Abstract
The SLC4A11 gene mutations cause a variety of genetic corneal diseases, including congenital hereditary endothelial dystrophy 2 (CHED2), Harboyan syndrome, some cases of Fuchs' endothelial dystrophy (FECD), and possibly familial keratoconus. Three NH2-terminal variants of the human SLC4A11 gene, named SLC4A11-A, -B, and -C are known. The SLC4A11-B variant has been the focus of previous studies. Both the expression of the SLC4A11-C variant in the cornea and its functional properties have not been characterized, and therefore its potential pathophysiological role in corneal diseases remains to be explored. In the present study, we demonstrate that SLC4A11-C is the predominant SLC4A11 variant expressed in human corneal endothelial mRNA and that the transporter functions as an electrogenic H+(OH−) permeation pathway. Disulfonic stilbenes, including 4,4′-diisothiocyano-2,2′-stilbenedisulfonate (DIDS), 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS), and 4-acetamido-4′-isothiocyanato-stilbene-2,2′-disulfonate (SITS), which are known to bind covalently, increased SLC4A11-C-mediated H+(OH−) flux by 150–200% without having a significant effect in mock-transfected cells. Noncovalently interacting 4,4′-diaminostilbene-2,2′-disulfonate (DADS) was without effect. We tested the efficacy of DIDS on the functionally impaired R109H mutant (SLC4A11-C numbering) that causes CHED2. DIDS (1 mM) increased H+(OH−) flux through the mutant transporter by ∼40–90%. These studies provide a basis for future testing of more specific chemically modified dilsulfonic stilbenes as potential therapeutic agents to improve the functional impairment of specific SLC4A11 mutant transporters.
Keywords: proton, transport, CHED2, cornea, endothelial cell, DIDS
slc4a11 belongs to the slc4 family of HCO3−/CO32− transport proteins based on its amino acid sequence homology with other members (26, 43). There are three known NH2-terminal variants of the human SLC4A11 gene: SLC4A11-A, 918 amino acids (NP_001167561.1); SLC4A11-B, 891 (NP_114423.1); and SLC4A11-C, 875 (NP_001167560.1). SLC4A11-B was the first NH2-terminal variant cloned (originally called BTR1) (44) and was subsequently reported to function as an electrogenic Na(n)+-B(OH−)4− cotransporter (n ≥ 2) and accordingly renamed NaBC1 (43). In the absence of BO4− the transporter functioned as a cation (H+ or Na+) permeation pathway (43). More recent studies have suggested that SLC4A11-B does not transport borate or other ions but rather mediates water flux (59). Still other investigators have reported that SLC4A11-B functions as an 5-(N-ethyl-N-isopropyl)amiloride (EIPA)-inhibitable Na+-OH− cotransporter (or equivalently an Na+/H+ exchanger) (23, 40). The SLC4A11 transporter has been recently reported to be localized to the basolateral membrane of corneal endothelial cells (59).
Mutations in the SLC4A11 gene cause autosomal recessive congenital hereditary endothelial dystrophy 2 (CHED2) and Harboyan syndrome (CHED2 with progressive sensorineural deafness) (12, 52, 62). Some cases of late-onset autosomal dominant Fuchs' endothelial dystrophy (FECD) and possibly familial keratoconus are also associated with mutations in the transporter (37, 60, 63). Patients with CHED2 and Harboyan syndrome have corneal abnormalities present at birth or soon thereafter that are typically nonprogressive (2). Phenotypically the cornea is edematous with variable degrees of clouding. Corneal endothelial cell density varies from normal to decreased, and the cells are irregularly shaped with loss of their typical hexagonal pattern (29). Ultrastructurally, the cells can be multinucleated and contain dilated mitochondria (24). Other nonspecific changes involve the stroma (thickening, disorganized lamellae) and corneal epithelium (increased number of layers, edema of the basal epithelium) that are thought to be secondary phenomena due to loss of endothelial cell active transport (13). Certain patients with autosomal dominant FECD also have mutations in SLC4A11 (63). Histologically, endothelial cells are flattened and in various degrees of degeneration. The propensity of mutant SLC4A11 oligomers to be retained intracellularly may play a role in determining the age of onset and the inheritance pattern in CHED2 and FECD patients (60).
In the present study, to further address the role of the SLC4A11 gene in corneal health and disease, we first determined which of the three SLC4A11 gene transcripts is expressed in human corneal endothelial cells. Our results show unequivocally that the SLC4A11-C transcript is specifically expressed in these cells. Given that the functional properties of the SLC4A11-C variant had not previously been investigated, we characterized its activity and showed that it mediates the flux of H+(OH−). Disulfonic stilbenes, which are known to inhibit the activity of other SLC4 transporters, surprisingly significantly increased H+(OH−) permeation through SLC4A11-C. Importantly, the flux of H+(OH−) by the poorly functioning SLC4A11-C-R109H mutant (analogous to the previously reported SLC4A11-B-R125H mutant; see Ref. 22) was significantly improved by DIDS. Our data raise the possibility that future therapeutic approaches using disulfonic stilbenes that have been modified to improve their specificity may be a fruitful approach to treat patients with certain disease causing SLC4A11 mutations.
MATERIALS AND METHODS
Materials.
Site-directed mutagenesis kits were from Stratagene (La Jolla, CA); 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-AM), H2DIDS and SITS were from Invitrogen Life Technologies (Grand Island, NY); DIDS was from Santa Cruz Biotechnology (Dallas TX); all salts and buffers, valinomycin, gramicidin, DADS, EIPA, and NS-8593 were from Sigma (St. Louis, MO); and 4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide (BCTC) was from Cayman Chemical (Ann Arbor, MI). The stilbene disulfonates were stored as a powder and kept from light, and stock solutions were prepared freshly for each experiment. The stocks were never freeze-thawed, and dilutions from the stocks were made for each experiment.
SLC4A11-A, -B, and -C constructs.
SLC4A11-A was isolated from brain total RNA (Clontech, Hampshire, UK). SLC4A11-B was isolated from human kidney Marathon Ready cDNA (Clontech). SLC4A11-C was isolated from human corneal endothelial cell total RNA (a kind gift from M. I. Rosenblatt, Department of Ophthalmology, Weill Cornell Medical College). cDNA from brain and corneal endothelial RNA was generated using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen Life Technologies). Each of the three NH2-terminal variants was cloned into the pTT mammalian expression vector using XbaI (5′) and HindIII (3′) restriction enzymes (New England BioLabs). The following primers sets were used for each construct: 1) SLC4A11-A: sense 5′-ACTCTAGAATGGGCGTTTATGGCCCCCAGGAC-3′, antisense 5′-ATAAGCTTTCAAGGCCTGTGCTCAGCGTCCATGACAT-3′; 2) SLC4A11-B: sense 5′-ACTCTAGAATGAGCCAGGTCGGGGGGCGGGGAGACA-3′, antisense 5′-ATAAGCTTTCAAGGCCTGTGCTCAGCGTCCATGACAT-3′; and 3) SLC4A11-C: sense 5′-ACTCTAGAATGGCCGCGGCCACCAGGCGCGTGT-3′, antisense 5′-ATAAGCTTTCAAGGCCTGTGCTCAGCGTCCATGACAT-3′.
An SLC4A11-C construct with an inactivating R109H mutation was generated using the QuikChange Lighting Site-Directed Mutagenesis Kit (Agilent Technologies). All sequences were validated using the University of California Los Angeles genotyping and sequencing core using the BigDye terminator kit version 3.1 (Invitrogen Life Technologies) and resolved with a 3730 XL ABI sequencer (Applied Biosystems, Life Technologies).
Detection of SLC4A11 transcripts in human endothelial mRNA.
The expression of each NH2-terminal SLC4A11 variant in human corneal endothelial mRNA was determined by PCR using sense primers against the specific NH2-terminal region of each variant as follows: SLC4A11-A (predicted size 929 bp): sense 5′-ATGGGCGTTTATGGCCCCCAGGAC-3′, antisense 5′-TGGCGGAAGGCGATATCCGAGAACA-3′; SLC4A11-B (predicted size 848 bp): sense 5′-ATGAGCCAGGTCGGGGGGCGGGGAGACA-3′, antisense 5′-TGGCGGAAGGCGATATCCGAGAACA-3′; and SLC4A11-C (predicted size 800 bp): sense 5′-ATGGCCGCGGCCACCAGGCGCGTGT-3′, antisense 5′-TGGCGGAAGGCGATATCCGAGAACA-3′.
The primers were first tested by PCR amplifying the NH2-terminal region of each of the full-length SLC4A11 clones. To determine the expression of NH2-terminal SLC4A11 variants in corneal endothelial mRNA, cDNA was made using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen Life Technologies).
Transient expression in HEK 293 cells.
The constructs were transiently expressed in human embryonic kidney 293 (HEK 293) cells using Lipofectamine 2000 (Invitrogen Life Technologies). HEK 293 cells were plated on 60-mm dishes (Corning, Tewksbury MA) in 4 ml of Dulbecco's modified Eagle's medium that was supplemented with fetal bovine serum (10%), l-glutamine (200 mg/l), and penicillin-streptomycin. After seeding (24 h), the cells were transfected with Lipofectamine 2000 following the manufacturer's protocol with the modification that the transfection solution was removed after a 2-h exposure. The cells were grown at 37°C in a 5% CO2 atmosphere and harvested 24 h posttransfection for immunoblot analysis.
Sulfo-NHS-SS-biotin surface labeling.
Whole cell labeling with sulfo-NHS-SS-biotin was performed using the manufacturer's protocol (Pierce, Rockford, IL). In brief, transfected HEK 293 cells (60-mm plates) were washed three times with ice-cold PBS (pH 8.0) and incubated with 1.1 mM sulfo-NHS-SS-biotin in 2 ml of PBS (pH 8.0) for 30 min at 4°C. The reaction was stopped by adding 2 ml of 50 mM Tris buffer (containing 140 mM NaCl, pH 8.0) and incubated at 4°C for a further 10 min. The cells were then collected, washed three times with PBS (pH 8.0), and lysed in 500 μl of IPB buffer [5 mM EDTA, 150 mM NaCl, 1% (vol/vol) Igepal, 0.5% (wt/vol) sodium deoxycholate, 10 mM Tris·HCl, pH 7.5] containing protease inhibitors (Roche) on ice for 10 min. After a 10-min centrifugation (20,000 g at 4°C), the supernatant was collected and incubated for 4 h at 4°C with 50 μl of streptavidin-agarose resin on a rotating shaker. The resin was collected by brief centrifugation at 6,000 g and washed three times with IPB buffer. The bound proteins were eluted with 2× SDS sample buffer containing 2% β-mercaptoethanol heated at 60°C for 5 min.
