Abstract
Theiler’s murine encephalomyelitis virus (TMEV) can induce demyelination or myocarditis in susceptible mouse strains. A deficiency of NKT cells exacerbated TMEV-induced demyelinating disease (TMEV-IDD) in SJL/J and BALB/c mice. In C57BL/6 background, however, NKT-cell-deficient Jαt 18 KO mice remained as resistant to TMEV-IDD as wild-type mice. Echocardiography and histology showed that Jα18 KO mice developed more severe myocarditis (greater T cell infiltration and fibrosis) than wild-type mice, suggesting a protective role of NKT cells in myocarditis in C57BL/6 mice. Jα18 KO mice had higher cardiac viral RNA and anti-viral antibody titers, but had lower lymphoproliferation and IL-4 and IL-10 production.
Keywords: Tropism, Picornaviridae infection, CNS demyelinating disease, Multiple sclerosis, GDVII strain, Seizure
Introduction
Theiler’s murine encephalomyelitis virus (TMEV) is a non-enveloped, positive-sense, single-stranded RNA virus that belongs to the family Picornaviridae, genus Cardiovirus. TMEV is divided into two subgroups, GDVII and Theiler’s original (TO), based on their neurovirulence. GDVII virus can cause acute fatal polioencephalitis in all mouse strains and most GDVII virus-infected mice die within 10 days of infection without induction of anti-viral T cell or antibody responses (Tsunoda et al., 1996). The TO subgroup viruses, such as the Daniels (DA) and BeAn strains, can induce a biphasic disease in the central nervous system (CNS) of susceptible mouse strains, such as SJL/J mice (Jarousse et al., 1998). DA virus infects and kills neurons in the CNS, leading to acute polioencephalitis with axonal degeneration in all mouse strains during the acute phase, around 1 week post infection (p.i.), while only susceptible mouse strains develop a progressive demyelinating disease with viral persistence in the CNS during the chronic phase, around 1 month p.i. (Sato et al., 2013). In contrast, resistant mouse strains, such as C57BL/6 and BALB/c mice can eradicate the virus and do not develop the chronic disease (Inoue et al., 1996, Tsunoda et al., 2008). Since TMEV-induced demyelinating disease (TMEV-IDD) in susceptible mice resembles multiple sclerosis (MS) both clinically and histologically, TMEV has been widely used as an animal model of MS (Martinez et al., 2013, Sato et al., 2011a). Although, outside the CNS, TMEV can cause viremia during the acute phase and has been shown to infect the gastrointestinal tract, skeletal muscle, and cardiac muscle (Daniels et al., 1952, Mi et al., 2006), there were only a few reports investigating the pathogenesis of TMEV infection outside the CNS (Gómez et al., 1996, Rames, 1995).
Natural killer T (NKT) cells are a subset of lymphocytes and express an invariant T cell receptor (TCR) (Godfrey et al., 2010) that recognizes glycolipid antigens presented by CD1d molecules (Berzofsky and Terabe, 2008). The TCR of NKT cells is composed of an invariant TCR α chain, Vα14Jα18 in mice and Vα14Jα18 in humans, which is associated with a limited set of TCR β chains, Vβ8.2, Vβ7, or Vβ2 in mice and Vβ 11 in humans (Bendelac et al., 2007, Godfrey, Stankovic, 2010). There have been several mechanistic approaches to determine the role of NKT cells. Jα18 knockout (KO) mice, anti-Vα14 TCR antibody (Ito et al., 1991), and CD1d KO mice (Smiley et al., 1997) have been used to reduce or deplete NKT cells (Tsunoda et al., 2009), while Vα14Jα18 transgenic mice (Bendelac et al., 1996) and administration of NKT cell-specific ligands (Wu et al., 2010), such as α-galactosylceramide, have been used to enhance their functions.
Since NKT cells can produce both pro-inflammatory and anti-inflammatory cytokines, such as interferon (IFN)-γ and interleukin (IL)-4, respectively, NKT cells have been shown to play either a protective or detrimental role in viral infections (Diana and Lehuen, 2009). Previously, we demonstrated that NKT cells played a protective role in TMEV-IDD, both in susceptible SJL/J and resistant BALB/c mice without involvement of the general organs. During the chronic phase of TMEV infection, NKT cell-depleted SJL/J mice, by injection of anti-Vα14 TCR antibody, developed more severe demyelination in the spinal cord compared with control mice (Tsunoda, Tanaka, 2009). Similarly, although infected wild-type BALB/c mice were resistant to TMEV-IDD, CD1d KO BALB/c mice that are deficient in CD1d-restricted cells, including NKT cells, developed TMEV-IDD (Tsunoda, Tanaka, 2008). On the other hand, NKT cells have been shown to play a detrimental role in other viral models, including dengue virus-infected Jα18 KO mice (Renneson et al., 2011) and sendai virus-infected CD1d KO mice (Kim et al., 2008).
Myocarditis is an inflammatory disease in the heart muscle and is caused by viral infections in many cases (38–80%) (Archard et al., 1987, Bowles et al., 2003, Kühl et al., 2005, Liu and Baughman, 2011). Epidemiologically, myocarditis is a major cause of sudden death in young adults, accounting for up to 20% of cases (Doolan et al., 2004, Fabre and Sheppard, 2006, Feldman and McNamara, 2000). Clinically, however, the incidence of myocarditis remains unknown, since some patients show no clinical signs or symptoms, and noninvasive standard diagnostic tests or criteria for myocarditis are not available (Fairweather et al., 2012, Yajima, 2011). Several viruses have been shown to cause myocarditis, including adenovirus, coxsackievirus B3 (CVB3), parvovirus B19, and hepatitis C virus, in humans (Cooper, 2009, Huber et al., 1998). Although the precise pathomechanisms of viral myocarditis are unclear, viral myocarditis has been proposed to be a triphasic disease, composed of phases I, II, and III (Kawai, 1999, Liu and Mason, 2001, Martinez et al., 2012). Phase I is characterized by active viral replication in the myocardium; human fulminant myocarditis may correspond to this phase (Yajima and Knowlton, 2009). Following initial viral replication, the virus can infect the heart tissue at low levels, where infiltration of immune cells in the heart induces tissue damage (immunopathology, phase II) (Whitton and Feuer, 2004). When the damage in phases I and II is severe, it leads to cardiac fibrosis and remodeling in the presence or absence of viral genome or inflammation (phase III) (Cooper, 2009). Although there have been a few reports investigating TMEV-induced myocarditis in mice (Gómez, Rinehart, 1996, Rames, 1995), the precise pathomechanism is unclear.
In this study, to further clarify the role of NKT cells in TMEV-IDD, we used Jα18 KO mice on the genetic background of TMEV-resistant C57BL/6 mice. Unexpectedly, unlike our previous reports, Jα 18 KO mice remained resistant to TMEV-IDD, suggesting that NKT cells had no major role in the CNS virus infection in C57BL/6 mice. Inadvertently, when we investigated the involvement of general organs, we found that TMEV-infected Jα 18 KO mice developed more severe myocarditis compared with wild-type C57BL/6 mice. In the heart, TMEV-infected Jα 18 KO mice had higher levels of viral RNA in phase I, more active inflammation in phase II, and larger fibrotic areas in phase III, compared with wild-type C57BL/6 mice. Using echocardiography, during the course of TMEV infection, we also found a higher ratio of mice with high intensity lesions in Jα 18 KO mice compared with wild-type C57BL/6 mice. Thus, using NKT cell-depleted or NKT KO mice, we found that NKT cells play a different role depending on the mouse strain or organ.
Materials and Methods
Animal experiments
Four to eight week-old wild-type C57BL/6 mice (Harlan laboratories, Indianapolis, IN) and Jα18 KO mice on the C57BL/6 mouse background (Cui et al., 1997) [maintained in an animal breeding facility of Louisiana State University Health Sciences Center (LSUHSC)] were infected intracerebrally with 2 × 105 plaque forming units (PFU) of the DA strain of TMEV or 0.1 to 1,000 PFU of the GDVII strain of TMEV as described previously (Tsunoda, Tanaka, 2008). In some experiments, we also infected wild-type C57BL/6 and Jα 18 KO mice with 2 × 107 PFU of the DA strain of TMEV intraperitoneally. The mice were weighed and monitored daily. Weight and survival rate differences between the two mouse strains were analyzed by Student's t test and χ2 test, respectively. Seizure activity was graded using the Racine scale as follows: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; and stage 5, rearing and falling (Benkovic et al., 2004, Racine, 1972). Lethal dose (LD)50 was calculated using the Reed and Muench calculation of the 50% end point (Burleson et al., 1992).
Animals were maintained under specific pathogen-free conditions in our animal care facility at LSUHSC. All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of LSUHSC and performed according to the criteria outlined by the National Institutes of Health (NIH).
Neuropathology
Mice were perfused with phosphate-buffered saline (PBS) followed by a 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) solution in PBS. The CNS tissues were harvested and fixed with 4% paraformaldehyde. The spinal cords and brains were divided into 10 to 12 transversal segments and five coronal slabs, respectively, and embedded in paraffin. Four-µm-thick sections were made using an HM 325 Rotary Microtome (Thermo Scientific Inc., Waltham, MA) and were stained with Luxol fast blue (Solvent blue 38; Sigma-Aldrich) for myelin visualization. Histological scoring of the CNS sections was conducted as described previously (Sato, Martinez, 2013). Brain sections were scored for meningitis (0, no meningitis; 1, mild cellular infiltrates; 2, moderate cellular infiltrates; 3, severe cellular infiltrates), perivascular cuffing (0, no cuffing; 1, 1 to 10 lesions; 2, 11 to 20 lesions; 3, 21 to 30 lesions; 4, 31 to 40 lesions; 5, over 40 lesions), and demyelination (0, no demyelination; 1, mild demyelination; 2, moderate demyelination; 3, severe demyelination). Each score from the brain was combined for a maximum score of 11 per mouse. For scoring of spinal cord sections, each spinal cord section was divided into four quadrants: the ventral funiculus, the dorsal funiculus, and each lateral funiculus. Any quadrant containing meningitis, perivascular cuffing, or demyelination was given a score of 1 in that pathological class. The total number of positive quadrants for each pathological class was determined and then divided by the total number of quadrants present on the slide and multiplied by 100 to give the percent involvement for each pathological class (Tsunoda, Tanaka, 2008, Tsunoda, Tanaka, 2009).
