Abstract
We have previously demonstrated that aging reduces the compensatory vasodilator response during hypoxic exercise due to blunted nitric oxide (NO) signaling. Recent evidence suggests that NO bioavailability can be augmented by dietary nitrate through the nitrate-nitrite pathway. Thus we tested the hypothesis that acute dietary nitrate supplementation increases the compensatory vasodilator response to hypoxic exercise, particularly in older adults. Thirteen young (25 ± 1 yr) and 12 older (64 ± 2 yr) adults performed rhythmic forearm exercise at 20% of maximum voluntary contraction during normoxia and hypoxia (∼80% O2 saturation); both before (control) and 3 h after beetroot juice (BR) consumption. Forearm vascular conductance (FVC; ml·min−1·100 mmHg−1) was calculated from forearm blood flow (ml/min) and blood pressure (mmHg). Compensatory vasodilation was defined as the relative increase in FVC due to hypoxic exercise (i.e., % increase compared with respective normoxic exercise trial). Plasma nitrite was determined from venous blood samples obtained before the control trials and each of the exercise trials (normoxia and hypoxia) after BR. Consumption of BR increased plasma nitrite in both young and older adults (P < 0.001). During the control condition, the compensatory vasodilator response to hypoxic exercise was attenuated in older compared with young adults (3.8 ± 1.7% vs. 14.2 ± 1.2%, P < 0.001). Following BR consumption, compensatory vasodilation did not change in young (13.7 ± 3.3%, P = 0.81) adults but was substantially augmented in older adults (11.4 ± 2.1%, P < 0.01). Our data suggest that acute dietary nitrate supplementation increases the compensatory vasodilator response to hypoxic exercise in older but not young adults.
Keywords: aging, vasodilation, hypoxia, exercise, dietary nitrate
hypoxia can have profound influences on the circulation, including vasodilation in skeletal muscle vascular beds. In humans, acute exposure to moderate hypoxia in combination with submaximal exercise produces a compensatory vasodilation and augmented blood flow in contracting skeletal muscles relative to the same level of exercise under normoxic conditions (7, 28, 54). In a series of studies we explored a number of potential mechanisms that might contribute to the compensatory vasodilation observed during hypoxic exercise. These studies have investigated the contribution of vasodilating substances such as nitric oxide (NO) and adenosine, the role of β-adrenergic receptors, and the interactions between sympathetic vasoconstriction and metabolic vasodilation in the regulation of vascular tone during hypoxic exercise (8, 10, 11, 65, 66). A synthesis of our findings clearly indicates that NO contributes to the compensatory vasodilator responses to exercise under conditions of reduced oxygen availability in young adults (8–10). However, we recently demonstrated that 1) the compensatory vasodilation during hypoxic exercise is reduced with aging and 2) this attenuated response is probably due to blunted NO signaling (13).
It is widely known that the l-arginine-NO synthase (NOS) pathway significantly contributes to the overall production of NO. Once synthesized, NO is rapidly oxidized to form nitrite (NO2−) and nitrate (NO3−). Until recently the inorganic anions NO2− and NO3− were considered inert end products of NO metabolism. However, accumulating evidence suggests that NO2− reduction (i.e., nitrate-nitrite-NO pathway) represents an alternative and differentially regulated system for NO generation that operates in parallel with the classic l-arginine-NOS pathway (5, 41–43). Interestingly, the nitrate-nitrite-NO pathway is greatly enhanced under hypoxic conditions (61). Additionally, recent evidence in experimental animals suggests that dietary nitrate supplementation increases skeletal muscle blood flow and vasodilation during exercise (22). Therefore, increasing plasma NO2− and NO3− levels in humans may enhance NO bioavailability and, subsequently, the hyperemic and vasodilator responses to exercise. With this information as background we aimed to examine the effects of acute dietary nitrate supplementation on the local control of skeletal muscle blood flow and vasodilation during exercise in young and older adults. We hypothesized that acute dietary nitrate supplementation via beetroot (BR) juice would augment blood flow and vasodilation in contracting skeletal muscle, particularly under hypoxic conditions. Moreover, we hypothesized that the improvement in the compensatory vasodilator response to hypoxic exercise would be greater in older adults than in young adults.
METHODS
Subjects.
A total of 13 young and 12 older healthy subjects volunteered to participate in the study. Subjects completed written informed consent and were healthy, nonobese (body mass index ≤30 kg/m−2), nonsmokers, not taking any vasoactive medications, and were sedentary to moderately active. Two older subjects were taking aspirin (withheld 5 days prior to study) and seven older subjects reported taking a daily vitamin (withheld 3 days prior to study). Studies were performed after an overnight fast and refraining from exercise and caffeine for at least 24 h. Subjects were also instructed to refrain from using antibacterial mouthwash or chewing gum the morning of the study. This was to avoid any possible interference with the processing of NO3− and NO2− in the saliva or stomach (23, 48). Young female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (45, 46). All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy. All study protocols were approved by the University of Iowa Institutional Review Board and were performed according to the Declaration of Helsinki.
Forearm exercise.
Subjects performed rhythmic forearm exercise with a handgrip device using the nondominant arm at 20% of each subject's maximal voluntary contraction (MVC). The weight was lifted 4 to 5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions per minute) using a metronome to ensure correct timing. The average weight used for forearm exercise was 9.7 ± 0.7 kg and 8.1 ± 0.6 kg for the young and older adults, respectively (P = 0.11).
