Abstract
Background
Sixty percent of cancer survivors are 65 years of age or older. Cancer and its treatments lead to cancer-related fatigue and many other side effects, in turn, creating substantial global side-effect burden (total burden from all side effects) which, ultimately, compromises functional independence and quality of life. Various modes of exercise, such as yoga, reduce cancer-related fatigue and global side-effect burden in younger cancer survivors, but no studies have specifically examined the effects of yoga on older cancer survivors.
Objectives
The purpose of this study was to assess the effects of a 4-week yoga intervention (Yoga for Cancer Survivors: YOCAS©®) on overall cancer-related fatigue, and due to its multidimensional nature, the subdomains of cancer-related fatigue (general, physical, emotional, and mental) and global side-effect burden in older cancer survivors.
Materials and Methods
We conducted a secondary analysis on data from a multicenter phase III randomized controlled clinical trial with 2 arms (standard care and standard care plus a 4-week YOCAS©® intervention). The sample for this secondary analysis was 97 older cancer survivors (≥ 60 years of age), between 2 months and 2 years post-treatment, who participated in the original trial.
Results
Participants in the YOCAS©® intervention arm reported significantly lower cancer-related fatigue, physical fatigue, mental fatigue, and global side-effect burden than participants in the standard care arm following the 4-week intervention period (p<0.05).
Conclusions
YOCAS©® is an effective standardized yoga intervention for reducing cancer-related fatigue, physical fatigue, mental fatigue, and global side-effect burden among older cancer survivors.
Keywords: cancer-related fatigue, side-effects, cancer, yoga, exercise
INTRODUCTION
Cancer is largely a disease affecting older adults [1]. Seventy-two percent of cancer survivors are 60 years of age and older [1]. Cancer treatments lead to a number of side effects in older adults including cancer-related fatigue and global side-effect burden (an aggregate indicator of the summative impact of all side effects stemming from cancer and its treatments including both total number and severity). High levels of cancer-related fatigue and global side effect burden lead to functional decline [2–4]. Although side effects can be detrimental to the physical and psychological functioning of all cancer survivors, older cancer survivors, due to additional age-related declines, may have more difficulty recovering from treatment-related side effects [3]. In addition, aging in the absence of a cancer history is associated with a decline in physical and psychological function, including sarcopenia, reduced strength,5 reduced bone mineral density [6], lower functional capacity [7–8], arthralgias [9], depressive symptoms [10], anxiety [11], and cognitive difficulties [12]. Cancer and its treatments can exacerbate these common decrements in function and lead to additional impairments [3, 13–14]. Cancer survivors also report engaging in less physical activity and lower levels of physical activity associated with reduced functional ability than those without a history of cancer [15].
Exercise interventions have been deemed beneficial for improving a number of outcomes in cancer survivors. Yoga, a specific type of exercise, has been found to improve a number of outcomes in cancer survivors including cancer-related fatigue, insomnia, depression, hot flash severity, joint pain as well as other side effects [16–21]. To date, however, no research has focused on using a yoga intervention to reduce cancer-related fatigue and global side-effect burden in older cancer survivors, despite the promising outcomes of trials conducted in cancer survivors who are younger. It is imperative to develop safe and feasible interventions that improve cancer-related fatigue and global side-effect burden that meet the unique needs of older cancer survivors so they can recover effectively and resume normal lives following cancer treatments [22]. Therefore, the purpose of this study was to perform a secondary analyses from a previously published clinical trial to assess the effects of a 4-week yoga intervention (Yoga for Cancer Survivors: YOCAS©®) on cancer-related fatigue and global side-effect burden in older cancer survivors [16]
MATERIALS AND METHODS
Study Background
A large, multi-site, randomized controlled trial to assess the efficacy of YOCAS©® for improving sleep quality and cancer-related fatigue and quality of life in cancer survivors experiencing persistent sleep disturbance was conducted through the University of Rochester Cancer Center (URCC) Community Clinical Oncology Programs (CCOP) Research Base. Twelve locations throughout the United States were used for recruitment of 410 participants between 2007 and 2010. The ages of participants in the original study ranged from 26 to 99. This is a secondary data analysis of a subsample of older cancer survivors who participated in the original trial. Results from the original study have been published elsewhere [16].
