Abstract
Background
Despite tremendous growth in research examining the role of cognitive bias in addictive behaviors, scant consideration has been paid to the close association between smoking and drinking behavior. This study sought to determine whether an association between smoking and drinking could be observed at an implicit level using a novel cognitive bias task, as well as characterize the relationship between performance on this task and clinically relevant variables (i.e., heaviness of use/dependence).
Methods
Individuals (N = 51) with a range of smoking and drinking patterns completed a modified Stroop task in which participants identified the color of drinking, smoking and neutral words that were each preceded by drinking, smoking or neutral picture primes. Participants also provided information regarding the heaviness of their smoking and drinking behavior and completed self-report measures of alcohol and nicotine dependence.
Results
Response times to smoking and drinking words were significantly slowed following the presentation of either smoking or drinking picture primes. This effect did not differ across subgroups. However, the strength of the coupling between smoking and drinking prime effects was greater among heavier drinkers, who also exhibited a concordant looser coupling of the effects of smoking and drinking primes on smoking words.
Conclusions
Associations between smoking and drinking can be observed at an implicit level and may be strongest for heavier drinkers.
Keywords: alcohol, tobacco, cognition, attentional bias
1. INTRODUCTION
There exists a close linkage between alcohol and tobacco use across numerous levels of analysis (Funk et al., 2006; McKee and Weinberger, 2013). Research exploring the factors responsible for this association have identified many potential candidates, including shared genetic risk and neural circuitry (Connor et al., 2007; Li et al., 2007), common experiences and personality traits (Elliott et al., 2014; Jackson et al., 2002; VanderVeen et al., 2013), as well as the impact of combined alcohol and nicotine on mood, cognition and substance use motivation (Braun et al., 2012; Oliver et al., 2013; Ralevski et al., 2012). Once a pattern of dual alcohol and nicotine use is established, associative conditioning processes may contribute to its maintenance (Drobes, 2002). Notably, these factors do not necessarily represent competing explanations. Indeed, each likely plays a contributing role in driving the association between alcohol and tobacco use, as do a number of other factors that have yet to be discovered.
Although research supports the presence of cognitive associations between alcohol and tobacco use among dual users (Monti et al., 1995), there has been comparatively little research in this area. This is particularly surprising given the increasing attention being given to information processing biases in recent theoretical accounts of addiction (Franken, 2003; McCusker, 2001; Ryan, 2002). A central component of these theories is the notion that repeated exposure to drugs of abuse increases their salience, resulting in cognitive systems prioritizing the processing of drug-related stimuli over alternatives (Berridge and Aldridge, 2008; Robinson and Berridge, 2008). Extensive efforts have been undertaken to understand this cognitive processing bias in both alcohol and nicotine dependence (Bradley et al., 2004; Ehrman et al., 2002; Field et al., 2013; Munafò et al., 2003; Townshend and Duka, 2001). These biases appear to have relationships with numerous other constructs relevant to addictive behavior, including craving (Field et al., 2009) and impulsivity (Coskunpinar and Cyders, 2013). It has also been suggested the relationship between attentional bias and craving may be mutually excitatory (Franken, 2003). That is, attentional bias may enhance craving by drawing attention to drug cues in the environment that would otherwise pass unnoticed and/or delaying attentional disengagement from drug cues once established. In turn, this enhanced craving may increase attentional bias (Smeets et al., 2009). Furthermore, attentional bias may promote the cognitive elaborations that have been both theoretically and empirically linked to drug use (Kavanagh et al., 2005; May et al., 2014). Indeed, attentional bias has also been shown to predict treatment outcome in both alcohol and nicotine dependence (Cox et al., 2002; Powell et al., 2010; Waters et al., 2003b). Accordingly, interventions designed to directly modify attentional bias have been developed and some have shown promise for helping to promote abstinence (e.g., McGeary et al., 2014; Schoenmakers et al., 2010).
A variety of laboratory tasks have been modified in order to study cognitive biases in drug-cue processing, including visual probe (Ehrman et al., 2002), flicker change blindness (Jones et al., 2003), N-back (Evans et al., 2011), visual search (Oliver and Drobes, 2012) and attentional blink paradigms (Chanon et al., 2010). Perhaps the most widely used task has been the addiction Stroop task (Cox et al., 2006). This task is a modified version of the classic Stroop paradigm (Stroop, 1935), in which individuals must identify the color of both addiction-relevant words and words derived from a “neutral” control category, with the assumption that slower responses to addiction relevant words is due to unintended processing of substance-related information (i.e., an inability to ignore the semantic content of the word). Its utility for measuring cognitive processes relevant to addiction is well-established (Field and Cox, 2008) and it appears to carry psychometric advantages over other measures (Ataya et al., 2012).
Examinations of cognitive biases to multiple types of drugs within the same study have been rare, but are necessary to fully understand the nature and specificity of drug-related cognitive biases (McCarthy and Thompsen, 2006). Similarly, there is evidence suggesting patterns of cognitive bias may differ among dual users (e.g., Cohn et al., 2014), but studies rarely report on the presence of co-occurring addictions. The present study sought to build on the attentional bias literature by explicitly seeking to examine cognitive associations between drinking and smoking through further modification of an addiction Stroop task. The modified version included both smoking and drinking words, as well as words from a neutral category. In addition, each word was preceded by a drinking, smoking or neutral image designed to activate cognitive schema relevant to that substance and potentially cause further delay in response time due to increased processing of salient words (i.e., a priming effect). The use of primes for this purpose has been studied extensively within traditional, affective and addiction Stroop tasks (Kramer and Goldman, 2003; Segal and Gemar, 1997; Stewart et al., 2002). The inclusion of both alcohol and smoking primes and target words enables examination of implicit associations between alcohol and tobacco. We hypothesized that relative to neutral primes, drinking and smoking primes would slow response times on both same-drug (i.e., drinking prime/drinking word and smoking prime/smoking word) and cross-drug (i.e., drinking prime/smoking word and smoking prime/drinking word) trials, but would not impact response time to neutral word trials. We also conducted a number of exploratory analyses aimed at determining: 1) whether effects differed as a function of substance dependence or usage patterns, 2) the correlation between the effects of same-drug and cross-drug primes, and 3) whether this correlation differed as a function of substance dependence or usage patterns.
