Abstract
ATP-sensitive K+ (KATP) channels regulate plasma membrane excitability. The Kir6.1/SUR2B isoform of KATP channels is expressed in vascular smooth muscles and plays an important role in vascular tone regulation. This KATP channel is targeted by several reactive species. One of them is methylglyoxal (MGO), which is overly produced with persistent hyperglycemia and contributes to diabetic vascular complications. We have previously found that MGO causes posttranscriptional inhibition of the KATP channel, aggravating vascular tone regulation. Here we show evidence for the underlying molecular mechanisms. We screened microRNA databases and found several candidates. Of them, miR-9a-3p, increased its expression level by ∼240% when the cultured smooth muscle cell line was exposed to micromolar concentrations of MGO. Treatments with exogenous miR-9a-3p downregulated the SUR2B but not Kir6.1 mRNA. Antisense nucleotides of miR-9a-3p alleviated the effects of MGO. Quantitative PCR showed that the targeting sites of the miR-9a-3p were likely to be in the coding region of SUR2B. The effects of miR-9a-3p were mostly eliminated when the potential targeting site in SUR2B was site-specifically mutated. Our functional assays showed that KATP currents were impaired by miR-9a-3p induced with MGO treatment. These results suggest that MGO exposure raises the expression of miR-9a-3p, which subsequently downregulates the SUR2B mRNA, compromising KATP channel function in vascular smooth muscle.
Keywords: microRNA, ATP-sensitive potassium channel, diabetes
diabetes mellitus is a major challenge to biomedical sciences (12). Diabetes causes metabolic alterations leading to excessive production of various intermediary metabolites (4). One of them is methylglyoxal (MGO), a highly reactive carbonyl species (RCS) (2, 16, 39). MGO can react with proteins, nucleotides, and lipids, damaging these molecules and promoting inflammation and cell injuries (10, 17, 26). Normally, MGO is rapidly detoxified by metabolic and redox enzymes so that it is maintained at a rather low level (37). Under diabetic conditions, however, the increased production of precursor molecules and impaired carbonyl detoxification system result in overproduction and accumulation of this RCS (38). The imbalance in the production and clearance of RCS then leads to carbonyl stress, which is known to play an important role in the development of diabetic complications, especially in the vasculature (1, 7, 11).
In the vasculature, an increase in MGO levels can impair structure and function of the vascular walls by acting on the vascular smooth muscle (VSM), endothelium, or both. This in turn disrupts the signaling network in these cells, triggers structural remodeling of the vascular wall, propagates vascular inflammation, and causes vascular dysfunction (40–42). Indeed, our recent studies have shown that an important molecular target of MGO in the vasculature is the ATP-sensitive K+ (KATP) channel (44).
The vascular KATP channel consisting of Kir6.1 and SUR2B subunit is expressed in VSM cells. This KATP channel is modulated by a number of vasoactive substances and metabolites (45). Changes in channel activity in altered metabolic states affect membrane excitability as well as vascular tones and permeability (3, 21, 36). Disruption of the KATP channel has severe consequences as shown in KATP channel-knockout mice (9, 13, 22, 27). In diabetic patients, KATP channel-dependent vasodilation is impaired, suggesting that KATP channel dysfunction occurs under diabetic conditions (14, 28). In our recent studies, we have found that a prolonged exposure to MGO brings about inhibition of the KATP channel and augmentation of vasoconstriction responses (44). However, the underlying mechanisms for the MGO-induced inhibition of KATP channel are still unclear.
MicroRNAs (miRs) are important regulators of gene expression especially in diseased conditions. In eukaryotes, miRs are encoded by nuclear DNA and transcribed as longer hairpin transcripts known as pre-miRs. After processed by the Drosha and Dicer enzymes, one strand of the hairpin duplex is loaded to an Argonaute family protein to form the core of miR-induced silencing complex that subsequently functions via base-pairing with complementary sequences of the target mRNA to regulate the mRNA life and its capability of translation. In the process, the binding of miRs to the target mRNA is important for their recognition. Most miR binding sites sufficient for the transcript silencing are located in the 3′-untranslated region (3′-UTR), while some are in the coding sequence (CDS). Our previous studies have suggested that MGO acts on the CDS of SUR2B mRNA, impairing their stability as well as KATP channel activity (44). Thus, it is possible that under diabetic conditions, the reactive carbonyl stress raises the expression of miRs that subsequently target the KATP channel, and impair the vascular tone regulation. To test this hypothesis, we performed the present studies.
MATERIALS AND METHODS
Reagents.
Antibodies against Kir6.1 and GAPDH were purchased from Sigma-Aldrich (St. Louis, MO). Polyclonal antibodies against SUR2B were purchased from Santa Cruz Biotechnology (Dallas, TX). All other chemicals and reagents were purchased from common commercial sources. Reagents were prepared in stocks with high-concentration in double-distilled water or DMSO. The final concentration of DMSO in experiments was <0.1% (vol/vol), which was tested to have no detectable effects.
Cell culture.
HEK293 cells (CRL-1573; American Type Culture Collection, Manassas, VA) and rat VSM cells (A10 cell line, CRL-1476; American Type Culture Collection) were cultured in complete DMEM (10% FBS) in a 5% CO2 atmosphere at 37°C. Two to four generations were used for experiments.
Cell transfection.
After growth arrest, the cells were transfected using lipofectamine. Subsequently, the cells were switched to complete DMEM (10% FBS) and cultured in a 5% CO2 atmosphere at 37°C for another 24–48 h.
Bioinformatics prediction of miR targets.