Participants
For this post-hoc study, participants met the following criteria: a) consented and completed the parent study; b) were 60 years of age or older; c) provided evaluable data on the Multidimensional Fatigue Symptom Inventory – Short Form (MFSI-SF) [23] and Symptom Inventory [24]; d) diagnosis of any type of cancer; e) received standard treatment (surgery, chemotherapy, radiation therapy, or a combination) in the past; f) completed standard treatment between 2 and 24 months prior to enrollment; g) reported persistent sleep disturbance (≥ 3 on 11- point scale, with 0 = no sleep disturbance and 10 = worst possible sleep disturbance); and h) were able to read and understand English. Participants were excluded if they were regularly participating in yoga, defined as one or more sessions per week currently or in the past 3 months, if they had a confirmed diagnosis of sleep apnea or metastatic cancer, and if they were currently receiving standard cancer treatments defined as surgery, chemotherapy, or radiation therapy. Participants in both study arms underwent assessments twice during the study period: 1) during the week prior to the intervention period, and 2) during the week following the 4-week intervention period.
Intervention
Participants were randomized into one of two arms: 1) standard care, or 2) standard care plus the 4-week YOCAS©® intervention. Randomization was stratified by gender and baseline level of sleep disturbance. Stratification by level of sleep disturbance was determined by sleep disturbance self-report on an 11 point scale with 0 = no sleep disturbance and 10 = worst possible sleep disturbance. Participants were stratified in two levels: self-reported sleep disturbance scores of less than or equal to 5 or self-reported sleep disturbance scores of greater than 5. A cut-off of 5 was chosen because it was expected that of those that qualified for the study with a self-reported sleep disturbance score of ≥3, many would not report sleep-disturbance scores closer to 10, equivalent to the worst possible sleep disturbance, but rather, near the middle of the 11-point scale. Therefore, we expected a cut-off of less than or equal to 5 to leave us with about half of our participants falling below and half falling above that self-reported value for sleep disturbance. A computer-generated random numbers table with blocks of 2, for an allocation ratio of 1:1, was used to determine group assignment. Study coordinators recruited participants at the various CCOPs, obtained written informed consent from participants, and registered the participants individually using a website that generated a follow-up email which was sent to the URCC CCOP and the specific CCOP site from which the participant was recruited. The follow-up email included group assignment. The study coordinator was therefore blinded to group assignment until written informed consent was obtained. The principal investigator and the biostatistician at the URCC CCOP Research Base were blinded to study condition allocation. Cancer survivors were recruited in cohorts of 20 to 30 to allow for group participation in YOCAS©® sessions.
Participants assigned to the standard care plus the 4-week YOCAS©® intervention were expected to attend YOCAS©® sessions 2 days per week, each lasting 75 minutes, during the 4 week intervention for a total of 8 sessions. Sessions were held in a group setting, in small regional cancer centers or yoga studios. No make-up sessions were offered. The YOCAS©® program is a standardized program that consists of breathing exercises, postures, and mindfulness exercises. The breathing exercises included slow, controlled, diaphragmatic breaths and breathing coordinated with movement. The postures included 16 gentle Hatha and Restorative yoga poses, of which there are seated, standing, transitional, and supine poses. The meditation exercises included meditation, visualization, and affirmation. Although the specific intensity of this standardized yoga intervention has not been established, hatha and restorative yoga are considered low intensity [< 3 metabolic equivalents (METs)] [25–26].
All instructors were Yoga Alliance Registered and underwent a 2-hour formal, intense training session with the study principal investigator. In addition, they were provided with a detailed instructor manual and DVD to standardize the intervention. Instructors were required to teach the classes exactly as described. They were not allowed to add or remove breathing exercises or postures; however, postures could be modified as needed. A study coordinator at each site performed a random, independent observation of sessions in order to verify the content was being taught as planned. Sessions were provided free of charge to participants.
