Abstract
Neutrophilic infiltration is a leading contributor to pathology in a number of pulmonary disease states including cystic fibrosis. Hepoxilin A3 (HXA3) is a chemotactic eicosanoid shown to mediate the transepithelial passage of neutrophils in response to infection in several model systems and at multiple mucosal surfaces. Another well known eicosanoid mediating general neutrophil chemotaxis is leukotriene B4 (LTB4). We sought to distinguish the roles of each eicosanoid in the context of infection of lung epithelial monolayers by Pseudomonas aeruginosa. Using human and mouse in vitro transwell model systems, we utilized a combination of biosynthetic inhibitors, receptor antagonists, as well as mutant sources of neutrophils to assess the contribution of each chemoattractant in driving neutrophil transepithelial migration. We found that following chemotaxis to epithelial-derived HXA3 signals, neutrophil-derived LTB4 is required to amplify the magnitude of neutrophil migration. LTB4 signaling is not required for migration to HXA3 signals, but LTB4 generation by migrated neutrophils plays a significant role in augmenting the initial HXA3-mediated migration. We conclude that HXA3 and LTB4 serve independent roles to collectively coordinate an effective neutrophilic transepithelial migratory response.
Keywords: Neutrophils, Lung, Lipid Mediators, Chemotaxis, Inflammation
Introduction
Neutrophils are a critical component of the innate immune system and are indispensable for the clearance of many bacterial infections (1). However, uncontrolled neutrophil responses can lead to excessive inflammation and pathology, as is seen in cystic fibrosis (CF) (2, 3). CF is a congenital disorder defined by mutation of the CFTR gene. Abnormal ion regulation by dysfunctional CFTR results in chronic infection of the airway, most commonly with Pseudomonas aeruginosa (4), which can infect 70–80% of patients (5). Airway inflammation in patients with CF is dominated by persistent neutrophil infiltration and is associated with severe loss of function, respiratory failure, and ultimately mortality.
A key therapeutic strategy for treatment of CF is restraining neutrophil migration to the airspace (6). Neutrophil chemotaxis is a complex, orchestrated process involving the coordinated actions of selectins, integrins, and chemotactic signals as diverse as chemokines (IL-8), lipid mediators (LTB4), complement factors (C5a), matrix breakdown products (PGP), and bacterial products (fMLP) (7, 8). Unique among known neutrophil chemoattractants is hepoxilin A3 (HXA3). HXA3 is a lipid mediator produced by mucosal epithelium and secreted apically into lumenal spaces (9) where a chemotactic gradient is formed through the tight junction complexes and recruits neutrophils across mucosal epithelial surfaces (10). Its production is triggered by pathogenic bacteria (11) and plays a necessary role in chemotaxis across both pulmonary and intestinal epithelial surfaces in vitro (9, 12). Inhibition of the HXA3 signaling pathway also has profound affects in vivo, resulting in reduced neutrophilic pathology in models of inflammatory bowel disease (13), and reduced systemic disease in a model of pneumonia (14).
HXA3-mediated chemotaxis is only a part of a coordinated recruitment cascade that must first mobilize neutrophils from the blood stream, across the endothelium, and through the basement membrane before reaching the basolateral surface of the epithelial border. Many chemoattractants have been implicated as necessary for efficient neutrophil migration in models of pulmonary inflammation. The epithelial-derived CXC chemokine interleukin 8 (IL-8) effectively drives neutrophils from the blood into the tissue, but is directed basolaterally and is not sufficient to drive neutrophils across the epithelium (15, 16). C5a is a complement component and anaphylatoxin that plays a role in neutrophil recruitment in a number of pulmonary inflammatory conditions (17, 18). Leukotriene B4 (LTB4) is a very well studied eicosanoid that has long been known for driving the chemotaxis of neutrophils as well as other leukocytes, and has been implicated in a number of biological mechanisms (19, 20).
Despite the diversity of neutrophil chemoattractants that have been identified, an integrated picture involving the specific roles and sources of each chemoattractant has yet to emerge. Considering the large number of chemotactic signals that neutrophils may encounter in an inflammatory scenario, multistep navigation is necessary for successful homing (21). Chemotactic signals are prioritized by down-regulating the expression of alternative chemotactic receptors (22, 23), suggesting that the management of multiple simultaneous signals is an important part of neutrophil biology. Further, neutrophils can encourage their own migration by promoting microvascular leakage through the production of cytokine mediators (24). Once in the tissue, neutrophils cluster at sites of infection or damage. This swarming behavior organizes neutrophil localization within tissues (25, 26) and relies heavily on the production of neutrophil-derived LTB4 (27). In a model of rheumatoid arthritis, neutrophils and synovial tissue coordinate multiple chemotactic signals to manage waves of neutrophil recruitment (28–30). Expanding our understanding of neutrophil recruitment cascades across epithelial surfaces may allow for the development of therapeutic strategies for the treatment of patients with cystic fibrosis.
Given the complex nature of neutrophil recruitment mechanisms, as well as the tendency for LTB4 to serve as an amplifying mediator, we sought to determine if LTB4 played a role in bacterial-induced, HXA3-mediated neutrophil transepithelial migration. HXA3 and LTB4 are both eicosanoid neutrophil chemoattractants generated by the lipoxygenase family of enzymes (19, 31, 32). HXA3 plays a discrete role in mediating transepithelial migration, while LTB4 serves in a broad variety of functions as a leukocyte chemoattractant. We used and developed inverted transwell models of transepithelial migration to investigate the role of LTB4 in HXA3-mediated chemotaxis. We describe an axis of amplified migration that relies on neutrophil-derived LTB4 to magnify neutrophil migration following an initial response to HXA3-associated signals. This work consolidates HXA3-mediated transepithelial migration with the larger body of neutrophil migration. The relationship between HXA3 and LTB4 provides possible therapeutic targets for the modulation of neutrophil-associated immunopathology of the airway.
Materials and Methods
Bacterial Strains
Pseudomonas aeruginosa strain PAO1 was grown aerobically in Luria-Bertani broth at 37°C overnight. Prior to infection, cultures were washed and resuspended in Hank’s balanced salt solution (HBSS) and resuspended to a concentration 6×107 CFU/mL of HBSS.
Human Neutrophil Isolation
Neutrophils were isolated from whole blood collected from healthy human volunteers (IRB protocol #1999P007782) as previously detailed (33). Briefly, blood was anti-coagulated with acid citrate/dextrose. Buffy coat was obtained by centrifugation at 400 × g at room temperature. Plasma and mononuclear cells were removed by aspiration, and the majority of red blood cells were removed by 2% gelatin sedimentation. Residual RBC were removed by lysis using a cold ammonium chloride buffer. After lysis, neutrophils were washed and resuspended in HBSS without calcium or magnesium (HBSS−).
Bone Marrow Collection
C57BL/6J wild type, Ltb4r1−/−, and Alox5−/− were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in specific pathogen free conditions. Ltbr4r1−/− animals were homozygous for a targeted disruption of the LTB4 receptor BLT1 (34), while Alox5−/− animals were homozygous for a targeted disruption of the LTB4 biosynthetic enzyme 5-lixpoxygenase (35). Experiments were performed on female mice aged 6–8 weeks according to the guidelines of the Institutional Animal Care and Use Committee of Massachusetts General Hospital. Femurs and tibia were collected and flushed with HBSS− to collect bone marrow. RBC were lysed using a cold ammonium chloride solution. After lysis, remaining bone marrow was washed, and counted immediately prior to use.
