Abstract
High-frequency spinal cord stimulation (HF-SCS) is a novel technique of inspiratory muscle activation involving stimulation of spinal cord pathways, which may have application as a method to provide inspiratory muscle pacing in ventilator-dependent patients with spinal cord injury. The purpose of the present study was to compare the spatial distribution of motor drive to the parasternal intercostal muscles during spontaneous breathing with that occurring during HF-SCS. In nine anesthetized dogs, HF-SCS was applied at the T2 spinal level. Fine-wire recording electrodes were used to assess single motor unit (SMU) pattern of activation in the medial bundles of the 2nd and 4th and lateral bundles of the 2nd interspaces during spontaneous breathing and HF-SCS following C1 spinal section. Stimulus amplitude during HF-SCS was adjusted such that inspired volumes matched that occurring during spontaneous breathing (protocol 1). During HF-SCS mean peak SMU firing frequency was highest in the medial bundles of the 2nd interspace (17.1 ± 0.6 Hz) and significantly lower in the lateral bundles of the 2nd interspace (13.5 ± 0.5 Hz) and medial bundles of the 4th (15.2 ± 0.7 Hz) (P < 0.05 for each comparison). Similar rostrocaudal and mediolateral gradients of activity were observed during spontaneous breathing prior to C1 section. Since rib cage movement was greater and peak discharge frequencies of the SMUs higher during HF-SCS compared with spontaneous breathing, stimulus amplitude during HF-SCS was adjusted such that rib cage movement matched that occurring during spontaneous breathing (protocol 2). Under this protocol, mean peak SMU frequencies and rostrocaudal and mediolateral gradients of activity during HF-SCS were not significantly different compared with spontaneous breathing. This study demonstrates that 1) the topographic pattern of electrical activation of the parasternal intercostal muscles during HF-SCS is similar to that occurring during spontaneous breathing, and 2) differential spatial distribution of parasternal intercostal activation does not depend upon differential descending synaptic input from supraspinal centers.
Keywords: breathing, spinal cord injury, spinal cord stimulation
during spontaneous breathing in both animals and humans, the distribution of neural drive to the inspiratory intercostal muscles follows specific patterns. Inspiratory drive to the external intercostal muscles is greater in the rostral compared with the caudal segments and greater in the dorsal compared with the ventral portion of the rib cage, in both animals (9, 12, 27) and humans (3, 5, 32). Inspiratory drive to the parasternal muscles is also greater in the rostral compared with the caudal segments in both animals (7, 8, 26) and humans (3, 16, 22). With regard to mediolateral patterns of inspiratory drive to the parasternal intercostal muscles, however, differences exist between animals and humans. While inspiratory drive is greater in the medial regions of the muscle and decreases progressively toward the costochondral junction in animals, drive is uniform across these regions in humans. Remarkably, in each of these muscle groups, the level of inspiratory drive matches the mechanical advantage of each portion of these muscles, i.e., inspiratory drive is greater in regions where there is a greater mechanical advantage and unchanged where none exists (9, 28).
The mechanism of the spatial distribution of intercostal muscle activation is not well understood. In recent studies in animals, we demonstrated that the spatial distribution of external muscle activation does not depend upon synaptic inputs from central sources but that the neural circuitry responsible for the specific pattern of external intercostal muscle activation resides at a spinal cord level (13). This study was accomplished by using a novel method of electrical stimulation of the inspiratory muscles, i.e., high-frequency spinal cord stimulation (HF-SCS) in a C2 spinal cord preparation (10–14, 25). This technique involves the stimulation of spinal cord tracts located on the ventral surface of the spinal cord which synapse with the inspiratory motoneuron pools. By this method, the stimulus is processed within the motoneuron pools resulting in activation of both the diaphragm and inspiratory intercostal muscles at physiological firing frequencies. Moreover, HF-SCS results in an asynchronous pattern of EMG activity resembling spontaneous breathing.
In the present investigation, we sought to perform a similar evaluation of the distribution of electrical activation to the parasternal intercostal muscles. In general, motoneuron recruitment is determined by both intrinsic membrane excitability and the quantity and the quality of synaptic connections which further lead to differential muscle activation. Unlike the external intercostal muscles, the parasternal muscles have a paucity of proprioceptors which could potentially mediate differential activation between intercostal segments. This fact increases the likelihood that differences in synaptic inputs from supraspinal sources play a significant role in the differential activation of parasternal bundles.
To assess the potential role of supraspinal influences on the spatial distribution of parasternal activation, the pattern of parasternal muscle activation was assessed during spontaneous breathing and compared with that occurring during HF-SCS following spinal cord section at the C1 level. We hypothesized that the spatial distribution of neural drive to the parasternal muscles is preserved during HF-SCS indicating that the circuitry for the differential drive to these muscles resides within the spinal cord.
MATERIALS AND METHODS
All studies were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University, Cleveland, OH.
Studies were performed on nine dogs (mean weight 20.3 ± 2.2 kg). All animals were initially anesthetized with pentobarbital sodium (25 mg/kg) administered intravenously. Additional doses (1–2 mg/kg) were provided, as needed to suppress corneal reflexes and response to noxious stimuli. Animals were euthanized at the completion of the experiments with pentobarbital (100 mg/kg iv).
Following a midline cervical tracheal incision, a large-bore cuffed endotracheal tube (10 mm ID) was sutured into the trachea in the midcervical region. A femoral vein catheter was placed to administer fluid and additional doses of anesthesia. Blood pressure and heart rate were monitored (Waveline Pro Multi-Function Monitor, DRE, Louisville KY) via a femoral arterial catheter. Body temperature was maintained at 38 ± 0.5°C with a heating blanket (Harvard Apparatus, Holliston, MA). End-tidal Pco2 was monitored at the trachea and oxygen saturation from the earlobe (Waveline Pro Multi-Function Monitor). Tidal volume was recorded by electrical integration of the flow signal from a pneumotachograph (Series 3700, Hans Rudolph, Shawnee, KS).
