Abstract
Rapid enhancement of phagocyte functionality is a hallmark of neutrophil priming. GeneChip analyses unveiled elevated CD54, dectin-2, and IL-1β mRNA expression by neutrophils isolated from inflammatory sites. In fact, CD54 and dectin-2 protein expression was detected on neutrophils recovered from skin, peritoneal and lung inflammation lesions, but not on those in bone marrow or peripheral blood. Neutrophils elevated CD54 and dectin-2 mRNA during migration in Boyden chambers and acquired CD54 and dectin-2 surface expression after subsequent exposure to GM-CSF. Neutrophils purified from IL-1β promoter-driven DsRed transgenic mice acquired DsRed signals during cell migration or exposure to GM-CSF. CD54 and dectin-2 were expressed by DsRed+ (but not DsRed–) neutrophils in GM-CSF-supplemented culture, and neutrophils recovered from inflammatory sites exhibited strong DsRed signals. The dynamic process of neutrophil priming was then studied in chemically induced inflammatory skin lesions by monitoring DsRed expression under confocal microscopy. A majority (>80%) of Ly6G+ neutrophils expressed DsRed, and those DsRed+/Ly6G+ cells exhibited crawling motion with a higher velocity compared to the DsRed–/Ly6G+ counterpart. This is the first report showing motile behaviors of primed neutrophils in living animals. We propose that neutrophil priming occurs in a sequential manner with rapid enhancement of phagocyte functionality followed by CD54 and dectin-2 mRNA and protein expression, IL-1β promoter activation, and accelerated motility. Not only do these findings provide a new conceptual framework for our understanding of the process of neutrophil priming, they also unveil new insights into the pathophysiology of many inflammatory disorders characterized by neutrophil infiltration.
INTRODUCTION
Neutrophils are the most abundant leukocytes in blood circulation and serve as the first line of defense against microbial invasion by extruding neutrophil extracellular traps, engulfing microorganisms, producing reactive oxygen species (ROS), and releasing various enzymes via degranulation (1-3). However, circulating neutrophils exhibit limited antimicrobial activity in the steady state – they must be pre-instructed by microbial or endogenous agents to exert maximal phagocyte functionality as measured by bacterial uptake and respiratory burst (4,5). This process known as “priming” is a key event whereby neutrophil responsiveness to an activating stimulus is markedly augmented by prior exposure to a priming agent. Although various agents (e.g., microbial products, chemoattractants, and inflammatory cytokines) can induce neutrophil priming, they do not elicit phagocyte functionality on their own unless applied at extremely high concentrations (6). These agents can prime neutrophils in relatively short periods, ranging from several seconds (e.g., ATP) to 120 min (e.g., LPS and GM-CSF) (7-11). Not only do primed neutrophils exhibit markedly enhanced phagocytosis and ROS production upon encountering microorganisms, they also change surface phenotype (6,7,12). Most of these functional and phenotypic changes occur in the absence of de novo biosynthesis (13-16). For example, inflammatory cytokines augment respiratory burst by phosphorylating NADPH oxidase components (e.g., p47phox) (2,5,17,18). ROS production can also be enhanced via mobilization of flavocytochrome b558 from granules to plasma and phagosomal membranes (14,19,20). Exocytosis of secretory vesicles may result in elevated surface expression of fMLP receptor, CD11b, CD35, CD66b, and Fcγ receptors (13-15,21). Conversely, CD62L surface expression is diminished via enzymatic shedding (22,23). In essence, neutrophil priming is generally regarded as a rapid process requiring no gene transcription or translation. Interestingly, neutrophils treated in vitro with LPS or G-CSF showed enhanced ROS production even when tested 24 h after priming (24). Likewise, after in vivo infusion of endotoxin, circulating neutrophils exhibited augmented respiratory burst upon PMA stimulation and this phenotype was maintained for longer than 24 h (25). These observations imply that neutrophil priming may not necessarily be a rapid and transient process. In the present study, we sought to identify phenotypic and functional changes occurring in a late phase of neutrophil priming.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Construction and characterization of the pIL1-DsRed transgenic mice are described elsewhere (26). Both male and female animals (10-30 weeks old) were used in the experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Toledo and performed in accordance with the National Institutes of Health guidelines.
Antibodies and flow cytometry
All fluorescently conjugated mAbs were purchased from BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), and Miltenyi Biotec (Auburn, CA). Samples were first incubated on ice for 15 min with anti-CD16/32 mAb (2.4G2) for Fc blocking, and then stained on ice for 30 min with mAbs against the following antigens: CD11b (M1/70), CD11c (N418), CD48 (HM48-1), CD54 (3E2), dectin-2 (KVa7-6E7), 7/4 (7/4), Gr-1 (RB6-8C5), and Ly6G (1A8). The samples were analyzed with the FACSCalibur (BD Biosciences), in which only propidium iodide-negative populations were included in the analyses. The data were analyzed by the FlowJo software (Tree Star Inc., Ashland, OR).
Culture media
Cells were cultured in “complete” RPMI medium – RPMI 1640 (Hyclone, Logan, UT) supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM L-glutamine (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), 100 μM nonessential amino acids (Hyclone), 10 mM HEPES (Hyclone), 50 μM β-mercaptoethanol (Gibco, Grand Island, NY), 100 U/ml penicillin (Hyclone), 100 μg/ml streptomcycin (Hyclone), and 0.25 μg/ml amphotericin B (Hyclone).
Cell purification
Gr-1high/CD48– neutrophils were sorted from bone marrow (BM) cells isolated from C57BL/6 mice by FACSAria II (BD Biosciences) as described previously (27). We reported recently that peritoneal exudate cell (PEC) samples harvested from thioglycollate-induced peritoneal lesions contained Ly6G+/CD11c+ neutrophil-dendritic cell hybrids in addition to Ly6G+/CD11c– conventional neutrophils and Ly6G–/CD11c+ traditional dendritic cells (28). Thus, the Ly6G+/CD11c– population was sorted from PEC samples as “neutrophils” in the present study. DsRed–/Gr-1high/CD48– neutrophils were sorted from BM cells isolated from pIL1-DsRed reporter mice. In some experiments, those BM neutrophils were cultured (1 × 106 cells/ml) for 16 h in complete RPMI supplemented with 10 ng/ml GM-CSF (R&D Systems, Minneapolis, MN). DsRed+ and DsRed– populations were then sorted from the cultures. All sorted populations showed >98.5% purity in post-sorting analyses.
ROS production assay
Cells were incubated for 20 min at 37°C with 200 μM luminol (Acros Organics, Morris Plains, NJ) and 50 U/ml horseradish peroxidase (Sigma-Aldrich) and then plated onto 96-well plates. After adding fMLP (Sigma-Aldrich) at the final concentrations of 10 or 100 μM, chemiluminescence was immediately measured using the FLUOstar Omega microplate reader (BMG Labtech, Cary, NC).
Bacterial uptake assay
The E. coli K-12 strain expressing GFP cDNA under the control of lac promoter was used to measure bacterial uptake by neutrophils. After 1 h incubation at multiplicity of infection (MOI) of 10, unbound bacteria were removed by centrifugation over FBS. Samples were then examined for GFP signals with the FACSCalibur (BD Biosciences) as before (27).
Chemotaxis assay
Chemotaxis assay was performed using 24-well plates with 6.5-mm (3 μm pore size) Transwell inserts (Corning Costar, Tewksbury, MA). After FACS purification, neutrophils were suspended in complete RPMI medium at a density of 1 × 107 cells/ml and added to the upper chamber (100 μl/well). Complete RPMI containing 30 nM of recombinant CXCL1 and/or CXCL2 (both from PeproTech, Rocky Hill, NJ) was added to the lower chamber (600 μl/well). After 2 h incubation at 37°C, “migrated” cells were harvested from the lower chambers. The cells remaining in the upper chambers were harvested as “unmigrated” cells. The samples were examined for cell numbers and viability by FACS, and the level of migration (% migration) was then calculated by dividing the number of migrated cells by the total cell numbers recovered from the well (i.e., migrated cells plus unmigrated cells).
mRNA expression analyses
Gene expression profiles were examined as described previously (27). Total RNA samples were extracted from FACS-purified BM neutrophils with Trizol (Invitrogen, Grand Island, NY), purified with the RNeasy Mini Kit (QIAGEN, Valencia, CA), and then hybridized with Affymetrix Mouse Genome 2.0 Array (Genome Explorations, Memphis, TN). The datasets of these BM neutrophils (deposited in the Gene Expression Omnibus under accession number GSE 53826, http://www.ncbi.nlm.nih.gov/geo/) were compared to publicly available microarray datasets (GSE 24102) for PEC neutrophils purified from casein-induced acute peritonitis lesions (29). Real-time PCR was performed using the LightCycler II (Roche Diagnostics, Indianapolis, IN) from the first-strand cDNA generated from the total RNA with the Superscript III First-Strand Synthesis System (Invitrogen). IL-1β primers were purchased from QIAGEN. Other primer pairs were custom prepared by Integrated DNA Technologies using the following sequences: β-actin forward, TGTGATGGTGGGAATGGGTCAGAA; β-actin reverse, TGTGGTGCCAGATCTTCTCCATGT; CD54 forward, TCCTAAAATGACCTGCAGACG; CD54 reverse, AGTTTTATGGCCTCCTCCTGA; Dectin-2 forward, ACCCCTGACCTTCTGAACATACAC; Dectin-2 reverse, TGAGCCCCCATCTGAACACA; DsRed forward, GACGGCTCCTTCATCTACAAG; DsRed reverse, CTTGTGGATCTCGCCCTTC.
