicity (Slotkin, 1999, 2004). Pups were weighed, litters were re-randomized within treatment groups and dams were rotated among litters every few days to distribute differential effects of maternal caretaking equally among all litters, making sure that all the pups in a given litter were from the same treatment group to avoid the possibility that the dams might distinguish among pups with different treatments; cross-fostering, by itself, has no impact (Nyirenda et al., 2001). Animals were weaned on PN21.
On PN4, 2 hr after the last chlorpyrifos injection, 6 pups (3 male, 3 female, with no more than one of each sex from a given litter) from each group were decapitated and whole brain dissected for determination of cholinesterase activity. Subsequently, on PN30, 60, 100 and 150, additional animals were decapitated and brain regions were dissected for determination of ACh synaptic markers: frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum, midbrain and brainstem. The two cortical regions were sectioned at the midline and the left half used for the current determinations. The right halves of the cortical regions were reserved for future studies, along with the cerebellum, which is sparse in ACh projections. Tissues were frozen in liquid nitrogen and stored at −80°C until assayed. For the ACh synaptic markers, each treatment group comprised 12 animals at each age point, equally divided into males and females, with each final litter assignment contributing no more than one male and one female to any of the treatment groups.
Assays
Assays were conducted on each individual tissue, so that each determination represented a value from the corresponding brain region of one animal. For cholinesterase determinations, tissues were thawed and homogenized (Polytron, Brinkmann Instruments, Westbury, NY) in ice-cold 50 mM Tris (pH 7.4), and aliquots of the homogenate were withdrawn for measurement of total protein (Smith et al., 1985) and cholinesterase activity (Ellman et al., 1961). For the latter, the homogenate was diluted in 0.5% Triton X100, 0.1 M Na2HPO4/KH2PO4 (pH 8) and left on ice for 15 min to allow the Triton X100 to solubilize membrane-associated cholinesterase. Homogenates were sedimented at 40,000 ×g for 15 min and aliquots of the supernatant solution were added to final concentrations of 0.5 mM acetylthiocholine iodide and 0.33 mM 5,5′-dithiobis(2-nitrobenzoic acid) in the same buffer without Triton. Assays were incubated at room temperature for 4, 8, 12, 16 and 20 min, and the enzyme activity was assessed from the linear portion of the time course, reading the absorbance at 415 nm. The assay was standardized by using mercaptoethanol standards and calculated relative to total protein.
For the determinations of nAChR binding, HC3 binding and ChAT activity, tissues were thawed in 79 volumes of ice-cold 10 mM sodium-potassium phosphate buffer (pH 7.4) and homogenized with a Polytron (Brinkmann Instruments, Westbury, NY). Duplicate aliquots of the homogenate were assayed for ChAT using established procedures (Qiao et al., 2003a, 2004). Each tube contained 50 μM [14C]acetyl-coenzyme A as a substrate and activity was determined as the amount of labeled ACh produced relative to tissue protein (Smith et al., 1985). For measurements of HC3 binding, the cell membrane fraction was prepared from an aliquot of the same tissue homogenate by sedimentation at 40,000 ×g for 15 min. The pellet was resuspended and washed, and the resultant pellet was assayed with established procedures (Qiao et al., 2003a, 2004), using a ligand concentration of 2 nM [3H]HC3 with or without 10 μM unlabeled HC3 to displace specific binding. Determinations of nAChR binding were carried out in another aliquot, each assay containing 1 nM [3H]cytisine with or without 10 μM nicotine to displace specific binding (Slotkin et al., 2008). Binding for both ligands was calculated relative to the membrane protein concentration.
Data analysis
Because of the large number of potential comparisons in a study of this type, it was important to avoid the increased probability of a type I statistical error that would result from multiple tests on the data set. We adopted a strategy where all the values in the entire study were first evaluated in a global, multivariate ANOVA that would identify main treatment effects that might be detected considering all brain regions, ages and both sexes, and all three dependent measures of ACh synaptic function; this represents a single statistical test of the entire study, so that a selected critical value of p < 0.05 is not compromised by multiple tests. Then, from the interactions of treatment with the other factors, we were justified in subdividing the data into more easily-grasped subsets, each of which was then tested with a lower-order ANOVA, still incorporating all the other remaining factors. Where interactions remained, this led to subsequent subdivisions, ultimately stopping wherever treatment effects remained without further interactions. This procedure thus provides protection against increased type I errors at every level, with interactions justifying subdivisions and protecting the lower-order tests. In the initial, global ANOVA, we simultaneously evaluated all the factors (the four treatment groups, the six brain regions, the four age points, both sexes) and the three neurochemical measures that were all related to ACh synapses, (nAChR binding, HC3 binding, ChAT; nested as repeated measures, since all three determinations were derived from the same sample), with the data log-transformed because of heterogeneous variance among regions, ages and measures. This test identified interactions of treatment with the other variables, triggering subdivisions into lower-order ANOVAs to evaluate treatments that differed from the corresponding control. Among these were the interactions of treatment with the three dependent ACh measures (hereafter, designated simply as “measures”), connoting differences in the impact of treatment on nAChR binding, HC3 binding, or ChAT, necessitating separate consideration of each neurochemical endpoint. As permitted by the interaction terms, individual groups that differed from control were identified with Fisher’s Protected Least Significant Difference Test.
Significance was assumed at the level of p < 0.05. However, for interactions at p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables, a procedure recommended for multivariate analyses where variance contributed by interactive variables can often obscure significant underlying main effects (Snedecor and Cochran, 1967). The criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of main effects of the treatments. In the few cases where this was done, we verified that the interaction of interest was either significant at p < 0.05 in a higher-order test, or that a significant interaction was present with an alternative subdivision of the data, since the multiple interactions of treatment with the other variables enabled alternative subdivisions of the data. Nevertheless, for completeness, we report all the interactions for which 0.1 < p < 0.05, whether or not these were required for subdivision of the data. Finally, where treatment effects were not interactive with other variables, we report only the main treatment effects without performing lower-order analyses of individual values.
Data were compiled as means and standard errors. To enable ready visualization of treatment effects across different regions, ages and measures, the results for the ACh synaptic measures are given as the percent change from control values. Statistical procedures were always conducted on the original data, with log transforms because of heterogeneous variance as noted above. In addition, the log-transform evaluates the treatment differences as a proportion to control values, rather than as an arithmetic difference. This was important because of technical limitations: on any single day, we could conduct assays for all treatment groups and both sexes, but only for one region at one age point. Accordingly, representing the data as proportional differences (percent control) enables a full comparison of treatment effects and treatment interactions with all the other variables, even though absolute values for the controls cannot be compared across regions and ages. Graphs were scaled to encompass the different dynamic ranges of the changes in the various parameters. The absolute values for each set of determinations appear in the Supplemental Tables.
There were two separate issues addressed in the data analysis. First, we determined whether the effects of nicotine, chlorpyrifos, or the combination, differed from control values and from each other. Second, we wanted to assess whether the effects of the combination were additive (sum of individual effects of nicotine and chlorpyrifos), or non-additive (synergistic, less-than-additive or antagonistic). The first issue required an ANOVA regarding the four treatment groups as one factor (“treatment”), followed by post-hoc comparisons for intergroup differences. The second issue required that the nicotine and chlorpyrifos treatments be considered as two separate factors, with the interaction term (nicotine × chlorpyrifos) thus testing for additivity (not significant if additive, significant if non-additive).