To identify the candidate miRs that potentially targeted rat Kir6.1 (Kcnj8), we used the miRWalk database (https://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html) that is supported by eight miRNA prediction programs on 3′-UTRs of all known genes of the human, mouse, and rat. A probability distribution of random matches of a subsequence (miR 5′-end sequence) in the given sequence was calculated by using Poisson distribution, where a low probability implies a significant hit (20, 35). The default P value (0.05), the mirSVR score ≤ 0 (33), plus at least six complementarity seeds were used for miR/Kcnj8 alignment. A number of miRs potentially target Kir6.1 3′-UTR. To limit the number we used the following criteria: 1) their high conservation across a wide range of mammals and 2) their potential involvement in diabetes in existing literature. On the basis of these criteria, three potential Kir6.1-targeting miRs were selected for further screening. Similarly, six potential miRs for SUR2B CDS targeting were predicted using the same database. The targeting site conservation was examined among rats, mice, and humans by comparing NCBI blastn alignment as the similar searches with human KCNJ8/ABCC9 showing no miRs matches in the same 3′-UTR/coding region.
Synthesis of miR-9a-3p and anti-miR-9a-3p.
Chemically synthesized and optimized double-strand nucleotides (m-9) were designed to mimic endogenous mature miR-9a-3p in VSM and scrambled RNAs (scmiR) was synthesized as negative control (Qiagen, Sample & Assay Technologies, Valencia, CA). Single-strand antisense oligonucleotides (anti-9) complementary to the mature miR-9a-3p were synthesized to specifically target and knock down endogenous miR-9a-3p in VSM (Sigma-Aldrich).
Construction of Kir6.1 and SUR2B mRNA expression vectors.
The cDNAs encoding rat Kir6.1 mRNA (GenBank no. D42145.1) and mouse SUR2B mRNA isoform (GenBank no. D86038, mRNA isoform NM_011511) were cloned and inserted into pcDNA3.1 (a eukaryotic expression vector), respectively, as previously described (44), which were named with Kir and SUR, respectively.
Site-directed mutagenesis.
The mutated pcDNA3.1 construct of the SUR2B (M-SUR) was obtained using the Stratagene QuikChange mutagenesis (New England BioLabs, Ipswich, MA) and used for real-time quantitative PCR (qPCR) and patch studies. The mutagenic oligonucleotide primer pair was designed according to the desired mutation in its seed match sequences. Briefly, the M-SUR was cloned with the primer pair (forward: CCCTAAATTACTTTTGGCCTTATTCCTGTACTGG; reverse: CCAGTACAGGAATAAGGCCAAAAGTAATTTAGGG), containing two site mutations in position 622 and 625 of SUR2B mRNA (Fig. 1B).
Fig. 1.
Mutation sites in Kir6.1 and SUR2B mRNA. A: evolutionary conservation of miR-9a-3p among mammals. B: sequences of SUR2B mRNA (SUR). C: sequences of mutated SUR2B mRNA (M-SUR). Mutated sites were numbered in SUR2B mRNA. Some omitted sequences are represented with “N”. Underlined letters are targeting sites for miR-9a-3p.
Heterologous expression of KATP channel.
KATP channels were heterologously expressed in HEK293 cells as previously described (44). Briefly, the constructions of Kir6.1 and SUR2B mRNA expression vectors were cotransfected to the HEK293 cells (ratio of 1:3), and the pEGFP-N2 (Clontech, Palo Alto, CA) was transfected together to identify the positively transfected cells. One day after transfection, the cells were again transfected with synthesized m-9 or anti-9. After another day in culture, the cells were used for electrophysiological studies and luciferase analysis.
Patch-clamp studies.
The single-cell voltage clamp was used to record whole cell KATP currents in cells transfected with different agents. Patch-clamp protocols were performed as previously described (44). Briefly, the patch pipettes were made with 1.2 mm borosilicate glass capillaries (with resistance of 2–5 MΩ). Current records were filtered with low-pass (2 kHz, Bessel 4-pole filter, −3 dB), digitized (20 kHz, 16-bit resolution), and analyzed with Clampfit 9 software (Axon Instruments, Union City, CA) The bath solution contained (in mM) 10 KCl, 135 potassium gluconate, 5 EGTA, 5 glucose, and 10 HEPES (pH 7.4). The pipette solution contained (in mM) 133.0 K+ gluconate, 10.0 KCl, 5.0 EGTA, 5.0 glucose, 1 K2ATP, 0.5 NaADP, and 10.0 HEPES (pH 7.4). The final Mg2+ concentration was adjusted to 1 mM. The membrane potential was held at 0 mV and stepped to −80 mV every 3–4 s.
Real-time quantitative PCR.
The qPCR analysis was performed with high-capacity cDNA Reverse Transcription Kit and Fast SYBR Green Master Mix (Applied Biosystems, Life Technologies, New York, NY) following the manufacturer's instructions. Primers specific for Kir6.1, SUR2B, miRs, RNU6B, and GAPDH were synthesized from Sigma Genesis (Sigma). The qPCR was performed with a Fast Real-time PCR system (Applied Biosystems 7500) for 40 cycles. The fold increase relative to control samples was determined by the 2−2ΔΔCT method. Expression levels of target mRNAs were determined using total RNA from A10 or HEK cells. GAPDH was used as internal control for SUR2B mRNA expression. RNU6 was used for normalization of miR-9a-3p expression.
Western blot analysis.
Proteins extracted from A10 and HEK cells were detected using a standard Western blot protocol. GAPDH was used as an internal control.
Statistical methods.