Cytokine screening
A cytokine library was constructed by purchasing a total of 66 recombinant cytokines and chemokines from PeproTech – biological activities have been confirmed for many of these cytokines in our previous screening studies (30,31). The tested cytokines include: activin A, activin B, adiponectin, a proliferation-inducing ligand, BM stroma-derived growth factor 20, bone morphogenic protein 2, 4, 7 and 13, cardiotrophin-1, CXCL1, CXCL2, epidermal growth factor, fibroblast growth factor 1, 2 and 9, FMS-like tyrosine kinase 3 ligand, G-CSF, GM-CSF, insulin-like growth factor 1, IFN-α, IFN-β, IFN-γ and IFN-λ2, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-17A, IL-17F, IL-18, IL-21, IL-22, IL-23, IL-31, IL-33, keratinocyte growth factor, leukemia inhibitory factor, M-CSF, β-nerve growth factor, neuropoietin, noggin, platelet-derived growth factor-BB, prolactin, resistin, resistin-like molecule α and β, soluble receptor activator of NF-κB ligand, stem cell factor, thrombopoietin, transforming growth factor β1, β2 and β3, TNF-α, TNF ligand superfamily 14, and vascular endothelial growth factor. DsRed–/Gr-1high/ CD48– neutrophils purified from the BM of pIL1-DsRed reporter mice were cultured for 24 h in round-bottom 96-well plates (2 × 104 cells/well) in complete RPMI medium supplemented with each of the above cytokines added at 10 ng/ml. To measure baseline levels, some of the above BM neutrophils were cultured as above in the absence of added cytokines. Samples were then analyzed in a semi-automated fashion using the FACSCalibur equipped with HTP sampler device (BD Biosciences).
Inflammatory disease models
Acute peritonitis was induced by i.p. injection of 1 ml of 3% thioglycollate (BD Biosciences), and PEC samples were collected 24 or 48 h later by washing the peritoneal cavity with 7 ml ice-cold PBS (28). Acute lung inflammation was induced by intratracheal instillation of 40 μl of German cockroach frass extracts (1 mg/ml), and bronchial alveolar lavage (BAL) samples were harvested 24 or 48 h later by washing the airway with 1 ml ice-cold phenol red-free HBSS (28). Acute skin inflammation was induced by topical application of 12.5 μl of 1.25% oxazolone (OX, Sigma-Aldrich) onto the right ear (26). The treated ears showed significant (P < 0.01) swelling compared with the left ears of the same animals painted with vehicle alone. After 24 or 48 h, the animals were sacrificed to harvest the entire ears. Samples were incubated for 45 min at 37°C with 0.5% dispase II (Roche Diagnostics), and the epidermal and dermal compartments were then gently separated at the dermo-epidermal junction using a pair of forceps. The epidermal specimens were incubated for 10 min at 37°C with 0.3% trypsin (Worthington, Lakewood, NJ) and 0.1% DNase I (Roche Diagnostics). The dermal specimens were incubated for 60 min at 37°C with 1,000 U/ml collagenase IV (Worthington). The resulting single cell suspensions were analyzed for surface phenotype and DsRed expression by FACS (26).
Intravital confocal imaging
Motile behaviors of leukocytes were studied in OX-induced inflammatory skin lesions as described previously (26,32). Immediately before and 8 h after topical application of OX, pIL1-DsRed mice received i.v. injection of AF647-conjugated anti-Ly6G mAb at a low dose (5 μg/animal/injection) to label neutrophils (33). The blood vessels were visualized by i.v. injection of FITC-conjugated dextran (3 mg/animal, 150 kDa, Sigma-Aldrich). Anesthetized mice were placed onto an imaging stage to mount the tip of the ear, ventral side down, and images of DsRed+ cells and Ly6G+ cells were recorded using an Olympus FV1000 confocal microscope (Olympus, Center Valley, PA). Three dimensional image sets were created by scanning the ear skin from the stratum corneum (the outermost layer readily localizable based on its strong autofluorescence signals) with x, y, z volumes of 317 × 317 × 30 μm (40× objective), 635 × 635 × 30 μm (20× objective), or 1270 × 1270 × 50 μm (10× objective) at 2 μm z-steps. In time-lapse imaging experiments, three dimensional images were recorded every 2 or 4 min for up to 8 h. Datasets were then processed using Metamorph (Universal Imaging, Downington, PA) and Photoshop CS2 software (Adobe, San Jose, CA). Metamorph software was also used to track individual cells, to generate migratory paths, and calculate the directionality and velocity of migration.
Statistical analyses
Microarray analysis was performed in duplicates and all other experimental analyses were performed with at least triplicate samples. All experiments were repeated at least three times to assess reproducibility. Comparisons between two groups were performed using a two-tailed Student's t-test, and more than two groups were compared by analysis of variance.
RESULTS
Signature genes expressed by fully primed neutrophils recovered from inflammatory sites
As an experimental approach to study late-phase changes associated with neutrophil priming, we induced acute sterile peritonitis by i.p. injection of thioglycollate. Consistent to previous reports (34,35), neutrophils purified from PEC samples exhibited typical polymorphonuclear morphology characterized by segmented nuclei, whereas control neutrophils purified from the BM of non-treated mice showed ring-shaped nuclei. Both populations uniformly expressed all tested markers of neutrophils (e.g., Ly6G, Ly6C, 7/4, and CD11b) as we observed before (27,28). Augmented ROS production in response to fMLP has been widely used as the “gold standard” to test neutrophil priming (7). An enhanced capacity to phagocytose bacteria is another functional parameter of primed neutrophils (12,36,37). Thus, we compared the above two neutrophil populations for priming status by measuring ROS production and bacterial uptake. Without fMLP stimulation, ROS production was not detectable in either population. Upon stimulation with 10 μM or 100 μM fMLP, PEC neutrophils exhibited significantly augmented ROS production compared to BM neutrophils (Fig. 1A). Moreover, the former population was far more efficient in the uptake of GFP-producing E.coli (Fig. 1B, 1C). Thus, neutrophils isolated from peritonitis lesions indeed exhibited characteristic features of priming, validating our approach.
Figure 1. Signature genes expressed by fully primed neutrophils recruited to inflammatory sites.
Gr-1high/CD48– neutrophils purified from BM and Ly6G+/CD11c– neutrophils purified from PEC 24 h after thioglycollate treatment of C57BL/6 mice were compared for ROS production (A), bacterial uptake (B and C), and gene expression profiles (E). (A) After incubation with luminol and horseradish peroxidase, the cells were stimulated with 10 or 100 μM fMLP, and chemiluminescence was measured immediately thereafter. The data shown are the means ± SD (n = 3) of relative fluorescence units (RFU). *** P < 0.001 between the peak values of the two populations. (B and C) Cells were incubated for 1 h at 37°C in the presence or absence of GFP-E.coli (MOI = 10) and then examined for GFP signals. The data shown are representative histograms (B) and means ± SD (n = 3) of the mean fluorescence intensities (MFI) (C). *** P < 0.001. (D) Gene expression profiles for our BM neutrophil preparation (A) were compared to the publicly available dataset for PEC neutrophils harvested from casein-induced peritonitis lesions (GSE 24102). Only genes showing over 2-fold changes between the two populations are shown in the heat map. (E) BM neutrophils and PEC neutrophils purified from thioglycollate-induced peritonitis lesions (A) were compared for CD54, decrtin-2, and IL-1β mRNA expression by real-time PCR. Expression levels were then normalized to β-actin mRNA. Data shown are the magnitudes of differences (means ± SD, n = 3) between the two neutrophil populations. *** P < 0.001. (F-H) Various neutrophil preparations were stained with mAb against CD54 or dectin-2 (yellow histograms) or isotype-matched control IgG (blue lines) and analyzed by FACS. The tested samples include FACS-purified BM neutrophils (F), PEC neutrophils isolated 24 or 48 h after thioglycollate injection (G), and BAL neutrophils isolated 24 or 48 h after intratracheal instillation of cockroach allergen (H). For PEC and BAL neutrophil preparations (G and H), staining profiles were examined within the Ly6G+/CD11c– gated population. Data are representative of three independent experiments.