Materials
Animals were purchased from Charles River Laboratories (Raleigh, NC) and osmotic minipumps from Durect Corp. (Cupertino, CA). Bacteriostatic water was obtained from Abbott Laboratories (N. Chicago, IL) and PerkinElmer Life Sciences (Boston, MA) was the source for radioligands: [3H]HC3 (specific activity, 125 Ci/mmol), [3H]cytisine (specific activity 35 Ci/mmol) and [14C]acetyl-coenzyme A (specific activity 6.7 mCi/mmol). Sigma Chemical Co. (St. Louis, MO) was the source for all other reagents.
RESULTS
Maternal, litter and growth effects
Nicotine infusions during pregnancy resulted in a small, but statistically significant reduction in maternal weight gain, representing about a 7–10 g overall difference from the total gain of 110 g seen in the controls (Supplement Figure 1). The effect was immediately apparent in the first few days after pump implantation, after which weight gains were parallel in the two groups. There were no differences in the proportion of dams giving birth (100% for both), litter size (11.8 ± 0.4 for control, 11.4 ± 0.3 for nicotine), sex distribution (47 ± 2% male for control, 48 ± 2% for nicotine), or birth weight (7.4 ± 0.1 g for both groups, measured on PN1). Neither nicotine nor chlorpyrifos, alone or in combination, produced any significant reductions in growth of the offspring (Supplement Table 1). In fact, there was a small, but significant increase in body weights in the groups receiving chlorpyrifos alone or the combination of nicotine + chlorpyrifos: p < 0.0002 for the main treatment effect; control vs. nicotine not significant; control vs. chlorpyrifos, p < 0.0001 (net 4% increase); control vs. nicotine + chlorpyrifos, p < 0.0005 (net 1% increase). We did not find any deficits in brain region weights, and in fact, the only statistically significant difference was at one age point (PN100), restricted to about a 3% overall increase for the groups receiving either nicotine alone or the combined treatment (Supplement Table 2).
Cholinesterase activity
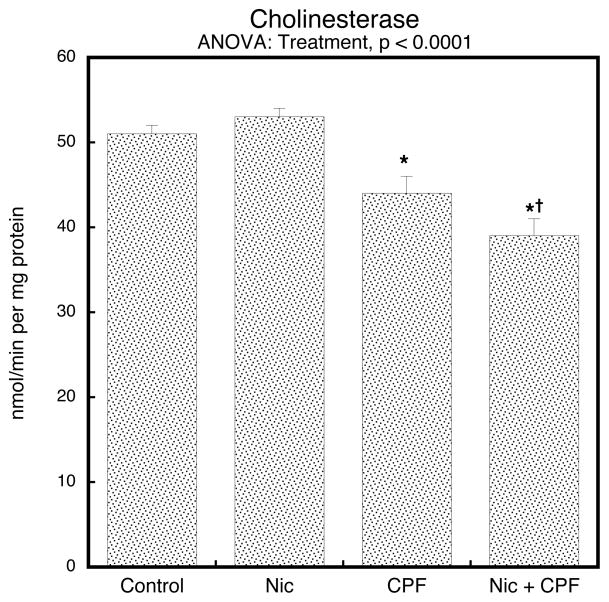
To determine whether prenatal nicotine could affect the cholinesterase inhibition evoked by postnatal chlorpyrifos treatment, we evaluated brain cholinesterase activity on PN4, 2 hr after the last injection, corresponding to the time of peak inhibition (Dam et al., 2000). In keeping with earlier studies (Song et al., 1997), the postnatal chlorpyrifos regimen used here produced small (<15%), but statistically significant inhibition of brain cholinesterase (Figure 1). By itself, nicotine had no discernible effect on cholinesterase, but it did enhance the response to chlorpyrifos: the net reduction of the combined treatment was about 25% compared to the group receiving nicotine alone (nicotine × chlorpyrifos interaction, p < 0.04); note that results were combined for males and females because of the absence of a treatment × sex interaction.
Figure 1.
Treatment effects on cholinesterase activity in whole brain on PN4, 2 hours after the final injection of chlorpyrifos (CPF) or vehicle (mean ± SE, n=6). Inhibition by chlorpyrifos was significantly enhanced by prenatal nicotine (Nic) treatment. One-factor ANOVA for the four treatment groups is shown at the top of the panel; asterisks denote the groups that differ significantly from the control value and the dagger indicates that the Nic+CPF group is different from CPF alone. Two-factor ANOVA showed a significant nicotine × chlorpyrifos interaction (p < 0.04), indicating the 25% decrease comparing Nic+CPF to nicotine alone is significantly greater than the 14% decrease evoked by CPF alone (CPF vs. Control). Values were combined for males and females because of the absence of a treatment × sex interaction.
Global statistical analyses of ACh synaptic markers
Multivariate ANOVA incorporating all four treatment groups, brain region, age, sex, and the three ACh synaptic measures (nAChR binding, HC3 binding, ChAT activity) indicated a main effect of the treatments (p < 0.0001) that interacted with all the other variables: p < 0.0001 for treatment × measure, p < 0.007 for treatment × measure × age, p < 0.02 for treatment × measure × sex, p < 0.02 for treatment × measure × region, p < 0.09 for treatment × age × region, and p < 0.009 for treatment × measure × age × region. The main treatment effect reflected a net elevation evoked by nicotine (p < 0.005 vs. control), a nonsignificant overall increase caused by chlorpyrifos (not significant vs. control but significantly lower than nicotine, p < 0.005), but a net decrease for the combination treatment (p < 0.03 vs. control, p < 0.0001 vs. nicotine, p < 0.03 vs. chlorpyrifos). The significant interactions of treatment with the other variables indicated that treatment effects differed among brain regions, between ages, and between the sexes, requiring corresopnding subdivisions of the results. We then compared each of the three treatments (nicotine alone, chlorpyrifos alone, nicotine + chlorpyrifos) individually to the control group, and again found interactions of treatment with variables for each: nicotine, p < 0.0001 for treatment, p < 0.0001 for treatment × measure, p < 0.0001 for treatment × measure × age, p < 0.02 for treatment × measure × region, p < 0.06 for treatment × age × region, and p < 0.007 for treatment × measure × age × region; chlorpyrifos, p < 0.02 for treatment × measure × age, p < 0.0004 for treatment × measure × sex, p < 0.03 for treatment × measure × sex × age, p < 0.1 for treatment × measure × age × region, p < 0.08 for treatment × sex × age, and p < 0.1 for treatment × sex × region; nicotine + chlorpyrifos, p < 0.02 for treatment × measure, p < 0.02 for treatment × measure × age, p < 0.02 for treatment × measure × region, p < 0.0004 for treatment × age × region, and p < 0.007 for treatment × measure × age × region. Since we found treatment interactions with the remaining variables after this subdivision, we separated the values by treatment and measure and then examined corresponding lower-order tests of treatment effects and treatment interactions with region, age and sex. Each subsection below begins with the ANOVA appropriate to that particular biomarker.
