Abstract
Aging is associated with motor declines that lead to functional limitations and disability, necessitating the development of therapies to slow or reverse these events. We tested the hypothesis that sodium nitrite supplementation attenuates declines in motor function in older C57BL/6 mice. Motor function was assessed using a battery of tests (grip strength, open-field distance, rota-rod endurance) in old animals (age 20–24 mo) at baseline and after 8 wk of sodium nitrite (old nitrite, n = 22, 50 mg/liter) or no treatment (old control, n = 40), and in young reference animals (3 mo, n = 87). Eight weeks of sodium nitrite supplementation improved grip strength (old nitrite, +12.0 ± 14.9% vs. old control, +1.5 ± 15.2%, P < 0.05) and open field distance (old nitrite, +9.5 ± 7.7%, P < 0.01 vs. old control, −28.1 ± 2.0%) and completely restored rota-rod endurance-run time (old nitrite, +3.2 ± 7.1%, P < 0.01 vs. old control, −21.5 ± 7.2%; old nitrite after treatment P > 0.05 vs. young reference). Inflammatory cytokines were markedly increased in quadriceps of old compared with young reference animals (by ELISA, interleukin-1β [IL-1β] 3.86 ± 2.34 vs. 1.11 ± 0.74, P < 0.05; interferon-gamma [INF-γ] 8.31 ± 1.59 vs. 3.99 ± 2.59, P < 0.01; tumor necrosis factor-alpha [TNF-α] 1.69 ± 0.44 vs. 0.76 ± 0.30 pg/ml, P < 0.01), but were reduced to young reference levels after treatment (old nitrite, IL-1β 0.67 ± 0.95; INF-γ 5.22 ± 2.01, TNF-α 1.21 ± 0.39 pg/ml, P < 0.05 vs. old control, P > 0.05 vs. young reference). Cytokine expression and treatment (old nitrite vs. old control) predicted strength (R2 = 0.822, P < 0.001, IL-1β, INF-γ, group), open field distance (R2 = 0.574, P < 0.01, IL-1β, group) and endurance run time (R2 = 0.477, P < 0.05, INF-γ). Our results suggest that sodium nitrite improves motor function in old mice, in part by reducing low-grade inflammation in muscle.
Keywords: frailty, physical function, nitrite, nitric oxide, inflammation, mouse
advancing age is associated with declines in integrative motor function that increase the risk of functional limitations and disability (20, 23, 53, 54). In the absence of effective intervention, this will contribute to marked, perhaps unsustainable, increases in future healthcare burden (42, 47). Therefore, identifying treatments that preserve motor function with aging is of the highest priority. Such interventions would broadly help to extend the period of life associated with independence, productivity, and well-being while compressing dysfunction and disability into a shorter period closer to the end of life (21, 30, 31).
The systemic factors contributing to age-related declines in motor function are largely unknown, but reduced nitric oxide (NO) bioavailability and signaling may play an important role (24, 41, 61). One mechanism linking reduced NO signaling to a decline in motor function is chronic low-grade inflammation (11, 56, 57). Proinflammatory mediators increase with age (6, 18, 19, 33) and are associated with functional limitations (5, 49) and disability in older adults (15), as well as frailty and mortality in mouse models of aging (32).
Boosting the bioavailability of nitrite, which is both an oxidation product and an immediate precursor of NO, is a promising intervention for improving NO signaling and physiological function with aging (29, 40). In old animals, sodium nitrite supplementation in the drinking water improves vascular NO bioavailability, endothelial function, and arterial stiffness (16, 57), and reduces vascular inflammatory cytokine expression to levels observed in young animals (57). These findings support the view that nitrite supplementation exerts a potent anti-inflammatory effect that is associated with improved physiological function with aging (56). However, the efficacy of oral sodium nitrite supplementation as a potential later-life intervention to slow or reverse declines in motor function or to reduce proinflammatory mediators in skeletal muscle of older animals is unknown.
In the present study, we hypothesized that oral sodium nitrite supplementation would either improve or slow motor dysfunction in older male mice while exerting anti-inflammatory effects in skeletal muscle. To test this hypothesis, we assessed motor function in a large cohort of young reference animals and in old mice before and after 8 wk of either oral sodium nitrite supplementation or normal drinking water. We also measured skeletal muscle inflammatory cytokines following the treatment period and developed regression models to determine the contribution of treatment and muscle inflammatory profile to motor function in old animals. Male mice were used to test our hypothesis in this initial intervention study because we recently established motor assessments with aging in this group (27) and had a large group of phenotyped young male animals to serve as reference controls.