2. METHODS
2.1 Participants
Individuals (N = 51) who were current users of both alcohol and cigarettes were recruited from the local community as part of a larger study designed to examine the combined effects of alcohol, nicotine and cues on motivation to smoke and drink. The present sample includes only those individuals who completed a modified addiction Stroop task (described below) as part of their baseline session for the study. At the time of scheduling, participants had to report consuming between 1 and 50 drinks per week and smoking at least one cigarette on four or more days per week. All participants were between the ages of 21 and 55, had been smoking regularly for the past two years with a stable smoking pattern in the most recent year, were not actively attempting to quit smoking, and were not regular users of alternative tobacco products.
2.2 Procedures
The session began with informed consent procedures, followed by breath alcohol (BrAC) and carbon monoxide level (CO) readings. BrAC was required to be zero for participation. As light/non-daily smokers were eligible for inclusion, there were no requirements imposed regarding CO level. Next, participants provided a urine specimen that was subjected to a toxicology screen (required to be negative for all drugs except marijuana), cotinine test (required to be > 0) and pregnancy test (females only; required to be negative). A brief medical exam was conducted, including a blood draw for liver enzyme analysis. As the primary study included laboratory sessions involving alcohol administration, participants whose liver enzymes were outside normal limits were excluded from further participation. Similarly, a brief psychological diagnostic interview to assess for current depressive episodes, manic episodes, panic disorder, psychosis, alcohol dependence and drug dependence (SCID-I; First et al., 2012) was conducted and participants who met criteria for any disorder besides alcohol dependence were excluded. Lastly, participants completed a brief interview to assess their recent alcohol use, as well as a battery of self-report measures and computer tasks. Additional details on measures/tasks relevant to the present study are provided below.
2.3 Self-Report and Interview Measures
Single-item questions were used to assess basic demographic information (e.g. age, race, income). A single item asked participants to identify their preferred type of alcohol beverage (beer, liquor, wine) for purposes of tailoring the images and words used in the Stroop task (see below). An abbreviated medical history was also obtained to confirm the absence of exclusionary medical conditions. A detailed smoking history (e.g., cigarettes per day, age at initiation) was obtained; including a brief self-report of nicotine dependence - the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). Alcohol dependence was assessed using the full 25-item version of the Alcohol Dependence Scale (ADS; Skinner and Allen, 1982) but was scored according to the reduced 9-item version developed for community samples (Kahler et al., 2003). In this latter scoring method, 9 items from the original ADS are recoded into binary (yes/no) outcomes and then summed, resulting in a 0–9 scale in which higher numbers reflect greater alcohol dependence. The internal consistencies of both the FTND and the 9-item ADS were in the acceptable range (α’s = .72–.74). Additional details regarding participants’ recent drinking behavior was collected via a Timeline Followback interview (TLFB; Sobell and Sobell, 1995) that assessed alcohol use over the previous 30 days, though drinking statistics were computed based on only the previous 28 day to prevent the inclusion of additional weekend days from impacting estimates of drinking behavior.
2.4 Cross-Primed Stroop Task
This modified Stroop task consisted of a series of trials involving the presentation of drinking, smoking and neutral “prime” images, followed by drinking, smoking and neutral words presented in colored (red, green, or blue), uppercase text. Neutral images and words consisted of common household items (e.g., tools, kitchen utensils). In order to enhance the personal relevance and salience of the stimuli, all drinking images and a subset of drinking words were tailored to participants’ stated beverage preference (beer, liquor or wine). Due to the minimal perceptual differences among cigarette types and the complexity of developing balanced word lists that could adequately address preferences, a single set of smoking images and words was used. Each trial consisted of: 1) a fixation cross (500 ms duration), 2) a blank screen (500 ms), 3) the prime image (800 ms), 4) a blank screen (500 ms) and 5) the target word (2000 ms). Participants were instructed to respond as quickly as possible by identifying the color of the word via buttons labeled “R”, “G” and “B”. The task was programmed in Superlab Version 4.0.7b and participants used an RB-730 Response Pad (Cedrus Corporation; San Pedro, CA). A total of 90 trials were presented, divided evenly among nine different trial types that fully crossed the three prime categories with the three word categories. To ensure an even dispersion of trial types over the course of the task, trials were divided into ten blocks with one of each trial type included in each block. Participants were randomly assigned to one of five pseudorandom block orders and trials within each block were presented in a random order for each participant. Images and words in the present study were drawn from stimuli used in previous studies of cue reactivity and attentional bias (Brandon et al., 2011; Evans et al., 2011; Gilbert and Rabinovich, 1999; Stritzke et al., 2004) as well as public online databases. A diverse set of 10 images were selected as primes for each category. Images were selected to deliberately allow significant variance in complexity (i.e., simple foreground objects versus complex scenes) and perceptual characteristics (e.g., brightness, contrast), though efforts were made to achieve rough equivalence across categories. Six out of the ten images in all but one category contained some sort of human element (e.g., hand holding a drink/cigarette, scene containing people). The only exception was the wine category, which had five images with a human element. Words also varied significantly across several dimensions, including length and orthographic frequency, though again efforts were made to ensure balance across categories. Five of ten drinking words were tailored to beverage preference, with the balance being words that were applicable to all beverage preferences (e.g., “alcohol”). (The following words were used in the present study: General Drinking-ALCOHOL, BAR, BARTENDER, DRINK, TAVERN; Beer Preference – ALEHOUSE, BEER, BREW, BREWERY, LAGER; Liquor Preference – COCKTAIL, MALT, SHOT, TEQUILA, VODKA; Wine Preference – CORK, FERMENT, PINOT, VINEYARD, WINE; Smoking – ASH, CIGARETTE, DRAG, FILTER, MATCH, MENTHOL, NICOTINE, PUFF, SMOKE, TOBACCO; Neutral – BOLTS, COMPASS, FORKS, HAMMER, KEY, NAIL, PICK, SKILLET, UMBRELLA, YARDSTICK. Images used in the present study are available from the corresponding author.)