Via a laminectomy incision at the T4 level, an eight-plate electrode lead with 4-mm contacts (AD-TEDH Medical Instrument, Racine, WI) was positioned under direct vision on the ventral surface of the spinal cord and advanced to the T2 level [see previous references (10–14) for detailed description]. Electrical stimulation was provided with square-wave pulse stimulator (model S88, Grass Technologies, West Warwick, RI) equipped with a stimulus isolation unit (SIU5, Grass Technologies). Stimulus train duration during HF-SCS was set at 1.4 s since this duration approximated that occurring during spontaneous breathing.
Respiratory displacements of the chest wall were monitored by inductance plethysmography (Braebon Medical Kanata, ON, Canada). The effort belts were placed around the rib cage at the lower border of the sternum and around the abdomen at the level of the umbilicus, respectively. Gains of the two signals were adjusted during spontaneous efforts following airway occlusion.
Bipolar Teflon-coated, stainless steel fine-wire electrodes, uninsulated at their terminal ∼5 mm, were used to assess multiunit inspiratory muscle EMG recordings of the parasternal muscles (medial and lateral bundles of the 2nd interspace and medial bundles of the 4th interspace). Electrodes in the medial bundles were positioned ∼1 cm lateral to the sternum whereas the electrodes in the lateral bundles were positioned ∼1 cm medial to the costochondral junctions. Single motor units (SMUs) were also monitored to further characterize inspiratory muscle activation. SMU recordings were made using Teflon-coated stainless steel electrodes with an uninsulated portion of ∼1 mm, to provide greater selectivity. EMG potentials were amplified (1,000–10,000 times) and filtered (30 Hz to 5.0 kHz) (Grass Technologies P511 Amplifier, AstroMed, West Warwick, RI). Recordings were made with the animal in the supine posture to facilitate access to the parasternal intercostal muscles. All recordings were monitored and stored for offline analysis on a computer utilizing a data acquisition and analyzing system (Spike2 with 1401 interface, Cambridge Electronic Design, Cambridge, UK).
After measurements were made during spontaneous breathing, the cervical spinal cord was sectioned at the C1 level in each animal with watchmaker forceps. A hook forceps was passed across the area of transection to verify complete spinal cord section.
Protocol 1 (n = 6).
To assess their pattern of activation, EMG recording electrodes were positioned in each of the parasternal muscles. Multiunit and SMU measurements of each muscle were initially taken during spontaneous breathing. Following C1 section, EMG measurements were made during HF-SCS (300 Hz; 0.2-ms pulse width) at the T2 level; stimulus amplitude was adjusted to approximate the magnitude of inspired volume observed during spontaneous breathing (mean stimulus amplitude of 0.72 ± 0.08 mA). Five to 10 sites were sampled from each muscle for SMU recordings. At each site, one to four SMUs were distinguished on the basis of their size and distinct morphology. Several breaths were analyzed at each site.
Protocol 2 (n = 3).
Similar to our prior studies (12) and preliminary results of this study, the rib cage contribution to inspired volume was greater during HF-SCS compared with spontaneous breathing; stimulus amplitude was adjusted to approximate the magnitude of rib cage movement observed during spontaneous breathing (mean stimulus amplitude, 0.31 ± 0.07 mA). With this protocol, we sought to determine the distribution of inspiratory activity to the parasternal muscles under conditions in which activation was more comparable to spontaneous breathing. In all other respects, the protocol procedure was the same as protocol 1.
Data analysis.
During spontaneous breathing, the time of onset of multiunit EMG of the parasternal muscles (To) was determined relative to the onset of inspiratory flow. During HF-SCS, the onset of multiunit EMG was determined relative to the onset of the stimulus pulse. The flow signal was used to determine inspiratory time (Ti). To was also expressed as a percentage of Ti. Values were averaged over five consecutive breaths.
Mean peak discharge frequencies of SMUs were assessed over several consecutive breaths. The discharge frequency of each SMU during spontaneous breathing and HF-SCS was plotted against the number of motor units recorded.
Comparisons were made, where applicable, using one-way ANOVA and post hoc Newman-Keuls tests. A P value of < 0.05 was taken as representing statistical significance. All data are reported as means ± SE.
RESULTS
During spontaneous breathing, a total of 295 motor units were recorded (mean 49 units for each of the six animals, range 16–70) and 349 motor units during HF-SCS (mean 39 units for each of the 9 animals, range 11–74). Overall 644 units were recorded under both conditions. Blood pressure remained stable throughout the period of study in each animal (mean > 80 mmHg).
Protocol 1 (n = 6).
The inspired volumes achieved during HF-SCS, during which parasternal EMG measurements were made, were not significantly different from those occurring during spontaneous breathing (252 ± 7 vs. 270 ± 5 ml, NS) (Table 1).
Table 1.
Mean inspired volume and rib cage contribution to inspired volume during spontaneous breathing and HF-SCS
| Tidal Volume Matching (n = 6) |
Rib Cage Contribution Matching (n = 3) |
||
|---|---|---|---|
| Spontaneous Breathing | HF-SCS | HF-SCS | |
| Tidal volume, ml | 252 ± 7 | 270 ± 5 | 136 ± 2* |
| Volume/kg, ml/kg | 13.5 ± 0.3 | 14.5 ± 0.2 | 5.8 ± 0.1* |
| Rib cage contribution | |||
| ml | 96.0 ± 10.3 | 135.1 ± 2.8* | 90.3 ± 6.5 |
| % | 38.9 ± 3.8 | 51.0 ± 2.0* | 64.0 ± 2.5* |
Values are means ± SE.