METHODS
Animal Husbandry and Experimental Groups
Male C57BL/6 mice were obtained from the National Institute on Aging rodent colony at 19 mo of age. All mice were housed in an animal care facility at the University of Colorado Boulder on a 12:12 light:dark cycle. Upon arrival mice were ear-punched for identification and housed in groups (approximately 3–4 per cage). Mice were acclimated to our existing colony in the animal care facility for 4 wk with normal rodent chow and water provided ad libitum. Motor behavior was assessed at baseline (20–24 mo of age) and after an 8-wk experimental period in time control (old control, n = 40) and sodium nitrite-treated (old nitrite, n = 22) mice. The control animals continued drinking regular water and the treated animals received sodium nitrite-supplemented water at a dose (50 mg/liter) previously reported to have therapeutic efficacy in old animals (16, 56, 57). Water intake was monitored four times per week. Motor function tests were administered in all animals before and after the 8-wk intervention period. An additional cohort of young adult C57BL/6 mice obtained from Charles River (young reference, n = 87, 3 mo of age) served as reference animals and were administered a behavioral battery at a single time point (27). A subset of animals from each cohort was killed (old control and old nitrite n = 10 each, young reference n = 6) for skeletal muscle and tissue collection. The University of Colorado Boulder Animal Care and Use Committee approved all animal procedures.
Experimental Measurements
Motor function and home cage activity.
Several motor function tests taken from our recently published battery (27) were administered over a 3-day period. Forelimb grip strength (day 1), open field locomotor activity (day 1), and accelerating (day 2) and endurance (day 3) rota-rod run ability were assessed as described in detail previously (27). Briefly, grip strength was measured as the average force recorded at forepaw release over five trials using a custom grip strength device that included a force transducer attached to a trapeze grip. Open field distance (locomotor activity) was determined as the total distance traveled during 5 min in a novel arena, quantified offline from a video-recorder using multiarena video tracking software (EthoVision XT; Noldus Information Technology, Leesburg, VA). Latency to fall from an accelerating rota-rod (Ugo Basile, Comerio, Italy) over three trials was used to determine the speeds set during the endurance rota-rod trial. The endurance rota-rod test consisted of four consecutive phases corresponding to 25% (refresh, 2 min), 50% (warm-up, 5 min), 75% (endurance phase 1, 10 min or until fall), and 100% (endurance phase 2, 20 min or until fall) of maximum achieved accelerating rota-rod speed. The latency to fall was recorded during the endurance phases, and distance run across all phases was calculated.
Home cage activity in old animals was determined by telemetry in a subset of old nitrite and old control animals (n = 12 per group). Transmitters were surgically implanted in the intraperitoneal cavity via ventral incision to detect core body temperature and gross home cage activity (TA-F10 transmitters; Data Sciences International, St. Paul, MN). Home cage activity and body temperature were determined over 24 h following 8-wk treatment periods. Animals with implanted transmitters also underwent motor function assessments. For technical and logistical reasons, transmitters were not implanted in the young reference animals.
Muscle mass and size.
Following the 8-wk treatment period, the quadriceps and gastrocnemius muscles were dissected and weighed, and mass was recorded. Quadriceps muscles were cut in half across the muscle belly. One half was placed in Eppendorf tubes and snap-frozen in liquid nitrogen and stored at −80°C until extraction of protein, and the other half was fixed in formaldehyde, paraffin-embedded, and sectioned midbelly. Sections were stained with hematoxylin and eosin (Fisher, NH) and imaged using a photomicroscope (TS100 Nikon Eclipse) to determine quadriceps cross-sectional area.
Skeletal muscle inflammatory cytokines.
Quadriceps muscles were homogenized in ice-cold radioimmunoprecipitation assay lysis buffer containing protease and phosphatase inhibitors (Protease Inhibitor Cocktail Tablet [Roche, Indianapolis, IN] and 0.01% phosphatase inhibitor cocktail [Sigma, St Louis, MO]). Equal amounts of protein (15 μg) were used to determine concentrations of the proinflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and interferon gamma (IFN-γ) by multiplex ELISA (Searchlight Mouse Inflammatory Cytokine Kit; Aushon Biosystems; Billerica, MA) following the manufacturer's instructions. Tumor necrosis factor alpha (TNF-α) levels were determined separately by ELISA (Mouse TNF-α ELISA Max Deluxe Sets; BioLegend, San Diego, CA) per the manufacturer's instructions but with a modified standard curve to include five additional points between 0.2 and 7.8 pg/ml per the manufacturer's recommendation to achieve greater resolution. With the addition of lower-concentration standards we were able to generate a standard curve with R2 > 0.99.
Plasma nitrite and nitrate.
Plasma was obtained at death by centrifugation at 800 g for 10 min in the subsample of young reference, old control, and old nitrite mice. Plasma nitrite and nitrate were analyzed using a dedicated high-throughput HPLC system (EiCom ENO-20) as described in detail elsewhere (7–9).