Cell Culture
Transwell inserts with 3μm pores were purchased from Corning Life Sciences (Corning, NY). Inverted monolayers were prepared as previously described (33). Briefly, transwell inserts were flipped to an inverted position, collagen coated, then seeded with either mouse lung epithelial cells (MLE12) or human lung epithelial cells (H292) on the underside of the transwell. Epithelial cells were allowed to attach overnight then flipped back into transwells containing media and grown inverted until functional monolayers were established. Epithelial cells were maintained in DMEM/F12 (1:1) culture medium with 10% heat inactivated serum and 1X antibiotics for three to five days for MLE12 cultures or greater than seven days for H292 cultures. Functional monolayers were confirmed by the maintenance of fluid resistance between apical and basolateral compartments. In addition, all drug and infection conditions were assayed to assess their impact on epithelial viability and barrier integrity in independent experiments. Cell viability was assayed by MTT assay (Life Technologies; Carlsbad, CA). Barrier integrity was assessed by tracking the translocation of horse-radish peroxidase from the basolateral to apical compartment as previously described (12). None of the infection or drug treatment conditions significantly impacted epithelial cell viability or barrier integrity.
Transmigration Assays
Lung epithelial monolayers were grown on 24-well or 96-well inverted transwell inserts (Corning Life Sciences). Transwells were first washed and equilibrated in HBSS for 30 minutes prior to infection. After equilibration, transwells were again inverted and the apical surface of the epithelial cells was infected with 25μL of 6×107 bacteria/mL for 1h at 37°C, or mock infected with HBSS. After infection, transwells were washed and prepared for migration. PAO1-infected cells were placed in wells containing HBSS, as were HBSS control wells. Chemotactic gradients of IL-8 (100ng/mL, eBioscience; San Diego, CA), LTB4 (5ng/mL, Enzo Life Sciences, Farmingdale, NY), and mouse recombinant C5a (100ng/mL, R&D Systems; Minneapolis, MN) were prepared in HBSS and provided at the apical chamber. Lipid fractions from epithelial supernatants were prepared as described below and serially diluted for optimal dosing. In antagonist and apical soluble epoxide hydrolase (sEH) experiments, LY2239832 (Cayman Chemical; Ann Arbor, MI) or recombinant sEH (prepared by C. Morriseau as previously described (36, 37)) was also added to the apical chamber at the indicated concentrations. Neutrophils (2×105/96w transwell, 1×106/24w transwell) or whole bone marrow cells (8×105/96w transwell, 2×106/24w transwell) were supplied to the basolateral chamber and incubated for 2h at 37°C. After a 2h migration, the transwells were removed, and the apical well was assayed for neutrophil content by myeloperoxidase assay (33). In cases where different neutrophil or bone marrow sources were used in the same assay, standard curves were used to control myeloperoxidase variation between sources. Migration values were normalized to HBSS controls.
Inhibitors
Epithelial drug treatments were performed immediately prior to infection. Epithelial cells were washed three times, and incubated with zileuton (Sigma-Aldrich; St. Louis, MO), CDC (Enzo Life Sciences), MK886 (Enzo Life Sciences), or NS398 (Enzo Life Sciences) at the indicated concentration for 1h at 37°C in HBSS. After drug treatment, epithelial cells were washed three times then infected, as above. Untreated neutrophils were then added for migration. In experiments calling for drug-treatment of neutrophils, neutrophils (5×107/mL) were suspended in zileuton, CDC, MK886 or vehicle controls at the indicated concentrations for 1h at 37°C in HBSS−. Immediately prior to migration, neutrophils were resuspended in HBSS containing inhibitors. Neutrophil viability was confirmed by trypan blue exclusion.
Lipid extractions
Human H292 and murine MLE12 lung epithelial cells were seeded in a 162cm2 flask and grown to confluence. Confluent monolayers were washed and infected with PAO1 at 6×107 bacteria/mL in HBSS or mock infected for 1h at 37°C. The monolayers were then washed 3 times and incubated for an additional 2h in HBSS at 37°C. Supernatants were collected, and acidified to pH 5. In experiments where apical compartments were extracted for lipids, the plates were spun down to pellet neutrophils, and the apical supernatant was collected and acidified to pH 5. Acidified supernatants were poured through a Supelco Discovery DSC-18 SPE column (Sigma-Aldrich) and eluted with methanol. This lipid fraction in methanol was dried under a stream of nitrogen to 100μL and stored at −80°C for further processing. Immediately prior to the experiment, lipid fractions extracted from either epithelial cultures or from the apical supernatant of co-cultured epithelium and neutrophils were resuspended in 1mL of methanol, dried under a stream of nitrogen, then finally resuspended in HBSS for use in our assays. Lipid preparations used in epoxide hydrolase studies were incubated with 100μg/mL sEH or vehicle control for 2h at 30°C prior to bioactivity assessment. Lipid fractions were serially diluted for optimal dosing.
Calcium Mobilization Assays
Neutrophils or whole bone marrow were stained with the calcium indicator Fluo-4 AM (Life Technologies) at a final concentration of 1μM for 30 min at 37°C, then washed in HBSS−. Whole bone marrow was further stained with anti-Ly6G-APC (eBioscience) for 20 min on ice in order to identify neutrophils specifically. Stained cells were resuspended to a concentration of 2.5 ×106/mL. LY223982 treated neutrophils were incubated for 30 min at 2.5μg/mL prior to analysis by flow cytometry. Immediately prior to collection, stained cells were stimulated with LTB4 (5ng/mL), IL-8 (100ng/mL), HBSS−, or extracted lipid preparations from infected or mock-infected epithelial cells. Lipid preparations were suspended to 400μL of HBSS− then diluted 10-fold in neutrophil suspensions. Lipid stimulated cells were stimulated with at least 3 independent lipid preparations in each condition. Median fluorescent intensity was calculated for gated neutrophils.
Flow cytometry
Migrated mouse neutrophils were collected from the apical compartment following 2h migration. The cell suspension was blocked with fetal bovine serum and anti-CD16/CD32 (1:200, BD Biosciences; San Jose, CA) for 20 min on ice. Suspension was then stained with anti-CD45-PE (eBioscience), and anti-Ly6G-APC (eBioscience), and washed. Data was collected on a Accuri C6 flow cytometer (BD Biosciences), and analyzed with FlowJo analysis software (FlowJo; Ashland, OR).
Mixed BM migrations
Bone marrow from C57BL/6J wild type, Alox5−/−, and Ltb4r1−/− mice was collected and processed as above. Whole bone marrow was differentially stained with CFSE (BioLegend; San Diego, CA) at a concentration of 0.25μM or 2.5μM and mixed in equal proportion. Mixed cell suspensions were then used as a neutrophil source in the transmigration assay. The apical compartment was collected following a 2h migration, and the cell suspension was stained as above. CFSE+, Ly6G+ cells were gated and the relative proportion of each population was calculated. Relative enrichment was calculated a previously described (38). Briefly, for wild type (WT) enrichment over knock out (KO), a normalization value (N) was calculated as (% KO/% WT) in the input mixed bone marrow suspension. Percent enrichment was then calculated for each migrated sample as follows: [(% WT × N) − (% KO)]/(% WT × N) × 100.