For purposes of analysis, objective indices of perceptual characteristics of interest were extracted from images using MATLAB. Brightness and contrast were computed by converting the image to greyscale and taking the mean and standard deviation (respectively) of the resulting matrix of pixels. Color images were then saved as JPEG files and compressed, with the compression ratio (compressed file size/uncompressed file size) used as an index of complexity (Forsythe et al., 2008). All images were coded for the presence/absence of a human element. Words were coded for length (number of letters) and whether or not they were tailored to participants’ preferred beverage. Orthographic frequency statistics were obtained using MCWord (Medler and Binder, 2005). One word (“PINOT”) was not included among the 66,372 unique words in this database so its orthographic frequency was imputed as zero.
2.5 Data Processing and Analysis
Analyses employed a crossed random effects model (Baayen et al., 2008; Locker et al., 2007). This approach carries numerous advantages over traditional repeated measures analysis, including the ability to handle continuous covariates and the use of maximum likelihood estimation to better account for missing data at the trial level (including inaccurate responses). The use of mixed models also provides the ability to examine and control for trial-level effects (Waters et al., 2005). Critically, inclusion of random effect terms for images and words in addition to participants provides a way to account for the fact that images and words are also drawn from a larger population of potential images and words. Traditional analytic approaches for attentional bias treat these items as fixed effects. Other fields of research have frequently noted the potential for spurious findings due to systematic bias in the selection of stimuli included in the study (Locker et al., 2007), but this issue has been given only cursory consideration within the attentional bias literature. Given the lack of previous research and therefore exploratory nature of examination of trial-level effects, a full model was run that included random effects for participant, image and word, as well as fixed effects for prime type, word type, their interaction and all possible trial-level covariates. Insignificant predictors were backed out in a second step, in order to produce a more parsimonious model. Potential moderators (e.g., dependence level) were then examined individually to determine if they interacted with the experimental effects.
Only after this point were response times on accurate trials averaged for each prime/word combination (as in traditional analyses of attentional bias data). In order to characterize the association between the effects of smoking and drinking primes for each word type, partial correlations were computed with response time on neutral prime trials for the appropriate word type included as a covariate. A multiple regression framework was used to determine if the strength of this association varied as a function of individual differences in dependence levels or alcohol/cigarette use. Response times for same-drug and neutral prime trials were entered into a model predicting response time on cross-drug prime trials separately for each word type of interest (drinking, smoking). Interactions between the individual difference variables of interest and same-drug response times were then tested in separate regression models.
3. RESULTS
3.1 Sample Characteristics
Sample characteristics are presented in Table 1. Overall, participants appear reflective of a broad range of smoking and drinking patterns. There was no evidence of a correlation between alcohol dependence and nicotine dependence (r = −.129, p = .366) and a modest positive correlation between cigarettes per day and drinks per week (r = .340, p = .015).
Table 1.
Sample Characteristics
| Variable | Percentage or Mean (SD) |
Range |
|---|---|---|
| Demographics | ||
| Sex (% Female) | 31.4% | --- |
| Race | --- | |
| Black/African-American | 27.5% | --- |
| White/Caucasian | 72.5% | --- |
| Ethnicity (% Hispanic) | 5.9% | --- |
| Income (% <$20,000) | 45.1% | --- |
| Age | 34.2 (9.8) | 21–54 |
| Years of Education | 13.2 (2.9) | 2–18 |
| Drinking | ||
| Preferred Alcohol Beverage: | ||
| Beer | 51.0 | --- |
| Liquor | 39.2 | --- |
| Wine | 9.8 | --- |
| Alcohol Use Diagnoses: | ||
| No Diagnosis | 61.2% | --- |
| Abuse | 20.4% | --- |
| Dependence | 18.4% | --- |
| Drinks per Week | 16.3 (11.4) | 1–47 |
| Drinks per Drinking Day | 4.65 (2.17) | 1–12 |
| Percent Drinking Days | 52.2 (28.2) | 5–100 |
| ADS-9 | 3.5 (2.5) | 0–8 |
| Smoking | ||
| Cigarettes Per Day | 15.0 (7.9) | 2–40 |
| Years of Regular Smoking | 15.5 (9.9) | 2–36 |
| FTND | 4.2 (2.6) | 0–9 |
Note. ADS-9 = Alcohol Dependence Scale – 9 Item Version; FTND =Fagerström Test for Nicotine Dependence.
3.2 Stroop Task Psychometric Properties
Accuracy on the task was high, with participants responding correctly on 98.2% of trials, incorrectly on 1.0% of trials and failing to respond or responding outside the allotted time window on 0.8% of trials. It was decided a priori to exclude response times more than 3 SDs above or below the trial type mean (computed separately for each individual) or below an absolute cutoff of 200 ms, but no trials met this criterion. The final sample for analysis included 4,508 discrete trials disbursed among 51 participants, with no more than 10 trials missing for any given participant.