Participants in the standard care control condition continued to receive follow-up care as needed from their oncologists and primary care providers. They received the same amount of contact time and attention from study staff, aside from attendance in the YOCAS©® classes, as those assigned to the YOCAS©® intervention arm, including contact time during the assessments and being given the opportunity to contact study coordinators throughout the study for any reason. Participants in the standard care control arm were offered the opportunity to participate in a 4 week YOCAS©® program following their participation in the study, free of charge.
This study received approval from and the Institutional Review Boards at the University of Rochester and each of the participating sites. All participants provided written informed consent prior to participation.
Measures
Demographic and Medical Information
Clinical and demographic information was collected using On-Study Data/Participant Record and Clinical Record Information forms by the study coordinator. Race and ethnicity were categorized according to the National Cancer Institute Cancer Therapy Reporting Program criteria for clinical trials.
Cancer-Related Fatigue
Cancer-related fatigue was assessed using the MFSI-SF. The MFSI-SF is a 30-item scale developed specifically for cancer-related fatigue assessment. Respondents rate their level of cancer-related fatigue during the previous 7 days. The MFSI-SF provides a total cancer-related fatigue score as well as subscales for the following domains: general, physical, emotional, mental, and vigor. Each of the 30 items is scored from 0 to 4, with 0=not at all and 4=a great deal. The subscale scores can range from 0 to 24, with higher scores on the general, physical, emotional, and mental subscales indicating greater levels of cancer-related fatigue. The vigor subscale is reverse scored as higher responses on the vigor subscale indicate greater vigor. The total MFSI-SF cancer-related fatigue score can range from −24 to 96. The MFSI-SF has been psychometrically validated in a sample of 304 patients with cancer. It has been deemed reliable and valid in this population [27–28]. Participants completed the MFSI-SF at home during the baseline week and the post-intervention week.
Symptom Inventory
Global side-effect burden was assessed using the Clinical Symptom Inventory. The global side-effect burden score takes into account 12 side effects commonly experienced by cancer survivors during and following treatment. Side-effect severity for each of the following side-effects was rated, based on severity, on an 11-point scale with 0 = not present to 10 = as bad as you can imagine: pain, fatigue, nausea, sleep problems, feelings of depression, shortness of breath, memory loss, weight loss, hair loss, difficulty concentrating, hot flashes, and skin problems. The global side-effect burden score, which is the sum of the severity of all side-effects, can range from 0 to 120. The Symptom Inventory has been validated in numerous studies of patients with cancer [24]. Participants completed the Symptom Inventory at home during the baseline week and the post-intervention week.
Adverse Events
Unexpected, serious, life-threatening and fatal adverse events were monitored using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and reported to the URCC Data Safety Monitoring Committee.
Statistical Analyses
Analyses were performed with SPSS Version 19 and JMP Version 9. Clinical and demographic variables were examined using t-tests for continuous variables and chi-squared tests for categorical variables to determine means, standard errors, frequencies, and percentages, as appropriate, within and between the two YOCAS©® and standard care groups. Analysis of covariance (ANCOVA) was used to compare mean cancer-related fatigue total and sub-domain scores at post-intervention by intervention arm (YOCAS©® versus standard care), controlling for age and baseline cancer-related fatigue total and sub-domain scores, respectively. ANCOVA was used to compare the mean global side-effect burden at post-intervention by intervention arm, controlling for age and baseline global side effect burden. All analyses were conducted based on intent to treat.