HF-SCS, high-frequency spinal cord stimulation.
P < 0.05 compared with spontaneous breathing.
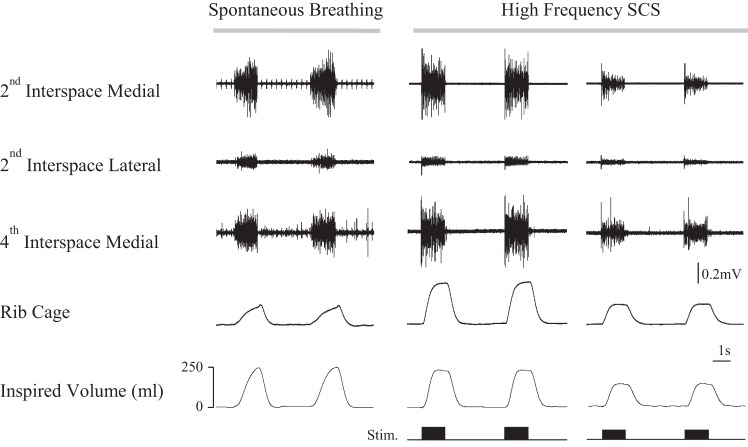
The pattern of multiunit EMG activity of the parasternal intercostal muscles during spontaneous breathing (left panel) and during HF-SCS with matching of inspired volume (middle panel) is shown for a single representative animal in Fig. 1. During spontaneous breathing and HF-SCS, the parasternal muscles of the 2nd interspace (medial and lateral) and 4th interspace were electrically active. Similar to spontaneous breathing, HF-SCS results in an asynchronous pattern of parasternal activation at each of the recording sites. During spontaneous breathing, the onset of the parasternal muscle activity in each muscle was delayed relative to the onset of inspiratory flow.
Fig. 1.
Multiunit EMG recordings form the parasternal intercostal muscles during spontaneous breathing and high-frequency spinal cord stimulation (HF-SCS) in one animal. From top to bottom, tracings represent multiunit EMG recordings of the parasternal intercostal muscles from the medial bundles of the 2nd interspace, lateral bundles of the 2nd interspace, and medial bundles of the 4th interspace and inspired volume. Recordings were obtained during spontaneous breathing (left panel) and HF-SCS with matching of inspired volume to spontaneous breathing (middle panel) and during HF-SCS with matching of rib cage expansion to spontaneous breathing (right panel). See text for further explanation.
Delays in timing during spontaneous breathing (relative to the onset of inspiratory flow and percentage of inspiratory time) and HF-SCS (relative to stimulus onset) are shown in Table 2. During spontaneous breathing, the onset of parasternal EMG in the medial bundles of the 2nd interspace was 301 ± 37 ms from the onset of inspiratory flow and To/Ti of 18 ± 2%. The onset of parasternal activity in the lateral bundles of the 2nd interspace and medial bundles of the 4th interspace were also significantly delayed relative to the onset of activity in the medial bundles of the 2nd interspace. During HF-SCS, the onset of parasternal intercostal EMG in the medal bundles of the 2nd interspace occurred following a delay of 4.1 ± 0.3 ms from the onset of stimulation. Moreover, the onset of activity in the lateral bundles of the 2nd interspace and medial bundles of the 4th interspace were delayed relative to the onset of activity in the medial bundles of the 2nd interspace (P < 0.05). The onset of inspiratory flow during HF-SCS expressed relative to the onset of the stimulus occurred with a mean delay of 83.9 ± 2.4 ms.
Table 2.
Delays in onset of parasternal intercostal EMG activity during spontaneous breathing and HF- SCS
| HF-SCS |
||||
|---|---|---|---|---|
| Spontaneous Breathing (n = 6) |
Tidal volume matching (n = 6) |
Rib cage contribution matching (n = 3) |
||
| Delay, ms | To/TI, % | Delay, ms | Delay, ms | |
| 2nd interspace medial | 301 ± 37 | 18 ± 2 | 4.1 ± 0.3 | 4.4 ± 0.2 |
| 2nd interspace lateral | 563 ± 58* | 30 ± 2* | 5.4 ± 0.5* | 6.1 ± 0.4* |
| 4th interspace medial | 576 ± 52* | 33 ± 2* | 4.9 ± 0.2* | 5.3 ± 0.4* |
Values are means ± SE.