Real-Time PCR
Mice were perfused with PBS 4, 7 days, and 4 weeks p.i. The hearts were harvested, frozen with liquid nitrogen, and then homogenized in TRI-reagent (Molecular Research Center, Inc., Cincinnati, OH), using a Polytron PT1200E homogenizer (Kinematica AG, Luzern, Switzerland). RNA was isolated using a Qiagen RNeasy mini kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s instruction. We reverse-transcribed 1 µg of total RNA into cDNA, using the ImProm-II™ Reverse Transcription System (Promega, Corp. Madison, WI). Using 50 ng of cDNA, real-time PCR was conducted with RT2 Fast SYBR Green/Flourescein qPCR Master kit (Qiagen) and MyiQ™2 Real Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA). A primer pair for a capsid protein VP2 of TMEV was used to detect the viral RNA in the heart, while a primer pair for glyceraldehyde-3-phosphate dehydrogenase (Gapd) or phosphoglycerate kinase 1 (Pgk1) (Real Time Primers, LLC, Elkins Park, PA) was used as as a housekeeping gene. The primer pair sequences for VP2 were forward (5’ -TGGTCGACTCTGTGGTTACG-3’) and reverse (5’-GCCGGTCTTGCAAAGATAGT-3’) (Deb et al., 2009). Viral RNA levels between the two mouse strains were statistically analyzed by Student's t test or the Mann-Whitney U test.
Lymphoproliferative assay
Mice were killed 1 week, 1 month, and 2 months p.i. Mononuclear cells (MNCs) were isolated from the spleen using Histopaque®-1083 (Sigma-Aldrich). MNCs were cultured with RPMI 1640 medium (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (Mediatech), 2 mM L-glutamine (Mediatech), 50 µM β-mercaptoethanol (Sigma-Aldrich), and 1% antibiotic-antimycotic solution (Mediatech), at 2 × 105 cells/well in 96-well plates (Corning, Inc., Corning, NY) and stimulated with TMEV at a multiplicity of infection (MOI) of 5, 2 × 105 cells/well of DA-infected antigen presenting cells (DA-APCs), or 2 × 105 cells/well of sham-infected antigen presenting cells (nAPCs) for 5 days. DA-APCs were made from whole spleen cells infected in vitro with DA virus at an MOI of 1, while nAPCs were made without virus. DA-APCs and nAPCs were irradiated with 2,000 rads using a 137Cs irradiator (J.L. Shepherd & Associates, San Fernando, CA). To assess the levels of lymphoproliferative responses, [3H]thymidine (PerkinElmer, Inc., Waltham, MA) was added in the culture at a concentration of 1 µCi /well for the last 24 hours. MNCs were harvested on Reeves Angel 934AH filters (Brandel, Gaithersburg, MD) using a PHD™ Harvester (Brandel). The incorporated radioactivity was measured by a Wallac 1409 Liquid Scintillation Counter (PerkinElmer). All cultures were performed in triplicate and the data were expressed as stimulation indexes [experimental counts per minute (cpm)/control cpm] (Martinez et al., 2014a).
Enzyme-linked immunosorbent assay (ELISA) for anti-TMEV antibody and cytokines
Peripheral blood was collected from TMEV-infected mice 1 week and 1 month p.i. The levels of serum anti-TMEV antibodies were assessed by ELISA as described previously. Ninety-six-well flat-bottom Nunc-Immuno plates (Thermo Fisher Scientific) were coated with 10 µg/ml of TMEV antigen overnight. After blocking with 10% FBS and 0.2% Tween® 20 (Thermo Fisher Scientific), serial dilutions of sera were added to the plates and incubated for 90 minutes at room temperature. Following washing, a peroxidase-conjugated anti-mouse IgG (H + L) (Life Technologies, Gaithersburg, MD) was added to the plates for 90 minutes. Immunoreactive complexes were detected with o-phenylendiamine dihydrochloride (Sigma-Aldrich) and absorbance was read at 492 nm on a Multiskan MCC/340 Microplate Reader (Thermo Fisher Scientific) (Martinez, Karlsson, 2014a).
For cytokine assays, mice were killed 1 week, 1 month and 2 months p.i. MNCs isolated from the spleens of TMEV-infected mice were cultured at 8 × 106 cells/well in 6-well-plates (Corning) and stimulated with 5 µg/ml of concanavalin A for 48 hours. The culture supernatants were harvested and stored at −80°C until examined. The concentrations of IL-4, IL-10, and IFN-γ in the culture supernatants were quantified by ELISA (BD Biosciences, San Diego, CA), according to the manufacturer’s instructions (Fernando et al., 2014).
Cardiac pathology
Mice were killed 4 days, 1 week, and 4 weeks p.i. The heart tissues from the perfused mice were harvested and fixed with 4% paraformaldehyde. The heart was sliced into 1-mm-thick transverse sections and embedded in paraffin. Four-µm-thick heart sections were stained with hematoxylin (Electron Microscopy Sciences, Hatfield, PA) and eosin (Thermo Fisher Scientific, Inc., Rochester, NY) to visualize the general structure, or picrosirius red (ScyTek Laboratories, Inc., Logan, UT) to detect fibrosis (collagen I and III). The fibrotic areas were quantified by Image-Pro® Plus Version 6.3 (Media Cybernetics, Inc., Rockville, MD). CD3+ T cell infiltration was visualized by immunohistochemistry with anti-CD3 antibody (DAKO corp., Carpinteria, CA), using the avidin-biotin-peroxidase complex (ABC) technique (Vector, Burlingame, CA), following antigen retrieval with a Vector® Antigen Unmasking Solution (Vector Laboratories, Inc., Burlingame, CA) (Tsunoda et al., 2007). The numbers of CD3+ T cells were counted in the section at the papillary muscle level, where the two papillary muscles were seen.
Echocardiography
Using the Vevo 770 system with a 707B transducer (VisualSonics Inc., Toronto, Canada), we conducted trans-thoracic echocardiography prior to TMEV infection, and 4, 7, 10, 14, and 28 days p.i. Mice were anesthetized with isoflurane and thorax fur was shaved and removed using a hair remover (Nair; Church & Dwight Co., Inc., Princeton, NJ). We placed mice on the animal handling platform, monitoring the animal's electrocardiogram, heart rate, and temperature. Left ventricular (LV) fraction shortening (FS) and ejection fraction (EF) were measured in LV short axis view (Tajiri et al., 2012). Outflow and valve diameter of the aorta, pulmonary artery, and mitral valve inflow were also measured. We recorded high intensity lesions in the heart wall and compared the incidence between wild-type C57BL/6 and Jα18 KO mice using χ2 test.
Results
Jα18 KO mice remain resistant to TMEV-IDD
After intracerebral DA virus infection, the virus predominantly infects neurons in the brain and induces acute polioencephalitis during the acute phase, 1 week p.i., in all mouse strains. During the chronic phase, 1 month p.i., only susceptible mouse strains developed demyelination in the spinal cord (Martinez et al., 2014b, Sato et al., 2011b). We infected wild-type C57BL/6 and Jα18 KO mice with DA virus intracerebrally and monitored weight changes and clinical signs of TMEV-IDD, such as spastic paralysis, waddling gait, and impaired righting reflexes. Infected Jα18 KO mice remained as resistant to TMEV-IDD as wild-type C57BL/6 mice; no clinical signs of TMEV-IDD were seen in 17 wild-type C57BL/6 or 21 Jα18 KO infected mice (data not shown). During the acute phase, since TMEV can cause seizures in C57BL/6 mice, we also evaluated TMEV-induced seizures in wild-type C57BL/6 and Jα18 KO mice using the Racine scale. We found that wild-type C57BL/6 mice had seizures from days 3 to 9, while Jα18 KO mice had seizures from days 4 to 8. There was no statistical difference in the incidence or severity of seizures between the two mouse strains (Supplementary Figure 1A and B). With the exception of seizures, both wild-type C57BL/6 and Jα18 KO mice did not show any clinical signs and remained asymptomatic during the 2-month observation period (Supplementary Figure 2A).