To identify priming-associated changes occurring at transcriptional levels, we next compared a publicly available microarray dataset for murine neutrophils purified from casein-induced acute peritonitis lesions (GSE 24102) (29) with our own microarray dataset for neutrophils freshly purified from BM. We identified a cluster of 4,033 genes showing >2-fold higher expression by the PEC neutrophils (Fig. 1D). From this cluster, we focused our subsequent analyses on the genes encoding cell surface molecules, cytokines or transcription factors. In real-time PCR analyses, we then selected 38 genes showing significantly higher mRNA expression in the PEC neutrophil population purified from thioglycollate-induced peritonitis lesions compared to BM neutrophils. From this list, we were able to confirm elevated protein expression of 13 genes, including Icam1 (CD54), Clec4n (dectin-2), and IL1b (IL-1β). Real-time PCR showed significantly higher mRNA expression of CD54 (> 100-fold), dectin-2 (> 1,000-fold), and IL-1β (> 30-fold) in PEC neutrophils compared to BM neutrophils (Fig. 1E). CD54 protein and dectin-2 protein, which were undetectable on BM neutrophils (Fig. 1F), were found on the surface of PEC neutrophils recovered 24 h after thioglycollate injection (Fig. 1G). We also observed similar changes in a lung inflammation model – both CD54 and dectin-2 were found on neutrophils in BAL samples harvested 48 h after intra-tracheal instillation of cockroach allergen (Fig. 1H). Thus, fully primed neutrophils recovered from inflammatory sites are characterized by CD54 and dectin-2 expression at both mRNA and protein levels.
CD54 and dectin-2 expression by neutrophils in a late phase of priming
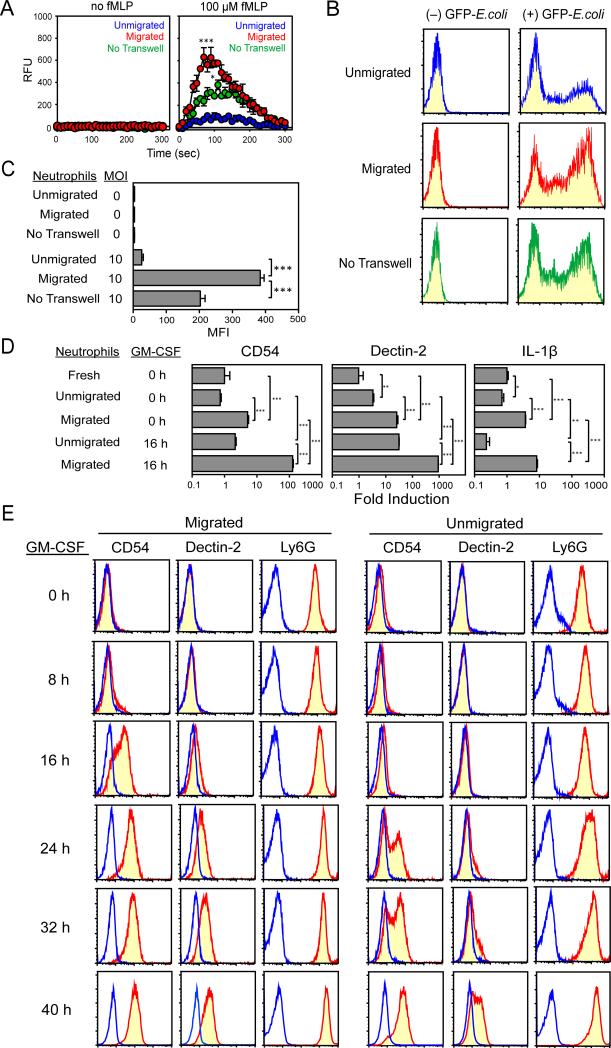
We reasoned that neutrophils might acquire CD54 and dectin-2 expression during the process of migration to inflammatory sites and/or after exposure to inflammatory cytokines. To test the first part, we employed a standard Boyden chamber method as the simplest model (38). When tested individually, two prototypic neutrophil chemokines, CXCL1 and CXCL2 (39,40), induced significant migration of BM neutrophils in dose-dependent fashions (Supplemental Fig 1A). When combined together, they induced even more robust migration (Supplemental Fig 1B), corroborating with a previous report (41). Importantly, significant fractions (50-70%) of neutrophils remained “unmigrated” in the upper chambers even when CXCL1 and/or CXCL2 were added to the lower chambers at the highest tested concentrations or in combination. This implied functional heterogeneity between the migrated neutrophil population and the unmigrated counterpart. Because CXCL1 and CXCL2 were both detected at relatively high levels in various inflammatory lesions (42-45), we chose to add both chemokines to the lower chamber to mimic neutrophil migration under pathogenic conditions. After the 2 h-chemotaxis assay, we then harvested the migrated cells from the lower chambers and the “unmigrated” cells from the upper chambers and compared their states of priming by measuring ROS production and bacterial uptake. Upon stimulation with 100 μM of fMLP, migrated cells exhibited higher ROS production compared to unmigrated cells (Fig. 2A). Likewise, migrated cells exhibited more efficient bacterial uptake (Fig. 2B, 2C). To determine whether cell migration was required for these changes, we cultured neutrophils for 2 h with the same two chemokines, but in conventional culture plates (“no tranwell” panels in Fig. 2A through 2C). We observed that simple exposure to CXCL1 and CXCL2 was not sufficient to induce the maximal augmentation of ROS production and bacterial uptake at the levels observed with migrated neutrophils. Thus, we concluded that neutrophils acquire augmented phagocyte functionality during chemotactic migration.
Figure 2. CD54 and dectin-2 expression occurs in neutrophils during migration and upon exposure to GM-CSF.
Gr-1high/CD48– neutrophils purified from the BM of C57BL/6 mice were placed into the upper chambers, and CXCL1 and CXCL2 (30 nM each) were added into the lower chambers. After 2 h incubation, migrated and unmigrated cells were harvested from the lower and upper chambers, respectively, to measure ROS production (A), bacterial uptake (B and C), mRNA expression of CD54, dectin-2 and IL-1β by real-time PCR (D), and surface expression of CD54, dectin-2, and Ly6G (E). In parallel, the purified neutrophils were cultured for 2 h in conventional tissue culture plates (without transwell inserts) in the presence of the same two chemokines to serve as a control and then examined for ROS production (A) and bacterial uptake (B and C). Some of the migrated and unmigrated cells harvested from the Boyden chambers were further cultured with GM-CSF (10 ng/ml) for up to 40 h and then examined for mRNA expression (D) and surface phenotype (E). Data shown are means ± SD from triplicate samples. * P < 0.05; ** P < 0.01; *** P < 0.001. In panel A, * P < 0.05 between migrated and control cells; *** P < 0.001 between migrated and unmigrated cells. All the data are representative of at least three independent experiments.
At the end of the 2 h-chemotaxis assay, migrated neutrophils (recovered from the lower chambers) also showed elevated mRNA expression for CD54 (~7-fold), dectin-2 (~8-fold), and IL-1β (~5-fold) compared to unmigrated neutrophils (0 h time-point in Fig. 2D). Simple exposure to the CXCL1 and CXCL2 was not sufficient to produce the same changes. At the protein level, neither CD54 nor dectin-2 was detectable on migrated neutrophils (0 h time-point in Fig. 2E). To mimic conditions at inflammatory sites, we next exposed the two neutrophil populations to an inflammatory cytokine GM-CSF for up to 40 h. When tested after 16 h culturing with GM-CSF, migrated neutrophils exhibited markedly (>50-fold) elevated CD54, dectin-2, and IL-1β mRNA expression compared to the unmigrated counterpart (16 h time-point in Fig. 2D). Interestingly, unmigrated neutrophils failed to elevate IL-1β mRNA expression even after 16 h exposure to GM-CSF, even though they slightly elevated CD54 and dectin-2 mRNA expression during this period. Consistent with the mRNA data, surface expression of CD54 and dectin-2 became detectable in the migrated population after 16 and 24 h exposure to GM-CSF, respectively (16 h and 24 h time-points in Fig. 2E). It should be pointed out, however, that unmigrated neutrophils began to express CD54 and dectin-2 at modest levels at later time-points after incubation with GM-CSF (24-40 h time-points in Fig. 2E). Moreover, when tested after 16 h exposure to GM-CSF, both migrated and unmigrated neutrophil populations showed robust ROS production and bacterial uptake at comparable levels (Supplemental Fig. 2). We interpreted these data to suggest that neutrophil priming occurs in a sequential manner. Neutrophils first acquire elevated phagocyte functionality during chemotactic migration – this process occurs relatively rapidly within 2 h. The migrated neutrophils subsequently acquire CD54 and dectin-2 expression when exposed to GM-CSF – this process occurs more slowly with a peak at 16-24 h. It should be stated that neutrophil priming could also take place in a migration-independent manner after prolonged exposure to GM-CSF.