Data are expressed as means ± SE. Comparisons of data were accomplished by one-way ANOVA followed by post hoc Dunnett's test or Student's t-test. The differences between means were considered significantly different when P ≤ 0.05.
RESULTS
Expression profiling of candidate miRNAs in reactive carbonyl stress.
In diabetic patients, persistent hyperglycemia leads to overproduction of MGO to ∼400 μM (23, 30). In our previous study, we found that exposure to 300 μM MGO causes disruption of vascular KATP channels (44). Therefore, we used this concentration of MGO in the present study.
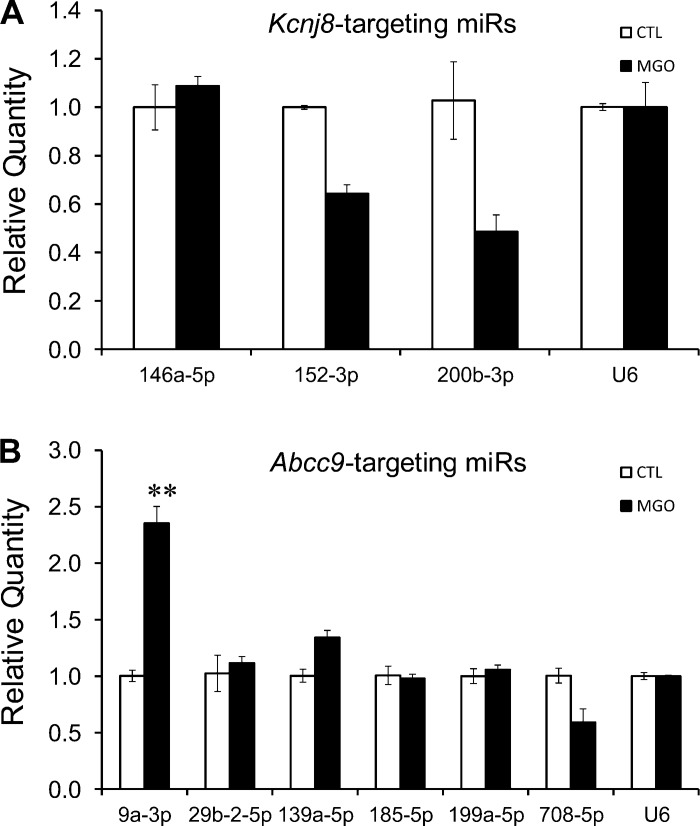
We first screened the potential miR candidates targeting mRNAs of rat Kir6.1 and SUR2B subunits using bioinformatics, as our previous experiments were mostly done in the rat A10 VSM line. On the basis of their mammalian conservation and potential involvement in diabetes in existing literature, nine diabetes-associated miRs were found. Among them, three miRs were potentially targeting Kir6.1, and six miRs were potentially targeting SUR2B. Subsequently, the expression profiles of the nine mature miRs were determined in A10 VSM line following MGO exposure. Our qPCR analysis showed that the MGO treatment resulted in a significant increase of miR-9a-3p level by 2.4 ± 0.2-folds (n = 3 separated experiments with 3–6 samples each), while none of the other miRs increased their expression significantly (Fig. 2). We did not further study miRs that showed reductions in their expression as according to current literature they did not seem to have a direct effect on KATP channel inhibition by MGO exposure. Because of this and because miR-9a-3p is highly conserved in mammals (Fig. 1A), all further studies were performed on miR-9a-3p.
Fig. 2.
Profiling of methylglyoxal (MGO)-regulated microRNAs (miRs) in A10 cells. A10 cells were treated with 300 μM MGO for 6 h. Real-time quantitative PCR (qPCR) analysis was then performed to show changes in expression levels of candidate miRs targeting Kir6.1 (A) and SUR2B mRNA (B). RNU6 (U6) was used for normalization. **P < 0.01 (n = 3 separate experiments with 3–6 samples each). 9a-3p, miR-9a-3p; 29b-2-5p, miR-29b-2-5p; 139a-5p, miR-139a-5p; 146a-5p, miR-146a-5p; 152-3p, miR-152-3p; 185-5p, miR-185-5p; 199a-5p, miR-199a-5p; 200b-3p, miR-200b-3p; 708-5p, miR-708-5p. Ctl, control.
Inhibition of KATP channel expression by MGO and miR-9a-3p.
To show whether miR-9a-3p affects KATP channel expression, one chemically synthesized and optimized double-strand nucleotide (m-9) was designed with an identical sequence to the mature endogenous miR-9a-3p. Also synthesized was one single-strand nucleotide complementary to the mature miR-9a-3p (anti-9). We then transfected the A10 cells with these synthetic nucleotides and studied their effects on the expression of SUR2B subunit after MGO exposure.