Heart rate and systemic blood pressure.
Heart rate was recorded via continuous three-lead electrocardiogram, and systemic blood pressure was assessed (beat to beat) with a finger plethysmograph (Nexfin; Edwards Lifesciences, Irvine, CA) on the nonexercising hand.
Forearm blood flow.
Brachial artery mean blood velocity and diameter were determined with a 12-MHz linear-array Doppler probe (model M12L; Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured with a probe insonation angle previously calibrated to 60°. Measured brachial velocity wave forms were synchronized to a data acquisition system (WinDaq; DATAQ Instruments, Akron, OH) via a Doppler audio transformer (26). Brachial artery diameter measurements were obtained at end diastole between contractions during steady-state conditions. Forearm blood flow (FBF) was calculated as the product of mean blood velocity (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min). Mean blood velocity measurements included the velocity profiles across the entire duty cycle (both contraction and relaxation phases).
Systemic hypoxia.
Subjects were instrumented with a tight-fitting oronasal facemask and a self-regulating partial rebreathe system that clamps end-tidal CO2 at baseline levels despite large changes in minute ventilation and was used to help generate hypoxic conditions (4, 10, 13). The target arterial O2 saturation (assessed via pulse oximetry) for the hypoxia trials was ∼80%. The amount of O2 provided in the inspiratory gas was controlled by mixing N2 with medical air via an anesthesia gas blender. End-tidal carbon dioxide concentrations were monitored throughout each trial (Cardiocap/5; Datex-Ohmeda, Louisville, CO).
Nitrite analysis.
Venous blood was sampled in the nonexercising arm via an 18- or 20-gauge, 5-cm catheter. Venous blood for the determination of circulating [NO2−] was collected in tubes containing EDTA and immediately underwent centrifugation at 3,000 rpm for 15 min. Plasma was separated into Eppendorf tubes and immediately frozen at −80°C for later analysis of [NO2−]. All measurements of plasma [NO2−] were performed within 30 min of thawing using a Sievers chemiluminescence NO analyzer (NOA 280i; Sievers Instruments, Boulder, CO). [NO2−] was determined by addition of plasma samples to potassium iodide in acetic acid at room temperature in a gas-sealed purging vessel.
Experimental design.
Each subject completed four separate trials (two normoxia; two hypoxia) in a supine position in a temperature-controlled room. Each trial consisted of a resting baseline condition (2 min) followed by rhythmic forearm exercise (5 min) at 20% MVC. The first two trials were considered the control trials and were performed during normoxia and normocapnic hypoxia. Exposure to normoxia or hypoxia was alternated and randomized. All hypoxia trails included a 2-min normoxia (prehypoxia) period immediately before the level of inspired O2 was titrated to achieve an arterial O2 saturation of ∼80%.
Normoxic and hypoxic trials were repeated 3 h after consumption of 500 ml of a commercially available nitrate-rich BR (Biotta, Carmel IN). The measured NO3− content in the batch of BR used in the current study was 18,708 μmol/liter. The 3-h time frame was based on available data suggesting that plasma NO3− and NO2− levels peak ∼2 and 3 h after BR consumption, respectively (31, 67). Due to plasma NO3− and NO2− being elevated in the systemic circulation for up to 5 h after consumption of BR (63), the exercise trials following BR consumption were always performed last. A rest period of at least 20 min was allowed between trials under each condition (control and BR).
Seven (5 men/2 women) of the 12 older adults also completed a placebo control trial on a separate day. The experimental procedures and experimental design were identical to those outlined above, with the exception of 1) 140 ml of a concentrated nitrate-depleted BR (Beet It, Heartbeet Ltd.) and ∼360 ml of bottled water (Ice Mountain; Nestle, Stamford, CT) were consumed in place of the 500 ml of nitrate-rich BR and 2) blood draws were not performed. The measured NO3− content in the batch of the nitrate-depleted BR used for the placebo control trials was 280 μmol/liter. The nitrate-depleted BR trials were included during the review process of the original protocol described above and therefore were not used in a double-blind crossover design. That is, the subjects were kept blind to the idea that the BR was depleted of nitrate, whereas the investigators involved in the study were aware of the placebo condition.
Data analysis and statistics.
Data were collected at 250 Hz and analyzed offline with signal processing software (WinDaq; DATAQ Instruments). Mean arterial pressure (MAP) was derived from the Nexfin pressure waveform and heart rate was determined from the electrocardiogram. End-tidal CO2, O2 saturation (pulse oximetry; SpO2), heart rate, MAP, and FBF were determined by averaging values over the final minute of rest and exercise for each trial. Forearm vascular conductance (FVC) was calculated as (FBF/arterial pressure) × 100 and expressed as ml·min−1·100 mmHg−1. Plasma [NO2−] was determined from blood samples obtained prior to the control trials (baseline) and each of the exercise trials (normoxia and hypoxia) following consumption of the BR.
All values are expressed as means ± SE. ANOVA was used to analyze baseline differences between age groups. To determine the effect of acute nitrate supplementation on skeletal muscle blood flow and vasodilation at rest and during exercise in young and older adults under normoxic and hypoxic conditions, the differences in FBF and FVC were determined via repeated measures ANOVA. All other hemodynamic variables and plasma [NO2−] were also assessed via repeated measures ANOVA. Appropriate post hoc analysis determined where statistical differences occurred. When significance was detected, Tukey's post hoc analysis was used to identify differences between groups. Statistical difference was set a priori at P < 0.05. To further examine the effects of aging and nitrate supplementation on the compensatory vasodilator response to hypoxic exercise we compared via ANOVA the absolute and relative change in FVC (steady-state FVC during hypoxic exercise; steady-state FVC during normoxic exercise) between the young and older adults under each respective condition (with and without BR). Finally, the absolute and relative compensatory vasodilator responses to hypoxic exercise in the seven older adults who performed the placebo trials were compared with their respective responses during the nitrate-rich BR trials via a paired t-test.
