Abstract
Troponin I (TnI) variant Pro82Ser (cTnIP82S) was initially considered a disease-causing mutation; however, later studies suggested the contrary. We tested the hypothesis of whether a causal link exists between cTnIP82S and cardiac structural and functional remodeling, such as during aging or chronic pressure overload. A cardiac-specific transgenic (Tg) mouse model of cTnIP82S was created to test this hypothesis. During aging, Tg cTnIP82S displayed diastolic dysfunction, characterized by longer isovolumetric relaxation time, and impaired ejection and relaxation time. In young, Tg mice in vivo pressure-volume loops and intact trabecular preparations revealed normal cardiac contractility at baseline. However, upon β-adrenergic stimulation, a blunted contractile reserve and no hastening in left ventricle relaxation were evident in vivo, whereas, in isolated muscles, Ca2+ transient amplitude isoproterenol dose-response was blunted. In addition, when exposed to chronic pressure overload, Tg mice show exacerbated hypertrophy and decreased contractility compared with age-matched non-Tg littermates. At the molecular level, this mutation significantly impairs myofilament cooperative activation. Importantly, this occurs in the absence of alterations in TnI or myosin-binding protein C phosphorylation. The cTnIP82S variant occurs near a region of interactions with troponin T; therefore, structural changes in this region could explain its meaningful effects on myofilament cooperativity. Our data indicate that cTnIP82S mutation modifies age-dependent diastolic dysfunction and impairs overall contractility after β-adrenergic stimulation or chronic pressure overload. Thus cTnIP82S variant should be regarded as a disease-modifying factor for dysfunction and adverse remodeling with aging and chronic pressure overload.
Keywords: cardiac troponin I mutation, transgenic mouse, hypertrophy, diastolic dysfunction
familial hypertrophic cardiomyopathy (HCM) is the most common inherited cardiovascular disease. In general, it is associated with autosomal dominant inheritance, and a number of animal models have replicated the human phenotype. Sarcomere mutations lead to the development of HCM and predispose to sudden cardiac death. Up to ∼5–6% of familial HCM patients may have either double (>1 mutation in same gene) or compound (>1 mutated gene) genotypes (17).
Cardiac troponin I (cTnI) is a key sarcomere protein that regulates myocardial contraction and relaxation by acting as a molecular switch (28). Mutations in cTnI may result in HCM, restrictive (RCM), or dilated cardiomyopathy (DCM) phenotypes (12, 21, 46). However, the role of cTnIP82S mutations in elderly patients with late-onset hypertrophy remains uncertain. Initial studies showed that ∼20% of late-onset hypertrophy cases have an identifiable genetic cause and concluded that cTnIP82S was a disease-causing mutation (36). However, a study on a larger cohort of HCM families concluded that, because cTnIP82S was present in 3% of Afro-Caribbean controls, it was likely to be a non-disease-causing polymorphic variant (32). Unfortunately, conducting a phenotype-genotype segregation analysis has been prohibitive due to the small size of cTnIP82S families studied to date.
We found that a heterozygous proline to serine mutation at cTnI (cTnIP82S) mutation is present in 3% of apparently normal African Americans subjects (11) (from a normal population control panel, Coriell Institute for Medical Research Biorepository); however, in a small cohort of hypertensive young African American men, cTnIP82S significantly correlated with increased left ventricle (LV) mass (4). In addition, cTnIP82S variant coexistence with MHY7 R453S mutation was associated with a severe phenotype in an African American female patient (11). Thus it is plausible that cTnIP82S could promote or exacerbate cardiac dysfunction, particularly under conditions of hemodynamic stress, such as that present during chronic hypertension, where afterload is persistently elevated. Yet, despite TnIP82S association with late-onset HCM, a cause-effect relationship between this mutation and the appearance of cardiac dysfunction, especially under conditions of acute or chronic hemodynamic stress, remains to be firmly established.
This study was designed to address the functional impact of TnIP82S. Using a transgenic (Tg) mouse model of cTnIP82S, here we assessed the functional effects of the cTnIP82S sequence variant on whole heart and in isolated muscle function. We hypothesized that cTnIP82S variant causes cardiac dysfunction during aging and with imposition of chronic stress. Using isolated intact cardiac trabeculae, tissue Doppler imaging (TDI) and pressure-volume relationships, we document a late-onset of cardiac dysfunction. Furthermore, young male TgcTnIP82S showed a blunted response to β-adrenergic stimulation and an exacerbated hypertrophic response when pressure overload was imposed by transverse aortic constriction (TAC).
METHODS
Tg model.
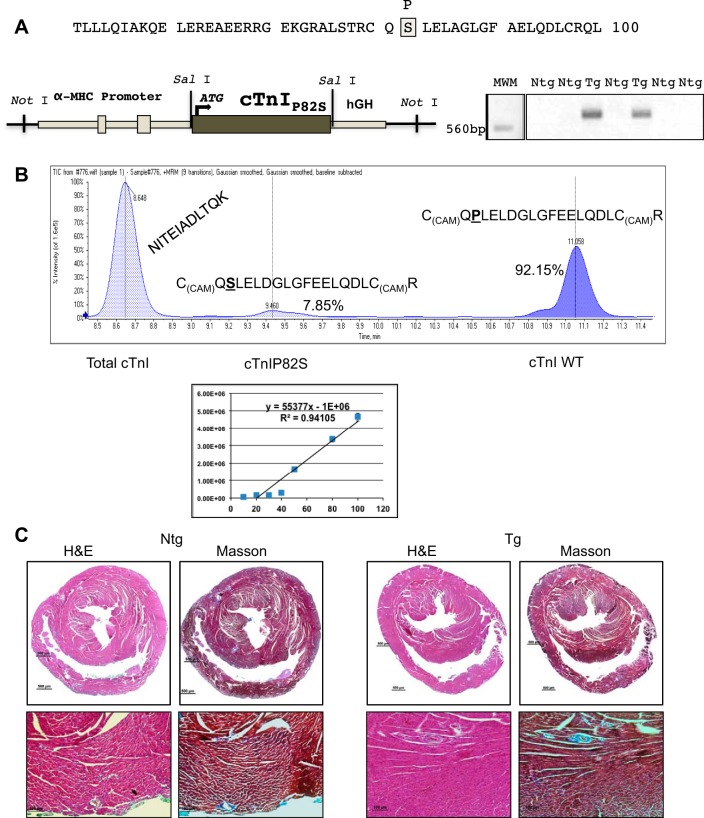
The cDNA for rat cTnI was subjected to site-directed mutagenesis (Stratagene, San Diego, CA) to mutate proline 82 to serine (cTnIP82S), confirmed by sequencing and then ligated into the Tg expression vector downstream of the mouse α-myosin heavy chain (MHC) promoter (Fig. 1A) (kind gift of Dr. J. Robbins). To generate multiple lines of TgcTnIP82S, a linearized vector was used to inject pronuclear embryos (C57BL6 X A/J), as previously described (34), and crossbred for at least six generations to C57BL6. Genotyping was performed by PCR (Fig. 1A), with forward G05 5′-ATG-GCG-GAT-GAG-AGC-AGC-GAT-G-3′ and reverse AG05 5′-CAA-TGT-CCT-CCT-TCT-TCA-CCT-GCT-TG-3′ primers. A total of three mouse lines were established: two were used for the majority of experiment (270 and 273), although a third establish line (265) was also included in the echocardiographic analysis of aged mice. All protocols were performed in accordance with the “Guide for the Use and Care of Laboratory Animals” published by the National Institutes of Health and approval of the Institutional Animal Care and Use Committee.
Fig. 1.
