Abstract
Down Syndrome (DS) is a highly complex developmental genetic disorder caused by trisomy for human chromosome 21 (Hsa21). All individuals with DS exhibit some degree of brain structural changes and cognitive impairment; mouse models such as Ts65Dn have been instrumental in understanding the underlying mechanisms. Several phenotypes of DS might arise from a reduced response of trisomic cells to the Sonic Hedgehog (SHH) growth factor. If all trisomic cells show a similar reduced response to SHH, then up-regulation of the pathway in trisomic cells might ameliorate multiple DS phenotypes. We crossed Ptch1tm1Mps/+ mice, in which the canonical SHH pathway is expected to be up-regulated in every SHH-responsive cell due to the loss of function of one allele of the pathway suppressor, Ptch1, to the Ts65Dn DS model and assessed the progeny for possible rescue of multiple DS-related phenotypes. Down-regulation of Ptch produced several previously unreported effects on development by itself, complicating interpretation of some phenotypes, and a number structural or behavioral effects of trisomy were not compensated by SHH signaling. However, a deficit in a nest-building task was partially restored in Ts;Ptch+/− mice, as were structural anomalies of the cerebellum in Ts65Dn mice. These results extend the body of evidence indicating that reduced response to SHH in trisomic cells and tissues contributes to various aspects of the trisomic phenotype.
Keywords: Down syndrome, Development, Hedgehog, Brain, Behavior
1. Introduction
Trisomy for Hsa21 results in Down Syndrome (DS) which is among the most complex survivable genetic disorders (Lana-Elola et al., 2011). Individuals with DS always display some degree of intellectual disability and brain dysmorphology (Das and Reeves, 2011). Phenotypes of DS are believed to result from combinations of genetic perturbations caused by excess expression of dosage sensitive genes from Hsa21 during development and adulthood (Lana-Elola et al., 2011). Genetic studies have focused on discovering alterations in the function of specific pathways and the expression of individual genes to discover targets for drug intervention (Korbel et al., 2009).
Mouse models with segmental trisomy for regions orthologous to Hsa21 have been utilized to study the effects of excess gene dosage of Hsa21 homologs on developmental processes, structure and cognition (Das and Reeves, 2011). Ts65Dn, a mouse model of DS, displays cognitive deficits (Das and Reeves, 2011) and brain dysmorphology (Baxter et al., 2000) that parallel deficits seen in people with DS. Both humans with DS and Ts65Dn mice display hypoplasia of the cerebellum, due primarily to defects in the response of trisomic cerebellar granule cell precursors (GCPs) to Sonic Hedgehog (SHH) in the perinatal period (Baxter et al., 2000; Roper et al., 2006).
We injected pups on the day of birth (P0) with SAG, a small molecule that binds to Smo to activate the SHH pathway. This single treatment was able to restore the cerebellar size and granule cell (GC) density to euploid levels throughout life (Das et al., 2013; Roper et al., 2006).The improvement in cerebellar structure coincided with an improvement in the performance of the trisomic mice in the Morris water maze (MWM) (Das et al., 2013), a learning and memory task linked to hippocampal and cerebellar function (D’Hooge and De Deyn, 2001).
The SHH pathway is involved in the patterning, growth and survival of many cells and tissues during embryogenesis and early development (Villavicencio et al., 2000) and is especially important in the developing central nervous system (Matise and Wang, 2011). If the deficit in response to SHH seen in trisomic GCPs is present to a similar degree in all SHH responsive trisomic cells, then this deficit may contribute to many of the phenotypes seen in DS. This hypothesis is supported by the demonstration that a single acute SHH pathway stimulation can overcome a mitogenic deficit in cerebellar precursor neurons and normalize cerebellar structure in Ts6Dn mice (Vaillant and Monard, 2009). Ptch1 is the main repressor of the canonical SHH pathway (Currier et al., 2012). To test our hypothesis that a SHH response deficit underlies multiple DS phenotypes, we utilized a genetic model of widespread pathway up-regulation, the Ptch1tm1Mps/+ (Ptch1+/−) mouse, which carries a LacZ “knockin” to the first and second exons of Ptch1 (Goodrich et al., 1997). These mice transcribe a Ptch mRNA lacking one transmembrane domain which would invert the protein in the membrane if it is actually synthesized. Transcription is presumed to occur through an alternative ATG start site although the details of this transcript and possible protein products have not been reported. The biological effects are manifest, however. These mice evidence an increased response to SHH in all SHH responsive cells that have been examined throughout embryonic, neonatal and tumor development (Goodrich et al., 1997; Matsuo et al., 2013). Homozygous knockin mice die in early gestation and heterozygotes have a range of problems, most notably a very high frequency of medulloblastoma. Here we crossed the Ptch1+/− mouse to Ts65Dn and examined the progeny for changes in behavioral and cerebellar phenotypes seen in Ts65Dn mice that parallel phenotypes seen in humans with DS.
2. Results
2.1. Canonical SHH pathway expression
We crossed Ptch+/− onto trisomic, Ts65Dn mice to generate four genotypes: Euploid mice with two normal copies of the Ptch1 gene (Eu;Wt), Euploid mice heterozygous for a lacZ knockin that produced Ptch1tm1Mps/+ (Eu;Ptch+/−) (Goodrich et al., 1997), Ts65Dn;Wildtype (Ts;Wt), and Ts65Dn;Ptch1tm1Mps/+ (Ts;Ptch+/−). These mice were examined for a number of behavioral and structural phenotypes that are affected in Ts65Dn mice that do or may arise from perturbations in SHH signaling as a result of trisomy (Appendix: Supplementary Table S1). Craniofacial development in these mice is considered elsewhere (Singh et al., in preparation).
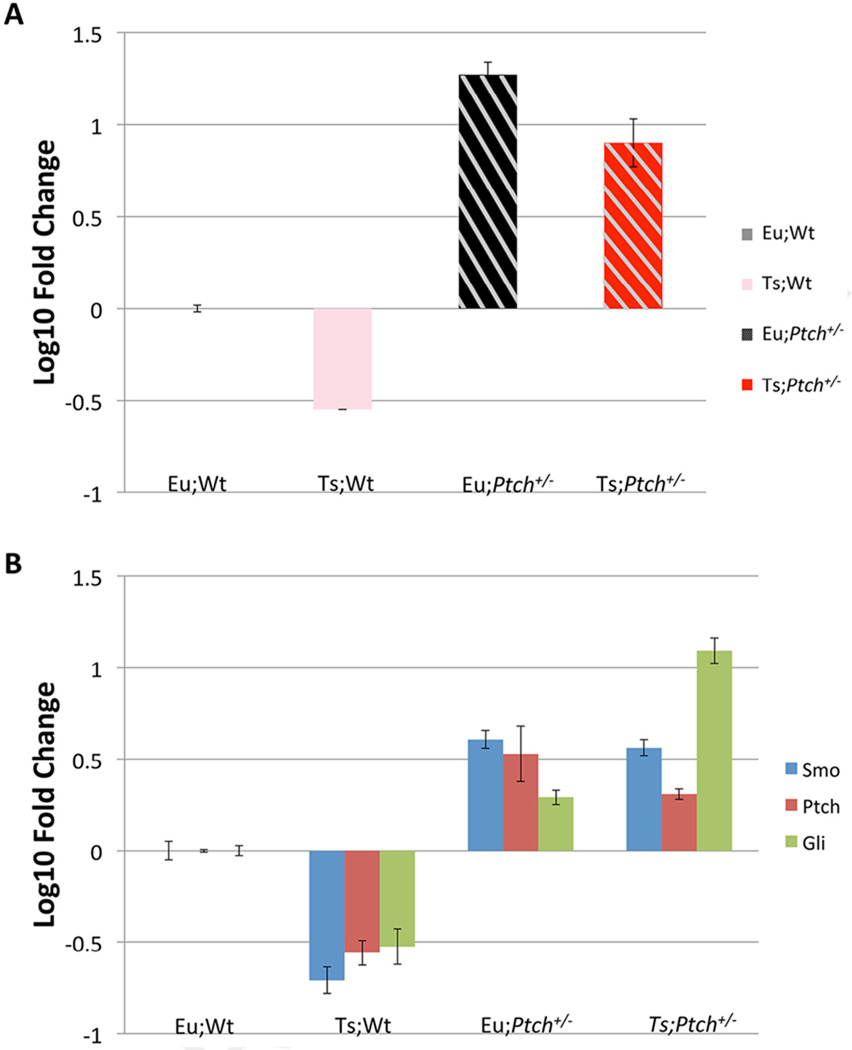
We used TaqMan® to analyze mRNA levels of canonical HH pathway components in the four genotypes. SHH levels were lower in Ts65Dn than in euploid (Fig. 1A), consistent with previous observations of reduced cerebellar growth and of the deficit in cranial neural crest generation and proliferation that contributes to hypoplasia of the mid-face skeleton in Ts65Dn mice (Roper et al., 2006, 2009). As predicted, the pathway appeared to be up-regulated in mice with only one functional copy of Ptch, with elevated mRNA levels of Gli1 and Smo as predicted from the reduction in pathway suppression (Goodrich et al., 1997; Oliver et al., 2003). However, steady state Ptch mRNA was also elevated in adult cerebellum of mice carrying the null allele. The relationship of pathway elements is not straight forward for a number of reasons, including a negative feedback loop producing more Ptch when the canonical pathway is up-regulated, the fact that each of these pathway elements can be regulated independently of the others through non-canonical signaling, the complexity of cell types in the cerebellum and measurement at the level of RNA (Ingham et al., 2011). We observe a new equilibrium with respect to transcript levels of principal pathway components in adult cerebellum (Fig. 1). The level of Ptch mRNA in mice with one functional allele is increased relative to euploid, and this increase is somewhat greater in Eu;Ptch+/− than in Ts;Ptch+/− (Fig. 1B). It will be important to see how this increase in message level is reflected at the protein level and in different activation states of the component proteins. This is the first demonstration of hedgehog pathway mRNA levels in DS model mice.