RESULTS
Subject characteristics are summarized in Table 1. Although young and older subjects were of similar height and weight, older adults had a higher body mass index (P < 0.05). Forearm volume (P = 0.76) and MVC (P = 0.10) did not differ between the young and older subjects.
Table 1.
Subject characteristics
| Variable | Young | Older |
|---|---|---|
| Age, yr | 25 ± 1 | 64 ± 2* |
| Men/Women | 10/3 | 9/3 |
| Height, cm | 179 ± 3 | 174 ± 3 |
| Weight, kg | 74 ± 3 | 77 ± 2 |
| BMI, kg/m2 | 23.1 ± 0.6 | 25.5 ± 0.7* |
| FAV, ml | 1,015 ± 69 | 966 ± 48 |
| MVC, kg | 48 ± 3 | 40 ± 3 |
Values are means ± SE.
BMI, body mass index; FAV, forearm volume; MVC, maximal voluntary contraction.
P < 0.05 vs. young.
Effects of BR on plasma [NO2−].
Due to catheter-related issues, complete blood samples (all three time points; baseline and both the normoxia and hypoxia trials following BR consumption) were collected in only 20 (10 in each age group) of the 25 subjects. In both young and older adults plasma [NO2−] was substantially greater (approximately threefold) prior to each of the exercise trials (normoxia and hypoxia) following consumption of BR compared with baseline values (pre-BR consumption) (Fig. 1).
Fig. 1.
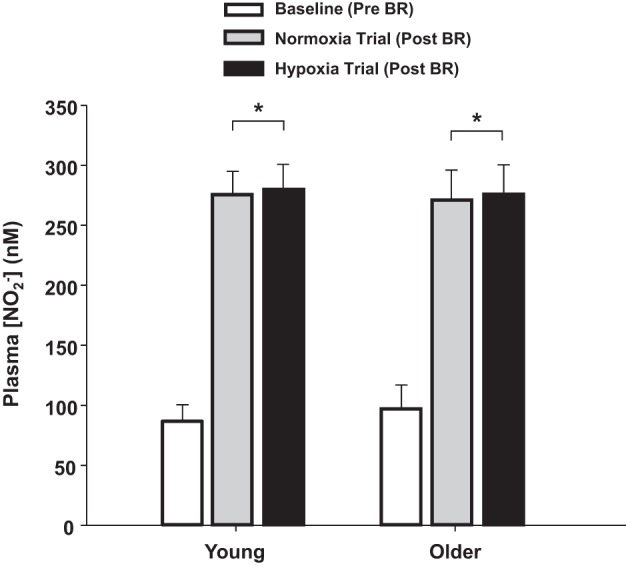
Plasma levels of nitrite (NO2−) before the control trials (baseline) and each of the exercise trials (normoxia and hypoxia) following consumption of beetroot juice (BR). Acute consumption of BR resulted in substantial increases in plasma [NO2−] in both young and older adults. *P < 0.001 vs. baseline.
Systemic hemodynamic responses.
The group data (means ± SE) for hemodynamic responses due to combined forearm exercise and hypoxia under each condition are presented in Table 2. Under normoxic conditions older adults demonstrated a lower SpO2 and end-tidal CO2 compared with their young counterparts (P < 0.05). As expected, SpO2 was decreased and heart rate increased as a consequence of systemic hypoxia and incremental forearm exercise in both the young and older adults (P < 0.05). Despite higher MAP values under resting conditions in older adults, the magnitude of change in MAP during exercise (both normoxia and hypoxia) was similar between age groups. By design, end-tidal CO2 was maintained during all conditions within each age group. Nitrate supplementation did not alter MAP, SpO2, or end-tidal CO2 at rest or during exercise under normoxic or hypoxic conditions in the young or older adults. However, resting heart rate during normoxic conditions was lower following BR consumption in both age groups (P < 0.05).
Table 2.