TgcTnIP82S mouse model displays normal histology. A: a fragment of cardiac troponin I (cTnI) cDNA where the mutation was introduced is shown in a gray shaded box. This mutant was cloned into a Sal I site downstream murine α-myosin heavy chain (MHC) promoter. Right: DNA 1% agarose gel showing two PCR products using primers specific for transgenic (Tg) mice illustrates two founders, from left to right, 270 and 273, positive genotype. B, top: a representative total ion chromatogram from a multiple reaction monitoring (MRM) assay quantifying the relative content of wild-type (WT) cTnI and cTnI P82S peptides with respect to total cTnI. Bottom: a representative calibration curve for mutant cTnIP82S peptide. A serial dilutions of mutant peptide were used to produce a 7-point calibration curve at 10, 20, 30, 40, 50, 80, and 100 pmol/μl for mutant P82S peptide [C(CAM)QSLELDGLGFEELQDLC(CAM)R̂]. C: TgcTnIP82S normal histology in representative hematoxylin and eosin (H&E), and lack of fibrosis in Masson's staining (×40 top row, and ×100 bottom row). Ntg, nontransgenic.
Q-trap nano-liquid chromatography tandem mass spectrometry and multiple reaction monitoring analysis.
Multiple reaction monitoring (MRM) analysis was performed to determine the content of cTnIP82S in Tg mice. Myofilaments were isolated, as previously described (38). The mixture of peptides from the in gel digestion of cTnI proteins was reconstituted with 20 μl of HPLC water containing 0.1% formic acid. MRM analyses were performed on a 4000 QTRAP hybrid triple quadrupole/linear IT mass spectrometer (AB SCIEX), as previously described (50), operating with Analyst 1.4.2 software scheduled experiments in positive ion mode. Peak detection and quantification of peak area were determined with Multiquant software version 2.0 and also inspected manually to ensure correct peak identification and quantification (See Fig. 1B). Results are shown as mean in triplicates. For statistical analysis of data, Student's t-test was performed with significance set at P < 0.05. Synthesized internal standards peptides of wild-type (WT) [C(CAM)QPLELDGLGFEELQDLC(CAM)R̂] and mutant cTnI P82S [C(CAM)QSLELDGLGFEELQDLC (CAM) R̂] had an N15 stable isotope label and were produced by solid-phase peptide synthesis (New England Peptide). A dilution series of peptides was used to produce seven-point calibration curves at 10, 20, 30, 40, 50, 80, and 100 pmol/μl. A control peptide (NITEIADLTQK) was used to determine the total quantity of cTnI in the sample in a series dilution of light/heavy peptides, which were made to produce six-point calibration curves at 0.05, 0.1, 0.25, 05, 1.0, and 2.5 fmol/μl with matrix comprising a pool digest of WT and mutant samples used. The mutant expression was calculated as a percentage of the ratio to WT cTnI (See Fig. 1B).
Echocardiography.
M-mode echocardiography and Tissue-Doppler imaging of the medial mitral annular velocities were performed in conscious animals at baseline, and at different time points after TAC, using an Acuson Sequoia C256 (Malvern, PA) equipped with a 15-MHz transducer, as previously described (6).
Chronic pressure overload model.
TAC was performed on anesthetized and mechanically ventilated mice, as previously described (6). Serial echocardiography, including the estimation of LV mass, and physiological studies were performed on these mice.
Histology.
Mice were euthanized with a pentobarbital overdose (75 mg/kg) intraperitoneal injection. Hearts were harvested, quickly rinsed in PBS (pH 8.0), then fixed in 4% formaldehyde in PBS (pH 8.0), paraffin-embedded, sectioned (8–10 μm thick), and stained with hematoxylin and eosin and Masson's trichrome, as previously described (34).
Isolated cardiac muscle studies.
Male or female mice (3–9 mo old) were anesthetized with pentobarbital (50 mg/kg) and heparinized (100 U) intraperitoneally. Hearts were excised and perfused retrogradely (∼15 ml/min) with dissecting Krebs-Henseleit (K-H) solution equilibrated with 95% O2 and 5% CO2. The dissecting K-H solution is composed of the following (in mM): 120 NaCl, 20 NaHCO3, 5 KCl, 1.2 MgCl, 10 glucose, 0.5 CaCl2, and 20 2,3-butanedione monoximine, pH 7.35–7.45, at room temperature (21–22°C). Trabeculae or small papillary muscles were quickly dissected from hearts and mounted between a force transducer and a micromanipulator. Force was measured by a custom-made basket attached to a force transducer (SI Heidelberg), as previously described (38). Force was expressed in milli-Newtons per millimeter squared (mN/mm2) of cross-sectional area. Muscles size average was as follows, width = 0.17 ± 0.03 mm, thickness = 0.28 ± 0.04 mm, length = 1 ± 0.12 mm, and cross-sectional area = 0.04 ± 0.01 mm2. The muscles underwent isometric contractions superfused with no 2,3-butanedione monoximine K-H solution at a rate of 10–12 ml/min, with a voltage stimulus of 3.5–5 V, pacing frequency of 0.5 Hz, at room temperature (21–22°C), and external Ca2+ concentration of 2.0 mM. The resting length was set such that resting force was 10–15% of total force development (optimal muscle length). This resting muscle length, corresponding to a resting sarcomeric length of 2.2–2.3 μm, as determined previously by laser diffraction, was maintained throughout the experiment. Muscles were allowed to stabilize for 30–45 min, before diffusionally loaded with 50 μg fura 2-AM (Invitrogen), as previously described (23). Calcium transients were calculated by the ratio of fluorescent signals excited at 340 and 380 nm, and emitted at 510 nm, using a photomultiplier tube PMT (R1527, Hamamatsu, Japan). The ratio of calcium transients amplitude was calculated after subtracting background fluorescence at 340 and 380 nm and expressed as arbitrary units. For skinned fiber studies, similar muscles were exposed for 5–10 min to 1% Triton X-100 in relaxing solution. Skinned steady-state force-extracellular Ca2+ concentration relations were obtained as previously described (13), fit to the Hill equation to yield Fmax, or maximal Ca2+-activated force; ECa502+, the extracellular Ca2+ concentration required for 50% of maximal activation; and the Hill coefficient (n Hill).
LV function pressure-volume studies.
Pressure-volume relationships were assessed in young mice at baseline and after isoproterenol infusion, and at 10 wk post-TAC, as previously described (2, 6).
Protein analysis.
Myofilaments were isolated from snap-frozen ventricles, as previously described (38), and separated by 4–12% bis-tris Novex SDS-PAGE (Invitrogen) or by Mn-Phos-tag (Phos-tag Consortium, Japan) acrylamide gels, as previously described (22, 26). Western blots were performed using antibodies against cTnI 1:5,000 (I-87, Spectral Diagnostics), phospho-troponin I (TnI) (Ser23/Ser24) 1:2,000 (Cell Signaling, Danvers, MA), and myosin-binding protein C (MyBP-C) 1:10,000 (kind gift of Dr. S. Sadayappan).
Molecular modeling cTnIP82S mutation.
The molecular models of TnI WT and TnIPro82Ser were rendered using the Kortemme Lab web server (https://kortemmelab.ucsf.edu/backrub/cgi-bin/rosettaweb.py?query=index). This server utilizes the “backrub” method (7) for flexible protein backbone modeling implemented in Rosetta (41). To predict the structural effects of a single point mutation, we used the human cTnI protein database identifier (PDB ID: 1J1D) (43). The resulting molecular model of hcTnIPro82Ser and WT human cardiac cTnI PDB files was downloaded, visualized, and aligned in PyMOL version 1.5 for iPad.
Statistics.
Statistical analysis of the data was performed using Student's t-test and two-way ANOVA with repeated measurements (twitching muscles relaxation, Ca2+ transients kinetics, and echocardiography baseline and serial post-TAC analysis). A value of P < 0.05 was used to indicate significant differences between control and Tg mice. Pooled data were expressed as means ± SE.
RESULTS
Tg cTnIP82S protein expression by MRM.
To detect a mutant protein that has no tags, we developed a MRM assay using radiolabeled synthetic peptides as internal standards (50). We isolated myofilaments from three snap-frozen hearts for non-Tg (Ntg) and each Tg line (270 and 273). These samples were processed in parallel to quantify the expression of cTnIP82S by mass spectrometry. The MRM assay was analyzed by a 4000 QTRAP hybrid triple quadrupole/linear IT mass spectrometer (AB SCIEX). Our results revealed that the stoichiometry of cTnIP82S Tg replacement was 7.85% (7.57% ± 0.29 for line 270 and 8.13% ± 0.06 for line 273) (Fig. 1B).
cTnIP82S expression impairs diastolic function of aged-mice.