Fig. 1.
Effects of Ptch1 haploinsufficiency on mRNA levels of expression of canonical Hedgehog pathway components suggest complex stoichiometric regulation in genetic models relative to euploid. (A) Shh mRNA levels are increased in both Ts65Dn and Euploid mice carrying one allele of Ptch1 and decreased in Ts;Wt mice. (B) Ptch1+/− mice express higher levels of Smo, Ptch1 and Gli1 than euploid in adult cerebellum regardless of ploidy, while pathway components are reduced in trisomic mice. Log10 expression of Shh and three Shh pathway elements; Smo, Ptch1 and Gli1 from TaqMan analysis of RNA from whole cerebellum. All samples were normalized initially to Gapdh. Data represent means of the combination of 3 technical replicates containing quadruplicates in each session for each genotype.
2.2. Loss of Ptch1 affects birth frequency, survival and weight
We found that the frequencies of the four genotypes at birth were significantly altered from Mendelian ratios (p = 6.1e-7, χ2) with more Eu;Wt and fewer Ts;Ptch+/− mice than expected (p < 0.05, SR; Table 1). At the end of the behavioral testing, 4 to 5.5 months of age, there was also a significant difference in survival across genotypes (p = 3.8e-4, χ2; p = 1e-4, FET; Table 1). Based on overall survival of all mice surveyed, a larger proportion of Eu;Wt mice survived to the completion of the behavior tests (p < 0.05, SR; Table 1). Additionally, a smaller than expected proportion of Eu;Ptch+/− mice survived to the completion of the tests (p < 0.05, SR; Table 1). Pair-wise comparisons found that a significantly larger fraction of Eu;Wt survived than either Ts;Ptch+/− or Eu;Ptch+/− (p < 0.01, FET) and a somewhat larger proportion than Ts;Wt (p < 0.1, FET).
Table 1.
Population statistics for all genotypes.
| Genotype | Percent frequency at birth |
Percent frequency survivala |
Average weight at 1 week (g) ± standard error |
|---|---|---|---|
| Eu;Wt | 42% | 98% | 4.2 ± 0.1 |
| Eu;Ptch1+/− | 22% | 77% | 4.4 ± 0.1 |
| Ts;Wt | 23% | 86% | 3.0 ± 0.1 |
| Ts;Ptch1+/− | 13% | 78% | 3.3 ± 0.1 |
Survival recorded to completion of behavioral testing (4–5.5 months of age).
Both haploinsufficiency of Ptch1 and Ts65Dn are known to affect body size (Goodrich et al., 1997; Roper et al., 2006). We found that, in agreement with their previously reported effects, trisomy significantly reduced body size and haploinsufficiency of Ptch1 significantly increased body size in the first week of life (p = 7.6e-23 and p = 0.015, respectively, ANOVA) (Table 1). Genotype overall also significantly affected body size (p = 8.0e-23, ANOVA) and planned comparisons found that Ts; Ptch+/− mice weighed significantly less than both euploid groups (p < 0.001) and trended toward a higher weight than Ts; Wt mice (p < 0.1) (Table 1; Appendix: Supplementary Table S1).
2.3. Trisomy does not alter tumor development in Ptch1+/− mice
Between 14% and 30% of all Ptch1+/− mice spontaneously develop cerebellar tumors, medulloblastoma (MB), depending in part on genetic background (Goodrich et al., 1997; Wetmore et al., 2000). A single report indicates that MB is rare among people with DS (Satge et al., 2013). We found no impact of trisomy on overall frequency of MB in the Ptch1+/− mice that took part in our behavioral study (euploid = 29%; trisomic = 30%; age 10–24 weeks). A subset of those mice found to have tumors upon dissection were asymptomatic at death. The frequency of tumors in these mice also did not differ significantly (euploid = 19%; trisomic = 26%). None of the mice with tumors at the end of their life was found to perform consistently outside the range of others of their genotype in any behavioral test. Thus we found no reason to remove these animals from behavior analyses.
2.4. Chronic up-regulation of the SHH pathway does not ameliorate most behavioral deficits in Ts65Dn
2.4.1. Haploinsufficiency of Ptch1 differentially affects motor learning depending on mouse ploidy
We first examined the mice using an accelerating rotarod test, which has been used frequently as a measure of motor learning (Mandillo et al., 2008). Humans with DS have difficulties with fine motor skills and motor planning (Mon-Williams et al., 2001; Spano et al., 1999; Vicari, 2006), but these deficits have proven difficult to replicate consistently in mouse models of DS. Ts65Dn mice have been found by some to perform as well as or better than euploid mice in this test (Baxter et al., 2000; Hyde et al., 2001). In contrast, Tc1 mice, which have a transchromosomic Hsa21, perform consistently worse than euploid littermates in an accelerating rod trial (Duchon et al., 2011a; Galante et al., 2009). We performed this test to determine whether haploinsufficiency of Ptch1 affected motor learning, and whether Ts65Dn would show an affect using the acceleration paradigm that found a deficit in Tc1 mice (Galante et al., 2009).
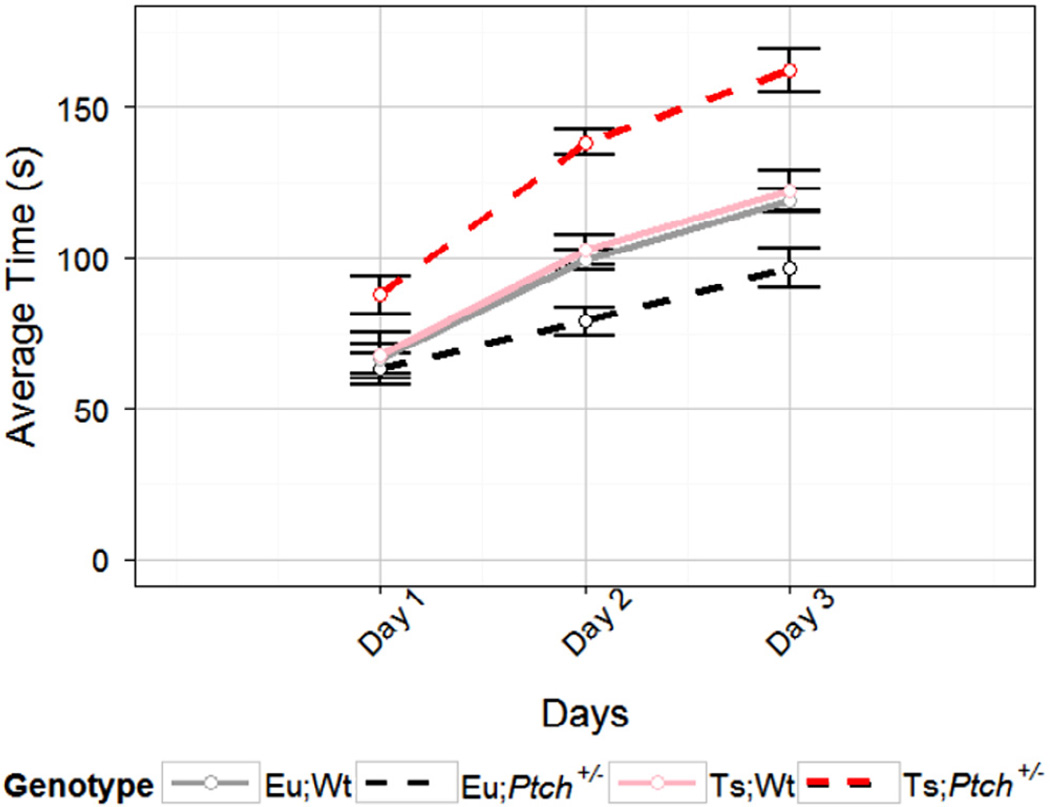
All mice showed significant improvement in ability and motor learning over the course of the test (p[GG] = 1.6e-23, RM ANOVA; p < 1e-4, LME) and there were no significant interactions between training day and genetic factors (Fig. 2). As we observed previously (Baxter et al., 2000), Ts;Wt performed as well as Eu;Wt mice in accelerating rotarod experiments. Ts;Ptch1+/− demonstrated improved performance compared to Ts;Wt, while Eu;Ptch1+/− demonstrated reduced performance compared to Eu;Wt. This differential effect was reflected in a significant interaction between ploidy and mutation status (p = 0.022, RM ANOVA; p = 0.02, LME). We also found that genotype overall had a significant impact on performance (p = 0.0065, RM ANOVA; p = 0.005, LME) and that Ts;Ptch1+/− mice performed significantly better than all other mice (p < 0.05, PLN).
Fig. 2.
