Abstract
A simple, reliable and sensitive high-performance liquid chromatography tandem mass spectrometry method (HPLC-MS/MS) was established for simultaneous analyses of the following 5 steroid saponins in rat plasma after the single dose administration of total steroid saponins extracted from the rhizome of Dioscorea zingiberensis C.H.Wright for the first time. Protodioscin, huangjiangsu A, zingiberensis new saponin, dioscin, and gracillin were quantified using ginsenoside Rb1 as the internal standard (IS). The plasma samples were pretreated by a single step acetonitrile-mediated protein precipitation. The chromatographic separation was performed on an Inersil ODS-3 C18 column (250 mm × 4.6 mm, 5 μm) with the mobile phase composed of acetonitrile and water containing 0.1% formic acid under a gradient elution mode at 0.2 mL min−1 using a microsplit after the eluent from the HPLC apparatus. The quantification was accomplished on a triple quadrupole tandem mass spectrometer using the multiple reaction monitoring (MRM) in the positive ionization mode. The above five analytes were stable under sample storage and preparation conditions applied in the present study. The linearity, precision, accuracy, and recoveries of the analysis confirmed the requirements for quality-control purposes. After validation, this proposed method was successfully adopted to investigate the pharmacokinetic parameters of these five analytes.
Keywords: HPLC-MS/MS, total steroid saponins, Dioscorea zingiberensis C.H.Wright, MRM, pharmacokinetic parameters
1. Introduction
Dioscorea zingiberensis C.H.Wright (huangjiang in Chinese), is an important and widely used medicinal herb in Traditional Chinese Medicine (TCM) for a long time. It has been applied for the treatment of various diseases, such as cough, anthrax, rheumatoid arthritis, sprain as well as cardiac diseases [1,2]. Plentiful phytochemical investigations of this plant revealed that total steroid saponins (TSSN) extracted from the rhizomes of D. zingiberensis are the major potential bioactive constituents [3]. Extensive pharmacological studies indicated that TSSN and their monomers could reduce the risk of cardiovascular diseases through increasing coronary blood flow, improving peripheral circulation as well as depressing platelet aggregation [4–6]. They also have in vitro anti-cancer activities through inhibiting the proliferation of cells such as Hela cells, HL-60 cell lines and HepG2 cells [7–13]. Therefore, D. zingiberensis is a promising therapeutic agent.
Several analytical methods have been established for the determination of various steroid saponins in D. zingiberensis such as protodioscin, huangjiangsu A, zingiberensis new saponin, dioscin, and gracillin in the herb medicine using high-performance liquid chromatography coupled with evaporative light scattering detection (HPLC-ELSD), liquid chromatography mass spectrometry (LC-MS), and liquid chromatography tandem mass spectrometry (LC-MS/MS) [14–16]. However, no analytical methods have been reported for simultaneous determination of these five steroid saponins in the crude extract from the D. zingiberensis in biological samples. During the last decade, triple-quadrupole mass spectrometer coupled with multiple reaction monitoring (MRM) interfaced with HPLC has become a powerful analytical tool in quantifying saponins in biological fluids in drug development due to its high sensitivity and specificity [17,18].
In the present study, a rapid and sensitive HPLC-ESI-MS/MS method was established and validated for the simultaneous quantitation of five saponins (protodioscin, huangjiangsu A, zingiberensis new saponin, dioscin and gracillin) in rat plasma for the first time. The proposed method was applied to study these five active components pharmacokinetically in rats after the oral administration of TSSN. It was expected that the results of the work could provide some useful information about the action mechanism, further pre-clinical studies, and elucidating its pharmacokinetic behaviors of TSSN.
2. Materials and methods
2.1. Materials and reagents
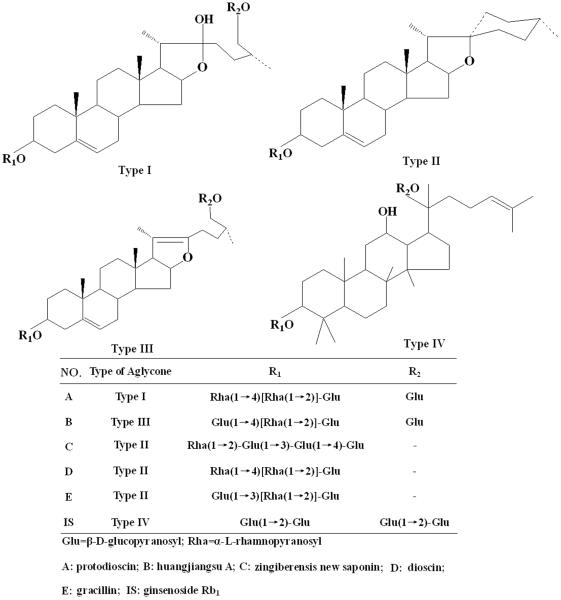
The dried rhizomes of D. zingiberensis were provided by the Yangtze River Pharmaceutical Industry Co., Ltd. (Jiangsu, China) and authenticated by Professor Wenji Sun (Northwest University, Xi'an, China). The voucher specimens (Voucher NO. HJ20100925-10) were available in the Biomedicine Key Laboratory of Shaanxi Province (Xi'an, China). The reference standards of protodioscin (A) [19], huangjiangsu A (B) [20], zingiberensis new saponin (C) [21], dioscin (D) [22], and gracillin (E) [23] were isolated and purified in our laboratory. Their structures were confirmed by MS and 13C NMR analysis, the corresponding spectroscopy data were presented in Table 1, and their purities were over 98% determined using the HPLC-ELSD. The internal standard (IS, 98% purity) ginsenoside Rb1 was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Chemical structures of these six compounds were presented in Fig. 1. Acetonitrile (ACN) was of HPLC grade and obtained from Tianjin Damao Co. Ltd. (Tianjin, China). Deionized water was purified using a Milli-Q water purification system (18.2Ω, Millipore, Bedford, MA, USA). Other reagents were all of analytical grade.