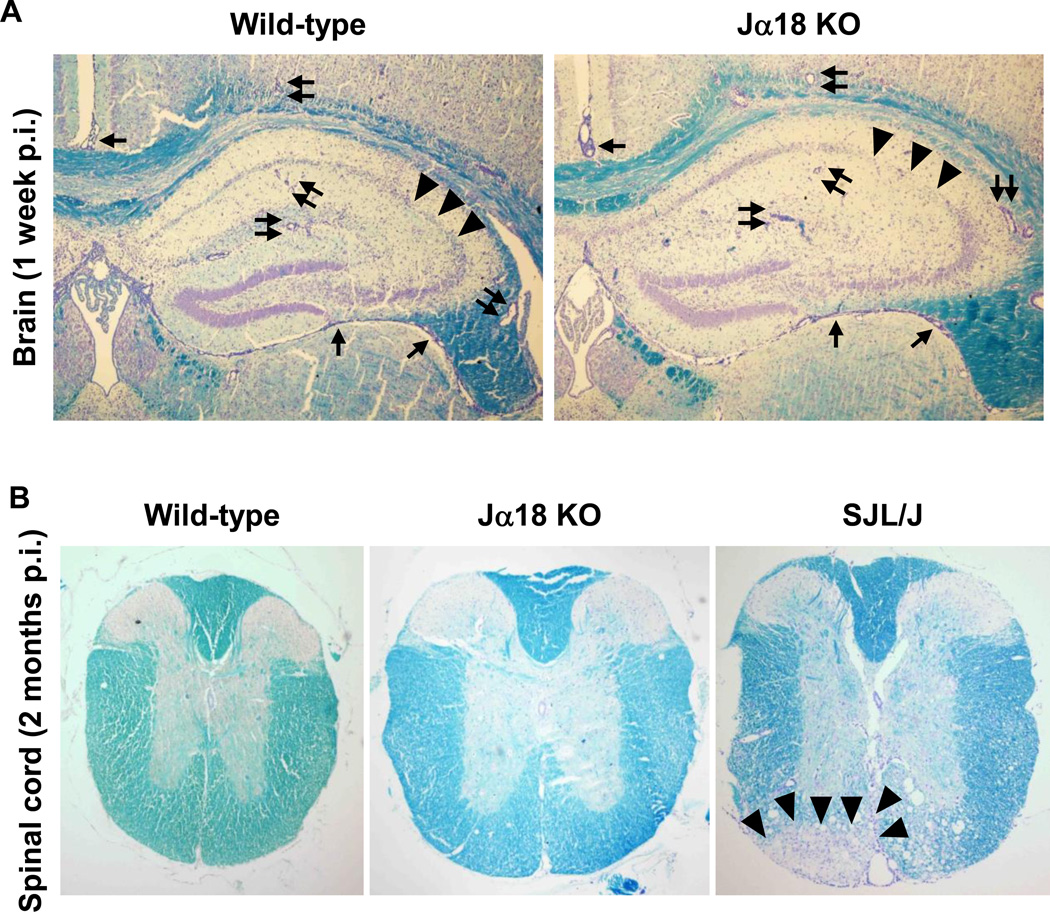
Histologically, during the acute phase, 1 week p.i., both mouse strains developed polioencephalitis with severe meningitis and perivascular cuffing (inflammation), which were composed of mainly MNCs. Most lesions were found in the gray matter of the brain, particularly in the hippocampus (Figure 1A). There were no differences in the pathology scores in the brain between the two mouse strains: the mean ± standard error of the mean (SEM) of brain pathology scores in wild-type C57BL/6 and Jα18 KO mice were 7.3 ± 0.3 and 7.0 ± 0.5 (P = 0.62), respectively (Supplementary Figure 2B). Inflammation in the brain subsided 1 and 2 months p.i., and mild inflammation was observed only in a few mice of both mouse strains (Supplementary Figure 2B). In the spinal cord, we found similar levels of overall pathology, meningitis, and perivascular cuffing between the two mouse strains 1 week p.i. (e.g., mean overall pathology scores ± SEM: wild-type C57BL/6, 27.8 ± 10.7; Jα18 KO, 26.4 ± 6.4, P = 0.90, Supplementary Figure 2C). During the chronic phase, DA virus has been known to cause demyelination in the spinal cords of susceptible SJL/J mice (Figure IB). We did not find any neuropathology in the spinal cords of wild-type C57BL/6 or Jα18 KO mice 2 months p.i. (Figure IB), although we had expected that some of the infected Jα18 KO mice would develop perivascular cuffing or demyelination during the chronic phase (Tsunoda, Tanaka, 2008).
Figure 1.
Neuropathology of wild-type C57BL/6 and Jα18 knockout (KO) mice, 1 week and 2 months post infection (p.i.). Wild-type C57BL/6 and Jα18 KO mice were infected intracerebrally with the Daniels (DA) strain of Theiler’s murine encephalomyelitis virus (TMEV). (A) During the acute phase of TMEV infection, 1 week p.i., wild-type C57BL/6 and Jα18 KO mice had similar levels of meningitis (arrows), perivascular cuffing (paired arrows), and neuronal loss (arrowheads) in the hippocampus. Sections were representatives of six wild-type C57BL/6 and 10 Jα18 KO mice. Magnification, 35×. (B) During the chronic phase of TMEV infection, 2 months p.i., neither wild-type C57BL/6 nor Jα18 KO mice developed lesions in the spinal cord, while susceptible SJL/J mice developed severe demyelination (arrowheads) in the ventral funiculus of the spinal cord. Sections were representatives of 12 wild-type C57BL/6 and 21 Jα18 KO mice. Magnification, 23×. (A, B) Luxol fast blue staining.
Jα18 KO mice have lower cellular immune responses compared with wild-type C57BL/6 mice
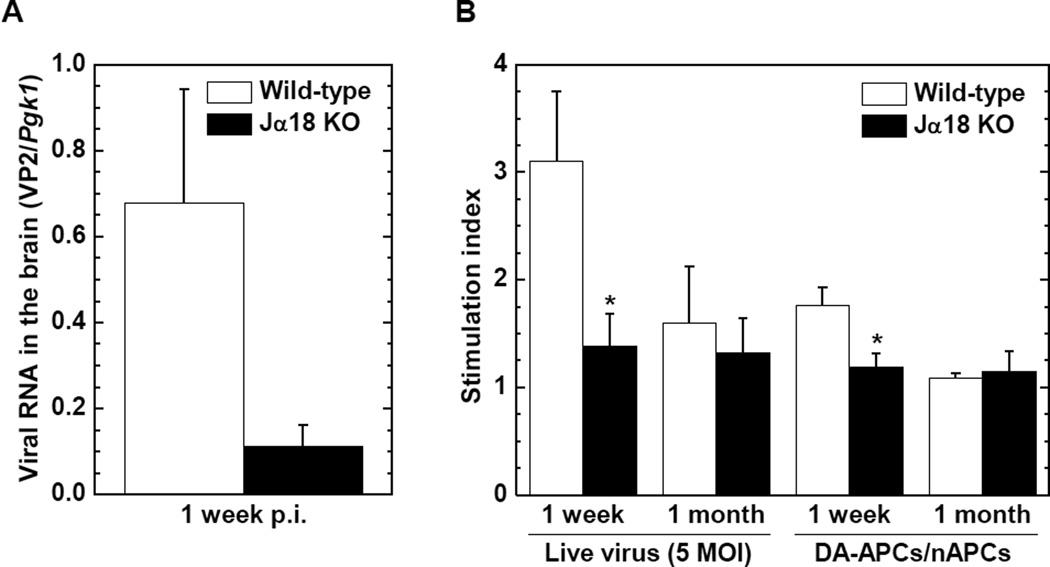
Since NKT cells can contribute to the induction of anti-viral immune responses (Diana and Lehuen, 2009), we compared viral replication in the brain between wild-type C57BL/6 and Jα18 KO mice 1 week p.i. The levels of viral RNA in the brain tended to be lower in Jα18 KO mice than in wild-type C57BL/6 mice (P = 0.07, Figure 2A). There was no correlation between viral RNA levels in the brain versus the severity (or incidence) of seizures (P > 0.05, Supplementary Figure 1C and D). Since the lower viral load could be explained by greater anti-viral immune responses, we compared anti-TMEV lymphoproliferative responses (cellular immunity) between the two mouse strains. Interestingly, however, Jα18 KO mice had significantly lower levels of TMEV-specific lymphoproliferation 1 week p.i. compared with wild-type C57BL/6 mice (P < 0.05, Figure 2B), while there was no significant difference in lymphoproliferative responses to TMEV between the two mouse strains 1 month p.i.
Figure 2.
Viral replication in the brain, and TMEV-specific cellular immune responses in infected wild-type C57BL/6 and Jα18 KO mice. (A) The levels of viral RNA in the brain 1 week p.i. were higher in wild-type C57BL/6 (open column) mice compared with Jα18 KO (closed column) mice, although it did not reach statistical differences (P = 0.07). Viral genome was semi-quantified by real-time PCR using a pair of primers for a capsid protein VP2 of TMEV. Phosphoglycerate kinase 1 (Pgk1) was used as a housekeeping gene for normalization. (B) Jα18 KO mice had significantly lower levels of lymphoproliferative responses to TMEV compared with wild-type C57BL/6 mice, 1 week p.i. (*, P < 0.05, Student's t test). Mononuclear cells (MNCs) isolated from the spleen were stimulated with TMEV at a multiplicity of infection (MOI) of 5, DA-infected antigen presenting cells (DA-APCs), or sham-infected antigen presenting cells (nAPCs). Lymphoproliferative responses were quantified by [3H]thymidine incorporation assays and were expressed as stimulation indexes [experimental counts per minute (cpm)/control cpm]. Results are mean ± standard error of the mean (SEM) from 6 to 20 mice/group/time point.