P < 0.05 compared with 2nd interspace medial during spontaneous breathing and HF-SCS, respectively
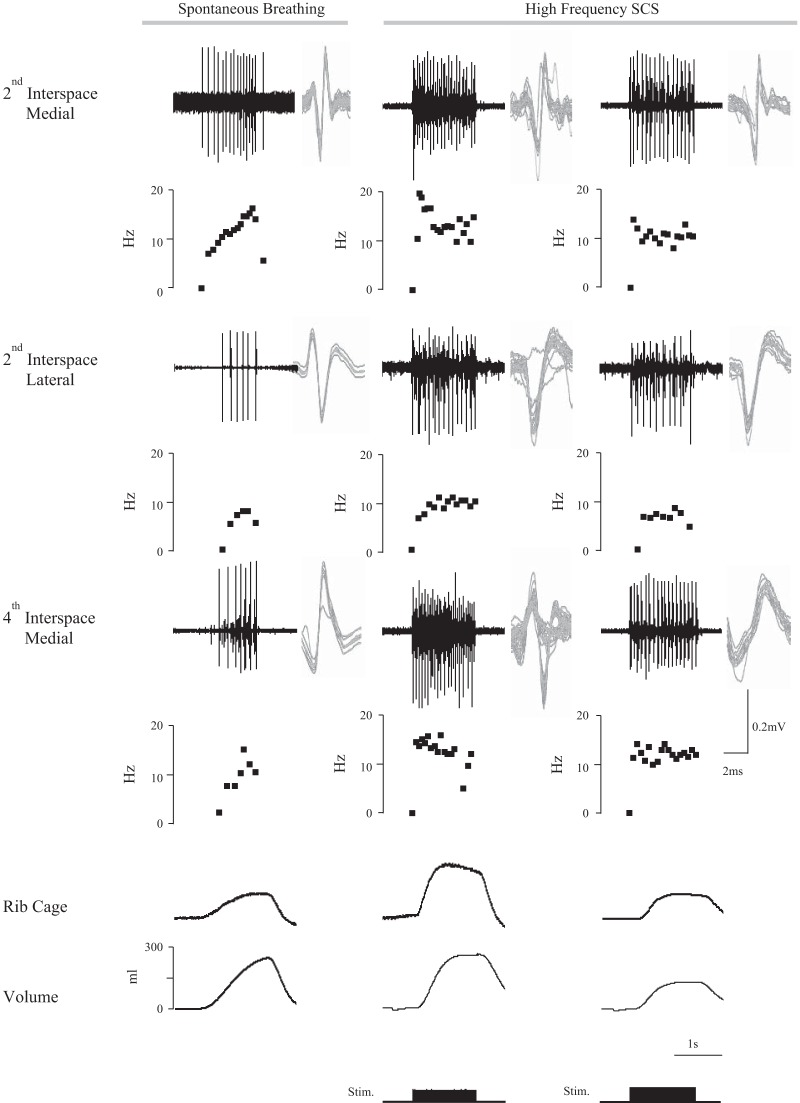
SMU activities of the parasternal intercostal muscles during spontaneous breathing and HF-SCS are shown for one animal in Fig. 2. The action potentials from the SMUs are superimposed to the right of each tracing for each muscle, thus confirming their similar morphology. In a graph below each tracing, the instantaneous firing frequencies of the motor units are provided. With matching of inspired volumes (middle panel), the firing frequencies during HF-SCS for each of the parasternal intercostal muscle bundles, EMG recordings are somewhat higher than those occurring during spontaneous breathing. As shown in the lower portion of Fig. 2 (left and middle panels), these firing frequencies during HF-SCS are associated with a greater rib cage contribution to inspired volume compared with spontaneous breathing (Table 1).
Fig. 2.
Single motor unit recordings from the parasternal intercostal muscles during spontaneous breathing and HF-SCS in one animal. From top to bottom, tracings represent single motor unit EMG recordings of the parasternal intercostal muscles from the medial bundles of the 2nd interspace, lateral bundles of the 2nd interspace, and medial bundles of the 4th interspace, the instantaneous frequency plot of that unit, and the corresponding inspired volume. Recordings were obtained during spontaneous breathing (left panel) and HF-SCS with matching of inspired volume to that observed during spontaneous breathing (middle panel) and during HF-SCS with matching of rib cage expansion to that observed during spontaneous breathing (right panel). All the action potentials from each motor unit are superimposed on the right of each tracing. See text for further explanation.
Histograms of the discharge frequencies of all recorded single motor units identified are provided in Fig. 3. Mean peak discharge frequencies of the parasternal SMUs in the medial bundles of the 2nd interspace were 17.1 ± 0.6 Hz during HF-SCS and 13.3 ± 0.4 Hz during spontaneous breathing (P < 0.05). Mean peak firing frequencies of the parasternal SMUs in the lateral bundles of the 2nd interspace were 13.5 ± 0.5 Hz during HF-SCS and 8.8 ± 0.2 Hz during spontaneous breathing (P < 0.05). Mean peak discharge frequencies of the SMUs in the medial bundles of the parasternal muscle in the 4th interspace were 15.2 ± 0.7 Hz during HF-SCS and 11.4 ± 0.3 Hz during spontaneous breathing (P < 0.05). During both HF-SCS and spontaneous breathing, mean peak discharge frequencies of the SMUs in the medial bundles of 2nd interspace were significantly higher than that of the lateral bundles of the parasternal in the 2nd interspace and medial bundles of the parasternal in the 4th interspace (P < 0.05 for each comparison).
Fig. 3.