Table 1.
The MS/MS parameters for the detection of five steroid saponins and internal standard (IS) in rat plasma.
| Compounds | Ionization mode | Precursor→Product Q1 mass→Q3 mass (m/z) |
Lose ion fragments | Collision energy (eV) |
|---|---|---|---|---|
| Protodioscin | [M+H]+ | 1050.2→888.0 | Glu | 22 |
| Huangjiangsu A | [M+H]+ | 1048.1→886.0 | Glu | 26 |
| Zingiberensis newsaponin | [M+H]+ | 1048.2→739.9 | Rha-Glu | 24 |
| Dioscin | [M+H]+ | 870.0→577.7 | 2Rha | 18 |
| Gracillin | [M+H]+ | 886.0→723.9 | Glu | 20 |
| Ginsenoside Rb1 | [M+H]+ | 1110.3→948.2 | Glu | 28 |
Figure 1.
The chemical structures of five analytes and ginsenoside Rb1 (IS).
2.2. Animals
Healthy adult male Sprague-Dawley rats were supplied by the Experimental Animal Center of the Fourth Military Medical University (Xi'an, China). All the animals were kept at constant temperature (24 ± 1°C) and humidity (55–65%) under a normal 12 h:12 h light/dark cycle. Prior to the experiments, animals were allowed to free access to water and food for a week. All the procedures were approved by the Animal Ethics Committee of the Fourth Military Medical University (approved on February 2, 2014, NO. FMMU 2013-06) and in strict accordance with the recommendations of local Animal Ethical Committee (approved on January 1, 2012, NO. 3245/2012) for minimizing animal suffering.
2.3. Instruments and HPLC-MS/MS conditions
Chromatographic analyses were carried out on a Waters 2695 serious liquid chromatography system (Waters, Milford, MA, USA) composed of a quaternary pump, an on-line vacuum degasser, an autosampler, and a column temperature controller. The samples were separated on an Inersil ODS-3 column (250 × 4.6 mm, 5 μm), and eluted with acetonitrile (A) and 0.1% formic acid (v/v) water (B) under a linear gradient mode. The mobile phase program was as follows: 0–10 min, 30% A; 10–24 min, from 30% to 40% A; 24–25 min, from 40%–70% A; 25–35 min, from 70% to 80% A. The sample injection volume was 10 μL and the column temperature was kept constant at 25°C during all these procedures.
The mass spectrometric detection was performed on a Varian triple-quadrupole mass spectrometer (320-MS) equipped with an electrospray ionization (ESI) interface connected to the HPLC system. The positive mass scan MRM mode was applied in this study. A portion of the column effluent (0.2 mL min−1) was delivered into the ion source of mass spectrometer after a microsplit. The internal standard (IS) optimized mass parameters used in this study were as follows: The voltages of capillary and cone were −4.5 Kv, the source temperature was 350°C, and the desolvation temperature was 400°C. Nitrogen was employed as the desolvation at a flow rate of 8 L min−1. The argon was used as the collision gas with an approximate pressure 3.05 × 10−3 mbar. The control of equipment, data acquisition and analysis were performed on the Varian MS workstation software.
2.4. Extract sample preparation
Dried raw material of D. zingiberensis (2.9 Kg) was powdered and extracted with 37 liter 70% ethanol for three times. The ethanol solution was combined and evaporated to dryness under reduced pressure with a rotary evaporator, and the residue was redissolved in water, and subjected to centrifugation. The supernatant was separated on a D-101 macroporous resin column, by eluting with deionized water followed by 60% ethanol. The ethanol effluent was dried under reduced pressure to obtain 125 g of total steroid saponins (TSSN) for the subsequent experimental use.
2.5. Rat plasma sample preparation
All plasma samples were thawed at room temperature before analysis. A 200 μL aliquot of each rat sample and 20 μL (2.03 μg mL−1) of IS were delivered to a 1.5 mL heparinized eppendorf tube. In order to precipitate protein, 600 μL acetonitrile was added. After vortexing vigorously for 1 min, the sample was centrifuged at 5000 rpm for 15 min. Then, the supernatant was transferred to a clean heparinized eppendorf tube, and blown to dryness under a gentle stream of nitrogen at 40°C. The residue was dissolved in 100 μL methanol solution, and centrifuged at 5000 rpm for 15 min. After that, the supernatant was separated and transferred to an autosampler vial. An aliquot of 10 μL was injected into the HPLC-ESI-MS/MS system for analysis.
2.6. Preparation of standard solution and quality control samples
The accurate amounts of five standard steroids and IS were separately weighed and dissolved in a 5 mL volumetric flask with methanol to prepare the final standard stock solutions at concentration of 0.41 mg mL−1 for A, 0.42 mg mL−1 for B, 0.41 mg mL−1 for C, 0.40 mg mL−1 for D, 0.39 mg mL−1 for E and 0.40 mg mL−1 for IS, respectively. All these solutions were kept at 4°C, and returned at room temperature before use. Subsequently, the mixed solutions were prepared by diluting the five standard stock solutions with methanol to obtain the ideal concentrations. In order to achieve a series of concentration calibration standards, the desired volumes of mixed solutions were spiked into the blank control plasma and diluted with methanol. This process was dealt with as described in the plasma sample preparation. The IS standard solution was kept at the concentration of 40 ng mL−1.
For the validation of the method, the quality control (QC) sample were independently prepared at three different concentrations of 800, 400, and 100 ng mL−1 (namely high, medium, and low levels, respectively) in the same procedure as the calibration standards treatment.