Statistics
Data are presented as means ± SE in figures, or means ± SD in tables and text. Statistical analysis was performed with SPSS software (v22; IBM, Somers, NY); the α-level for all statistical analyses was set at 0.05. Prior to primary analysis of the motor function outcomes, normality of each variable was assessed with the Kolmogorov-Smirnov test. No outliers (>3 SD) were identified for motor function tasks; one outlier was identified in the old control group for TNF-α (11.0 pg/ml) and was replaced with the group mean (2.93 pg/ml). Multivariate analysis of variance confirmed age-related motor function declines in young reference compared with old control and old nitrite animals at baseline; differences for each functional outcome were confirmed with univariate analyses and post hoc Tukey's honestly significant difference (HSD) for means comparison. Group differences in motor function with 8-wk sodium nitrite supplementation or normal drinking water were determined by multivariate mixed-model ANOVA (within factor, time; between factor, treatment) followed by univariate comparisons and post hoc Tukey's HSD. Differences among young reference, old control, and old nitrite (posttreatment only) for body mass, muscle mass, and proinflammatory cytokines were compared by ANOVA followed by Tukey's HSD post hoc tests. Differences in home cage activity and temperature between old control and old nitrite groups were determined by t-test, and relations between home cage activity and motor functions were determined by Pearson correlation. The contribution of proinflammatory cytokines and treatment group to the change in motor function outcomes was determined by backward, linear regression equations, with separate models run to predict grip strength, open field distance, and endurance run time. An absence of multicollinearity for the explanatory variables was verified by variance inflation factor and tolerance.
RESULTS
Animal Characteristics
Water intake was not different between old control (4.48 ± 1.16 ml/mouse/day) and old nitrite animals (4.36 ± 1.29 ml/mouse/day). Nitrite supplemented animals consumed an average of 0.22 ± 0.07 mg sodium nitrite per day (6.65 ± 2.1 mg/kg body mass). Age-related differences were observed in body mass (P < 0.05), but muscle mass (mean of right and left quadriceps and gastrocnemius) and quadriceps cross-sectional area were not statistically different between young reference and old animals (P > 0.05, Table 1). Body mass, muscle mass and cross-sectional area were not different between old control and old nitrite-treated animals (Table 1, P > 0.05). Sodium nitrite treatment had no effect on home cage activity (old control 20.9 ± 3.1, old nitrite 21.5 ± 3.8 average counts/day) or core body temperature (old control 36.2 ± 1.50°C, old nitrite 36.1 ± 0.95°C) in old animals (P > 0.05, both).
Table 1.
Skeletal muscle size and mass
| Young Reference | Old Control | Old Nitrite | |
|---|---|---|---|
| Body mass, g | 30.1 ± 2.1* | 33.6 ± 3.2 | 33.1 ± 2.4 |
| Muscle mass, mg | |||
| Quadriceps | 170 ± 30 | 145 ± 21 | 169 ± 32 |
| Gastrocnemius | 146 ± 13 | 135 ± 31 | 134 ± 22 |
| Cross-sectional area, mm2 | |||
| Quadriceps | 22.1 ± 1.31 | 17.2 ± 4.66 | 18.6 ± 4.30 |
P < 0.05 between young reference (n = 6) and old animals (n = 10 per group).
Sodium Nitrite Improves Motor Function in Old Mice
Multivariate analysis revealed age-related declines in motor function in old compared with young reference animals (P < 0.001), and significant time × treatment interaction indicated an effect of sodium nitrite on motor function (grip strength measures, open-field distance, and rota-rod performance) in old C57BL/6 mice (P < 0.001). No differences were observed between old control and old nitrite mice at baseline (P > 0.05), or between mice with or without surgically implanted transmitters (Table 2, P > 0.05 all). Home cage activity was not significantly correlated with motor functions (P > 0.05 all, Table 3), indicating that habitual activity was not related to motor performance.
Table 2.
Motor function in old male C57BL/6 with and without surgically implanted transmitters
| Transmitters | No Transmitters | |
|---|---|---|
| Grip strength, g | 98.5 ± 16.4 | 94.8 ± 14.4 |
| Open field distance, cm | 1,610 ± 510 | 1,478 ± 520 |
| Accelerating rota-rod, s | 167 ± 59 | 168 ± 63 |
| Endurance run time, min | 10.6 ± 6.9 | 11.2 ± 7.5 |
| Endurance run distance, m | 14.3 ± 7.3 | 15.2 ± 8.2 |
All nonsignificant (P > 0.05); n = 24 transmitters, 38 no transmitters.
Table 3.
Pearson correlations between home cage activity and motor functions
| Grip Strength | Open Field Distance | Endurance Run Time | Endurance Run Distance | |
|---|---|---|---|---|
| Home cage activity | 0.33 | −0.17 | −0.27 | −0.23 |
All correlations between home cage activity and motor functions were not significant (P > 0.05); n = 24.
Grip strength.
Grip strength (P < 0.001, Fig. 1A) and grip strength normalized to body mass were reduced in old animals at baseline compared with young reference mice (body mass-normalized grip strength; old 2.75 ± 0.45 g, young 5.58 ± 0.55 g; P < 0.001). Grip strength did not change over 8 wk in old control (2.75 ± 0.43 to 2.83 ± 0.44 g, P > 0.05), but increased in old nitrite (2.81 ± 0.45 to 3.25 ± 0.45 g, P < 0.05) mice.