Leukotriene Measurement
Leukotriene B4 detection assays were purchased from Cayman Chemical and performed according to manufacturer’s instruction. LC/MS/MS was performed by a triple quadruple linear ion trap system (3200 QTRAP; AB SCIEX; Framingham, MA) as previously described (39, 40).
Statistics
Results are expressed as mean ± standard deviation. Data is representative of at least three independent data points per condition, and multiple experiments yielding similar results. Single comparisons were evaluated using unpaired two-tailed Student’s t test. Where it is noted, data was analyzed by 2-Way ANOVA with bonferroni post test. p values ≤ 0.05 were considered to be significant.
Results
BLT1 antagonism disrupts PAO1 mediated migration
In order to discriminate migration by either hepoxilin A3 (HXA3) or leukotriene B4 (LTB4) signaling, we relied on a well described transepithelial transwell model system (9, 33). Briefly, human lung epithelium is grown on the underside of a transwell filter with 3μm pores. Epithelial cells spontaneously form a polarized monolayer with distinct apical and basolateral compartments (Fig 1a). The epithelium is infected at the apical surface and bacteria is subsequently washed away. Neutrophils are supplied to the basolateral compartment and, in the presence of chemotactic signals, migrate through the filters and into the apical space. After migration, the transwell is discarded and neutrophil migration is measured by myeloperoxidase activity. In this model, P. aeruginosa infection has been demonstrated to induce endogenous epithelial production of HXA3, resulting in the migration of neutrophils in a basolateral to apical direction (12, 41). Imposed apical to basolateral gradients of LTB4 or IL-8 are also capable of driving neutrophil transepithelial migration. All chemotactic signals produced a robust migratory response (Fig 1b).
Figure 1. PAO1-induced transepithelial neutrophil migration is disrupted by antagonism of the LTB4 receptor, BLT1.
(A) A model schematic of transepithelial neutrophil migration using lung epithelia grown on inverted transwells is shown. (B) Relative migration to PAO1-induced signals as well as control chemoattractants was quantified by myeloperoxidase assay. (C) Migration to PAO1-induced signals or control gradients was assayed in the presence of an apical gradient of LY223982. Data is represented as migration as a percent of vehicle control. Significance values were calculated by ANOVA. * symbols indicate significant change between IL-8 gradient and PAO1-induced migration. § symbols indicate significant change between PAO1-induced migration and LTB4 gradient. (D) H292 monolayers grown on inverted transwells were treated with pharmacological inhibitors to 12-lipoxygenase (CDC), 5-lipoxygenase (zileuton), or 5-lipoxygenase activating protein (MK886) prior to infection or the addition of neutrophils. Data is shown as a percentage of migrating cells with respect to vehicle controls. All data is represented as mean ± standard deviation and are representative of multiple independent experiments. * or § p < 0.05, ** or §§ p < 0.001.
LY223982 is a highly specific antagonist to BLT1, the primary chemotactic receptor for leukotriene B4 on neutrophils (42). LY223982 was added to the apical space during migration in order to specifically antagonize LTB4 mediated signaling (Fig 1c). As anticipated, we observed significant inhibition of LTB4-mediated migration, whereas IL-8 mediated migration was not significantly affected by the LTB4 receptor antagonist. Notably, PAO1-induced migration, mediated by HXA3, was also impacted by the LTB4 receptor antagonist, albeit to a lesser extent than LTB4 gradients at multiple doses. These data suggest that BLT1 antagonism specifically and partially inhibited PAO1-induced migration.
Lung epithelial cells respond to infection with the production of HXA3 as well as other eicosanoids such as PGE2 (39), however we have been unable to detect the presence of LTB4 in the culture supernatant of either infected or uninfected epithelial cells. Furthermore, PAO1-mediated migration was very strongly reduced when epithelial cells were pretreated with CDC, an inhibitor of 12-lipoxygenase (the HXA3 biosynthetic enzyme), but not zileuton, an inhibitor of 5-lipoxygenase (the LTB4 biosynthetic enzyme) (Fig 1d). Additionally, 5-lipoxygenase activating protein (FLAP) inhibition with MK886 had no significant effect on migration when targeting epithelial cells (Fig 1d).
While BLT1 seems to play a role in PAO1-induced neutrophil transepithelial migration, LTB4 was not detectable in epithelial supernatants, and treatment of the epithelium with 5-lipoxygenase inhibitors did not significantly impact the ability of neutrophils to migrate. Since previous evidence suggests that LTB4 is not a relevant epithelial-derived chemotactic signal in our model, we considered two possible hypotheses to explain these results; (1) HXA3 interacts with the LTB4 receptor BLT1 to signal neutrophils, or (2) LTB4 is produced by migrating neutrophils and contributes to the magnitude of the transepithelial response.
BLT1 Antagonism does not inhibit PAO1-associated Calcium Signals
Given the importance of BLT1 for a robust migratory response to HXA3, we sought to determine whether HXA3 may rely on BLT1 for signaling of neutrophils. The receptor for HXA3 is unknown, but has been described as a G-protein coupled receptor (GPCR) that induces calcium influx upon stimulation (9, 10). Because GPCRs can heterodimerize in order to modulate signaling (43) and BLT1 has multiple agonists (44), we considered whether BLT1 may be a required component of HXA3 mediated migration.
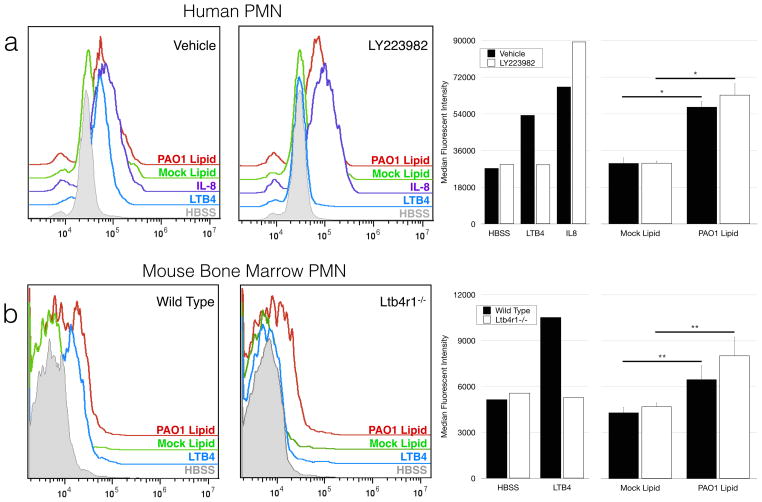
In order to determine whether BLT1 was required for HXA3-associated signaling on neutrophils, the calcium indicator Fluo-4 AM was used to measure calcium mobilization in response to GPCR stimulation by various chemoattractants. Calcium mobilization was strongly induced by LTB4 as well as IL-8 (Fig 2a). In order to assess the calcium mobilization to HXA3-associated signals, we used lipid extracts from PAO1-infected epithelial supernatants. These supernatants are enriched for HXA3 and retain chemotactic activity dependent on HXA3 (41). PAO1 lipids induced a strong calcium mobilization in neutrophils, while mock infected lipids had no detectable effect (Fig 2a).