Because most neutrophils isolated from BM are relatively young (27,46), one might argue that the above changes simply reflected differentiation, but not priming. To test this, we examined circulating neutrophils, which are considered to be fully differentiated (34). Neutrophils freshly isolated from peripheral blood expressed neither CD54 nor dectin-2 on the surface, but acquired both when cultured for 16-24 h in the presence of GM-CSF (Fig. 3A). This time-course was somewhat different from that observed with BM neutrophils, especially the unmigrated population, which required 24-40 h culturing with GM-CSF to acquire CD54 and dectin-2 surface expression. It has been reported that BM neutrophils are fully functional, but less responsive to fMLP stimulation compared to circulating neutrophils (34). Thus, it is tempting to speculate that BM neutrophils may differ from peripheral blood neutrophils in their state of maturation. Nevertheless, our results have identified CD54 and dectin-2 expression as a previously unrecognized phenotypic change occurring in a late phase of neutrophil priming.
Figure 3. Late phase changes associated with priming of peripheral blood neutrophils.
(A) Peripheral blood leukocytes isolated from C57BL/6 mice were cultured for 0-32 h with 10 ng/ml GM-CSF and then examined for surface expression of CD54 and dectin-2 by neutrophils. The staining profiles for CD54 or dectin-2 within the Ly6G+/CD11c– gated population are shown. Blue lines indicate staining profiles with isotype-matched control IgG. Data are representative of three independent experiments. (B and C) Peripheral blood leukocytes harvested from pIL-DsRed reporter mice (red) or from WT mice (blue) were examined for DsRed expression before and after 24 h culture with 10 ng/ml GM-CSF. The data shown are representative histograms within the Ly6G+/CD11C– gated population (B) and means ± SD (from triplicate samples) of % DsRed+ cells and MFI values of DsRed signals in the samples from pIL1-DsRed mice (C). ** P < 0.01; *** P < 0.001.
IL-1β promoter activation as a marker of neutrophil priming
IL-1β was another signature gene elevated in neutrophils recovered from inflammatory lesions (Fig. 1E). We recently constructed transgenic mouse expressing DsRed reporter gene under the control of the mouse IL-1β promoter (pIL1-DsRed) (26). We reasoned that these transgenic mice might allow us to monitor the dynamic process of neutrophil priming. In fact, DsRed fluorescence signals were detected in only a small fraction (3.0 ± 0.9%, n = 3) of neutrophils isolated from BM (Fig. 4A, 4B). By contrast, PEC neutrophils recovered from thioglycollate-induced peritonitis lesions and BAL neutrophils isolated from cockroach allergen-induced inflammatory lung lesions both uniformly expressed DsRed at much higher levels. Thus, neutrophils acquire DsRed expression (i.e., IL-1β promoter activation) as they are recruited to inflammatory sites.
Figure 4. DsRed expression (i.e., IL-1β promoter activation) by migrating neutrophils.
(A and B) Various neutrophil samples harvested from pIL1-DsRed mice (red) or from WT mice (blue) were examined for DsRed expression by FACS. Tested samples include FACS-purified BM neutrophils, PEC neutrophils isolated 24 h after thioglycollate injection, and BAL neutrophils isolated 24 h after intratracheal instillation of cockroach allergen. The data shown are representative histograms (A) and means ± SD (from triplicate samples) of % DsRed+ cells and MFI values of DsRed signals in samples harvested from pIL1-DsRed mice (B). ** P < 0.01; *** P < 0.001. (C and D) DsRed–/Gr-1high/CD48– neutrophils purified from the BM of pIL1-DsRed mice (red) or WT mice (blue) were allowed to migrate toward CXCL1 and CXCL2 in the Boyden chambers. After 2 h incubation, migrated and unmigrated neutrophils were harvested from the lower and upper chambers, respectively, to examine DsRed expression by FACS. The data shown are representative histograms (C) and means ± SD (from triplicate samples) of % DsRed+ cells and MFI values of DsRed signals in the samples from pIL1-DsRed mice (D). *** P < 0.001. (E and F) DsRed–/Gr-1high/CD48– neutrophils purified from the BM of pIL1-DsRed mice (red) or WT mice (blue) plated onto the upper chambers, and two chemokines (CXCL1 and CXCL2) or PBS alone were added to the lower chamber. After 2 h incubation, migrated and unmigrated neutrophils were harvested from the lower and upper chambers, respectively, to examine DsRed expression by FACS. Alternatively, the same BM neutrophil preparations were cultured for 2 h in conventional tissue culture plates (without transwell inserts) in the presence or absence of the same two chemokines. The data shown are representative histograms (E) and means ± SD (n = 6) of % DsRed+ cells and MFI values of DsRed signals in the samples from pIL1-DsRed mice (F). ** P < 0.01; *** P < 0.001. All the results are representative of at least three independent experiments.
We observed previously that pIL1-DsRed mice did not differ significantly from wild-type (WT) mice in the tempo or magnitude of neutrophil recruitment to inflammatory skin lesions (26). Likewise, pIL1-DsRed mice were found to be comparable to WT mice in the numbers of neutrophils recovered from acute peritonitis lesions and inflammatory lung lesions (Supplemental Fig. 3A). Furthermore, neutrophils purified from pIL1-DsRed mice were comparable to those from WT mice in their in vitro migratory behaviors toward CXCL1 and CXCL2 (Supplemental Fig. 3B). A key question then concerned whether DsRed expression would occur in association with neutrophil migration. To test this, we allowed BM neutrophils purified from pIL1-DsRed mice to migrate toward CXCL1 and CXCL2 in Boyden chambers. Over 70% of migrated cells expressed DsRed fluorescence signals at high levels, whereas only 25% of unmigrated cells expressed DsRed at much lower levels (Fig. 4C, 4D). To study the requirement of chemokine gradients, we examined DsRed expression by a relatively small population of neutrophils that had migrated into the lower chamber in the absence of added chemokines. Those randomly migrated cells exhibited marked DsRed expression at the same frequency and level as observed in the cells that had migrated toward CXCL1 and CXCL2 (Fig. 4E, 4F). Moreover, simple exposure of neutrophils to the same chemokines in conventional culture plates (without transwell inserts) failed to elevate DsRed expression (bottom panels in Fig. 4E, 4F). It should be pointed out, however, that DsRed expression could also take place in a migration-independent fashion with a minor population of unmigrated cells exhibiting modest DsRed signals. Nevertheless, it appears reasonable to conclude that DsRed expression by neutrophils is closely associated with the migratory behavior.
Upon extravasation, neutrophils are exposed to a wide variety of cytokines present in inflammatory sites, including GM-CSF, TNF-α, and IL-1β (1,2,47). To study cytokine-dependent regulation of DsRed expression, we cultured BM neutrophils purified from pIL1-DsRed mice for 24 h in the presence of each of 66 different cytokines or chemokines. At the tested concentration (10 ng/ml), GM-CSF and IL-18 were the only two cytokines that elevated DsRed expression above the baseline level (mean + 3SD observed in control cultures treated with vehicle alone) in a reproducible manner (Fig. 5A). When the same cytokines were tested in a 4.5 h screening assay, a classical neutrophil priming cytokine TNF-α was identified as a hit elevating DsRed expression above the baseline level. However, TNF-α failed to show DsRed induction in the above 24 h screening assay, consistent with the report that TNF-α elevates IL-1β mRNA expression in neutrophils, but only transiently with a peak at 1 h (48). GM-CSF triggered DsRed expression in a dose- and time-dependent fashion (Fig. 5B). A small amount (0.03 ng/ml) of GM-CSF was sufficient to induce the maximum DsRed expression, and a significant increase in DsRed signals became already detectable within 2 h after exposure to GM-CSF. After 24-48 h exposure to GM-CSF, more than 70% of neutrophils exhibited DsRed signals. Only modest DsRed signals were detected in minor populations (10-30%) of the neutrophils freshly isolated from peripheral blood (Fig. 3B, 3C). When cultured for 24 h in the presence of GM-CSF, however, virtually all cells uniformly exhibited DsRed signals at much higher levels. Thus, we have concluded that GM-CSF is a potent cytokine triggering neutrophil priming as assessed by IL-1β promoter activation.