In addition to analyses where the treatment was considered as a single ANOVA factor with four categories (control, nicotine, chlorpyrifos, nicotine + chlorpyrifos), we also examined a global test where the treatments were regarded as two separate dimensions (prenatal treatment, postnatal treatment) so as to detect interactions between nicotine and chlorpyrifos treatments that would indicate synergistic, less-than additive or antagonistic effects. We found a significant interaction of nicotine × chlorpyrifos (p < 0.0001), reflecting the fact that the group receiving combined treatment had lower values across all the synaptic markers than would have been expected from summation of the individual effects of nicotine and chlorpyrifos. This interaction also depended on the other variables: nicotine × chlorpyrifos × measure × sex (p < 0.03), nicotine × chlorpyrifos × measure × sex × region (p < 0.02). Accordingly, we performed similar analyses for each of the lower-order groupings to look for specific cholinergic biomarkers showing non-additive interactions of nicotine and chlorpyrifos treatment.
nAChR binding
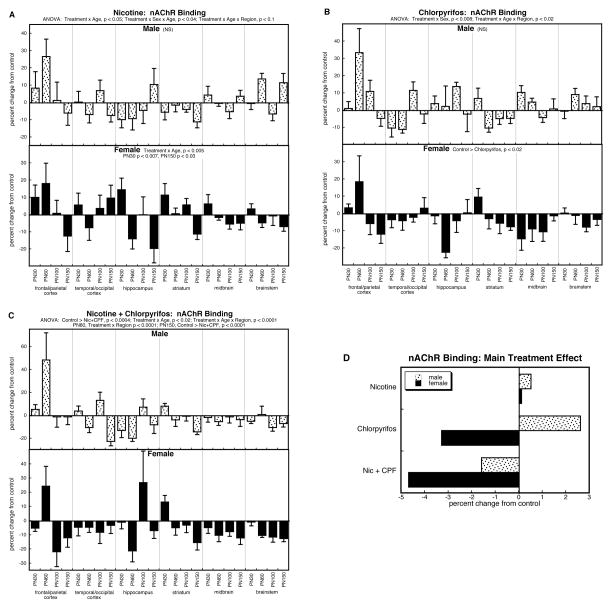
For nAChR binding, ANOVA across all factors identified a main treatment effect (p < 0.002) that depended on all the other variables: p < 0.05 for treatment × sex, p < 0.05 for treatment × sex × age, and p < 0.004 for treatment × age × region. The main treatment effect reflected the fact that the group receiving combined treatment had significantly lower overall values than any of the other groups: p < 0.0007 vs. control, p < 0.03 vs. nicotine, p < 0.02 vs. chlorpyrifos. In light of the main treatment effect and interaction of treatment with the other variables, we examined each treatment separately.
By itself, prenatal nicotine exposure had variable effects on nAChR binding in males, resulting in a small overall increase that was not significant (Figure 2A); in females, nicotine produced initial increases in nAChR binding (PN30, p < 0.007), an effect that eventually waned, with emergence of deficits in full adulthood (PN150, p < 0.03). For postnatal chlorpyrifos exposure, males showed a tendency toward overall increases in nAChR binding that did not achieve statistical significance, whereas once again, there was a net, significant reduction in females (Fig. 2B). Notably, though, animals receiving both prenatal nicotine treatment and postnatal chlorpyrifos exposure displayed an overall reduction in nAChR binding that extended across both males and females (Fig. 2C). Superimposed on the net effect, there were significant age and regional differences, with increases predominating in adolescence, regressing downward to eventual deficits in adulthood: increases in the striatum on PN30 (p < 0.02) and the frontal/parietal cortex on PN60 (p < 0.03), but decreases on PN150 in the temporal/occipital cortex (p < 0.03), the striatum (p < 0.0006), and the brainstem (p < 0.009).
Figure 2.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on nAChR binding. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table 3. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (A,B) Effects on specific regions and ages are stated above or within the panels where appropriate. For (C), the interactions of treatment × age and treatment × age × region permitted further subdivision which identified significant differences for the frontal/parietal cortex on PN60 (p < 0.03), the temporal/occipital cortex on PN150 (p < 0.03), the hippocampus on PN60 (p < 0.02), the striatum on PN30 (p < 0.02) and PN150 (p < 0.006), the midbrain on PN60 (p < 0.05), and the brainstem on PN100 (p < 0.02) and PN150 (p < 0.009). Panel (D) shows the simple main treatment effects, collapsed across all the other variables.
In light of the significant interaction of treatment × age × region (p < 0.004) in the overall ANOVA for nAChR binding, we also examined the results for all treatments subdivided by regions rather than sex. There were significant treatment × age interactions in two of the regions containing nerve terminals: frontal/parietal cortex (p < 0.03) and striatum (p < 0.006). Again, these reflected early increases that regressed to become subsequent deficits. For the frontal/parietal cortex, there was a significant elevation in nAChR binding on PN60, with all three treatments distinguishable from control values (p < 0.05 for each); for the striatum, there was a significant treatment-related reduction on PN150 (p < 0.006), with individual significance for the nicotine group (p < 0.02) and the group receiving both nicotine and chlorpyrifos (p < 0.0007).
To illustrate the differences in the main treatment effects among the three groups, we calculated the mean values for nAChR binding, collapsed across all the interactive variables (Fig. 2D). This simplified picture dilutes the effects seen in specific regions or at particular ages, by averaging them with data points for which there was no effect or an opposite effect, so that the absolute magnitude becomes smaller. Despite these limitations, there was an obvious overall pattern: the group receiving the combined exposure had lower values than those predicted from the individual effects of nicotine or chlorpyrifos. This was verified by ANOVA treating nicotine and chlorpyrifos as two separate factors (nicotine × chlorpyrifos interaction, p < 0.05), indicative of an outcome that differed significantly from simple summation of the individual effects. For males, whereas the net effect of nicotine or chlorpyrifos individually showed overall increases relative to control values, the group receiving the combined treatment instead showed a reduction in nAChR binding. For females, nicotine produced a very slight increase when values were collapsed across regions and ages, whereas chlorpyrifos evoked a clear, overall reduction; however, the group receiving the combined treatment showed an even stronger reduction in nAChR binding.