Figure 2. BLT1 Antagonism does not inhibit PAO1-associated signaling of neutrophils.
(A) Isolated human neutrophils were stained with the calcium indicator dye Fluo-4 AM, and incubated in the presence of LY223982 (2.5μg/mL) or vehicle control for 30 min. Neutrophils were then stimulated with either control chemoattractants or lipids extracted from mock or PAO1-infected H292 epithelium. Histograms are gated on live cells. (B) Mouse bone marrow from wild type or Ltb4r1−/− mice was stained with Fluo-4 AM as well as anti-Ly6G antibodies. Bone marrow preparations were stimulated as above. Histograms are gated on Ly6G+ cells. Shown are representative histograms gated on neutrophils, as well as quantified median fluorescent intensity. Data is represented as mean response to at least 3 lipid preparations ± standard deviation. Data is representative of multiple independent experiments.* p < 0.05, ** p < 0.001.
Calcium mobilization associated with LTB4 signaling was strongly inhibited in the presence of the specific BLT1 receptor antagonist LY223982, whereas responses to IL-8 were not diminished (Fig 2a). Mobilization in response to lipids collected from PAO1-infected epithelium was not impacted by the BLT1 receptor antagonist, suggesting that BLT1 is not required for HXA3-associated signaling in neutrophils.
In a similar experiment, bone marrow neutrophils were isolated from mice deficient for BLT1 (Ltb4r1−/−). Wild type mice responded to all chemotactic signals with calcium mobilization (Fig 2b). As expected, mice lacking the BLT1 gene were incapable of responding to LTB4 signaling, but were fully competent in responding to PAO1-induced epithelial-derived lipid extracts. Taken together, these data suggest that BLT1 is not a necessary component of HXA3 signaling, and therefore BLT1 may play a role in complementing HXA3 signaling to drive neutrophil transepithelial migration.
HXA3 signaling is a conserved mechanism in a mouse in vitro model of transepithelial migration
Previous work has demonstrated an important role for HXA3 in neutrophil transepithelial migration in mouse models. HXA3 has been detected in response to Pseudomonas infection (41), and disruption of HXA3 signaling significantly disrupts neutrophil migration in models of gastrointestinal (13), and pulmonary inflammation (14) in mice. We adapted our transwell migration model to a fully mouse in vitro model in order to leverage available genetic tools and explore the functional implications of leukotriene signaling in a HXA3 mediated migration model. Briefly, the mouse lung epithelial cell line, MLE12, was grown as inverted monolayers on transwell supports. As a source of neutrophils, whole primary mouse bone marrow was supplied to the basolateral compartment. Bone marrow neutrophils have previously been shown to be a functionally competent model of neutrophil migration (45).
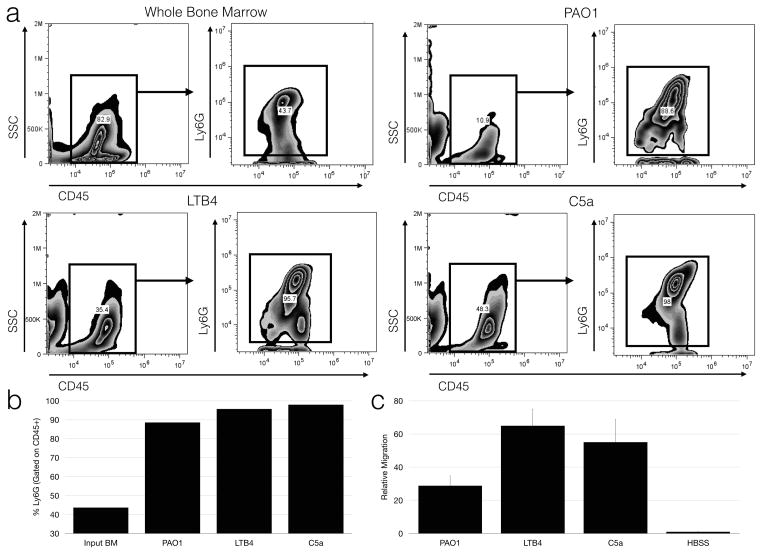
Neutrophil transmigration was measured by myeloperoxidase activity as well as flow cytometry (Fig 3). Flow cytometry analysis of the apical compartment demonstrated bone marrow neutrophils were selectively enriched in response to pseudomonas infection as well as imposed gradients of leukotriene B4 and C5a (Fig 3a, 3b), supporting the functional specificity of the transmigration model. Myeloperoxidase was readily detectable in apical compartments following migration to infection and chemotactic gradients (Fig 3c).
Figure 3. A mouse in vitro model of transepithelial migration selectively recruits bone marrow neutrophils.
(A) Representative dot plots of whole bone marrow (upper left) used as a source of neutrophils, and of migrated cells after PAO1 infection (upper right) are shown. Cells migrating to LTB4 (lower left) and C5a (lower right) were also analyzed. Cells were initially gated on CD45 to exclude cells of epithelial origin and other debris, then gated on the Ly6G. (B) Percentage of neutrophils among CD45+ cells in the apical chamber following migration to various chemotactic stimuli was calculated. Data represent six pooled individual wells and is representative of three replicate experiments. (C) Relative migration was measured by myeloperoxidase assay, shown as mean ± standard deviation and is representative of at least 3 independent experiments.
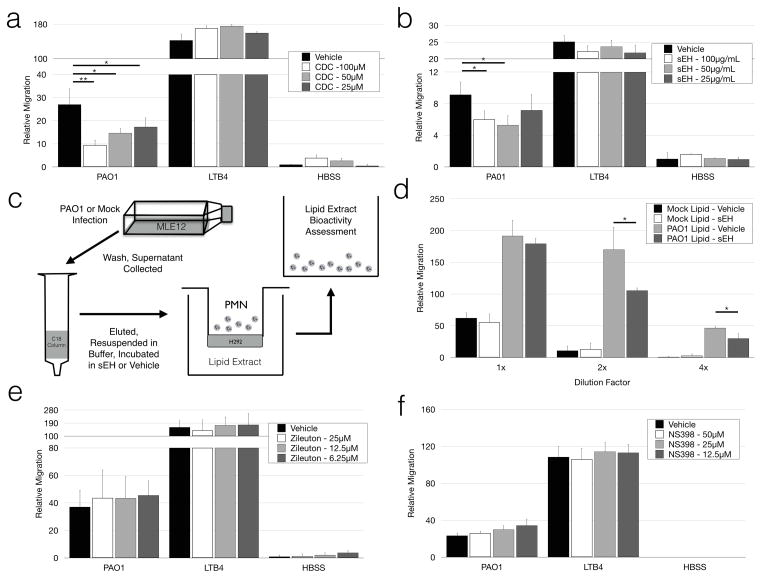
We wanted to determine whether disruption of HXA3 signaling pathways was conserved in our in vitro mouse model. First, we targeted biosynthesis of HXA3 in epithelial cells with the 12-lipoxygenase inhibitor CDC. CDC treatment of the epithelium significantly reduced migration in response to pseudomonas infection, but not a leukotriene B4 gradient (Fig 4a).