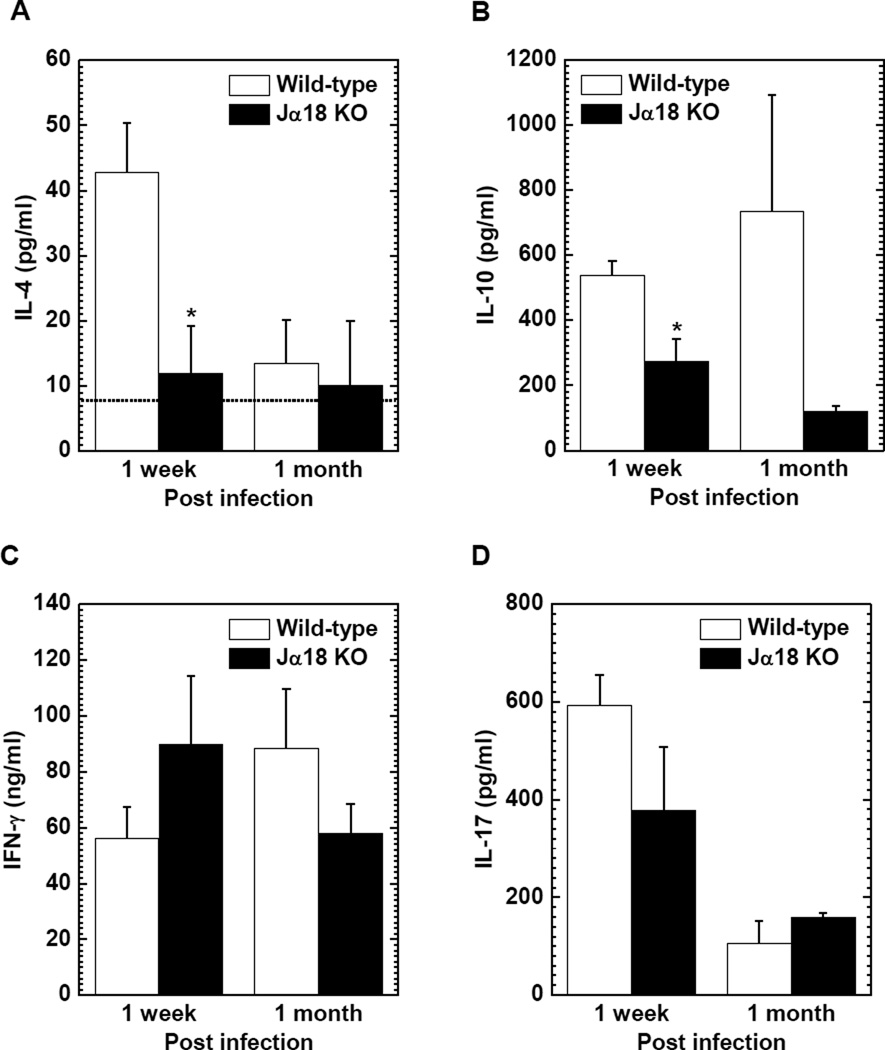
We also compared the levels of anti-inflammatory IL-4 and IL-10 production, and pro-inflammatory IFN-γ and IL-17 production between wild-type C57BL/6 and Jα18 KO mice 1 week and 1 month p.i. (Figure 3). Jα18 KO mice produced significantly less IL-4 compared with wild-type C57BL/6 mice 1 week p.i. (mean IL-4 ± SEM: wild-type C57BL/6, 42.8 ± 7.5 pg/ml; Jα18 KO, 11.9 ± 7.3 pg/ml, P < 0.05, Figure 3A), while IL-4 production was low in both mouse strains 1 month p.i. Jα18 KO mice produced less IL-10 compared with wild-type C57BL/6 mice at both time points, particularly 1 week p.i. (mean IL-10 ± SEM: wild-type C57BL/6, 536.5 ± 45.1 pg/ml; Jα18 KO, 272.2 ±70.1 pg/ml, p < 0.05, Figure 3B). On the other hand, there was no consistent trend in the production of IFN-γ and IL-17 between the two mouse strains (Figure 3C and D).
Figure 3.
Cytokine production in TMEV-infected wild-type C57BL/6 and Jα18 KO mice. (A) Jα18 KO (closed column) mice produced significantly lower levels of interleukin (IL)-4 compared with wild-type C57BL/6 (open column) mice 1 week p.i., while IL-4 production 1 month p.i. was around the detection limit of the enzyme-linked immunosorbent assay (ELISA) kit (dotted line). (B) The levels of IL-10 production were lower in Jα18 KO mice compared with wild-type C57BL/6 mice 1 week (*, P < 0.05) and 1 month p.i. (C) High levels of interferon (IFN)-γ production were detected in both wild-type C57BL/6 and Jα18 KO mice; there were no statistical differences between the two mouse strains. (D) No consistent trend was seen in IL-17 production between wild-type C57BL/6 and Jα18 KO mice. (A–D) MNCs were isolated from the spleen and stimulated with concanavalin A. The levels of cytokine production were assessed by ELISA. Results are mean ± SEM from 6 to 21 mice/group/time point.
NKT cells play a detrimental role in neurovirulent GDVII infection
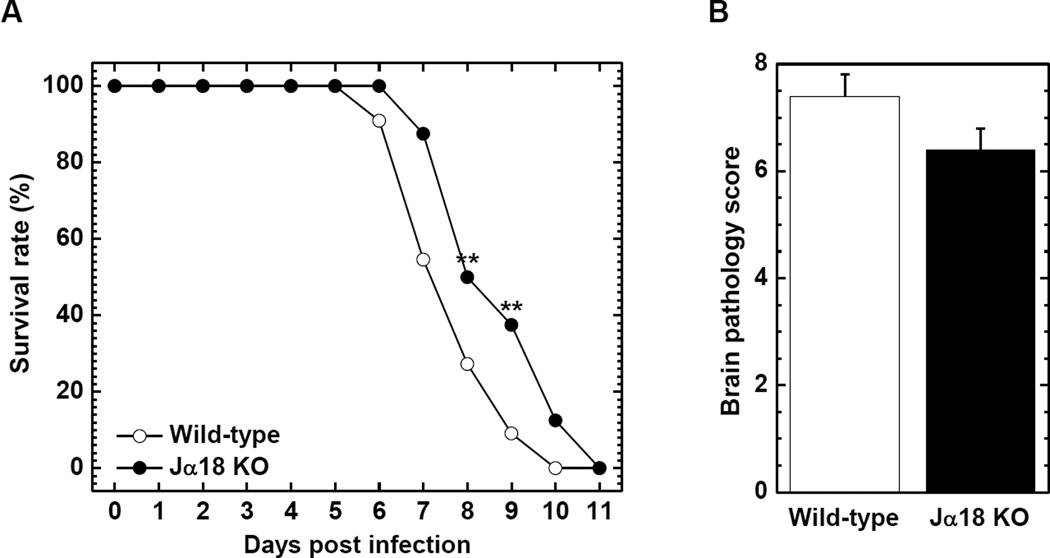
In the above experiments, we observed different immune responses between wild-type C57BL/6 and Jα18 KO mice. To further examine the role of NKT cells in TMEV infection, we used the GDVII strain of TMEV, which is highly neurovirulent. Unlike DA virus, GDVII virus causes acute fatal polioencephalitis in mice; most infected mice die within 10 days of infection. In our previous report using CD1d KO mice, we found that NKT cells played a protective role in GDVII virus infection. To our surprise, in this study, we saw opposite results, where NKT cells were detrimental in GDVII virus infection. The LD50 was higher in Jα18 KO compared with wild-type C57BL/6 mice; wild-type C57BL/6, 0.398 PFU; Jα18 KO, 1.58 PFU (Table 1). Among mice infected with 10 to 1,000 PFU of GDVII virus, most mice started to show clinical signs, such as seizures, weight loss, ruffled fur, and hunched posture 4 and 5 days p.i. In mice infected with 10 PFU of GDVII virus, the survival rate was significantly higher in Jα18 KO mice compared with wild-type C57BL/6 mice 8 and 9 days p.i. (P < 0.01, Figure 4A). Jα18 KO mice also tended to have lower brain pathology scores (Figure 4B) and reduced weight loss compared with wild-type C57BL/6 mice (Supplementary Figure 3A).
Table 1.
Mortality and survival day of wild-type C57BL/6 and Jα18 knockout (KO) mice in GDVII virus infection1
| PFU2 | Wild-type C57BL/6 |
Jα18 KO |
||
|---|---|---|---|---|
| Mortality3 | Mean survival day4 | Mortality | Mean survival day | |
| 1,000 | 6/6 | 5.4 ±0.3 | 10/10 | 5.4 ±0.4 |
| 100 | 4/4 | 6.0 ±0.4 | 8/8 | 6.0 ±0.5 |
| 10 | 11/11 | 6.8 ±0.4 | 8/8 | 7.9 ±0.5 |
| 1 | 5/6 | 8.0 ±1.0 | 5/8 | 8.7 ±0.3 |
| 0.1 | 0/5 | N/A5 | 0/10 | N/A |
Mice were infected with the GDVII strain of Theiler’s murine encephalomyelitis virus (TMEV).
Plaque forming units (PFU) of virus inoculated.
Numbers of dead mice / total numbers of infected mice.
Mean survival day ± standard error of the mean (SEM) following viral infection.
N/A, not applicable.
Figure 4.
The survival rate and brain pathology of wild-type C57BL/6 and Jα18 KO mice infected with the GDVII strain of TMEV. Mice were infected intracerebrally with 10 plaque forming units (PFU) of neurovirulent GDVII virus. (A) Jα18 KO (●) mice had significantly higher survival rates 8 and 9 days p.i. compared with wild-type C57BL/6 (○) mice. **, P < 0.01, χ2 test. (B) Infected Jα18 KO mice (closed column) tended to have lower brain pathology scores compared with wild-type C57BL/6 mice (open column). (A, B) Each group was composed of 8 to 11 mice.