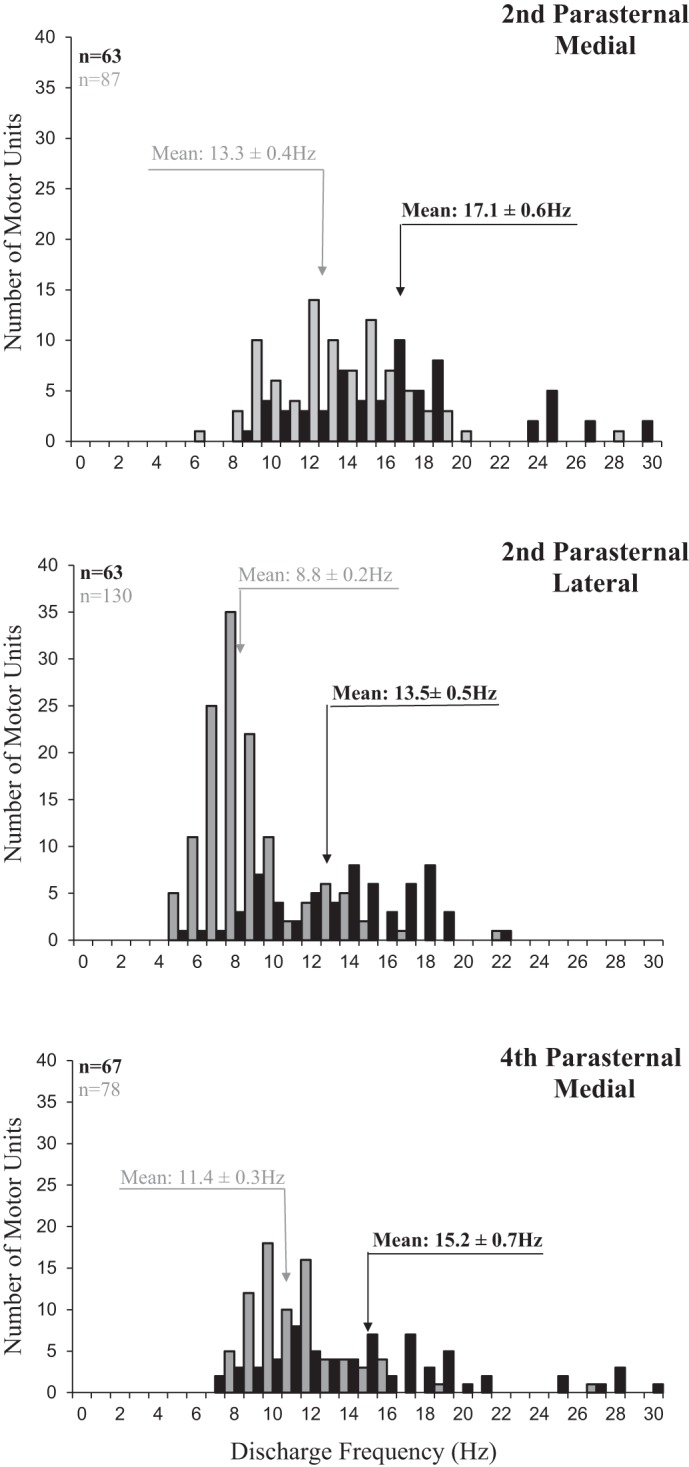
Histograms of the peak discharge frequencies of all single motor units identified in the medial bundles of the 2nd interspace (top panel), lateral bundles of the 2nd interspace (middle panel), and medial bundles of the 4th interspace (bottom panel) during spontaneous breathing (gray bars) and during HF-SCS (dark bars). Bin width, 1 Hz. Recordings were obtained under conditions in which inspired volume during HF-SCS was matched to inspired volumes recorded during spontaneous breathing by adjustment of stimulus amplitude. See text for further explanation.
When expressed as a percentage of the mean peak firing frequencies of the SMUs in the medial bundles of the parasternal muscle in the 2nd interspace under each condition, the mean peak firing frequencies of the SMUs of the parasternal muscle in the lateral bundles of the 2nd interspace and medial bundles of the 4th interspace during HF-SCS and spontaneous breathing were not significantly different.
Protocol 2 (n = 3).
Mean inspired volumes during spontaneous breathing and HF-SCS during which EMG measurements were recorded are presented in Table 1. Under the conditions of this protocol, the absolute rib cage contribution to inspired volumes during HF-SCS was well matched and not significantly different from that occurring during spontaneous breathing. Inspired volumes during spontaneous breathing were significantly greater than those occurring during HF-SCS (P < 0.05).
Multiunit EMG and single motor unit activities during spontaneous breathing and HF-SCS are shown for one animal in Figs. 1 and 2, respectively. With this protocol of matched rib cage contribution, firing frequencies of motor units appeared somewhat lower for each bundles of the parasternal muscles compared with the middle panel, but were similar to spontaneous breathing (left panel).
Histograms of the discharge frequencies of all motor units identified in the parasternals in the 2nd (medial and lateral) and medial bundle of the 4th interspace are provided in Fig. 4. Mean peak discharge frequencies of the SMUs in the medial bundle of the 2nd interspace were 13.3 ± 0.4 Hz during spontaneous breathing and 14.3 ± 0.5 Hz during HF-SCS (NS). During both spontaneous breathing and HF-SCS, mean peak discharge frequencies of the SMUs in the lateral bundles of the 2nd interspace and medial bundles of the 4th interspace were significantly lower than that of the medial bundles of the 2nd interspace (P < 0.05 for each comparison).
Fig. 4.
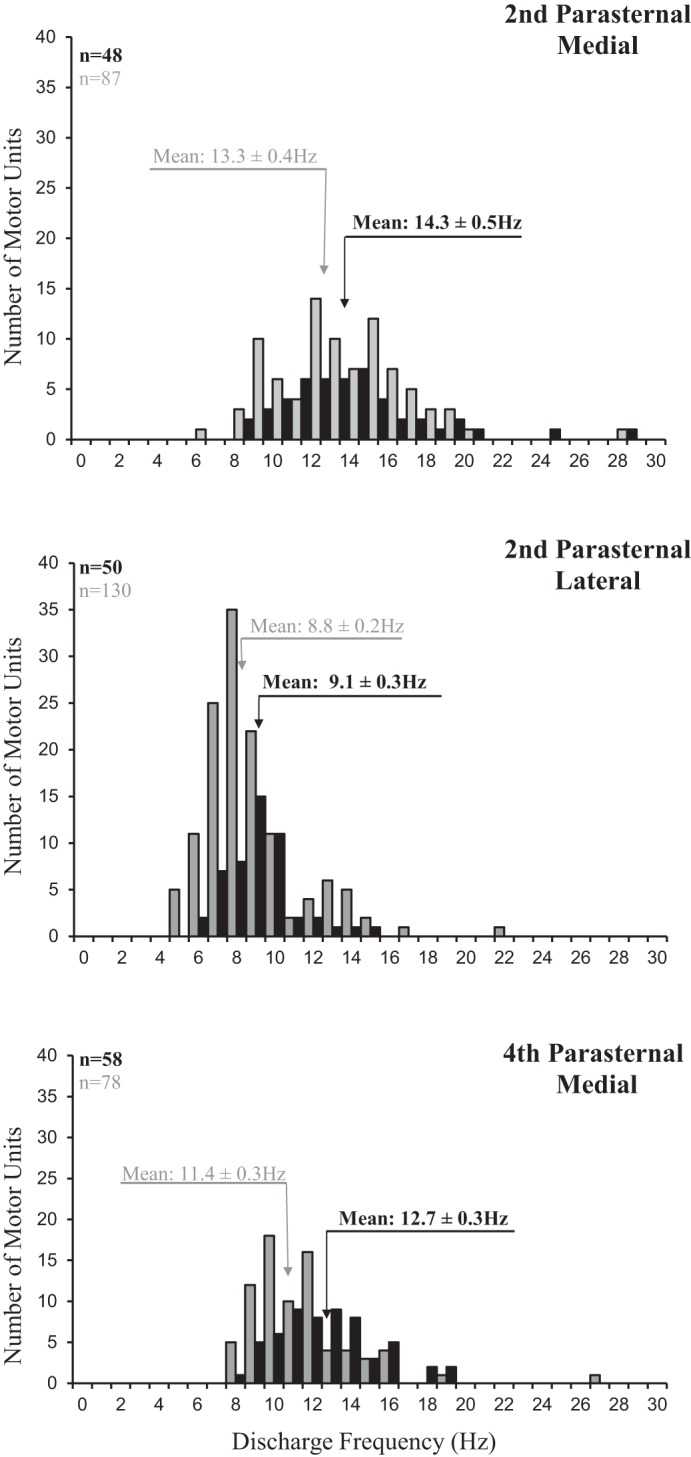
Histograms of the peak discharge frequencies of all single motor units identified in the medial bundles of the 2nd interspace (top panel), lateral bundles of the 2nd interspace (middle panel), and medial bundles of the 4th interspace (bottom panel) during spontaneous breathing (gray bars) and during HF-SCS (dark bars). Bin width, 1 Hz. Recordings were obtained under conditions in which rib cage expansion during HF-SCS was matched to that recorded during spontaneous breathing by adjustment of stimulus amplitude. See text for further explanation.
When expressed as a percentage of the mean peak firing frequencies of the SMUs in the medial bundles of the 2nd interspace under each condition, mean peak firing frequencies of the SMUs in the lateral bundles of the 2nd interspace and medial bundles of the 4th interspace during spontaneous breathing and HF-SCS were not significantly different. In addition, the percentage reductions in firing frequencies during both spontaneous breathing and HF-SCS were not significantly different compared with protocol 1 in which inspired volumes were matched.
DISCUSSION