SDS-PAGE and immunoblotting.
The protein samples were resolved on 7.5% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The biotinylated protein expression levels were assessed by probing the blot with a previously characterized rabbit polyclonal antihuman SLC4A11 COOH-terminal polyclonal antibody (32) at 1:3,000 dilution in a buffer containing Tween 20 [0.1% (vol/vol); 137 mM NaCl, 20 mM Tris, pH 7.5, containing 5% (wt/vol) nonfat milk]. For GAPDH detection, the protein samples were resolved on 4–20% polyacrylamide gels, and the GAPDH (A-3) antibody (Santa Cruz Biotechnology) was used at 1:5,000 dilution.
Immunocytochemistry.
The cells were grown on 35-mm glass bottom dishes (MatTek, Ashland, MA). Posttransfection with Lipofectamine 2000 (24 h), the dishes were rinsed with PBS (140 mM NaCl, 3 mM KCl, 6.5 mM Na2HPO4, and 1.5 mM KH2PO4, pH 7.4) and permeabilized with 1 ml of methanol for 2 min before immunostaining. Following a PBS wash, the cells were incubated with the SLC4A11 antibody (1:100 dilution in PBS) (32) for 30 min at room temperature. After being rinsed in PBS, the cells were further incubated for 30 min at room temperature with the Alexa Fluor 594 goat anti-rabbit secondary antibody (1:500 in PBS; Invitrogen Life Technologies). The cells were then rinsed three times with PBS, and images were acquired with a PXL charge-coupled device camera (model CH1; Photometrics, Tucson, AZ) coupled to a Nikon Microphot-FXA epifluorescence microscope (Melville, NY).
Measurement of intracellular pH.
To monitor intracellular pH (pHi), HEK 293 cells grown on coated cover slips were transfected using Lipofectamine 2000 and studied 24 h posttransfection. Typically ∼80% of cells were transfected in each experiment judged by anti-SLC4A11 staining. The cells were loaded (20 min) in a custom-designed chamber with the esterified fluorescent pHi probe BCECF-AM, and pHi was measured using a previously described microscope-fluorometer (25). The bathing solution (150 μl) was continuously perfused over the cell monolayer at 2 ml/min at 37°C. The data from ∼200 cells were averaged in each experiment. The intracellular fluorescence data were calibrated at the end of each experiment with nigericin (26 μM) and valinomycin (5 μM) present in all calibration solutions to equilibrate intracellular and extracellular K+ and pH (50). The ion composition of the BCECF-AM loading solution varied depending on the experimental protocol used. To calculate the flux of H+, pHi values were converted to Hin+. Intrinsic cell buffer capacity (βi), calculated as Δ[NH4+]/Δ[Hin+], was measured in HEPES buffer using NH4+ addition/removal experiments over a range of intracellular H+ (Hin+) values. The rate of change of [Hin+] or d[Hin+]/dt was calculated in the initial 10–15 s following a solution change. The flux of H+ in units of micromolar per second was calculated as βi × [dHin+/dt]. In all experiments the solutions contained EIPA (30 μM) to block endogenous Na+/H+ exchange unless otherwise stated. Because the transport of H+ and OH− are indistinguishable, we refer to the flux as H+(OH−) flux.
State-state pHi.
Steady-state pHi was measured in a physiological HCO3−-buffered solution, pH 7.4 (in mM: 115 NaCl, 2.5 K2HPO4, 1 CaCl2, 1 MgCl2, 5 dextrose, and 25 Na HCO3/5% CO2). In separate studies, pHi was measured in a HCO3−-free, 5 mM HEPES-buffered solution. The experiments were done in the presence and absence of 30 µM EIPA.
Plasma membrane hyperpolarization.
The cells were bathed in 110 mM KCl, 30 mM tetramethylammonium chloride (TMACl), 5 mM HEPES, and 30 μM EIPA, pH 7.4. Following an equilibration period of ∼25 min, the effect of hyperpolarizing the plasma membrane voltage on pHi was assessed by acutely changing the decreasing external K+ concentration to 5 mM KCl with 135 mM TMACl, 5 mM HEPES, and 30 μM EIPA, pH 7.4, in the presence or absence of the K+ ionophore valinomycin (5 μM). Additional plasma membrane-hyperpolarizing experiments were performed in cells initially bathed in 140 NaCl, 5 mM HEPES, and 30 μM EIPA, pH 7.4. Following an equilibration period of ∼25 min, the bathing solution was switched to a zero Na+ solution containing 140 TMACl, 5 mM HEPES, and 30 μM EIPA, pH 7.4, plus the Na+ ionophore gramicidin (6 μM).
Na+ removal/readdition.
Na+ removal/addition experiments were performed in HEPES-buffered solutions while endogenous Na+/H+ activity was blocked with EIPA (30 μM). The cell membrane was chemically voltage clamped using 110 mM K+-valinomycin (5 μM) in a solution also containing 100 mM NaCl and 5 mM HEPES, pH 7.4. Following an ∼25-min equilibration period, the bathing solution was switched to a Na+-free solution containing 100 mM TMACl with 110 mM KCl, 5 mM HEPES, and 5 μM valinomycin, pH 7.4. The cells were then bathed in the original Na+-containing solution. Additional experiments were performed in 110 mM K+-valinomycn (5 μM) chemically voltage-clamped Cl−-free solutions to block any endogenous Cl−/OH− exchange process(es) from masking potential Na+-driven pHi changes. In the Cl−-free experiments, the cells were preincubated before the pHi measurements for 1 h at 370C in the cell incubator in a zero Cl− solution containing 115 mM sodium gluconate, 2.5 mM K2HPO4, 7 mM calcium gluconate, 2 mM magnesium gluconate, 5 mM dextrose, and 25 mM NaHCO3/5% CO2, pH 7.4. During the experimental period, the cell membrane was chemically voltage clamped using 110 mM K+-valinomycin (5 μM) with a solution containing 110 mM potassium gluconate, 100 mM sodium gluconate, and 5 mM HEPES, pH 7.4. In the Na+ removal period, the solution bathing the cells contained 110 mM potassium gluconate, 100 mM tetramethylammonium hydroxide (TMAOH), 100 mM d-(+)-gluconic acid δ-lactone, 5 mM HEPES, and 5 μM valinomycin, pH 7.4. The cells were then reexposed to the initial Na+-containing solution. In experiments to examine the effect of borate, the cells were bathed in 110 mM KCl, 90 mM NaCl, 10 mM B(OH)3, 10 mM NaOH, 5 mM HEPES, and 5 μM valinomycin, pH 7.4. Following an ∼25-min equilibration period the bathing solution was switched to an Na+-free solution containing 110 mM KCl, 90 mM TMACl, 10 mM B(OH)3, 10 mM TMAOH, 5 mM HEPES, and 5 μM valinomycin, pH 7.4. The cells were then subsequently bathed in the original Na+-containing solution.
H+(OH−) flux following an acute decrease in pHi at constant external pH.
The cells were chemically voltage clamped using 110 M K+-valinomycin (5 μM) and bathed in a solution containing 110 mM KCl, 10 mM NH4Cl, 100 mM NaCl, 5 μM valinomycin, and 30 μM EIPA, pH 7.4.1 pHi was then decreased to ∼6.7 (Hin+-Hout+ gradient ∼160 nM) by switching to an NH4+-free solution containing 110 mM KCl, 100 mM NaCl, 10 mM TMACl, 5 mM HEPES, 5 μM valinomycin, and 30 μM EIPA, pH 7.4, and the magnitude of H+(OH−) flux was quantitated in the initial period of pHi recovery. Similar experiments were also done under 110 mM K+-valinomycin chemically voltage-clamped conditions in the absence of Na+ using the following solutions: 110 mM KCl, 10 mM NH4Cl, 100 mM TMACl, 5 μM valinomycin, and 30 μM EIPA, pH 7.4, followed by NH4+ removal with a solution containing 110 mM KCl, 110 mM TMACl, 5 μM valinomycin, and 30 μM EIPA, pH 7.4. Finally, experiments were done (following 1 h Cl−-free preincubation) in the absence of Na+ and Cl− with 110 mM potassium gluconate, 10 mM NH4OH, 100 mM TMAOH, 110 mM d-(+)-gluconic acid δ-lactone, and 5 mM HEPES, pH 7.4, followed by an NH4+-free solution containing 110 mM potassium gluconate, 110 mM TMAOH, 110 mM d-(+)-gluconic acid δ-lactone, and 5 mM HEPES, pH 7.4.
H+(OH−) flux following an acute decrease in pHi at various plasma membrane potentials.
The cells were equilibrated in 110 mM KCl, 10 mM NH4Cl, 100 mM TMACl, 5 mM HEPES, 5 μM valinomycin, and 30 μM EIPA, pH 7.4. H+(OH−) flux through the wild-type and mutant transporter was then monitored at various plasma membrane potentials in the initial recovery phase following the acute decrease of pHi to ∼6.7. In these experiments NH4+ was removed while simultaneously changing the external solution K+ concentration acutely in the presence of valinomycin to hyperpolarize or depolarize the plasma membrane potential. The NH4+-free solution (NH4Cl substituted with equimolar TMACl) contained 5 mM HEPES, 5 μM valinomycin, and 30 μM EIPA, pH 7.4, with varying KCl concentrations (5–220 mM; KCl substituted with equimolar TMACl). In the previous experiments where the cells were chemically voltage clamped with 110 K+-valinomycin, the use of 100 mM NaCl [or equimolar Na+ or TMA+ (Na+ substitute) salts] necessitated that the solutions were hypertonic. Accordingly, in the following experiments, the flux of H+(OH−) was assessed in cells bathed in 110 mM KCl, 20 mM TMACl, 10 mM NH4Cl, 5 mM HEPES, 5 μM valinomycin, and 30 μM EIPA, pH 7.4. pHi was decreased to ∼6.7 by switching to an NH4+-free solution containing 110 mM KCl, 30 mM TMACl, 5 mM HEPES, 5 μM valinomycin, and 30 μM EIPA, pH 7.4, and H+(OH−) flux was quantitated in the initial period of pHi recovery. The experiments were repeated with NH4+ removed while simultaneously decreasing the external solution K+ concentration to 5 mM K+ (10 mM NH4Cl and 105 mM KCl substituted with equimolar TMACl) in the presence of valinomycin to hyperpolarize the membrane potential, and H+(OH−) flux was measured in the initial period of pHi recovery.