RESULTS
Baseline Characteristics of Participants
See Figure 1, CONSORT Participant Diagram. A total of 97 cancer survivors were 60 years of age or older and included in this study. A majority of participants were white (98%), female (94%), and breast cancer survivors (65%). Participants also reported a history of hematologic (14%), gynecologic (6%), genitourinary (6%), lung (3%), gastrointestinal (3%), and brain (2%) cancers. The mean age of participants in each group differed with the standard care participants being slightly younger (64.81 versus 67.91 years of age, p = 0.02). See Table 1 for participant demographics and characteristics. There were no significant differences between participants in the YOCAS©® intervention arm and the participants in the standard care arm at baseline in cancer-related fatigue total score or on subdomain scores or global side-effect burden.
Figure 1.
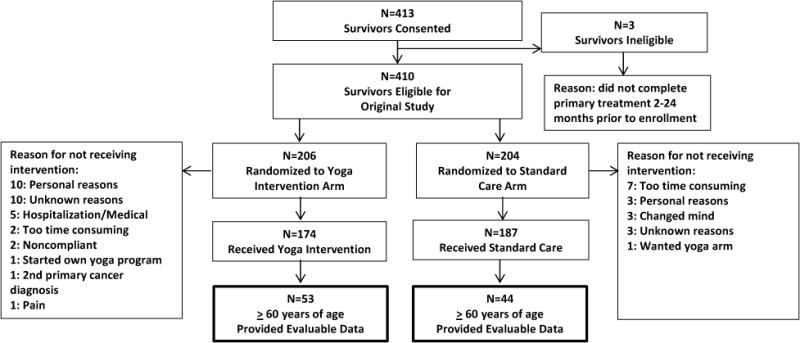
Consort Participant Diagram.
Table 1.
Participant Demographics and Characteristics
| Variable | Yoga (N=53) |
Standard Care (N=44) |
p-value |
|---|---|---|---|
| Female, n (%) | 48 (91) | 43 (98) | 0.15 |
| Age – yr. (mean ± standard error) | 67.91 ± 1.05 | 64.81 ± 0.59 | 0.02 |
| Race, n (%) | |||
| White | 52 (98) | 43 (98) | 0.89 |
| Other | 1 (2) | 1 (2) | |
| Currently employed, n (%) | 34 (64) | 29 (66) | 0.86 |
| Marital status, n (%) | 0.06 | ||
| Married/long-term relationship | 34 (64) | 31 (71) | |
| Divorced or separated | 3 (6) | 10 (23) | |
| Single | 6 (11) | 1 (2) | |
| Widowed | 10 (19) | 2 (4) | |
| Education, n (%) | 0.95 | ||
| Completed ≥ 4 years of college | 28 (53) | 21 (49) | |
| Completed < 4 years of college | 15 (28) | 13 (30) | |
| High school graduate | 10 (19) | 10 (23) | |
| Cancer type, n (%) | 0.11 | ||
| Breast | 31 (58) | 32 (73) | |
| Others | 22 (42) | 12 (27) | |
| Cancer stage, n (%) | 0.61 | ||
| Stage 0 | 3 (6) | 3 (7) | |
| Stage I | 15 (28) | 22 (50) | |
| Stage II | 16 (30) | 11 (25) | |
| Stage III | 10 (19) | 6 (14) | |
| Stage IV | 3 (6) | 1 (1) | |
| Unknown | 6 (11) | 1 (1) | |
| Previous treatment, n (%) | |||
| Surgery | 41 (77) | 37 (84) | 0.41 |
| Chemotherapy | 30 (57) | 25 (57) | 0.98 |
| Radiation therapy | 34 (64) | 34 (77) | 0.11 |
Attendance and Adherence
Attendance records showed the participants in the YOCAS©® intervention arm attended an average of 6.2 of the 8 sessions prescribed. Participants missed classes for reasons including having other commitments and traveling, among others. The average dose of yoga for over the 4-week intervention was 465 of 600 prescribed minutes.
Cancer-Related Fatigue
Following the 4-week intervention, participants in the YOCAS©® intervention group reported a significantly lower level of cancer-related fatigue as assessed using the MFSI-SF total score compared to participants in the standard care arm (p = 0.03). Participants in the YOCAS©® intervention arm also reported significantly less physical fatigue (p < 0.01), mental fatigue (p < 0.01), as assessed with the MFSI-SF subdomain scores. See Table 2 for cancer-related fatigue total and sub-domain scores by group.