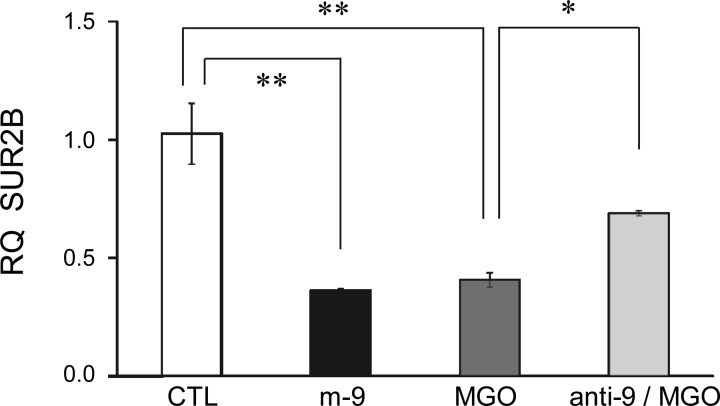
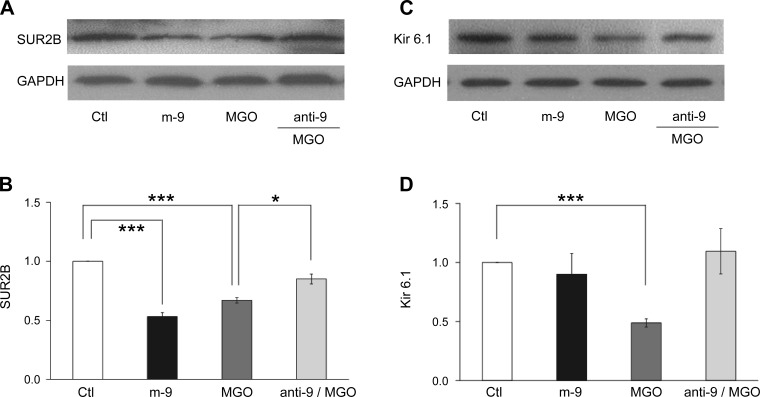
Our qPCR analysis showed that the m-9 transfection caused suppression of the SUR2B mRNA expression (Fig. 3), which resembled the effect of MGO. Meanwhile, the effect of MGO was markedly diminished when the cells were transfected with anti-9 (Fig. 3). Consistent with qPCR results, Western blot analysis showed that m-9 inhibited SUR2B expression at the protein level, while anti-9 partially blocked the MGO effect (Fig. 4, A and B). To determine whether m-9 also acts on Kir6.1 in the same conditions, we tested the expression of Kir6.1 at protein level. Our data showed that m-9 had no effect on Kir 6.1 protein expression (Fig. 4, C and D). These results suggest that miR-9a-3p regulates vascular KATP channel expression, which appears necessary for MGO to produce its effect.
Fig. 3.
Inhibition of SUR2B mRNA expression by MGO and exogenous miR-9a-3p. After transfection with m-9, A10 cells were cultured for 12–24 h. Cells transfected with anti-9 were also treated with 300 μM MGO and cultured under the same conditions. A marked reduction in the relative quantity (RQ) of SUR2B mRNA was found in cells transfected with m-9, to the similar degree as the effect of 300 μM MGO treatment. The effect of MGO was significantly reduced in cells transfected with anti-9. Cells transfected with scrambled RNA (scmiR) were used as control. GAPDH was used for internal normalization. *P < 0.05; **P < 0.01 (n = 4).
Fig. 4.
Effects of miR-9a-3p on MGO-induced inhibition of SUR2B/Kir6.1 expression at the protein level. After transfection with m-9, A10 cells were cultured for 12–24 h. Cells transfected with anti-9 were also treated with 300 μM MGO and cultured under the same conditions. Cells transfected with scmiR were used as negative control, and GAPDH was used for normalization. A and C: Western blot assay of expression of protein levels of SUR2B (A) and Kir6.1 (C). B and D: bar graphs represent the photodensity of each protein. *P < 0.05; ***P < 0.001 (n = 3–4).
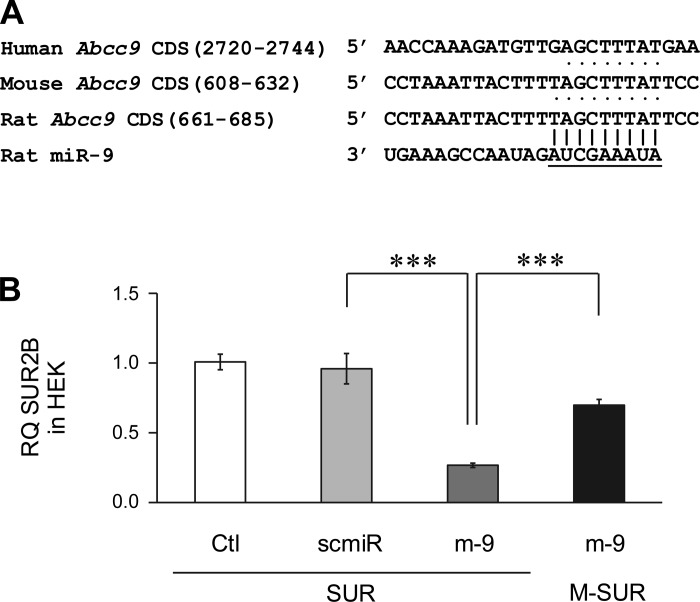
Targeting at the CDS of SUR2B.
Our bioinformatics analysis showed that a 9 seed-nucleotide region in the position 661–685 of rat SUR2B mRNA matches the miR-9a-3p, which also can be recognized in mouse (608–632) and human (2720–2744) SUR2B mRNAs (Fig. 5A). Several constructs were made to demonstrate whether such a potential binding site interacts directly with miR-9a-3p. The mouse SUR2B mRNA was cloned into the pcDNA3.1 vector (SUR). Site-directed mutagenesis of two nucleotides at positions 622 and 625 without changing the amino acids (M-SUR) was also carried out using the same vector (Fig. 1C). We then cotransfected the HEK293 cells together with the M-SUR and m-9. The cotransfection of HEK293 cells with the SUR alone or scmiR was used as negative control. As shown in Fig. 5B, the cotransfection of HEK293 cells with the SUR and m-9 resulted in downregulation of SUR mRNA expression compared with controls, which is consistent with our findings in the A10 cells. This effect of m-9 was abrogated when the miR-9a-3p binding site was mutated (Fig. 5B). Therefore, these results suggest that miR-9a-3p is likely to target the SUR2B CDS, and the position 608–632 seems to be an important target site.