Figure 5. DsRed expression as an inducible marker of neutrophil priming.
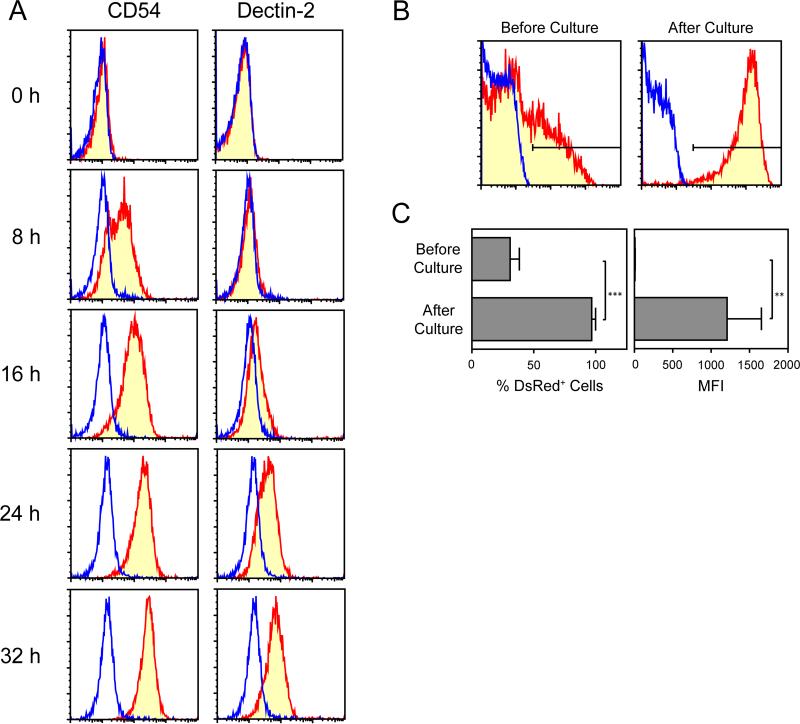
(A) DsRed–/Gr-1high/CD48– neutrophils purified from the BM of pIL1-DsRed mice were cultured in 96-well plates for 24 h in the presence of each of 66 different cytokines and chemokines (added at 10 ng/ml) and then examined for DsRed expression by FACS. Each dot represents the % of DsRed+ cells emerging in the culture supplemented with a given cytokine. Baseline levels (means ± 3SD observed in control cultures without added cytokines) are shown on the top. The cytokines that elevated the % of DsRed+ cells above the baseline levels are indicated in red. The data shown are representative of three independent screening experiments with similar results. (B) DsRed–/Gr-1high/CD48– neutrophils purified from the BM of pIL1-DsRed mice were cultured in 96-well plates for 24 h in the presence of the indicated concentrations of GM-CSF (left panel) or for the indicated periods in the presence of 10 ng/ml GM-CSF (right panel). The samples were then examined for DsRed expression by FACS (means ± SD, n = 3). *** P < 0.001 compared to the baseline without added GM-CSF (left panel) or at time 0 (right panel). (C) DsRed–/Gr-1high/CD48– neutrophils purified from the BM of pIL1-DsRed mice were cultured for 16 h in the presence of 10 ng/ml GM-CSF. The DsRed+ population (bottom column) and DsRed– population (middle column) sorted from these cultures by FACS were then examined for DsRed, CD54, and dectin-2 mRNA expression by real-time PCR. DsRed–/Gr-1high/CD48– neutrophils freshly purified from the BM of pIL1-DsRed mice were examined in parallel without culturing to serve as a control (top column). The data shown are means ± SD from triplicate samples. *** P < 0.001. (D) DsRed–/Gr-1high/CD48– neutrophils purified from the BM of pIL1-DsRed mice were cultured for 40 h in the presence of 10 ng/ml GM-CSF. The DsRed+ population (top) and DsRed– population (bottom) were then examined for surface expression of CD54 and dectin-2. Blue lines indicate the staining profiles with isotype-matched control IgG. The data shown are representative of three independent experiments.
Our data also suggested that DsRed signals might serve as an easy-to-measure indicator of neutrophil priming. To test this, we cultured the BM neutrophils from pIL1-DsRed mice for 16 h in the presence of GM-CSF and then sorted them into DsRed+ and DsRed– populations by FACS. To assess the efficiency of our cell sorting procedures, we measured DsRed mRNA expression as “quality control”. As expected, the DsRed+ population showed 30-fold higher DsRed mRNA expression compared to the DsRed– population (Fig. 5C). Importantly, DsRed+ neutrophils expressed CD54 and dectin-2 mRNA at much higher (>45-fold) levels compared to the DsRed– counterpart. Furthermore, surface expression of CD54 and dectin-2 was observed only on the DsRed+ population (Fig. 5D). On the other hand, DsRed+ and DsRed– populations purified from GM-CSF-supplemented cultures exhibited comparable levels of ROS production after fMLP stimulation (DsRed+ and DsRed– panels in Supplemental Fig. 3C). As aside, when BM neutrophils purified from WT mice were tested in parallel, they exhibited a similar level of ROS production (WT panels in Supplemental Fig. 3C). Taken all together, our results indicated that DsRed expression could be used as a marker of fully primed neutrophils characterized by CD54 and dectin-2 expression.
Intravital visualization of neutrophil priming in inflammatory lesions
We reasoned that one might be able to visualize the process of neutrophil priming in living animals by monitoring DsRed signals. To test this, we recorded images of DsRed+ cells in experimentally induced skin inflammatory lesions under confocal microscopy. We topically applied the skin sensitizer OX on the right ear of pIL1-DsRed mice and vehicle alone in the left ear of the same animal. FITC-conjugated dextran was i.v. injected to demarcate blood vessels. Almost no DsRed+ cells were detectable in the skin of untreated mice, consistent with our previous report (26). A relatively small, but significant, number of DsRed+ cells became detectable 12 h after OX treatment (Fig. 6A, upper right panel). A markedly increased number of DsRed+ cells emerged 24 h after OX treatment, and most of them were found in the extravascular space (Fig. 6A, lower right panel). By contrast, only a few, if any, DsRed+ cells were observed in the control ear treated with vehicle alone (Fig. 6A, left panels).
Figure 6. Emergence of DsRed+/Ly6G+ neutrophils in chemically induced inflammatory skin lesions.
(A through C) pIL1-DsRed mice received topical application of OX (right ear) or vehicle alone (left ear). (A) After 12 or 24 h, the mice were anesthetized and examined for fluorescence signals under confocal microscopy. To demarcate blood vessels, FITC-dextran was injected i.v. immediately before imaging. The data shown are representative of at least three independent experiments. Bar = 100 μm. (B and C) Epidermal and dermal cell suspensions were prepared from ear skin 24 h after topical application of OX or vehicle alone to examine DsRed expression by FACS. Some of the samples were stained with mAb against CD11b, Ly6G, and 7/4 or with control IgG (C). The data shown are representative of at least three independent experiments.
We reported previously that most of the DsRed+ cells emerging in OX-induced inflammatory skin lesions expressed Gr-1, which is expressed by neutrophils, inflammatory monocytes, and myeloid-derived suppressor cells (26). To further determine the identity of those DsRed+ cells, we tested their surface phenotype more definitively. We harvested ear skin samples 24 h after treatment, separated them into epidermal and dermal compartments, and prepared single cell suspensions. Very few DsRed+ cells were found in epidermal or dermal cell suspensions prepared from the vehicle-treated ear. By contrast, strong DsRed signals were detected iñ5% of epidermal cells and ~15% dermal cells isolated from the OX-treated ear (Fig. 6B). Importantly, an overwhelming majority (>90%) of DsRed+ epidermal cells expressed CD11b, Ly6G, and 7/4, indicating that they are mostly primed neutrophils (Fig. 6C, upper panels). Moreover, CD54 and dectin-2 were both detected on the surface of the Ly6G+ neutrophil population isolated from the epidermal compartment after OX painting (Supplemental Fig. 4). By contract, we failed to detect CD54+/Ly6G+ or dectin-2+/Ly6G+ cells in control skin treated with vehicle alone. DsRed+ dermal cells uniformly expressed CD11b and 7/4, but only a portion (~30%) of them displayed Ly6G (Fig. 6C, lower panels). Compared to Ly6G (known to be expressed exclusively by neutrophils), 7/4 has been detected on not only neutrophils, but also inflammatory monocytes and activated macrophages (49,50). Thus, we concluded that DsRed expression occurs almost exclusively in fully primed neutrophils in the epidermis, whereas other leukocyte populations also express DsRed in the dermal compartment under inflammatory conditions.