Table 2.
Cancer-related fatigue and global side-effect burden scores at post-intervention in participants who were 60 years of age or older.
| Variable | Yoga (N=53) |
Standard Care (N=44) |
p-value |
|---|---|---|---|
| Total cancer-related fatigue | 9.00 ± 2.22 | 14.50 ± 2.99 | 0.03 |
| Cancer-related fatigue subdomains: | |||
| General | 6.91 ± 0.63 | 9.43 ± 0.88 | 0.06 |
| Physical | 4.43 ± 0.52 | 6.65 ± 0.68 | 0.007 |
| Emotional | 4.87 ± 0.60 | 4.91 ± 0.67 | 0.96 |
| Mental | 4.79 ± 0.46 | 6.02 ± 0.66 | 0.006 |
| Vigor | 12.00 ± 0.70 | 12.41 ± 0.73 | 0.26 |
| Global side-effect burden | 17.19 + 1.54 | 21.70 + 2.10 | 0.009 |
Global Side-Effect Burden
Following the 4-week intervention, participants in the YOCAS©® intervention group reported a significantly lower level of global side-effect burden as assessed using the Symptom Inventory total score compared to participants in the standard care arm (p < 0.01). See Table 2 for global side-effect burden scores by group.
DISCUSSION AND CONCLUSION
This secondary data analysis provides evidence that a yoga intervention may be very both feasible and beneficial for older cancer survivors as a means to reduce cancer-related fatigue and global side effect burden following cancer and its treatments. Older cancer survivors reported significantly less cancer-related fatigue and global side-effect burden following the 4-week YOCAS©® intervention compared to participants in the standard care condition. Significant improvements were seen in cancer-related fatigue, the physical and mental domains of cancer-related fatigue, and global side-effect burden. These results provide a promising foundation from which to develop exercise interventions, yoga interventions specifically, that target the unique needs of older cancer survivors during and following treatment.
These results are especially promising due to the relative lack of evidence supporting the safety and efficacy of exercise interventions that target older cancer survivors. Cancer-related fatigue and global side-effect burden can both lead to a reduction in functional ability. Older cancer survivors are at the greatest risk of experiencing a reduction in functional ability during and following cancer treatments, they are less likely to recover from the resulting functional declines, and these reductions in functional ability can ultimately lead to the inability for older cancer survivors to live independently. This investigation into the benefits of yoga for older cancer survivors builds on previous research that has provided evidence for beneficial outcomes resulting from yoga interventions in cancer survivors [17–21, 29–33]. Yoga has led to a number of improvements in cancer survivors, although not specifically in older cancer survivors, including reduced cancer-related fatigue, sleep disturbances, depression, hot flash severity, and joint pain [16–21]. We can now conclude that yoga can improve cancer-related fatigue and global side-effect burden in cancer survivors 60 years of age and older.
Previous yoga interventions in cancer survivors have varied in a number of ways, including the specific type of yoga used and dose of yoga prescribed. Yoga interventions for cancer survivors have lasted from 10 days to 12 weeks and have been prescribed to cancer survivors during and following cancer treatment [29–33]. Many studies have used relatively small sample sizes, often including approximately 50 participants. The YOCAS©® intervention was specifically designed for cancer survivors and despite its relatively short duration of 4 weeks, resulted in improvements in cancer-specific outcomes. It is difficult to hypothesize how older cancer survivors may respond to other forms of yoga but future research using other safe and appropriate forms of yoga in this population is warranted. This investigation was unique as very few exercise intervention studies have focused specifically on older cancer survivors. In addition, our results provide evidence that older cancer survivors, like younger cancer survivors, can see significant improvements form even a low to moderate intensity intervention such as YOCAS©®.