Fig. 1.
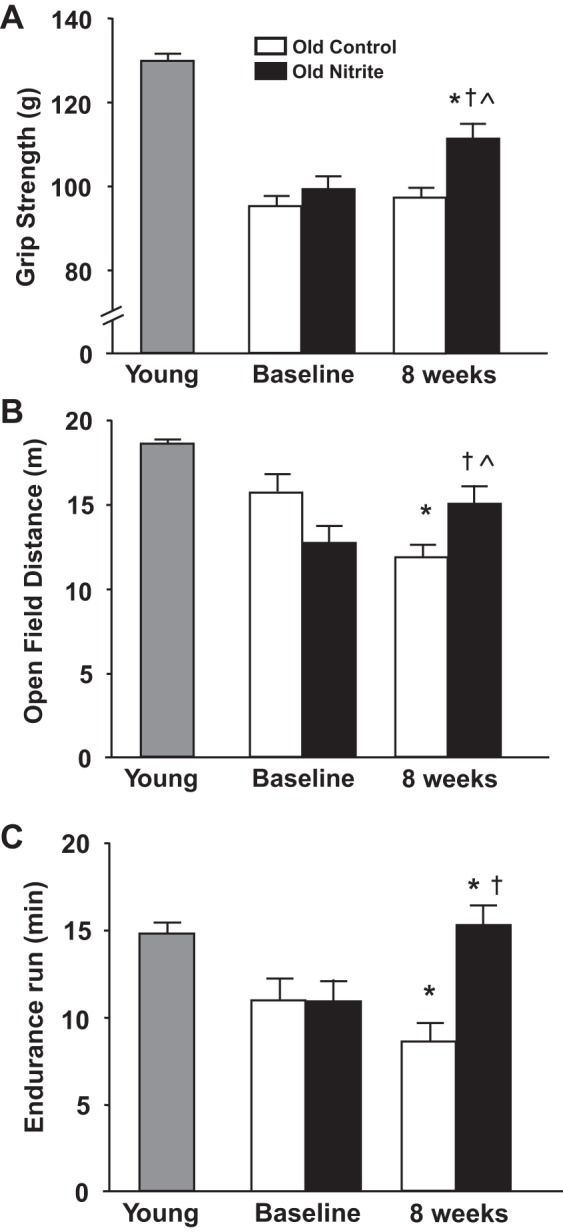
Primary motor function outcomes in young reference animals (grid-lined bar, n = 87), old control (open bars, n = 40), and old nitrite (black bars, n = 22) animals before and after 8 wk of drinking regular water or sodium nitrite-supplemented water (50 mg/liter). Motor functions are displayed for three primary motor outcomes: A, grip strength; B, distance traveled over 5 min in a novel open arena; and C, time run during an endurance rota-rod run fixed to 75% and 100% of maximal run speeds. *P < 0.05 from baseline; †P < 0.05 old control to old nitrite mice at 8 wk; ^P < 0.05 old nitrite mice at 8 wk compared with young reference mice.
Open-field distance.
Distance traveled in a novel open arena was less in old compared with young reference animals (P < 0.001, Fig. 1B) and declined further over 8 wk in old control animals (P < 0.05). Nitrite treatment attenuated the reduction in distance over the 8-wk study period (old nitrite, P > 0.05, baseline vs. posttreatment).
Rota-rod testing.
Latency to fall from a constantly accelerating rota-rod was reduced in old compared with young reference animals at baseline (old 168 ± 61 s, young 240 ± 57 s, P < 0.05). No differences were observed with treatment or time in old control (160.9 ± 61.3 to 167.2 ± 55.4 s) and old nitrite (175.8 ± 63.7 to 184.6 ± 46.5 s) animals during the 8-wk experimental period (P > 0.05, both). Baseline impairments were observed in endurance run time (P < 0.001, Fig. 1C) and distance in old mice compared with young reference (old 14.9 ± 7.9, young 24.4 ± 9.9 m, P < 0.001), and further declines were observed over 8 wk in old control animals (14.6 ± 8.5 to 11.3 ± 5.9 m, P < 0.05). Sodium nitrite supplementation improved endurance run distance (14.5 ± 5.7 to 17.8 ± 5.9 m, P < 0.01) and restored endurance run time to that observed in young reference animals (P < 0.001 vs. old control, P > 0.05 vs. young reference, Fig. 1C).
Sodium Nitrite Ameliorates Inflammation in Skeletal Muscle of Old Mice
Protein expression of the proinflammatory cytokines IL-1β, IL-6, INF-γ, and TNF-α was increased in quadriceps muscle of old compared with young reference mice. Sodium nitrite reduced expression levels of IL-1β, INF-γ, and TNF-α in skeletal muscle in old mice, normalizing expression levels to those observed in young reference (P < 0.05, Fig. 2). IL-6 expression was not significantly different among the groups (P > 0.05, Fig. 2).