To mark neutrophils in living animals, we employed a protocol developed by Yipp and Kubes (33). Briefly, a small amount of AF647-conjugated anti-Ly6G mAb was administered before and after OX application, and FITC-dextran was injected before imaging to demarcate blood vessels. At 24 h after OX application, numerous DsRed+/Ly6G+ cells were found under confocal microscopy (Fig. 7A). By counting >800 cells expressing DsRed and/or Ly6G in the extravascular space, we estimated that a majority (82.0 ± 1.5%) of the cells were DsRed+/Ly6G+ cells (i.e., primed neutrophils). We also found relatively small numbers of DsRed–/Ly6G+ cells (9.4 ± 2.4%) and DsRed+/Ly6G– cells (8.6 ± 1.8%) – the former population most likely represented resting neutrophils, whereas the latter perhaps included inflammatory monocytes and activated macrophages. Interestingly, Ly6G+ neutrophils emerging in inflamed skin exhibited typical amoeba-like crawling movement in time-lapse videos (supplemental Video 1). To compare motile behaviors between the DsRed+/Ly6G+ versus DsRed–/Ly6G+ neutrophil populations, we tracked their migratory paths (Fig. 7B). The chemotactic index values reflecting the directionality of migration (51) were relatively low in both DsRed+/Ly6G+ neutrophils (0.23 ± 0.15, n = 169) and DsRed–/Ly6G+ neutrophils (0.21 ± 0.15, n = 51). Likewise, track plot analyses unveiled that both populations exhibited “random” migration once entering the extravascular space (Fig. 7C). Interestingly, the DsRed+/Ly6G+ population exhibited a significantly higher velocity compared to the DsRed–/Ly6G+ counterpart (Fig. 7D). Thus, neutrophil priming is associated with accelerated motility in inflammatory lesions.
Figure 7. Visualization of motile activities of DsRed+/Ly6G+ neutrophils in inflammatory skin lesions.
(A through E) pIL1-DsRed mice received topical application of OX and i.v. injection of AF647-Ly6G mAb immediately before or 8 h after OX application. The mice were anesthetized and examined under confocal microscopy. To demarcate blood vessels, FITC-dextran was i.v. injected immediately before imaging. (A) Static images were recorded at 24 h after OX treatment to show three leukocyte populations in the extravascular space, i.e., DsRed+/Ly6G+ cells (asterisks), DsRed–/Ly6G+ cells (triangles), and DsRed+/Ly6G– cells (arrows). Bar = 20 μm. (B-D) A time-lapse movie (see supplemental Video 1) was recorded every 2 min for 40 min starting at 24 h after OX application. DsRed+/Ly6G+ cells (red) and DsRed–/Ly6G+ cells (blue) were compared for migratory paths (B), track plots (C), and velocity (D) using Metamorph software. (D) Bars indicate the mean velocity values. *** P < 0.001. (E) Starting at different time-points after OX application, a series of time-lapse movies was recorded (see the supplemental Videos 2 through 4). At the indicated time-points, the numbers of DsRed+/Ly6G+ cells and DsRed–/Ly6G+ cells were counted using Metamorph software. The data shown are the cell numbers/mm2 (means ± SD) calculated from three consecutive images. (F) Static images were created from a time-lapse movie recorded from 11 to 14.5 hours after OX painting (supplemental Video 5). The arrow indicates a Ly6G+ cell that acquired DsRed expression in the extravascular space during the observation period. Bar = 50 μm.
To understand the time-kinetics of neutrophil priming, we next recorded a series of time-lapse videos starting at different time-points after OX application (supplemental Videos 2-4). DsRed–/Ly6G+ cells (i.e., resting neutrophils) became detectable in the extravascular space as early as 8-12 h after OX treatment, and their numbers remained relatively low thereafter (Fig. 7E and supplemental Videos 3-4). By contrast, the number of DsRed+/Ly6G+ cells (i.e., primed neutrophils) increased progressively at later time points, starting at 14-16 h after OX application. During this period, we observed that some of the DsRed–/Ly6G+ cells gradually acquired DsRed signals after recruitment to the extravascular space (Fig. 7F and supplemental Video 5). At 16-20 h, most of the extravascular Ly6G+ neutrophils were scored “primed” based on DsRed expression. Our data unveil for the first time the magnitude, tempo, and location of neutrophil priming occurring at inflammatory lesions, thus, providing new insights into the pathophysiology of many inflammatory disorders characterized by neutrophil infiltration.
DISCUSSION
Neutrophil priming is generally viewed as a relatively rapid event in which neutrophils elevate their phagocyte functionality (as measured by ROS production and bacterial uptake) via mechanisms requiring no de novo biosynthesis. Here we report that this early event is followed by previously unrecognized changes occurring at transcriptional levels. To recapitulate the essence of our findings, fully primed neutrophils isolated from inflammatory lesions exhibited elevated CD54, dectin-2, and IL-1β mRNA expression, surface expression of CD54 and dectin-2 proteins, and IL-1β promoter activation (as measured by DsRed signals). Interestingly, cell migration was closely associated with enhanced phagocyte functionality, elevated CD54 and dectin-2 mRNA expression, and IL-1β promoter activation. On the other hand, all these changes were also inducible by long-term exposure to GM-CSF, which was, indeed, required for surface expression of CD54 and dectin-2. By monitoring DsRed expression by in vivo labeled Ly6G+ cells in pIL1-DsRed reporter mice, we were able to visualize the dynamic progress of neutrophil priming in living animals. Strikingly, a majority (>80%) of Ly6G+ neutrophils in the extravascular space expressed DsRed signals at inflammatory lesions, and those DsRed+ neutrophils showed a higher velocity compared to the DsRed– counterpart. Thus, we propose that fully primed neutrophils recruited to inflammatory sites are characterized by CD54 and dectin-2 expression, IL-1β promoter activation, and accelerated motility.
Neutrophil priming is known to be associated with elevated surface expression of selected markers (e.g., CD11b, CD35, and CD66b) – such changes mediated by exocytosis of the secretory vesicles occur rapidly (within 10-15 min) after exposure to priming agents (14,21). By contrast, CD54 and dectin-2 expression occurred much more slowly. A modest (up to 10-fold) increase in CD54 and dectin-2 mRNA expression was observed in the neutrophil population recovered from the lower chamber after the 2 h chemotaxis assay. When those migrated neutrophils were exposed to GM-CSF for 16-24 h, they showed more striking elevation of CD54 mRNA (~100-fold) and dectin-2 mRNA (~1,000-fold) and surface expression of CD54 and dectin-2 proteins. Importantly, CD54 and dectin-2 are both detectable on the surface of neutrophils isolated from experimentally induced inflammatory lesions, indicating that they can serve as markers of fully primed neutrophils.
Molecular interaction between CD54 on endothelial cells and CD11b/CD18 expressed by neutrophils is well known to promote neutrophil extravasation by mediating firm adhesion (52). Though neutrophils are generally regarded to lack CD54 expression, CD54+ neutrophils have been detected in peripheral blood from patients with rheumatoid arthritis, atherosclerosis, and sarcoidosis (53,54). Some of the neutrophils that have been recruited to the site of inflammation migrate back into the blood circulation (55). Interestingly, neutrophils have been shown to acquire CD54 expression during this process of reverse endothelial transmigration (53,56). Thus, it is tempting to speculate that CD54 on primed neutrophils may facilitate their motility via binding to fibrinogen and other putative ligands (57,58). Dectin-2 is a C-type lectin known to be expressed almost exclusively by dendritic cells and macrophages and to mediate their fungal recognition (59). Relatively modest expression of dectin-2 has been detected on neutrophils recovered from zymosan-induced peritonitis lesions (60). Further studies are required to define the functional roles for CD54 and dectin-2 expressed by neutrophils.