In light of recent concerns regarding the reliability of attentional bias tasks (Ataya et al., 2012), we computed Cronbach’s α for the response times of each trial type. To accommodate the sporadic missing data while computing these values, response times for trials with inaccurate or absent responses were imputed using the mean response time for trials of the same type for that participant. Internal consistency of response times was good across all trial types (α’s = .88 – .91) and was not substantially improved by the removal of any individual trials (largest improvement was .004). Response times did not differ across block order assignments [F (4, 46.0) = 1.44, p = .237], nor as a function of alcohol preference [F (2, 48.2) = 0.87, p = .427].
3.3 Stroop Task Outcomes
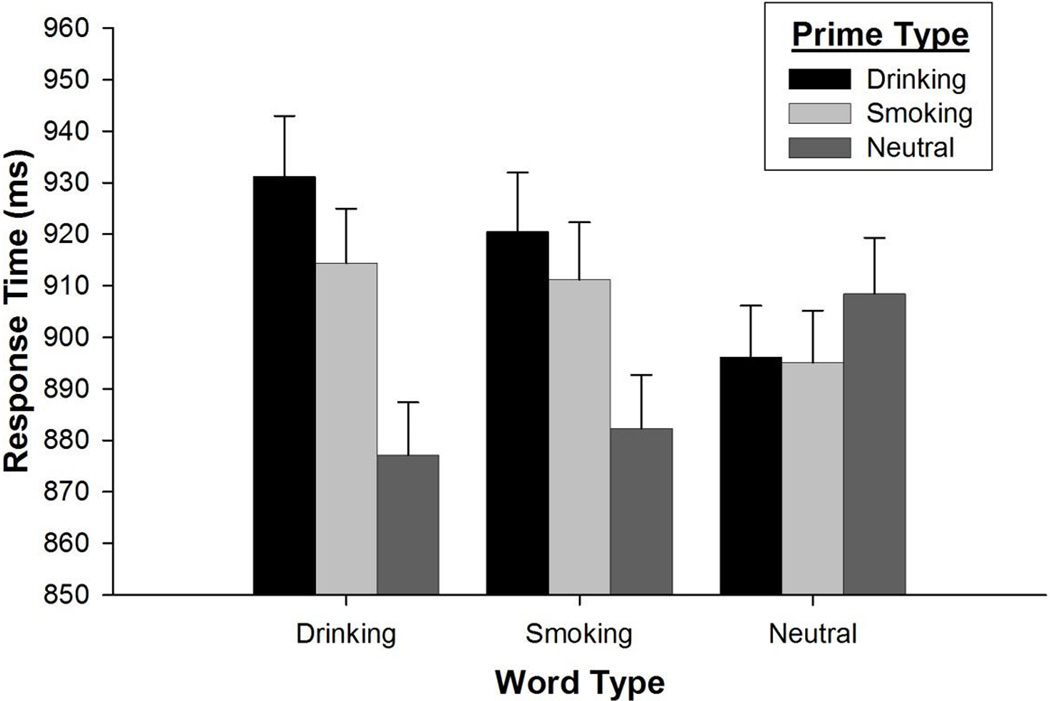
A preliminary model examined the effects of prime and word types after controlling for several possible extraneous trial-level effects (e.g., picture brightness, word length, etc.). Results from this model indicated the vast majority of these variables were unrelated to response time. The sole exceptions were a highly significant effect indicating response times decreased across trials [F (1, 4140.0) = 148.10, p < .001] and a trend-level finding indicating somewhat slower response times on trials with preference-tailored words [F (1, 37.7) = 3.54, p = .068]. The analysis was then repeated with other predictors removed from the model, producing a more parsimonious model with equivocal fit [Χ2 (6) = 1.21, p = .976]. Results from the final model indicated the presence of a highly significant prime type×word type interaction [F (4, 4371.5) = 4.34, p = .002]. Parameter estimates from both models are presented in Table 2. When this interaction was broken down by word type, a significant effect of prime type was found for drinking words [F (2, 33.7) = 4.74, p = .015], with both drinking (p = .004) and smoking (p = .055) primes resulting in slowed response times relative to neutral primes. A similar, albeit weaker effect of prime type was observed for smoking words [F (2, 29.62) = 3.02, p = .064], with drinking (p = .022) and smoking (p = .097) primes again resulting in slowed response times relative to neutral primes. Neutral words were unaffected by primes [F (2, 27.25) = 0.31, p = .738]. Experimental effects are illustrated in Figure 1.
Table 2.
Parameter Estimates from Crossed Random Effects Model
| Full Model | Final Model | |
|---|---|---|
| Est (SE) | Est (SE) | |
| Fixed Effects | ||
| Intercept | 676.768*** (2.992) | 677.654*** (2.788) |
| Experimental Effects | ||
| Alcohol Prime | 0.009 (1.639) | −0.605 (1.305) |
| Smoking Prime | −0.374 (1.787) | −1.076 (1.369) |
| Alcohol Word | −4.265* (1.624) | −4.307** (1.574) |
| Smoking Word | −2.513† (1.370) | −2.549† (1.377) |
| Alc Prime × Alc Word | 5.922*** (1.613) | 5.926*** (1.612) |
| Alc Prime × Smk Word | 4.565** (1.609) | 4.565** (1.609) |
| Smk Prime × Alc Word | 4.918** (1.615) | 4.919** (1.615) |
| Smk Prime × Smk Word | 4.174** (1.609) | 4.175** (1.609) |
| Task Variables | ||
| Trial Number | −0.130*** (0.011) | −0.130*** (0.011) |
| Prime Variables | ||
| Contains Human | 0.676 (0.951) | ----- |
| Brightness | 1.578 (3.502) | ----- |
| Contrast | −0.967 (7.575) | ----- |
| Complexity | −0.829 (1.612) | ----- |
| Word Variables | ||
| Preference Tailored | 2.630† (1.492) | 2.598† (1.382) |
| Length | 0.088 (0.262) | ----- |
| Orthographic Frequency | −0.005 (0.016) | ----- |
| Random Effects | ||
| Residual | 3.252*** (0.070) | 3.251*** (0.070) |
| Subject Intercept | 3.332*** (0.667) | 3.331*** (0.667) |
| Prime Intercept | 0.027† (0.014) | 0.030* (0.015) |
| Word Intercept | 0.028† (0.015) | 0.029* (0.014) |
| Fit Statistics | ||
| −2 Log Likelihood | −2371.98 | −2370.77 |
| # Estimated Parameters | 21 | 15 |
Note. Due to log-scaling of the DV, parameter estimates and standard errors have been multiplied by 100.