Fig. 2.
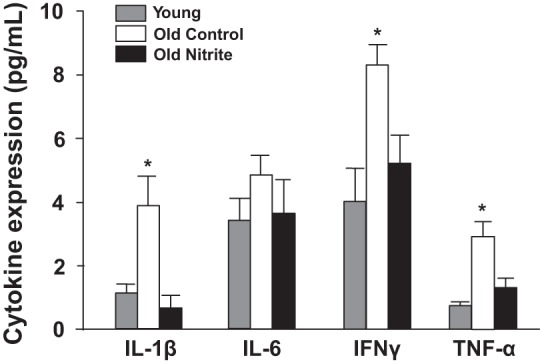
Quadriceps inflammatory cytokine expression (ELISA) in young reference (n = 6), old control (n = 10), and old nitrite (n = 10; 8 wk of 50 mg/liter or regular drinking water) mice. IL-1β, interleukin-1 beta; IL-6, interleukin-6; INF-γ, interferon gamma; TNF-α, tumor necrosis factor alpha.*P < 0.05 compared with young reference animals.
Inflammatory Cytokines Predict Improvements in Motor Function in Old Animals
The relative contribution of skeletal muscle inflammatory cytokines and treatment to gains in motor function was assessed with backward linear regression models. Improvements in motor function were significantly explained by selective inflammatory cytokines (Table 4). Improvements in grip strength were significantly explained by INF-γ and TNF-α (overall model R2 = 0.843, P < 0.05). Distance traveled in an open field arena was explained by IL-1β (partial r, P < 0.05; overall model R2 = 0.452, P < 0.05). Increases in endurance run time were explained by TNF-α and INF-γ (overall model R2 = 0.429, P < 0.05). In each model, greater improvements in function were negatively related to markers of inflammation (Table 4), with the exception of IL-6. Although the latter was included as an overall predictor in each model, it was not statistically significant as an individual explanatory variable (P > 0.05). Of the cytokines assessed, TNF-α and INF-γ were most consistent in predicting gains in function as revealed by significant partial correlations within each model. Moreover, nitrite supplementation was an important explanatory variable in describing functional gains in each model (P < 0.05 each model, Table 4). Power achieved in each model was greater than 90%, and a maximum variance inflation factor of 0.884 and minimum tolerance of 1.275 was observed, thus confirming minimal multicollinearity among explanatory variables.
Table 4.
Regression models demonstrating relations between skeletal muscle cytokine expression and motor functions in old C57BL/6 mice
| Beta | Standard Error | Partial Correlations | |
|---|---|---|---|
| Grip strength (R2 = 0.843, P < 0.001) | |||
| IL-6 | 2.98 | 1.56 | 0.420 |
| INF-γ | −3.22* | 1.14 | −0.566 |
| TNF-α | −11.4* | 2.48 | −0.744 |
| Treatment group | −14.7* | 2.88 | 0.778 |
| Open field distance (R2 = 0.452, P < 0.05) | |||
| IL-6 | 47.7 | 66.0 | 0.173 |
| IL-1β | −88.9* | 74.0 | −0.280 |
| TNF-α | −96.7 | 135 | −0.171 |
| Treatment group | −255.3* | 125 | −0.443 |
| Endurance run time (R2 = 0.429, P < 0.05) | |||
| TNF-α | −178* | 88.9 | −0.447 |
| IL-6 | 114.8 | 56.7 | 0.452 |
| INF-γ | −95.7* | 41.0 | −0.504 |
| Treatment group | 183* | 105 | 0.400 |
Beta and partial r = P < 0.05. Constants include grip strength =164.6, open field distance = 2282, and endurance run time = 913.2.
Plasma Nitrite and Nitrate
Plasma nitrite concentrations were greater in the old nitrite than in the old control and young reference animals (P < 0.05, Fig. 3). In contrast, plasma nitrate was not different between old nitrite (38.3 ± 12.2 μM), old control (38.8 ± 12.6 μM), and young reference mice (35.7 ± 11.4 μM).
Fig. 3.
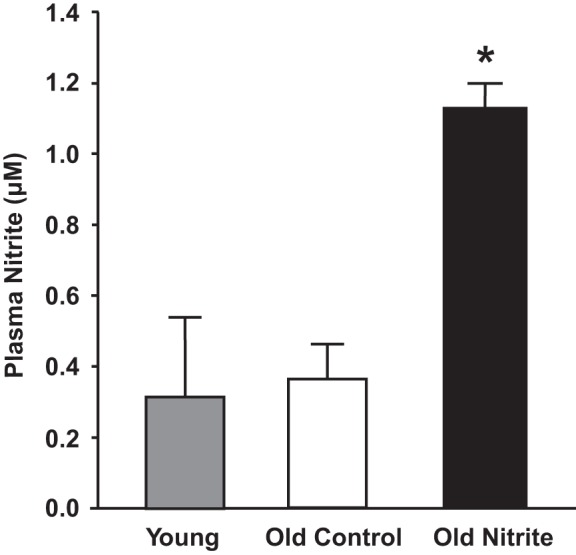
Plasma nitrite (μM) in young reference, and old control and old nitrite (8 wk of 50 mg/liter or regular drinking water) mice. *P < 0.05 compared with young reference and old control animals.