Fig. 5.
Targeting sites in SUR2B mRNAs by miR-9a-3p. A: seed match regions of miR-9a-3p to the SUR2B mRNA. The seed regions were numbered in positions of SUR2B mRNA. The conserved seed matches in different species are represented with dots. B: qPCR assay for SUR2B mRNA reduction by CDS targeting. Cotransfection of HEK293 cells with m-9 and the SUR2B mRNA construct (SUR) led to significant inhibition of SUR2B expression levels. Such inhibition was abrogated when cells were cotransfected with m-9 and M-SUR (2 site mutations in SUR2B mRNA). ***P < 0.001 (n = 3).
Inhibition of functional KATP currents by miR-9a-3p.
To prove that exogenous m-9 and anti-9 had a functional impact on KATP channels, we studied its effects on heterologously expressed Kir6.1/SUR2B channels in HEK293 cells, in which KATP currents were relatively large and sufficient for a long-term analysis. Equal high concentrations of K+ (145 mM) were applied to both sides of the membranes. The membrane potential was held at 0 mV and stepped to −80 mV every 3 s in voltage clamp. Pinacidil (Pin), a specific KATP opener, and glibenclamide (Glib), a KATP inhibitor, were used to set a window of KATP channel activity. At the basal level, KATP channel activity was low. Exposure to 10 μM Pin strongly activated KATP currents, which were subsequently suppressed by 10 μM Glib (Fig. 6A).
Fig. 6.
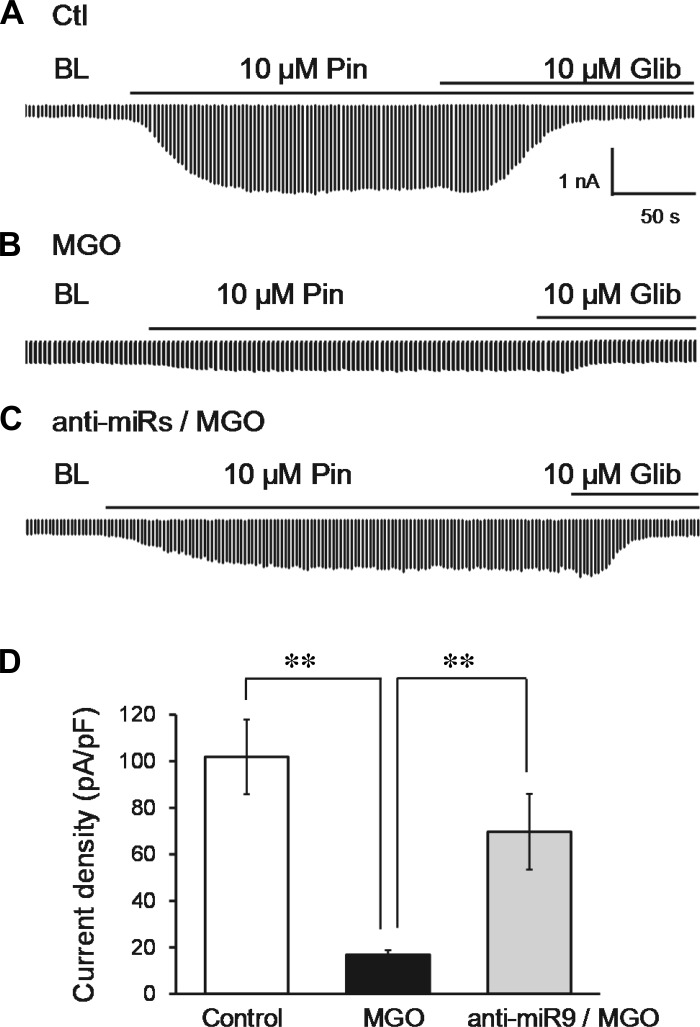
Effects of MGO and miR-9a-3p on functional ATP-sensitive K+ (KATP) currents. One day after Kir6.1/SUR2B channels were expressed in HEK293 cells, the cells transfected with anti-9 were treated with 300 μM MGO and cultured for 12–24 h. Cells transfected with scmiR were used as negative control. A: KATP currents were recorded using symmetric K+ concentrations of internal and bath solutions. Inward K+ currents were elicited with voltage commands from 0 to −80 mV every 3 s. KATP currents were strongly activated by 10 μM pinacidil (Pin) and were inhibited by 10 μM glibenclamide (Glib). BL, baseline. B: MGO treatment significantly suppressed KATP current in HEK cells. C: MGO-induced reduction of KATP currents was reversed in anti-9-transfected cells. D: current density is represented in the bar graph. **P < 0.01 (n = 8–10 cells).
Consistent with our previous study (44), a marked inhibition in KATP currents occurred after cells were treated with MGO for 12 h (Fig. 6B). In cells transfected with anti-9, the MGO effect was drastically attenuated (Fig. 6, C and D).
A significant inhibition in KATP currents was found after the cells were cotransfected with m-9 (Fig. 7B), to the degree similar to the effect of MGO on KATP currents (Fig. 6B). KATP current inhibition was reversed partially when the binding sites for miR-9a-3p were mutated in SUR2B (M-SUR) (Fig. 7, C and D). In contrast, scmiR had no effect on KATP channel activity (data not shown).