H+(OH−) flux following an acute change in external pH.
The external solution pH was decreased from pH 7.4 to various values (6.2–6.4) and after an equilibration period was returned to 7.4. H+(OH−) flux was measured immediately following the decrease in external pH, and also following the subsequent increase in extracellular pH. The plasma membrane voltage was chemically clamped throughout the experiments in cells bathed in 110 mM KCl, 30 mM TMACl, 5 mM HEPES, 5 μM valinomycin, and 30 μM EIPA. The magnitude of H+(OH−) flux at various H+in-H+out gradient values was also assessed. In separate experiments, the effect of DIDS (1 mM) on H+(OH−) flux following a change in external pH was also measured. In these studies the cells were preincubated in the cell culture incubator for 1 h at 37°C in a solution containing 115 mM NaCl, 2.5 mM K2HPO4, 1 mM CaCl2, 1 mM MgCl2, 5 mM dextrose, and 25 mM NaHCO3/5% CO2, pH 7.4, before initiating the H+(OH−) flux measurements. In addition, the dose dependence of DIDS (1–1,000 μM) stimulation of SLC4A11-C H+(OH−) flux was assessed. In separate experiments using the same 1-h preincubation protocol, e.g., DIDS, H2DIDS, and SITS were not present during the experimental measurements, the effect of other covalently binding disulfonic stilbenes (H2DIDS and SITS) was measured. Finally, the effect of the noncovalently interacting disulfonic stilbene DADS (1 mM) was examined, with the compound present in all solutions throughout the experiments.
Statistical analysis.
Statistical analysis was performed using one-way ANOVA followed by Tukey's test. Results are reported as means ± SE. P < 0.05 was considered significant.
RESULTS
Expression of SLC4A11 transcripts in human corneal endothelium.
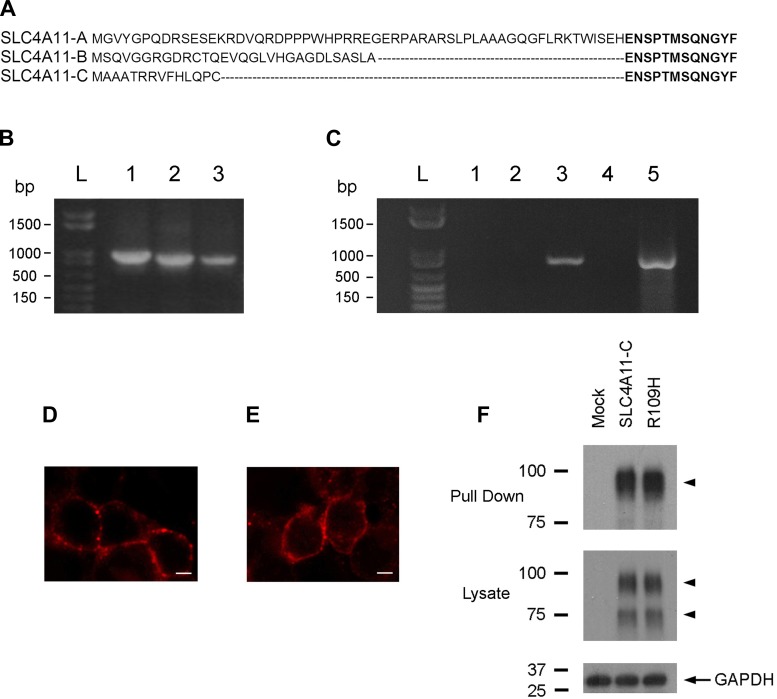
Figure 1A shows the specific NH2-terminal sequence of the three known human SLC4A11 NH2-terminal variants. We first tested the PCR primers for their ability to amplify each variant using the full-length clones as a template (Fig. 1B). We then used the same PCR primers to detect each of the variants in human corneal endothelial cell cDNA, and, as shown in Fig. 1C, only SLC4A11-C could be detected. Our results suggest that, in humans, SLC4A11-C is the major if not the only transcript expressed in the human corneal endothelium.
Fig. 1.
A: amino acid alignment of the NH2-terminal sequences of SLC4A11-A, -B, and -C. B: specific sense PCR primers were used in control experiments to amplify a region containing the unique sequence of each NH2-terminal variant. PCR products were amplified from full-length constructs as templates that had been cloned into pTT mammalian expression vectors. Lane 1, SLC4A11-A; lane 2, SLC4A11-B; and lane 3, SLC4A11-C. C: the same variant-specific PCR primers were then used to determine the expression of SLC4A11-A, -B, and -C using human corneal endothelial cell cDNA as a template. Lane 1, SLC4A11-A; lane 2, SLC4A11-B; lane 3, SLC4A11-C; lane 4, no RT (control); and lane 5, GAPDH primers. D–F: membrane-processing analysis of wild-type SLC4A11-C and R109H mutant proteins. Representative fluorescence images of SLC4A11-C (D) and the R109H mutant (E) expressed in human embryonic kidney (HEK) 293 cells showing a plasma membrane staining pattern. Scale bar: 5 μm. F: plasma membrane proteins were labeled using sulfo-NHS-SS-biotin and pulled down using streptavidin-agarose resin. Immunoblots of pulled-down proteins and whole cell lysates are shown. The lanes represent HEK 293 cells that were transfected with an empty pTT vector used for mock transfection, SLC4A11-C, or the R109H mutant and labeled after 24 h expression. Protein samples were resolved on SDS-PAGE, and the blots were probed with antibodies against the SLC4A11 COOH-terminus (pull-down and whole cell lysates; arrowheads, SLC4A11-C) and GAPDH (whole cell lysates).
HEK 293 cell expression of SLC411-C and the R109H mutant.
Immunocytochemistry studies using a previously characterized rabbit polyclonal antibody to the SLC4A11 COOH-terminus (32) showed that wild-type and mutant proteins, when transiently expressed in HEK 293 cells, had a plasma membrane staining pattern (Fig. 1, D and E). As shown in the representative immunoblot depicted in Fig. 1F of biotinylated plasma membrane proteins probed with the same antibody, SLC4A11-C and the R109H mutant were similarly expressed on the plasma membrane.
Steady-state pHi.
As shown in Table 1, steady-state pHi in mock-transfected and SLC4A11-C-expressing cells bathed in physiological HCO3−-buffered solutions was not significantly different. Inhibition of endogenous HEK 293 Na+/H+ exchange activity with EIPA (30 μM) in HCO3−-buffered solutions lowered steady-state pHi in both groups; however, there was no significant difference between mock-transfected and SLC4A11-C-expressing cells. In contrast, in HEK 293 cells bathed in HEPES-buffered solutions to block pHi regulation by potential endogenous HEK 293 HCO3−/CO32− transporter(s), steady-state pHi was significantly lower in SLC4A11-C-expressing cells in either the presence or absence of EIPA. A significant difference was also detected between mock-transfected and SLC4A11-C-expressing cells in the presence of EIPA. In each group, steady-state pHi was decreased further because of the inhibition of endogenous Na+/H+ exchange. These findings suggest that SLC4A11-C functions as an acid loader (or base efflux pathway) without requiring the presence of HCO3−/CO32− as a substrate and that the transporter is active in the presence of EIPA (30 μM).
Table 1.
Steady-state pHi
| Steady-State pHi |
||||
|---|---|---|---|---|
| HCO3− | HCO3− + EIPA | HEPES | HEPES + EIPA | |
| Mock | 7.26 ± 0.04 | 7.13 ± 0.03 | 7.29 ± 0.04 | 7.02 ± 0.05 |
| n | 12 | 8 | 12 | 8 |
| SLC4A11-C | 7.19 ± 0.04 | 7.07 ± 0.03 | 7.11 ± 0.03 | 6.93 ± 0.03 |
| n | 12 | 9 | 12 | 9 |
| P value | NS | NS | 0.005 | 0.002 |
n, No. of experiments. EIPA, 5-(N-ethyl-N-isopropyl)amiloride; NS, not significant.
Effect of plasma membrane hyperpolarization on SLC4A11-C H+(OH−) flux.