2.7. Method validation
The established method validation was evaluated according to the following guidelines published by the US Food and Drug Administration (FDA) for the Bioanalytical Method Validation.
2.7.1. Specificity and Selectivity
In order to investigate the potential interference from endogenous compounds co-eluting with five steroid saponins and IS, the blank rat plasma samples, plasma samples spiked with analytes and IS, and plasma samples after an oral dosage at 10 h were examined from 6 different batch sources.
2.7.2. Linearity of calibration curve, limit of detection (LOD) and limit of quantitation (LLOQ)
The calibration curves of steroid saponins A, B, C, D, and E were assayed each at appropriate eight concentrations. The linearity of each standard calibration curve was constructed by plotting the peak areas ratios (y) of analyte to the IS versus the corresponding normal concentrations (x) using the weighted least-squares linear regression (1/x2 as a weighting factor) in duplicate analysis on three consecutive days. The LOD (signal-to-noise <3) was defined as the amount that could be detected, while the LLOQ (signal-to-noise >10) as the lowest concentration point of calibration curve at which the accuracy (relative error, RE) within 20% and the precision below 20% can be considered acceptable. They were evaluated by analyzing samples in six replicates.
2.7.3. Precision and accuracy
The intra-day precision and accuracy for the established method were determined by analyzing the six replicates of three batch QC samples on a single day, and the six replicates of QC samples were tested once a day for 3 consecutive days to assay the inter-day precision and accuracy, respectively. The relative standard deviation (RSD) was used to express the precision, while the relative error (RE) for the accuracy which was calculated by comparing the measured concentration with its true value. The variation of the precision and accuracy within 15% was accepted.
2.7.4. Stability
The stability of five analytes in rat plasma during the sample storing and processing procedures was evaluated by the QC samples in six replicates. The freeze-thaw stability test was performed after 3 cycles within 3 days (The preparative plasma samples were frozen at −80°C for 24 h and thawed at the room temperature in each cycle). The post-preparative stability was assayed the extracted QC samples left in the auto vial (4°C) for 24 h. The short-term stability was assessed after the stored QC samples exposed to ambient temperature for 4 h. The long-term stability was studied by testing the QC samples after a period of 4 weeks storage at −80°C.
2.7.5. Extraction recovery and matrix effect
The extraction recovery and matrix effect were determined by the three different QC sample levels in six replicates. The extraction recovery was calculated by comparing the peak area ratios of analytes/IS spiked into the pre-extracted rat plasma samples to that of post-extracted ones according to the reported method, while the matrix effect by comparing the ratios of analytes/IS spiked into the post-extracted rat plasma samples with that of corresponding equivalent concentrations of the pure standard solution reconstituted in methanol.
2.7.6. Drug administration and applications in pharmacokinetics studies
The validated method was successfully applied to the pharmacokinetic study of rat plasma. Before the experiment, the rats had free access to water but without food for 12 h. Six Sprague-Dawley rats were orally administrated with a single dose of 15 g kg−1 (equivalent to 90.37 mg A, 70.33 mg B, 87.1 mg C, 121.8 mg D, and 59.26 mg E) TSSN dissolved in deionized water. Blood sample (400 μL) were immediately collected from each rat under ether anesthesia into the heparinized eppendorf tube by puncturing the retro orbital sinus at the intervals of 0 h (prior to dosing), 1 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, and 24 h after dosing. The following major pharmacokinetic parameters were calculated by the computer program kinetica (4.4.1): the peak plasma concentration (Cmax); the time to peak concentration (Tmax); the elimination half-life (t1/2); the terminal elimination rate constant (ke); mean residence time (MRT); clearance (CL); the area under the plasma concentration-time curve from time 0 to the time of the last measurable concentration (AUC0-t); and from time 0 to infinity (AUC0-∞). The results were expressed as mean ± standard deviation (SD). All the data were analyzed and evaluated with SPASS (19.0) software.
3. Results and discussion
3.1. Selection of internal standard
The choice of an ideal IS is important for the analysis of biological samples. Several compounds including ginsenoside Re, ginsenoside Rb1, swertiamarin and gentiopicroside were investigated in our experiment. Considering the satisfactory resolution with endogenous interferences, better extraction efficiency, and a similar chromatographic retention time, ginsenoside Rb1 was finally chosen as the IS.
3.2. Optimization of rat plasma sample preparation
Plasma, a complex biological matrix, could prevent the drugs and their metabolites from detection. Therefore, prior to injection into HPLC column, removal of proteins and potential endogenous interferences is necessary. To avoid loss of target compounds in this clean-up process, direct protein precipitation with organic reagent is considered as a fast and simple way through disrupting protein-drug binding with minimum steps. For receiving a superior recovery of analytes and limiting the matrix effects from endogenous interferences, three kinds of organic solvents of ethyl acetate, methanol, and acetonitrile were investigated. After several preliminary experiments, the acetonitrile was selected as an efficient solvent to prepare the plasma due to its high extraction recovery and intense signal for these five steroid saponins and IS. Subsequently, different volumes (200, 400, and 600 μL) were also examined. The results revealed that suitable extract efficiency can be obtained by adding 600 μL aliquot of acetonitrile to 200 μL plasma sample.
3.3. Optimization of HPLC and MS parameters
In order to achieve high sensitivity, good resolution with adjacent peaks in appropriate retention time for simultaneous analysis of five components and IS, several different mobile phases composed of methanol-water and acetonitrile-water in various proportions were tested and compared. The results indicated that acetonitrile-water system produced better response than the methanol-water. An addition of a small proportion of formic acid to the mobile phrase as a modifier further improved the peak shape, decreased tailing of the chromatographic peak, and also increased the ionization efficiency of the analyte. Because of a wide range in polarity for the determined compounds, the gradient elution mode was applied as shown in section 2.3. Therefore, acetonitrile (A) and water containing 0.1% formic acid (B) solvent was adopted as the optimal solvent system in the current study.