To delineate whether cTnIP82S murine heart expression replicates the signs of hypertrophy and/or cardiac fibrosis, cardiac function was evaluated in aged Tg and Ntg mice (14.5 ± 1.8 mo old). For the initial characterization of this Tg model, we used M-mode echocardiography and TDI. Interestingly, TDI of aged Tg mice consistently displayed an ∼19% increase in isovolumetric relaxation time (IVRT) (Ntg 24.06 ± 0.12 ms vs. Tg 28.64 ± 0.18 ms, P = 0.022), increased Tei index (Ntg 1.25 ± 0.03 vs. Tg 1.46 ± 0.04, P = 0.025), and normal ejection time and isovolumetric contraction time (Table 1). In addition, the dynamics of tissue Doppler waves velocities were analyzed [as early diastolic myocardial velocity (Ea) and late diastolic myocardial velocity (Aa) cm/s] and their respective Ea-to-Aa ratios. In agreement with Tei index, Ea was significantly slower, and Ea-to-Aa ratios significantly larger in Tg (Table 1), also indicative of diastolic dysfunction. In contrast, there were no echocardiographic differences between Tg and Ntg littermates at younger age (4.2 ± 0.02 mo old) (Table 2). Interestingly, no signs of overt hypertrophy or fibrosis were evident in old or young Tg mice, as determined by heart-to-body weight ratios (Table 1) and by light microscopy analysis of hematoxylin and eosin and Masson's stained slides (Fig. 1C). Thus the selective impairment of diastolic function in aged mice, in the absence of cellular hypertrophy or fibrosis, suggests that cTnIP82S expression might alter myofilament properties and diastolic function in an age-dependent manner without findings suggestive of a typical HCM phenotype.
Table 1.
Aged-mice contractility and LV chamber dimensions by M-mode, tissue Doppler echocardiography, and gross pathology
| Ntg | TgcTnIP82S | |
|---|---|---|
| n | 15 | 14 |
| Age, mo | 13.8 ± 1.2 | 15.2 ± 2.1 |
| HR, beats/min | 677 ± 33 | 682 ± 34 |
| LVEDD, mm | 2.86 ± 0.03 | 2.88 ± 0.04 |
| LVESD, mm | 1.21 ± 0.03 | 1.24 ± 0.04 |
| FS, % | 57.85 ± 0.15 | 57.16 ± 0.16 |
| IVCT, ms | 16.73 ± 0.09 | 17.00 ± 0.01 |
| ET, ms | 33.00 ± 0.12 | 31.64 ± 0.13 |
| IVRT, ms | 24.06 ± 0.12 | 28.64 ± 0.18* |
| Tei index | 1.25 ± 0.03 | 1.46 ± 0.04* |
| Ea, cm/s | 0.06 ± 0.01 | 0.05 ± 0.01* |
| Aa, cm/s | 0.05 ± 0.01 | 0.07 ± 0.01 |
| Ea/Aa ratio | 1.35 ± 0.05 | 0.78 ± 0.05* |
| Heart weight, mg | 152 ± 15 | 147 ± 7 |
| Heart weight/body weight ratio, mg/g | 4.68 ± 2.41 | 4.81 ± 2.4 |
Values are averages ± SE; n, no. of mice.
Ntg, nontransgenic; HR, heart rate; LVEDD, left ventricle end-diastolic dimension; LVESD, left ventricle end-systolic dimension; FS, fractional shortening; IVCT, isovolumetric contraction time; ET, ejection time; IVRT, isovolumetric relaxation time; Tei index = (IVCT + IVRT)/ET; Ea, tissue Doppler imaging early diastolic myocardial velocity; Aa, tissue Doppler imaging late diastolic myocardial velocity.
P value ≤ 0.05.
Table 2.
Young mice (17 wk) contractility and LV chamber dimensions by M-mode and tissue Doppler echocardiography
| Ntg | TgcTnIP82S | |
|---|---|---|
| n | 8 | 9 |
| HR, beats/min | 711 ± 12 | 688 ± 5 |
| LVEDD, mm | 2.94 ± 0.05 | 2.93 ± 0.04 |
| LVESD, mm | 1.14 ± 0.03 | 1.12 ± 0.03 |
| FS, % | 61.3 ± 0.15 | 69.9 ± 0.55 |
| IVCT, ms | 16.43 ± 0.15 | 17.67 ± 0.13 |
| ET, ms | 43.71 ± 0.25 | 43.33 ± 0.2 |
| IVRT, ms | 19.29 ± 0.18 | 20.33 ± 0.15 |
| Tei index | 0.82 ± 0.04 | 0.88 ± 0.03 |
| Ea, cm/s | 0.06 ± 0.003 | 0.09 ± 0.015 |
| Aa, cm/s | 0.05 ± 0.004 | 0.06 ± 0.011 |
| Ea/Aa ratio | 1.45 ± 0.1 | 1.36 ± 0.1 |
| LV mass | 91.5 ± 3.27 | 95.99 ± 4.68 |
Values are averages ± SE; n, no. of mice.
LV mass = 1.055 × [(IVSD + LVEDD + LVPWTED)3] − (LVEDD)3, where IVSD is interventricular septal thickness at end-diastole, and LVPWTED is LV posterior wall thickness at end diastole.
P value ≤ 0.05.
cTnI proline 82 serine presence blunts acute β-adrenergic response.
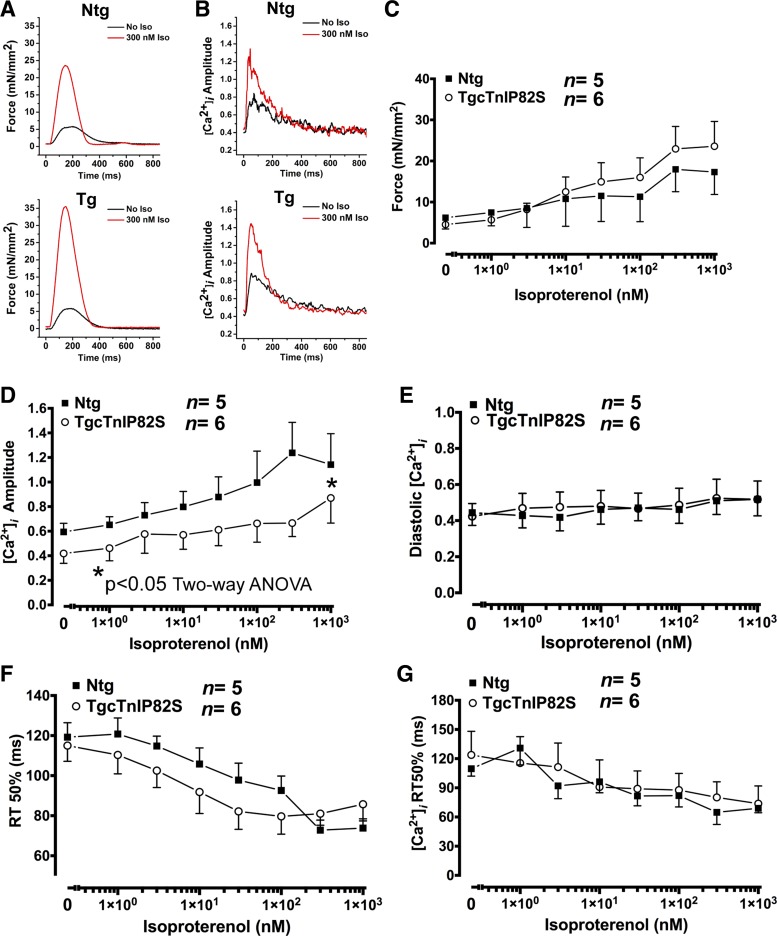
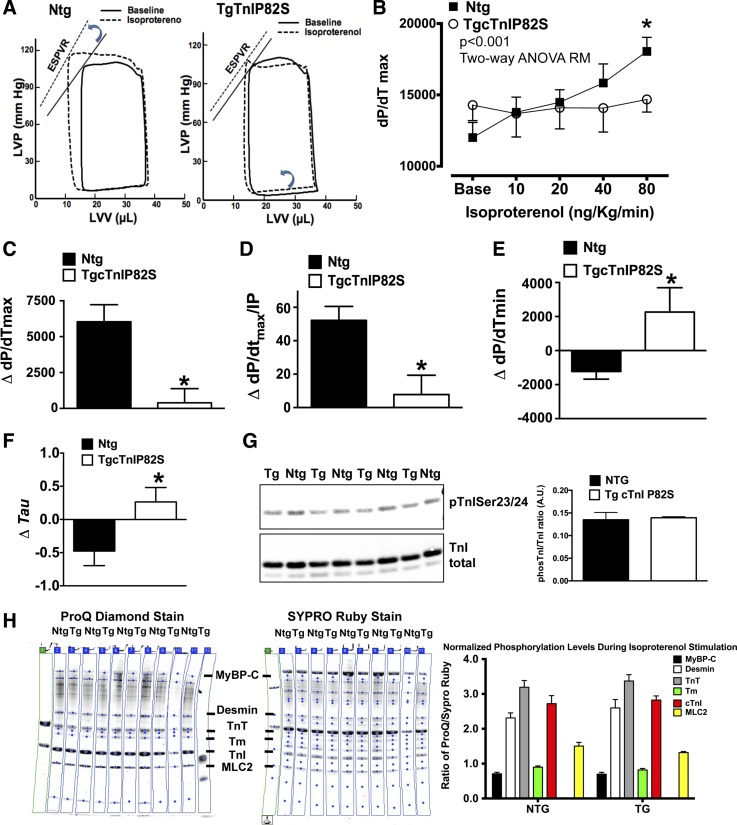
Acute β-adrenergic stimulation greatly enhances cardiac pump function, via augmented inotropy/lusitropy, representing a major adaptive response for the heart to cope with increased workload. To assess whether cTnIP82S has an adverse impact on heart contractile reserve, we challenged Tg and Ntg mice with acute β-adrenergic stimulation. Intact twitching cardiac muscle and corresponding Ca2+ transient representative tracings are shown at baseline and after isoproterenol treatment (Fig. 2, A and B). Although at baseline, developed force and Ca2+ transients amplitude were not different between Ntg and Tg mice (Fig. 2, C and D), during a isoproterenol dose-dependent response Tg mice exhibited a blunted β-adrenergic-dependent increase of Ca2+ transient amplitude (Fig. 2D). On the contrary, β-adrenergic-dependent force development augmentation (Fig. 2C), Ca2+ transients diastolic levels (Fig. 2E), β-adrenergic-dependent