HC3 binding
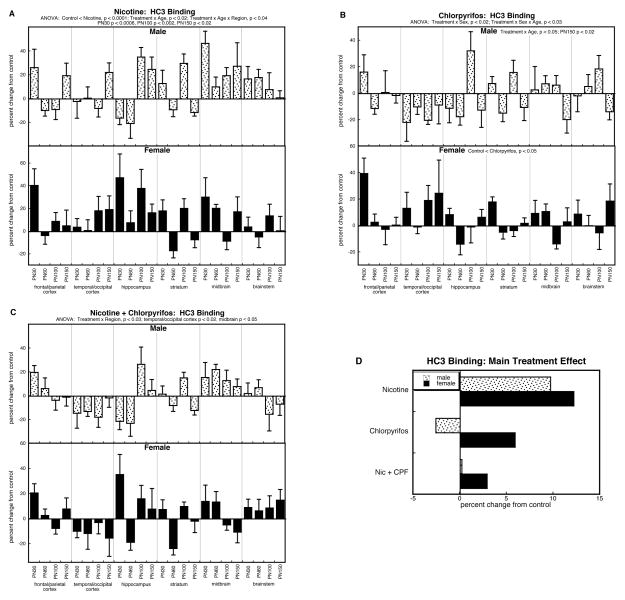
The global ANOVA for HC3 binding showed main effects of treatment (p < .0001) and interactions with sex and age (treatment × sex, p < 0.1; treatment × sex × age, p < 0.04). The main treatment effect reflected the fact that the nicotine group had higher values than all others: p < 0.0001 vs. control, p < 0.0002 vs. chlorpyrifos, p < 0.0002 vs. the group receiving combined treatment. These overall main effects were reflected in the analyses of the individual treatment differences. For prenatal nicotine treatment, we found a significant overall elevation involving both males and females (Figure 3A), with superimposed age- and regional selectivities. The treatment × age interaction (p < 0.02) reflected individually significant elevations on PN30 (p < 0.0006), PN100 (p < 0.002) and PN150 (p < 0.02); the treatment × age × region interaction involved two age points, PN100 (hippocampus, p < 0.003; striatum, p < 0.003) and PN150 (hippocampus, p < 0.02; midbrain, p < 0.0002), once again indicating progression with age, rather than just persistence of an initial effect.
Figure 3.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on HC3 binding. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table 4. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (B). Effects on specific regions and ages are stated above or within the panels where appropriate. Panel (D) shows the simple main treatment effects, collapsed across all the other variables.
Chlorpyrifos had more selective effects on HC3 binding, reflected in a significant sex difference in the treatment response (treatment × sex, p < 0.02; Figure 3B). In males, the chlorpyrifos effect was age-dependent (treatment × age, p < 0.05), with progressively greater reductions, achieving statistical significance by PN150. In contrast, females showed a net overall increase in HC3 binding.
The group receiving the combined treatment of prenatal nicotine followed by postnatal chlorpyrifos, exhibited a pattern distinct from that seen with either treatment alone, characterized by regional selectivity (treatment × region, p < 0.03) rather than a specific age-course or overall adulthood. Overall, though, the changes were blunted when compared to the more robust overall effects seen with nicotine or chlorpyrifos alone.
As before, we collapsed the main treatment effects across all the interactive variables to illustrate these points (Fig. 3D). The overall elevations in HC3 binding caused by prenatal nicotine treatment were readily evident, whereas the net effects of chlorpyrifos were distinctly smaller, with opposite directions for males (decrease) and females (increase). Notably, the group receiving combined treatment had the smallest changes, entirely distinguishable from the summation of the individual treatment effects, as demonstrated by a significant two-factor interaction term (nicotine × chlorpyrifos, p < 0.003). For males, there was virtually no overall treatment difference when values were collapsed across region and age, whereas there had been a robust increase in the group receiving just nicotine alone; for females, both nicotine and chlorpyrifos individually produced greater increases than were seen with the combination treatment, rather than an even larger effect that would be expected from summation of the two individual treatment responses.
ChAT activity
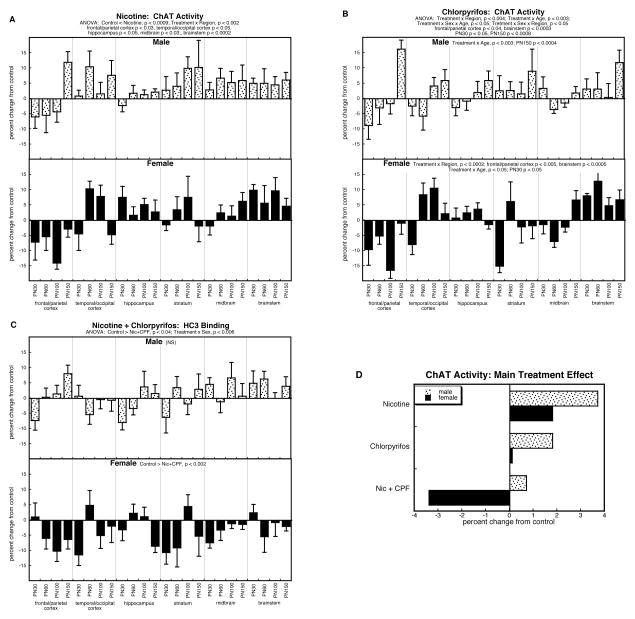
For ChAT, the multivariate ANOVA confirmed a main treatment effect (p < 0.0001) that was interactive with all the other variables: p < 0.05 for treatment × age, p < 0.07 for treatment × sex, and p < 0.003 for treatment × region. The main treatment effect reflected overall increases caused by either nicotine or chlorpyrifos alone, whereas the combined treatment group showed a net reduction rather than the increase seen with either of the individual treatments: control < nicotine, p < 0.0001; con < chlorpyrifos, p < 0.02; nicotine > combination treatment, p < 0.0001; chlorpyrifos > combination treatment, p < 0.0001.
For the group receiving prenatal nicotine treatment, we found a significant overall elevation (p < 0.0009) in ChAT activity extending across both males and females (Figure 4A), albeit with regional selectivity (treatment × region, p < 0.002). The interaction reflected the fact that, despite the significant elevation overall, the frontal/parietal cortex actually displayed a reduction in ChAT, whereas increases were seen in the other regions.
Figure 4.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on ChAT activity. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table 5. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (B,C). Effects on specific regions and ages are stated above or within the panels where appropriate. Panel (D) shows the simple main treatment effects, collapsed across all the other variables.
By itself, postnatal chlorpyrifos treatment had more complex effects, characterized by sex, region and age-dependent differences (Figure 4B). In males, the chief differences reflected a progressive change with age (treatment × age, p < 0.003) characterized once again by the eventual emergence of overall increases on PN150 after earlier stages of smaller effects. In females, the primary disparities reflected regional differences (treatment × region, p < 0.0002), with a net decrease in the frontal/parietal cortex (p < 0.005) but an increase in the brainstem (p < 0.0005). The less significant treatment × age interaction in females (p < 0.05) reflected an overall lowering on PN30 (p < 0.05), despite the fact that the values in the brainstem were increased even at that age point.
For ChAT activity, as for the other synaptic parameters, the combination exposure group showed effects unlike those of the individual treatments (Figure 4C), characterized by a significant overall reduction (p < 0.04 for the main treatment effect) that was highly sex-selective (treatment × sex, p < 0.006). In males, we did not find either an overall elevation, as had been seen with nicotine alone, nor an age progression resulting in eventual increases, as was the case for chlorpyrifos. In females, there was an overall decrease in ChAT evoked by the combined treatment (p < 0.002), opposite to the increase seen with nicotine alone, and lacking the regional selectivity seen for chlorpyrifos.