ESI in both positive and negative ion modes were carried out in the study to obtain intense signal and lower background noise. For steroid saponins and IS, higher response and more information were produced in the positive mode than in the negative mode. Thus, the ion source was selected in the positive mode during the whole analytical procedure. Some other MS parameters such as source temperature and the collision energy of collision-induced decomposition (CID) were also optimized to achieve sensitivity and abundant ion fragments. The mass parameters and major sensitive transitions selected for quantification of five steroid saponins and IS in MRM were listed in Table 2 and Fig. 2.
Table 2.
The calibration curves, LOD, and LLOQ of this method for five steroid saponins in rat plasma.
| Compounds | Regression equation | Correlation coefficient (R2) | Linear range (ng mL−1) | LLOQ (ng mL−1) | LOD (ng mL−1) |
|---|---|---|---|---|---|
| Protodioscin | y=0.2776x+0.4156 | 0.9918 | 0.246~800 | 0.246 | 0.08 |
| Huangjiangsu A | y=0.2698x+0.4368 | 0.9975 | 0.504~800 | 0.504 | 0.157 |
| Zingiberensis newsaponin | y=0.7300x+0.3233 | 0.9984 | 0.123~800 | 0.123 | 0.05 |
| Dioscin | y=0.2621x+0.5764 | 0.9964 | 0.08~800 | 0.08 | 0.02 |
| Gracillin | y=0.5114x+0.5883 | 0.9989 | 0.078~800 | 0.078 | 0.019 |
Figure 2.
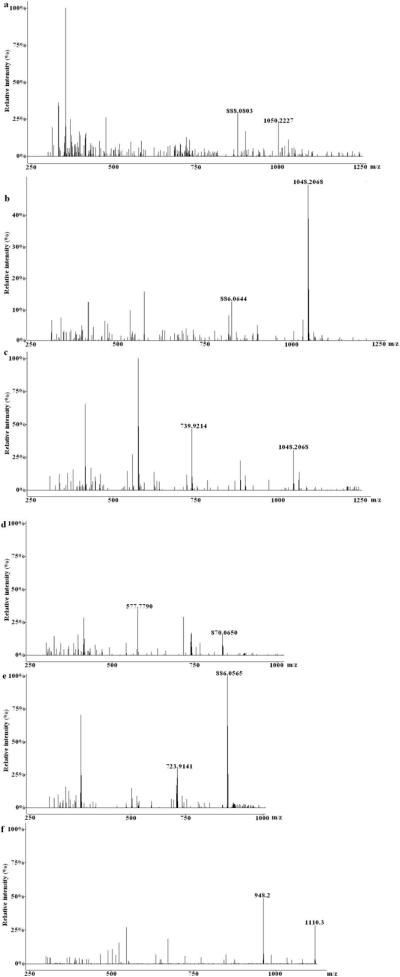
The full scan product ion spectrums of five analytes and ginsenoside Rb1 (IS).
(a): Protodioscin; (b): Huangjiangsu A; (c): Zingiberensis newsaponin; (d): Dioscin; (e): Gracillin; (f): Ginsenoside Rb1
3.4. Method validation
3.4.1. Selectivity
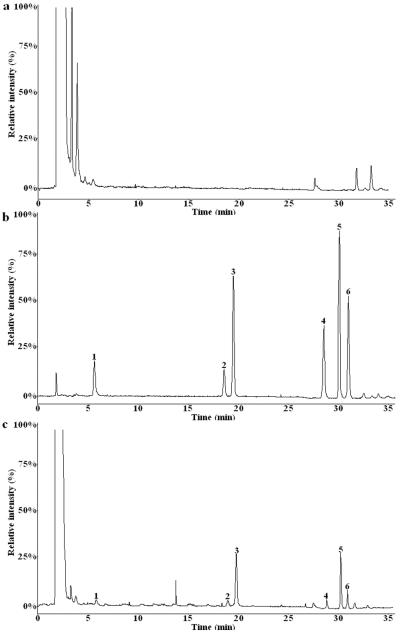
The representative MRM chromatograms of the blank plasma, blank plasma spiked with standards A, B, C, D, E, and IS, and plasma sample at 10 h after oral administration of the TSSN extract were presented in Fig. 3. As shown in the chromatograms, the analytes were well separated with excellent resolution and good peak shapes. There were no obvious interferences from the endogenous substances or metabolites at the respective retention position of analytes.
Figure 3.
The typical HPLC-MS and MRM chromatograms of five analytes and ginsenoside Rb1 (IS) in rat plasma. 1: Protodioscin; 2: Huangjiangsu A; 3: Ginsenoside Rb1; 4: Zingiberensis newsaponin; 5: Dioscin; 6: Gracillin
(a): Blank control plasma; (b): Blank control plasma spiked with standard samples and IS; (c): Plasma sample obtained after single oral dose administration of total steroid saponins extract (15 g Kg−1) at 12 h.
3.4.2. Linearity, LOD, and LLOQ
The typical equations of calibration curves and linearity ranges for the five analytes are listed in Table 3. The results revealed that it had a good correlation between the ratio of peak area and concentration within the linearity ranges, and it was enough for the pharmacokinetic study following the oral administration of TSSN extract. The LODs (S/N=3) for the standards A, B, C, D, and E were 0.08, 0.157, 0.05, 0.02, and 0.019 ng mL−1, while the LLOQs were 0.246, 0.504, 0.123, 0.08, and 0.078 ng mL−1, respectively.