Hemodynamic responses at rest and with forearm exercise during normoxia and hypoxia before and after beetroot juice consumption
| Normoxia |
Hypoxia |
|||
|---|---|---|---|---|
| Rest | 20% MVC | Rest | 20% MVC | |
| Young (n = 13) | ||||
| Control (before beetroot juice) | ||||
| Mean arterial pressure, mmHg, | 91 ± 1 | 98 ± 2§ | 92 ± 1 | 99 ± 1§ |
| Heart rate, beats/min | 62 ± 2 | 70 ± 3§ | 76 ± 4* | 83 ± 4*§ |
| SpO2, % | 99 ± 0 | 99 ± 0 | 80 ± 1* | 81 ± 1* |
| End-tidal CO2, mmHg | 36 ± 1 | 37 ± 1 | 35 ± 1 | 36 ± 1 |
| After beetroot juice | ||||
| Mean arterial pressure, mmHg | 90 ± 1 | 98 ± 1§ | 92 ± 1 | 100 ± 1§ |
| Heart rate, beats/min | 56 ± 2‡ | 68 ± 3§ | 73 ± 3* | 84 ± 3*§ |
| SpO2, % | 99 ± 0 | 99 ± 0 | 81 ± 1* | 79 ± 1* |
| End-tidal CO2, mmHg | 37 ± 1 | 37 ± 1 | 35 ± 1 | 36 ± 1 |
| Older (n = 12) | ||||
| Control (before beetroot juice) | ||||
| Mean arterial pressure, mmHg | 95 ± 2† | 104 ± 2†§ | 98 ± 2† | 105 ± 2†§ |
| Heart rate, beats/min | 65 ± 2 | 70 ± 2§ | 72 ± 3* | 77 ± 3*§ |
| SpO2, % | 97 ± 1† | 97 ± 0† | 80 ± 1* | 80 ± 1* |
| End-tidal CO2, mmHg | 33 ± 1† | 33 ± 1† | 32 ± 1 | 33 ± 1 |
| After beetroot juice | ||||
| Mean arterial pressure, mmHg | 95 ± 2† | 104 ± 2†§ | 96 ± 2† | 104 ± 2†§ |
| Heart rate, beats/min | 60 ± 2‡ | 68 ± 3§ | 69 ± 3* | 74 ± 3*§ |
| SpO2, % | 97 ± 1† | 98 ± 0† | 80 ± 1* | 79 ± 1* |
| End-tidal CO2, mmHg | 33 ± 1† | 34 ± 1† | 32 ± 1 | 33 ± 1 |
MVC, maximal voluntary contraction.
Values are means ± SE.
Main effect of hypoxia, P < 0.05;
P < 0.05 vs. young;
P < 0.05 vs. control,
P < 0.01 vs. rest.
Forearm exercise.
Table 3 shows (means ± SE) forearm hemodynamics at rest and during normoxic and hypoxic exercise before and after BR consumption. In the young adults, FBF and FVC during hypoxic exercise were higher compared with normoxic exercise under both control and post-BR conditions (main effect of hypoxia, P < 0.05, Table 3). In the older adults, FBF and FVC did not differ between normoxic and hypoxic exercise under control conditions (P = 0.08 for both). However, the FBF and FVC response to hypoxic exercise post-BR was substantially greater compared with normoxic exercise under the same condition in older adults (P < 0.001, Table 3). In turn, FBF and FVC during hypoxic exercise post-BR were greater compared with values during hypoxic exercise under control conditions (P < 0.01). Although older adults demonstrated lower hypoxic exercise FBF and FVC responses compared with their younger counterparts under control conditions (P < 0.05), these age-related differences in hypoxic exercise FBF and FVC were not observed following BR consumption (P = 0.25 and 0.17, respectively).
Table 3.
Forearm hemodynamics at rest and with forearm exercise during normoxia and hypoxia before and after beetroot juice consumption
| Rest | 20% | |
|---|---|---|
| Young (n = 13) | ||
| Forearm blood flow, ml/min | ||
| Control, before beetroot juice | ||
| Normoxia | 66 ± 9 | 305 ± 19 |
| Hypoxia | 69 ± 7 | 350 ± 22* |
| After beetroot juice | ||
| Normoxia | 62 ± 10 | 312 ± 21 |
| Hypoxia | 67 ± 8 | 362 ± 28* |
| Forearm vascular conductance, ml·min−1·100mmHg−1 | ||
| Control, before beetroot juice | ||
| Normoxia | 72 ± 10 | 308 ± 17 |
| Hypoxia | 75 ± 7 | 352 ± 21* |
| After beetroot juice | ||
| Normoxia | 68 ± 10 | 319 ± 21 |
| Hypoxia | 73 ± 8 | 361 ± 25* |
| Older (n = 12) | ||
| Forearm blood flow, ml/min | ||
| Control, before beetroot juice | ||
| Normoxia | 59 ± 6 | 271 ± 30 |
| Hypoxia | 60 ± 7 | 284 ± 31† |
| After beetroot juice | ||
| Normoxia | 52 ± 6 | 282 ± 31 |
| Hypoxia | 55 ± 6 | 315 ± 33*‡ |
| Forearm vascular conductance, ml·min−1·100mmHg−1 | ||
| Control, before beetroot juice | ||
| Normoxia | 63 ± 7 | 262 ± 29 |
| Hypoxia | 62 ± 7 | 271 ± 30 |
| After beetroot juice | ||
| Normoxia | 55 ± 7 | 276 ± 32 |
| Hypoxia | 57 ± 6 | 305 ± 34*‡ |
Values are means ± SE.
Main effect of hypoxia, P < 0.05;
P < 0.05 vs. young;
P < 0.01 vs. control.
Dietary nitrate and compensatory vasodilatation.
Similar to several of our previous studies (8, 12, 13), we also examined the compensatory vasodilator response (steady-state FVC during hypoxic exercise; steady-state FVC during normoxic exercise) to better understand the effect of acute dietary nitrate supplementation on muscle blood flow during hypoxic exercise in both young and older adults. Under control conditions both the absolute (9.3 ± 4.5 vs. 44.3 ± 5.3 ml·min−1·100 mmHg−1, P < 0.001) and relative (3.8 ± 1.7 vs. 14.2 ± 1.2%, P < 0.001) compensatory vasodilator response to hypoxic exercise was attenuated in the older compared with young adults (Fig. 2, A and B). The compensatory vasodilator responses (both absolute and relative) did not change following consumption of BR in the young adults (Fig. 2, A and B). Conversely, acute nitrate supplementation augmented the absolute (29.1 ± 5.6 ml·min−1·100 mmHg−1) and relative (11.4 ± 2.1%) compensatory vasodilation in the older adults (P < 0.01, Fig. 2, A and B). Moreover, the change in FVC between the two hypoxic conditions (control vs. BR) was related to the increase in plasma [NO2−] in older adults (r = 0.69, P < 0.05, Fig. 3), whereas no relationship was observed in the young adults (r = 0.28, P = 0.43).