Despite the promising results from this investigation, it is not without limitations. The large majority of participants were white, female breast cancer survivors who were relatively well educated, limiting generalizability. Participants and study coordinators were not blinded to the intervention arm. Participants were also aware of the outcomes of interest. A yoga placebo was not used in this study design. There is no way to ensure that the yoga instructors always led the yoga sessions exactly as planned. Additionally, participants were aware of the outcomes of interest because the informed consent included both the name of the study, alluding to the research hypothesis, as well as information specific to why the study was being conducted. This investigation was not specifically designed with the primary aim of assessing the effects of the yoga intervention on cancer-related fatigue and global side-effect burden, nor was the original study designed to assess the effects of yoga on only those who were 60 years of age and older.
Strengths include the fact that we conducted a randomized clinical trial using a yoga intervention that is well described and can be scalable in many other settings. Participants reported enjoyment and satisfaction in participating and reported no adverse events as the intervention was well tolerated, feasible, and safe. This low to moderate intensity exercise intervention is effective in the segment of our cancer survivor population that is in the greater need of interventions that can improve functional capacity and prolong the ability to live independently than younger individuals due to the presence of more comorbidities and treatment side effect burden. Future intervention studies need to be designed to specifically address the unique needs of older cancer survivors.
In conclusion, our results indicate that YOCAS©®, which includes restorative and hatha postures, improves cancer-related fatigue and global side-effect burden in cancer survivors who are 60 years of age and older. It is one of the largest investigations to date into the effects of an exercise intervention on cancer-related fatigue and global side-effect burden in older cancer survivors, and to our knowledge, the only that has focused on yoga. Further randomized controlled trials are needed to determine the dose of yoga that is most beneficial for this population and to assess the effects of yoga on long-term outcomes such as the ability to live independently and even recurrence and survival rates in participants. Future studies should use a double-blind design and include a yoga placebo arm as a control condition.
Acknowledgments
This work was supported by NCI U10CA037420 with supplemental funding from OCCAM, NCI K07CA120025, NCI R25CA102618.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: All authors have no conflicts of interest to disclose.
Trial Registration: Clinicaltrials.gov: NCT00397930
AUTHOR CONTRIBUTIONS
Study Concepts: LK Sprod, ID Fernandez, R Block, KM Mustian
Study Design: LK Sprod, ID Fernandez, MC Janelsins, LJ Peppone, R Block, KM Mustian
Data Acquisition: JN Atkins, J Giguere, KM Mustian
Quality Control of Data and Algorithms: LK Sprod, KM Mustian, JN Atkins, J Giguere
Data Analysis and Interpretation: LK Sprod, ID Fernandez, MC Janelsins, LJ Peppone, R Block, KM Mustian
Statistical Analysis: LK Sprod, KM Mustian
Manuscript Preparation, Editing, and Review: LK Sprod, ID Fernandez, MC Janelsins, LJ Peppone, JN Atkins, J Giguere, R Block, KM Mustian
References
- 1.Rowland JH, Bellizzi KM. Cancer survivors and survivorship research: a reflection on today’s successes and tomorrow’s challenges. Hematology/oncology clinics of North America. 2008;22:181–200. v. doi: 10.1016/j.hoc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. Journal of the American Geriatrics Society. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 3.Deimling GT, Arendt JA, Kypriotakis G, et al. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(Suppl 2):S289–92. doi: 10.1111/j.1532-5415.2009.02515.x. [DOI] [PubMed] [Google Scholar]
- 4.Deimling GT, Sterns S, Bowman KF, et al. Functioning and activity participation restrictions among older adult, long-term cancer survivors. Cancer Investigation. 2007;25:106–116. doi: 10.1080/07357900701224813. [DOI] [PubMed] [Google Scholar]
- 5.Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61:157–67. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- 6.Suh TT, Lyles KW. Osteoporosis considerations in the frail elderly. Curr Opin Rheumatol. 2003;15:481–6. doi: 10.1097/00002281-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Barnard RJ, Grimditch GK, Wilmore JH. Physiological characteristics of sprint and endurance Masters runners. Med Sci Sports. 1979;11:167–71. [PubMed] [Google Scholar]