Table 3.
Intra-day and inter-day precision and accuracy results of five steroid saponins in rat plasma at three different QC levels ( ± SD).
| Compounds | Spiked concentration (ng mL−1) | Intra-day (n=6) | Inter-day (n= 18) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Measured concentration (ng mL−1) | Precision (RSD, %) | Accuracy (RE, %) | Measured concentration (ng mL−1) | Precision (RSD, %) | Accuracy (RE, %) | ||
| Protodioscin | 100 | 95.22 ± 0.52 | 0.55 | −4.78 | 98.32 ± 1.21 | 1.23 | −1.68 |
| 400 | 390.13 ± 3.63 | 0.93 | −2.47 | 351.22 ± 4.35 | 1.24 | −12.19 | |
| 800 | 790.44 ± 4.91 | 0.62 | −1.20 | 755.46 ± 7.19 | 0.95 | −5.57 | |
| Huangjiangsu A | 100 | 89.87 ± 5.23 | 5.82 | −10.13 | 98.57 ± 3.59 | 3.64 | −1.43 |
| 400 | 378.42 ± 4.84 | 1.28 | −5.40 | 394.13 ± 4.83 | 1.23 | −1.47 | |
| 800 | 789.66 ± 3.27 | 0.41 | −1.30 | 787.06 ± 4.31 | 0.55 | −1.62 | |
| Zingiberensis newsaponin | 100 | 97.56 ± 3.74 | 3.83 | −2.44 | 96.57 ± 4.03 | 4.17 | −3.43 |
| 400 | 392.75 ± 4.23 | 1.08 | −1.81 | 393.64 ± 4.54 | 1.15 | −1.59 | |
| 800 | 754.24 ± 4.91 | 0.65 | −5.72 | 810.68 ± 3.97 | 0.49 | 1.34 | |
| Dioscin | 100 | 103.12 ± 1.35 | 1.31 | 3.12 | 101.25 ± 5.57 | 5.50 | 1.25 |
| 400 | 388.69 ± 2.95 | 0.76 | −2.83 | 410.64 ± 5.66 | 1.38 | 2.66 | |
| 800 | 781.83 ± 3.32 | 0.42 | −2.27 | 765.41 ± 3.72 | 0.49 | −4.32 | |
| Gracillin | 100 | 93.98 ± 4.21 | 4.48 | −6.02 | 109.53 ± 3.62 | 3.31 | 9.53 |
| 400 | 382.72 ± 2.46 | 0.64 | −4.32 | 387.06 ± 4.86 | 1.26 | −3.24 | |
| 800 | 837.60 ± 3.52 | 0.42 | 4.70 | 832.25 ± 3.71 | 0.45 | 4.03 | |
3.4.3. Precision and accuracy
The data for evaluating the intra- and inter-day precision and accuracy of the five steroid saponins are listed in Table 4. The intra- and inter-day precision (RSD) were at the range of 0.42~5.82% and 0.45~5.5%, while the accuracy (RE) at the range of 1.2~10.13 and 1.25~12.19 at their absolute values, respectively. The results indicated that the established method yielded satisfactory reproducibility.
Table 4.
The stability of five steroid saponins in rat plasma at three different QC levels (n=6, ± s).
| Compounds | Spiked concentration (ng mL−1) | Three freeze-thaw cycles stability | Short-term Stability (room temperature) | Long-term Stability (−80 °C for 4 weeks) | Auto vial (post-preparative) | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Measured concentration (ng mL−1) | Accuracy (RE, %) | Measured concentration (ng mL−1) | Accuracy (RE, %) | Measured concentration (ng mL−1) | Accuracy (RE, %) | Measured concentration (ng mL−1) | Accuracy (RE, %) | ||
| Protodioscin | 100 | 97.84 ± 1.31 | −2.16 | 100.67 ± 1.21 | 0.67 | 103.09 ± 1.71 | 3.09 | 97.04 ± 1.51 | −2.96 |
| 400 | 395.72 ± 2.91 | −1.07 | 394.33 ± 2.87 | −1.42 | 396.9 ± 2.32 | −0.77 | 392.81 ± 1.1 | −1.8 | |
| 800 | 794.15 ± 3.51 | −0.73 | 794.76 ± 21.18 | −0.65 | 814.5 ± 2.17 | 1.81 | 802.43 ± 1.62 | 0.3 | |
| Huangjiangsu A | 100 | 101.72 ± 1.02 | 1.72 | 97.87 ± 1.61 | −2.13 | 97.61 ± 1.62 | −2.39 | 100.52 ± 1.58 | 0.52 |
| 400 | 396.76 ± 3.21 | −0.81 | 395.19 ± 2.86 | −1.2 | 407.12 ± 1.14 | 1.78 | 388.85 ± 0.54 | −2.79 | |
| 800 | 808.81 ± 4.52 | 1.1 | 791.25 ± 10.61 | −1.1 | 793.21 ± 3.29 | −0.85 | 808.84 ± 4.37 | 1.11 | |
| Zingiberensis newsaponins | 100 | 98.21 ± 2.81 | −1.79 | 97.57 ± 1.11 | −2.43 | 94.51 ± 2.81 | −5.49 | 97.6 ± 1.12 | −2.4 |
| 400 | 405.24 ± 3.54 | 1.31 | 394.42 ± 2.46 | −1.4 | 394.47 ± 2.27 | −1.38 | 395.07 ± 1.94 | −1.23 | |
| 800 | 785.61 ± 5.27 | −1.8 | 793.01 ± 7.56 | −0.87 | 803.27 ± 3.76 | 0.41 | 798.12 ± 3.65 | −0.23 | |
| Dioscin | 100 | 98.06 ± 1.93 | −1.94 | 98.79 ± 1.8 | −1.21 | 100.21 ± 1.11 | 0.21 | 96.04 ± 1.31 | −3.96 |
| 400 | 409.87 ± 3.61 | 2.47 | 403.51 ± 1.16 | 0.88 | 396.81 ± 1.04 | −0.8 | 385.27 ± 2.01 | −3.68 | |
| 800 | 793.34 ± 5.08 | −0.83 | 789.22 ± 5.39 | −1.35 | 794.52 ± 6.05 | −0.69 | 794.42 ± 4.54 | −0.7 | |
| Gracillin | 100 | 102.04 ± 1.52 | 2.04 | 101.41 ± 1.67 | 1.41 | 99.20 ± 2.98 | −0.8 | 101.68 ± 0.19 | 1.68 |
| 400 | 398.61 ± 4.21 | −0.34 | 396.12 ± 6.33 | −0.97 | 397.62 ± 1.52 | −0.6 | 406.21 ± 1.24 | 1.55 | |
| 800 | 789.14 ± 3.6 | −1.36 | 812.75 ± 8.12 | 1.59 | 796.15 ± 7.18 | −0.48 | 789.22 ± 8.34 | −1.35 | |
3.4.4. Stability
The data for assaying the stability of the five steroid saponins are presented in Table 5. The accuracy of freeze-thaw, post-preparative, short-term, and long-term stability were less 20% which was in an acceptable range. The results displayed that the five reference standards dissolved in rat plasma were stable under the applied conditions throughout the entire analysis.