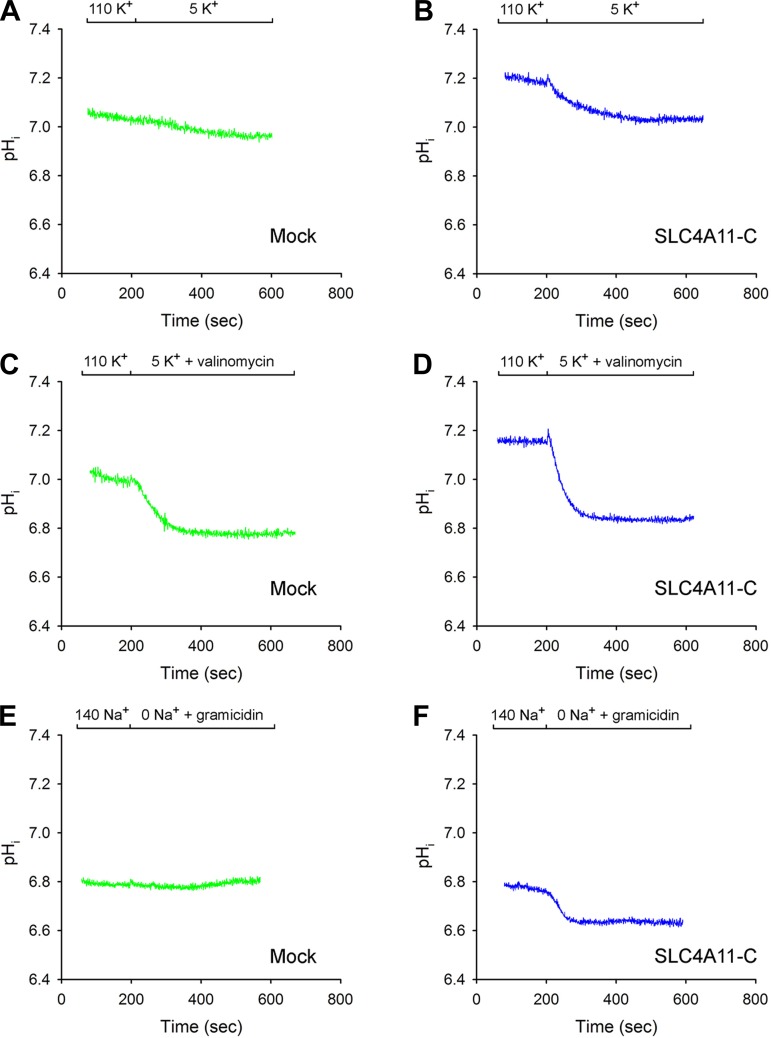
Experiments were next done to determine whether changes in the cell membrane voltage can drive SLC4A11-C H+(OH−) flux (Fig. 2, A-F). The plasma membrane voltage was initially acutely hyperpolarized by decreasing the extracellular K+ from 110 to 5 mM in the absence or presence of the K+ ionophore valinomycin, while endogenous Na+/H+ activity was blocked with EIPA (30 μM). As shown in Fig. 2A, in mock-transfected cells in the absence of valinomycin, there was minimal change in pHi following the change in extracellular K+ with an H+(OH−) flux of 3.61 ± 1.07 μM/s (n = 4), whereas, in SLC4A11-C-expressing cells, the H+(OH−) flux was 14.8 ± 2.89 μM/s (n = 4, P < 0.01; Fig. 2B). When the extracellular solution was switched to 5 mM K+ plus valinomycin to increase the magnitude of the plasma membrane hyperpolarization, pHi decreased in mock-transfected cells (Fig. 2C) with an H+(OH−) flux of 37.4 ± 4.16 μM/s (n = 4); however; in SLC4A11-C-expressing cells (Fig. 2D), the flux was significantly greater (66.1 ± 6.08 μM/s, n = 4, P < 0.01). The results indicate that HEK 293 cells possess an endogenous background H+(OH−) permeation pathway stimulated by the acute plasma membrane hyperpolarization that could be detected in the presence of valinomycin. Moreover, the finding that SLC4A11-C H+(OH−) flux was increased in the presence of valinomycin indicates that an electroneutral K+/H+ exchange process is not mediating the observed pHi changes. Additional confirmatory experiments were done to hyperpolarize the membrane voltage using an alternate approach that did not require K+ concentration changes with valinomycin. Extracellular Na+ removal experiments were performed with the Na+ ionophore while blocking endogenous Na+/H+ exchange activity with EIPA. As shown in Fig. 2E in mock-transfected cells, there was no significant change in pHi following removal of extracellular Na+ [H+(OH−) flux: 2.84 ± 0.59, n = 6]. In contrast, in SLC4A11-C-expressing cells (Fig. 2F), Na+ removal plus gramicidin caused a decrease in pHi [H+(OH−) flux: 36.1 ± 0.81, n = 4, P < 0.001]. These findings together strongly suggest that SLC4A11-C mediates electrogenic H+(OH−) flux.
Fig. 2.
A–D: plasma membrane hyperpolarization. Decrease of extracellular K+ in the absence or the presence of the K+ ionophore valinomycin. Extracellular K+ was decreased from 110 to 5 mM K+ in mock-transfected cells (A and C) and SLC4A11-C-expressing cells (B and D). Extracellular K+ was acutely decreased from 110 to 5 mM K+ in the absence (A and B) or presence (C and D) of valinomycin. E and F: plasma membrane hyperpolarization following a decrease in extracellular Na+ with the Na+ ionophore gramicidin. Extracellular Na+ was acutely removed in the presence of gramicidin in mock-transfected cells (E) or in SLC4A11-C-expressing cells (F).
Effect of Na+-induced changes on SLC4A11-C H+(OH−) flux.
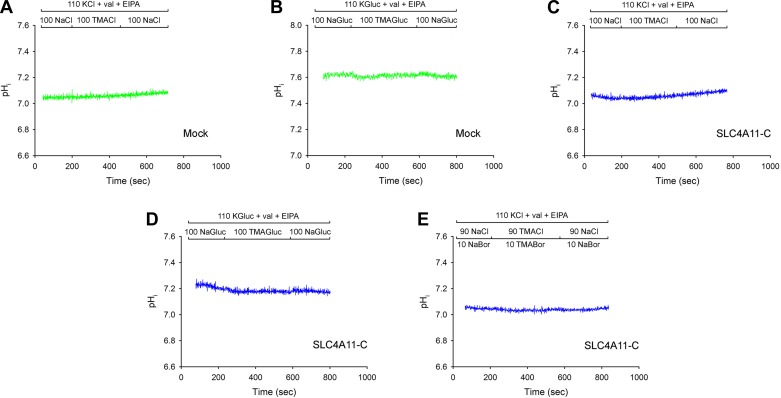
To further examine whether the electrogenic H+(OH−) flux mediated by SLC4A11-C is obligatorily coupled to Na+, additional Na+ removal/readdition experiments were performed in HEPES-buffered solutions while endogenous Na+/H+ activity was blocked with EIPA (30 μM) with the cell membrane voltage chemically clamped using 110 mM K+-valinomycin solutions. As shown in Fig. 3, A and C, removal and subsequent readdition of extracellular Na+ did not drive SLC4A11-C-mediated H+(OH−) flux, indicating that H+(OH−) flux is not obligatorily coupled to Na+. To ensure that endogenous background HEK 293 Cl−-coupled H+/base transport process(es) were not masking any potential pHi changes in these experiments, and in addition to determine whether SLC4A11-C transport is coupled to Cl− or requires the presence of Cl−, the studies were repeated under Cl−-free conditions. Following 1 h of incubation in the cell culture incubator at 37°C in a Cl−-free HCO3−-buffered solution, the Na+ removal and readdition experiments were repeated (Fig. 3, B and D). In these experiments, the initial pHi was significantly higher in mock-transfected cells, 7.65 ± 0.09 (n = 4) vs. 7.25 ± 0.07 (n = 4, P < 0.02), likely due to the reversal of endogenous HEK 293 Cl−/base exchange transport process(es) while providing evidence that SLC4A11-C acts as an acid loader (or base efflux pathway) under these Cl−-free conditions. Moreover, as shown in Fig. 3, B and D, as in the presence of Cl−, under Cl−-free conditions while the plasma membrane was chemically voltage clamped, removal and subsequent readdition of extracellular Na+ did not alter pHi. These studies further indicate that SLC4A11-C H+(OH−) flux is not obligatorily coupled to Na+ or Cl−. Additional experiments as shown in Fig. 3E demonstrate that pHi was not altered during Na+ removal and readdition studies performed in the presence of 10 mM borate.
Fig. 3.
A–E: lack of effect of changes in extracellular Na+ on intracellular pH (pHi) and SLC4A11-C-mediated H+(OH−) flux under K+-valinomycin voltage-clamped conditions in the presence of 5-(N-ethyl-N-isopropyl)amiloride (EIPA). Na+ removal/readdition experiments were performed with 110 mM K+- and valinomycin-containing solutions. Na+ removal/readdition in the presence of Cl−: mock-transfected cells (A) and SLC4A11-C-expressing cells (C). Na+ removal/readdition in the absence of Cl−: mock-transfected cells (B) and SLC4A11-C expressing cells (D). Na+ removal/readdition in the presence of borate (10 mM): SLC4A11-C-exressing cells (E).
SLC4A11-C and mutant R109H H+(OH−) flux following an acute decrease in pHi at constant external pH in the presence and absence of Na+ and Cl−.
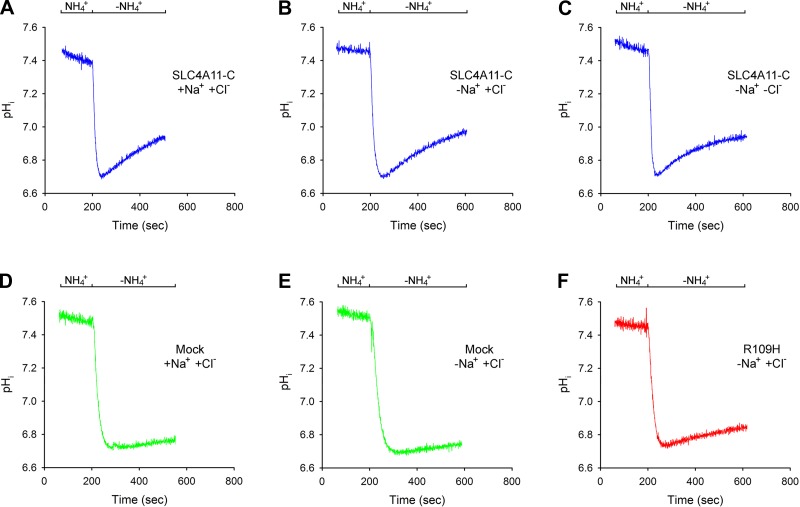
The previous Na+ removal/readdition experiments in Cl−-containing and Cl−-free solutions suggested that SLC4A11-C H+(OH−) flux is not obligatorily coupled to Na+ or Cl−. To address this question further, pHi in SLC4A11-C-expressing cells was acutely decreased following NH4+ addition and subsequent removal, and the spontaneous initial rate of pHi recovery was monitored and compared with mock-transfected cells (Fig. 4, A-F). In these experiments, the plasma membrane voltage was chemically voltage clamped throughout in 110 mM K+- and valinomycin-containing solutions. pHi was decreased following NH4+ removal to ∼6.7 while endogenous Na+/H+ activity was blocked with EIPA (30 μM). As shown in Fig. 4A, in SLC4A11-C-expressing cells, following intracellular acidification in the presence of Na+ and Cl−, pHi increased briskly with a calculated H+(OH−) flux of 23.9 ± 1.44 μM/s (n = 5). In mock-transfected cells, pHi increased significantly more slowly (Fig. 4D), with an H+(OH−) flux of 5.58 ± 0.82 μM/s (n = 3, P < 0.01). SLC4A11-C H+(OH−) flux was unaffected by the removal of Na+ (Fig. 4B) or Cl− from the solutions (Fig. 4C): Na+ free, 24.5 ± 1.29 μM/s (n = 5); Na+ and Cl− free, 22.2 ± 1.82 μM/s (n = 5). As shown in Fig. 4F, R109H mutant H+(OH−) flux was significantly decreased compared with the wild-type transporter [8.95 ± 1.80 μM/s (n = 6, P < 0.01)], almost to the level of mock-transfected cells.
Fig. 4.