IL-1β is another signature gene elevated in primed neutrophils. This finding may not be surprising because neutrophils are known to produce IL-1β mRNA and/or protein in response to various stimuli, including inflammatory cytokines and bacterial products (61). Neutrophils isolated from pIL1-DsRed mice acquired DsRed expression when tested after chemotactic migration toward CXCL1 and CXCL2. Interestingly, DsRed expression was observed after random migration (in the absence of chemokine gradients), but not after exposure to the same chemokines (in the absence of transwell inserts). This implies that IL-1β promoter activation is closely coupled to the process of cell migration. By testing 66 cytokines and chemokines, we have identified GM-CSF as a potent inducer of DsRed expression by neutrophils, and GM-CSF was found to trigger all the changes associated with neutrophil priming. This implies that IL-1β promoter activation is also inducible by selected cytokines in a migration-independent manner. Importantly, mRNA expression and surface expression of CD54 and dectin-2 occurred almost exclusively within the DsRed+ neutrophil population. Thus, we propose that IL-1β promoter activation represents a previously unrecognized change associated with neutrophil priming. Because DsRed+ neutrophils were found in experimentally induced inflammatory lesions, one might predict the presence of GM-CSF in such lesions. In fact, GM-CSF has been detected in OX-induced inflammatory skin lesion (62), thioglycollate-induced peritonitis lesion (28), and cockroach allergen-induced inflammatory lung lesion (63).
Working with pIL1-DsRed reporter mice, we have visualized the process of neutrophil priming in living animals under confocal microscopy. The initial wave of small numbers of DsRed–/Ly6G+ neutrophils emerging at inflammatory sites was followed by progressive increases in DsRed+/Ly6G+ neutrophils – this most likely represents in situ neutrophil priming because some of the DsRed–/Ly6G+ cells gained DsRed signals after recruitment into the extravascular space. Although DsRed+/Ly6G+ neutrophils and DsRed–/Ly6G+ neutrophils both exhibited amoeba-like random migration, the former showed a significantly higher velocity. Working with lysozyme M promoter-driven GFP transgenic mice (in which GFP is expressed by neutrophils and activated monocytes), Kreisel et al. observed that GFP+ neutrophils were rapidly recruited to experimentally induced lung inflammatory lesions, where they migrated randomly with a mean velocity of 7-9 μm/min (64). By injecting AF647-conjugated anti-TNF-α mAb to these animals, Finsterbusch et al. further demonstrated that neutrophils rapidly release TNF-α as they transmigrate through vascular endothelial cells in response to chemoattractants (65). After in vivo labeling of neutrophils with AF750-conjugated anti-Gr-1 mAb, Yipp and Kubes observed that Gr-1+ neutrophils exhibited a crawling motion with a mean velocity of 6 μm/min in skin lesions infected with Staphylococcus aureus (33). Working with transgenic mice expressing a cytoplasmic fluorescence resonance energy transfer biosensor, Mizuno et al. demonstrated marked activation of ERK in neutrophils during adhesion to and migration through vascular endothelial cells in inflamed intestine (66). Because most of the neutrophils found in the extravascular space exhibited DsRed signals, one may assume that TNF-α release and ERK activation most likely occur predominantly in the primed neutrophil population. Out study together with these intravital imaging studies now provide new insights into dynamic behavior and function of neutrophils recruited to inflammatory lesions.
The present study also unveiled significant heterogeneity among neutrophils. Only fractions of neutrophils isolated from BM incorporated GFP-expressing E. coli or migrated toward CXCL1 and CXCL2. Migrated neutrophils, but not unmigrated neutrophils, showed elevated IL-1β mRNA expression (as measured by RT-PCR) and robust IL-1β promoter activation (as measured by DsRed expression). Furthermore, BM neutrophils differed from the peripheral blood counterpart in the kinetics for GM-CSF-induced acquisition of CD54 and dectin-2 surface expression. Our observations are consistent to the previous findings in human peripheral blood neutrophils, demonstrating striking heterogeneity in terms of maturity, functionality, and phenotype (67-69). It will be interesting to determine whether full priming defined by surface expression of CD54 and dectin-2 and IL-1β promoter activation occurs only in mature neutrophils or most preferentially within a certain subset of neutrophils.
It is equally important to state the limitations of this study. First, functional significance of CD54 and dectin-2 expression by primed neutrophils remains to be elucidated. Second, we have not addressed underlying mechanisms for the observed heterogeneity among neutrophils. Third, of the >4,000 genes that were significantly elevated in the primed neutrophils, we only studied Icam1, Clec4n, and IL1b. Because this cluster also included several transcription factors, it would be interesting to determine their potential roles in the process of neutrophil priming. Finally, our study was limited to murine neutrophils. On the other hand, elevated CD54 mRNA in human neutrophils can be found in the publicly available database. CD54 mRNA expression is markedly (>30-fold) elevated in human peripheral blood neutrophils after in vitro treatment with GM-CSF plus IFN-γ or with LPS alone (GSE 22103) (70). Moreover, human neutrophils isolated from the inflamed airway after endotoxin challenge show >5-fold higher CD54 mRNA expression compared to circulating neutrophils (GSE 2322) (71). Likewise, human neutrophils have been shown to acquire dectin-2 mRNA and protein expression after in vitro stimulation with IL-6 and IL-23 (72). Studies are in progress in our laboratory to determine late phase changes associated with priming of human neutrophils.
In conclusion, we have demonstrated that neutrophil priming occurs in a sequential manner. The changes currently known to occur rapidly and transiently in the absence of de novo biosynthesis are followed by transcription of a relative large number of genes, gradual and sustained expression of CD54 and dectin-2, and accelerated cellular motility. We believe that the present study provides a novel conceptual framework for our understanding of the process of neutrophil priming. Moreover, the pIL1-DsRed reporter system can serve as a powerful tool to study the behavior and function of primed neutrophils in living animals.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Robert Blumenthal (University of Toledo) and Dr. Kristen Page (National Institutes of Health) for providing the GFP-expressing E.coli and German cockroach frass extracts, respectively.
This work was supported by grants from the NIH and the Bill and Melinda Gates Foundation.
Abbreviations used in this article
- BAL
bronchial alveolar lavage
- BM
bone marrow
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- OX
oxazolone
- PEC
peritoneal exudate cell
- RFU
relative fluorescence units
- ROS
reactive oxygen species
- WT
wild-type
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 2.Nauseef WM, Borregaard N. Neutrophils at work. Nat. Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 3.Raftery MJ, Lalwani P, Krautkrmer E, Peters T, Scharffetter-Kochanek K, Kruger R, Hofmann J, Seeger K, Kruger DH, Schonrich G. beta2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014;211:1485–1497. doi: 10.1084/jem.20131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology. (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 5.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi SD, Voyich JM, Burlak C, Deleo FR. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. (Warsz. ) 2005;53:505–517. [PubMed] [Google Scholar]
- 7.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin. Sci. (Lond) 1998;94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 8.Kuhns DB, Wright DG, Nath J, Kaplan SS, Basford RE. ATP induces transient elevations of [Ca2+]i in human neutrophils and primes these cells for enhanced O2-generation. Lab Invest. 1988;58:448–453. [PubMed] [Google Scholar]
- 9.Weisbart RH, Golde DW, Gasson JC. Biosynthetic human GM-CSF modulates the number and affinity of neutrophil f-Met-Leu-Phe receptors. J. Immunol. 1986;137:3584–3587. [PubMed] [Google Scholar]
- 10.Tennenberg SD, Fey DE, Lieser MJ. Oxidative priming of neutrophils by interferon-gamma. J. Leukoc. Biol. 1993;53:301–308. doi: 10.1002/jlb.53.3.301. [DOI] [PubMed] [Google Scholar]
- 11.Guthrie LA, McPhail LC, Henson PM, Johnston RB., Jr. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J. Exp. Med. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainard P, Riollet C, Poutrel B, Paape MJ. Phagocytosis and killing of Staphylococcus aureus by bovine neutrophils after priming by tumor necrosis factor- alpha and the des-arginine derivative of C5a. Am. J. Vet. Res. 2000;61:951–959. doi: 10.2460/ajvr.2000.61.951. [DOI] [PubMed] [Google Scholar]
- 13.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol. Cell Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Ward RA, Nakamura M, McLeish KR. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J. Biol. Chem. 2000;275:36713–36719. doi: 10.1074/jbc.M003017200. [DOI] [PubMed] [Google Scholar]