Table 5.
The mean extraction recoveries and matrix effects of five steroid saponins in rat plasma at three different QC levels (n=6).
| Conpounds | Spiked Concentration (ng mL−1) | Mean extraction recovery | Mean matrix effects | ||
|---|---|---|---|---|---|
|
| |||||
| ± SD | RSD (%) | ± SD | RSD (%) | ||
| Protodioscin | 100 | 66.41 ± 3.16 | 4.76 | 88.83 ± 4.27 | 4.81 |
| 400 | 55.23 ± 3.27 | 5.92 | 89.15 ± 4.61 | 5.17 | |
| 800 | 70.12 ± 4.33 | 6.18 | 92.43 ± 3.34 | 3.61 | |
| Huangjiangsu A | 100 | 69.95 ± 3.82 | 5.46 | 89.55 ± 4.86 | 5.43 |
| 400 | 61.53 ± 4.78 | 7.77 | 89.81 ± 4.03 | 4.49 | |
| 800 | 69.87 ± 2.95 | 4.22 | 85.33 ± 1.65 | 1.93 | |
| Zingiberensis newsaponins | 100 | 68.09 ± 3.12 | 4.58 | 89.81 ± 2.23 | 2.48 |
| 400 | 68.47 ± 8.67 | 12.66 | 86.45 ± 3.77 | 4.36 | |
| 800 | 70.26 ± 3.12 | 4.44 | 86.56 ± 5.73 | 6.62 | |
| Dioscin | 100 | 53.91 ± 4.04 | 7.49 | 85.45 ± 3.25 | 3.8 |
| 400 | 68.23 ± 3.27 | 4.79 | 84.09 ± 3.87 | 4.6 | |
| 800 | 62.47 ± 4.51 | 7.22 | 83.42 ± 2.24 | 2.69 | |
| Gracillin | 100 | 65.31 ± 2.36 | 3.61 | 86.28 ± 4.67 | 5.41 |
| 400 | 60.74 ± 2.35 | 3.87 | 88.73 ± 3.78 | 4.26 | |
| 800 | 68.91 ± 3.13 | 4.54 | 82.31 ± 2.45 | 2.98 | |
3.4.5. Extraction recovery and matrix effect
The results of the analysis on the extraction recovery and matrix effect are summarized in Table 6. The average extraction recoveries of the investigated steroid saponins were found to range from 53.91 to 70.26% with RSD below 12.66%, which revealed that the extraction procedure was reproducible, consistent, and acceptable. The mean matrix effect was in the range of 82.31~92.43% with promising RSD below 6.62%, indicating that the matrix effect from endogenous substances can be negligible.
Table 6.
Pharmacokinetic parameters of five steroid saponins in rats after single oral administration of total extract from Dioscorea zingiberensis C.H.Wright (n=6, mean ± SD).