acceleration of twitching dynamics, and Ca2+ transient kinetics of decay (Fig. 2, F and G) showed no differences between Ntg and Tg mice. To assess the impact of this finding at the whole organ level, in vivo ventricular pressure volume studies, at baseline and upon β-adrenergic stimulation, were performed in Ntg and Tg littermates. These studies confirmed and further extended the findings of isolated muscles studies. Representative pressure-volume plots, at baseline and after isoproterenol infusion, illustrate the lack of systolic and diastolic response to β-adrenergic stimulation (Fig. 3A). The overall isoproterenol dose-response curve shows that Tg mice displayed a blunted β-adrenergic contractile reserve, where maximal rate constant of pressure rise (dP/dtmax) dose response was basically flat (Fig. 3B). The overall response to β-adrenergic stimulation was also assessed by delta change in contractility, defined as the difference in contractility between baseline (without isoproterenol) and at the highest isoproterenol dose. Systolic response was clearly impaired, as evidenced by an ∼15-fold lower delta for dP/dtmax in Tg mice (Ntg 6,040.5 ± 1,186 mmHg/s vs. Tg 393.3 ± 985 mmHg/s, n = 6 vs. n = 5, P < 0.05) (Fig. 3C) and a significantly lower delta for dP/dtmax normalized to instantaneous pressure in Tg (Fig. 3D) (Ntg 52.2 ± 8.4 vs. Tg 7.8 ± 10.4, n = 4 vs. n = 4, P < 0.05). On the other hand, diastolic response to β-adrenergic stimulation was “negative”, meaning that Tg mice rates of relaxation become slower rather than faster, delta for minimum rate constant of pressure rise (dP/dtmin) (Ntg −1,231 ± 450 mmHg/s vs. Tg +2,266.7 ± 1,430 mmHg/s, n = 6 vs. n = 5, P < 0.05) (Fig. 3E) and delta for isovolumetric relaxation constant (τ) (Ntg −0.47 ± 0.21 vs. Tg +0.26 ± 0.21, n = 6 vs. n = 5, P < 0.05) (Fig. 3F). These negative effects on relaxation rates did not seem to be mediated by altered myofilament phosphorylation in response to β-adrenergic stimulation. Myofilament phosphorylation pattern was estimated by SDS-PAGE followed by phosphor-TnI Ser23/24 WB (Fig. 3G) or by ProQ Diamond and Sypro Ruby staining (Fig. 3H). These results suggest that cTnIP82S blunts augmentation of Ca2+ transient amplitude and whole organ contractility in response to acute β-adrenergic stimulation.
Fig. 2.
Intact twitching cardiac muscle shows blunted β-adrenergic dependent increase of Ca2+ transient amplitude. Representative twitch force (A) and calcium transients tracings (B) of Ntg and Tg, during isoproterenol (Iso) dose response at baseline (black) and 300 nM (red). C: developed force in response to increased dose of Iso (0 to 103 nM) for Ntg (solid squares) and Tg (open circles) isolated cardiac muscles. D: systolic Ca2+ transients amplitude (fura 2-AM, F1/F0) were significantly decreased in Tg, despite β-adrenergic stimulation. *P < 0.05, two-way ANOVA. E: diastolic Ca2+ transients in response to Iso. F: corresponding acceleration of force relaxation time (RT) to 50%. G: Ca2+ transient acceleration of decay to 50%. Values are means ± SE. [Ca2+]i, intracellular Ca2+ transients.
Fig. 3.
In vivo β-adrenergic dose-response is blunted in TgcTnIP82S. A: representative Ntg (n = 6) and Tg (n = 5) pressure-volume (PV) loops at baseline (solid line) and at highest point of Iso dose-response (10, 20, 40, and 80 ng·kg−1·min−1) (dashed line). Notice on left, a leftward and upward shift of Ntg PV loop, typical of adequate contractile adrenergic response, whereas the right shows the failure of TgcTnIP82S to respond to β-adrenergic stimulation. LVP, left ventricular pressure; LVV, left ventricular volume; ESPVR, end-systolic PV relationship. B: mean results for Iso dose-response of maximal rate constant of pressure rise (dP/dtmax). TgcTnIP82S mice show a blunted dose-dependent augmentation of systolic function vs. Ntg. *P < 0.001 is for group interaction terms analysis by two-way ANOVA repeated measures (RM). C: change (Δ) in dP/dtmax. D: Δmaximal rate of pressure rise normalized to instantaneous pressure (dP/dtmax/IP). E: Δpeak negative dP/dt (dP/dtmin). F: Δisovolumetric relaxation constant (τ), which was calculated by logistic regression. G: myofilament from Ntg and TgcTnIP82S that were subject to Iso dose-response showed no difference in TnI phosphorylation of Ser23/24. H: myofilament preparations from Ntg and TgcTnIP82S that were subject to Iso dose-response were determined by Pro-Q staining (left) and normalized to total protein content, determined by Sypro Ruby (middle). Comparison of normalized phosphorylation levels expressed as ratio of ProQ/Sypro Ruby signals. Phosphorylaton pattern is not significantly different (right). MyBP-C, myosin-binding protein C; MLC, myosin light chain. Values are means ± SE.
cTnI proline 82 serine exacerbates pressure overload-induced maladaptive hypertrophy.