TMEV-infected Jα18 KO mice develop myocarditis
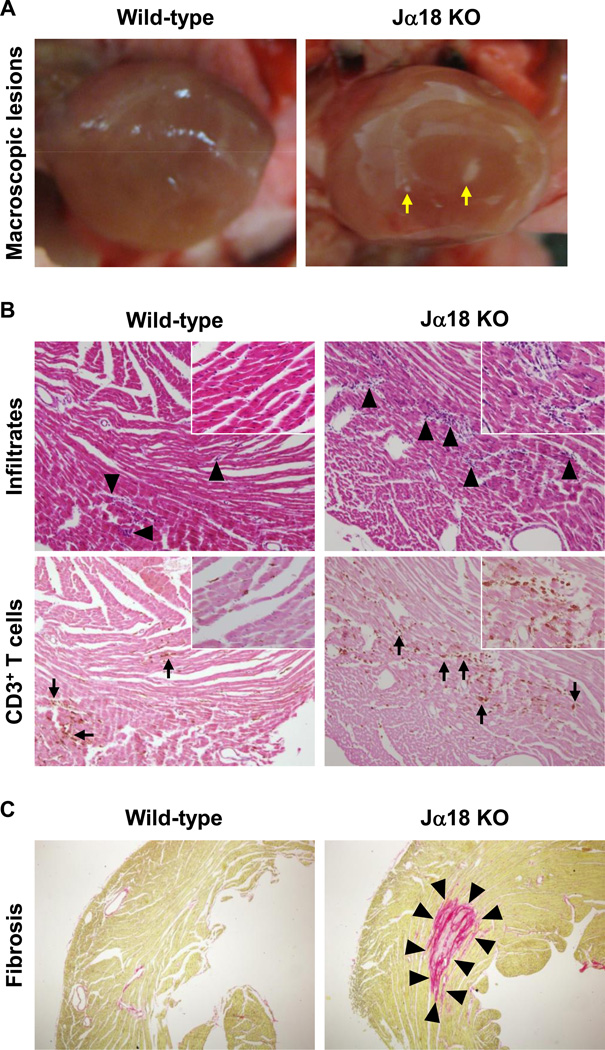
Inadvertently, during the course of the above experiments, we observed that some Jα18 KO mice had macroscopic focal lesions in the heart, when we perfused mice transcardially. Since all the above infections were induced with intracerebral viral inoculation, we tested whether the induction of cardiac lesions could be changed by the route of injection, comparing intracerebral versus intraperitoneal injection. We found that intraperitoneal viral injection induced more severe cardiac lesions compared with intracerebral injection (data not shown). Thus, we decided to infect mice with DA virus intraperitoneally for further studies, monitored weight changes, and evaluated clinical signs of heart failure, such as lower activity and edema, in infected wild-type C57BL/6 and Jα18 KO mice. We did not find statistical differences in weight changes between the two mouse strains (Supplementary Figure 3B) and no mice developed clinical signs. Histologically, we found that infected Jα18 KO mice had macroscopic multiple focal lesions in the heart, while no wild-type C57BL/6 mice have macroscopic lesions in the heart (Figure 5A). Microscopically, although inflammatory cell infiltration was seen in the hearts of both mouse strains, more cell infiltration was observed in Jα18 KO mice compared with wild-type C57BL/6 mice (Figure 5B). The inflammatory lesions were smaller than 1 mm and all the lesions were in the myocardium; the cell infiltration was confined to the focal areas of myocardium and was mainly composed of MNCs. Using immunohistochemistry against CD3 (T cell marker, Figure 5B) and picrosirius red staining (specific for collagen I and III, Figure 5C), we further characterized TMEV-induced myocarditis. We found that substantial T cell infiltration was seen only 1 week p.i. (Figure 5B) but not 4 days or 1 month p.i. Fibrosis was detectable as early as 1 week p.i. and progressed during the course of TMEV infection (Figure 5C). Quantification of T cells and fibrosis in the heart showed that Jα18 KO mice had higher T cell infiltration 1 week p.i. and larger fibrotic areas 1 week and 1 month p.i. compared with wild-type C57BL/6 mice (Supplementary Figure 4). However, there was no statistical difference in the T cell infiltration or in the areas of fibrosis between the two mouse strains, because of high variations of T cell number and fibrotic areas even among mice within the same group. This is most likely due to the multifocal, not diffuse, nature of the myocarditis lesions induced by TMEV, where both the number and size of lesions were inconsistent among the histology sections.
Figure 5.
Cardiac pathology of TMEV-infected wild-type C57BL/6 and Jα18 KO mice. Wild-type C57BL/6 and Jα18 KO mice were infected intraperitoneally with the DA strain of TMEV. (A) Jα18 KO mice had macroscopic multiple focal lesions (arrows) in the heart 1 month p.i., while no wild-type C57BL/6 mice had macroscopic lesions. (B) Histologically, we found more inflammatory cell infiltrates (arrowheads) in the hearts of Jα18 KO mice compared with wild-type C57BL/6 mice 1 week p.i. by hematoxylin and eosin (H & E) staining. Consecutive sections immunostained with antibody against CD3 (T cell marker) showed that cell infiltrates were mainly composed of CD3+ T cells (arrows). Sections were representatives of 12 wild-type C57BL/6 and 17 Jα18 KO mice. Magnification, 51×; inset, 92×. (C) Picrosirius red staining visualized large fibrotic lesions (arrowheads) in the hearts of infected Jα18 KO mice but not in wild-type C57BL/6 mice, 1 month p.i. Sections were representatives of 8 wild-type C57BL/6 and 13 Jα18 KO mice. Magnification, 20×.
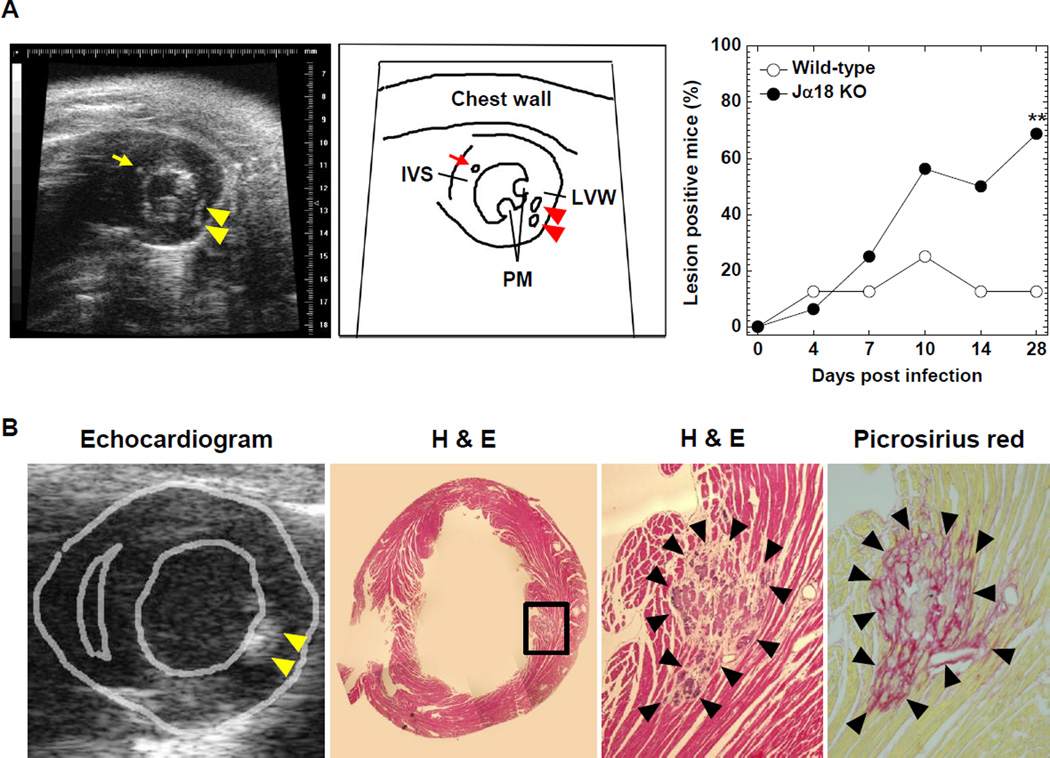
TMEV-infected Jα18 KO mice have high intensity echocardiogram lesions
We also conducted a longitudinal study of TMEV-induced myocarditis using echocardiography. We found multiple high intensity lesions in the LV wall and interventricular septum of infected mice (Figure 6A and Supplementary Video 1), while we were not able to observe the entire right ventricle wall because of the limited acoustic window. High intensity lesions were detectable as early as 4 days p.i. and the size of high intensity lesions was less than 1 mm. The number of the lesions increased in Jα18 KO mice during the 1-month observation period. Twenty-eight days p.i., 68.8% (11 of 16 mice) of Jα18 KO mice had high intensity lesions, while 12.5% (1 of 8 mice) of wild-type C57BL/6 mice had high intensity lesions (Figure 6A). There were statistical abnormalities in the incidence of mice with high intensity lesions between wild-type C57BL/6 and Jα18 KO mice (P < 0.05). Functionally, however, there were no abnormalities in cardiac functions, including FS, EF and other parameters, in both mouse strains (data not shown). Histologically, the high intensity lesions corresponded to basophilic degeneration, calcification, and fibrotic changes in the heart (Figure 6B). We confirmed no high intensity lesions in the mice prior to TMEV infection and age-matched uninfected control mice (data not shown).
Figure 6.
Echocardiograms and heart sections stained with H & E or picrosirius red of mice infected with TMEV. (A) The left and middle panels show the short axis view at the papillary muscle (PM) level in an echocardiogram and the scheme, respectively. Some TMEV-infected wild-type C57BL/6 and Jα18 KO mice had high intensity lesions in the interventricular septum (IVS, arrow) and left ventricular wall (LVW, arrowheads). The incidence of mice with high intensity lesions in the heart was higher in Jα18 KO mice, compared with wild-type C57BL/6 mice during the 1-month observation period (**P < 0.01, χ2 test). (B) H & E and picrosirius red staining (magnification, 52×) showed that high intensity lesions in echocardiograms corresponded to basophilic degeneration, calcification, and fibrosis in the hearts of mice with TMEV-induced myocarditis. The picture showing the morphology of the heart (second from the left) is a composite picture. (A, B) Echocardiograms are representative of 8 to 16 mice/group/time point.