DISCUSSION
The key finding of this study was that 8 wk of sodium nitrite supplementation in old male C57BL/6 mice improved several domains of motor function and restored endurance run times compared with those of young animals. Aging is associated with declines in motor function, which leads to an increased risk of functional limitation, loss of independence, and disability (1, 20, 23, 60). Therefore, these preclinical findings suggest that moderate supplementation with sodium nitrite has translational potential to prevent or improve age-related motor declines in humans. Importantly, the present results also provide novel insight into a key mechanism by which sodium nitrite may improve motor function with age (i.e., by ameliorating chronic, low-grade inflammation in skeletal muscle). These effects of nitrite supplementation were observed in the absence of significant changes of habitual activity, body mass, or skeletal muscle mass or size.
Age-Related Motor Dysfunction: Effects of Nitrite Supplementation
The present results are consistent with previous reports from our laboratory (27) and others (10, 22, 32) demonstrating that declines in motor function develop with advancing age in male C57BL/6 mice, as indicated by impaired performance on behavioral tasks. Most importantly, sodium nitrite supplementation initiated later in life improved grip strength, attenuated the decline in open-field activity, and restored endurance capacity to levels observed in young mice. Improvements in multiple subdomains of function with oral sodium nitrite supplementation in the present study suggest that NO signaling, or possibly the independent biological signaling effects of nitrite, may be an important modulating influence on age-related motor functions. Of interest, motor function gains in old mice in our study were independent of statistically significant changes in skeletal muscle mass or skeletal muscle cross-sectional area. Importantly, improvements in motor function were also independent of habitual cage activity, thereby indicating that the gains in motor function were not due to increased daily activity with nitrite supplementation.
Recently it was shown that 7-day supplementation with dietary nitrate, a precursor to nitrite and NO (29, 40), in young C57BL/6 male mice was sufficient to induce a leftward shift in the force-frequency curve in the extensor digitorum longus, such that greater contractile forces could be exerted at submaximal frequencies of stimulation (25). Furthermore, 6 wk of treatment in 18-mo-old female C57BL/6 mice with an NO donor, isosorbide dinitrate, improved vascular density and sarcolemma integrity in skeletal muscle, particularly when coupled with voluntary exercise (38). These previous findings suggest that supplementation with NO precursors may have beneficial effects on skeletal muscle physiology in mice. In the present study, we are the first to show in a behavioral model of motor function that sodium nitrite improves multiple subdomains of motor function and restores endurance run time compared with that observed in young animals. More broadly, our findings show that sodium nitrite improves motor function in a setting of reduced baseline function, and the functional gains observed may reflect clinically relevant subdomains of human motor function (27). This suggests that nitrite supplementation may be effective for prevention or treatment of motor dysfunction with primary aging in humans.
Previous work from our laboratory demonstrated that plasma nitrite concentrations are reduced with aging in this model (male C57BL/6 mice), and that 3 wk of sodium nitrite supplementation in the drinking water at a concentration identical to that used in the present study markedly increased plasma nitrite in both young and old mice (57). Although we did not observe an age-related decline in plasma nitrites or nitrates, the observed increase in circulating nitrite with sodium nitrite supplementation was associated with improvements in motor function in older animals. This concept is consistent with the fact that inorganic nitrate and nitrite consumed in the diet are reduced to NO (29, 43), which exerts biological effects in numerous systems, including vascular (16, 57), neural (14, 48, 50, 51), and motor/skeletal muscle (13, 35, 59). It is possible, for example, that nitrite supplementation boosts NO bioavailability, which could directly enhance blood flow and vascularization (38, 57), thereby improving systemic function. Additionally, nitrite may have direct biological effects that are independent of reduction to NO (29), and nitrite supplementation could alter gene and protein expression or activity, with physiological benefits (8, 56, 58).
Findings from several investigations indicate that dietary nitrate may also have significant physiological effects in humans, suggesting a translational potential of sodium nitrite on motor function. In young adults, dietary nitrate consumption increased plasma nitrite levels, and this was associated with decreased muscle oxygen costs during submaximal and maximal exercise (36, 37) and increased exercise tolerance and time to exhaustion (3, 4, 34), possibly resulting from improved mitochondrial efficiency (35). However, limited studies exist replicating these results in older adults. Kelly et al. (28) found that 2.5 days of high nitrate beetroot juice consumption reduced blood pressure and accelerated V̇o2 kinetics, but these changes did not correspond to enhanced walking and cognitive performance in men and women aged 60–70 yr. The present work suggests that perhaps longer moderate treatment with biologically active sodium nitrite affects motor functions in rodents. Thus sodium nitrite may improve motor functions and performance when administered for a longer duration in older men and women. However, given potential differences in metabolism between rodents and humans, this remains to be tested experimentally.