The results of this study demonstrate that, like the external intercostal muscles, the spatial distribution of inspiratory activity to the parasternal intercostal muscles during HF-SCS in a C1 spinal cord preparation is similar to that occurring during spontaneous breathing. The relative spatial distribution of parasternal intercostal activity, i.e., the electrical activation of the medial bundles of the 2nd interspace being greater than both the lateral bundles of the same space and medial bundles of the 4th interspace, was present in the same proportion during HF-SCS and similar to that occurring during spontaneous breathing. Activation of the medial bundles also preceded the onset of the lateral bundles of parasternal activation in the 2nd interspace under both conditions. In further support of the physiological nature of HF-SCS as a means of inspiratory muscle activation, these results indicate that the mediolateral and rostrocaudal gradients of parasternal activation during HF-SCS are similar to those occurring during spontaneous breathing. These results strongly suggest the neural circuitry responsible for the differential activation of the parasternal muscles resides at the level of the spinal cord and does not require supraspinal inputs.
Study limitations.
The peak discharge frequencies of the parasternal intercostal muscles during HF-SCS were significantly higher than those occurring during spontaneous breathing despite matching of inspired volumes. This finding is consistent with our previous analyses of the pattern of activation of the external intercostal muscles in which peak discharge frequencies of this muscle group were significantly higher during HF-SCS compared with spontaneous breathing at the same inspired volumes (12). As in this prior study, we determined that the rib cage contribution to inspired volume was significantly higher during HF-SCS compared with spontaneous breathing (51.0 ± 2.0% vs, 38.9 ± 3.8%). Greater rib cage movement would be expected to require a higher level of neural drive, explaining the higher intercostal firing frequencies. As in our prior study, the amplitude of HF-SCS was adjusted such that the absolute rib cage contribution to inspired volume was matched and not significantly different from that occurring during spontaneous breathing. Under these conditions, mean peak firing frequencies of each portion of the parasternal muscles during HF-SCS was not significantly different compared with that occurring spontaneous breathing. Importantly, the mediolateral and rostrocaudal gradients of electrical activation of the parasternal intercostal muscles at both the initial and subsequently reduced levels of HF-SCS were preserved. As a result, we demonstrated that the differential activation of the parasternal muscles during HF-SCS was preserved at two different levels of inspired volume generation.
Comparison to previous studies.
The distribution of neural drive to the parasternal intercostal muscles has been investigated previously in animal studies (7, 8, 26). In studies performed in dogs, the peak heights of integrated EMG activities during spontaneous breathing were compared to the magnitude of the EMG signal during tetanic stimulation of the internal intercostal nerves in their respective interspaces in an attempt to provide a quantitative assessment of the degree of muscle activation (26). By this methodology, parasternal intercostal inspiratory activity was greater in the third compared with the fifth interspace and smallest in the seventh interspace. Moreover, distribution of parasternal muscle activation was maintained during hypercapnia, inspiratory resistive loading, following phrenicotomy, and after deafferentation of the rib cage. The spatial distribution of parasternal activity observed in the present study is qualitatively similar to these previous studies in animals. Of note, the spatial distribution of motor drive to the parasternal muscles was also investigated in humans by measurements of peak motor unit firing frequencies, as in the present study. As with the animal studies, discharge frequencies of motor units were greater in the upper compared with the lower interspaces (2, 3, 16, 22). Unlike the animals, the peak discharge frequencies were similar in the medial and lateral bundles in the 2nd interspace (16).