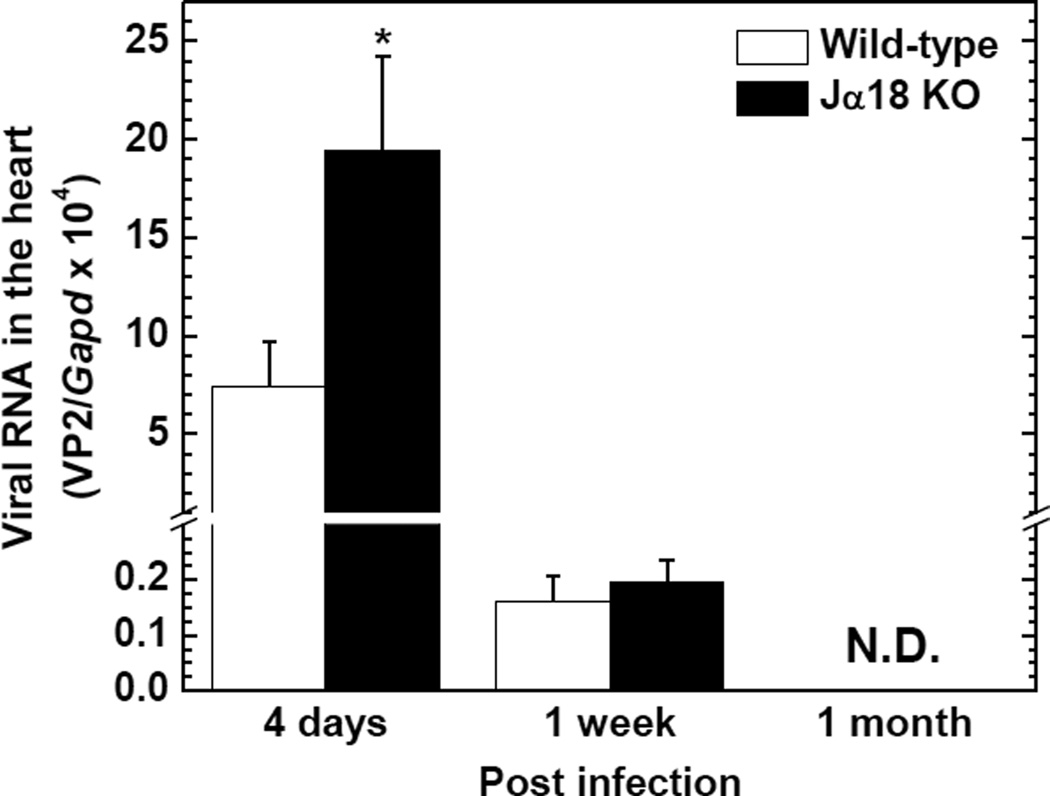
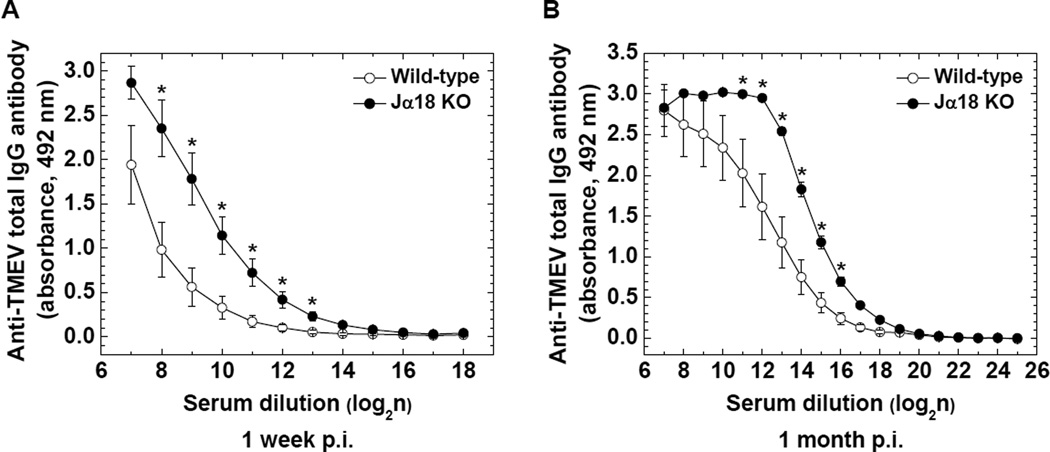
We compared viral replication in the hearts between wild-type C57BL/6 and Jα18 KO mice 4 days, 1 week, and 1 month p.i., using quantitative real-time PCR. Jα18 KO mice had 2.6 times (P < 0.05) and 1.2 times higher levels of viral RNA compared with wild-type C57BL/6 mice 4 days and 1 week p.i., respectively (Figure 7). The amount of viral RNA declined over the time course in both strains and was undetectable 1 month p.i. Since humoral immunity (antibody) has been shown to play a role in not only viral clearance but also pathogenesis of viral myocarditis, we also compared serum anti-TMEV antibody titers between the two mouse strains. Jα18 KO mice had significantly higher titers of anti-TMEV antibody in sera 1 week and 1 month p.i. compared with wild-type C57BL/6 mice (P < 0.05, Figure 8A and B). Lastly, we biochemically attempted to quantify cardiomyocyte damage, using ELISA for cardiac troponin I (cardiac-specific protein) in sera; the presence of cardiac troponin in sera indicates cardiomyocyte damage. However, in both wild-type C57BL/6 and Jα18 KO mice, serum troponin I was undetectable during the time course of TMEV infection (data not shown).
Figure 7.
Viral replication in the hearts of TMEV-infected wild-type C57BL/6 and Jα18 KO mice. The levels of viral RNA in the heart were significantly higher in Jα18 KO mice 4 days p.i. compared with wild-type C57BL/6 mice (*P < 0.05, Mann-Whitney U-test). Viral genome was semi-quantified by real-time PCR using a primer pair for the capsid protein VP2 of TMEV. Glyceraldehyde-3-phosphate dehydrogenase (Gapd) was used as a housekeeping gene for normalization. Each group was composed of 3 to 10 mice/group/time point. N.D., not detectable.
Figure 8.
Humoral immune responses in wild-type C57BL/6 and Jα18 KO mice following TMEV infection. Jα18 KO (●) mice had significantly higher anti-TMEV IgG antibody responses compared with wild-type C57BL/6 (○) mice 1 week (A) and 1 month (B) p.i. (*P < 0.05, Student's t test). The levels of serum anti-TMEV antibody were quantified by ELISA. Results are mean ± SEM from six to seven mice/group/time point.
Discussion
We previously reported that NKT cells played a protective role in both TMEV-IDD induced with DA virus infection and acute polioencephalitis induced with neurovirulent GDVII virus infection in two studies (Tsunoda, Tanaka, 2008, Tsunoda, Tanaka, 2009). We demonstrated that, in DA virus infection, susceptible SJL/J mice administered anti-Vα14 TCR antibody, which depletes NKT cells, had more severe TMEV-IDD in the spinal cord during the chronic phase (Tsunoda, Tanaka, 2009). We also demonstrated that, while wild-type BALB/c mice were resistant to TMEV-IDD, CD1d KO mice on the BALB/c mouse background developed an inflammatory demyelinating disease in the spinal cord (Tsunoda, Tanaka, 2008). In neurovirulent GDVII virus infection, CDld KO mice had a lower LD50 and survival rate compared with wild-type BALB/c mice. Thus, we initially anticipated that Jα18 KO mice would become susceptible to TMEV-IDD. However, DA virus-infected Jα18 KO mice on the C57BL/6 mouse background remained resistant to TMEV-IDD as wild-type C57BL/6 mice. In neurovirulent GDVII virus infection, the higher LD50 and survival rate showed that Jα18 KO mice were more resistant compared with wild-type C57BL/6 mice. Thus, the presence of NKT cells was not protective in mice on the C57BL/6 mouse background infected with DA virus, and the presence of NKT cells may even play a detrimental role in GDVII virus infection. Since GDVII virus has been shown to cause acute fatal polioencephalitis due to higher viral pathology (viral replication and neuronal apoptosis) without induction of acquired immunity (Tsunoda, Iwasaki, 1996, Tsunoda et al., 1997, Tsunoda, Libbey, 2007), alteration of overall innate immunity by a lack of NKT cells [a subset of innate-like lymphocytes (Van Kaer et al., 2013)] may affect viral pathology, resulting in the expanded lifespan of Jα18 KO mice. It is interesting that our three studies focusing on the function of NKT cells resulted in different outcomes (Table 2).
Table 2.
Contrasting roles of NKT cells in three inbred mouse strains during TMEV infection1
| Experiment | Mouse strain |
MHC2 | Demyelination | GDVII encephalitis |
Myocarditis | Reference |
|---|---|---|---|---|---|---|
| Vα14 Ab3 | SJL/J | H2s | Protective | Protective | No role | (Tsunoda, Tanaka, 2009) |
| CDld KO4 | BALB/c | H2d | Protective | Protective | No role | (Tsunoda, Tanaka, 2008) |
| Jα18 K05 | C57BL/6 | H2b | No role | Detrimental | Protective | Current study |
Mice were infected with the DA or GDVII strain of TMEV.
Haplotype of major histocompatibility complex (MHC).
Depletion of Vα14+ NKT cells by anti-Vα14 T cell receptor (TCR) antibody (Ab).
Deficiency of CD1d-restricted NKT cells.
Deficiency of Vα14+Jα18+ NKT cells.
A major factor for these differences may be the mouse strains that have different susceptibilities to TMEV infection and/or different immunological functions (Sellers et al., 2012). C57BL/6 and BALB/c mice are resistant to TMEV-IDD, while SJL/J mice are susceptible to TMEV-IDD. The cytokine profiles of C57BL/6 and SJL/J mice have been shown to be biased toward T helper (Th) 1 cytokines, including IFN-γ, while that of BALB/c mice is biased toward Th2 cytokines, such as IL-4 and IL-10. C57BL/6 mice have been shown to express high levels of NKl.l molecules, while BALB/c and SJL/J mice have lower levels or are deficient of NK1.1 expression (Giorda et al., 1992, Kaminsky et al., 1983, Singh et al., 2005). Since these factors can affect diverse immune-mediated diseases, this may explain the different susceptibilities to CNS and cardiac diseases induced by TMEV.
Another factor that could contribute to the different disease susceptibilities among the three mouse strains is NKT cell subtypes. CD1d-restricted NKT cells have been divided into two subtypes. The classical type of NKT cells, known as type I NKT or invariant NKT cells, express an invariant TCR and recognize glycolipid antigens, such as α-galactosylceramide, while type II NKT cells express a variant TCR and recognize some hydrophobic antigens (Adams and Luoma, 2012, Berzofsky and Terabe, 2008, Godfrey, Stankovic, 2010). Jα18 KO C57BL/6 and anti-Vα14 TCR antibody-treated SJL/J mice are deficient and depleted of type I NKT cells, respectively, while CD1d KO BALB/c mice are deficient in both type I and II NKT cells. Since Jα18 KO mice have been shown to have different susceptibilities to some microbial infections from CD1d KO mice (Huber et al., 2003, Smiley et al., 2005), these different approaches of NKT cell depletion could result in different effects on the two subtypes of CD1d-restricted NKT cells in microbial infections, including viral infections. Thus, although type II NKT cells are a smaller population (Jahng et al., 2004), the presence or absence of type II NKT cells may alter the susceptibilities to TMEV-IDD among the three mouse strains.