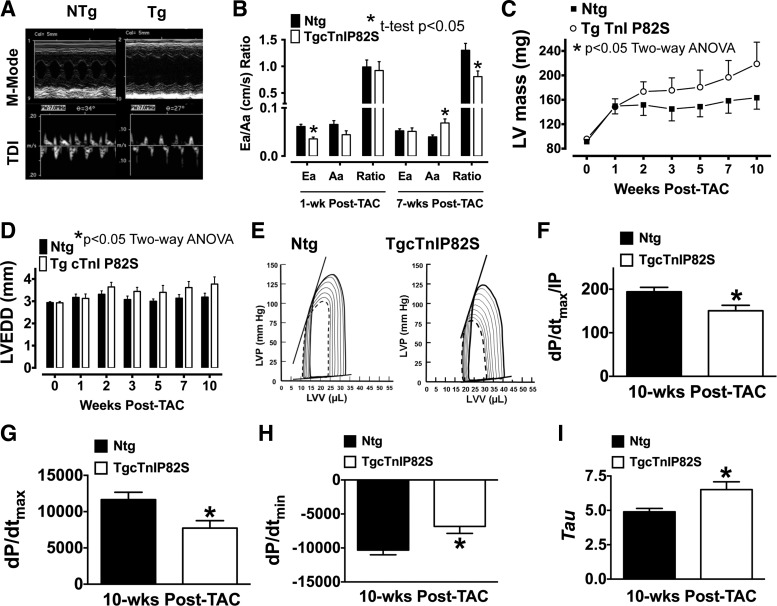
Because cTnIP82S has been potentially associated with elderly onset HCM in humans, we tested if cTnIP82S exacerbated hypertrophy when the Tg mice were subjected to chronic pressure overload by TAC. To this end, cardiac function was assessed by echocardiography at baseline (pre-TAC) and followed at 1, 2, 3, 5, 7, and 10 wk post-TAC. Interestingly, our data revealed that, although 4-mo-old Tg mice appear normal, at 1 wk post-TAC, they displayed a significant increase in IVRT; see representative M-mode and TDI echocardiography (Fig. 4A) (Ntg 28.7 ± 0.39 ms vs. Tg 35.57 ± 0.44 ms, n = 8 vs. n = 7, P < 0.05), which is suggestive of diastolic dysfunction (Fig. 4B). In addition, 7 wk post-TAC Tg mice showed, by means of echocardiography, that genotype exacerbated hypertrophic response (Fig. 4C), where LV mass (Ntg 158.35 ± 1.3 mg vs. Tg 196.79 ± 1.48 mg, n = 8 vs. n = 7, P < 0.05 two-way ANOVA) and LV chamber dilatation were significantly increased (Fig. 4D). Furthermore, terminal LV pressure-volume studies (see representative traces in Fig. 4E) revealed that cTnIP82S mice had impaired overall systolic, as judged by dP/dtmax normalized to instantaneous pressure (Fig. 4F) or by dP/dtmax (Fig. 4G), and abnormal diastolic function, as evidenced by reduced dP/dtmin (Fig. 4H) and prolonged τ (Fig. 4I), compared with Ntg after chronic pressure overload (See Table 3). These results suggest that cTnIP82S amplifies hypertrophic response to TAC, worsens adverse negative chamber remodeling, and exacerbates TAC-induced heart failure.
Fig. 4.
cTnIP82S exacerbates hypertrophic response and decreases contractility during chronic pressure-overload. A: representative M-mode and tissue Doppler imaging (TDI) echocardiograms of Ntg and Tg 1-wk post-transverse aortic constriction (TAC). B: reduced transmitral flow dynamics are evident in Tg mice (*t-test, P < 0.05). Ea, early diastolic myocardial velocity; Aa, late diastolic myocardial velocity. Left ventricular (LV) mass (C) and LV chamber dilatation (D) are significantly influenced by Tg genotype (*two-way ANOVA, P < 0.05). LVEDD, left ventricular end diastolic dimension. E: representative PV loops of Ntg and Tg 10 wk post-TAC at baseline (dashed line) and after imposing afterload (dashed line), respectively. Note that, although there is a right and upward shift of PV loop (contrary to a typical adequate contractility response), Ntg mice (n = 6) have overall better baseline and afterload contractility. This is clearly illustrated in F–I, showing PV loop parameters that were significantly changed in Tg mice (n = 6). F: dP/dtmax/IP. G: dP/dtmax. H: dP/dtmin. I: τ. *P < 0.05. Values are means ± SE.
Table 3.
LV pressure-volume measurements of Ntg and TgcTnIP82S mice at 10 wk post-transverse aortic constriction
| Ntg | TgcTnIP82S | |
|---|---|---|
| n | 6 | 6 |
| LVVES, μl | 13.68 ± 2.17 | 23.10 ± 2.40* |
| EF, % | 58.7 ± 5.30 | 39.8 ± 1.84* |
| dP/dtmax, mmHg/s | 11651 ± 1012 | 7727 ± 1023* |
| dP/dtmin, mmHg/s | −11,886 ± 694 | −6,833 ± 1022* |
| SW | 2,378 ± 215 | 1,659 ± 237* |
| τ | 4.9 ± 0.248 | 6.51 ± 0.568* |
| dP/dtmax/IP | 194 ± 10.2 | 150 ± 12.6* |
Values are averages ± SE; n, no. of mice.
LVVES, left ventricle volume at end-systole; EF, ejection fraction; dP/dtmax, maximal rate constant of pressure rise; dP/dtmin, minimum rate constant of pressure rise; SW, stroke work; τ, isovolumetric relaxation constant calculated by logistic regression; dP/dtmax/IP, dP/dt max normalized to instantaneous pressure.
P value ≤ 0.05.
cTnIP82S selectively impairs myofilament cooperativity.
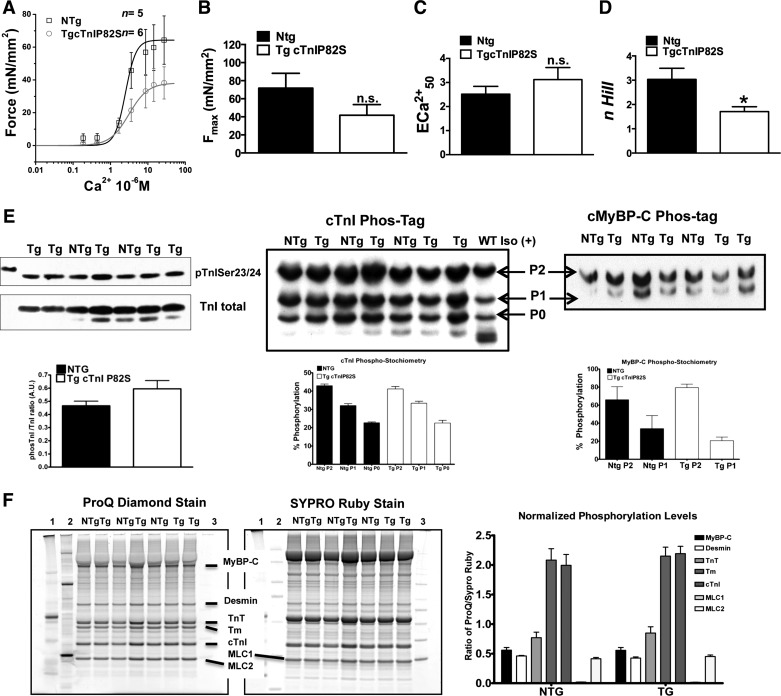
To gain further insights into the impact of cTnIP82S variant on myofilament function, we evaluated force-calcium relationships in skinned fibers (Fig. 5A). Surprisingly, Tg mice exhibited a marked depression in myofilament cooperativity, as estimated by the n Hill coefficient (Tg 1.72 ± 0.17 vs. Ntg 3.09 ± 0.44, n = 7 vs. n = 5, P < 0.05) (Fig. 5D), while no significant effect was observed on myofilament calcium sensitivity (ECa502+) (Fig. 5C) or Fmax (Fig. 5B). In addition, this effect could not be attributed to changes in myofilament phosphorylation pattern, as estimated by phosTag SDS-PAGE followed by TnI and MyBP-C Western blot or by ProQ Diamond SDS-PAGE staining (Fig. 5E).
Fig. 5.