Figure 4. PAO1-induced transepithelial migration in an MLE12 transwell model relies on HXA3 and 12-LO signaling.
(A) Relative migration was quantified after MLE12 monolayers were incubated with the 12-lipoxygenase inhibitor CDC at multiple concentrations prior to infection. (B) Soluble epoxide hydrolase (sEH) was added to the apical compartment of transwells at multiple doses during migration to assess interference with neutrophil migration. (C) A schematic of lipid extraction and assessment of lipid chemotactic bioactivity is shown. (D) Lipids collected from MLE12 epithelium were incubated in the presence of sEH (100μg/mL) or vehicle control for 2h prior to evaluating their bioactivity. Lipids were serially diluted to evaluate bioactivity within a functional window. MLE12 transwells were incubated in the presence of zileuton (E) and NS398 (F) at multiple doses prior to infection. Relative migration was calculated as a function of total myeloperoxidase activity following migration. Data is represented as mean ± standard deviation, and is representative of multiple independently run experiments. * p < 0.05, ** p < 0.001.
In addition to targeting HXA3 biosynthesis with the use of pharmacological inhibitors, HXA3 signaling can be modified by directly degrading HXA3 with soluble epoxide hydrolase (sEH) (41). An epoxide ring is a unique structural feature of HXA3, and its hydrolysis converts HXA3 to the non-chemotactic degradative product trioxilin A3 (46, 47). Incubation of the apical compartment with sEH during migration was sufficient to significantly impair neutrophil migration (Fig 4b). In order to further validate sEH targeting of HXA3 lipids, we extracted and concentrated the lipid fraction from infected and uninfected MLE12 monolayers. These extracted lipids were then assayed for their chemotactic bioactivity following incubation with soluble epoxide hydrolase (Fig 4c). Incubation of lipid extracts with sEH significantly reduced their chemotactic bioactivity (Fig 4d).
This PAO1-induced chemotaxis was not affected when the epithelium was incubated with selective inhibitors for either 5-lipoxygenase (Fig 4e), or cyclo-oxygenase-2 (Fig 4f). Taken together, these data suggest that bacterial-induced, HXA3-dependent neutrophil transepithelial migration is conserved in this murine in vitro model.
Neutrophil-derived LTB4 signaling plays a significant role in HXA3 mediated migration
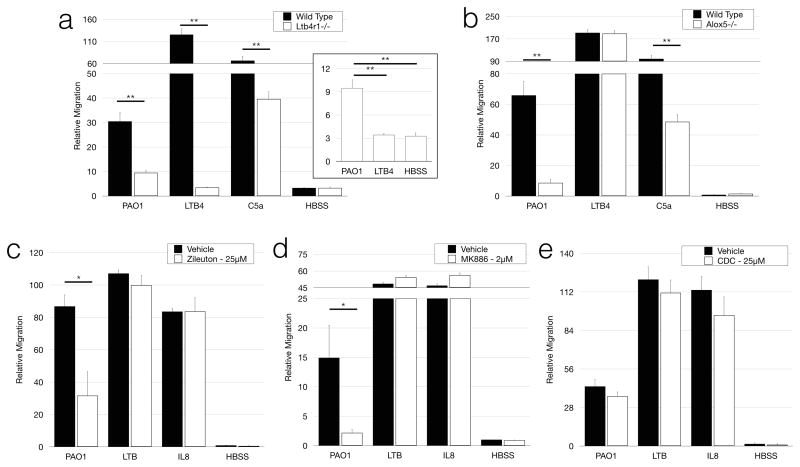
Having established that epithelial-derived signaling in our mouse in vitro model is consistent with prior work, we investigated the role of LTB4 signaling by neutrophils in response to HXA3-mediated migration. Ltb4r1−/− neutrophils were severely deficient in migrating to gradients of leukotriene B4, and were not significantly distinguishable from buffer controls (Fig 5a). PAO1-induced migration was also severely impaired using Ltb4r1−/− bone marrow, but responses were significantly higher than either LTB4 responses or buffer controls (Fig 5a, inset). This suggests that BLT1 is not absolutely necessary for PAO1-associated migration, but that it plays a key role in amplifying the neutrophil response to such stimuli. Interestingly, we saw a similar, though less pronounced, BLT1 dependency in C5a mediated migration, suggesting that this is a mechanism that may be broadly used by neutrophils to amplify select chemotactic signals.
Figure 5. Neutrophil-derived LTB4 signaling plays a significant role in migration to HXA3 signals.
Migration of Ltb4r1−/− (A) and Alox5−/− (B) bone marrow was evaluated in a mouse in vitro transwell model of neutrophil transepithelial migration. Human neutrophils were incubated in the presence of zileuton (C), MK886 (D), or CDC (E) prior to migration in a human in vitro transwell model of transepithelial migration. Data are represented as mean ± standard deviation and represent multiple independently run experiments. * p < 0.05, ** p < 0.001.
Neutrophils are capable of releasing a variety of inflammatory factors following stimulation, including LTB4 (48). We sought to determine whether neutrophil-derived LTB4 was responsible for the BLT1 dependent migration we observed. We tested bone marrow isolated from 5- lipoxygenase deficient mice (Alox5−/−) for their ability to respond to PAO1-induced signals (Fig. 5b). We again found a significant deficit in PAO1-induced and C5a-mediated migration, further implicating a role for neutrophil-generated LTB4 in transepithelial migration to some chemotactic signals. Alox5−/− bone marrow was fully responsive to gradients of LTB4.
Neutrophil-associated 5-lipoxygenase activity was also important in mediating robust chemotaxis in response to PAO1 infection in the human in vitro model. Human neutrophils were treated with either zileuton (Fig 5c) or MK886 (Fig 5d). Unlike drug treatment of the epithelium (Fig 1d, 4e, 4f), targeting the neutrophil directly significantly impaired the ability of neutrophils to respond to PAO1 infection. Notably, neither LTB4− nor IL-8-mediated migration relied on 5-lipoxygenase-associated amplification for robust migration in this model. Finally, treatment of neutrophils with the 12-lipoxygenase inhibitor CDC had no significant affect on migration (Fig 5e), in contrast to the significant inhibitory effect of CDC when pre-treating the epithelium (Fig 1d). Taken together, these data strongly suggest that neutrophil-associated 5-lipoxygenase activity is critical for robust response to epithelial-derived HXA3.
PMN-derived LTB4 contributes to chemotactic bioactivity in HXA3-induced migration
We suspected that migrated neutrophils likely produced LTB4 in order to amplify initial HXA3 chemotactic signaling. In order to test whether PMN-derived LTB4 was detectable in apical compartments and amplified migratory responses to HXA3, we repeated our transepithelial migration assay in the presence and absence of neutrophils (Fig 6a). First, we assayed this initial apical supernatant for the presence of leukotriene B4. LTB4 was only detected in supernatants containing neutrophils, and was significantly higher in samples migrated in response to PAO1-induced signals (Fig 6b). LTB4 levels did not correlate with myeloperoxidase levels (Fig 6b, inset). By correcting the amount of detectable LTB4 for the amount of migration, we observe that LTB4 release was much more strongly induced in response to PAO1-associated signals (Fig 6c). The presence of LTB4 was confirmed by LC/MS/MS. LTB4 detected in neutrophil containing samples migrating to PAO1-infection totaled 189.6 ± 30.5pg/mL. No LTB4 was detected in response to mock infection or in the absence of neutrophils.