Haploinsufficiency of Ptch1 improves motor learning ability in Ts65Dn mice and reduces motor learning ability in euploid mice (p = 0.022, RM ANOVA; p = 0.02, LME). Trace of the latency to fall for each genotype on each day of the increasing speed rotarod protocol averaged over four trials per day. Error bars are Morey corrected SEM. Eu;Wt n = 21, Eu;Ptch+/− n = 15, Ts;Wt n = 17, Ts;Ptch+/− n = 16.
2.4.2. Haploinsufficiency of Ptch1 does not affect Y-maze performance in Ts65Dn or Euploid mice
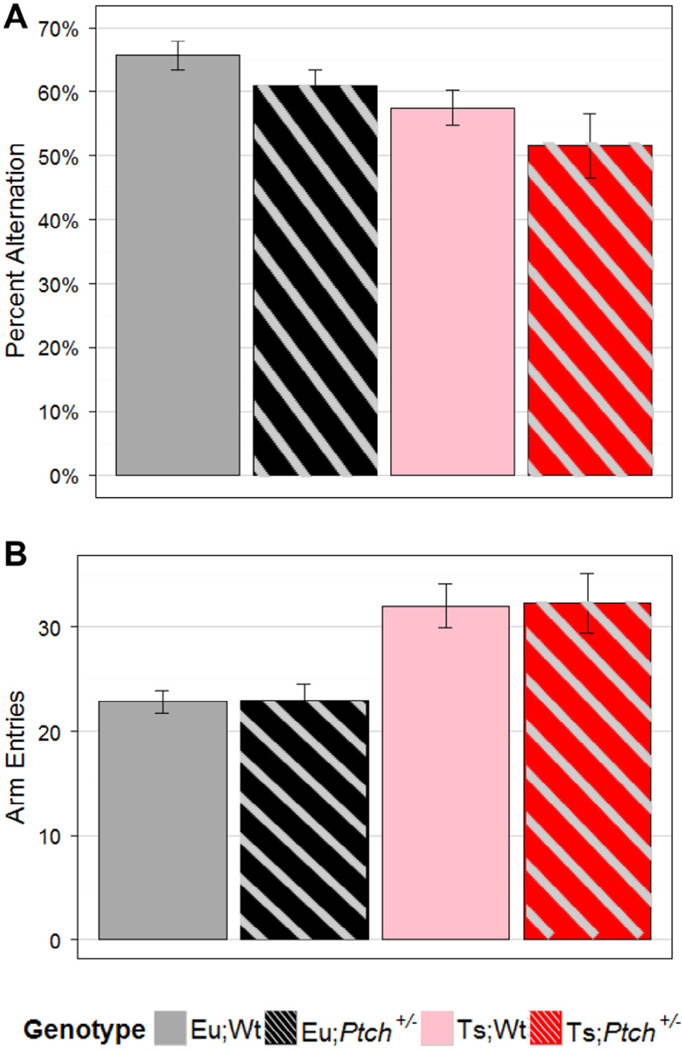
We next assessed the impact of reduced Ptch1 expression (SHH pathway up-regulation) on performance in the Y-maze. This is a non-aversive task that is considered a measure of active retrograde working spatial memory and does not require strong training, relying instead on the innate curiosity of the mouse to explore new territory (Mandillo et al., 2008). Ts65Dn mice display a consistent deficit in this task (reviewed (Das and Reeves, 2011)) and this outcome was unaffected by treatment with SAG at P0 (Das et al., 2013). Here, we found a significant effect of overall genotype on the outcomes of the percent alternation and arm entries in the Y-maze (p = 9e-4, R-MANOVA; Fig. 3). Trisomic mice had fewer complete alternations with more overall exploratory activity than did euploid mice (Fig. 3). Haploinsufficiency of Ptch1 did not significantly alter this phenotype.
Fig. 3.
Haploinsufficiency of Ptch1 does not improve Y-maze performance of Ts65Dn mice. (A) Average percent alternations and (B) average arm entries are plotted by genotype. Trisomy significantly decreased percent alternations (p = 0.0045, ANOVA) and increased arm entries (p = 6e-5, KW, post-hoc α = 0.05) while haploinsufficiency of Ptch1 had no significant effects. Error bars are SEM. Eu;Wt n = 33, Eu;Ptch+/− n = 21, Ts;Wt n = 20, Ts;Ptch+/− n = 11.
All Ts65Dn mice had significantly fewer complete alternations than all euploid mice (55% vs. 64%, p = 0.0045, ANOVA) and there was a trend toward lower performance comparing Ptch1+/− to wildtype mice (58% vs. 63%, p = 0.080, ANOVA), but no significant interaction between ploidy status and mutation status (Fig. 3A). We observed a significant impact of genotype overall on percent alternation, as well (p = 0.015, ANOVA). Ts;Ptch1+/− mice had significantly fewer alternations than Eu;Wt (52% vs. 66%, p < 0.05, PLN) and somewhat fewer than Eu;Ptch1+/− mice (52% vs. 61%, p < 0.1, PLN) (Fig. 3A).
We found a significant impact of genotype on overall activity as measured by total arm entries (p = 6e-5, KW). Posthoc comparisons determined that trisomic mice had significantly more arm entries than euploid mice regardless of genotype at Ptch1 (32 entries each vs. 23 entries each; p < 0.05; Fig. 3B).These results indicate that the lower percent alternations observed in the Ts65Dn mice was not a result of lower levels of exploration. Haploinsufficiency of Ptch1 had no impact on the characteristic hyperactivity of Ts65Dn mice.
2.4.3. Reduced retention of contextual and cued memory in Ts; Ptch1+/− mice
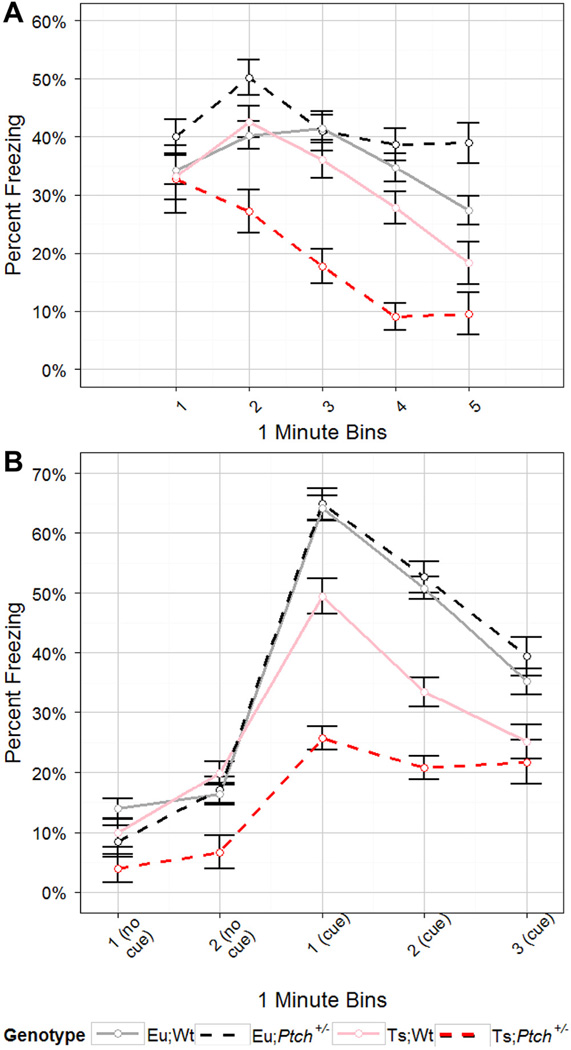
We investigated the effect of the Ptch1 mutation on performance in a fear conditioning (FC) paradigm. This is an aversive task that pairs a context (conditioned context; CC) and a noise (conditioned stimulus; CS) to an electrical shock (unconditioned stimulus; US) (Gerlai, 1998).The US generates a natural fearful response manifested as freezing behavior. Following training, both the CC and the CS can separately elicit the same freezing response in the absence of the US (Gerlai, 1998). Memory of the CS and CC rely on somewhat different brain regions; memory of the CC is thought to rely more heavily on hippocampal and cerebellar function (Kim and Jung, 2006; Sanders et al., 2003).Ts65Dn mice generally display lower levels of freezing in the CC (contextual fear) than do euploid mice (reviewed (Das and Reeves, 2011)) and response to the CS (cued fear) varies in different studies (Bianchi et al., 2010; Costa et al., 2010; Faizi et al., 2011; Salehi et al., 2009). We found a significant interaction between ploidy, mutation, and training time on the training day of FC (p[GG] = 0.0023, RM ANOVA; p = 1e-4, LME) and an interaction between genotype and training time (p[GG] = 1.9e-7, RM ANOVA; p < 1e-4, LME) (Appendix: Supplementary Fig. S2). Post-hoc-comparisons found no significant differences in percent freezing between any genotypes in the final two time bins, indicating that while learning progressed differently for each genotype, the final level of learning or freezing was similar for all four genotypes (Appendix: Supplementary Fig. S2).
In the contextual fear test, trisomy significantly decreased the freezing response relative to both euploid groups over time (p[GG] = 0.0051, RM ANOVA; p = 0.008, LME; Fig. 4A). Haploinsufficiency of Ptch1 trended toward a differential effect, decreasing memory retention in the trisomic animals but showing no effect in euploid animals (p = 0.067, RM ANOVA; p = 0.06, LME). Genotype overall also had a significant impact on retention of fear memory over time in the contextual fear trial (p[GG] = 0.014 RM ANOVA; p = 0.006, LME). Ts;Ptch1+/− mice showed significantly lower memory retention over time compared to all other genotypes (p < 0.05, PLN).