Inflammation
The present findings are consistent with previous work from our laboratory (57) and others (6, 32) showing that inflammation increases with age as indicated by increases in proinflammatory cytokine concentrations in old compared with young control mice. In this study, we showed that sodium nitrite treatment initiated later in life completely reversed the increase in proinflammatory cytokines in skeletal muscle of old mice to levels observed in young reference animals. This is consistent with the results of a previous investigation by our laboratory in which 3 wk of sodium nitrite supplementation normalized inflammatory cytokine profiles in the aorta to those of young animals (57).
Importantly, the results of the present study are the first to demonstrate that reductions in inflammatory cytokines are significantly associated with improvements in motor performance. Indeed, we found that expression of IL-1β, INF-γ, and TNF-α in skeletal muscle had a strong inverse relation to motor function. Moreover, along with treatment effects, the concentrations of these cytokines explained much of the improvements in motor function with sodium nitrite supplementation. The fact that IL-6 expression did not follow this pattern may be due to the complex signaling of this cytokine in skeletal muscle (45). Taken together, our results provide the first evidence of a potent anti-inflammatory effect of sodium nitrite treatment in vivo in aging skeletal muscle, and that this effect is strongly associated with improvements in motor function.
Limitations and Future Directions
Although the current study suggests that motor function may be improved by sodium nitrite supplementation in old C57BL/6 male mice, future work is needed to determine whether these findings extend to females (12, 44) or different rodent strains (2, 17, 46). Female animals and additional strains were excluded from the present investigation for improved internal consistency with our newly established battery of motor function tests developed with male C57BL/6 inbred mice that included young reference values. Additionally, future investigation should examine the potential influence of nitrite supplementation on other possible mechanisms that may have contributed to improvements in motor performance and inflammatory profiles, including enhanced blood flow and mitochondrial and muscle energetics. For example, sodium nitrite supplementation may increase skeletal muscle mitochondrial efficiency, as does dietary nitrate in humans (35), and this could, in turn, mitigate mitochondrial-related inflammatory responses (39, 55) and improve endurance capacity. Moreover, other dietary or pharmacological approaches for enhancing systemic nitrite and NO bioavailability may have similar beneficial effects as those presented here for sodium nitrite supplementation in the drinking water (25, 38). Finally, clinical trials should be conducted using appropriate doses of sodium nitrite to ensure the safety and efficacy of long-term supplementation on motor functions in older adults. As nitrite is scavenged by oxyhemoglobin resulting in the formation of methemoglobin, future clinical trials should include careful monitoring of methemoglobin. However, completed trials in humans suggest that nitrite administration is not associated with clinically significant elevations in this marker (26, 52).
Conclusion
The results of this study show for the first time that late-life oral supplementation with sodium nitrite improves motor function in old C57BL/6 male mice and restores endurance run time to that of young animals. Our findings also provide evidence that improvements in motor function may be mediated in part by the anti-inflammatory influences of sodium nitrite on aged skeletal muscle with chronic low-grade inflammation. Moreover, improvements in functional capability and inflammatory profiles were independent of changes in skeletal muscle mass, skeletal muscle cross-sectional area, and habitual activity. Overall, these findings establish an experimental basis for translational research aimed at determining the efficacy of sodium nitrite therapy for improving motor function and reducing the risk of disability and loss of independence associated with aging in humans. Sodium nitrite treatment may also have efficacy for improving motor function in age-related disease states or other conditions associated with baseline deficits in motor function.
GRANTS
Support for this study was provided by National Institute on Aging Grants R37 AG-013038, 5T32 AG-000279-12, and F31 AG-047784.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.N.J., R.A.G.-R., A.L.S., R.M.E., and D.R.S. conception and design of research; J.N.J., L.C.J., M.L.B., N.E.d.P., H.J.B., H.J., and N.S.B. performed experiments; J.N.J., R.A.G.-R., L.C.J., M.L.B., N.E.d.P., H.J.B., H.J., and N.S.B. analyzed data; J.N.J., R.A.G.-R., A.L.S., N.S.B., R.M.E., and D.R.S. interpreted results of experiments; J.N.J. and R.A.G.-R. prepared figures; J.N.J. drafted manuscript; J.N.J., R.A.G.-R., L.C.J., M.L.B., N.E.d.P., H.J.B., A.L.S., N.S.B., R.M.E., and D.R.S. edited and revised manuscript; J.N.J., R.A.G.-R., L.C.J., M.L.B., N.E.d.P., H.J.B., H.J., A.L.S., N.S.B., R.M.E., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mike Pont Carpentry, LLC (Boulder, CO), for design, development, and construction of customized experimental apparatus. Additional thanks to Trent Evans for assistance in skeletal muscle histology, Steward Webber for transmitter surgical assistance, and Jonathan Herrera for assistance with ELISA.