As in previous animal investigations (7, 26), we also found that the onset of activation of the parasternal muscles varies with interspace during spontaneous breathing, i.e., onset of activity in the upper interspaces precedes that of the lower interspaces and activity in the medial precedes activity in the lateral interspaces. Comparison of the onset of activation between the medial and middle (lateral bundle in present study) parasternal bundles in the 3rd interspace was assessed quantitatively by DeTroyer et al. (7) who found a mean delay of 176 ms. Given the fact that the medial and lateral bundles were compared in the present study, this value is in the same range of the mean delay of 262 ms observed herein. Consistent with our previous investigation of the external intercostal muscles, the onsets of each of the parasternal bundles during HF-SCS occurred with very short delays. As with spontaneous breathing, however, activation of the medial bundles of the parasternal muscle in the 2nd interspace preceded activity in the lateral bundles of the 2nd interspace and medial bundles of the 4th interspace when expressed relative to the onset of the stimulus pulse. The virtual simultaneous activation of the parasternal bundles with HF-SCS is not likely to have any potential clinical impact since we have demonstrated in previous animal studies that this method can provide long-term ventilator support without evidence of fatigue and at very low stimulus amplitude.
While the pattern of intercostal muscle activation is of some physiological interest, the true significance of these findings lies in the fact that the differential pattern of parasternal activation matches their differential mechanical advantage (33). Using changes in length in response to passive inflation and muscle fiber orientation, Wilson and DeTroyer (33) determined that the mechanical advantage of the parasternal bundles also decreased progressively between the cephalad and caudal interspaces and between the medial and lateral bundles of the same interspace, in animal studies. Of interest, the model predicted no mechanical advantage between the medial and lateral bundles of the parasternal muscles in humans, and indeed, as we mentioned above, there was no detectable difference in neural drive between medial and middle bundles of these muscles. From a teleological standpoint, this precise matching of degree of neural activation and mechanical advantage would serve to minimize energy expenditure involved in the work of breathing.
Mechanism of parasternal differential activation.
Since the present investigation was performed in a C1 section animal preparation, it is clear that the neural circuitry responsible for the differential distribution of neural drive to the parasternal muscles exists at a spinal level. Like the external intercostal muscles, therefore, differential activation of the parasternal muscles is not dependent upon differential descending synaptic input from supraspinal centers.
While the external intercostal muscles have a high density of muscle spindles which may, in fact, mediate the differential activation of these muscles, the parasternal muscles have relatively few proprioreceptors, making this mechanism less likely (6, 15). Moreover, previous investigators monitored parasternal activation patterns before and after sectioning the dorsal roots (8). Since dorsal rhizotomy had no effect on differential parasternal activation, the potential role of proprioceptors (including muscle spindles and tendon organs) in mediating this phenomenon was considered nonexistent. These data combined with the results of the present study suggest that the spatial distribution of parasternal activation is not due to mechanoreceptor feedback and therefore must be due to other mechanisms.
Another potential mechanism of differential parasternal activation relates to the intrinsic properties of the parasternal motoneurons. Motor units with lower axonal conduction velocities and slow-twitch oxidative muscle fibers (Type I) correlate with smaller motoneuron size, are more excitable, and are therefore recruited first (19–21). This is followed by recruitment of motor units with progressively larger higher-threshold motoneurons with greater axonal conduction velocities innervating fast-twitch glycolytic muscle fibers. By this mechanism, motor unit recruitment proceeds in an orderly fashion from slow to fast units (Henneman's size principle). With regard to the external intercostal muscles, some studies have demonstrated that areas of the external intercostal muscles with high levels of drive had a higher proportion of Type I fibers (18). These motor units would be expected to have smaller motoneurons and therefore be activated preferentially. Other studies however have failed to demonstrate regional differences in fiber type composition (8, 26). With regard to the parasternal muscles, DeTroyer et al. (8) evaluated this potential mechanism in dogs and, consistent with other studies (26), found a high percentage of Type I fibers in the range of 55–60%. However, there were no discernible differences in the topographic distribution of fiber type composition across the rib cage. These investigators concluded, therefore, that difference in motoneuron size is an unlikely explanation of the regional differences in parasternal activation.
Other motoneuron properties may also be responsible for differential activation of the parasternal motoneurons. One consideration is the role of persistent inward currents (PICs) which are mediated by calcium channels, particularly in the motoneuron dendrites (4, 23). It has been suggested that motoneurons innervating different areas of the intercostal musculature have greater amounts of these channels and/or different degrees of modulation of the PICs by descending monoaminergic inputs. In fact, Zhan et al. (34) have demonstrated that different portions of the parasternal motoneuron population have differences in serotonergic inputs. The number of 5-hydroxytryptamine (5-HT) terminals and their density were greater in the motoneurons innervating the medial compared with the lateral regions of the parasternal muscles. This phenomenon may result in enhanced motoneuron excitability and lower activation thresholds of the motoneurons in the medial compared with the lateral regions of the parasternal muscle accounting for the earlier and greater activation. Interestingly, serotonin is a well-known modulator of PICs. Moreover, serotonergic synaptic input is important in facilitating automatic rhythmic motor behaviors including breathing.
Finally, another potential mechanism relates to a spinal premotoneuronal network of interneurons. In support of this possibility, large numbers of respiratory interneurons with inspiratory firing patterns have been identified in the lower cervical and thoracic spinal cord in animal studies (1, 24, 29–31).
Clinical implications.
The fact that HF-SCS results in inspiratory drive to both the external intercostal (12) and parasternal muscles that resembles spontaneous breathing strengthens the concept that this method results in physiological activation of the inspiratory muscles. Taken together with the previously described evidence of physiological activation (10–14), this technique may be a useful method to restore breathing in patients with ventilator-dependent tetraplegia. It should be mentioned, however, that these studies were performed in an acute animal preparation in which descending bulbospinal axons may remain functional and could be activated during electrical stimulation (17).