Fig. 2.
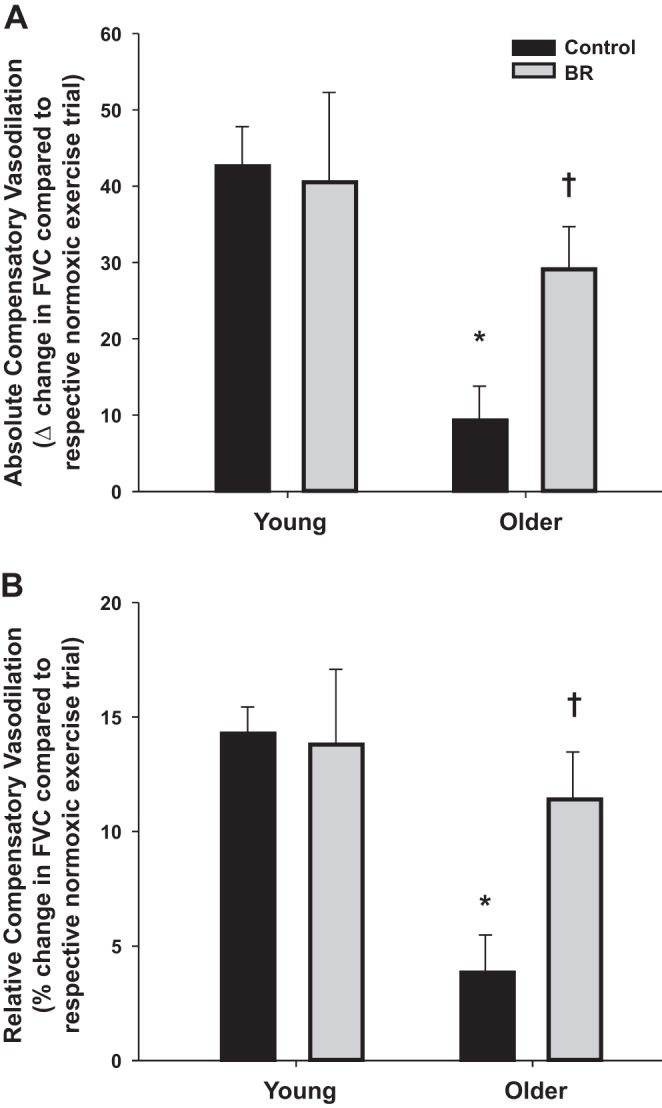
Absolute (A) and relative (B) compensatory vasodilator responses [change in forearm vascular conductance (FVC) compared with respective normoxic exercise condition] to hypoxic exercise before and after consumption of BR. The compensatory vasodilator response to hypoxic exercise was substantially attenuated in older compared with young adults under control conditions (pre-BR consumption). Acute dietary nitrate (via BR) enhanced the compensatory vasodilator response to hypoxic exercise in older, but not young adults. *P < 0.001 vs. young; †P < 0.01 vs. control (pre-BR consumption).
Fig. 3.
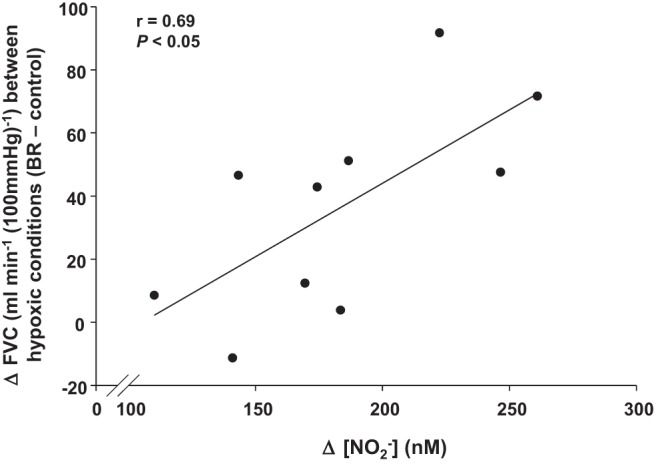
Relationship between the change in FVC during hypoxic exercise with BR (compared with control trial) and the increase in plasma [NO2−] following BR supplementation in older adults (n = 10).
Placebo trials.
In the seven older adults who completed the placebo trials, FBF and FVC did not differ between normoxic and hypoxic exercise under control conditions (prior to consumption of nitrate-depleted BR). Both absolute (9.7 ± 2.4 vs. 10.8 ± 2.7 ml·min−1·100 mmHg−1; P = 0.36) and relative (3.1 ± 0.8 vs. 3.7 ± 0.8%; P = 0.18, Fig. 4A) compensatory vasodilation were unchanged following consumption of the nitrate-depleted BR. Conversely the absolute (10.8 ± 2.7 vs. 36.3 ± 5.7 ml·min−1·100 mmHg−1) and relative (3.7 ± 0.8 vs. 14.1 ± 1.4%, Fig. 4, A and B) compensatory vasodilator responses to hypoxic exercise were substantially less during the nitrate-depleted BR (placebo) trials compared with the nitrate-rich BR trials in the seven older subjects who completed both study days (P < 0.01 for both).