- 8.Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33:877–88. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 9.Chen DY, Hsieh TY, Chen YM, et al. Proinflammatory cytokine profiles of patients with elderly-onset rheumatoid arthritis: a comparison with younger-onset disease. Gerontology. 2009;55:250–8. doi: 10.1159/000164393. [DOI] [PubMed] [Google Scholar]
- 10.Russo A, Cesari M, Onder G, et al. Depression and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE Study) J Geriatr Psychiatry Neurol. 2007;20:131–7. doi: 10.1177/0891988707301865. [DOI] [PubMed] [Google Scholar]
- 11.Thalmann A, Morfeld M, Benthien A. Anxiety and Depression in the Elderly – Results of a Regional Examination. Gesundheitswesen. 2010 doi: 10.1055/s-0030-1269839. [DOI] [PubMed] [Google Scholar]
- 12.Dal Forno G, Kawas CH. Cognitive problems in the elderly. Curr Opin Neurol. 1995;8:256–61. doi: 10.1097/00019052-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mohile SG, Fan L, Reeve E, et al. Association of cancer with geriatric syndromes in older medicare beneficiaries. J Clin Oncol. 2011;29:1458–64. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101:1206–15. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprod LKMS, Fan L, Janelsins MC, Peppone LJ, Chandwani K, Morrow GR, Mustian KM. Physical Activity Participation and Functional Limitations in Geriatric Cancer Survivors. Journal of Clinical Oncology supplement. 2012 [Google Scholar]
- 16.Mustian KMSL, Palesh OG, Janelsins MC, Peppone LJ, Chandwani K, Reddy PS, Melnik MK, Heckler CE, Morrow GR. A Multi-Center Randomized Clinical Trial of Yoga for Sleep Quality Among Cancer Survivors. Journal of Clinical Oncology. 2013;31:3233–41. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadiraja SH, Rao MR, Nagendra RH, et al. Effects of yoga on symptom management in breast cancer patients: A randomized controlled trial. International journal of yoga. 2009;2:73–9. doi: 10.4103/0973-6131.60048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–60. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 19.Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2009;17:1301–9. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 20.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011 doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho-oncology. 2009;18:360–8. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satariano WA, Ragheb NE, Branch LG, et al. Difficulties in physical functioning reported by middle-aged and elderly women with breast cancer: a case-control comparison. Journal of gerontology. 1990;45:M3–11. doi: 10.1093/geronj/45.1.m3. [DOI] [PubMed] [Google Scholar]
- 23.Stein KDMS, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Ray US, Pathak A, Tomer OS. Hatha yoga practices: energy expenditure, respiratory changes and intensity of exercise. Evidence-based complementary and alternative medicine : eCAM. 2011;2011:241294. doi: 10.1093/ecam/neq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagins M, Moore W, Rundle A. Does practicing hatha yoga satisfy recommendations for intensity of physical activity which improves and maintains health and cardiovascular fitness? BMC complementary and alternative medicine. 2007;7:40. doi: 10.1186/1472-6882-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 28.Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. Journal of Pain and Symptom Management. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banasik J, Williams H, Haberman M, et al. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. Journal of the American Academy of Nurse Practitioners. 2011;23:135–42. doi: 10.1111/j.1745-7599.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integrative cancer therapies. 2007;6:242–50. doi: 10.1177/1534735407306214. [DOI] [PubMed] [Google Scholar]
- 31.Danhauer SC, Tooze JA, Farmer DF, et al. Restorative yoga for women with ovarian or breast cancer: findings from a pilot study. Journal of the Society for Integrative Oncology. 2008;6:47–58. [PubMed] [Google Scholar]
- 32.Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integrative cancer therapies. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 33.Vadiraja HS, Rao MR, Nagarathna R, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complementary therapies in medicine. 2009;17:274–80. doi: 10.1016/j.ctim.2009.06.004. [DOI] [PubMed] [Google Scholar]