Again, the unique response in the group receiving both nicotine and chlorpyrifos was readily demonstrable by collapsing the treatment effects across regions and ages (Figure 4D). In males, both nicotine and chlorpyrifos elicited net increases that were larger than those seen with the combination treatment. In females, nicotine produced a clear overall increase and chlorpyrifos produced a minuscule increase, whereas the group receiving combined exposures showed a change in the opposite direction, i.e. a net decrease in ChAT. Accordingly, the nicotine × chlorpyrifos interaction was highly significant (p < 0.0001), representing lower values with the combination relative to the those expected from summation of the individual treatment effects.
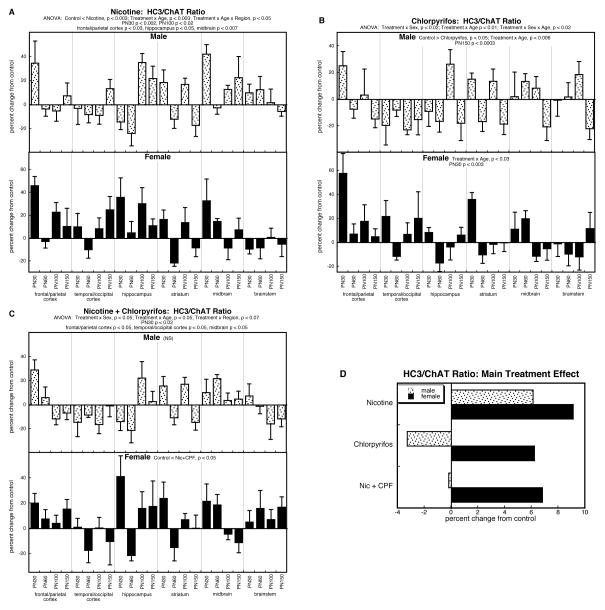
HC3/ChAT ratio
Multivariate ANOVA also indicated treatment effects and interactions for the HC3/ChAT ratio: p < 0.007 for the main treatment effect, p < 0.02 for treatment × age, p < 0.08 for treatment × sex, and p < 0.05 for treatment × sex × age. The main treatment effect reflected overall higher values for the nicotine group compared to all others: p < 0.005 vs. control, p < 0.002 vs. chlorpyrifos, p < 0.0001 vs. the combined treatment group.
By itself, prenatal nicotine treatment evoked an overall increase in the HC3/ChAT ratio (main treatment effect, p < 0.003) that also displayed age- and regional dependence (treatment × age, p < 0.003; treatment × age × region, p < 0.05; Figure 5A). The time course again reflected a progressive change, from predominant increases in adolescence (PN30, p < 0.002) and young adulthood (PN100, p < 0.02), that regressed by full adulthood (not significant by PN150); the regional selectivity originated in significant increases in the frontal/parietal cortex (p < 0.03), hippocampus (p < 0.05) and midbrain (p < 0.007).
Figure 5.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on the HC3/ChAT ratio. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table 6. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests for each sex were carried out only where there was a treatment × sex interaction (B,C). Effects on specific regions and ages are stated above or within the panels where appropriate. Panel (D) shows the simple main treatment effects, collapsed across all the other variables.
The effects of postnatal chlorpyrifos were dominated by sex-dependent differences (treatment × sex, p < 0.02) superimposed on age-related effects (treatment × age, p < 0.01; treatment × sex × age, p < 0.02; Figure 5B). In males, chlorpyrifos elicited an overall reduction in the ratio (main treatment effect, p < 0.05) that progressed with age to emerge most strongly in full adulthood (treatment × age interaction, p < 0.006; individually significant on PN150, p < 0.0009). In females, there was also an age-dependent effect (treatment × age, p < 0.03), reflecting a downward trend; in this case, though, there were early elevations (PN30, p < 0.003) so that values subsequently regressed toward normal in adulthood. Thus, both sexes showed a declining value with age, progressing from normal to subnormal in males, and from elevated to normal in females.
As was true for the effects of the combination treatment on the individual measures of HC3 binding and ChAT activity, the HC3/ChAT ratio showed alterations unlike those of the two individual treatments (Figure 5C). There were significant sex differences (treatment × sex, p < 0.05) that reflected an overall nonsignificant change in males but a net elevation in females (p < 0.05). However, the net differences, regardless of sex, were influenced by age and region (treatment × age, p < 0.05; treatment × region, p < 0.07). The age effect once again represented a progressive change, characterized by an initial elevation (PN30, p < 0.02) that regressed over time. The regional selectivity involved significant increases in the frontal/parietal cortex and midbrain, but reductions in the temporal/occipital cortex (all significant at p < 0.05).
The main treatment effects, collapsed across region and age, again illustrate some of the essential features (Figure 5D). Nicotine treatment produced overall elevations in both males and females, whereas chlorpyrifos elicited decreases in males and increases in females. Nevertheless, the combination treatment again showed smaller changes than would have been expected from simple summation of the two individual sets of treatment effects, with essentially no overall change in males and an increase in females that was smaller than that seen with nicotine alone, and clearly less than would be expected from summation of the individually robust increases seen with nicotine or chlorpyrifos. Thus, the combination treatment once again produced overall lower values than expected from additive responses. However, because of the complex relationship with sex, age and region, treating the nicotine and chlorpyrifos as two factors in the ANOVA produced significant interactions with the other variables: p < 0.04 for nicotine × chlorpyrifos × age, p < 0.05 for nicotine × chlorpyrifos × sex × region. For the age interaction, the strongest deviation of the combination group from summation of individual effects, was seen on PN30 (nicotine × chlorpyrifos, p < 0.04): 30% expected increase (18% for nicotine + 12% for chlorpyrifos) compared to an observed 12% increase for the combination treatment. For the separation by region, there were significant interactions of the two treatment factors for frontal/parietal cortex (p < 0.03; 20% expected increase vs. 5% observed increase) and temporal/occipital cortex (p < 0.05; no change vs. 8% observed decrease). Separation by sex revealed nicotine × chlorpyrifos interactions for males on PN100 (p < 0.05; 17% expected increase vs. 0% increase) and females on PN30 (p < 0.02; 45% expected increase vs. 19% observed increase). Thus, for every subdivision, the nicotine × chlorpyrifos interaction represented a lower value for the combination treatment than expected from summation of the individual effects of nicotine and chlorpyrifos.
DISCUSSION
Our findings show that prenatal nicotine administration alters the subsequent developmental neurotoxicity of chlorpyrifos in a complex manner, comprising sex-specific, regional and temporal effects on the expression of the synaptic proteins that most closely delineate ACh function. Despite that complexity, there was a clear overall pattern. The combined exposure always lowered values relative to those of the individual treatments or to the values expected from simple additive effects, producing a unique, global interference with emergence of the ACh phenotype, an effect not seen with nicotine or chlorpyrifos alone. Depending upon sex, age and region, nicotine or chlorpyrifos by themselves evoked both increases and decreases in expression of nAChRs, the high-affinity presynaptic choline transporter and ChAT. Consequently, where an individual treatment evoked an increase in a given parameter, the superimposed reduction from combined treatment appeared to “protect” by restoring those values closer to normal. However, our results show quite clearly that this is probably not protection against the initial brain injury, but rather a failure of adaptive responses: in every case, the effects on ACh parameters showed progressive changes between adolescence and adulthood, not persistence of an initial effect. This conclusion is reinforced by similar findings in our previous work with the combination of prenatal dexamethasone and postnatal chlorpyrifos, which found that apparent protection against the effect of chlorpyrifos on ACh and serotonin pathways actually connoted interference with compensatory adaptations, thus worsening the outcomes for synaptic and behavioral performance (Levin et al., 2014; Slotkin et al., 2013, 2014a).