Fig. 4.
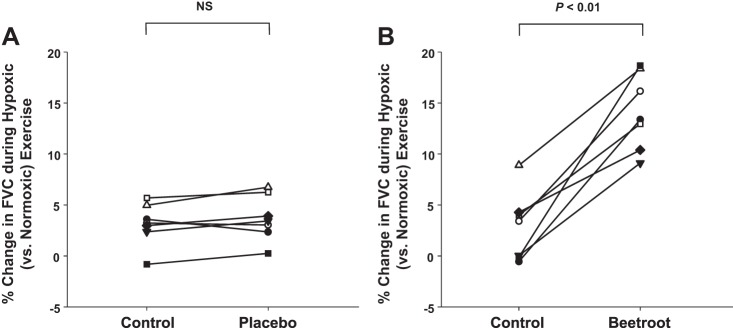
Individual compensatory vasodilator responses (relative change) to hypoxic exercise in a subset of older adults (n = 7) before and after nitrate-depleted BR (placebo) (A) and nitrate-rich BR (B). Compensatory vasodilation during hypoxic exercise was unchanged in the placebo trial (control vs. nitrate-depleted BR) and was substantially less than during the nitrate-rich BR trials.
DISCUSSION
In recent years, data have accumulated to suggest that dietary nitrate supplementation can have physiological and therapeutic effects in both animal models and humans [see review by Weitzberg and Lundberg (64)]. Briefly, several of the reported physiological benefits of acute oral nitrate supplementation in young and older humans include but are not limited to improvements in 1) exercise efficiency and tolerance (2, 3, 30, 35, 37), 2) measures of endothelial function and vascular stiffness (25, 52, 63), and 3) blood pressure (29, 30, 36, 37, 62, 63).
To our knowledge, this is the first study to investigate the effects of inorganic dietary nitrate on exercising skeletal muscle blood flow in humans. Our primary findings are that 1) acute dietary nitrate supplementation enhances skeletal muscle blood flow and vasodilation in older adults during hypoxic but not normoxic exercise, and 2) the blood flow and vasodilator responses to forearm exercise under normoxic and hypoxic conditions are unaltered in young adults following acute dietary nitrate supplementation. Taken together, the results suggest that acutely elevating plasma [NO2−] via BR consumption has a beneficial effect on the exercise hyperemic and vasodilator responses to exercise in older adults under conditions of reduced oxygen availability.
Aging and vasodilator responses to exercise: normoxia vs. hypoxia.
It is commonly believed that the control of blood flow to dynamically contracting skeletal muscle is altered with normal aging during submaximal exercise. This belief is supported by several studies that have demonstrated that the hyperemic and vasodilator responses to exercise are attenuated in older adults during submaximal forearm and leg exercise under normoxic conditions (32, 34, 38, 47, 49–51). Additionally, the age-related differences have been attributed in part to less NO-mediated vasodilation in older adults (55). However, it should be noted that other studies have failed to identify any age-associated differences in the hyperemic and vasodilator response during or immediately following dynamic forearm exercise (19, 27, 40). In the current study we did not observe any age-related differences in FBF (P = 0.19) or FVC (P = 0.11) during normoxic exercise under control conditions. Of particular interest to the current study, there was a trend for an increase in FBF (271 ± 30 vs. 282 ± 31 ml/min, P = 0.12) and FVC (262 ± 29 vs. 276 ± 32 ml·min−1·100 mmHg−1, P = 0.07) response to exercise under normoxic conditions in older adults following acute dietary nitrate supplementation; however, these changes were not significant (Table 3).
Although the overall purpose of the current study was to examine the effects of dietary nitrate supplementation on muscle blood flow, we were particularly interested in the compensatory vasodilator response to hypoxic exercise in older adults. This interest was primarily driven by evidence that suggests 1) aging appears to affect the hyperemic and vasodilator response to exercise to a greater extent under hypoxic compared with normoxic conditions (13), and 2) the nitrate-nitrite-NO pathway appears to be enhanced under hypoxic conditions in humans (15, 44). Despite acute dietary nitrate having no effect on hypoxic-mediated blood flow and vasodilation during exercise in young adults (discussed below), older adults demonstrated substantial increases in compensatory vasodilation following consumption of BR. In fact, the age-related differences in the compensatory vasodilator response to hypoxic exercise observed under control conditions (pre-BR) were abolished following BR supplementation (Fig. 2). Furthermore, we found a positive correlation between the change in plasma [NO2−] following BR supplementation and the change in FVC between the two hypoxic exercise conditions (control vs. BR). That is, older adults with the largest increase in plasma [NO2−] demonstrated the greatest increase in FVC from the control to post-BR hypoxic exercise trials (Fig. 3). Finally, the inclusion of placebo (nitrate-depleted BR) trials in a subset of older adults supports the idea that nitrate, as opposed to other components of BR (i.e., antioxidants), is likely the major contributor to the enhanced compensatory vasodilation during hypoxic exercise (Fig. 3).
Potential mechanisms for nitrite-induced compensatory vasodilation.