CONCLUSIONS
The present investigation demonstrates that HF-SCS results in the same spatial distribution of inspiratory drive to the parasternal muscles as that occurring during spontaneous breathing. Since these studies were performed in a C1 spinal preparation, we also show that the spatial distribution of inspiratory drive to the parasternal muscles is not dependent upon input from central medullopontine centers. Given the previously reported findings related to the spatial distribution of drive to the inspiratory intercostal muscles, we conclude that the pattern of inspiratory drive to the major groups of rib cage muscles can be replicated by HF-SCS and that this pattern is not dependent on input from central sources. HF-SCS holds promise as a more physiological method of providing inspiratory muscle activation in patients with ventilatory-dependent tetraplegia.
GRANTS
This study was funded by National Institutes of Health Grant R01-NS-064157.
DISCLOSURES
A. F. DiMarco is a Founder of and has a significant financial interest in Synapse BioMedical, Inc., a manufacturer of diaphragm pacing systems, and holds patents for spinal cord stimulation to restore cough and respiration. A patent concerning high-frequency spinal cord stimulation is pending. K. E. Kowalski has no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Author contributions: A.F.D. and K.E.K. conception and design of research; A.F.D. and K.E.K. performed experiments; A.F.D. and K.E.K. analyzed data; A.F.D. and K.E.K. interpreted results of experiments; A.F.D. and K.E.K. prepared figures; A.F.D. and K.E.K. drafted manuscript; A.F.D. and K.E.K. edited and revised manuscript; A.F.D. and K.E.K. approved final version of manuscript.
REFERENCES
- 1.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol 159: 115–126, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Butler JE, Gandevia SC. The output from human inspiratory motoneurone pools. J Physiol 586: 1257–1264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci 21: 4059–4065, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Troyer A, Gorman RB, Gandevia SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol 546: 943–954, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev 85: 717–756, 2005. [DOI] [PubMed] [Google Scholar]
- 7.De Troyer A, Legrand A. Inhomogeneous activation of the parasternal intercostals during breathing. J Appl Physiol 79: 55–62, 1995. [DOI] [PubMed] [Google Scholar]
- 8.De Troyer A, Legrand A, Gayan-Ramirez G, Cappello M, Decramer M. On the mechanism of the mediolateral gradient of parasternal activation. J Appl Physiol 80: 1490–1494, 1996. [DOI] [PubMed] [Google Scholar]
- 9.De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. J Physiol 518: 283–289, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol 107: 662–669, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMarco AF, Kowalski KE. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir Physiol Neurobiol 171: 218–224, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMarco AF, Kowalski KE. Distribution of electrical activation to the external intercostal muscles during high frequency spinal cord stimulation in dogs. J Physiol 589: 1383–1395, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMarco AF, Kowalski KE. Spinal pathways mediating phrenic activation during high frequency spinal cord stimulation. Respir Physiol Neurobiol 186: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMarco AF, Kowalski KE. Activation of inspiratory muscles via spinal cord stimulation. Respir Physiol Neurobiol 189: 483–449, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duron B, Jung-Caillol MC, Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 152: 171–192 1978. [DOI] [PubMed] [Google Scholar]
- 16.Gandevia SC, Hudson AL, Gorman RB, Butler JE, De Troyer A. Spatial distribution of inspiratory drive to the parasternal intercostal muscles in humans. J Physiol 573: 263–275, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandevia SC, Kirkwood PA. Spinal breathing: stimulation and surprises. J Physiol 589: 2661–2662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer JJ, Martin TP. Distribution of muscle fiber types and EMG activity in cat intercostal muscles. J Appl Physiol 69: 1208–1211, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. [DOI] [PubMed] [Google Scholar]
- 20.Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Handbook of Physiology. The Nervous System. Motor Control Bethesda, MD: Am. Physiol Soc., 1981, vol. II, sect. 1, pts. 1 and 2, p. 423–508. [Google Scholar]
- 21.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965. [DOI] [PubMed] [Google Scholar]
- 22.Hudson AL, Gandevia SC, Butler JE. Common rostrocaudal gradient of output from human intercostal motoneurones during voluntary and automatic breathing. Respir Physiol Neurobiol 175: 20–28, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. In: Brain Mechanisms for the Integration of Posture and Movement. Progress in Brain Research, edited by Mori S, Stuart DG, Weisendanger M. Amsterdam, Netherlands: Elsevier, 2004, vol. 143, p. 75–95. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol 395: 161–192, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalski KE, Hsieh YH, Dick TE, DiMarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 247: 689–693, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legrand A, Brancatisano A, Decramer M, De Troyer A. Rostrocaudal gradient of electrical activation in the parasternal intercostal muscles of the dog. J Physiol 495: 247–254, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legrand A, De Troyer A. Spatial distribution of external and internal intercostal activity in dogs. J Physiol 518: 291–300, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legrand A, Wilson TA, De Troyer AD. Mediolateral gradient of mechanical advantage in the canine parasternal intercostals. J Appl Physiol 80: 2097–2101, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res 61: 625–637, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95: 477–487, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Palisses R, Pers'egol L, Viala D. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord of the decorticate rabbit. Exp Brain Res 78: 624–632, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol 102: 772–780, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Wilson TA, De Troyer A. Respiratory effect of the intercostal muscles in the dog. J Appl Physiol 75: 2636–2645, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Zhan WZ, Mantilla CB, Zhan P, Bitton A, Prakash YS, De Troyer A, Sieck GC. Regional differences in serotonergic input to canine parasternal intercostal motoneurons. J Appl Physiol 88: 1581–1589, 2000. [DOI] [PubMed] [Google Scholar]