Steady-state force-Ca2+ relationships in skinned fibers. A: pool raw force data averaged and fitted to modified Hill equation: Ntg (n = 5), Tg (n = 6). B: averaged maximal Ca2+ activated force (Fmax). Note the trend of Tg to reduced Fmax. ns, Nonsignificant. C: Ca2+ sensitivity of Tg is modestly increased. ECa502+, extracellular Ca2+ concentration required for 50% of maximal activation D: myofilament cooperativity (n Hill, Hill coefficient) is markedly reduced in Tg muscles. *P < 0.05. E, left: TnI phosphorylation on Ser23/24 is not affected. PhosTag-SDS-PAGE show that phosphorylation stoichometry is unchanged for TnI (middle) and MyBP-C (right). F: phosphorylation of myofilament preparations from Ntg and TgcTnIP82S were determined by Pro-Q staining (left) and normalized to total protein content, determined by Sypro Ruby (middle). Molecular weight markers are labeled 1 (Precision-C, Bio-Rad), 2 (Peppermint Stick, Inv), and 3 (MagicMark XP, Inv) with myofilament protein migration highlighted by a line. Right: comparison of normalized phosphorylation levels expressed as ratio of ProQ/Sypro Ruby signals. Phosphorylation pattern is not significantly different. Values are means ± SE.
DISCUSSION
Here we report three major novel findings. First, cTnIP82S induces a late-onset diastolic dysfunction, without the presence of hypertrophy, tissue disarray, or fibrosis. The second major observation is that, in younger Tg mice, this mutation blunts β-adrenergic response and exacerbates maladaptive hypertrophy as well as pump failure in pressure-overloaded hearts. Finally, at the myofilament level, cTnIP82S selectively impairs cooperativity and, consequently, overall cardiac contractility.
cTnIP82S leads to age-dependent diastolic dysfunction.
Since its first identification, cTnIP82S has been considered a potential disease-causing variant (36). However, later studies suggested that it was a benign polymorphic variant (31). Previously, our group reported a patient with MHY7 R453S and TNNI3 cTnIP82S compound mutations, had severe disease (11). Based on this association as well as the confirmation that this is a variant present in ∼3% of the African American population, we hypothesized that cTnIP82S could be a relatively common disease-causing mutation or a disease modifier. In this study, we provide strong evidence that, despite low levels of protein expression, cTnIP82S has adverse effects on cardiac function, and that these effects seem to be amplified by hemodynamic stress and by advanced age. Echocardiography confirmed altered diastolic dysfunction in aging mice. However, notably, cardiac dysfunction occurred in the absence of evidence for adverse hypertrophy or cardiac fibrosis. Intriguingly, present data confirmed previous observations showing the occurrence of diastolic dysfunction (as indexed by TDI) before the onset of hypertrophy or tissue abnormalities. This sequence of events was documented in several animal models of HCM (3, 10, 15, 42), RCM (8), and even in patients with β-MHC (16), MyBP-C (5, 29, 35), TnI (30), and troponin T (TnT) (35) mutations. Given the consistency of this observation in different experimental and human cardiomyopathy contexts, diastolic dysfunction detected by TDI has been proposed as an early diagnosis (16, 35) or prognosis tool (24) in genotype (+) but hypertrophy-negative individuals. While diastolic dysfunction appears in aged cTnIP82S mice, it is also notable the profound effect of increased afterload in the cTnIP82S mice, which have more severe systolic and diastolic dysfunction when TAC is performed in younger mice. In the latter, recapitulating the full adverse features of the cTnIP82S mutation required the presence of sustained hemodynamic stress. Overall, these data suggest that TnIP82S could predispose a small but significant percent of the African American population to cardiac diastolic dysfunction late in life and to more profound effects if exposed to additional stressors earlier in adulthood. Given the compelling evidence of increased risk of hypertension in African Americans (9), this may be quite important on a population-wide basis.
β-Adrenergic stimulation and increased afterload precipitate cardiac dysfunction in Tg cTnIP82S mice.
Diastolic dysfunction is a silent early feature, common to HCM, RCM, and DCM, that can often be uncovered by exercise and dobutamine echo test (40, 49). Accordingly, baseline echocardiography in young adult (17–19 wk) Tg cTnIP82S did not reveal any abnormalities. However, under β-adrenergic stimulation, LV pressure-volume analysis uncovered an underlying cardiac dysfunction. Interestingly, this was true even at maximal concentrations of isoproterenol. Our findings are in agreement with others; since Tg TnT(I79N) mice also displayed minimal hypertrophy with no fibrosis under basal conditions, normal whole heart contractility in the presence of abnormal myofilament properties, such as increased myofilament Ca2+ sensitivity and Mg-ATPase activity (9). However, when challenged with β-adrenergic stimulation, Tg TnT(I79N) showed no significant systolic and diastolic response. Chronic β-adrenergic stimulation resulted in overall cardiac dysfunction and sudden cardiac death by ventricular arrhythmias (25). Likewise, TgcTnIP82S excitation-contraction coupling analysis uncovered that β-adrenergic-dependent increase of Ca2+ transient amplitude was significantly lessened in Tg muscles. In addition, when TAC was induced to increase afterload, there was a prolongation of IVRT at 1 wk after the procedure, which suggests a deficient compensatory hemodynamic response; however, by 7 wk post-TAC, a progressive chamber remodeling and exacerbated LV hypertrophy was evident. Our findings are in contrast with those of HCM model α-MHC (R403Q) Tg mouse. Although Tg α-MHC403/+ mice also displayed abnormal excitation contraction coupling and reduced myofilament cooperativity (14), when subjected to TAC the mice did not develop more LV hypertrophy than Ntg mice (39). While these differences can be attributed to diverse strain genetic backgrounds, cTnIP82S may confer a particular susceptibility to LV hypertrophy with coexisting pressure overload, such as uncontrolled systemic hypertension.
Our data suggests that cTnIP82S might recapitulate some of the phenotypic features, both at the myofilament and whole heart levels, of previously reported HCM mouse models where TnT or α-MHC function is compromised.
β-Adrenergic response, TnI interactions, and myofilament cooperativity.
Site-specific phosphorylation of cTnI, in concert with MyBP-C, is one of the major regulators of systolic and diastolic kinetic properties, both at rest and during β-adrenergic stimulation (18, 19, 45). β-Adrenergic stimulation increases Ca2+ transients through a rapid phosphorylation and functional activation of key calcium handling proteins (27). Although Tg cTnIP82S did increase their calcium transient amplitude in response to isoproterenol, the steepness of their β-adrenergic dose response was significantly blunted. Steady-state force-Ca2+ studies in skinned fibers showed a clear isolated defect in myofilament cooperativity (n Hill) in Tg cTnIP82S, whereas myofilament Ca2+ sensitivity (ECa502+) and Fmax were minimally lower, but did not reach statistical significance. Mechanistically, the myofilament cooperativity defect could not be attributed to abnormalities in myofilament phosphorylation patterns. In fact, there were no changes in phosphorylation of cTnI and MyBP-C (See Fig. 5E). For example, phos-Tag acrylamide SDS-PAGE followed by immunoblots demonstrated no significant differences in phosphorylation stoichiometry. Similarly, phosphorylation at cTnI Ser23/Ser24 was unchanged between Ntg and Tg, at baseline or during isoproterenol stimulation. Previously, it has been suggested that cardiac muscle relaxation rates are dictated by myofilament intrinsic properties (20). Thus, because the Ca2+ transient's amplitude was overall lower but produced similar force, it is possible that myofilaments might be sensitized to Ca2+ in the concentration range of dynamic twitching muscles, without showing evident changes in ECa502+ estimated by skinned muscles during steady-state force-Ca2+ relationships (38). Such increase in myofilaments Ca2+ sensitivity could contribute significantly to the impairment of diastolic function found in the intact Tg cTnIP82S heart. A TnI mutant (TnIR193H) increases myofilament ECa502+ in skinned muscles during steady-state force-Ca2+ relationships, and it is associated with RCM and severe diastolic dysfunction. However, a recent Tg TnIR193H mouse model study demonstrated impaired myofilament cooperativity with normal myofilament Ca2+ sensitivity in skinned muscles steady-state force-Ca2+ relationships. Their steady-state findings were in sharp contrast with the phenotype observed in dynamic twitching muscles with clear blunted Ca2+ transients amplitude and slower kinetics of decay, which ultimately explain the in vivo elevated diastolic pressure and diastolic dysfunction (8).