Cardioviruses have been known to cause myocarditis in mice and other animals. Although TMEV is a member of the genus Cardiovirus and has been reported to infect the heart in some mouse strains (Gómez, Rinehart, 1996), there have been a few reports investigating the pathomechanism of TMEV-induced myocarditis (Rames, 1995). In this study, we showed different susceptibilities to TMEV-induced myocarditis between wild-type C57BL/6 and Jα18 KO mice. In the heart, 4 days p.i., Jα18 KO mice had higher viral replication compared with wild-type C57BL/6 mice. This could result in more severe cardiomyocyte damage by direct viral infection (viral pathology) at this phase of TMEV infection, corresponding to phase I of viral myocarditis in humans (Martinez, Sato, 2012, Omura et al., 2014). In phase II (1 week p.i.), we found more severe inflammation in the hearts of Jα18 KO mice compared with wild-type C57BL/6 mice. This suggests that severe viral pathology in phase I may lead to severe immunopathology in phase II by recruiting a larger amount of immune cells to the hearts in Jα18 KO mice. Then, the greater amount of cardiomyocyte damage in phases I and II could result in larger fibrotic changes in phase III (1 month p.i.). Despite substantial viral replication and inflammation, all mice in both strains survived and showed no obvious clinical signs of heart failure, such as edema and reduced cardiac function. This suggests that the TMEV-induced myocarditis model may mimic asymptomatic myocarditis, which has been reported in humans as a major cause of sudden death (Blauwet and Cooper, 2010, Eckart et al., 2004, Liu and Baughman, 2011).
In our model, the higher initial viral load in phase I was associated with more severe inflammation in phase II, although the levels of viral replication and inflammation have sometimes been correlated negatively in other model systems (Tsunoda, Kurtz, 1997, Tsunoda, Libbey, 2007). Our findings are similar to what has been reported in another myocarditis model induced by CVB3, where high viral replication was associated with high levels of cardiac inflammation (Fairweather, Stafford, 2012, Wang et al., 2014, Yajima, 2011). On the other hand, Liu et al. reported that, during infections with a nonmyocarditic CVB3 variant, Jα18 KO mice developed severe myocarditis, while wild-type C57BL/6 mice developed minimal inflammation in the heart, although both mouse strains had similar viral titers in the heart (Liu et al., 2013). It is intriguing that Jα18 KO mice developed more severe myocarditis in both TMEV and CVB3 models, while the levels of viral replication increased only in the TMEV model, but not in the CVB3 model. This discrepancy between the two models could be due to distinct roles of other cell types, such as regulatory T cells and γδ T cells (Howe et al., 2007, Martinez, Karlsson, 2014a, Olson et al., 2009, Liu et al., 2013).
During the acute phase of TMEV (DA virus) infection, Jα18 KO mice had lower levels of TMEV-specific lymphoproliferation (cellular immunity) and lower levels of viral RNA in the CNS, while Jα18 KO mice had higher levels of anti-TMEV antibody production (humoral immunity) and higher levels of viral RNA in the heart, compared with wild-type C57BL/6 mice. In theory, a higher level of viral replication would induce a greater antiviral cellular immune response to eradicate the virus. Thus, the lower lymphoproliferation in Jα18 KO mice could be due to the smaller viral load. Alternatively, anti-viral cellular and humoral immune responses could contribute to viral clearance in the heart and CNS, respectively. In the general organs, including the heart, expression of major histocompatibility complex (MHC) molecules is necessary for cellular immune responses, since anti-viral T cells recognize viral antigen/MHC complexes on infected cells, leading to viral clearance. For example, in the CVB3 myocarditis model, cellular immune responses mediated by T cells mainly contribute to viral clearance (Whitton and Feuer, 2004). On the other hand, neurons, which are predominantly infected with TMEV in the CNS during the acute phase, have been shown to express no or lower levels of MHC (Joly et al., 1991). Here, for example, in Sindbis virus infection, passive transfer of anti-viral T cells into severe combined immunodeficiency (SCID) mice, which have a lack of functional T and B cells, had no effect on viral replication in neurons, while passive administration of anti-viral antibodies into SCID mice resulted in viral clearance from the CNS (Levine et al., 1991). This can be explained by anti-viral antibodies in the CNS, which contribute to (1) suppression of viral spread by neutralizing extracellular viruses released from infected cells as well as (2) intracellular viral clearance, since antibodies can be transported to the cytoplasm of neurons (Greenlee et al., 2010). In TMEV infection, passive administration of anti-viral neutralizing antibodies after TMEV infection significantly increased the survival of infected nude mice, which cannot produce anti-viral immune responses (Fujinami et al., 1989). This suggests that anti-viral antibody could help TMEV clearance even in virus-infected neurons in the CNS. On the other hand, anti-TMEV antibody has been shown to cross-react with host antigens, exacerbating immunopathology in the CNS (Yamada et al., 1990). Thus, high anti-TMEV antibody titers in Jα18 KO mice may also contribute to immunopathology in the heart as reported in CVB3-induced myocarditis.
In phase II of human viral myocarditis, pro-inflammatory Th1 cytokines, including IFN-γ, have been reported to contribute to immune-mediated tissue damage (immunopathology) (Yuan et al., 2010). Furthermore, we have demonstrated that Thl immune responses may play a pathogenic role in a TMEV-induced myocarditis model, using another susceptible mouse strain, C3H mice (Omura et al., 2014). In our current study, in phase II of TMEV-induced myocarditis, Jα18 KO mice had about 6-times higher levels of Th1 immune responses compared with wild-type C57BL/6 mice [Th1 (IFN-γ)/Th2 (IL-4) ratio 1 week p.i.: wild-type C57BL/6, 1.3; Jα18 KO mice, 7.6]. In contrast, at the same time point, wild-type C57BL/6 mice produced significantly higher anti-inflammatory IL-4 and IL-10 (Th2 cytokines) compared with Jα18 KO mice. Consistent with the pro-inflammatory Th1 cytokine profile of Jα18 KO mice, the incidence of mice with high intensity lesions in echocardiography was higher in Jα18 KO mice compared with wild-type C57BL/6 mice. NKT cells have been shown to be a major source of anti-inflammatory Th2 cytokines (Matsuda et al., 2008), which can suppress Th1 cell activation as well as Th1 cell differentiation by regulating functions of antigen-presenting cells (O'Garra, 1998). Thus, NKT cells may play a protective role in TMEV-induced myocarditis by regulating the Th1/Th2 immune balance due to production of anti-inflammatory Th2 cytokines. These findings in our TMEV myocarditis model may be a more clinically relevant myocarditis model compared with other models, such as the CVB3 myocarditis model, where IFN-γ and IL-4 have been shown to be protective and detrimental, respectively. Thus, our TMEV myocarditis model could provide relevant information of human viral myocarditis.
In summary, following TMEV (DA virus) infection, Jα18 KO mice remained as resistant to TMEV-IDD as wild-type C57BL/6 mice, while Jα18 KO mice developed more severe myocarditis compared with wild-type C57BL/6 mice. In TMEV-induced myocarditis, Jα18 KO mice had higher levels of viral RNA in phase I, more active inflammation in phase II, and larger fibrotic areas in phase III, compared with wild-type C57BL/6 mice. Using echocardiography, we also found a higher incidence of myocarditis in Jα18 KO mice compared with wild-type C57BL/6 mice. Thus, using NKT cell-depleted or NKT KO mice, we found that NKT cells could play a different role depending on the mouse strain or organ.
Supplementary Material
Highlights.