SLC4A11-C and mutant R109H mediated H+(OH−) flux following an acute decrease in pHi. NH4+ removal experiments were done under chemically voltage-clamped conditions with 110 mM K+- and valinomycin-containing solutions in the presence or absence of Na+ and Cl−. pHi was decreased following NH4+ removal to ∼6.7, and the H+(OH−) flux was determined during the initial phase of pHi recovery. A: Na+- and Cl−-containing solutions: SLC4A11-C-expressing cells; B: Na+-free Cl−-containing solutions: SLC4A11-C-expressing cells; C: Na+-free Cl−-free solutions: SLC4A11-C-expressing cells; D: Na+- and Cl−-containing solutions: mock-transfected cells; E: Na+-free Cl−-containing solutions: mock-transfected cells; F: Na+-free Cl−-containing solutions: R109H mutant-expressing cells.
Dependence of H+(OH−) flux on plasma membrane potential.
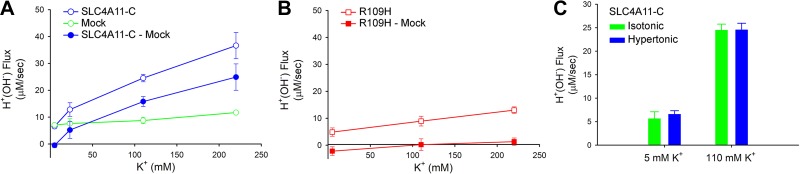
The prior plasma membrane hyperpolarization experiments suggested that SLC4A11-C transports H+(OH−) electrogenically. Further studies were performed to characterize the dependence of SLC4A11-C-mediated H+(OH−) flux on the plasma membrane potential. The intracellular K+ concentration was initially set to 110 mM in a solution containing 110 mM external K+-valinomycin. pHi was acutely decreased to ∼6.7 following NH4+ removal while simultaneously varying the extracellular K+ concentration with valinomycin to generate a range of plasma membrane potentials. The dependence of H+(OH−) flux on the extracellular K+ concentration is shown in Fig. 5A. The difference between the H+(OH−) flux in SLC4A11-C-expressing cells and mock-transfected cells is shown in the subtraction data depicted in Fig. 5A. In separate experiments, the H+(OH−) flux was measured at various extracellular K+ concentrations in cells transfected with the functionally impaired R109H mutant. As shown in Fig. 5B, the results were essentially comparable to those obtained in mock-transfected cells demonstrating significant loss of electrogenic H+(OH−) flux by the mutant transporter. To determine whether hypertonicity affects SLC4A11-C activity, the experiments were repeated at different plasma membrane potentials in isotonic solutions using a K+ concentration of 5 and 110 mM. As shown in Fig. 5C, the flux was not significantly different from the results obtained in hypertonic solutions.
Fig. 5.
SLC4A11-C and R109H mediated H+(OH−) flux dependence on the plasma membrane potential. The intracellular K+ was initially clamped at 110 mM with valinomycin. pHi was decreased to ∼6.7 following NH4+ removal while the extracellular K+ concentration was simultaneously changed to various values in the presence of valinomycin to vary the plasma membrane potential. H+(OH−) flux was determined during the initial phase of pHi recovery. A: SLC4A11-C H+(OH−) flux (blue closed circles) was determined by subtracting the endogenous HEK 293 cell flux in mock-transfected cells [green open circles: 5 mM (n = 4), 23.2 mM (n = 4), 110 mM (n = 4), and 220 mM (n = 4)] from the flux obtained in SLC4A11-C-expressing cells [blue open circles: 5 mM (n = 4), 23.2 mM (n = 4), 110 mM (n = 6), and 220 mM (n = 9)]. B: similar experiments were done in cells expressing the functionally impaired R109H mutant. Red open squares, flux in R109H-expressing cells [5 mM (n = 4), 110 mM (n = 6), and 220 mM (n = 4)]; red closed squares, R109H mutant flux following subtraction of mock-transfected cell data. C: data summary of SLC4A11-C H+(OH−) flux in isotonic and hypertonic solutions following acute intracellular acidification at two different predicted plasma membrane potentials. The results show that a change in tonicity did not significantly alter SLC4A11-C H+(OH−) flux. 5 mM K+: isotonic, n = 12; hypertonic, n = 4, P = not significant (NS). 110 mM K+: isotonic, n = 5; hypertonic, n = 6, P = NS.
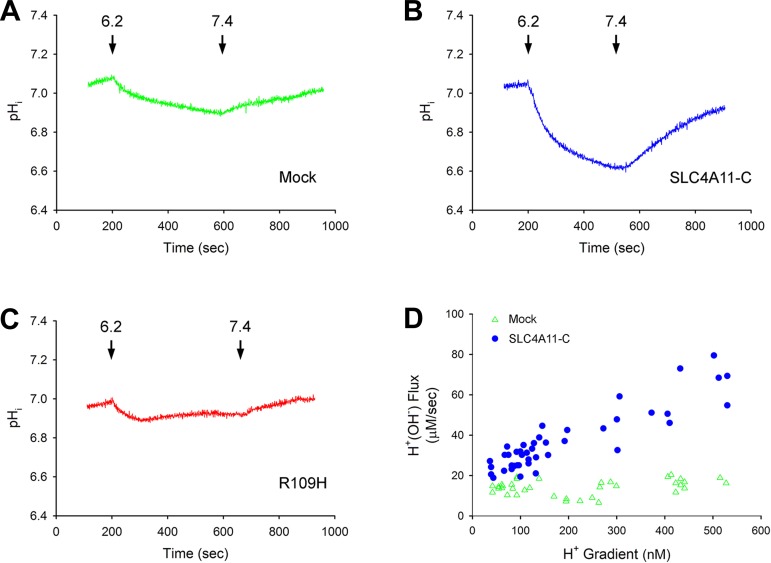
H+(OH−) flux following an acute change in external pH; dependence of H+(OH−) flux on the H+in-H+out gradient; modulation of SLC4A11-C, mutant R109H, and SLC4A11-B H+(OH−) flux by DIDS.
In the previous protocols, an H+in-H+out gradient of ∼160 nM was used to drive SLC4A11-C H+(OH−) flux and was generated by acutely decreasing pHi to ∼6.7 following NH4+ removal, keeping the extracellular pH constant at 7.4. To study a wide range of bidirectional H+in-H+out gradients, the extracellular pH was decreased acutely from 7.4 to 6.2–6.4, and, following the decrease in pHi to various values, the extracellular pH was subsequently returned to 7.4. The cells were chemically voltage clamped with 110 mM K+-valinomycin in the absence of Na+ with EIPA present throughout. Typical experiments depicting the effect of changing the external pH from 7.4 to 6.2 and returning the external pH to 7.4 are shown in Fig. 6, A–C. Figure 6D shows the dependence of H+(OH−) flux on the H+in-H+out gradient. Because the H+(OH−) flux and H+in-H+out gradients were in both directions, absolute values are depicted. Independent of the H+ gradient or flux direction, the data fell on the same line. The difference between the H+(OH−) flux in SLC4A11-C-expressing cells and mock-transfected cells was dependent on the absolute value of the magnitude of the H+in-H+out gradient.2
Fig. 6.
Effect of acute changes in extracellular pH on H+(OH−) flux. A: mock-transfected cells; B: SLC4A11-C-expressing cells; C: R109H-expressing cells. After a steady-state period, the extracellular pH was decreased to 6.2 to induce mock-transfected, SLC4A11-C, and R109H mutant H+(OH−) flux. The extracellular pH was then returned to 7.4, driving H+(OH−) flux in the reverse direction. The cells were chemically voltage clamped with 110 mM K+-valinomycin throughout. D: H+(OH−) flux vs. the H+in-H+out gradient in mock-expressing and SLC4A11-C-transfected cells. Following a steady-state period, the extracellular pH was decreased from 7.4 to various values (6.2–6.4), which created a range of H+in-H+out gradients for inducing SLC4A11-C H+(OH−) flux. The extracellular pH was then returned to 7.4, which drove H+(OH−) flux in the reverse direction at various H+in-H+out gradients. The cells were chemically voltage clamped with 110 mM K+-valinomycin throughout the experiments. Experiments were done in mock-transfected (open green triangles) and SLC4A11-C-expressing (closed blue circles) cells. Given that the flux was bidirectional depending on the direction of the H+in-H+out gradient, depicted is the absolute value of individual data points.