Fig. 4.
Haploinsufficiency of Ptch1 reduces retention of contextual and cued memory in Ts65Dn mice. Trace of average percent freezing in the fear conditioning paradigm as one minute bins of time for each genotype over the course of the (A) contextual trial day and (B) cued trial day. Each bin refers to the minute preceding the stated time in minutes. Haploinsufficiency of Ptch1 correlated with slightly worse (decreased) freezing levels in Ts65Dn in the contextual trial (p = 0.067, RM ANOVA; p = 0.06, LME) and caused a significant further reduction in freezing in trisomic animals in the cued test (p = 0.048, RM ANOVA; p = 0.04, LME) while having no impact on the euploid animals. Error bars are Morey corrected SEM. Eu;Wt n = 23, Eu;Ptch+/− n = 20, Ts;Wt n = 20, Ts;Ptch+/− n = 11.
In cued fear, the loss of a single Ptch1 locus was correlated with a significant further decrease in performance of trisomic animals while having no impact on behavior in euploid animals (p = 0.048, RM ANOVA; p = 0.04, LME; Fig. 4B). As in the context trial, genotype overall had a significant impact on retention of cued fear memory over time (p[GG] = 3.1e-6, RM ANOVA; p < 1e-4, LME) and Ts;Ptch1+/− had a significantly lower memory over time compared to all other genotypes (p < 0.05, PLN). Haploinsufficiency of Ptch1 thus increased the deficits in contextual and cued fear memory in Ts65Dn mice rather than ameliorating the effects of trisomy on these tasks.
2.4.4. Haploinsufficiency of Ptch1 reduces MWM performance in Ts65Dn and Euploid mice
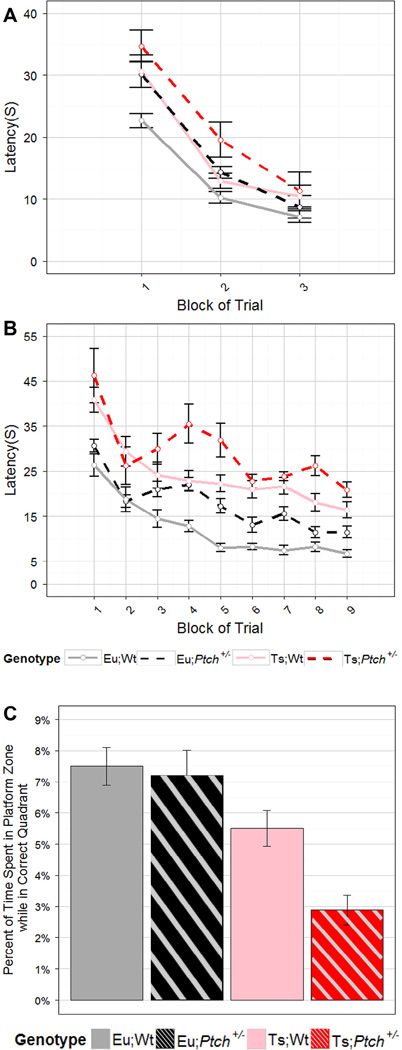
We examined the mice using the visible platform (VP), hidden platform (HP) and probe tests of the Morris water maze (MWM) (D’Hooge and De Deyn, 2001). The VP test examines praxic and taxic learning strategies (Fraenkel, 1961) which rely on several brain areas, but do not substantially involve hippocampal function (D’Hooge and De Deyn, 2001).Ts65Dn mice are usually found to perform comparably to euploid mice in this task (reviewed (Das and Reeves, 2011)). We found that all genotypes showed significant improvement in performance (reduced latency to platform) over the course of VP (p = 2.2e-32, RM ANOVA; p < 1e-4, LME) (Fig. 5A). Trisomy significantly increased latency to platform relative to both euploid groups (p = 0.017, RM ANOVA; p = 0.02, LME). Haploinsufficiency at the Ptch1 locus also led to significantly increased latency (p = 0.029, RM ANOVA; p = 0.02, LME). There was no interaction between ploidy and mutation status, suggesting that the reduction in performance caused by these two factors was additive. A significant difference in learning based on genotype was observed (p = 0.014, RM ANOVA; p = 0.01, LME) and Ts;Ptch1+/− mice performed significantly worse than Eu;Wt (p = 0.003, PLN; Fig. 5A). Post-hoc comparisons found no significant differences between genotypes in any single block of trials, indicating that neither trisomy nor the mutation caused difficulties in seeing visual cues or in swimming ability that would result in large differences in latency and preclude use of the HP and probe tests.
Fig. 5.
Haploinsufficiency of Ptch1 reduces MWM performance in Ts65Dn and Euploid mice. Trace of average latency to platform in each block of trials in (A) the VP test and (B) the HP test with Morey corrected SEM for error bars. (C) Average percent of time spent in the former platform location during the probe test while in the quadrant that contained the platform in the HP test with SEM for error bars. Trisomy reduced performance relative to euploid in the VP (p = 0.017, RM ANOVA; p = 0.02, LME), HP (over time, p = 0.055, RM ANOVA; p = 0.005, LME) and probe test (p = 1.4e-5, ANOVA). Loss of a single Ptch1 locus reduced performance relative to wildtype in the VP (p = 0.029, RM ANOVA; p = 0.02, LME), HP (over time, p = 0.037, RM ANOVA; p = 0.02, LME) and probe test (p = 0.014, ANOVA). There were no interaction effects in any tests and no significant differences in latency in the VP for the final block of trials. In the VP test: Eu;Wt n = 20, Eu;Ptch+/− n = 17, Ts;Wt n = 14, Ts;Ptch+/− n = 9. In the HP and probe test Eu;Wt n = 23, Eu;Ptch+/− n = 17, Ts;Wt n = 16, Ts;Ptch+/− n = 10.
The HP and probe trials of the MWM examine allocentric and egocentric learning strategies, which are thought to have strong input from the cerebellum (egocentric) and the hippocampus (allocentric) (Garthe and Kempermann, 2013; Kealy et al., 2008). Multiple studies have found a deficit in Ts65Dn performance in the HP and probe tests in the MWM (reviewed (Das and Reeves, 2011)); these deficits were corrected in adults with a stimulation of the SHH pathway by SAG at P0 (Das et al., 2013). In HP MWM task, all mice again showed improvement in performance over the course of the training; however, these improvements differed based on genotype. The presence of trisomy significantly increased latency to platform relative to all euploids over time (p = 0.0055, RM ANOVA; p = 0.005, LME; Fig. 5B). Haploinsufficiency of the Ptch1 locus also significantly increased latency relative to all wildtype mice over time (p = 0.037, RM ANOVA; p = 0.02, LME). As in VP, there was no interaction between ploidy and mutation, indicating an additive impact of these two factors in reduced ability to locate the hidden platform. In HP, the genotype overall also had a significant impact on the trend of learning (p = 0.010, RM ANOVA; p = 0.006, LME) and Ts;Ptch1+/− mice performed significantly worse over time compared to all other genotypes (p < 0.05, PLN; Fig. 5B). To confirm the accuracy of average latency to platform as a measure of search strategy we examined the average ratio of path efficiency in the HP and found similar overall results to those seen with latencies (Appendix: Supplementary Fig. S3). The path efficiencies were also highly correlated to the latencies (p < 2.2e-16, PCC; Appendix: Supplementary Table S1).
In the MWM probe test, there was no significant effect of genotype or ploidy or mutation status on percent of time spent in the quadrant containing the platform (Appendix: Supplementary Fig. S4A). All genotypes evidenced some memory of the former platform location by spending the majority of the trial in the quadrant containing the platform (Appendix: Supplementary Fig. S4A). We next examined time spent in the platform zone while in the correct quadrant. Mice would be expected to encounter a subset of the quadrant space by chance during the time spent in that quadrant. A larger percentage of the time spent in the former location of the platform would indicate a higher specificity of spatial learning and reference to spatial cues as well as a more optimal search strategy in these mice (Garthe and Kempermann, 2013; Morris, 1999; Vorhees and Williams, 2010). Additionally mice that quickly realize the absence of a platform and adopt a wider search strategy are not penalized by this measure as they would be with the more traditional normalization to total time of trial (Garthe and Kempermann, 2013; Morris, 1999; Vorhees and Williams, 2010).
We found that trisomy significantly reduced the normalized time spent in the platform zone (p = 1.4e-5, ANOVA; Fig. 5C). Haploinsufficiency of Ptch1 also significantly reduced the percentage of time spent in the platform zone while in the correct quadrant (p = 0.014, ANOVA). As in HP and VP, there was no interaction between ploidy and mutation status indicating an additive impact of these two factors on performance. The overall genotype also had a significant impact on the time spent in the area of the platform while in the quadrant containing the platform (p = 4.7e-5, ANOVA) and Ts;Ptch1+/− mice performed significantly worse than all other genotypes (p < 0.01, PLN). Similar results were found when examining platform site crossovers normalized to time spent in the quadrant containing the platform (Appendix: Supplementary Fig. S4B) and both of these measures were correlated to path efficiency to first platform zone entry in the probe trial (p = 0.001, PCC; Appendix: Supplementary Fig. S4C).