We found contrasting roles of NKT cells in the CNS and heart
Neurovirulence of the CNS of Theiler’s virus differed among NKT-deficient mouse strains
Changes in B-mode of echocardiography in viral myocarditis
Theiler’s virus induced fibrosis in the heart
NKT cells were associated with myocarditis susceptibility
Acknowledgments
This work was supported by fellowships (549-40-6173, F. Sato and 549-40-6172, S. Omura) from the Malcolm Feist Cardiovascular Research Endowment, Louisiana State University Health Sciences Center-Shreveport, and grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH, R21NS059724), the National Center for Research Resources of the NIH (5P20RR018724-10), and the National Institute of General Medical Sciences COBRE Grant (8P20GM103433-10, P30-GM110703). We thank Drs. Maureen N. Ajuebor, J. Steven Alexander, Fereidoon Shafiei, Liam A. Morris, and Madan M. Acharya for their many helpful discussions and Sadie Faith Pearson, Lesya Ekshyyan, Elaine A. Cliburn, and Christi L. Eugene for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams EJ, Luoma AM. The yin and yang of CD1d recognition. Nat Immunol. 2012;13:814–815. doi: 10.1038/ni.2401. [DOI] [PubMed] [Google Scholar]
- Archard LC, Richardson PJ, Olsen EGJ, Dubowitz V, Sewry C, Bowles NE. The role of Coxsackie B viruses in the pathogenesis of myocarditis, dilated cardiomyopathy and inflammatory muscle disease. Biochem Soc Symp. 1987;53:51–62. [PubMed] [Google Scholar]
- Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss H-P, McCarthy R, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- Burleson FG, Chambers TM, Wiedbrauk DL. Introduction to quantal virus assay. In: Burgleson FG, Chambers TM, Wiedbrauk DL, editors. Virology: a laboratory manual. San Diego, CA: Academic Press, Inc; 1992. pp. 53–57. [Google Scholar]
- Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Daniels JB, Pappenheimer AM, Richardson S. Observations on encephalomyelitis of mice (DA strain) J Exp Med. 1952;96:517–530. doi: 10.1084/jem.96.6.517. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb C, Lafrance-Corey RG, Zoecklein L, Papke L, Rodriguez M, Howe CL. Demyelinated axons and motor function are protected by genetic deletion of perforin in a mouse model of multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:1037–1048. doi: 10.1097/NEN.0b013e3181b5417e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana J, Lehuen A. NKT cells: Friend or foe during viral infections? Eur J Immunol. 2009;39:3283–3291. doi: 10.1002/eji.200939800. [DOI] [PubMed] [Google Scholar]
- Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110–112. doi: 10.5694/j.1326-5377.2004.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92:316–320. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Stafford KA, Sung YK. Update on coxsackievirus B3 myocarditis. Curr Opin Rheumatol. 2012;24:401–407. doi: 10.1097/BOR.0b013e328353372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- Fernando V, Omura S, Sato F, Kawai E, Martinez NE, Elliott SF, et al. Regulation of an autoimmune model for multiple sclerosis in Th2-biased GATA3 transgenic mice. Int J Mol Sci. 2014;15:1700–1718. doi: 10.3390/ijms15021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami RS, Rosenthal A, Lampert PW, Zurbriggen A, Yamada M. Survival of athymic (nu/nu) mice after Theiler's murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J Virol. 1989;63:2081–2087. doi: 10.1128/jvi.63.5.2081-2087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorda R, Weisberg EP, Ip TK, Trucco M. Genomic structure and strain-specific expression of the natural killer cell receptor NKR-P1. J Immunol. 1992;149:1957–1963. [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Gomez RM, Rinehart JE, Wollmann R, Roos RP. Theiler's murine encephalomyelitis virus-induced cardiac and skeletal muscle disease. J Virol. 1996;70:8926–8933. doi: 10.1128/jvi.70.12.8926-8933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee JE, Clawson SA, Hill KE, Wood BL, Tsunoda I, Carlson NG. Purkinje cell death after uptake of anti-Yo antibodies in cerebellar slice cultures. J Neuropathol Exp Neurol. 2010;69:997–1007. doi: 10.1097/NEN.0b013e3181f0c82b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Adelson JD, Rodriguez M. Absence of perforin expression confers axonal protection despite demyelination. Neurobiol Dis. 2007;25:354–359. doi: 10.1016/j.nbd.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Gauntt CJ, Sakkinen P. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res. 1998;51:35–80. doi: 10.1016/s0065-3527(08)60783-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- Inoue A, Koh C-S, Yahikozawa H, Yanagisawa N, Yagita H, Ishihara Y, et al. The level of tumor necrosis factor-α producing cells in the spinal cord correlates with the degree of Theiler's murine encephalomyelitis virus-induced demyelinating disease. Int Immunol. 1996;8:1001–1008. doi: 10.1093/intimm/8.7.1001. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ito T, Ishibashi K, Imai K, Koseki H, Ra CS, Fernandez E, et al. Monoclonal antibody against murine T cell receptor Vα14 cross-reacts with human CD3ε and detects disulfide-linked dimeric form. Int Immunol. 1991;3:991–995. doi: 10.1093/intimm/3.10.991. [DOI] [PubMed] [Google Scholar]
- Jahng A, Maricic I, Aguilera C, Cardell S, Haider RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarousse N, Syan S, Martinat C, Brahic M. The neurovirulence of the DA and GDVII strains of Theiler's virus correlates with their ability To infect cultured neurons. J Virol. 1998;72:7213–7220. doi: 10.1128/jvi.72.9.7213-7220.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E, Mucke L, Oldstone MBA. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Kaminsky SG, Nakamura I, Cudkowicz G. Selective defect of natural killer and killer cell activity against lymphomas in SJL mice: low responsiveness to interferon inducers. J Immunol. 1983;130:1980–1984. [PubMed] [Google Scholar]
- Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with "idiopathic" left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Liu P, Baughman KL. Myocarditis. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's Heart Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2011. pp. 1595–1610. [Google Scholar]
- Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- Liu W, Moussawi M, Roberts B, Boyson JE, Huber SA. Cross-regulation of T regulatory-cell response after coxsackievirus B3 infection by NKT and γδ T cells in the mouse. Am J Pathol. 2013;183:441–449. doi: 10.1016/j.ajpath.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NE, Karlsson F, Sato F, Kawai E, Omura S, Minagar A, et al. Protective and detrimental roles for regulatory T cells in a viral model for multiple sclerosis. Brain Pathol. 2014;24:436–451. doi: 10.1111/bpa.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NE, Sato F, Kawai E, Omura S, Chervenak RP, Tsunoda I. Regulatory T cells and Th17 cells in viral infections: implications for multiple sclerosis and myocarditis. Future Virol. 2012;7:593–608. doi: 10.2217/fvl.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NE, Sato F, Kawai E, Omura S, Takahashi S, Yoh K, et al. Th17-biased RORyt transgenic mice become susceptible to a viral model for multiple sclerosis. Brain Behav Immun. 2014b doi: 10.1016/j.bbi.2014.07.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NE, Sato F, Omura S, Minagar A, Alexander JS, Tsunoda I. Immunopathological patterns from EAE and Theiler's virus infection: Is multiple sclerosis a homogenous 1-stage or heterogenous 2-stage disease? Pathophysiology. 2013;20:71–84. doi: 10.1016/j.pathophys.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the mmune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Young CR, Storts RW, Steelman AJ, Meagher MW, Welsh CJR. Restraint stress facilitates systemic dissemination of Theiler's virus and alters its pathogenecity. Microb Pathog. 2006;41:133–143. doi: 10.1016/j.micpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- Olson CM, Jr, Bates TC, Izadi H, Radolf JD, Huber SA, Boyson JE, et al. Local production of IFN-γ by invariant NKT cells modulates acute Lyme carditis. J Immunol. 2009;182:3728–3734. doi: 10.4049/jimmunol.0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S, Kawai E, Sato F, Martinez NE, Chaitanya GV, Rollyson PA, et al. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ Cardiovasc Genet. 2014;7:444–454. doi: 10.1161/CIRCGENETICS.114.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rames DS. The etiopathogenesis of Theiler's murine encephalomyelitis virus (TMEV)-induced cardiomyopathy, including characterization of new strains of TMEV [Thesis/dissertation, Manuscript] College Station: Texas A & M University; 1995. [Google Scholar]
- Renneson J, Guabiraba R, Maillet I, Marques RE, Ivanov S, Fontaine J, et al. A detrimental role for invariant natural killer T cells in the pathogenesis of experimental dengue virus infection. Am J Pathol. 2011;179:1872–1883. doi: 10.1016/j.ajpath.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Martinez NE, Shahid M, Rose JW, Carlson NG, Tsunoda I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am J Pathol. 2013;183:1390–1396. doi: 10.1016/j.ajpath.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Omura S, Martinez NE, Tsunoda I. Animal models of multiple sclerosis. In: Minagar A, editor. Neuroinflammation. London, UK: Elsevier; 2011a. pp. 55–79. [Google Scholar]
- Sato F, Tanaka H, Hasanovic F, Tsunoda I. Theiler's virus infection: Pathophysiology of demyelination and neurodegeneration. Pathophysiology. 20l1b;18:31–41. doi: 10.1016/j.pathophys.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012;49:32–43. doi: 10.1177/0300985811429314. [DOI] [PubMed] [Google Scholar]
- Singh AK, Yang J-Q, Parekh VV, Wei J, Wang C-R, Joyce S, et al. The natural killer T cell ligand α-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur J Immunol. 2005;35:1143–1154. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [PubMed] [DOI] [PubMed] [Google Scholar]
- Smiley ST, Lanthier PA, Couper KN, Szaba FM, Boyson JE, Chen W, et al. Exacerbated susceptibility to infection-stimulated immunopathology in CD1d-deficient mice. J. Immunol. 2005;174:7904–7911. doi: 10.4049/jimmunol.174.12.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri K, Imanaka-Yoshida K, Matsubara A, Tsujimura Y, Hiroe M, Naka T, et al. Suppressor of cytokine signaling 1 DNA administration inhibits inflammatory and pathogenic responses in autoimmune myocarditis. J Immunol. 2012;189:2043–2053. doi: 10.4049/jimmunol.1103610. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler's murine encephalomyelitis virus. Acta Neuropathol. 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kurtz CIB, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Libbey JE, Fujinami RS. TGF-P1 suppresses T cell infiltration and VP2 puff B mutation enhances apoptosis in acute polioencephalitis induced by Theiler's virus. J Neuroimmunol. 2007;190:80–89. doi: 10.1016/j.jneuroim.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Fujinami RS. Regulatory role of CD1d in neurotropic virus infection. J Virol. 2008;82:10279–10289. doi: 10.1128/JVI.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Taniguchi M, Fujinami RS. Contrasting roles for Val4+ natural killer T cells in a viral model for multiple sclerosis. JNeurovirol. 2009;15:90–98. doi: 10.1080/13550280802400684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2013;34:50–58. doi: 10.1016/j.it.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li M, Xie Y, Yu Y, Liu G, Yu Y, et al. The frequency of invariant natural killer T cells correlates with the severity of myocarditis. Viral Immunol. 2014;27:88–95. doi: 10.1089/vim.2013.0078. [DOI] [PubMed] [Google Scholar]
- Whitton JL, Feuer R. Myocarditis, microbes and autoimmunity. Autoimmunity. 2004;37:375–386. doi: 10.1080/08916930410001713089. [DOI] [PubMed] [Google Scholar]
- Wu CY, Feng Y, Qian GC, Wu JH, Luo J, Wang Y, et al. α-Galactosylceramide protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Clin Exp Immunol. 2010;162:178–187. doi: 10.1111/j.1365-2249.2010.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T. Viral myocarditis: potential defense mechanisms within the cardiomyocyte against virus infection. Future Microbiol. 2011;6:551–566. doi: 10.2217/fmb.11.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Knowlton KU. Viral myocarditis: from the perspective of the virus. Circulation. 2009;119:2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- Yamada M, Zurbriggen A, Fujinami RS. Monoclonal antibody to Theiler's murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J Exp Med. 1990;171:1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yu M, Lin Q-W, Cao A-L, Yu X, Dong J-H, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185:4004–4010. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.