Figure 6. Neutrophils respond to PAO1-signals with significant upregulation of LTB4.
(A) A schematic model of the workflow evaluating production of LTB4 and lipid based bioactivity is shown. (B) The apical compartment was sampled after PMN or mock migration to PAO1 infection, IL-8 gradient, or HBSS controls. Samples were assayed for the presence of LTB4 by EIA. Relative migration of PMN containing wells was also measured by myeloperoxidase and is shown as inset. (C) LTB4 concentration of the apical compartment with neutrophil migrated samples was divided by relative neutrophil migration and normalized. Data are represented as LTB4 concentration of apical compartment as a function of relative neutrophil number. (D) Apical supernatant was collected from PAO1-infected, IL-8 migrated and HBSS control wells with and without neutrophils. This supernatant was extracted for lipid components and then evaluated for chemotactic bioactivity. Neutrophil-containing conditions are represented with circles. The inset figure shows conditions where no neutrophils were provided and is represented with triangles. Significance was calculated by ANOVA. (E) The apical compartment was sampled following migration to PAO1-infected lipid preparation, IL-8 gradient, or Mock-infected lipid preparation. Lipid samples were also directly sampled. LTB4 levels were measured by EIA. Relative migration of PMN was also assayed by myeloperoxidase and is shown inset. (F) LTB4 concentration of lipid migrated samples was divided by the relative neutrophil migration and normalized. Data are represented as LTB4 generated by relative neutrophil migration. (G) Transepithelial migration was performed using zileuton-treated neutrophils, vehicle-treated neutrophils, or without neutrophils. Apical compartment was sampled following migration and assayed for the presence of LTB4 by EIA. Data are presented as mean ± standard deviation. Data are representative of multiple independent experiments. L.D. denotes limit of detection of the assay. * p < 0.05, ** p < 0.001.
Apical supernatant was also collected and lipid components were extracted as before in order to assay their bioactivity. Previous lipid preparations (Fig 2, 4d) were prepared in the absence of neutrophils and represented lipids exclusively produced by the epithelium. In this case, the apical supernatant extract contains lipids from both epithelium and migrated neutrophils. Strong migratory bioactivity was detected in samples collected from wells containing neutrophils that had migrated to PAO1-induced signals (Fig 6d). Notably, while IL-8 recruited significantly more neutrophils in our assay, these neutrophils did not produce lipid-associated bioactivity above buffer background. This suggests that IL-8 does not induce significant levels of LTB4 in this model of transepithelial migration. Notably, we were able to detect a small amount of lipid bioactivity from PAO1-infected transwells lacking neutrophils (Fig 6d, inset). This activity likely represents epithelial HXA3.
In addition to infection-induced neutrophil transepithelial migration experiments, we also performed similar experiments in which the epithelium was not infected, but instead lipid preparations from mock or PAO1-infected epithelial cells were provided (Fig 6e, 6f). These lipids were prepared in the absence of neutrophils. LTB4 was not detectable in lipids collected from epithelial cells in response to PAO1 or mock infection (Fig 6e). When the HXA3-enriched lipids collected from infected epithelium are used as a chemotactic gradient, we observe heightened LTB4 generating capacity from migrating neutrophils compared to the response to a gradient of IL-8. Thus, the amplifying factor is preserved by lipid extraction of epithelial signals.
Lastly, we measured LTB4 levels from apical supernatants containing migrated neutrophils that had been treated with zileuton or vehicle control (Fig 6g). We confirmed that zileuton treatment of neutrophils significantly reduced the amount of LTB4 in apical supernatants, which is consistent with the observed reduction in migration of zileuton-treated neutrophil (Fig 5c).
LTB4 amplification skews recruitment in favor of BLT1 competent PMN
Our data suggest that neutrophils migrating across PAO1-infected epithelial monolayers respond by secreting LTB4, thereby amplifying chemotaxis and increasing the magnitude of the responding population of neutrophils. We hypothesized that this network effect could be more directly observed using mixed bone marrow migration assays. Briefly, two genotypes of bone marrow were differentially stained with CFSE and mixed in equal proportion. The mixed population was used as a source of neutrophils in our migration assay. Following a two hour migration, the apical compartment was sampled to determine the proportion of each population, the total migration, and total leukotriene B4 levels.
The first combination was a mix of wild type and Alox5−/− bone marrow. We did not observe preferential migration of either population in response to either infection with PAO1, or gradients of LTB4 or C5a (Fig 7a). We also observed very strong migration to all three chemotactic signals (Fig 7d). This is in contrast to the poor migration of Alox5−/− neutrophils alone (Fig 7d). The presence of wild type bone marrow rescues the ability of 5-lipoxygenase deficient neutrophils to respond. This suggests that the leukotriene produced by wild type neutrophils is capable of recruiting both genotypes of neutrophils. Indeed, we observe strong LTB4 levels in the apical compartment following migration of wild type and Alox5−/− bone marrow mixtures (Fig 7e).
Figure 7. LTB4 generation rescues 5-lipoxygenase deficiencies, but not BLT1 deficiencies.
Transepithelial migrations were performed using mixed bone marrow populations in response to infection, as well as LTB4 and C5a gradients. Each bone marrow population was differentially labeled with CFSE prior to migration and mixed at 1:1 ratio. After migration, the apical compartment was sampled and analyzed by flow cytometry to determine the relative proportion of each population. Combinations of (A) Alox5−/− with wild type bone marrow, (B) wild type with Ltb4r1−/− bone marrow, and (C) Alox5−/− with Ltb4r1−/− bone marrow was mixed in order to assess paracrine signaling affects. (D) Myeloperoxidase and (E) LTB4 levels were also measured from apical compartments following migration. Representative histograms, as well as quantified enrichment scores from at least 3 replicated experiments are shown. Histograms are gated on Ly6G+ neutrophils. Relative migration and LTB4 levels are shown as mean ± standard deviation. * p < 0.05. **p < 0.001.
We then migrated a combination of wild type and Ltb4r1−/− bone marrow. As expected, in all cases Ltb4r1−/− neutrophils were deficient in responding to the chemoattractants we tested (Fig 7b). Ltb4r1−/− neutrophils had virtually no detectable response to LTB4. Ltb4r1−/− neutrophils also represented a very small proportion of the cells responding to either infection or C5a gradients, resulting in a population heavily skewed towards wild type neutrophils. This skewing in favor of wild type neutrophils in PAO1-induced and C5a gradients was significantly lower than in response to LTB4 gradients, suggesting that Ltb4r1−/− neutrophils functionally respond to their primary gradients, but do not respond to amplifying signals. In all cases the relative migration of this combination of genotypes is significantly higher than the migration of Ltb4r1−/− neutrophils alone, although much lower than either wild type alone, or the combination of wild type and Alox5−/− (Fig 7d). In the case of PAO1 infection and C5a gradients, this likely reflects the limited number of neutrophils capable of responding to amplifying signals, despite the significant level of LTB4 present in the apical space (Fig 7e).