p < .001;
p < .01;
p < .05;
p < .10
Figure 1.
Response Time as a Function of Prime and Word Types
Note. Bars represent standard error of the mean.
3.4 Moderation of Stroop Task Outcomes
Substance use patterns and dependence levels were all examined as potential moderators of Stroop task outcomes. Participants were categorized as light (52.9% of participants) or heavy (47.1%) drinkers in accordance with the criteria set forth by the Substance Abuse and Mental Health Services Association (i.e. ≥ 5 drinks on ≥ 5 of the past 30 days; SAMHSA, 2003). Per the criteria established by the Centers for Disease Control and Prevention, light smoking (41.2% of participants) was defined as ≤ 15 cigarettes per day, whereas moderate-heavy smoking (58.8%) was defined as > 15 cigarettes per day (CDC, 2011). Neither of these variables, nor their interaction was found to moderate the effects of prime category, word category or the prime×word category interaction (all p’s > .05). The pattern of results was identical when continuous versions of smoking and drinking heaviness were analyzed (i.e. cigarettes per day, drinks per week). Similarly, nicotine dependence (FTND score), alcohol dependence (ADS-9 score) and their interaction had no moderating influence on any of the task effects (all p’s > .05).
3.5 Drinking-Smoking Prime Associations
Partial correlation coefficients revealed a strong positive association between response times on drinking prime/drinking word trials and smoking prime/drinking word trials after adjusting for response times on neutral prime/drinking word trials, r (48) = .448, p = .001. A positive association was also observed between response times on smoking prime/smoking word trials and drinking prime/smoking word trials after adjusting for response times on neutral prime/smoking word trials, r (48) = .298, p = .036.
3.6 Predictors of Drinking-Smoking Prime Associations
As mentioned previously, a series of regression models were run to examine whether the strength of the association between same-drug and cross-drug priming effects differed as a function of individual differences in dependence or usage characteristics. Continuous measures of dependence and usage were employed for these analyses. Results are presented in Table 3. Individuals with higher levels of alcohol dependence or heavier drinking had a stronger association between response times on drinking prime/drinking word trials and smoking prime/drinking word trials. That is, heavier (or more dependent) drinkers exhibited a tighter coupling of same-drug and cross-drug priming effects for drinking words. Individuals with higher levels of alcohol dependence also exhibited a weaker association between response times on smoking prime/smoking word trials and drinking prime/smoking word trials. Smoking variables were unrelated to the coupling of priming effects.
Table 3.
Regression Models Predicting Drinking-Smoking Associations
| Moderator Variables | Drinking Words | Smoking Words | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | |
| Drinking Variables | ||||||
| Alcohol Dependence | 4.08 | [0.76, 7.40] | .017 | −5.38 | [−9.61, −1.16] | .014 |
| Drinks Per Week | 1.09 | [0.37, 1.82] | .004 | −0.49 | [−1.51, 0.53] | .336 |
| Smoking Variables | ||||||
| Nicotine Dependence | −0.18 | [−3.01, 2.65] | .898 | 1.65 | [−1.88, 5.17] | .351 |
| Cigarettes Per Day | 0.57 | [−0.71, 1.85] | .377 | 0.20 | [−1.22, 1.63] | .775 |
Note. Parameters reflect interactive effects of each moderator variable with the concordant prime in predicting discordant prime trials of the specified word type. Main effects were included in the model but are not reported. Due to log scaling, estimates and CI’s have been multiplied by 100. Significant effects are bolded.
4. DISCUSSION
Taken together, the above results support an implicit association between drinking and smoking. Consistent with our hypotheses, both same-drug and cross-drug primes significantly delayed response latencies to drinking and smoking words. Response times decreased across the course of the task, which is suggestive of practice effects. There was also some evidence to support slower responses to words that were preference-tailored. Yet critically, the impact of same-drug and cross-drug primes was robust to these and other extraneous variables. Furthermore, the effects were robust across various levels of smoking and drinking severity, suggesting an omnipresent association between smoking and drinking behavior regardless of the heaviness of use. This result is not surprising, given alcohol and nicotine are frequently used together even among light/social users (Piasecki et al., 2011; Shiffman et al., 2012). However, the presence of this association even among lighter users is notable, as it may play a role in driving the co-occurring use that happens across the full range of alcohol and nicotine users.