- 15.Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J. Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 16.Uriarte SM, Rane MJ, Luerman GC, Barati MT, Ward RA, Nauseef WM, McLeish KR. Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J. Immunol. 2011;187:391–400. doi: 10.4049/jimmunol.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang PM, Dewas C, Gaudry M, Fay M, Pedruzzi E, Gougerot-Pocidalo MA, El BJ. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox). J. Biol. Chem. 1999;274:20704–20708. doi: 10.1074/jbc.274.29.20704. [DOI] [PubMed] [Google Scholar]
- 18.Dewas C, Dang PM, Gougerot-Pocidalo MA, El-Benna J. TNF-alpha induces phosphorylation of p47(phox) in human neutrophils: partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNF-alpha and granulocyte-macrophage colony-stimulating factor. J. Immunol. 2003;171:4392–4398. doi: 10.4049/jimmunol.171.8.4392. [DOI] [PubMed] [Google Scholar]
- 19.Deleo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, Takagi M, Mizutani S, Morio T. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat. Immunol. 2012;13:369–378. doi: 10.1038/ni.2234. [DOI] [PubMed] [Google Scholar]
- 21.Borregaard N, Kjeldsen L, Sengelov H, Diamond MS, Springer TA, Anderson HC, Kishimoto TK, Bainton DF. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J. Leukoc. Biol. 1994;56:80–87. doi: 10.1002/jlb.56.1.80. [DOI] [PubMed] [Google Scholar]
- 22.Walcheck B, Kahn J, Fisher JM, Wang BB, Fisk RS, Payan DG, Feehan C, Betageri R, Darlak K, Spatola AF, Kishimoto TK. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 24.Ichinose Y, Hara N, Ohta M, Aso H, Chikama H, Kawasaki M, Kubota I, Shimizu T, Yagawa K. Recombinant granulocyte colony-stimulating factor and lipopolysaccharide maintain the phenotype of and superoxide anion generation by neutrophils. Infect. Immun. 1990;58:1647–1652. doi: 10.1128/iai.58.6.1647-1652.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerasoli F, Jr., McKenna PJ, Rosolia DL, Albertine KH, Peters SP, Gee MH. Superoxide anion release from blood and bone marrow neutrophils is altered by endotoxemia. Circ. Res. 1990;67:154–165. doi: 10.1161/01.res.67.1.154. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima H, Ogawa Y, Miyazaki T, Tanaka H, Nishibu A, Takashima A. Intravital imaging of IL-1beta production in skin. J. Invest. Dermatol. 2010;130:1571–1580. doi: 10.1038/jid.2010.11. [DOI] [PubMed] [Google Scholar]
- 27.Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, Kotol PF, Chojnacki BJ, Miyazaki T, Gallo RL, Takashima A. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K, Blumenthal RM, Ward NL, Miyazaki T, Takashima A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690–1700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur. J Immunol. 2009;39:3331–3342. doi: 10.1002/eji.200939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Takashima A. Reciprocal Regulation of Development of Neutrophil-Dendritic Cell Hybrids in Mice by IL-4 and Interferon-Gamma. PLoS. One. 2013;8:e82929. doi: 10.1371/journal.pone.0082929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 33.Yipp BG, Kubes P. Antibodies against neutrophil LY6G do not inhibit leukocyte recruitment in mice in vivo. Blood. 2013;121:241–242. doi: 10.1182/blood-2012-09-454348. [DOI] [PubMed] [Google Scholar]
- 34.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 35.Braber S, Koelink PJ, Henricks PA, Jackson PL, Nijkamp FP, Garssen J, Kraneveld AD, Blalock JE, Folkerts G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am. J. Physiol Lung Cell Mol. Physiol. 2011;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldwin GC, Fuller ND, Roberts RL, Ho DD, Golde DW. Granulocyte-and granulocyte-macrophage colony-stimulating factors enhance neutrophil cytotoxicity toward HIV-infected cells. Blood. 1989;74:1673–1677. [PubMed] [Google Scholar]
- 37.Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J. Immunol. 2012;189:287–295. doi: 10.4049/jimmunol.1103564. [DOI] [PubMed] [Google Scholar]
- 38.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J. Exp. Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J. Leukoc. Biol. 1995;58:359–364. doi: 10.1002/jlb.58.3.359. [DOI] [PubMed] [Google Scholar]
- 40.Bozic CR, Kolakowski LF, Jr., Gerard NP, Garcia-Rodriguez C, von Uexkull- Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 41.Zwijnenburg PJ, Polfliet MM, Florquin S, van den Berg TK, Dijkstra CD, van Deventer SJ, Roord JJ, van der Poll T, van Furth AM. CXC-chemokines KC and macrophage inflammatory protein-2 (MIP-2) synergistically induce leukocyte recruitment to the central nervous system in rats. Immunol. Lett. 2003;85:1–4. doi: 10.1016/s0165-2478(02)00200-6. [DOI] [PubMed] [Google Scholar]
- 42.Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J. Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- 43.Brown CR, Blaho VA, Loiacono CM. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 2003;171:893–901. doi: 10.4049/jimmunol.171.2.893. [DOI] [PubMed] [Google Scholar]
- 44.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, Hickey MJ, Firth N, Weninger W. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat. Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends in Immunology. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marucha PT, Zeff RA, Kreutzer DL. Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J. Immunol. 1990;145:2932–2937. [PubMed] [Google Scholar]
- 49.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 50.Gordon S, Keshav S, Stein M. BCG-induced granuloma formation in murine tissues. Immunobiol. 1994;191:369–377. doi: 10.1016/S0171-2985(11)80442-0. [DOI] [PubMed] [Google Scholar]
- 51.Beltman JB, Maree AF, de Boer RJ. Analysing immune cell migration. Nat. Rev. Immunol. 2009;9:789–798. doi: 10.1038/nri2638. [DOI] [PubMed] [Google Scholar]
- 52.Springer TA. Traffic signals for lymphocyte recirculatin and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 53.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, Nash GB, Rainger GE. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 54.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch v. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 56.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 58.Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1505–1509. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 60.McDonald JU, Rosas M, Brown GD, Jones SA, Taylor PR. Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLoS. One. 2012;7:e45781. doi: 10.1371/journal.pone.0045781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 62.Tamura T, Matsubara M, Takada C, Hasegawa K, Suzuki K, Ohmori K, Karasawa A. Effects of olopatadine hydrochloride, an antihistamine drug, on skin inflammation induced by repeated topical application of oxazolone in mice. Br. J. Dermatol. 2004;151:1133–1142. doi: 10.1111/j.1365-2133.2004.06172.x. [DOI] [PubMed] [Google Scholar]
- 63.Page K, Zhou P, Ledford JR, Day SB, Lutfi R, Dienger K, Lewkowich IP. Early immunological response to German cockroach frass exposure induces a Th2/Th17 environment. J. Innate. Immun. 2010;3:167–179. doi: 10.1159/000320718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finsterbusch M, Voisin MB, Beyrau M, Williams TJ, Nourshargh S. Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF. J. Exp. Med. 2014;211:1307–1314. doi: 10.1084/jem.20132413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mizuno R, Kamioka Y, Kabashima K, Imajo M, Sumiyama K, Nakasho E, Ito T, Hamazaki Y, Okuchi Y, Sakai Y, Kiyokawa E, Matsuda M. In vivo imaging reveals PKA regulation of ERK activity during neutrophil recruitment to inflamed intestines. J. Exp. Med. 2014;211:1123–1136. doi: 10.1084/jem.20132112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open. Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pillay J, Ramakers BP, Kamp VM, Loi AL, Lam SW, Hietbrink F, Leenen LP, Tool AT, Pickkers P, Koenderman L. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J. Leukoc. Biol. 2010;88:211–220. doi: 10.1189/jlb.1209793. [DOI] [PubMed] [Google Scholar]
- 69.Welin A, Amirbeagi F, Christenson K, Bjorkman L, Bjornsdottir H, Forsman H, Dahlgren C, Karlsson A, Bylund J. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS. One. 2013;8:e69575. doi: 10.1371/journal.pone.0069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotz KT, Xiao W, Miller-Graziano C, Qian WJ, Russom A, Warner EA, Moldawer LL, De A, Bankey PE, Petritis BO, Camp DG, Rosenbach AE, Goverman J, Fagan SP, Brownstein BH, Irimia D, Xu W, Wilhelmy J, Mindrinos MN, Smith RD, Davis RW, Tompkins RG, Toner M. Clinical microfluidics for neutrophil genomics and proteomics. Nat. Med. 2010;16:1042–1047. doi: 10.1038/nm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coldren CD, Nick JA, Poch KR, Woolum MD, Fouty BW, O'Brien JM, Gruber MP, Zamora MR, Svetkauskaite D, Richter DA, He Q, Park JS, Overdier KH, Abraham E, Geraci MW. Functional and genomic changes induced by alveolar transmigration in human neutrophils. Am. J. Physiol Lung Cell Mol. Physiol. 2006;291:L1267–L1276. doi: 10.1152/ajplung.00097.2006. [DOI] [PubMed] [Google Scholar]
- 72.Taylor PR, Roy S, Leal SM, Jr., Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat. Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.