Lastly, we combined the two knock out genotypes, Alox5−/− and Ltb4r1−/−. We observed a skewing phenotype in favor of Alox5−/− neutrophils similar to the skewing in favor of wild type neutrophils when either is combined with Ltb4r1−/− neutrophils (Fig 7c). Predictably, Alox5−/− neutrophils were nearly all of the neutrophils responding to LTB4 gradients, but they also represented approximately 80–90% of the migrating population to both PAO1-infection and C5a gradients. The migratory response is elevated compared to either knock out genotype alone, and similar to the response the wild type/Ltb4r1−/− combination (Fig 7d). We also detected significant LTB4 levels in these wells (Fig 7e). Taken together, these data suggest that both genotypes can respond to PAO1 infection and C5a gradients. Ltb4r1−/− neutrophils, once migrated, produce LTB4 and recruit Alox5−/− neutrophils leading to a migrating population dominated by Alox5−/− neutrophils.
Discussion
A thorough understanding of the mechanisms and pathways of neutrophil recruitment across mucosal epithelium is of immense translational value. In the airspace alone, neutrophils play key clinical roles in the etiology of cystic fibrosis, bacterial pneumonia, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), and severe asthma, among others. Intervention strategies that manipulate the magnitude and severity of the neutrophil responses have long been recognized as key therapeutic tools in limiting neutrophil-mediated pathology (6).
HXA3-mediated chemotaxis is a necessary step in neutrophil transepithelial migration (10, 12). HXA3 production is unique in that it is directed into the lumen of mucosal spaces, and thus is relatively distal from the blood stream. Its immunological niche necessitates collaboration with other signals in a chemotactic cascade. Sampling of the mucosal lumen during inflammatory events often reveals the presence of many chemoattractants that may not provide appropriate context for their ultimate role in chemotaxis. In an effort to distinguish the role HXA3 plays from the many other relevant chemoattractants, we relied on a well described inverted transwell model system. Our immediate observation was that we were unable to disentangle HXA3 signaling from BLT1 receptor activity. Blockage of the BLT1 receptor significantly and persistently impacted the magnitude of PAO1-induced migration. This was not a result of epithelial LTB4 production. We were unable to detect LTB4 in epithelial supernatants in the presence or absence of infection, and manipulation of LTB4 biosynthetic pathways in epithelial cells had no impact on migration.
The HXA3 receptor is currently unknown, but HXA3 signaling relies on a G-protein coupled receptor, and induces calcium mobilization in neutrophils (9, 10). We investigated whether we were able to block HXA3 signaling by either antagonizing BLT1 or using BLT1 deficient neutrophils. We were able to efficiently block LTB4 signaling in neutrophils without diminishing responses to control stimuli or HXA3-enriched lipids. These samples can be experimentally paired with mock-infected lung epithelial supernatants, which do not contain HXA3, in order to gain insight into HXA3 signaling (41, 49). This maneuver is necessitated by limited availability of commercial sources of purified HXA3. While we cannot dismiss the possibility of an unknown infection-induced lipid source of calcium signaling, these results suggest that BLT1 antagonism is not directly impacting HXA3 signaling pathways, but likely plays an indirect role in this system.
In order to further leverage genetic tools in exploring this mechanism, we developed a parallel murine in vitro model. Whole bone marrow was used in place of isolated neutrophils in order to functionally demonstrate the selective migration of neutrophils. In all conditions, the responding populations were overwhelmingly Ly6G+ neutrophils, and were readily detectable by myeloperoxidase assay. In vivo mouse models have repeatedly demonstrated HXA3 and HXA3 signaling pathways as being a relevant and even critical pathway for neutrophil transepithelial recruitment (13, 14, 41). In this model, pseudomonas induced PMN transepithelial migration was also significantly reduced by targeting HXA3 specific pathways at the epithelium. Inhibition of HXA3 biosynthesis effectively reduced migration in this model, as in previous models. Meanwhile, inhibition of control eicosanoid synthesis pathways 5-lipoxygenase and cyclooxygenase-2 had no impact. Direct degradation of HXA3 using soluble epoxide hydrolase (41, 47) also effectively reduced PAO1-induced migration. Notably, sEH degradation of HXA3 was effective when targeting the apical space directly and may provide insight into the potential of therapeutically targeting the airway to modulate neutrophilic pathology. Taken together, these observations confirm that HXA3 signaling is a conserved pathway in this mouse model.
HXA3-mediated chemotaxis has not previously been associated with leukotriene B4 amplification. In both human and murine models, neutrophils deficient in either BLT1 signaling or 5-lipoxygenase activity were severely impaired in their ability to respond to PAO1 infection. Notably, migration to HXA3-associated signals was not entirely eliminated. PAO1-induced migration of Ltb4r1−/− and Alox5−/− neutrophils was above background. Inhibition of LTB4 biosynthetic pathways in human neutrophils produced similar results, while inhibition of neutrophil 12-lipoxygenase had no significant affect. These observations nicely integrate HXA3 with the existing body of literature. Neutrophil-derived leukotriene B4 functions as a signal relay for neutrophil localization in many models (21, 27, 48, 50), and functions to orchestrate neutrophil recruitment in models of rheumatoid arthritis (29, 51).
Release of LTB4 into the apical space was detectable when 5-lipoxygenase competent neutrophils migrated, and served to significantly enhance lipid associated bioactivity in these preparations. LTB4 levels did not correlate well with total neutrophil migration in response to all chemotactic stimuli, suggesting that this is not a general response by neutrophils that have migrated. By correcting the total LTB4 levels detected for the relative number of neutrophils quantified by myeloperoxidase, it is evident that Pseudomonas-induced migration was associated with significantly higher levels of leukotriene production than IL-8 gradient-induced migration.
Therefore, neutrophils do not blindly function as to amplify their own recruitment, but rely on signals that may be independently modulated. Both fMLP and C5a have been shown to stimulate LTB4 production in neutrophils (48), and here we see LTB4 production in response to HXA3-associated migration. In this model system, neutrophils are selectively recruited across the epithelium to apically directed gradients of HXA3 and LTB4. We cannot exclude the possibility that small contaminating populations of cells such as lymphocytes, monocytes, or other granulocytes may also release some LTB4, but such LTB4 production would need to predominantly occur in the apical compartment in order to reinforce the directed gradient. In vivo, resident immune populations may actively play a role in neutrophil recruitment. Further study is needed to transition these observations to account for the complexity of the lung environment in vivo.
It is not clear whether HXA3 signaling directly induces LTB4 production, or if other factors associated with the epithelial response are necessary. This inducing factor may be of bacterial or epithelial origin, but must persist through washing of bacteria and preparation of lipid extracts. Ultimately a better understanding of LTB4 triggering mechanisms will require studies employing a purified source of HXA3. What is evident is that LTB4 is a critical component extending and expanding the response to HXA3 migration.