The observed results also provide important methodological insight regarding the Stroop task. Previous research has demonstrated the presence of carry-over effects on similar tasks, with salient stimuli from previous trials slowing responses on future trials (Sharma and Money, 2010; Waters et al., 2005; Waters et al., 2003a). Coupled with more robust findings for blocked versions of the addiction Stroop task (Cox et al., 2006), this has led to the suggestion that Stroop effects are driven by cue reactivity and/or cognitive elaboration on previously presented salient stimuli rather than biased processing of the semantic content of the stimuli presently on the screen. Results from the primed Stroop task provides further support for this view, as delayed responses to substance-related words were limited to situations where they were preceded by substance-related images. Our results also indicated that participants responded more slowly to preference-tailored words. This finding was weak (p < .10), but it does stand in contrast to a prior study showing preference-tailoring did not impact addiction Stroop performance (Fridrici et al., 2013). However, the method of tailoring in that study involved the use of highly specific personalized stimuli (e.g., participant-generated words about a recent drinking episode), rather than broad class of alcohol beverages (e.g., beer, liquor, wine). This distinction may be critical, but further research is certainly needed before any definitive claims can be made. Regardless, the analytic approach employed in the present study may prove useful for examining the role that preference tailoring and other stimulus characteristics may play in attentional bias, an area of research that has received only limited consideration to date.
These findings were extended to examine the correlations between same-drug and cross-drug priming effects (i.e., the “tightness” of the association between smoking and drinking). Modest correlations were observed for this coupling for both smoking and drinking words. Importantly, greater alcohol dependence/use resulted in a tighter association between smoking and drinking prime effects on drinking words even though it did not impact overall response time. Prior research has shown that heavy drinking is associated with stronger associations among positive alcohol expectancies, presumably leading stimulus exposure to more readily activate a broad range of positive expectancies in heavy drinkers (Rather and Goldman, 1994). It is possible that our results reflect a similar consolidating process occurring for priming effects among heavy drinkers, with more consistent patterns of activation of drinking-related schema in response to diverse stimuli. Consolidation of these priming effects under the umbrella of drinking-related schema might in turn impair the formation of strong associations in other contexts, offering an explanation for the weaker association of drinking-smoking prime effects on smoking words among heavier drinkers. Despite a strong theoretical basis for examining consolidation of substance-related information processing in addition to the more traditional strength-of-activation approach (Goldman, 2002), research on cognitive bias and related indices (e.g., cue reactivity) have typically emphasized a strength-of-activation framework. Closer examination of the role of consolidation in these models may prove informative. If consolidation is indeed occurring, this would suggest that intervention effects might be improved by placing more emphasis on reducing the diversity of stimuli that activate drug-seeking behavior rather than focusing on the intensity of that behavior.
Surprisingly, cigarette use and dependence were unrelated to the association between the effects of smoking and drinking primes. This may have been due to the wealth of differences in the subjective and metabolic effects of the two drugs (Miyata et al., 2004; Wall et al., 2007). However, it is also plausible that differences in usage patterns across these two substances are responsible. Although extensive efforts were made to ensure substantial heterogeneity of dependence levels and substance use patterns within the present sample, these variables are not easily equated across drugs. Even the heavier drinkers in the present study generally did not consume alcohol every day, whereas the vast majority of participants were daily smokers. It is possible this difference resulted in a relative range restriction for smoking variables and effects would be more similar to alcohol dependence if the sample were extended to include a higher percentage of light/intermittent smokers.
Some important limitations of the present study exist. Most notably, the absence of a control group means that the cross-drug interference effect cannot unambiguously be attributed to dual substance use. Indeed, recent work suggests mere familiarity may play a role in driving attentional bias (Forestell et al., 2012; Lochbuehler et al., 2012; Oliver and Drobes, 2012); thus, frequent associations between smoking and drinking in the environment may well result in an identical pattern of findings among non-drinkers/non-smokers. The absence of active control images (e.g., arousing, highly valenced, non-substance stimuli) limits the conclusions that can be drawn regarding the specificity of these effects, though this same limitation could be levied at the overwhelming majority of prior studies of attentional bias. Finally, although our desire to examine individual differences as moderators of cognitive bias effects necessitated recruitment of participants with a broad range of substance use patterns, the relative heterogeneity of our sample limits the conclusions that can be drawn when comparing to previous attentional bias literature that examined more homogeneous groups.
Overall, the task developed for this study appears psychometrically sound and provides a framework that can easily be extended to examine associations between other relevant variables (e.g., affective stimuli; Drobes et al., 2006). These results provide continuing support for close associations between alcohol and tobacco and (to our knowledge) the first evidence that these associations can be measured at an implicit level. An obvious next step would include comparing task performance of alcohol and tobacco dual users to that of dual abstainers to ascertain the specificity of these effects. Of course, there is the potential for associations between alcohol and tobacco to develop even in the absence of substance use, as seen with alcohol use expectancies (Zogg et al., 2004). These findings also help to reemphasize the importance of evaluating use of both alcohol and tobacco when an individual pursues treatment for either substance. However, the presence of an implicit association suggests that even if participants do not consciously report these as trigger situations, they may nonetheless activate cross-drug cognitions. Whether these cognitions would ultimately pose a potential threat to continued abstinence remains an important question for further research to address. In the event that they do, interventions designed to modify attentional bias to drug cues might attain greater efficacy by targeting a broader range of cues (e.g. multiple drugs, distal cues) than have typically been included. Ultimately, it is our hope that the present study will provide a foundation for further research concerning implicit mechanisms of alcohol-nicotine associations.
A modified Stroop task was developed and appears psychometrically sound.
Smoking and drinking are closely associated even at an implicit level of analysis.
Associations between smoking and drinking may be strongest among heavy drinkers.
Acknowledgements
The authors would like to thank Diana Diaz and Sarah Eisel for their work on the project and the Survey Methods Core of Moffitt Cancer Center for their assistance with data management.