Average speed while mobile was assessed during the probe trial to ensure that differences in latencies or times in the tests were not due to slower swimming speeds. There was no significant difference in average speed when comparing genotype overall, however, when genotype was broken into two factors, the speed was found to be significantly reduced by a loss of a single Ptch1 locus (18 cm/s vs. 16 cm/s; p = 0.029, ANOVA; Appendix: Supplementary Fig. S4D). In the HP, the accuracy of the latencies as a measure of strategy was confirmed by assessment of path efficiency, indicating that this small difference in speed is likely irrelevant to the HP and VP tests (Fig. 5A, B and Appendix: Supplementary Fig. S3). In the probe test, percent time and entries measured were correlated with path efficiency to first platform entry, indicating they were a good measure of memory and search strategy (Fig. 5C and Appendix: Supplementary Fig. S4B, C).
2.4.5. Haploinsufficiency of Ptch1 partially rescues nesting behavior Ts65Dn mice
The final behavioral test examined the ability of singly housed mice to form a nest. Singly housed mice typically form a single organized nest in the corner of a cage (Deacon and Rawlins, 2005). Nesting strategy is considered to reflect context discrimination and spatial learning. This task has been linked to hippocampal function and may require input from the cerebellum (Antonawich et al., 1997; Deacon and Rawlins, 2005; DeLorey et al., 2008; Goorden et al., 2007; Lin et al., 2007).Ts65Dn mice have been shown to have a decreased ability to form a complete intact nest (Heller et al., 2009; Salehi et al., 2009).
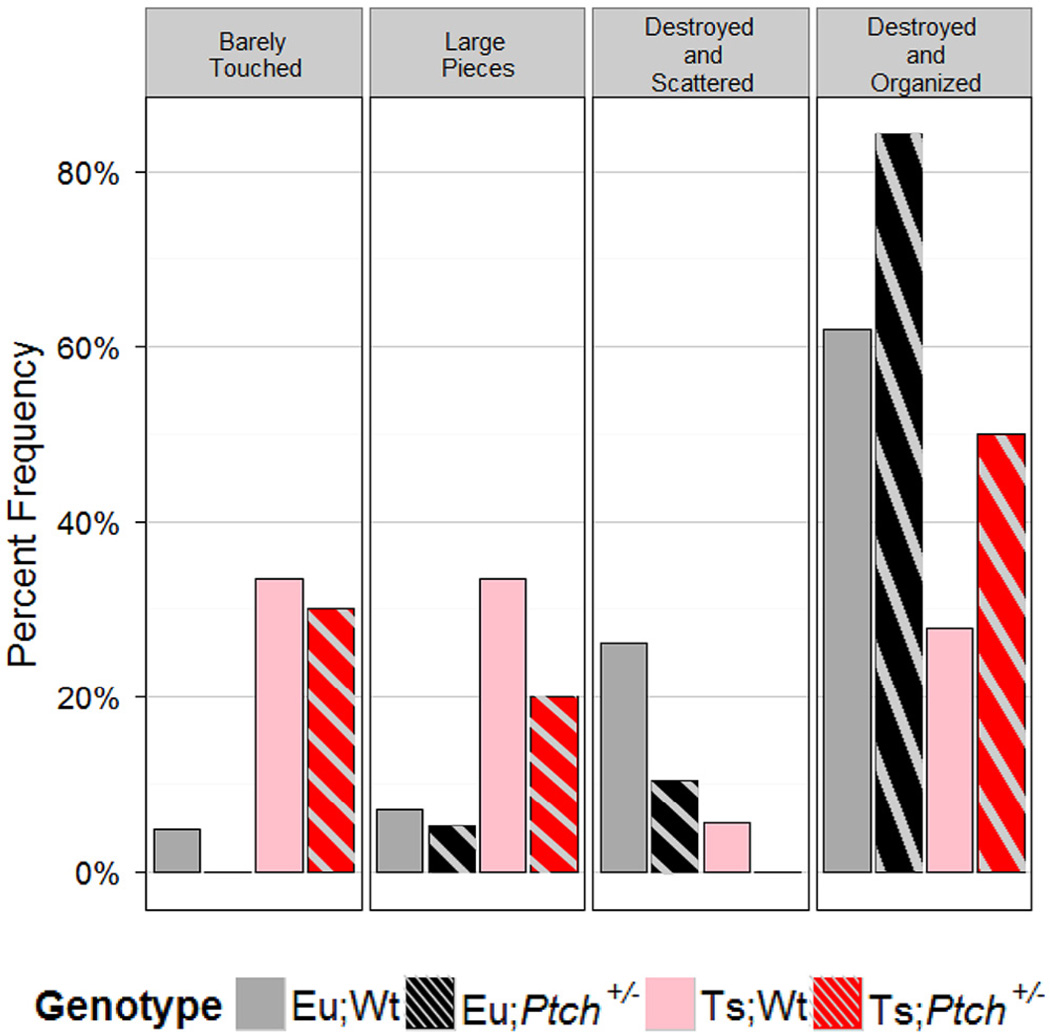
We found that there was a significant difference by genotype in the distribution of nesting behavior (p = 2e-4, χ2 and FET; Fig. 6). Ts;Wt mice exhibited poor nesting strategy, with a larger proportion of nesting squares barely touched or torn into large pieces (p < 0.05, SR). While the nest building ability of Ts;Wt mice was significantly worse than both euploid groups, Ts;Ptch1+/- displayed an intermediate ability between Ts;Wt and the two euploid groups and was not statistically distinguishable from either (p < 0.1, FET). Eu;Ptch1+/− and Eu;Wt did not differ in nesting ability. Overall, the animals grouped by ploidy in nesting behavior, though the addition of haploinsufficiency for Ptch1 somewhat improved behavior in Ts;Ptch1+/− mice.
Fig. 6.
Haploinsufficiency of Ptch1 partially rescues nesting behavior Ts65Dn mice. Percent frequency of mice exhibiting the indicated nesting behavior plotted by genotype (see Supplementary Figure S1 for a description of the categories of behavior). There was a significant difference by genotype in the distribution of nesting behavior (p = 2e-4 χ2 and FET). Ts;Wt mice were significantly more likely to exhibit a poor score (barely touched the nesting square or nesting square torn into large pieces) (p < 0.05, SR) and a trend toward impaired behavior was seen in Ts;Ptch+/− (p < 0.20, SR). Eu;Wt n = 42, Eu;Ptch+/− n = 19, Ts;Wt n = 18, Ts;Ptch+/− n = 10.
2.5. Haploinsufficiency of Ptch1 normalized cerebellar morphology in Ts65Dn mice
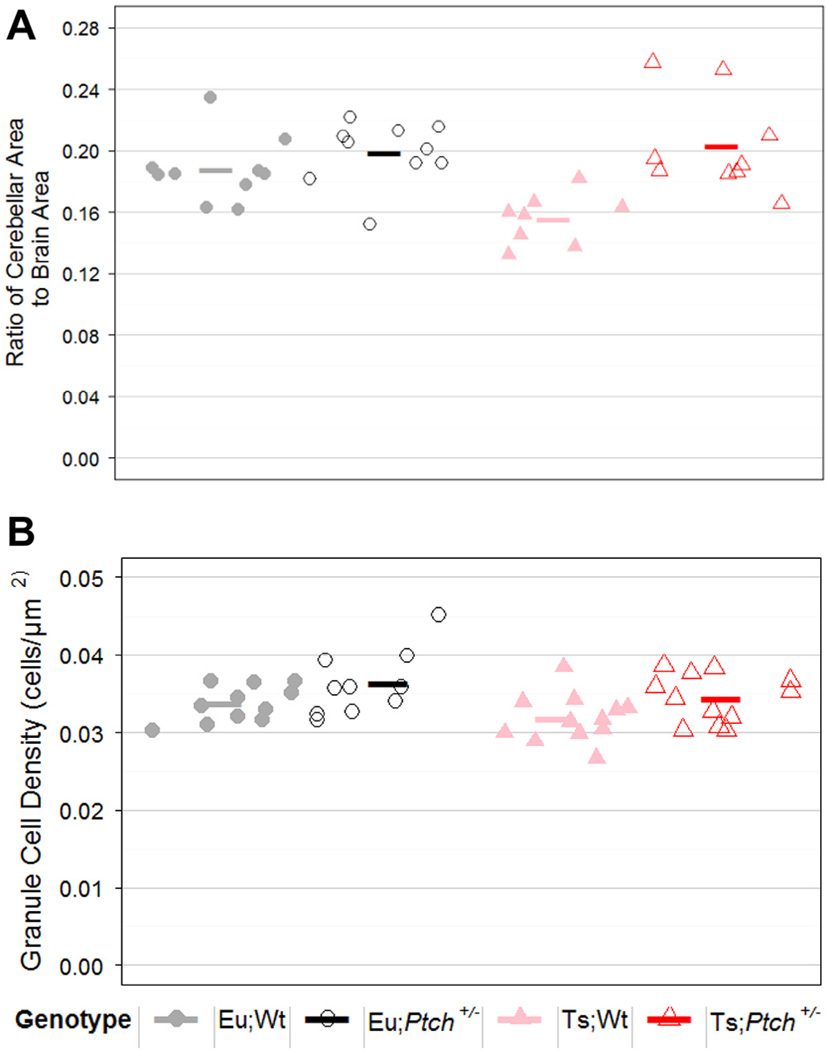
We determined cerebellar granule cell (GC) density and midline sagittal cerebellar area normalized to total brain area in adult mice from all four genotypes (Baxter et al., 2000; Das et al., 2013; Roper et al., 2006). Haploinsufficiency for Ptch1 eliminated the deficit in cerebellar cross sectional area in trisomic mice while having no significant effect on the euploid mice, a result reflected in a significant interaction effect between ploidy and mutation status (p = 0.012, RMANOVA; p = 0.007, LME; Fig. 7A; Table 2). A significant difference was also seen in the normalized cerebellar area based on genotype overall (p = 0.044, RM ANOVA; p = 1e-4, LME), where Ts;Ptch1+/− mice were significantly larger than Ts;Wt mice (p = 1e-4, PLN; Fig. 7A; Table 2).