REFERENCES
- 1.Atkinson HH, Cesari M, Kritchevsky SB, Penninx BW, Fried LP, Guralnik JM, Williamson JD. Predictors of combined cognitive and physical decline. J Am Geriatr Soc 53: 1197–1202, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Austad SN. Issues in the choice of genetic configuration for animal aging models. Exp Gerontol 32: 55–63, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 109: 135–148, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107: 1144–1155, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Blain H, Jaussent A, Beziat S, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Picot MC. Low serum IL-6 is associated with high 6-minute walking performance in asymptomatic women aged 20 to 70 years. Exp Gerontol 47: 143–148, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol 8: 131–136, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med 45: 468–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1: 290–297, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43: 645–657, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci 57: B193–B197, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Castiglione N, Rinaldo S, Giardina G, Stelitano V, Cutruzzola F. Nitrite and nitrite reductases: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 17: 684–716, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 509: 282–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerc P, Rigoulet M, Leverve X, Fontaine E. Nitric oxide increases oxidative phosphorylation efficiency. J Bioenerg Biomembr 39: 158–166, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol 32: 297–311, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639–646, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol 47: 588–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging 20: 167–176, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65: S173–S176, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92–105, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc 45: 92–100, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med 303: 130–135, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci 68: 1326–1336, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332: 556–561, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heffernan KS, Chale A, Hau C, Cloutier GJ, Phillips EM, Warner P, Nickerson H, Reid KF, Kuvin JT, Fielding RA. Systemic vascular function is associated with muscular power in older adults. J Aging Res 2012: 386–387, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol 590: 3575–3583, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunault CC, van Velzen AG, Sips AJ, Schothorst RC, Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol Lett 190: 48–53, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, Seals DR. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr) 36: 583–592, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol 304: R73–R83, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Kevil CG, Kolluru GK, Pattillo CB, Giordano T. Inorganic nitrite therapy: historical perspective and future directions. Free Radic Biol Med 51: 576–593, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol 48: 1–5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci 64: 209–212, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko F, Yu Q, Xue QL, Yao W, Brayton C, Yang H, Fedarko N, Walston J. Inflammation and mortality in a frail mouse model. Age (Dordr) 34: 705–715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 39: 687–699, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110: 591–600, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13: 149–159, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med 48: 342–347, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Leiter JR, Upadhaya R, Anderson JE. Nitric oxide and voluntary exercise together promote quadriceps hypertrophy and increase vascular density in female 18-mo-old mice. Am J Physiol Cell Physiol 302: C1306–C1315, 2012. [DOI] [PubMed] [Google Scholar]
- 39.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-Garcia C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13: 106–118, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol 5: 865–869, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda S, Otsuki T, Iemitsu M, Kamioka M, Sugawara J, Kuno S, Ajisaka R, Tanaka H. Effects of leg resistance training on arterial function in older men. Br J Sports Med 40: 867–869, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manton KG, Gu XL, Ullian A, Tolley HD, Headen AE Jr, Lowrimore G. Long-term economic growth stimulus of human capital preservation in the elderly. Proc Natl Acad Sci USA 106: 21080–21085, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxid Redox Signal 17: 422–432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol 40: 966–975, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 280: 4131–4148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nadon NL. Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging Cell 5: 9–15, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q 87: 842–862, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul V, Ekambaram P. Involvement of nitric oxide in learning & memory processes. Indian J Med Res 133: 471–478, 2011. [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira LS, Narciso FM, Oliveira DM, Coelho FM, Souza Dda G, Dias RC. Correlation between manual muscle strength and interleukin-6 (IL-6) plasma levels in elderly community-dwelling women. Arch Gerontol Geriatr 48: 313–316, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Piknova B, Kocharyan A, Schechter AN, Silva AC. The role of nitrite in neurovascular coupling. Brain Res 1407: 62–68, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitsikas N, Rigamonti AE, Cella SG, Sakellaridis N, Muller EE. The nitric oxide donor molsidomine antagonizes age-related memory deficits in the rat. Neurobiol Aging 26: 259–264, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Pluta RM, Oldfield EH, Bakhtian KD, Fathi AR, Smith RK, Devroom HL, Nahavandi M, Woo S, Figg WD, Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS One 6: e14504, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA 281: 558–560, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr) 34: 563–570, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci 69: 2999–3013, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sindler AL, Devan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol 116: 463–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh M, Arya A, Kumar R, Bhargava K, Sethy NK. Dietary nitrite attenuates oxidative stress and activates antioxidant genes in rat heart during hypobaric hypoxia. Nitric Oxide 26: 61–73, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA 305: 50–58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welsch MA, Dobrosielski DA, Arce-Esquivel AA, Wood RH, Ravussin E, Rowley C, Jazwinski SM. The association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc 40: 1237–1243, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