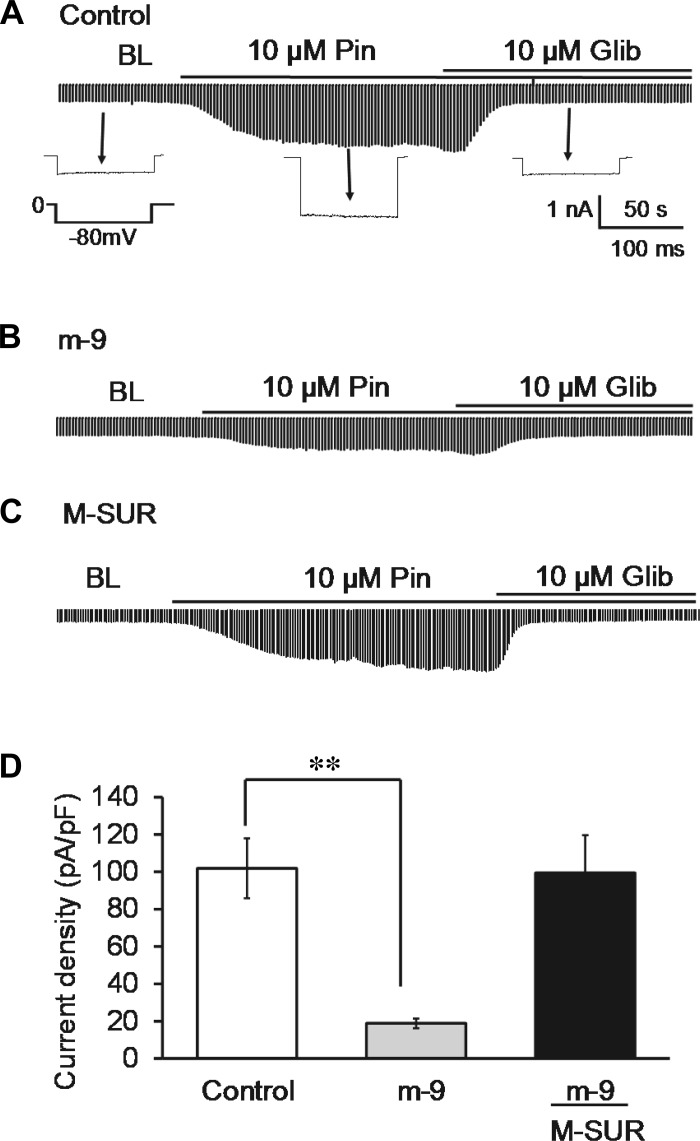
Fig. 7.
Inhibition of KATP currents by miR-9a-3p. Experiments were done as described in Fig. 6. Cells transfected with scmiR were used as negative control. A: KATP currents were recorded from the negative control (Ctl). B: Pin-induced currents became much smaller in m-9-transfected cells. C: m-9-induced current inhibition was abrogated when their targeting sites in SUR2B subunit were mutated. D: effect of miR-9a-3p on current density is represented in the bar graph. **P < 0.01 (n = 8–10 cells).
DISCUSSION
This is the first demonstration of regulation of KATP channel by miRs. The miR-9a-3p is upregulated in MGO-induced carbonyl stress. By targeting the CDS of SUR2B, the miR-9a-3p inhibits KATP channel expression, leading to a reduction in KATP channel activity.
In diabetic conditions, persistent hyperglycemia leads to overproduction of a variety of RCS including the highly reactive MGO, contributing to the development of diabetic complications. Acting on proteins, lipids, or nucleotides, excessive MGO can cause carbonyl stress and cell damage. Our previous studies have shown that MGO acts on KATP channels in VSM cells, causing instability of Kir6.1 and SUR2B mRNAs, in which the 3′-UTR of Kir6.1 and the CDS of SUR2B are likely to be targeted (44). As a result, a loss of functional KATP channels occurs followed by dysregulation of vascular tone (44).
Emerging evidence suggests that miRs contribute to the pathogenesis of diabetes and diabetic complications (5, 15, 31, 43). Since each miR has its potential targeting genes, information of miR involvement in diabetes is helpful for identification of the targeted molecules as well. The expression of these miRs may be upregulated by several pathological conditions in diabetes including the carbonyl stress. Therefore, it is reasonable to believe that the instability of Kir6.1 and SUR2B mRNAs shown in our previous studies is attributable to certain miRs.
With bioinformatics prediction, nine diabetes-associated miRs were selected for the profiling in carbonyl stress. With the information, we treated the A10 VSM cell line with 300 μM MGO, a concentration that is seen in serum of diabetic patients and also found effective for KATP disruption (44). With the MGO treatment, we have found that miR-9a-3p is upregulated, while none of the other eight miRs show a significant increase in their expression levels. miR-9a-3p is highly conserved in mammals and has potential targeting sites in the human SUR2B gene as well. Under diabetic conditions, miR-9 is suggested as being involved in insulin secretion by targeting Sirt1 in pancreatic β-islets (34). However, no previous study has reported that miR-9a-3p is regulated by RCS under diabetic conditions. Therefore, our current study provides the first evidence for the upregulation of miR-9a-3p in reactive carbonyl stress.
In A10 cells, we overexpressed m-9 with/without MGO treatment and found inverse correlations between the exogenous miR-9a-3p and SUR2B mRNA levels. Consistently, antagonizing the endogenous miR-9a-3p with anti-9 reversed the MGO-induced reduction of SUR2B expression. In HEK cells, our qPCR and Western analysis further proved that the CDS of SUR2B was directly targeted by miR-9a-3p. These results thus indicate that miR-9a-3p is involved in MGO-induced disruption of vascular KATP channels by targeting at the CDS of SUR2B.