Role of Funding Source: This study was funded by grant AA011157 from the National Institute on Alcohol Abuse and Alcoholism (Drobes). Additional support was provided by grant 13PRE14660076 from the American Heart Association (Oliver). Neither agency had any role in the design or conduct of the research beyond the provision of funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: JO conducted the literature review, analyzed the data and wrote the first draft of the manuscript. DD edited the manuscript. Both authors contributed to the design and conduct of the study and have approved the final version of the manuscript.
Conflicts of Interest: Dr. Drobes has been a paid expert witness in litigation against tobacco companies.
REFERENCES
- Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 121:148–151. doi: 10.1016/j.drugalcdep.2011.08.023. 201). [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008;59:390–412. [Google Scholar]
- Berridge KC, Aldridge JW. Decision utility, the brain, and pursuit of hedonic goals. Social Cogn. 2008;26:621–646. doi: 10.1521/soco.2008.26.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav. Pharmacol. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AR, Heinz AJ, Veilleux JC, Conrad M, Weber S, Wardle M, Greenstein J, Evatt D, Drobes D, Kassel JD. The separate and combined effects of alcohol and nicotine on anticipatory anxiety: a multidimensional analysis. Addict. Behav. 2012;37:485–491. doi: 10.1016/j.addbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- CDC. Decrease in smoking prevalence - Minnesota, 1999–2010. MMWR. 2011;60:138–141. [PubMed] [Google Scholar]
- Chanon VW, Sours CR, Boettiger CA. Attentional bias toward cigarette cues in active smokers. Psychopharmacology. 2010;212:309–320. doi: 10.1007/s00213-010-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn AM, Cobb C, Hagman BT, Cameron A, Ehlke S, Mitchell JN. Implicit alcohol cognitions in risky drinking nicotine users with and without co-morbid major depressive disorder. Addict. Behav. 2014;39:797–802. doi: 10.1016/j.addbeh.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Young RM, Lawford BR, Saunders JB, Ritchie TL, Noble EP. Heavy nicotine and alcohol use in alcohol dependence is associated with D2 dopamine receptor (DRD2) polymorphism. Addict. Behav. 2007;32:310–319. doi: 10.1016/j.addbeh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Cyders MA. Impulsivity and substance-related attentional bias: a meta-analytic review. Drug Alcohol Depend. 2013;133:1–14. doi: 10.1016/j.drugalcdep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: theoretical considerations and procedural recommendations. Psychol. Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol. Clin. Exp. Res. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Elibero A, Evans DE. Attentional bias for smoking and affective stimuli: a Stroop task study. Psychol. Addict. Behav. 2006;20:490–495. doi: 10.1037/0893-164X.20.4.490. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O'Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Stohl M, Wall MM, Keyes KM, Goodwin RD, Skodol AE, Krueger RF, Grant BF, Hasin DS. The risk for persistent adult alcohol and nicotine dependence: the role of childhood maltreatment. Addiction. 2014 doi: 10.1111/add.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Craig C, Oliver JA, Drobes DJ. The smoking N-back: a measure of biased cue processing at varying levels of cognitive load. Nicotine Tob. Res. 2011;13:88–93. doi: 10.1093/ntr/ntq214. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Mann B, Bennett GA, Bradley BP. Attentional biases in abstinent alcoholics and their association with craving. Psychol. Addict. Behav. 2013;27:71–80. doi: 10.1037/a0029626. [DOI] [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Clinician Version. Washington, DC: Administration Booklet: American Psychiatric Association; 2012. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I) [Google Scholar]
- Forestell CA, Dickter CL, Wright JD, Young CM. Clearing the smoke: parental influences on non-smokers' attentional biases to smoking-related cues. Psychol. Addict. Behav. 2012;26:638–643. doi: 10.1037/a0025096. [DOI] [PubMed] [Google Scholar]
- Forsythe A, Mulhern G, Sawey M. Confounds in pictorial sets: the role of complexity and familiarity in basic-level picture processing. Behav. Res. Methods. 2008;40:116–129. doi: 10.3758/brm.40.1.116. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Fridrici C, Leichsenring-Driessen C, Driessen M, Wingenfeld K, Kremer G, Beblo T. The individualized alcohol Stroop task: no attentional bias toward personalized stimuli in alcohol-dependents. Psychol. Addict. Behav. 2013;27:62–70. doi: 10.1037/a0029139. [DOI] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res. Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International Smoking Image Series (With Neutral Counterparts) Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- Goldman MS. Expectancy and risk for alcoholism: the unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcohol. Clin. Exp. Res. 2002;26:737–746. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002;97:517–531. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BT, Jones BC, Smith H, Copley N. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction. 2003;98:235–244. doi: 10.1046/j.1360-0443.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Stuart GL, Moore TM, Ramsey SE. Item functioning of the alcohol dependence scale in a high-risk sample. Drug Alcohol Depend. 2003;72:183–192. doi: 10.1016/s0376-8716(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychol Rev. 2005;112:446–467. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Kramer DA, Goldman MS. Using a modified Stroop task to implicitly discern the cognitive organization of alcohol expectancies. J. Abnorm. Psychol. 2003;112:171–175. [PubMed] [Google Scholar]
- Li TK, Volkow ND, Baler RD, Egli M. The biological bases of nicotine and alcohol co-addiction. Biol. Psychiatry. 2007;61:1–3. doi: 10.1016/j.biopsych.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lochbuehler K, Otten R, Voogd H, Engels RC. Parental smoking and children's attention to smoking cues. J. Psychopharmacol. 2012;26:1010–1016. doi: 10.1177/0269881112439254. [DOI] [PubMed] [Google Scholar]