As mentioned previously, we have demonstrated that NO contributes to hypoxic-mediated vasodilation during exercise (10), and this response is attenuated with aging, likely due to alterations in NO-mediated mechanisms (13). Theoretically, the reduction of NO3− and NO2− following BR supplementation leads to an increased NO bioavailability, which in turn, directly contributes to the beneficial effect of dietary nitrate supplementation on muscle blood flow in older adults observed in the current study. Along these lines, acute dietary nitrate supplementation increases plasma cyclic guanosine 5′-monophosphate levels, an indicator of generation of bioactive NO (29). In addition to the direct vasodilator effects of NO, an increase in bioavailable NO may also contribute to the reduction in reactive oxygen species (56), which subsequently improves vascular function. Indeed, scavenging of reactive oxygen species with intra-arterial ascorbic acid in older adults can improve skeletal muscle blood flow during forearm exercise (34), which has been primarily attributed to an increase in bioavailable NO (16). Moreover, 3 wk of sodium nitrite supplementation decreases arterial oxidative stress in older mice and subsequently improves vascular endothelial function (57). In addition to possessing antioxidant properties, sodium nitrite also has been shown to possibly work as an anti-inflammatory agent (57). Markers of oxidative stress and inflammation were not assessed in the current study, so we are unable to determine whether the improvements in compensatory vasodilation in older adults following acute dietary nitrate consumption were influenced in part by antioxidant and anti-inflammatory effects.
NO2− also has the potential to act as an independent signaling molecule and is involved with some of the same actions as NO. In this context, evidence from experimental animals suggests that NO2− that is not directly reduced to NO can modulate several downstream signaling pathways, including activation of soluble guanylyl cyclase (6). Additionally, recent evidence suggests that acute hypoxic vasodilation observed at rest in rats is an NO-mediated response facilitated through bioactive metabolites rather than free NO (60). In humans, intra-arterial infusions of nitrite can cause substantial increases in FBF at rest and during exercise, which is potentiated under hypoxic conditions (15, 44). Therefore, in addition to increasing NO bioavailability (via reduction), the elevated levels of NO2− following BR supplementation might also directly contribute to the regulation of blood flow in contracting skeletal muscle.
Lastly, both aging and hypoxia are associated with an increased sympathetic vasoconstrictor activity directed at the skeletal muscle at rest and during exercise (24, 58). Furthermore, evidence suggests that older adults have impairments in functional sympatholysis (ability to blunt sympathetic vasoconstriction) in the vascular beds of contracting skeletal muscle during dynamic exercise (18, 33, 47). Taken together, these age-related changes likely contribute to the reduced compensatory vasodilation during hypoxic exercise in older adults. Although the mechanisms for functional sympatholysis have not been fully elucidated, NO has been shown to inhibit sympathetic vasoconstriction in contracting skeletal muscle of experimental animals and humans (14, 17, 59). If functional sympatholysis is indeed mediated in part by NO, then increasing NO bioavailability (via NO2− reduction) could indirectly enhance muscle blood flow and vasodilation, as was observed in the older adults during hypoxic exercise.
Effects of BR on muscle blood flow and vasodilation in young adults.
The lack of change in exercising FBF and FVC (Table 3) following BR supplementation in young adults observed in the current study is in contrast to recent evidence in young rats (22). Ferguson and colleagues (22) demonstrated that 5 days of inorganic nitrate supplementation enhances total hindlimb muscle blood flow and vasodilation during treadmill running in rats. The discrepancy between the current findings and those of Ferguson et al. (22) could be simply due to species differences or the duration of BR supplementation (single bolus vs. 5 days). However, two other possibilities exist that may explain the discrepant findings. First, the improved muscle blood flow and vascular conductance in rats following BR supplementation was observed primarily in fast-twitch type IIb + d/x muscles of the hindlimb, which suggests a fiber-type selective effect of dietary nitrate on vascular control (22). The relevance of these findings to our data in humans is unclear because, unlike rodents, which have highly compartmentalized skeletal muscle containing predominantly fast, slow, or intermediate fiber types (1, 22), human skeletal muscle is mixed (39). Additionally, the fiber type distribution in the flexor muscles of the human hand and forearm involved with the work associated with handgrip type exercise are unknown.
Second, the lack of change in FBF and FVC following BR consumption in young adults in the current study might be related to the amount of dietary nitrate consumed. Along these lines, a high dose of dietary nitrate has been shown to be necessary to produce significant changes in exercising skeletal muscle blood flow and vasodilation in rats (21). However, the dose of BR administered in the current study was sufficient to elicit improvements in FBF and FVC in older adults and has also been shown to be effective in increasing tissue oxygenation during exercise in the lower limbs of patients with peripheral arterial disease (31). Moreover, the lack of an effect of BR on FBF and FVC (during both normoxic and hypoxic conditions) in the young adults is not likely due to issues related to the metabolism of NO3− to NO2−, because the magnitude of change in plasma [NO2−] after BR were similar between age groups. Taken together, we favor the idea that the lack of change in FBF and FVC following BR supplementation in the young adults is more of a function of them having normal blood flow and vasodilator responses to exercise and/or a sufficient constitutive NO production by endothelial NOS (eNOS) for normal physiological function prior to nitrate supplementation rather than issues related to the dose. Whereas older adults demonstrate a blunted vasodilator response to hypoxic exercise, likely due to alterations in the l-arginine-eNOS pathway (13), and therefore may be more amendable to an intervention aimed at improving NO bioavailability such as dietary nitrate supplementation.
Experimental considerations.