The SUR2B targeting by miR-9a-3p should have an impact on KATP channel activity. Our data indeed show that miR-9a-3p inhibits functional KATP currents to the degree similar to MGO treatment. The effects of MGO on KATP channel can partially be reversed when the endogenous miR-9a-3p was knocked down with anti-9, further supporting that miR-9a-3p mediates the modification of KATP channels in carbonyl stress. Our site-directed mutagenesis studies confirm that miR-9a-3p targets SUR2B mRNA at the CDS region.
In our previous studies, we have found that MGO causes instability of both Kir6.1 and SUR2B mRNAs (17). Although miR-9a-3p is likely to target SUR2B, the Kir6.1-targeting miR(s) remains to be demonstrated. Since none of the three conserved miRs that we have studied seems to be the player, and since miR-9a-3p does not affect Kir6.1 expression either, we speculate that the Kir6.1 may be targeted by miRs that are either nonconserved or missing in the database. Alternatively, the Kir6.1 inhibition by MGO may not be mediated by miRs at all.
Mouse models of loss-of-function of SUR2 genes have shown the critical role of the vascular KATP channel in the coronary circulation. KATP channel dysfunction in mice leads to coronary vasospasm and sudden death (9, 27). Notably, in SUR2−/− mice, loss of hyperpolarizing KATP current causes abnormally elevated [Ca2+]i, leading to a reduction in coronary artery vasospasm (9). Therefore, vascular KATP channel targeting by miR-9a-3p appears consistent with the adverse effects of MGO on vasculatures.
Recent studies indicate that the activity of the vascular isoform of KATP channels is necessary for the systemic responses in diabetes. The increased metabolic rate requires a corresponding elevation in cardiac output to meet the metabolic demands. Therefore, KATP-mediated vasodilation may act as compensatory role for several vital organs perfusion. Our studies indicate that miR-9a-3p modulates the KATP channel, which compromises the compensatory vasodilation in several vital organs and leads to inadequate perfusion, contributing to tissue hypoxia and cell injury. Therefore, our studies on miR- mediated KATP channel regulation in diabetes may help in the understanding of diabetic organ dysfunction.
A number of diabetes-associated miRs have been identified. Some miRs are involved in tissue dysfunction, including retina, kidney, peripheral nerves, heart, and the vasculature. For instance, some miRs modulate the renin-angiotensin-aldosterone system, like miR-181a, miR-663, miR-155, miR-29b, miR-129-3p, and miR-132, and oxidative stress like miR-377, miR-23a/b, miR-27a, miR-24, miR-335, miR-205, and miR-210 in diabetic nephropathy (19). Some miRs have been proved to be involved in vascular endothelial damage like miR-195, miR-503, and miR-146a in diabetic retinopathy (6, 8, 29), and some miRs like miR-34b, miR-34c, miR-199b, miR-210, miR-650, and miR-223 are shown to be dysregulated in diabetic ischemic heart failure patients (18). Therefore, our present study illustrating the role of MGO-induced miR-9a-3p in VSM cells helps in the understanding of the molecular mechanisms of vasculature dysfunction in diabetes.
At present, the mechanisms regulating miR expression and activity are still not fully understood. There is no information on the up- and downregulation of miRs by MGO in VSM cells either. Since the basic miR biogenesis pathways involve miR transcription, Drosha and Dicer processing, RNA editing, RNA modification, Argonaute loading, and RNA decay, MGO as a reactive carbonyl may act on one or some of these molecules affecting miR expression. Accumulating evidence suggests that aberrant DNA methylation of tumor suppressor genes occurs commonly in cancer, and events for miR hypermethylation/hypomethylation are found to play a role in human metastasis (24, 25). Indeed, MGO-mediated DNA demethylation has been found to alter the cellular redox balance in human cataract formation (32). Similar mechanisms may work in the VSM cells in reactive carbonyl stress. Thus, our findings in the present study may stimulate further studies of the regulation of miRs by RCS as well as the resulting vascular complications in diabetes.
Other unidentified targets may exist as miR-9a-3p sequence has multiple partial matches with Abcc9, Kir6.1, and other mRNAs. Indeed, miR-9a-3p seed match regions are found at three places in rat Abcc9 (675-AGCTTTAT-682, 5404-GCTTTAT-5398, and 5978-GCTTTAT-5972). Thus, we cannot rule out the possibility that other sites may also be targeted by miR-9a-3p in rat SUR2B and perhaps Kir6.1 as well.
In conclusion, the present study provides the first evidence for regulation of KATP channels by miRs. Our results indicate that miR-9a-3p plays an important role in the regulation of vascular KATP channels in reactive carbonyl stress, acting on the CDS of the SUR2B and inhibiting KATP channel activity. Therefore, the stabilization of miR-9a-3p levels may be a novel strategy for clinical treatment of diabetic vascular complications.
GRANTS
This work was supported by the National Institutes of Health (National Institute of Child Health and Human Development Grant HD-060959 and National Institute of Neurological Disorders and Stroke Grant NS-073875).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
SS.L., Y.W., X.J., and C.J. conception and design of research; SS.L., Y.W., and X.J. performed experiments; SS.L., Y.W., X.J., and C.J. analyzed data; SS.L., Y.W., X.J., and C.J. interpreted results of experiments; SS.L., Y.W., and X.J. prepared figures; SS.L., Y.W., X.J., and C.J. drafted manuscript; SS.L., Y.W., X.J., and C.J. edited and revised manuscript; C.J. approved final version of manuscript.