Each of these conclusions is bolstered by specific findings for the ACh synaptic variables. First, the overall reduction seen with combined exposure is evident from the main treatment effects collapsed across region and age. For nAChRs, nicotine by itself produced a small increase whereas chlorpyrifos elicited an increase in males and a decrease in females; with combined exposure, males showed a decrease that was not seen with either agent alone and females showed a larger decline than that seen with chlorpyrifos alone. For HC3 binding, nicotine again produced increases in both sexes, whereas chlorpyrifos produced an increase only in females; with combination exposure, the effect in males was reduced virtually to control values and females showed a smaller increase than with either agent alone. For ChAT, the individual treatments always produced an increase, larger in males than females; the combined exposure elicited a smaller increase in males and a large decrease in females.
Second, subdividing these treatment effects to consider temporal and regional patterns, we found multiple changes pointing to progressive changes in synaptic function. For nAChRs, the uniform finding was a progression from early increases to subsequent declines in expression, primarily involving nerve terminal regions in preference to cell bodies, pointing to regulatory adjustments at the synaptic level rather than loss of ACh neurons per se. For HC3 binding, the effects of chlorpyrifos alone showed a progression in males to produce significant deficits by PN150; it is this progressive change that was specifically blunted when chlorpyrifos was preceded by prenatal nicotine treatment. For ChAT activity, nicotine produced an overall net elevation, but one terminal region (frontal/parietal cortex) actually showed a significant decrease, pointing again to specific regulatory changes in nerve terminal zones rather than a generalized reduction throughout the brain; the same was true for chlorpyrifos in females (decreases in frontal/parietal cortex but increases in brainstem) and in males we also found a progressive change resulting in significant increases by PN150. Again, prenatal nicotine administration blunted the emergence of both the temporal and regional changes in ChAT that were elicited by the individual treatments.
Third, for the HC3/ChAT ratio, the overall increases caused by nicotine diminished with age, whereas the effects of chlorpyrifos increased with age in males and decreased with age in females. Again, the main principle is that there are progressive changes between adolescence and adulthood, not just persistent effects of an initial injury by each agent. Here, too, the combination treatment displayed comparable temporal and regional selectivities, superimposed on the net effect of producing overall lower values relative to those seen with the individual treatments. Accordingly, each subdivision of the data supports the main point, that the alterations in ACh synaptic proteins elicited by each treatment individually, or by the combined exposure, represents a specific set of adaptations that evolves over time after the initial brain injury.
As has been shown in prior studies of the developmental neurotoxicity of nicotine and chlorpyrifos individually (Aldridge et al., 2004; Levin et al., 2001, 2002; Rauh et al., 2012; Slotkin, 2005; Slotkin et al., 2001, 2013, 2014a; Slotkin and Seidler, 2011), we found a high degree of sex-selectivity of the effects of each agent and of the combination treatment. As presented in those earlier reports, this does not likely represent a difference in the initial effects of the toxicants but rather the sex-dependence of adaptive mechanisms, such as neural cell replacement. Again, then, the impact of prenatal nicotine on the subsequent developmental neurotoxicity of chlorpyrifos points to changes in the temporal pattern of regulatory adjustments to that injury. Nevertheless, we did measure cholinesterase activity to determine whether nicotine might change the initial toxicity of chlorpyrifos. We found a significantly greater initial inhibition of brain cholinesterase when chlorpyrifos was given to nicotine-exposed animals but the difference was quite small (25% inhibition vs. 15% inhibition), remaining well below the 70% inhibition required for signs of systemic toxicity (Clegg and van Gemert, 1999); notably, we did not observe any growth inhibition that would otherwise be expected from enhanced overall toxicity of chlorpyrifos. Furthermore, the effects on cholinesterase did not show sex-dependence, whereas the subsequent alterations in ACh synaptic markers did. It is thus unlikely that this initial difference in cholinesterase inhibition provides the mechanism underlying the unique effects of the combined exposure to nicotine and chlorpyrifos. Indeed, it may prove quite difficult to identify exactly how nicotine alters the subsequent response to chlorpyrifos. Nicotine shares many of the ACh-related targets for developmental neurotoxicity as does chlorpyrifos (Slotkin, 1999, 2004) but at the same time, nicotine has neuroprotective properties (Belluardo et al., 2000; Ferrea and Winterer, 2009; Kawamata and Shimohama, 2011). We already have some evidence with uniform neuronotypic cell cultures, demonstrating stage-specific damage or neuroprotection by nicotine, involving specific events in mitosis and neurodifferentiation (Qiao et al., 2003b, 2005; Slotkin et al., 2007b), but these distinctions will be hard to make in vivo, where cell populations are not in uniform stages of differentiation. In any case, though, enhanced initial injury cannot explain the temporal emergence of the changes in ACh synaptic proteins seen here, nor does it provide insight into sex-dependence of those effects.
In any biomarker study, it is important to relate the magnitude of the changes to functionally-relevant outcomes. We found highly significant changes in ACh synaptic parameters that showed consistency across multiple brain regions and age points but in general, the magnitude of the effects ranged from 10–40% difference from control values. These are likely to underestimate the actual impact on ACh synaptic function because the biochemical evaluations were conducted gross regional dissections, so that more robust effects on smaller subregions would have been diluted with inclusion of larger amounts of less affected areas. Indeed, our findings compare favorably with known treatments and disease states that target ACh systems. Upregulation of nAChRs in fetal brain amounts to about a 30% increase during the course of nicotine administration (Slotkin et al., 1987), compared to about 50% in older animals given high doses of nicotine over a long period (Trauth et al., 1999); in the latter, the effects are no longer evident within a few weeks after stopping nicotine treatment, whereas here, we found persistent effects ranging from 20–30%, months after the termination of fetal nicotine exposure. In freshly-obtained brain regions from patients with Alzheimer’s Disease, there is about a 30% decrease in ChAT (Slotkin et al., 1994). For HC3 binding, the magnitude of changes seen here are about half those seen in Alzheimer’s Disease (Pascual et al., 1991; Slotkin et al., 1994) or with complete tonic inhibition produced by differentiation of ACh neurons (Murrin et al., 1977; Simon et al., 1976). Most importantly, these effects on HC3 and ChAT are known to be mechanistically responsible for adverse effects on cognitive function (Levin et al., 2001; Slotkin, 2005). A second issue is raised simply by the scope of a large study in which there are hundreds of measurements and potentially, thousands of individual intergroup comparisons, almost akin to an “omics” study. In this case, it is critical to consider only those effects that are reinforced by consistency across multiple brain regions and/or time points, to protect against the possibility that any individual difference might represent a random finding. Here, our interpretations were restricted to conclusions reached from large groupings of data points using multivariate ANOVA, rather than on single-point comparisons. There is a trade-off: this conservative approach reduces the apparent magnitude of effects (combining data points showing large changes with those showing smaller changes), but provides the necessary protection against false-positive conclusions that would otherwise arise from more detailed analysis of individual data points.