Fig. 7.
Haploinsufficiency of Ptch1 normalized cerebellar morphology in Ts65Dn mice. Cerebella in Ts;Ptch+/− mice were larger and higher in GC density than Ts;Wt mice but did not differ from Eu;Wt mice. Graphical representation of (A) the average midline cerebellar area as a fraction of the total brain area for each mouse as well as the overall average ratio for each ploidy; (B) the average cerebellar GC density in cells per µm2 for each mouse as well as the overall average for each ploidy. For cerebellar area: Eu;Wt n = 10, Eu;Ptch+/− n = 10, Ts;Wt n = 8, Ts;Ptch+/− n = 9. For GC density: Eu;Wt n = 11, Eu;Ptch+/− n = 10, Ts;Wt n = 12, Ts;Ptch+/− n = 12.
Table 2.
Average cerebellar measurements as percent of Eu;Wt.
| Eu;Ptch1+/− | Ts;Wt | Ts;Ptch1+/− | |
|---|---|---|---|
| Cerebellar area | 105% | 85% | 107% |
| GC density | |||
| All folia | 105% | 94% | 102% |
| Folium 5 | 111% | 94% | 103% |
| Folium 6 | 109% | 92% | 100% |
| Folium 9 | 103% | 97% | 103% |
Reduced cerebellar cross sectional area in Ts65Dn and in DS has been correlated with changes in GC density (Baxter et al., 2000). We showed recently that this hypoplasia could be rescued by acute stimulation with a SHH pathway agonist at birth (Das et al., 2013). Here we found that chronic canonical pathway stimulation due to haploinsufficiency of Ptch1 also rescued GC density deficits in Ts65Dn mice. We observed a significant difference in GC density based on genotype overall (p = 0.015, ANOVA); Ts;Ptch1+/− mice had a significantly higher GC density than did Ts;Wt mice (p = 0.03, PLN). GC density was decreased significantly by trisomy (p = 0.042, ANOVA) and the presence of the Ptch1 mutation significantly increased GC density (p = 0.010, ANOVA) (Fig. 7B). We found no interaction effect between ploidy and mutation status, indicating that GC density was increased in Ptch1+/− mice regardless of ploidy.
To find the GC density we averaged density measurements from folia representing three different developmental zones (folia V, VI, IX) (White and Sillitoe, 2013). We found that both genotype and ploidy had a significant interaction effect with the folium examined (p[GG] = 0.029, RM ANOVA and p[GG] = 0.012, RM ANOVA, respectively) indicating that the magnitude of effect of trisomy may vary by developmental zone. In contrast, the presence of the Ptch1 mutation significantly increased GC density in all folia without an interaction effect (p = 0.010, RM ANOVA). Trisomy still significantly decreased density in each individual folium (p < 0.05, RM ANOVA and LME) and haploinsufficiency of Ptch still significantly increased density in each folium (p < 0.05, RM ANOVA and LME) (Appendix: Supplementary Fig. S5). Genotype also had a significant impact on GC density within each folium (p < 0.01, RM ANOVA and LME).Ts;Ptch1+/− mice had significantly higher GC density compared to Ts;Wt mice in folium V (p < 0.05, PLN) and a somewhat higher GC density in folia VI and IX (p < 0.1, PLN) (Appendix: Supplementary Fig. S5).Ts;Ptch1+/− mice also had a somewhat lower GC density compared to Eu;Ptch1+/− in all three folia (p < 0.1, PLN).
3. Discussion
Mutations in human PTCH1, PTCH2 or SUFU result in Basal Cell Nevus Syndrome (also known as Gorlin syndrome). As suggested by the name, a major feature of the syndrome is basal cell carcinoma, but it is also associated with MB and a range of other features, including at least one report of cognitive impairment (María et al., 2004). We demonstrate that chronic up-regulation of the SHH pathway in Ptch1+/− mice results in deficits in hippocampal- and cerebellar-related learning and memory as measured in the MWM and rotarod tasks. Previous rotarod results showing that Ts65Dn mice do as well or better than euploid in rotarod tasks remain unexplained; the results here, where reduced Ptch1 suppression of the SHH pathway has opposite effects in euploid and trisomic mice, do not immediately clarify the situation. It may be important that the poor rotarod performance of Eu;Ptch+/− mice is correlated with supernumerary GCs in the cerebellum. The MWM and rotarod results point to specific areas of the brain that are sensitive to SHH pathway disruption that may contribute to cognitive deficits in people with Gorlin or Down syndrome.
Reduced MB incidence has been reported in people with DS (Satge et al., 2013), however, we did not see a reduced incidence of MB in our Ts;Ptch1+/− mice, despite that fact that Ts65Dn mice are protected against some cancers (Sussan et al., 2008; Yang and Reeves, 2011). The Ptch1+/− mice develop a specific subtype of MB that frequently occurs with mutations in the SHH pathway in people (Matsuo et al., 2013). Trisomy 21 may be protective against the other subtypes which would account for the reduced incidence reported previously. Additionally, trisomy may not be protective against MB caused by germline loss of Ptch1. We could find no reported cases of haploinsufficiency of Ptch1 in an individual with DS.
The GCPs in newborn Ts65Dn mice lag in SHH stimulated proliferation resulting in a smaller cerebellar size and lower GC density in adult mice that parallels the situation in DS (Baxter et al., 2000; Roper et al., 2006).This deficit can be rescued by acute stimulation of the SHH pathway at birth (Das et al., 2013). Here we demonstrated that chronic up-regulation of the SHH pathway can also restore the cerebellar size and GC density in Ts65Dn mice to the levels of euploid mice. The rescue of GCP generation here is consistent with the earlier conclusion that acute up-regulation of the canonical pathway by SAG was responsible for rescue of the cerebellar hypoplasia phenotype in trisomic mice (Das et al., 2013). Increased GC density was seen with haploinsufficiency of Ptch1 in both euploid and trisomic animals in contrast to our observations with the SAG injections, but in keeping with previous findings in Ptch1+/− mice (Matsuo et al., 2013). Our results here support the supposition that the deficit in response to SHH in the GCPs is the result of a reduction in the response levels of the canonical SHH pathway.
In SAG-treated mice, rescue of cerebellar structure correlated with an increased ability in the MWM and a corresponding improvement in hippocampal LTP (Das et al., 2013).The absence of MWM correction here might suggest that the SAG effect was hippocampal and not cerebellar. This would be consistent with the fact that LTD from Purkinje cells showed only minor differences between Euploid and Ts65Dn mice and this was not affected by SAG. Further, a subtle but significant difference between euploid and Ts65Dn mice in a reverse training paradigm for the vestibulo-ocular reflex (VOR), which is known to depend on cerebellar function, was not ameliorated by SAG treatment (Gutierrez-Castellanos et al., 2013). These results might suggest that the improvement in learning and memory after SAG are less related to the improvement in cerebellar structure and point instead toward a role for the SHH pathway in hippocampal synaptogenesis, focusing translational studies of SAG in this direction.
Nest building, which is deficient in Ts65Dn (Heller et al., 2009; Salehi et al., 2009), was partially restored in the Ts;Ptch1+/− mice. This is a complex task that relies on context discrimination as mediated by the hippocampus with cerebellar input (Antonawich et al., 1997; Deacon and Rawlins, 2005; DeLorey et al., 2008; Goorden et al., 2007; Lin et al., 2007). Deficits in nest building ability in mouse models of other disorders have been linked with hypoplasia of cerebellar folia VI and VII which are part of the oculomotor vermis (DeLorey et al., 2008). Examination of the VOR in the Ts;Ptch1+/− mice compared to Ts;Wt might give an indication of whether the observed partial rescue of nesting ability is directly related to the rescue of cerebellar structure.
Cumulatively, our results are not consistent with a deficit in response to SHH in all trisomic cell populations in the CNS (Currier et al., 2012) (Appendix: Supplementary Table S1). The behavior results suggest that only some cell types in the CNS evidence an amelioration of trisomic phenotypes whereas others were not affected or were further impaired. We show that a structural deficit of the cerebellum that results from an impaired response to the mitogenic effects of SHH by GCPs is ameliorated by acute stimulation of the pathway (Roper et al., 2006), here, we see rescue by chronic stimulation, as well. SHH, however, can also act as a morphogen and act through non-canonical pathways. How SHH is able to play these many different roles throughout development is as yet incompletely understood. Different growth factors are co-expressed with SHH in different tissues. The exact stoichiometries of SHH relative to Ptch1, other receptors, and other growth factors that are necessary to signal a specific lineage differentiation or cellular proliferation remain to be elucidated (Varjosalo and Taipale, 2008; Villavicencio et al., 2000).