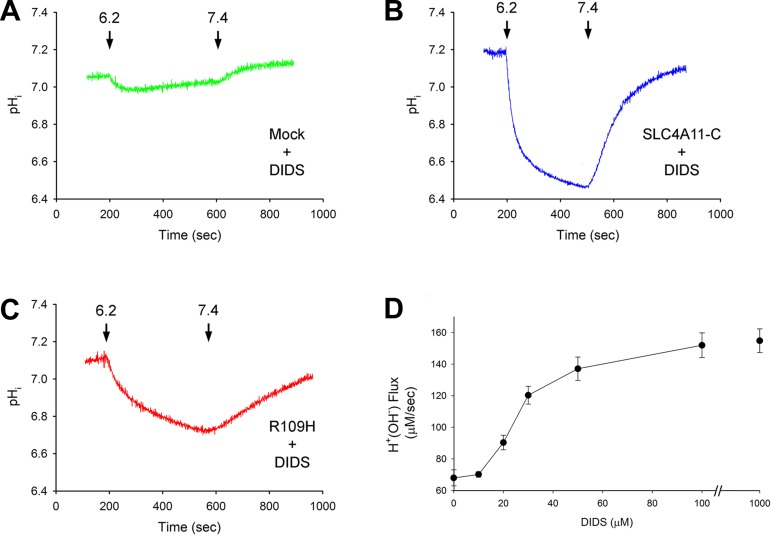
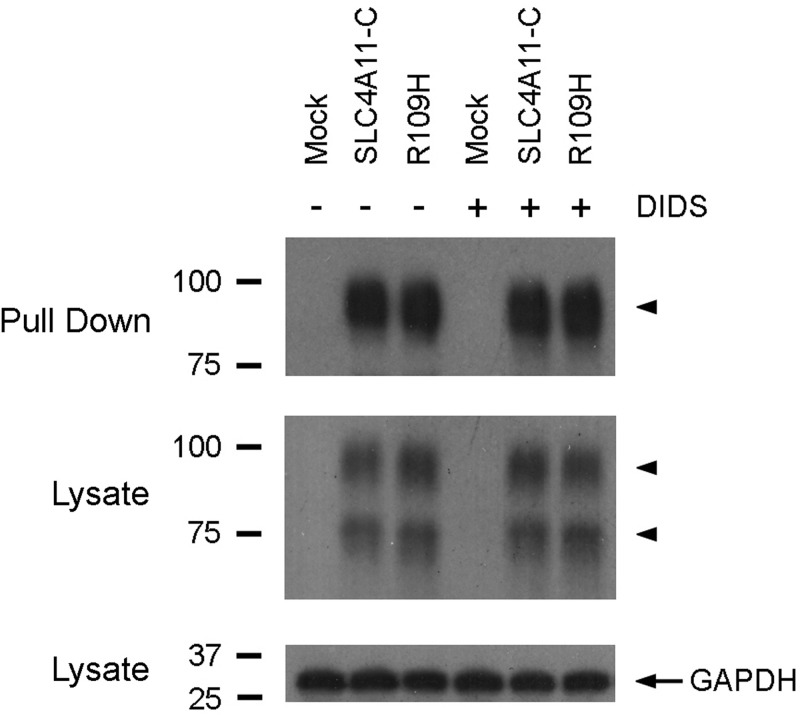
Disulfonic stilbene modulation of SLC4A11-C transport was investigated by preincubating the cells with DIDS for 1 h at 37°C. DIDS was not present during the experimental measurements. Extracellular pH was decreased from 7.4 to 6.2 and subsequently increased to 7.4. The cells were chemically voltage clamped with 110 mM K+-valinomycin in Na+-free solutions with EIPA present. The data are summarized in Table 2 and depicted in Fig. 7. Unlike mock-transfected cells (Fig. 7A), preexposure to 1 mM DIDS significantly increased the H+(OH−) flux in SLC4A11-C-expressing cells (Fig. 7B). Moreover, the data imply that DIDS was bound to the transporter given that the stimulation of H+(OH−) flux was detected following DIDS removal from the external solutions. Additional experiments demonstrated that the transport stimulation was not specific for DIDS, since preincubation with H2DIDS (1 mM) increased H+(OH−) flux in SLC4A11-C expressing cells (following a decrease in external pH from 7.4 to 6.2) from 62.4 ± 4.91 μM/s (n = 6) to 165 ± 11.0 μM/s (n = 4, P < 0.001); and SITS (1 mM) was also effective: 136 ± 6.34 μM/s (n = 4, P < 0.001). In contrast, 1 mM DADS, which only interacts noncovalently (7, 19), was therefore included in the external solutions throughout the experiments and failed to alter SLC4A11-C mediated H+(OH−) transport (data not shown).3 We next determined whether the R109H mutant H+(OH−) flux could also be stimulated by DIDS. As shown in Table 2 and Fig. 7C, 1 mM DIDS significantly increased the R109H mutant H+(OH−) flux. However, like the wild-type transporter, the activity of the R109H mutant was unaffected by DADS (data not shown). The dose-dependent effect of DIDS on SLC4A11-C H+(OH−) flux is shown in Fig. 7D, with half-maximal stimulation at 25.8 μM. In separate experiments, we determined whether DIDS altered the plasma membrane expression of the wild-type and mutant transporter, which potentially could have accounted for our findings. As shown in Fig. 8, in whole cell sulfo-NHS-SS-biotin surface labeling studies, the plasma membrane expression of SLC4A11-C and the R109H mutant was unaffected by DIDS (1 mM), indicating that the increase in H+(OH−) flux was caused by a change in transporter activity rather than increased plasma membrane expression.4 Finally, we examined SLC4A11-B activity to determine whether the effect of DIDS was unique to the SLC4A11-C variant. Following a decrease in external pH from 7.4 to 6.2 in SLC4A11-B-expressing cells, H+(OH−) flux increased significantly from 32.3 ± 1.54 μM/s (n = 3) to 82.9 ± 2.68 μM/s (n = 3, P < 0.001) after DIDS preincubation.
Table 2.
Effect of DIDS on H+(OH−) flux
| Mock | SLC4A11-C | R109H | DIDS Mock | DIDS SLC4A11-C | DIDS R109H | |
|---|---|---|---|---|---|---|
| Effect of DIDS on H+(OH−) Flux: pH 7.4 → pH 6.2 | ||||||
| Initial pHi | 7.02 ± 0.05 | 7.05 ± 0.043 | 7.05 ± 0.03 | 7.05 ± 0.03 | 7.21 ± 0.06 | 7.04 ± 0.03 |
| Minimum pHi | 6.88 ± 0.031 | 6.67 ± 0.043,4 | 6.90 ± 0.017 | 7.00 ± 0.03 | 6.51 ± 0.04 | 6.69 ± 0.03 |
| ΔpHi | 0.15 ± 0.031 | 0.38 ± 0.034,5 | 0.15 ± 0.038 | 0.06 ± 0.02 | 0.69 ± 0.02 | 0.35 ± 0.03 |
| Initial Hin+, nM | 95.9 ± 10.0 | 91.1 ± 7.173 | 89.2 ± 5.66 | 89.3 ± 6.44 | 64.1 ± 8.93 | 93.3 ± 5.73 |
| Minimum Hin+, nM | 133.4 ± 9.001 | 218.5 ± 17.94,6 | 125.4 ± 4.088 | 101.8 ± 6.96 | 310.6 ± 26.6 | 209 ± 12.9 |
| ΔHin+, nM | 37.6 ± 5.042 | 127.4 ± 13.64,5 | 36.2 ± 6.588 | 12.5 ± 5.07 | 246.6 ± 18.4 | 116 ± 12.6 |
| H+(OH−) flux, μM/s | 23.4 ± 5.96 | 62.4 ± 4.914,5 | 31.1 ± 3.159 | 23.8 ± 2.03 | 154.6 ± 7.47 | 43.4 ± 2.14 |
| n | 3 | 6 | 4 | 4 | 4 | 11 |
| Effect of DIDS on H+(OH−) Flux: pH 6.2 → pH 7.4 | ||||||
| Final pHi | 6.93 ± 0.042 | 6.99 ± 0.0310 | 6.99 ± 0.03 | 7.08 ± 0.02 | 7.13 ± 0.03 | 6.95 ± 0.02 |
| ΔpHi | 0.05 ± 0.01 | 0.32 ± 0.024,5 | 0.09 ± 0.017 | 0.08 ± 0.01 | 0.62 ± 0.02 | 0.27 ± 0.02 |
| Final Hin+, nM | 118.3 ± 9.912 | 103.3 ± 6.1810 | 102.8 ± 5.85 | 83.6 ± 4.85 | 74.3 ± 5.23 | 112.9 ± 5.77 |
| ΔHin+, nM | 15.1 ± 0.92 | 115.2 ± 12.34,5 | 22.5 ± 2.338 | 18.3 ± 3.15 | 236.4 ± 22.1 | 96.1 ± 11.0 |
| H+(OH−) flux, μM/s | 15.9 ± 0.67 | 36.2 ± 1.524,5 | 18.3 ± 2.687 | 14.0 ± 0.88 | 107.4 ± 4.61 | 34.1 ± 1.48 |
| n | 3 | 6 | 4 | 4 | 4 | 11 |
n, No. of experiments. DIDS, 4,4′-diisothiocyano-2,2′-stilbenedisulfonate. Note: the absolute values of the delta (Δ) and flux measurements are depicted. 1P < 0.05 vs. mock + DIDS. 2P < 0.02 vs. mock + DIDS. 3P < 0.05 vs. SLC4A11-C + DIDS. 4P < 0.01, SLC4A11-C vs. mock or R109H. 5P < 0.001 vs. SLC4A11-C + DIDS. 6P < 0.02 vs. SLC4A11-C + DIDS. 7P < 0.001 vs. R109H + DIDS. 8P < 0.005 vs. R109H + DIDS. 9P < 0.01 vs. R109H + DIDS. 10P < 0.01 vs. SLC4A11-C + DIDS.
Fig. 7.
Modulation of SLC4A11-C and mutant R109H H+(OH−) flux by 4,4′-diisothiocyano-2,2′-stilbenedisulfonate (DIDS) during changes in extracellular pH. The cells were preincubated with DIDS in the cell culture incubator for 1 h at 37°C before initiating the experiments in DIDS-free solutions. During the experiments, the cells were chemically voltage clamped with 110 mM K+-valinomycin. A: mock-transfected cells with DIDS preincubation; B: SLC4A11-C-expressing cells with DIDS preincubation; C: R109H-expressing cells with DIDS preincubation. D: dose dependence of DIDS stimulation of SLC4A11-C H+(OH−) flux. The cells were preincubated with various DIDS concentrations (0–1,000 μM). SLC4A11-C H+(OH−) flux was measured during the change of extracellular pH from 7.4 to 6.2 while the cells were chemically voltage clamped with 110 mM K+-valinomycin.
Fig. 8.
Immunoblot analysis of plasma membrane proteins that were labeled using sulfo-NHS-SS-biotin depicting membrane processing of SLC4A11-C and the R109H mutant in the absence or presence of DIDS (1 mM) preincubation. HEK 293 cells were transfected with the empty pTT vector (mock), SLC4A11-C, or the R109H mutant and labeled according to the manufacturer's protocol. Approximately 24 h posttransfection, the cells were preincubated with 1 mM DIDS for 1 h at 37°C in the cell culture incubator, and the plasma membrane proteins were then labeled with sulfo-NHS-SS-biotin and pulled down using streptavidin-agarose resin. Immunoblots of pulled-down proteins and whole cell lysates are shown. The lanes represent HEK 293 cells that were transfected with an empty pTT vector used for mock transfection, SLC4A11-C, or the R109H mutant and labeled after 24 h expression. Protein samples were resolved on SDS-PAGE, and the blots were probed with antibodies against the SLC4A11 COOH-terminus (pull-down and whole cell lysates; arrowheads, SLC4A11-C) and GAPDH (whole cell lysates).
DISCUSSION
Our findings demonstrate for the first time that the SLC4A11-C transcript is the predominant NH2-terminal variant expressed in human corneal endothelial cells and that the human SLC4A11-C transporter operates as an H+(OH−) permeation pathway. Moreover, SLC4A11-C mediates H+(OH−) transport (unlike other SLC4 transporters) in the absence of Na+, Cl−, and HCO3−/CO32−. Disulfonic stilbenes that previously have been shown to inhibit the transport of other SLC4 transporters (except NBCn1) stimulate H+(OH−) permeation through SLC4A11-C. Our findings are compatible with the hypothesis that covalently bound disulfonic stilbenes modify the surface electrostatic charge potentially near the entrance to the SLC4A11-C ion permeation pathway favoring enhanced H+(OH−) transport. The specific SLC4A11-C residues that interact with these compounds remain to be determined.