In a double-treatment study, such as the present one, there is a potential concern that stress caused by the prenatal treatment could contribute to adverse effects evoked by subsequent toxicant exposure. In the adult, concurrent stress augments the systemic toxicity and neurotoxicity of organophosphates (Basha and Poojary, 2011, 2012; Pung et al., 2006; Shaikh et al., 2003) but to our knowledge, there are no corresponding studies for stress in pregnancy. Here, the prenatal stressor was the surgical procedure to insert the minipumps. We minimized stress contributions by including control pump implants, followed by either postnatal vehicle or chlorpyrifos administration, thus permitting comparison of the doubly-exposed group to those receiving only the prenatal stressor. Furthermore, the surgery was carried out on the fourth day of gestation, two days prior to the implantation of the embryo in the uterine wall, so that there was no communication between maternal and fetal circulation at the time of surgery; indeed, any anomalies at that stage typically lead to failure of the embryo to implant or to spontaneous abortion, neither of which were noted here or in earlier studies with the same design (Slotkin et al., 2010; Slotkin and Seidler, 2010, 2011). Nevertheless, we cannot absolutely rule out a stress × drug interaction. To that end, we compared the effects of chlorpyrifos alone in the present study, which includes the factor of maternal surgical stress early in gestation, with those seen in an earlier series of experiments in which dams received milder stress (subcutaneous injections) late in gestation, followed by the same postnatal chlorpyrifos regimen (Slotkin et al., 2013). The chlorpyrifos effects showed no significant series × treatment interaction, indicating that any differences between the two series of studies were not distinguishable from random events; in both series, the main conclusion was that chlorpyrifos suppressed presynaptic ACh activity selectively in males.
A second concern is that, because rats are altricial, gestational nicotine administration exposure corresponds to only the first two trimesters of human brain development; no consistent models have yet been developed to maintain comparable nicotine levels through the first two postnatal weeks, as would be required to mimic pan-gestational human fetal exposure. Accordingly, nicotine effects seen in the rat model are minimized relative to the potential impact of maternal smoking or nicotine-delivery products on human fetal brain development. Again, we can make use of comparisons of the present results for nicotine alone with those in an earlier series with the same dose and initiation point, but where a different pump model was used that administered nicotine for nearly one additional week (Slotkin et al., 2010), thus extending exposure into the postnatal period via transfer in the milk (Ilett et al., 2003; Narayanan et al., 2002). In both studies, nicotine elicited increases across all three ACh synaptic markers, and again, we saw no series × treatment interaction that would point to a significant difference in nicotine’s effects between the two exposure paradigms.
In conclusion, the present results indicate that prenatal exposure to nicotine alters the subsequent developmental neurotoxicity of chlorpyrifos, with the combined exposure producing an outcome not seen with either agent alone, namely a global interference with the emergence of synaptic proteins characteristic of the ACh phenotype. Because both nicotine and chlorpyrifos are themselves developmental neurotoxicants that influence neurodifferentiation, the unique effect seen with double exposure is superimposed on the direct actions of each toxicant, producing a complex regional and temporal pattern of effects that can be interpreted only by studying a full time course and multiple brain regions containing ACh nerve terminals and cell bodies. Nevertheless, there is a clear-cut overall conclusion, namely that the interference with development of the ACh phenotype uniquely seen with the combined exposure is both “different” from, and “worse” than either treatment alone. When this is superimposed on increases in ACh synaptic parameters imposed by the individual treatments, the resultant effect may appear to be “better” because the values become closer to normal, but it would be erroneous to conclude that nicotine actually protects ACh circuits from the damage caused by chlorpyrifos. Further, the fact that the adverse effects show a clear-cut progression from adolescence to adulthood indicates that the interaction of nicotine and chlorpyrifos is not simply a greater degree of initial brain injury, but rather includes the subsequent adaptive changes to that injury. Accordingly, even where prenatal nicotine appears to diminish the response to chlorpyrifos, this does not necessarily connote protection from injury, but rather points to failed adaptation that could worsen the functional outcome of exposure. Indeed, this is precisely the case found in our prior study with dexamethasone, which likewise prevented adaptive responses to chlorpyrifos, resulting in more profound deterioration of synaptic function and greater behavioral disruption (Levin et al., 2014; Slotkin et al., 2013, 2014a); we are currently conducting studies to provide similar evaluations of the behavioral consequences of the combination of prenatal nicotine and postnatal chlorpyrifos exposure. Finally, this study provides a proof of principle that an individual’s “chemical history” of prenatal exposure can influence the subsequent sensitivity to commonly-encountered environmental neurotoxicants. Given that human exposures to nicotine and chlorpyrifos are widespread, our results have clear implications for the existence of a subpopulation with heightened neurotoxic vulnerability.
Supplementary Material
Nicotine (maternal smoking) and organophosphate pesticide coexposures are common
We gave nicotine to rats prenatally, followed by neonatal chlorpyrifos
Combined exposure uniquely suppressed development of acetylcholine synaptic proteins
Nicotine exposure may create a subpopulation that is vulnerable to neurotoxicants
Acknowledgments
Acknowledgments/disclaimers: The authors thank Jennifer Card, Ashley Stadler and Samantha Skavicus for technical assistance. Research was supported by NIH ES010356. TAS has received consultant income in the past three years from the following firms: Acorda Therapeutics (Ardsley NY), The Calwell Practice (Charleston WV), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), Alexander Hawes (San Jose, CA), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX), Shanahan Law Group (Raleigh NC), and Chaperone Therapeutics (Research Triangle Park, NC).