When assayed in isolation, neutrophils lacking key LTB4 signaling components were unable to migrate effectively. In combination with other genotypes, we are able to see that a relatively small proportion of migrated neutrophils can compensate for signaling deficiencies. When paired with wild type neutrophils, Alox5−/− neutrophils respond equally well, and the magnitude of the migratory response is comparable to wild type alone. Alox5−/− neutrophil migration is effectively compensated by having a source of LTB4. BLT1 deficiency, on the other hand is not readily compensated, and the result is a migratory response heavily skewed in favor of BLT1 competent neutrophils. This pattern is true even in the context of Alox5−/−/Ltb4r1−/− bone marrow mixtures. HXA3, as well as C5a, are capable of recruiting an initial wave of neutrophils. 5-lipoxygenase competent cells then produce LTB4, and recruit BLT1 competent cells to amplify the magnitude of the response. This compensation of genetic deficiency underscores the importance of neutrophil to neutrophil communication in mediating transepithelial migration.
Many current therapies rely on targeting neutrophil function in hopes of reducing morbidity associated with progressive lung damage (6). Non-steroidal anti-inflammatories such ibuprofen and BLT1 antagonists are targeted systemically with the goal of reducing neutrophil migration to the airspace (2, 52). Therapeutic doses are often associated with adverse events and poor adherence. Corticosteroid therapy is also effective at preventing lung damage in cystic fibrosis by limiting neutrophil migration, but is of limited value due to similar side effects (53). A strategy of limiting neutrophil migration to sites of disease has clear therapeutic value, but adverse events are often associated with systemic immunological disruption that is inherently problematic. Targeting therapies to the mucosal surface may provide the benefits of reducing airway pathology without broadly suppressing immunity. Our in vitro model suggests that both degradation of HXA3 and antagonism of BLT1 are effective at limiting neutrophil migration when targeted to the apical space. Further understanding of the mechanisms leading to mucosal breach of neutrophils during inflammatory insults and disease can lead to the development of novel therapeutic options for patients with cystic fibrosis, acute respiratory distress syndrome, pneumonia, COPD, and other neutrophil driven pulmonary insults. These observations are likely applicable to mucosal surfaces of the gastrointestinal tract, further expanding the impact to patients with any number of neutrophilic bowel disorders. Continued attention to the mechanisms driving mucosal breach of neutrophils may provide avenues for the treatment of disease associated with neutrophil-mediated pathology and opportunities to alleviate associated morbidities.
Acknowledgments
We thank Matthew Greenwood for technical assistance with LC/MS/MS analysis.
Abbreviations used in this paper
- PMN
polymorphonuclear cells (i.e. neutrophils)
- HXA3
hepoxilin A3
- LTB4
Leukotriene B4
- sEH
soluble epoxide hydrolase
Footnotes
This work has been supported financially by the NIH/NIAID (R01 AI095338) as well as the Cystic Fibrosis Foundation (PAZOS13F0). CM was supported financially by NIEHS RO1 ES002710.
References
- 1.Koh AY, Priebe GP, Ray C, van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 3.Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151:939–941. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]
- 4.Pier GB. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985;151:575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- 5.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 7.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 8.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 9.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 10.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick BA. Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. FEBS J. 2007;274:3513–3518. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- 12.Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- 13.Pazos M, Siccardi D, Mumy KL, Bien JD, Louie S, Shi HN, Gronert K, Mrsny RJ, McCormick BA. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J Immunol. 2008;181:8044–8052. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhowmick R, Tin Maung NH, Hurley BP, Ghanem EB, Gronert K, McCormick BA, Leong JM. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J Immunol. 2013;191:5115–5123. doi: 10.4049/jimmunol.1300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucharzik T, Hudson JT, Lügering A, Abbas JA, Bettini M, Lake JG, Evans ME, Ziegler TR, Merlin D, Madara JL, Williams IR. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565–1572. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 18.Höpken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 19.Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- 20.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 21.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomhave ED, Richardson RM, Didsbury JR, Menard L, Snyderman R, Ali H. Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J Immunol. 1994;153:3267–3275. [PubMed] [Google Scholar]
- 23.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finsterbusch M, Voisin M-B, Beyrau M, Williams TJ, Nourshargh S. Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF. J Exp Med. 2014;211:1307–1314. doi: 10.1084/jem.20132413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chtanova T, Schaeffer M, Han S-J, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, Robey EA. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng LG, Qin JS, Roediger B, Wang Y, Jain R, Cavanagh LL, Smith AL, Jones CA, de Veer M, Grimbaldeston MA, Meeusen EN, Weninger W. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol. 2011;131:2058–2068. doi: 10.1038/jid.2011.179. [DOI] [PubMed] [Google Scholar]
- 27.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91:207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace-Asciak CR, Reynaud D, Demin PM. Hepoxilins: a review on their enzymatic formation, metabolism and chemical synthesis. Lipids. 1995;30:107–114. doi: 10.1007/BF02538262. [DOI] [PubMed] [Google Scholar]
- 32.Pace-Asciak CR. Pathophysiology of the hepoxilins. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbalip.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Kusek ME, Pazos M, Pirzai W, Hurley BP. In vitro coculture assay to assess pathogen induced neutrophil trans-epithelial migration. J Vis Exp. 2014:e50823. doi: 10.3791/50823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tager AM, Dufour JH, Goodarzi K, Bercury SD, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 36.Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA. 2003;100:1558–1563. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 38.Byers AM, Kemball CC, Moser JM, Lukacher AE. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol. 2003;171:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
- 39.Hurley BP, Pirzai W, Mumy KL, Gronert K, McCormick BA. Selective eicosanoid-generating capacity of cytoplasmic phospholipase A2 in Pseudomonas aeruginosa-infected epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L286–94. doi: 10.1152/ajplung.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamang DL, Pirzai W, Priebe GP, Traficante DC, Pier GB, Falck JR, Morisseau C, Hammock BD, McCormick BA, Gronert K, Hurley BP. Hepoxilin A(3) facilitates neutrophilic breach of lipoxygenase-expressing airway epithelial barriers. J Immunol. 2012;189:4960–4969. doi: 10.4049/jimmunol.1201922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 43.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arita M, Ohira T, Sun Y-P, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 45.Boxio R, Bossenmeyer-Pourié C, Steinckwich N, Dournon C, Nüsse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 46.Pace-Asciak CR, Reynaud D, Demin P, Nigam S. The hepoxilins. A review. Adv Exp Med Biol. 1999;447:123–132. [PubMed] [Google Scholar]
- 47.Cronin A, Decker M, Arand M. Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase. J Lipid Res. 2011;52:712–719. doi: 10.1194/jlr.M009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahinden CA, Zingg J, Maly FE, de Weck AL. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988;167:1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubala SA, Patil SU, Shreffler WG, Hurley BP. Pathogen induced chemo-attractant hepoxilin A3 drives neutrophils, but not eosinophils across epithelial barriers. Prostaglandins Other Lipid Mediat. 2014;108:1–8. doi: 10.1016/j.prostaglandins.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev Cell. 2012;22:1079–1091. doi: 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc Natl Acad Sci USA. 2012;109:E3177–85. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Döring G, Bragonzi A, Paroni M, Aktürk F-F, Cigana C, Schmidt A, Gilpin D, Heyder S, Born T, Smaczny C, Kohlhäufl M, Wagner TOF, Loebinger MR, Bilton D, Tunney MM, Elborn JS, Pier GB, Konstan MW, Ulrich M. BIIL 284 reduces neutrophil numbers but increases P. aeruginosa bacteremia and inflammation in mouse lungs. J Cyst Fibros. 2014;13:156–163. doi: 10.1016/j.jcf.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126:515–523. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]