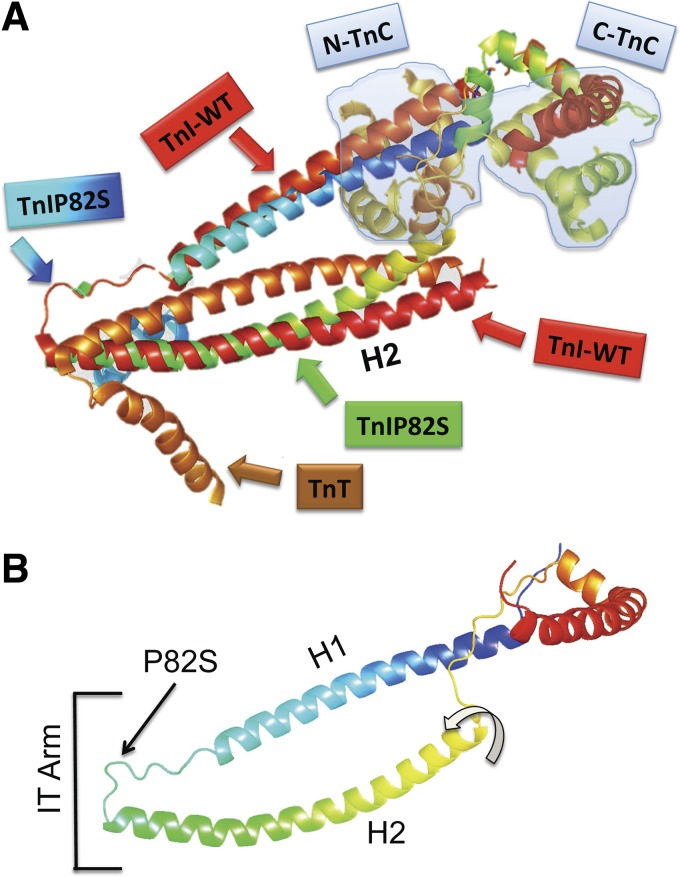
The cTnIPro82Ser missense mutation occurs in an evolutionally conserved proline residue that is localized in the hinge of IT arm and TnI-TnT interactions in H2 region (see Fig. 6) and thus likely to affect function. Our molecular modeling of Pro82Ser mutant show that this amino acid residue change may alter the hinge in the IT arm and set H2 TnI region further apart from TnT (See TnIWT and TnIPro82Ser overimposed structures, Fig. 6A). Similarly, the model of TnIPro82Ser of Ramachandran and colleagues (37) pointed out that serine substitution might result in a hydrogen bond interaction with the butyl ammonium nitrogen of Lys234 in TnT and perturb TnI-TnT interactions. Thus TnIPro82Ser appear to have similar structural and phenotypic effects as the dominant negative mutation in TnI Cys111Arg, which also decrease the binding affinity of TnI for TnT. Remarkably similar to the TnIPro82Ser mutation, cTnI Cys111Arg also impairs diastolic function and blunts the adrenergic response of cardiac muscle (1, 47, 48).
Fig. 6.
Molecular modeling of TnIP82S mutant. A: TnI WT and a structural model of TnIP82S are overimposed, maintaining the relation with TnT and TnC (light blue shaded). Cyan to blue gradient box and arrow show the proline → serine substitution. B: TnI P82S structural model and the functional domains. The hinge region of IT arm is where H1 and H2 join. Curved arrow shows the twist of H2 region would be further apart from TnT. To render the molecular models of TnI WT (PDB ID: 1J1D) and TnIP82S, we used the “backrub” method for flexible protein backbone modeling implemented in Rosetta.
Translational implications.
Our novel findings are of special clinical interest if we take into consideration that cTnIP82S variant is moderately prevalent (∼3%) in certain ethnic groups, such as African Americans (11) and/or Afro-Caribbean (32), and that familial HCM patients (∼5–6%) may have either double (>1 mutation in same gene) or compound (>1 mutated gene) genotypes (17). In particular, the Tg cTnIP82S mouse model ads mechanistic weight to our previous clinical observation that led us to speculate that a cTnIP82S + β-MHC R453S genotype was associated with a more severe phenotype (11). A phenotype-genotype cosegregation analysis would greatly help to understand the role of cTnIP82S in families with asymptomatic heterozygous carriers. This study holds potential in helping to clarify the clinical significance of cTnIP82S mutation when found in young asymptomatic, or in older, patients displaying LV hypertrophy or diastolic dysfunction.
Limitations.
This Tg cTnIP82S mouse model achieved modest TnI replacement in vivo, an MRM assay estimated an ∼7–8% proportion of mutant protein. The latter is a possible explanation for the cTnIP82S late-onset phenotype and cardiac dysfunction only under hemodynamic stress. The absolute level of expression of the mutant protein is generally unknown in human HCM; however, it has been demonstrated in murine models even low levels of mutant myofilament protein may lead to significant physiological effects (23, 33, 44). It is likely that several lines with increasing percentages of TnI replacement would be needed to assess the impact of higher levels of TnIP82S protein, although it is striking that even low expression leads to a late onset and stress-related phenotype, likely analogous to the “elderly onset” human HCM phenotype originally ascribed to this mutation.
Conclusions.
Our study reveals that sustained myocardial expression of cTnIP82S in mice is sufficient to cause late-onset cardiac diastolic dysfunction. Furthermore, cTnIP82S mutant blunts the heart's ability to respond during adrenergic stimulation and exacerbates maladaptive hypertrophy, as well as pump failure in response to pressure overload stress. The precise mechanism(s) underlying the development of late-onset HCM in the presence of a cTnIP82S mutation in humans is still unknown. Our data indicate that cTnIP82S is a significant myofilament mutation that may justify life-long monitoring of diastolic function in heterozygous carriers, as well as aggressive treatment of comorbid conditions, such as hypertension or coronary artery disease.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant RO1-HL-63038 to A. M. Murphy and The Johns Hopkins Proteomics Innovation Center in Heart Failure NIH Contract HHSN268201000032C. G. A. Ramirez-Correa was supported by the American Heart Association and The Lawrence and Florence A. DeGeorge Charitable Trust Scientist Developing Grant (AHA-12SDG9140008). V. Kooij and Y. Li were supported by AHA Mid-Atlantic postdoctoral fellowships, A. Frazier was supported by the Ruth L. Kirschstein-National Service Research Award T32HD4435 and the NIH Pediatric Loan Repayment Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.A.R.-C., A.H.F., V.K., W.D.G., E.T., D.B.F., and A.M.M. conception and design of research; G.A.R.-C., A.H.F., G.Z., P.Z., T.R., V.K., D.B., R.H., Y.L., X.S., and O.H.C. performed experiments; G.A.R.-C., A.H.F., G.Z., P.Z., T.R., V.K., D.B., G.A.S., N.S.L.-F., R.H., Y.L., X.S., O.H.C., E.T., D.B.F., and A.M.M. analyzed data; G.A.R.-C., A.H.F., G.Z., P.Z., T.R., V.K., D.B., G.A.S., N.S.L.-F., R.H., Y.L., X.S., W.D.G., O.H.C., E.T., D.B.F., and A.M.M. interpreted results of experiments; G.A.R.-C., A.H.F., G.Z., V.K., N.S.L.-F., X.S., O.H.C., and E.T. prepared figures; G.A.R.-C. and A.H.F. drafted manuscript; G.A.R.-C., A.H.F., P.Z., V.K., D.B., G.A.S., N.S.L.-F., R.H., Y.L., W.D.G., O.H.C., E.T., D.B.F., and A.M.M. edited and revised manuscript; G.A.R.-C., A.H.F., G.Z., P.Z., T.R., V.K., D.B., G.A.S., N.S.L.-F., R.H., Y.L., X.S., W.D.G., O.H.C., E.T., D.B.F., and A.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Robinson for expert technical assistance, and Dr. Nazareno Paolocci and Dr. Jonathan Kirk for helpful manuscript comments and suggestions. G. A. Ramirez-Correa thanks the American Heart Association (AHA) Minority Mentoring program for support. Preliminary data of this work have been presented in the AHA Basic Cardiovascular Sciences Scientific Meeting 2011.