The CHED2-causing SLC4A11-R125H mutation reported by Hemadevi et al. (22) (residue numbering was based on the SLC4A11-B sequence) was first shown by Vilas et al. (60) with SLC4A11-B-R125H and confirmed in the present study using the analogous SLC4A11-C-R109H mutant to have normal plasma membrane expression, suggesting that the mutation impairs the intrinsic activity of the transporter. Our results demonstrate for the first time that the R109H mutant has a severe impairment in H+(OH−) flux that was partially corrected by DIDS. Disulfonic stilbenes can interact reversibly with lysines (45), and those compounds with 4,4′-thiocyanate (SCN) groups (e.g., DIDS, H2DIDS, and SITS) are known to also covalently bind with lysine or cysteine residues on target proteins (20, 45, 47) and potentially react noncovalently with arginine (28). The 4,4′-SCN groups in DIDS are reported to be more reactive covalently than the equivalent groups in H2DIDS and SITS (7, 27). Despite these differences, DIDS, H2DIDS, and SITS were essentially equivalent in their stimulation of H+(OH−) flux by wild-type SLC4A11-C. The three compounds possess one (SITS) or two (H2DIDS, DIDS) SCN groups, whereas noncovalently interacting DADS (7, 19) with 4,4′-NH2 groups (lacking SCN groups) failed to stimulate H+(OH−) flux in both wild-type and mutant transporters. These results suggest that covalent binding of disulfonic stilbenes is required for its stimulatory effect. Covalent binding of DIDS has previously been hypothesized to account for its stimulation of a Na+ conductance through the electroneutral Na+-HCO3− cotransporter NBCn1 (8). In our studies, the specific steric and surface charge modification(s) required to improve R109H mutant activity are unknown but might be more stringent than the wild-type transporter.5 The finding that DIDS was clearly capable of improving R109H mutant H+(OH−) transport suggests the possibility that the development of more specific compounds with fewer effects on other endogenous anion transport pathways, for example, might become a promising therapeutic approach in patients with corneal diseases caused by other mutations that impair the activity of SLC4A11-C [assuming the loss of H+(OH−) transport is the underlying pathogenic mechanism involved].
Prior to this study, human SLC4A11-B has been the focus of all studies of the transport properties of the SLC4A11 gene product, since it was the first of the three NH2-terminal variants that was cloned (44). Various transport properties have been attributed to SLC4A11-B by several groups. Park et al. originally reported that SLC4A11-B, when expressed in Xenopus oocytes and HEK 293 cells, is an electrogenic Na(n)+-B(OH−)4− cotransporter (n ≥ 2) and a cation (Na+ or H+) permeation pathway in the absence of borate (42). Although there was some evidence in the literature that borate (boron) modulates cell growth, providing a potential mechanism for corneal diseases caused by SLC4A11 mutations (41), it has remained unclear what the specific role of borate was in corneal biology. Jalimarada et al. and Ogando et al. subsequently reexamined the activity of SLC4A11-B in primary cultures of bovine corneal endothelial cells, HEK 293 cells, and PS120 cells and failed to detect Na(n)+-B(OH−)4− cotransport. These authors reported that SLC4A11-B is an Na+-OH− cotransporter (equivalent to an Na+/H+ exchanger) and also acts as an NH4+ permeation pathway (23, 40). Their studies did not address the electrogenic properties of the transporter, control for potential Na+-induced changes in membrane voltage, or assess the Cl− dependence of the transport process. Vilas et al., using Xenopus oocytes as well as HEK 293 cell expression systems, concluded that SLC4A11-B does not transport ions but rather acts as a water permeation pathway (59). Because the aforementioned studies in the literature have assigned several different potential transport properties to the SLC4A11-B variant, it would be of interest in future experiments to determine which of these transport functions predominate. Our results involving the SLC4A11-C variant are most compatible with the finding by Park et al. that SLC4A11-B mediates H+(OH−) flux (42) yet differ in that borate failed to induce Na+-driven SLC4A11-C-mediated pHi transients. Our data cannot rule out the possibility that SLC4A11-C has additional yet to be described transport properties. Specifically, whether SLC4A11-C can mediate Na+-OH− cotransport (or Na+/H+ exchanger-like activity) that is EIPA inhibitable under voltage-clamped conditions, or transports water remains to be determined. It would also be of interest to determine the transport properties of the SLC4A11-A variant, which has thus far not been reported.
Disulfonic stilbenes, which are typically thought to act as inhibitors of the activity of various transporters (7), were shown for the first time in the present study to significantly enhance SLC4A11-C-mediated H+(OH−) transport. Moreover, SLC4A11-C was active in the presence of EIPA (30 μM). In this regard, our results differ in several aspects from reports in the literature with regards to the effect of disulfonic stilbenes and EIPA on SLC4A11-B transport. Park et al. first reported that SLC4A11-B-mediated ion transport was not inhibited by DIDS (500 μM) or EIPA (10 μM) but did not document whether SLC4A11-B transport was stimulated by DIDS. In contrast, Villas et al. reported that SLC4A11-B water transport was both 4,4′-dinitrostilbene-2,2′-disulfonate and H2DIDS sensitive (100 μM) and EIPA insensitive (5 μM) (59). Jalimarada et al. (23) reported that SLC4A11-B-mediated ion transport was insensitive to EIPA (0.25 μM), whereas Oganda et al. (40) found that 10 μM EIPA completely blocked the Na+-OH− cotransport activity they reported. The reason for these discrepant results remains to be clarified but are potentially due to the use of different expression systems, assays (with various signal-to-noise properties), and experimental conditions. Our data indicate unequivocally that SLC4A11-C mediates H+(OH−) transport in the presence of EIPA (30 μM) and is stimulated by disulfonic stilbenes. Given that compounds that modulate SLC4A11 transport likely interact with the same residues in the identical transmembrane region of the three variants, we examined the effect of disulfonic stilbenes on SLC4A11-B transport and showed that H+(OH−) flux was also increased significantly.
Our results do not shed any light as to whether SLC4A11-C transports H+ or OH−. This question is in general difficult to address given that: 1) the transport of each species has the same effect on pHi; 2) H+ or OH− have identical transcellular concentration gradients that are opposite in direction; and 3) they have identical Nernst potentials. One of the hypothesized mechanisms by which disulfonic stilbenes covalently bind is via the interaction of SCN groups with charged lysine residues on target proteins (7). Because of the potential neutralization of the positive charge on one or more lysine residues in SLC4A11-C and the fact that disulfonic stilbenes are negatively charged at physiological pH, our data favor an SLC4A11-C transport model where H+ being positively charged is the preferred species whose transport is enhanced. It is interesting, however, to note that experimental evidence exists in the literature to support OH− permeation in other systems such as (C6F5)2Hg planar phospholipid membranes (6) and freshwater algae Chara spp. channels (3, 33).
Based on our results, SLC4A11-C joins a large number of prokaryotic and mammalian H+-conducting proteins that either transport protons alone or couple H+ transport thermodynamically to the flux of other substrates. These proteins include voltage-gated H+ channels (10), channel rhodopson (4, 14), transient receptor potential cation channel subfamily M, member 7 (TRPM7; see Ref. 38), transient receptor potential cation channel subfamily V, member 1 (TRPV1; see Ref. 21), H+-coupled oligopeptide transporter OPT3 (17), excitatory amino acid transporter, member 4 (EAAT4; see Ref. 16), sodium glocose transporter, member 3 (SGLT3; see Ref. 5) SLC46A1 (56), Semliki Forest virus envelope protein (51), serotonin transporters (51), diphtheria toxin channels (49), sarco(endo)plasmic reticulum (36), Na+-K+-ATPase (58), and H+-K+-ATPase (1). Selective H+ conduction in these proteins is thought to be mediated either by a reversible protonation of carboxylates or by a Grotthuss-type (water-hopping) mechanism (10). A variant of the Grotthuss mechanism involves putative H+ hopping in channels that are occupied with ammonia appearing macroscopically as an anomalous large NH4+ permeability (53). Although preliminary topological characterization of SLC4A11 has been reported (61), the underlying mechanism of H+(OH−) permeation through SLC4A11-C will require more detailed structure-activity studies in the future and potentially the availability of specific inhibitors or agonists.
How the loss of SLC4A11-C in patients leads to the variety of corneal disease processes that are linked to mutations in the transporter is poorly understood. Several pathophysiological mechanisms have been implicated, which are not mutually exclusive that include apoptosis (30), decreased water permeation (59), and possibly ER stress (due to misfolded protein) (31, 60, 61). Given our findings, although speculative, it is also worth considering additional underlying pathogenic processes that might result from defective SLC4A11-C H+(OH−) permeation in corneal endothelial cells. In this regard, H+-transporting proteins play an important role in pHi regulation after acid loading (11), volume regulation (34), in setting the resting potential (15), and in extracellular pH regulation (18). One of the most thoroughly investigated systems involves the innate immunity NADPH oxidase response wherein voltage-gated H+ channels play a key role in charge compensation preventing cell membrane depolarization that would otherwise result from NADPH oxidase-mediated electron flow (35). Corneal epithelial and stromal fibroblasts express NADPH isoforms and have been demonstrated to produce the superoxide anion (O2·−) in a NADPH-dependent manner (39, 48). O2·− generated by NADPH is a member of the group of reactive oxygen species that is produced at low levels in a highly regulated manner because of its role in cell senescence apoptosis, cell proliferation, gene regulation, and signal transduction (55). Whether corneal endothelial cells express NADPH and the putative role of SLC4A11 in preventing dysregulated NADPH activity are potentially interesting avenues worth pursuing in future studies.
GRANTS
I. Kurtz was supported in part by funds from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-077162), the Allan Smidt Charitable Fund, the Factor Family Foundation, and the Arvey Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.K., R.A., N.A., D.N., and I.K. performed experiments; L.K. and I.K. analyzed data; L.K. and I.K. prepared figures; L.K., R.A., N.A., D.N., and I.K. approved final version of manuscript; I.K. conception and design of research; I.K. interpreted results of experiments; I.K. drafted manuscript; I.K. edited and revised manuscript.
Footnotes
Valimocycin is significantly more selective for K+ than NH4+ (46).
In SLC4A11-C expressing cells, during the acute decrease in extracellular pH from 7.4 to 6.2, inhibition of endogenous potentially expressed H+-conducting channels, including HVCN1 (Zn2+; see Refs. 10 and 54), TRPV1 (BCTC; see Ref. 57), and TRPM7 (NS-8593; see Ref. 9), had no significant effect on H+(OH−) flux in SLC4A11-C-expressing cells. Zn2+ (10 μM): 60.0 ± 9.22 μM/s (n = 3), P = not significant (NS); BCTC (0.5 μM): 64.3 ± 8.76 μM/s, (n = 3), P = NS; and NS-8593 (5 μM): 59.0 ± 4.0 μM/s (n = 3), P = NS.