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- ChAT
choline acetyltransferase
- HC3
hemicholinium-3
- nAChR
nicotinic acetylcholine receptor
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaça Y, Seidler FJ, Qiao D, Slotkin TA. Modeling the developmental neurotoxicity of nicotine in vitro: cell acquisition, growth and viability in PC12 cells. Dev Brain Res. 2005;154:239–246. doi: 10.1016/j.devbrainres.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha PM, Poojary A. Chlorpyrifos induced region specific vulnerability in rat CNS and modulation by age and cold stress: an interactive study. Neurochem Res. 2011;36:241–249. doi: 10.1007/s11064-010-0311-3. [DOI] [PubMed] [Google Scholar]
- Basha PM, Poojary A. Oxidative macromolecular alterations in the rat central nervous system in response to experimentally co-induced chlorpyrifos and cold stress: a comparative assessment in aging rats. Neurochem Res. 2012;37:335–348. doi: 10.1007/s11064-011-0617-9. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Blum M, Fuxe K. Central nicotinic receptors, neurotrophic factors and neuroprotection. Behav Brain Res. 2000;113:21–34. doi: 10.1016/s0166-4328(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health. 1999;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and Sudden Infant Death Syndrome. J Family Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Anders V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiat. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RAJ. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrea S, Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42:255–265. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VKY. Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J Appl Physiol. 2001;90:1968–1976. doi: 10.1152/jappl.2001.90.5.1968. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is upregulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Happe HK, Murrin LC. High-affinity choline transport regulation by drug administration during postnatal development. J Neurochem. 1992;58:2053–2059. doi: 10.1111/j.1471-4159.1992.tb10946.x. [DOI] [PubMed] [Google Scholar]
- Happe HK, Peters JL, Bergman DA, Murrin LC. Localization of nicotinic cholinergic receptors in rat brain: autoradiographic studies with [3H]cytisine. Neuroscience. 1994;62:929–944. doi: 10.1016/0306-4522(94)90484-7. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opinion Pediatr. 2008;20:184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Berger-Sweeney J. Cholinergic regulation of cortical development and plasticity: new twists to an old story. Perspect Dev Neurobiol. 1998;5:401–425. [PubMed] [Google Scholar]
- Ilett KF, Hale TW, Page-Sharp M, Kristensen JH, Kohan R, Hackett LP. Use of nicotine patches in breast-feeding mothers: transfer of nicotine and cotinine into human milk. Clin Pharmacol Ther. 2003;74:516–524. doi: 10.1016/j.clpt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer’s and Parkinson’s diseases. J Alzheimer Dis. 2011;24(Suppl 2):95–109. doi: 10.3233/JAD-2011-110173. [DOI] [PubMed] [Google Scholar]
- Klemm N, Kuhar MJ. Post-mortem changes in high affinity choline uptake. J Neurochem. 1979;32:1487–1494. doi: 10.1111/j.1471-4159.1979.tb11089.x. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Aldridge JE, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Disruption of rat forebrain development by glucocorticoids: critical perinatal periods for effects on neural cell acquisition and on cell signaling cascades mediating noradrenergic and cholinergic neurotransmitter/neurotrophic responses. Neuropsychopharmacology. 2005;30:1841–1855. doi: 10.1038/sj.npp.1300743. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107(Suppl 1):65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Cauley M, Johnson J, Cooper EM, Stapleton HM, Ferguson PL, Seidler FJ, Slotkin TA. Prenatal dexamethasone augments the neurobehavioral teratology of chlorpyrifos: significance for maternal stress and preterm labor. Neurotoxicol Teratol. 2014;41:35–42. doi: 10.1016/j.ntt.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol Appl Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Murrin LC, DeHaven RN, Kuhar MJ. On the relationship between (3H)choline uptake activation and (3H)acetylcholine release. J Neurochem. 1977;29:681–687. doi: 10.1111/j.1471-4159.1977.tb07786.x. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Wanyun Z, Haley NJ. Nicotine administration to rats: methodological considerations. Life Sci. 1987;40:1699–1708. doi: 10.1016/0024-3205(87)90020-8. [DOI] [PubMed] [Google Scholar]
- Narayanan U, Birru S, Vaglenova J, Breese CR. Nicotinic receptor expression following nicotine exposure via maternal milk. NeuroReport. 2002;13:961–963. doi: 10.1097/00001756-200205240-00012. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids: fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Pascual J, Fontán A, Zarranz JJ, Berciano J, Flórez J, Pazos A. High-affinity choline uptake carrier in Alzheimer’s Disease: implications for the cholinergic hypothesis of dementia. Brain Res. 1991;552:170–174. doi: 10.1016/0006-8993(91)90676-m. [DOI] [PubMed] [Google Scholar]
- Povey AC. Gene-environmental interactions and organophosphate toxicity. Toxicology. 2010;278:294–304. doi: 10.1016/j.tox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Pung T, Klein B, Blodgett D, Jortner B, Ehrich M. Examination of concurrent exposure to repeated stress and chlorpyrifos on cholinergic, glutamatergic, and monoamine neurotransmitter systems in rat forebrain regions. Intl J Toxicol. 2006;25:65–80. doi: 10.1080/10915810500527119. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Abreu-Villaça Y, Tate CA, Cousins MM, Slotkin TA. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Dev Brain Res. 2004;148:43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003a;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003b;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. 7-Year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. Brain anomalies in children exposed to a common organophosphate pesticide. Proc Natl Acad Sci. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MC, Seidler FJ, Qiao D, Tate CA, Cousins MM, Slotkin TA. Does pharmacotherapy for preterm labor sensitize the developing brain to environmental neurotoxicants? Cellular and synaptic effects of sequential exposure to terbutaline and chlorpyrifos in neonatal rats. Toxicol Appl Pharmacol. 2004;195:203–217. doi: 10.1016/j.taap.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Shaikh J, Karanth S, Chakraborty D, Pruett S, Pope CN. Effects of daily stress or repeated paraoxon exposures on subacute pyridostigmine toxicity in rats. Arch Toxicol. 2003;77:576–583. doi: 10.1007/s00204-003-0492-5. [DOI] [PubMed] [Google Scholar]
- Simon JR, Atweh S, Kuhar MJ. Sodium-dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J Neurochem. 1976;26:909–922. doi: 10.1111/j.1471-4159.1976.tb06472.x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Toxicity of Organophosphate and Carbamate Pesticides. San Diego: Elsevier Academic Press; 2005. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos; pp. 293–314. [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Infante A, Seidler FJ. Prenatal dexamethasone augments the sex-selective developmental neurotoxicity of chlorpyrifos: implications for vulnerability after pharmacotherapy for preterm labor. Neurotoxicol Teratol. 2013;37:1–12. doi: 10.1016/j.ntt.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Prenatal dexamethasone, as used in preterm labor, worsens the impact of postnatal chlorpyrifos exposure on serotonergic pathways. Brain Res Bull. 2014a;100:44–54. doi: 10.1016/j.brainresbull.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Stadler A, Levin ED, Seidler FJ. Effects of tobacco smoke on PC12 cell neurodifferentiation are distinct from those of nicotine or benzo[a]pyrene. Neurotoxicol Teratol. 2014b;43:19–24. doi: 10.1016/j.ntt.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at six months of age. Neuropsychopharmacology. 2007a;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007b;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Nemeroff CB, Bissette G, Seidler FJ. Overexpression of the high affinity choline transporter in cortical regions affected by Alzheimer’s Disease: evidence from rapid autopsy studies. J Clin Invest. 1994;94:696–702. doi: 10.1172/JCI117387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL. Development of [3H]nicotine binding sites in brain regions of rats exposed to nicotine prenatally via maternal injections or infusions. J Pharmacol Exp Ther. 1987;242:232–237. [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Seidler FJ. Additive and synergistic effects of fetal nicotine and dexamethasone exposure on cholinergic synaptic function in adolescence and adulthood: implications for the adverse consequences of maternal smoking and pharmacotherapy of preterm delivery. Brain Res Bull. 2010;81:552–560. doi: 10.1016/j.brainresbull.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: interactions of fetal nicotine and dexamethasone on serotonin and dopamine synaptic function in adolescence and adulthood. Brain Res Bull. 2010;82:124–134. doi: 10.1016/j.brainresbull.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: fetal nicotine exposure enhances the effect of late gestational dexamethasone treatment on noradrenergic circuits. Brain Res Bull. 2011;86:435–440. doi: 10.1016/j.brainresbull.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci. 1987;84:595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PR, Lindstrom J. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci. 1988;8:3395–3404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.