While impaired SHH response may underlie multiple developmental anomalies in trisomy, widespread up-regulation of the canonical SHH pathway by this mutation does not exhibit an effect on the majority of phenotypes examined here. The outcomes of the response deficits to SHH signaling may depend on the specific stoichiometries of Ptch1, SHH, and other factors so that the simple reduction in Ptch1+/− mice was not consistently effective. This pathway still presents a promising target for treatment of some specific DS phenotypes. For each cell type in DS where a deficit in SHH response is identified, there is potential to create a timed and targeted corrective stimulation that may serve to improve quality of life in people with DS.
4. Materials and methods
4.1. Mice
Ts65Dn females were crossed to Ptch1tm1Mps/+ males. Ts65Dn mice were obtained from the Jackson laboratory and maintained in the Reeves’ laboratory colony as a C57Bl/6J × C3H/ HeJ (B6 × C3H) advanced intercross. B6;129-Ptch1tm1Mps /J mice were obtained from the Jackson laboratory, backcrossed for five generations onto a B6 background and bred to C3H mice to create the F1 generation. The Ptch1tm1Mps/+ B6 × C3H mice were maintained in the Reeves’ laboratory colony as a B6 × C3H advanced intercross. All breeders were screened for the Pde6brd1 retinal degeneration locus to avoid homozygosity in the offspring.
Mice were genotyped by PCR for the break point in Ts65Dn (Duchon et al., 2011b; Reinholdt et al., 2011) and the neomycin cassette in Ptch1tm1Mps/+ (Goodrich et al., 1997) and weighed in the first week of life. For the birth frequency: Eu;Wt (n = 162), Eu;Ptch1+/− (n = 85),Ts;Wt (n = 88) and Ts;Ptch1+/− (n = 51). Further detail regarding the mice and the birth frequency and weight analysis is provided in Appendix: Supplementary Appendix S1. All procedures were reviewed, approved, and carried out in compliance with animal welfare guidelines approved by the Johns Hopkins University.
4.2. Gene expression
RNA was extracted from adult mouse cerebellum usingTrizol. q-RT PCR was performed using the following TaqMan assays (Applied Biosystems): mm00436527_m1 (Shh), Mm01306901_m1 (Ptch1), Mm00494645_m1 (Gli1), Mm01162710_m1 (Smo), and Mm99999915-g1 (Gapdh). All probes for the Shh pathway were labeled with FAM-MGB while the endogenous control, Gapdh, used VIC. All q-RT PCRs were conducted on the 75000 Real Time PCR Systems machine and analysis was done using the 7500 Real Time PCR systems software v2.0.5 and Excel.
4.3. Behavior tests
The tester was blind to genotype for all tests and histological assessments. A single tester performed all assessments. Codes were not broken for any group or test until all analyses were complete. Behavioral test were run on two cohorts of mice, aged 2.5–5.5 months, with approximately equal proportions of male and female mice to control for possible sex effects. We lacked sufficient numbers of each sex in each genotype category to accurately assess any possible interactions between sex and genotype. First group order of tests: novel object/novel location (NONL), rotarod; only rotarod is reported for this group. Second group order of tests: Y-maze; fear conditioning FC; Morris Water Maze (MWM); nesting. Only rotarod data was reliable and reported for the first group. A small subset of mice did not participate in MWM only or participated only in the nesting. Mice were removed from tests due to non-performance, death, or development of symptoms of a MB (Sanchez and Ruiz i Altaba, 2005; Wetmore et al., 2000). These mice were not removed from analysis of successfully completed tests. Survival was recorded until the end of behavioral testing (age 4–5.5 months). Littermates of the behavioral sets were maintained for survival analysis.
Mice were handled for a week prior to beginning testing for each of the two sets and for 5 days prior to each subsequent test with the exception of nesting. Researchers were blind to genotype throughout behavioral testing. Further detail on behavioral methods is provided in the Appendix: Supplementary Appendix S1.
Rotarod
Mice age: 3–4.2 months. Days 0–3 conducted as in Galante et al. (2009) 9 days after NONL. Y-maze: Mice age: 2.5–4 months. Conducted as in Das et al. (2013) in a Plexiglas apparatus. Mice tracked with ANY-maze (Stoelting co).
FC
Mice age: 3–4.5 months. FC training began 14–17 days after Y-maze. Training: 2 min acclimation CC, 5 min 5-trial delay method; CS-white noise, 30 s, 73–75 dB; US- 2 s, 0.7 mA; ITI, 30 s. Contextual trial: 24 hrs post-training, 5 min CC. Cued trial: 48 hrs post-training, 2 min NC, 3 min CS. Analyzed by FreezeScan (CleverSys).
MWM
Conducted in as in Das et al. (2013). Water height: 0.5 cm above platform. Water temperature range: 20–23 °C. Mice tracked with ANY-maze (Stoelting co). VP mice age: 3.5–5 months. HP mice age: 4–5.5 months. VP test as in Das et al. (2013) 14–16 days following FC. HP test as in Das et al. (2013) 12 days following the VP test. Probe trial as in Das et al. (2013) 1 day after HP.
Nesting
Mice age: 4 to 5.5 months. Nesting trials began 1–10 days after MWM. Mice were singly housed in a new cage with an intact nesting square for 24 hrs. Degree of nesting square use was categorized into one of four nesting categories for each mouse: nesting square barely touched, nesting square in large pieces, nesting square destroyed with organized nest, and nesting square destroyed and with disorganized nest (Appendix: Supplementary Fig. S1).
4.4. Histology and brain morphology
Mice were sacrificed as soon as MB symptoms developed or up to 2 weeks following the final test (age 4–5.5 months) as in Starbuck et al. (2014). The brains of a subset of mice from both behavioral data sets were processed and data collected for assessment of cerebellar area and GC density as described (Starbuck et al., 2014). Brains from Ptch1+/− mice, both euploid and Ts65Dn, were screened for the presence of large MBs. Mice with tumors disrupting the cerebellar architecture were eliminated from the histological assessment of cerebellar structure. Three brains containing MBs were prepared and sectioned as in Starbuck et al. (2014). The whole and half Ptch1+/− brains remaining were sectioned, stained with Lac-z, and examined for MBs. Further detail on the histological analysis is provided in Appendix: Supplementary Appendix S1.
4.5. Statistical analyses
All analyses were conducted in R and further detail is provided in the Appendix: Supplementary Appendix S1. Sex was not included as a factor in any analysis.
Categorical Data
Analyzed by Pearson’s Chi-squared test (χ2). Standardized residuals (SR) were used for post-hoc assessments. For low frequency groups confirmed with a Fisher’s Exact test (FET) followed by post-hoc pair-wise FETs (Bonferroni).
Continuous Data
Analyzed using linear models (ANOVA, repeat measure ANOVA (RM ANOVA), linear mixed-effects model (LME), Robust MANOVA (R-MANOVA), Kruskal–Wallis (KW)). Oneway linear analyses were based on genotype and two-way linear model analyses were based on ploidy and mutation. Planned contrasts (PLNs) of all the genotypes compared to Ts;Ptch1+/-mice used the one-way linear models. Post-hoc pair-wise comparisons (PHPC; Holm; α = 0.05) used the one-way linear models. Conformance to model assumptions was assessed by visual inspection of plots. Some data were transformed with a Box-Cox transformation (BC) to improve conformance. Greenhouse-Geisser sphericity corrected p-values (p[GG]) were reported in cases of violation of sphericity. All ANOVAs were calculated with Type 3 sums of squares.
Survival
Eu;Wt n = 97, Eu;Ptch1+/– n = 57, Ts;Wt n = 46 and Ts;Ptch1+/– n = 32.
Weight
Eu; Wt (n = 162), Eu; Ptch1+/– (n = 85), Ts; Wt (n = 88) and Ts; Ptch1+/– (n = 51)
Rotarod
One mouse was removed as an outlier. BC transformed data were analyzed with one-way and two-way RM ANOVAs confirmed with LMEs with one-way PLNs.
Y-maze
A Munzel and Brunner R-MANOVA was performed on ranked data. Percent alternation was analyzed by two and one-way ANOVAs with one-way PLNs. Arm entries was analyzed by one-way KW with post-hoc comparisons (Bonferroni; α = 0.05)
FC
BC transformed data were analyzed as in rotarod. For the training day PHPCs were performed instead of PLNs.
MWM
VP and HP BC transformed data were analyzed as in rotarod. For the VP test one-way PHPCs were also performed. Probe test data were analyzed as in Y-maze percent alternations. Percent of time spent in the platform zone and average mouse swimming speed were BC transformed. A Pearson’s correlation test (PCC) was used to compare the latency and path efficiency in the HP test and platform entries, percent of time spent in the platform zone, and path efficiency in the probe test.
Cerebellar Area
BC transformed data was analyzed as described for rotarod.
GC density
BC transformed overall data was analyzed by two-way and one-way RM ANOVAs. BC transformed average data were analyzed as described for Y-maze. Data within each folium were BC transformed, data were analyzed as described for rotarod.
Supplementary Material
Acknowledgements
We thank the Rodent Behavioral Testing Core at the Johns Hopkins University School of Medicine for their assistance in designing our behavioral studies. Dr. Fabain Fernandez provided insightful discussion. This work is presented in partial support of the requirements of the Ph.D. degree for TD.