| Parameters | Protodioscin | Huangjiangsu A | Zingiberensis newsaponins | Dioscin | Gracillin |
|---|---|---|---|---|---|
| Cmax (ng mL−1) | 29.18 ± 1.83 | 14.87 ± 1.46 | 14.87 ± 1.46 | 38.24 ± 0.63 | 130.86 ± 1.90 |
| Tmax (h) | 11.98 ± 2.45 | 11.43 ± 3.24 | 10.32 ± 2.17 | 10.29 ± 1.19 | 12.79 ± 2.49 |
| t1/2 (h) | 15.86 ± 2.13 | 11.04 ± 2.03 | 17.04 ± 2.03 | 12.79 ± 1.66 | 37.29 ± 1.82 |
| ke (h−1) | 0.044 ± 1.01 | 0.063 ± 1.98 | 0.041 ± 3.32 | 0.054 ± 2.84 | 0.019 ± 3.72 |
| MRT (h) | 18.15 ± 1.83 | 21.06 ± 2.30 | 48.61 ± 1.73 | 25.87 ± 0.74 | 57.99 ± 1.15 |
| CL (L h−1) | 0.21 ± 0.02 | 0.23 ± 0.02 | 0.06 ± 0.004 | 0.11 ± 0.01 | 0.01 ± 0.003 |
| AUC0-t (h ng mL−1) | 6473.11 ± 821.05 | 291.31 ± 21.14 | 291.31 ± 21.14 | 19990.91 ± 727.16 | 106902.70 ± 2118.13 |
| AUC0-∞ (h ng mL−1) | 9098.37 ± 1656.58 | 3 6747.44 ± 1504.58 | 6747.44 ± 1504.58 | 28379.48 ± 2066.52 | 469583.7 ± 24463.47 |
3.4.6. Pharmacokinetic analysis
The validated method was successfully adopted to simultaneously investigate the plasma concentrations of five steroid saponins in rats after oral administration of the total steroid saponins extracted from the rhizomes of Dioscorea zingiberensis C.H.Wright. The mean plasma concentration-time curves of five analytes are presented in Fig. 4, and their main pharmacokinetic parameters were calculated using the non-compartment mode by kinetica software as summarized in Table 7. As listed in this table, these five analytes had a long Tmax from 11.98 ± 2.45 h to 12.79 ± 2.49 h after a single dose of TSSN administration, which implied that steroid saponins has low adsorption in rat plasma. Other pharmacokinetic parameters such as t1/2 (more than 11.04 ± 2.03 h), ke (less than 0.063 ± 1.98 h), MRT (a long time and even reached 57.99 ± 1.15 h), and CL (less than 0.23 ± 0.02 h) were correlated closely with the low elimination and excretion rates. This absolutely low oral bioavailability of steroid saponins is probably due to their poor low permeability to cross through the intestinal epithelial membrane to enter the blood stream. In addition, the steroid saponin molecules consist of two parts, i.e. hydrophobic aglycone and sugar moieties, hence they exhibit low solubility in aqueous solutions. Also their molecular mass and high hydrogen-bonding capacity may be another reason for this phenomenon. The results of present study showed slightly different pharmacokinetic trends from the previous reports [7,24, 25]. The difference between the single dose and extract administration might induce drug-drug interactions by the coexisting ingredients in crude extract, hence generates individual pharmacokinetic characteristics. Double-peak absorption of steroid saponins A and B in the contention-curve were observed after the single dose administration of TSSN, while the other three compounds showed single peak absorption. This might be due to their different molecular structures in F ring [26]. A and B are furostanol saponin type because of F ring cleavage, whereas other three are spirostanol saponin forms with closed F ring. The spirostanol saponins are more stable than furostanol saponins. In the body, these saponins could display different metabolic rates leading to various absorption curves. Of course, the precise relationship between these two types of compounds with the different metabolic character should be further investigated.
Figure 4.
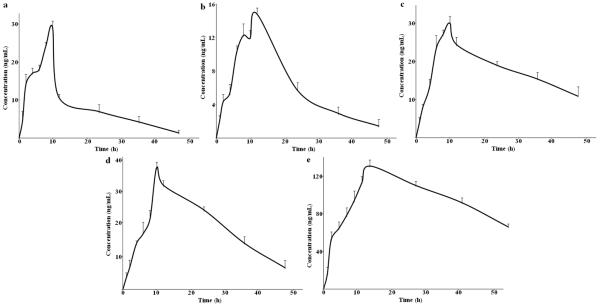
The mean plasma concentration-time curves of five analytes in six rats after single oral administration of total steroid saponins extract (15 g Kg−1). The values were expressed as mean ± standard errors for six rats.
(a): Protodioscin; (b): Huangjiangsu A; (c): Zingiberensis newsaponin; (d): Dioscin; (e): Gracillin
4. Conclusions
A simple, reproducible, and reliable HPLC-MS/MS method was successfully developed for the simultaneous quantification of steroid saponins A, B, C, D and E in the rat plasma for the first time. The method was applied to a pharmacokinetic study of these five steroid saponins after oral administration of the TSSN extract from the rhizome of D. zingiberensis to rats. What's more, this validated method demonstrated acceptable linearity, precision, accuracy, and adequate stability. These pharmacokinetic parameters could offer useful information for further investigation in clinical application of D. zingiberensis.
Acknowledge
The authors thank Prof. Zhongfu Wang of the Key Laboratory of Resource Biology and Biotechnology in western China and Juan Gao for assistance in ESI-MS experiments.