- Locker L, Jr, Hoffman L, Bovaird JA. On the use of multilevel modeling as an alternative to items analysis in psycholinguistic research. Behav. Res. Methods. 2007;39:723–730. doi: 10.3758/bf03192962. [DOI] [PubMed] [Google Scholar]
- May J, Kavanagh DJ, Andrade J. The Elaborated Intrusion Theory of desire: a 10-year retrospective and implications for addiction treatments. Addict. Behav. 2014 doi: 10.1016/j.addbeh.2014.09.016. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Thompsen DM. Implicit and explicit measures of alcohol and smoking cognitions. Psychol. Addict. Behav. 2006;20:436–444. doi: 10.1037/0893-164X.20.4.436. [DOI] [PubMed] [Google Scholar]
- McCusker CG. Cognitive biases and addiction: an evolution in theory and method. Addiction. 2001;96:47–56. doi: 10.1046/j.1360-0443.2001.961474.x. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Meadows SP, Amir N, Gibb BE. Computer-delivered, home-based, attentional retraining reduces drinking behavior in heavy drinkers. Psychol. Addict. Behav. 2014;28:559–562. doi: 10.1037/a0036086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH. How can we use our knowledge of alcohol-tobacco interactions to reduce alcohol use? Annu. Rev. Clin. Psychol. 2013;9:649–674. doi: 10.1146/annurev-clinpsy-050212-185549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An On-Line Orthographic Database of the English Language. 2005 2014, from http://www.neuro.mcw.edu/mcword/ [Google Scholar]
- Miyata H, Kono J, Ushijima S, Yanagita T, Miyasato K, Fukui K. Clinical features of nicotine dependence compared with those of alcohol, methamphetamine, and inhalant dependence. Ann. N. Y. Acad. Sci. 2004;1025:481–488. doi: 10.1196/annals.1316.059. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Alcohol and Tobacco: From Basic Science to Clinical Practice. Bethesda, MD: U.S. Department of Health and Human Services; 1995. Smoking Among Alcoholics During And After Treatment: Implications For Models, Treatment Strategies, And Policy. [Google Scholar]
- Munafò M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. J. Psychopharmacol. 2003;17:310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Blank MD, Van Rensburg KJ, MacQueen DA, Brandon TH, Drobes DJ. Nicotine interactions with low-dose alcohol: pharmacological influences on smoking and drinking motivation. J. Abnorm. Psychol. 2013;122:1154–1165. doi: 10.1037/a0034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA, Drobes DJ. Visual search and attentional bias for smoking cues: the role of familiarity. Exp. Clin. Psychopharmacol. 2012;20:489–496. doi: 10.1037/a0029519. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Rohrbaugh JW, Heath AC, Shiffman S, Sher KJ. The subjective effects of alcohol-tobacco co-use: an ecological momentary assessment investigation. J. Abnorm. Psychol. 2011;120:557–571. doi: 10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Perry EB, Jr, D'Souza DC, Bufis V, Elander J, Limoncelli D, Vendetti M, Dean E, Cooper TB, McKee S, Petrakis I. Preliminary findings on the interactive effects of IV ethanol and IV nicotine on human behavior and cognition: a laboratory study. Nicotine Tob. Res. 2012;14:596–606. doi: 10.1093/ntr/ntr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather BC, Goldman MS. Drinking-related differences in the memory organization of alcohol expectancies. Exp. Clin. Psychopharmacol. 1994;2:167–183. [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos. Transact. Roy. Soc. Lond. B. Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan F. Detected, selected, and sometimes neglected: cognitive processing of cues in addiction. Exp. Clin. Psychopharmacol. 2002;10:67–76. doi: 10.1037//1064-1297.10.2.67. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2002 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2003. [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend. 2010;109:30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Gemar M. Changes in cognitive organisation for negative self-referent material following cognitive behaviour therapy for depression: a primed Stroop study. Cogn. Emot. 1997;11:501–516. [Google Scholar]
- Sharma D, Money S. Carryover effects to addiction-associated stimuli in a group of marijuana and cocaine users. J. Psychopharmacol. 2010;24:1309–1316. doi: 10.1177/0269881109350079. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Exp. Clin. Psychopharmacol. 2012;20:264–277. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J. Abnorm. Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Smeets E, Roefs A, Jansen A. Experimentally induced chocolate craving leads to an attentional bias in increased distraction but not in speeded detection. Appetite. 2009;53:370–375. doi: 10.1016/j.appet.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users' Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- Stewart SH, Hall E, Wilkie H, Birch C. Affective priming of alcohol schema in coping and enhancement motivated drinkers. Cogn. Behav. Ther. 2002;31:68–80. [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol. Addict. Behav. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology. 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- VanderVeen JW, Cohen LM, Watson NL. Utilizing a multimodal assessment strategy to examine variations of impulsivity among young adults engaged in co-occurring smoking and binge drinking behaviors. Drug Alcohol Depend. 2013;127:150–155. doi: 10.1016/j.drugalcdep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Wall TL, Schoedel K, Ring HZ, Luczak SE, Katsuyoshi DM, Tyndale RF. Differences in pharmacogenetics of nicotine and alcohol metabolism: review and recommendations for future research. Nicotine Tob. Res. 2007;9(Suppl. 3):S459–474. doi: 10.1080/14622200701587045. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Franken IH, Schwartz JE. Generalizability of carry-over effects in the emotional Stroop task. Behav. Res. Ther. 2005;43:715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Wertz JM. Carry-over effects can modulate emotional Stroop effects. Cogn. Emot. 2003a;17:501–509. doi: 10.1080/02699930143000716. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003b;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Ma H, Dent CW, Stacy AW. Self-generated alcohol outcomes in 8th and 10th graders: exposure to vicarious sources of alcohol information. Addict. Behav. 2004;29:3–16. doi: 10.1016/s0306-4603(03)00088-1. [DOI] [PubMed] [Google Scholar]