Aging is associated with lower plasma and arterial concentrations of NO2− in mice (57). In the present study, age-related differences in basal levels of plasma NO2− were not observed in humans (Fig. 1). This could be interpreted as young and older adults having similar endogenous production of NO (via NOS enzymes). However, plasma levels of NO2− are strongly influenced by diet (53), which was not controlled for in our subjects (aside from fasting overnight). Therefore, the variability in dietary patterns among subjects in the days prior to each respective study day could have influenced the baseline NO2− values.
In the current study, we used a single dose of a commercially available BR (500 ml), which had a measured content of 18,708 μmol/liter NO3−. Using a single dose did not allow us to determine whether changes in muscle blood flow and vasodilation during normoxic and hypoxic exercise in humans were possibly dose-dependent. This is particularly important because varying doses of nitrate consumption have been shown to elicit different physiological responses, including blood flow in contracting muscles of rats (21). Moreover, tissue NO production from nitrite under hypoxic conditions occurs in a dose-dependent manner (20). Therefore, it might be possible to further augment the hyperemic and vasodilator responses to exercise with either higher doses or longer durations of nitrate supplementation.
Conclusions.
This study was the first to examine the effects of dietary nitrate supplementation on skeletal muscle blood flow and vasodilation during normoxic and hypoxic exercise in both young and older humans. Our results demonstrate that acute supplementation of dietary nitrate (via BR juice) has the potential to improve blood flow and vasodilation in the contracting skeletal muscle of older adults under hypoxic conditions. We believe these findings provide important information related to the potential therapeutic effects of dietary nitrate in improving vascular control and exercise hyperemia in populations that often demonstrate impairments in skeletal muscle blood flow and/or reduced NO bioavailability (i.e., diabetes, heart failure, vascular disease).
GRANTS
This research was supported by the American College of Sports Medicine Foundation and by National Heart, Lung, and Blood Institute Grant HL-105467.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.P.C. conception and design of research; D.P.C., D.P.T., C.T.G., A.C.S., and K.U. performed experiments; D.P.C., D.P.T., and C.T.G. analyzed data; D.P.C., D.P.T., C.T.G., A.C.S., and K.U. interpreted results of experiments; D.P.C. prepared figures; D.P.C. drafted manuscript; D.P.C., D.P.T., and K.U. edited and revised manuscript; D.P.C., D.P.T., C.T.G., A.C.S., and K.U. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the volunteers for their time, William Hughes for technical assistance, and Brett Wagner and Dr. Garry Buettner of the Radiation and Free Radical Research Core at the University of Iowa for technical assistance with the analysis of plasma [NO2−].
REFERENCES
- 1.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 109: 135–148, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107: 1144–1155, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Banzett RB, Garcia RT, Moosavi SH. Simple contrivance “clamps” end-tidal PCO2 and PO2 despite rapid changes in ventilation. J Appl Physiol 88: 1597–1600, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic Biol Med 41: 691–701, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1: 290–297, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284: R291–R303, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of beta-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol 110: 687–694, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol 111: 1527–1538, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol 107: 1128–1137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey DP, Shepherd JR, Joyner MJ. Sex and vasodilator responses to hypoxia at rest and during exercise. J Appl Physiol 116: 927–936, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540: 377–386, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem 283: 33927–33934, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Dose dependent effects of nitrate supplementation on cardiovascular control and microvascular oxygenation dynamics in healthy rats. Nitric Oxide 39: 51–58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hanada A, Sander M, Gonzalez-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol 551: 635–647, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiss C, Meyer C, Totzeck M, Hendgen-Cotta UB, Heinen Y, Luedike P, Keymel S, Ayoub N, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic Biol Med 52: 1767–1772, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol 474: 353–360, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyner MJ, Casey DP. Muscle blood flow, hypoxia, and hypoperfusion. J Appl Physiol 116: 852–857, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56: 274–281, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol 304: R73–R83, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol 110: 1582–1591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional alpha-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110: 591–600, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med 355: 2792–2793, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Limberg JK, Evans TD, Pegelow DF, Eldridge MW, Sebranek JJ, Proctor LT, Schrage WG. Heterogeneous vascular responses to hypoxic forearm exercise in young and older adults. Eur J Appl Physiol 112: 3087–3095, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res 89: 525–532, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, and Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol 5: 865–869, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol 25: 915–922, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 117: 670–677, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102: 1473–1476, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590: 6227–6236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AE, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med 46: 1068–1075, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol 63: 1584–1585, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Rava M, Varraso R, Decoster B, Huyvaert H, Le Moual N, Jacquemin B, Kunzli N, Kauffmann F, Zerimech F, Matran R, Nadif R. Plasma and exhaled breath condensate nitrite-nitrate level in relation to environmental exposures in adults in the EGEA study. Nitric Oxide 27: 169–175, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986. [DOI] [PubMed] [Google Scholar]
- 55.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sindler AL, Devan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol 116: 463–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umbrello M, Dyson A, Pinto BB, Fernandez BO, Simon V, Feelisch M, Singer M. Short-term hypoxic vasodilation in vivo is mediated by bioactive nitric oxide metabolites, rather than free nitric oxide derived from haemoglobin-mediated nitrite reduction. J Physiol 592: 1061–1075, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 29: 683–741, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. [DOI] [PubMed] [Google Scholar]
- 63.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr 33: 129–159, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol 115: 325–336, 2013. [DOI] [PubMed] [Google Scholar]