REFERENCES
- 1.Bansal S, Chawla D, Siddarth M, Banerjee BD, Madhu SV, Tripathi AK. A study on serum advanced glycation end products and its association with oxidative stress and paraoxonase activity in type 2 diabetic patients with vascular complications. Clin Biochem 46: 109–114, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Bourajjaj M, Stehouwer CD, van Hinsbergh VW, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem Soc Trans 31: 1400–1402, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 29: 312–316, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Buraczynska M, Zukowski P, Wacinski P, Ksiazek K, Zaluska W. Polymorphism in microRNA-196a2 contributes to the risk of cardiovascular disease in type 2 diabetes patients. J Diabetes Complications 28: 617–620, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation 123: 282–291, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Chang T, Wu L. Methylglyoxal, oxidative stress, and hypertension. Can J Physiol Pharmacol 84: 1229–1238, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 5: 949–966, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest 110: 203–208, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper KO, Witz G, Witmer CM. Mutagenicity and toxicity studies of several alpha,beta-unsaturated aldehydes in the Salmonella typhimurium mutagenicity assay. Environ Mutagen 9: 289–295, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Cooper RA. Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol 38: 49–68, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108: 1527–1532, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Croker B, Crozat K, Berger M, Xia Y, Sovath S, Schaffer L, Eleftherianos I, Imler JL, Beutler B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet 39: 1453–1460, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, Untereiner A, Wu L. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can J Physiol Pharmacol 88: 273–284, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Dumortier O, Hinault C, Gautier N, Patouraux S, Casamento V, Van Obberghen E. Maternal protein restriction leads to pancreatic failure in offspring: role of misexpressed microRNA-375. Diabetes 63: 3416–3427, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H, Cheeseman KH, Dianzani MU, Poli G, Slater TF. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J 208: 129–140, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang JL, Vaca CE. Development of a 32P-postlabelling method for the analysis of adducts arising through the reaction of acetaldehyde with 2′-deoxyguanosine-3′-monophosphate and DNA. Carcinogenesis 16: 2177–2185, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Greco S, Fasanaro P, Castelvecchio S, D'Alessandra Y, Arcelli D, Di Donato M, Malavazos A, Capogrossi MC, Menicanti L, Martelli F. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes 61: 1633–1641, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwara S, McClelland A, Kantharidis P. MicroRNA in diabetic nephropathy: renin angiotensin, aGE/RAGE, and oxidative stress pathway. J Diabetes Res 2013: 173783, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havilio M, Haddad Y, Smilansky Z. Intensity-based statistical scorer for tandem mass spectrometry. Anal Chem 75: 435–444, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Kane GC, Lam CF, O'Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J 20: 2271–2280, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, Basso E, Seraglia R, Traldi P. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med 41: 1166–1173, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-Cespedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, Gallagher WM, Eccles SA, Croce CM, Esteller M. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA 105: 13556–13561, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lujambio A, Esteller M. How epigenetics can explain human metastasis: a new role for microRNAs. Cell Cycle 8: 377–382, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res 148: 25–34, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 8: 466–472, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Miura H, Wachtel RE, Loberiza FR Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mortuza R, Feng B, Chakrabarti S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 57: 1037–1046, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Mukohda M, Yamawaki H, Okada M, Hara Y. Methylglyoxal enhances sodium nitroprusside-induced relaxation in rat aorta. J Pharmacol Sci 112: 176–183, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Osipova J, Fischer DC, Dangwal S, Volkmann I, Widera C, Schwarz K, Lorenzen JM, Schreiver C, Jacoby U, Heimhalt M, Thum T, Haffner D. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J Clin Endocrinol Metab 99: E1661–E1665, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Palsamy P, Bidasee KR, Ayaki M, Augusteyn RC, Chan JY, Shinohara T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radic Biol Med 72: 134–148, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson SM, Thompson JA, Ufkin ML, Sathyanarayana P, Liaw L, Congdon CB. Common features of microRNA target prediction tools. Front Genet 5: 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J 278: 1167–1174, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Sadygov RG, Yates JR 3rd. A hypergeometric probability model for protein identification and validation using tandem mass spectral data and protein sequence databases. Anal Chem 75: 3792–3798, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Shi WW, Yang Y, Shi Y, Jiang C. K(ATP) channel action in vascular tone regulation: from genetics to diseases. Sheng Li Xue Bao 64: 1–13, 2012. [PMC free article] [PubMed] [Google Scholar]
- 37.Sousa Silva M, Gomes RA, Ferreira AE, Ponces Freire A, Cordeiro C. The glyoxalase pathway: the first hundred years… and beyond. Biochem J 453: 1–15, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Thornalley PJ. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31: 1343–1348, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269: 1–11, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vander Jagt DL, Robinson B, Taylor KK, Hunsaker LA. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem 267: 4364–4369, 1992. [PubMed] [Google Scholar]
- 41.Vasdev S, Ford CA, Longerich L, Gadag V, Wadhawan S. Role of aldehydes in fructose induced hypertension. Mol Cell Biochem 181: 1–9, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Liu J, Wu L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem Pharmacol 77: 1709–1716, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Wang JY, Gao YB, Zhang N, Zou DW, Wang P, Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY, Yang JK. miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol 392: 163–172, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Li S, Konduru AS, Zhang S, Trower TC, Shi W, Cui N, Yu L, Wang Y, Zhu D, Jiang C. Prolonged exposure to methylglyoxal causes disruption of vascular KATP channel by mRNA instability. Am J Physiol Cell Physiol 303: C1045–C1054, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C25–C37, 1998. [DOI] [PubMed] [Google Scholar]