REFERENCES
- 1.Biesiadecki BJ, Schneider KL, Yu ZB, Chong SM, Jin JP. An R111C polymorphism in wild turkey cardiac troponin I accompanying the dilated cardiomyopathy-related abnormal splicing variant of cardiac troponin T with potentially compensatory effects. J Biol Chem 279: 13825–13832, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, Stull LB, Kass DA, Murphy AM. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol 292: H318–H325, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard E, Seidman C, Seidman JG, LeWinter M, Maughan D. Altered crossbridge kinetics in the αMHC(403/+) mouse model of familial hypertrophic cardiomyopathy. Circ Res 84: 475–483, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Brown AT, Frazier A, Dennison CR, Hill MN, Post WS, Robinson JC, Murphy AM. A Non-synonymous variant of the cardiac troponin I gene is associated with enhanced cardiac hypertrophy response to hypertension in young black men (Abstract). In: AHA Scientific Basis of Heart Failure in Children. Dallas, TX: American Heart Association, 2008. [Google Scholar]
- 5.Cardim N, Perrot A, Ferreira T, Pereira A, Osterziel KJ, Reis RP, Correia JF. Usefulness of Doppler myocardial imaging for identification of mutation carriers of familial hypertrophic cardiomyopathy. Am J Cardiol 90: 128–132, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res 109: 1410–1414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis IW, Arendall WB 3rd, Richardson DC, Richardson JS. The backrub motion: how protein backbone shrugs when a sidechain dances. Structure 14: 265–274, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Davis J, Yasuda S, Palpant NJ, Martindale J, Stevenson T, Converso K, Metzger JM. Diastolic dysfunction and thin filament dysregulation resulting from excitation-contraction uncoupling in a mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol 53: 446–457, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas JG, Bakris GL, Epstein M, Ferdinand KC, Ferrario C, Flack JM, Jamerson KA, Jones WE, Haywood J, Maxey R, Ofili EO, Saunders E, Schiffrin EL, Sica DA, Sowers JR, Vidt DG. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med 163: 525–541, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Fraysse B, Weinberger F, Bardswell SC, Cuello F, Vignier N, Geertz B, Starbatty J, Kramer E, Coirault C, Eschenhagen T, Kentish JC, Avkiran M, Carrier L. Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol 52: 1299–1307, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frazier A, Judge DP, Schulman SP, Johnson N, Holmes KW, Murphy AM. Familial hypertrophic cardiomyopathy associated with cardiac beta-myosin heavy chain and troponin I mutations. Pediatr Cardiol 29: 846–850, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Frazier AH, Ramirez-Correa GA, Murphy AM. Molecular mechanisms of sarcomere dysfunction in dilated and hypertrophic cardiomyopathy. Prog Pediatr Cardiol 31: 29–33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao WD, Murray CI, Tian Y, Zhong X, Dumond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res 111: 1002–1011, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao WD, Perez NG, Seidman CE, Seidman JG, Marban E. Altered cardiac excitation-contraction coupling in mutant mice with familial hypertrophic cardiomyopathy. J Clin Invest 103: 661–666, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgakopoulos D, Christe ME, Giewat M, Seidman CM, Seidman JG, Kass DA. The pathogenesis of familial hypertrophic cardiomyopathy: Early and evolving effects from an α-cardiac myosin heavy chain missense mutation. Nat Med 5: 327–330, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, Seidman CE, Solomon SD. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 105: 2992–2997, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hypertrophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet 42: e59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol 299: H1092–H1099, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol 299: H1741–H1749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen PM, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol 282: H499–H507, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, Hiroi S, Sasaoka T, Ohbuchi N, Nakamura T, Koyanagi T, Hwang TH, Choo JA, Chung KS, Hasegawa A, Nagai R, Okazaki O, Nakamura H, Matsuzaki M, Sakamoto T, Toshima H, Koga Y, Imaizumi T, Sasazuki T. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat Genet 16: 379–382, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics 5: 749–757, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kirk JA, MacGowan GA, Evans C, Smith SH, Warren CM, Mamidi R, Chandra M, Stewart AF, Solaro RJ, Shroff SG. Left ventricular and myocardial function in mice expressing constitutively pseudophosphorylated cardiac troponin I. Circ Res 105: 1232–1239, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitaoka H, Kubo T, Hayashi K, Yamasaki N, Matsumura Y, Furuno T, Doi YL. Tissue Doppler imaging and prognosis in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 14: 544–549, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, Housmans PR, Weissman NJ, Morad M, Potter JD. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem 276: 10039–10048, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Kooij V, Holewinski RJ, Murphy AM, Van Eyk JE. Characterization of the cardiac myosin binding protein-C phosphoproteome in healthy and failing human hearts. J Mol Cell Cardiol 60: 116–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature 298: 182–184, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res 94: 146–158, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Michels M, Soliman OI, Kofflard MJ, Hoedemaekers YM, Dooijes D, Majoor-Krakauer D, ten Cate FJ. Diastolic abnormalities as the first feature of hypertrophic cardiomyopathy in Dutch myosin-binding protein C founder mutations. JACC Cardiovasc imaging 2: 58–64, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen J, Kubo T, Duque M, Uribe W, Shaw A, Murphy R, Gimeno JR, Elliott P, McKenna WJ. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest 111: 209–216, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mogensen J, Murphy RT, Kubo T, Bahl A, Moon JC, Klausen IC, Elliott PM, McKenna WJ. Frequency and clinical expression of cardiac troponin I mutations in 748 consecutive families with hypertrophic cardiomyopathy. J Am Coll Cardiol 44: 2315–2325, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H, Burke M, Elliott PM, McKenna WJ. Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 44: 2033–2040, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol 536: 583–592, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marban E. Transgenic mouse model of stunned myocardium. Science 287: 488–491, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, Quinones MA, Roberts R, Marian AJ. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 104: 128–130, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niimura H, Patton KK, McKenna WJ, Soults J, Maron BJ, Seidman JG, Seidman CE. Sarcomere protein gene mutations in hypertrophic cardiomyopathy of the elderly. Circulation 105: 446–451, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran G, Kumar M, Selvi Rani D, Annanthapur V, Calambur N, Nallari P, Kaur P. An in silico analysis of troponin I mutations in hypertrophic cardiomyopathy of Indian origin. PLos One 8: e70704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez-Correa GA, Cortassa S, Stanley B, Gao WD, Murphy AM. Calcium sensitivity, force frequency relationship and cardiac troponin I: critical role of PKA and PKC phosphorylation sites. J Mol Cell Cardiol 48: 943–953, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt JP, Semsarian C, Arad M, Gannon J, Ahmad F, Duffy C, Lee RT, Seidman CE, Seidman JG. Consequences of pressure overload on sarcomere protein mutation-induced hypertrophic cardiomyopathy. Circulation 108: 1133–1138, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, Sevdalis E, Keren A, Pellerin D, McKenna WJ, Elliott PM. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart 94: 1288–1294, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Smith CA, Kortemme T. Predicting the tolerated sequences for proteins and protein interfaces using RosettaBackrub flexible backbone design. PLos One 6: e20451, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spindler M, Saupe KW, Christe ME, Sweeney HL, Seidman CE, Seidman JG, Ingwall JS. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest 101: 1775–1783, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature 424: 35–41, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Tardiff JC. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res 108: 765–782, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol 558: 927–941, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. N Engl J Med 364: 1643–1656, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Wei B, Gao J, Huang XP, Jin JP. Mutual rescues between two dominant negative mutations in cardiac troponin I and cardiac troponin T. J Biol Chem 285: 27806–27816, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei H, Jin JP. A dominantly negative mutation in cardiac troponin I at the interface with troponin T causes early remodeling in ventricular cardiomyocytes. Am J Physiol Cell Physiol 307: C338–C348, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu WC, Bhavsar JH, Aziz GF, Sadaniantz A. An overview of stress echocardiography in the study of patients with dilated or hypertrophic cardiomyopathy. Echocardiography 21: 467–475, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Zhang P, Kirk JA, Ji W, Dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation 126: 1828–1837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]