Reference
- [1].Li H, Huang W, Wen YQ, Gong GH, Zhao QB, Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H. Wright. Fitoterapia. 2010;81:1147–56. doi: 10.1016/j.fitote.2010.07.016. [DOI] [PubMed] [Google Scholar]
- [2].Qin Y, Wu XH, Huang W, Gong GH, Li D, He Y, et al. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis C.H.Wright in rodents. J Ethnopharmacol. 2009;126:543–50. doi: 10.1016/j.jep.2009.08.047. [DOI] [PubMed] [Google Scholar]
- [3].Zhang YQ, Tang LR, An X, Fu EH, Ma CF. Modification of cellulase and its application to extraction of diosgenin from Dioscorea zingiberensis C.H. Wright. Biochem Eng J. 2009;47:80–6. [Google Scholar]
- [4].Zhu YL, Huang W, Ni JR. A promising clean process for production of diosgenin from Dioscorea zingiberensis C.H. Wright. J Clean Prod. 2010;18:242–7. [Google Scholar]
- [5].Dong YS, Teng H, Qi SS, Liu L, Wang H, Zhao YK, et al. Pathways and kinetics analysis of biotransformation of Dioscorea zingiberensis by Aspergillus oryzae. Biochem Eng J. 2010;52:123–30. [Google Scholar]
- [6].Wei YL, Xu YS, Han X, Qi Y, Xu LN, Xu YW, et al. Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem Toxicol. 2013;59:118–28. doi: 10.1016/j.fct.2013.05.054. [DOI] [PubMed] [Google Scholar]
- [7].Wang BW, Ji SG, Zhang H, Zhao L, Lv L, Li YY, et al. Liquid chromatography tandem mass spectrometry in study of the pharmacokinetics of six steroidal saponins in rats. Steroids. 2013;78:1164–70. doi: 10.1016/j.steroids.2013.08.009. [DOI] [PubMed] [Google Scholar]
- [8].Man SL, Gao WY, Zhang YJ, Yan LL, Ma CY, Liu CX, et al. Antitumor and antimetastatic activities of Rhizoma paridis saponins. Steroids. 2009;74:1051–6. doi: 10.1016/j.steroids.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [9].Zhao Y, Kang LP, Liu YX, Liang YG, Tan DW. Steroidal saponins from the rhizome of Paris polyphylla and their cytotoxic activities. Planta Med. 2009;75:356–63. doi: 10.1055/s-0028-1088380. [DOI] [PubMed] [Google Scholar]
- [10].Guo L, Su J, Deng BW, Yu ZY, Kang LP, Zhao ZH. Active pharmaceutical ingredients and mechanisms underlying phasic myometrial contractions stimulated with the saponin extract from Paris polyphylla Sm. Var. yunnanensis used for abnormal uterine bleeding. Hum Reprod. 2008;23:964–71. doi: 10.1093/humrep/den001. [DOI] [PubMed] [Google Scholar]
- [11].Sun J, Liu BR, Hu WJ, Yu LX, Qian XP. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicine on human digestive tumor cell lines. Phytother Res. 2007;21:1102–4. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- [12].Man SL, Gao WY, Zhang YJ, Ma CY, Yang L. Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharm Res. 2011;34:43–50. doi: 10.1007/s12272-011-0105-4. [DOI] [PubMed] [Google Scholar]
- [13].Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao LB, Liu JS. Parissaponin II of Rhizoma paridis-a novel inducer of apoptosis in human ovarian cancer cells. Biosci Trends. 2012;6(4):201–11. doi: 10.5582/bst.2012.v6.4.201. [DOI] [PubMed] [Google Scholar]
- [14].Wang YH, Kang AL, Fan BJ, Sun WJ. HPLC-ELSD simultaneous determination of three components in root of Dioscorea zingiberensis C.H. Wright. Chin J Pharm Anal. 2009;29:739–42. [Google Scholar]
- [15].Zhang XX, Liang JR, Su Q, Xie RM, Sun WJ. Simultaneous content determination of five saponins in the rhizome of Dioscorea zingiberensis C.H. Wright by HPLC-ELSD. Chin J Pharm Anal. 2013;33:1235–58. [Google Scholar]
- [16].Zhu JB, Guo XJ, Fu SP, Zhang XL, Liang XM. Characterization of steroidal saponins in crude extracts from Dioscorea zingiberensis C.H. Wright by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Pharmaceut Biomed. 2010;53:462–74. doi: 10.1016/j.jpba.2010.05.019. [DOI] [PubMed] [Google Scholar]
- [17].Song M, Lee D, Lee T, Lee S. Determination of leelamine in mouse plasma by LC-MS/MS and its pharmacokinetics. J Chromatogr B. 2013;931:170–3. doi: 10.1016/j.jchromb.2013.05.018. [DOI] [PubMed] [Google Scholar]
- [18].Tao Y, Xu HY, Wang SS, Wang B, Zhang YC, Xiao XF, et al. Identification of the absorbed constituents after oral administration of Yuanhu Zhitong prescription extract and its pharmacokinetic study by rapid resolution liquid chromatography/quadrupole time-of-flight. J Chromatogr B. 2013;935:1–9. doi: 10.1016/j.jchromb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- [19].Dong M, Feng X, Wang B, Wu L, Ikejima T. Two novel furostanol saponins from the rhizomes of Dioscorea panthaica Prain et Burkill and their cytotoxic activity. Tetrahedron. 2001;64:17–22. [Google Scholar]
- [20].Sun WJ, Gao J, Tu GZ, Guo ZW, Zhang YM. A new steroidal saponin from Dioscorea zingiberensis C.H.Wright. Nat Prod Res. 2003;16:243–7. doi: 10.1080/1478641031000136997. [DOI] [PubMed] [Google Scholar]
- [21].Kang AL. Development and study on new drug of Huang Jiang Su. Northwest University; Xi'an: 2003. pp. 18–22. [Google Scholar]
- [22].Zheng QA, Zhang YJ, Li HZ, Yang CR. Steroidal saponins from fresh stem of Dracaena cochinchinensis. Steroids. 2004;69:111–9. doi: 10.1016/j.steroids.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [23].Tang SR, Wu YF, Pang ZJ. Identification and isolation of steroidal saponins of Dioscorea zingiberensis C.H.Wright. Acta Botanica Sinica. 1983;25:556–62. [Google Scholar]
- [24].Li K, Wang YW, Gu JK, Chen XY, Zhong DF. Determination of dioscin in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2005;817:271–5. doi: 10.1016/j.jchromb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- [25].Li K, Tang YB, Fawcett JP, Gu JK, Zhong DF. Characterization of the pharmacokinetics of dioscin in rat. Steroids. 2005;70:525–30. doi: 10.1016/j.steroids.2004.11.014. [DOI] [PubMed] [Google Scholar]
- [26].Viñas-Bravo O, Martinez-Pascual R, Vega-Baez JL, Gómez-Calvario V, Sandoval-Ramírez J, Montiel-Smith S, et al. Rapid conversion of spirostans into furostan skeletons at room temperature. Steroids. 2012;77:59–66. doi: 10.1016/j.steroids.2011.10.004. [DOI] [PubMed] [Google Scholar]