Grant funding
PHS award R01-HD038384; Lumind Foundation; Research Down Syndrome Foundation (RHR). These funding agencies had no role in the study design, data collection, analyses, preparation or publication of this manuscript. The information presented does not necessarily reflect their opinions.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.mod.2014.11.004.
REFERENCES
- Antonawich FJ, Melton CS, Wu P, Davis JN. Nesting and shredding behavior as an indicator of hippocampal ischemic damage. Brain Res. 1997;764(1–2):249–252. doi: 10.1016/s0006-8993(97)00488-5. [DOI] [PubMed] [Google Scholar]
- Baxter LL, Moran TH, Richtsmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum. Mol. Genet. 2000;9(2):195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- Bianchi P, et al. Early pharmacotherapy restores neurogenesis and cognitive performance in theTs65Dn mouse model for Down syndrome. J. Neurosci. 2010;30(26):8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Schmidt C, Davisson MT. Behavioral validation of the Ts65Dn mouse model for Down syndrome of a genetic background free of the retinal degeneration mutation Pde6b(rd1) Behav. Brain Res. 2010;206(1):52–62. doi: 10.1016/j.bbr.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier DG, Polk RC, Reeves RH. A Sonic hedgehog (Shh) response deficit in trisomic cells may be a common denominator for multiple features of Down syndrome. Prog. Brain Res. 2012;197:223–236. doi: 10.1016/B978-0-444-54299-1.00011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Reeves RH. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Dis. Model. Mech. 2011;4(5):596–606. doi: 10.1242/dmm.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, et al. Hedgehog agonist therapy corrects structural and cognitive deficits in a Down syndrome mouse model. Sci. Transl. Med. 2013;5(201):201ra120. doi: 10.1126/scitranslmed.3005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav. Brain Res. 2005;156(2):241–249. doi: 10.1016/j.bbr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav. Brain Res. 2008;187(2):207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Duchon A, et al. The telomeric part of the human chromosome 21 from Cstb to Prmt2 is not necessary for the locomotor and short-term memory deficits observed in the Tc1 mouse model of Down syndrome. Behav. Brain Res. 2011a;217(2):271–281. doi: 10.1016/j.bbr.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchon A, et al. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: relevance for modeling Down syndrome. Mamm. Genome. 2011b;22(11–12):674–684. doi: 10.1007/s00335-011-9356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizi M, et al. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: activation of beta1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol. Dis. 2011;43(2):397–413. doi: 10.1016/j.nbd.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel GS. In: The Orientation of Animals, Kineses, Taxes and Compass Reactions. Gunn DL, editor. New York: Dover Publications; 1961. [Google Scholar]
- Galante M, et al. Impairments in motor coordination without major changes in cerebellar plasticity in the Tc1 mouse model of Down syndrome. Hum. Mol. Genet. 2009;18(8):1449–1463. doi: 10.1093/hmg/ddp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Kempermann G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci. 2013;7:63. doi: 10.3389/fnins.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and a lesion study. Behav. Brain Res. 1998;95(2):191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Goorden SM, vanWoerden GM, van derWeerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann. Neurol. 2007;62(6):648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Castellanos N, et al. Size does not always matter: Ts65Dn Down syndrome mice show cerebellum-dependent motor learning deficits that cannot be rescued by postnatal SAG treatment. J. Neurosci. 2013;33(39):15408–15413. doi: 10.1523/JNEUROSCI.2198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HC, et al. Long term repair of learning disability through short-term reduction of CNS inhibition. In: Schmorrow D, Estabrooke I, Grootjen M, editors. Foundations of Augmented Cognition. Neuroergonomics and Operational Neuroscience. Vol. 5638. Springer Berlin Heidelberg: 2009. pp. 818–825. Lecture Notes in Computer Science. [Google Scholar]
- Hyde LA, Crnic LS, Pollock A, Bickford PC. Motor learning in Ts65Dn mice, a model for Down syndrome. Dev. Psychobiol. 2001;38(1):33–45. doi: 10.1002/1098-2302(2001)38:1<33::aid-dev3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011;12(6):393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- Kealy J, et al. The effects of overtraining in the Morris water maze on allocentric and egocentric learning strategies in rats. Behav. Brain Res. 2008;192(2):259–263. doi: 10.1016/j.bbr.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci. Biobehav. Rev. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbel JO, et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc. Natl. Acad. Sci. U.S.A. 2009;106(29):12031–12036. doi: 10.1073/pnas.0813248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana-Elola E, Watson-Scales SD, Fisher EM, Tybulewicz VL. Down syndrome: searching for the genetic culprits. Dis. Model. Mech. 2011;4(5):586–595. doi: 10.1242/dmm.008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Chen G, Kuang H, Wang D, Tsien JZ. Neural encoding of the concept of nest in the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 2007;104(14):6066–6071. doi: 10.1073/pnas.0701106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandillo S, et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol. Genomics. 2008;34(3):243–255. doi: 10.1152/physiolgenomics.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- María J, et al. Síndrome névico baso-celular. Presentación de seis casos y revisión de la literatura Basal cell nevus syndrome. Presentation of six cases and literature review. Med. Oral Patol. Oral Cir. Bucal. 2004;10:57–66. [PubMed] [Google Scholar]
- Matise MP, Wang H. Sonic hedgehog signaling in the developing CNS where it has been and where it is going. Curr. Top. Dev. Biol. 2011;97:75–117. doi: 10.1016/B978-0-12-385975-4.00010-3. [DOI] [PubMed] [Google Scholar]
- Matsuo S, et al. Thickened area of external granular layer and Ki-67 positive focus are early events of medulloblastoma in Ptch1(+)/(−) mice. Exp. Toxicol. Pathol. 2013;65(6):863–873. doi: 10.1016/j.etp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Mon-Williams M, et al. The preparation of reach to grasp movements in adults with Down syndrome. Hum. Mov. Sci. 2001;20(4–5):587–602. doi: 10.1016/s0167-9457(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Morris RJ. In: The Probe Test: Is It Always a Measure of Spatial Memory for Transgenic Mice? Baker R, editor. UK & HVS Image; 1999. < Watermaze.org>. [Google Scholar]
- Oliver TG, et al. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc. Natl. Acad. Sci. U.S.A. 2003;100(12):7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt LG, et al. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm. Genome. 2011;22(11–12):685–691. doi: 10.1007/s00335-011-9357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, et al. Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103(5):1452–1456. doi: 10.1073/pnas.0510750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, St John HK, Philip J, Lawler A, Reeves RH. Perinatal loss of Ts65Dn Down syndrome mice. Genetics. 2006;172(1):437–443. doi: 10.1534/genetics.105.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, VanHorn JF, Cain CC, Reeves RH. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech. Dev. 2009;126(3–4):212–219. doi: 10.1016/j.mod.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, et al. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci. Transl. Med. 2009;1(7):7ra17. doi: 10.1126/scitranslmed.3000258. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Ruiz i Altaba A. In vivo inhibition of endogenous brain tumors through systemic interference of Hedgehog signaling in mice. Mech. Dev. 2005;122(2):223–230. doi: 10.1016/j.mod.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur. J. Pharmacol. 2003;463(1–3):217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Satge D, et al. A very rare cancer in Down syndrome: medulloblastoma. Epidemiological data from 13 countries. J. Neurooncol. 2013;112(1):107–114. doi: 10.1007/s11060-012-1041-y. [DOI] [PubMed] [Google Scholar]
- Spano M, et al. Motor and perceptual-motor competence in children with Down syndrome: variation in performance with age. Eur. J. Paediatr. Neurol. 1999;3(1):7–13. doi: 10.1053/ejpn.1999.0173. [DOI] [PubMed] [Google Scholar]
- Starbuck JM, Dutka T, Ratliff TS, Reeves RH, Richtsmeier JT. Overlapping trisomies for human chromosome 21 orthologs produce similar effects on skull and brain morphology of Dp(16)1Yey and Ts65Dn mice. Am. J. Med. Genet. A. 2014;164:1981–1990. doi: 10.1002/ajmg.a.36594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan TE, Annan Yang FL, Ostrowski MC, Reeves RH. Trisomy represses ApcMin-mediated tumours in mouse models of Down’s syndrome. Nature. 2008;451(7174):73–75. doi: 10.1038/nature06446. [DOI] [PubMed] [Google Scholar]
- Vaillant C, Monard D. SHH pathway and cerebellar development. Cerebellum. 2009;8(3):291–301. doi: 10.1007/s12311-009-0094-8. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Vicari S. Motor development and neuropsychological patterns in persons with Down syndrome. Behav. Genet. 2006;36(3):355–364. doi: 10.1007/s10519-006-9057-8. [DOI] [PubMed] [Google Scholar]
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am. J. Hum. Genet. 2000;67(5):1047–1054. doi: 10.1016/s0002-9297(07)62934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2010;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore C, Eberhart DE, Curran T. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched the normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched 1. Cancer Res. 2000;60:2239–2246. [PubMed] [Google Scholar]
- White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip. Rev. Dev. Biol. 2013;2(1):149–164. doi: 10.1002/wdev.65. [DOI] [PubMed] [Google Scholar]
- Yang A, Reeves RH. Increased survival following tumorigenesis in Ts65Dn mice that model Down syndrome. Cancer Res. 2011;71(10):3573–3581. doi: 10.1158/0008-5472.CAN-10-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.