In the DADS experiments, unlike the other studies involving preincubation with disulfonic stilbenes, EIPA and DADS were both present in all solutions. In preliminary experiments using absorbance measurements, 1 mM DADS and 30 μM EIPA were not found to interact significantly in the solutions used in these studies (data not shown).
It is likely that the DIDS covalent interaction is far more selective and restricted than sulfo-NHS-SS-biotin. Assuming DIDS binds covalently in our experiments during the preincubation period with SLC4A11-C lysine(s), it is likely that only a few DIDS-interacting lysines are involved, leaving the remaining exposed lysines free to interact with sulfo-NHS-SS-biotin. In addition, DIDS may only interact covalently with SLC4A11-C nonlysine residues, leaving all exposed lysines free to interact with sulfo-NHS-SS-biotin.
Our data cannot rule out the possibility that disulfonic stilbenes and/or their breakdown products also interact with another protein(s) that directly or indirectly regulates SLC4A11-C and the R109H mutant.
REFERENCES
- 1.Abe K, Tani K, Friedrich T, Fujiyoshi Y. Cryo-EM structure of gastric H+,K+-ATPase with a single occupied cation-binding site. Proc Natl Acad Sci USA 109: 18401–18406, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldave AJ, Han J, Frausto RF. Genetics of the corneal endothelial dystrophies: an evidence-based review. Clin Genet 84: 109–119, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Khazaaly S, Beilby MJ. Zinc ions block H+/OH− channels in Chara australis. Plant Cell Environ 35: 1380–1392, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Berndt A, Prigge M, Gradmann D, Hegemann P. Two open states with progressive proton selectivities in the branched channelrhodopsin-2 photocycle. Biophys J 98: 753–761, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi L, Diez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One 5: e10241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaustein RO, Finkelstein A. A hydroxide ion carrier in planar phospholipid bilayer membranes: (C6F5)2Hg (dipentafluorophenylmercury). Biochim Biophys Acta 946: 221–226, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol Cell Physiol 262: C803–C827, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Chubanov V, Mederos y, Schnitzler M., Meissner M, Schafer S, Abstiens K, Hofmann T, Gudermann T. Natural and synthetic modulators of SK (Kca2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol 166: 1357–1376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeCoursey TE. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the HV family. Physiol Rev 93: 599–652, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J Cell Biol 133: 1391–1402, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet 44: 322–326, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers N, Modis L, Moller-Pedersen T. A morphological and functional study of congenital hereditary endothelial dystrophy. Acta Ophthalmol Scand 76: 314–318, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer K, Kuhne J, Ritter E, Berndt A, Wolf S, Freier E, Bartl F, Hegemann P, Gerwert K. In channelrhodopsin-2 Glu-90 is crucial for ion selectivity and is deprotonated during the photocycle. J Biol Chem 287: 6904–6911, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Chemaly A, Guinamard R, Demion M, Fares N, Jebara V, Faivre JF, Bois P. A voltage-activated proton current in human cardiac fibroblasts. Biochem Biophys Res Commun 340: 512–516, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fairman WA, Sonders MS, Murdoch GH, Amara SG. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat Neurosci 1: 105–113, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Fei YJ, Romero MF, Krause M, Liu JC, Huang W, Ganapathy V, Leibach FH. A novel H+-coupled oligopeptide transporter (OPT3) from Caenorhabditis elegans with a predominant function as a H+ channel and an exclusive expression in neurons. J Biol Chem 275: 9563–9571, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Fischer H. Function of proton channels in lung epithelia. Wiley Interdis Rev Membr Trans Signal 1: 247–258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa T, Virag L, Sawanobori T, Hiraoka M. Stilbene disulfonates block ATP-sensitive K+ channels in guinea pig ventricular myocytes. J Membr Biol 136: 289–302, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Gatto C, Lutsenko S, Kaplan JH. Chemical modification with dihydro-4,4′-diisothiocyanostilbene-2,2′-disulfonate reveals the distance between K480 and K501 in the ATP-binding domain of the Na,K-ATPase. Arch Biochem Biophys 340: 90–100, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Hellwig N, Plant TD, Janson W, Schafer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem 279: 34553–34561, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hemadevi B, Veitia RA, Srinivasan M, Arunkumar J, Prajna NV, Lesaffre C, Sundaresan P. Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy. Arch Ophthalmol 126: 700–708, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54: 4330–4340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judisch GF, Maumenee IH. Clinical differentiation of recessive congenital hereditary endothelial dystrophy and dominant hereditary endothelial dystrophy. Am J Ophthalmol 85: 606–612, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz I. Apical Na+/H+ antiporter and glycolysis-dependent H+-ATPase regulate intracellular pH in the rabbit S3 proximal tubule. J Clin Invest 80: 928–935, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz I. SLC4 Sodium-driven bicarbonate transporters. In: The Kidney: Physiology and Pathophysiology, edited by Alperin RJ, Caplan M, Moe OW. Boston, MA: Elsevier, 2013, p. 1837–1860. [Google Scholar]
- 27.Lepke S, Fasold H, Pring M, Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4′-diisothiocyano stilbene-2,2′-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol 29: 147–177, 1976. [DOI] [PubMed] [Google Scholar]
- 28.Linsdell P, Hanrahan JW. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a mammalian cell line and its regulation by a critical pore residue. J Physiol 496: 687–693, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisch W, Seitz B. Corneal Dystrophies. Basel, Switzerland: Karger, 2011, vol. 48, p. 1–160. [Google Scholar]
- 30.Liu J, Seet LF, Koh LW, Venkatraman A, Venkataraman D, Mohan RR, Praetorius J, Bonanno JA, Aung T, Vithana EN. Depletion of SLC4A11 causes cell death by apoptosis in an immortalized human corneal endothelial cell line. Invest Ophthalmol Vis Sci 53: 3270–3279, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loganathan SK, Casey JR. Corneal dystrophy-causing SLC4A11 mutants: suitability for folding-correction therapy. Hum Mutat 35: 1082–1092, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem 284: 26882–26896, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas WJ. Alkaline band formation in Chara corallina: due to OH efflux or H influx? Plant Physiol 63: 248–254, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morihata H, Nakamura F, Tsutada T, Kuno M. Potentiation of a voltage-gated proton current in acidosis-induced swelling of rat microglia. J Neurosci 20: 7220–7227, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta 1757: 996–1011, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Musgaard M, Thogersen L, Schiott B. Protonation states of important acidic residues in the central Ca2+ ion binding sites of the Ca2+-ATPase: a molecular modeling study. Biochemistry 50: 11109–11120, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Nowak DM, Karolak JA, Kubiak J, Gut M, Pitarque JA, Molinari A, Bejjani BA, Gajecka M. Substitution at IL1RN and deletion at SLC4A11 segregating with phenotype in familial keratoconus. Invest Ophthalmol Vis Sci 54: 2207–2215, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Numata T, Okada Y. Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem 283: 15097–15103, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Brien WJ, Krema C, Heimann T, Zhao H. Expression of NADPH oxidase in rabbit corneal epithelial and stromal cells in culture. Invest Ophthalmol Vis Sci 47: 853–863, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na+ permeable pHi regulator. Am J Physiol Cell Physiol 305: C716–C727, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park M, Li Q, Shcheynikov N, Muallem S, Zeng W. Borate transport and cell growth and proliferation. Not only in plants. Cell Cycle 4: 24–26, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun 282: 1103–1109, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Passow H, Wood PG, Lepke S, Müller H, Sovak M. Exploration of the functional significance of the stilbene disulfonate binding site in mouse band 3 by site-directed mutagenesis. Biophys J 62: 98–100, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pressman BC. Biological applications of ionophores. Ann Rev Biochem 45: 501–530, 1976. [DOI] [PubMed] [Google Scholar]
- 47.Proks P, Jones P, Ashcroft FM. Interaction of stilbene disulphonates with cloned KATP channels. Br J Pharmacol 132: 973–982, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizvi F, Heimann T, O'Brien WJ. Expression of NADPH oxidase (NOX) 5 in rabbit corneal stromal cells. PLoS One 7: e34440, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandvig K, Olsnes S. Diphtheria toxin-induced channels in Vero cells selective for monovalent cations. J Biol Chem 263: 12352–12359, 1988. [PubMed] [Google Scholar]
- 50.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436: 424–427, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Schlegel A, Omar A, Jentsch P, Morell A, Kempf C. Semliki Forest virus envelope proteins function as proton channels. Biosci Rep 11: 243–255, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Siddiqui S, Zenteno JC, Rice A, Chacon-Camacho O, Naylor SG, Rivera-de la Parra D, Spokes DM, James N, Toomes C, Inglehearn CF, Ali M. Congenital hereditary endothelial dystrophy caused by SLC4A11 mutations progresses to Harboyan syndrome. Cornea 33: 247–251, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda K, Barry PH, Gage PW. Effects of ammonium ions on endplate channels. J Gen Physiol 75: 589–613, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeshita K, Sakata S, Yamashita E, Fujiwara Y, Kawanabe A, Kurokawa T, Okochi Y, Matsuda M, Narita H, Okamura Y, Nakagawa A. X-ray crystal structure of voltage-gated proton channel. Nat Struc Mol Biol 21: 352–357, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antiox Redox Sig 10: 1343–1374, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 284: 17846–17857, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, Malik S, Whittemore ER, Hodges D. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties. I. in vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther 306: 377–386, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Vedovato N, Gadsby DC. Route, mechanism, and implications of proton import during Na+/K+ exchange by native Na+/K+-ATPase pumps. J Gen Physiol 143: 449–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet 22: 4579–4590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilas GL, Loganathan SK, Quon A, Sundaresan P, Vithana EN, Casey J. Oligomerization of SLC4A11 protein and the severity of FECD and CHED2 corneal dystrophies caused by SLC4A11 mutations. Hum Mutat 33: 419–428, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Vilas GL, Morgan PE, Loganathan SK, Quon A, Casey JR. A biochemical framework for SLC4A11, the plasma membrane protein defective in corneal dystrophies. Biochemistry 50: 2157–2169, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38: 755–757, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet 17: 656–666, 2008. [DOI] [PubMed] [Google Scholar]