Abstract
Cytochrome P-450 epoxygenase-derived epoxyeicosatrienoic acids (EETs) exert diverse biological activities, which include potent vasodilatory, anti-inflammatory, antiapoptotic, and antioxidatant effects, and cardiovascular protection. Liver has abundant epoxygenase expression and high levels of EET production; however, the roles of epoxygenases in liver diseases remain to be elucidated. In this study, we investigated the protection against high-fat diet-induced nonalcoholic fatty liver disease (NAFLD) in mice with endothelial-specific CYP2J2 overexpression (Tie2-CYP2J2-Tr). After 24 wk of high-fat diet, Tie2-CYP2J2-Tr mice displayed attenuated NAFLD compared with controls. Tie2-CYP2J2-Tr mice showed significantly decreased plasma triglyceride levels and liver lipid accumulation, improved liver function, reduced inflammatory responses, and less increase in hepatic oxidative stress than wild-type control mice. These effects were associated with inhibition of NF-κB/JNK signaling pathway activation and enhancement of the antioxidant defense system in Tie2-CYP2J2-Tr mice in vivo. We also demonstrated that 14,15-EET treatment protected HepG2 cells against palmitic acid-induced inflammation and oxidative stress. 14,15-EET attenuated palmitic acid-induced changes in NF-κB/JNK signaling pathways, malondialdehyde generation, glutathione levels, reactive oxygen species production, and NADPH oxidase and antioxidant enzyme expression in HepG2 cells in vitro. Together, these results highlight a new role for CYP epoxygenase-derived EETs in lipotoxicity-related inflammation and oxidative stress and reveal a new molecular mechanism underlying EETs-mediated anti-inflammatory and antioxidant effects that could aid in the design of new therapies for the prevention and treatment of NAFLD.
Keywords: CYP2J2, EETs, nonalcoholic fatty liver disease, palmitic acid, inflammation, oxidative stress
nonalcoholic fatty liver disease (NAFLD) is a prevalent chronic liver disease worldwide. There are no established treatments for NAFLD beyond management of comorbidity and weight loss. Unfortunately, weight loss has a poor long-term success rate, which emphasizes the need to validate alternative approaches to slow NAFLD progression (25). Despite the disease severity and prevalence, there are still no approved pharmaceutical treatments for NAFLD. The mechanisms involved in the pathogenesis of NAFLD are not yet fully understood. The most widely accepted theory is the “two-hit hypothesis”. The first hit, an accumulation of lipids in cells called steatosis, is believed to prime the liver to more severe liver pathologies. Hepatic steatosis renders the liver more susceptible to second hits comprised of inflammatory insults or oxidative stress (10).
Excess storage of fat in liver is the hallmark of NAFLD, which refers to a wide histological spectrum of liver diseases ranging from hepatic steatosis to steatohepatitis, hepatic fibrosis, and cirrhosis (44). Steatosis-induced inflammation, called steatohepatitis, results from recruitment and activation of inflammatory cells in the liver. NAFLD progresses when reactive oxygen species (ROS) and several immunomodulatory factors contribute to necroinflammation and hepatocellular injury (9). Activation of NF-κB increases expression of proinflammatory cytokines and chemokines that are key factors in high-fat diet-induced liver injury mice (21). The JNK/stress-activated protein kinase pathway is activated by numerous cellular stresses. Although JNK has been implicated in mediating inflammatory signaling, its role in NAFLD progression has not been fully elucidated (19).
Oxidative stress is an imbalance between prooxidant and antioxidant mechanisms that is caused by either increased formation of free radicals or diminution of antioxidant activity. Oxidative stress is frequently cited as a central mechanism of hepatocellular injury in NAFLD, which correlates with the accumulation of lipid peroxidation products, appearance of mitochondrial dysfunction, and elevation of proinflammatory cytokines (36). The prooxidant state that NAFLD patients exhibit occurs concomitantly with a significant decrease in the antioxidant capacity of the liver (40). The glutathione (GSH) antioxidant system is critical for counteracting oxidative stress-induced intracellular injury. A critical role for mitochondrial GSH in NAFLD development is suggested by data showing that GSH depletion sensitizes hepatocytes to inflammatory cytokines (16). The involvement of oxidative stress suggests that antioxidants could potentially mitigate the “second-hit” by the enhanced quenching or degradation of ROS in hepatic tissue.
The cytochrome P-450 (P450)-derived epoxyeicosatrienoic acids (EETs) represent a class of lipid mediators with cytoprotective properties and potent anti-inflammatory effects. EETs exert potent anti-inflammatory properties in the vasculature by inhibiting NF-κB activation in endothelial cells (28). 14,15-EET administration attenuates IκBα degradation after TNFα treatment in human airway smooth muscle cells (26). CYP2J2 gene transfection or exogenous administration of 8,9-EET abolished Hcy-induced NF-κB and IκBα activation in mouse aortic endothelial cells (27). Increased endothelial EET biosynthesis or globally decreased soluble epoxide hydrolase (sEH)-mediated EET hydrolysis attenuates endotoxin-induced activation of NF-κB signaling, chemokine and cytokine expression, and neutrophil infiltration in lung (12). Liu et al. had recently demonstrated that sEH inhibition could alleviate high-fat diet-induced hepatic steatosis, which might involve its anti-inflammatory effect in adipose tissue and direct inhibition in liver(23). Thus, the CYP epoxygenase-EET-sEH system may serve as a viable anti-inflammatory therapeutic strategy. Our previous study demonstrated that 11,12-EET inhibited arsenic trioxide (ATO)-induced apoptosis through a mechanism that involved induction of antioxidant proteins and attenuation of ROS-mediated mitochondrial dysfunction (22).
EETs have well-established effects in cardiovascular, renal, and inflammatory diseases in murine models, but their role NAFLD has not been fully investigated. The anti-inflammatory and antioxidatant properties of EETs make CYP epoxygenases a potential therapeutic target for treatment of many complications associated with NAFLD. Thus, we tested the hypothesis that endothelial-specific overexpression of CYP2J2 and the resultant increase in EET production would decrease hepatic inflammation, oxidative stress, and injury responses implicated in the progression of a mouse model of NAFLD induced by high-fat diet.
MATERIALS AND METHODS
Materials.
4-HNE (4-hydroxy-2-nonenal) antibody was purchased from Abcam (Cambridge, MA). All other antibodies used in this study were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The 14,15-DHET ELISA Kit was from Detroit R&D (Detroit, MI). All other chemicals and reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Animals.
All experimental protocols complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Tongji Medical Collage, Huazhong University of Science and Technology. Mice with transgenic, endothelial-specific CYP2J2 overexpression (Tie2-CYP2J2-Tr) on a pure C57BL/6 genetic background were obtained from Dr. Darryl Zeldin's colony at the National Institute of Environmental Health Science (RTP, NC) and were genotyped using PCR-based methods. All animals were housed (4 per cage) in a temperature- (25 ± 3°C) and humidity- (50 ± 20%) controlled room with a 12:12-h light-dark cycle. Until 8 wk of age, all mice were fed standard laboratory chow and water ad libitum. At 8 wk of age, male Tie2-CYP2J2-Tr and wild-type (WT) littermate control mice were fed a high-fat diet (HFD; 60% kcal% fat; Beijing HFK Bio-Technology, China) or normal chow ad libitum plus water for 24 wk. All animals were euthanized after 24 wk with pentobarbital sodium (50 mg/kg body wt). Livers were collected, frozen with liquid nitrogen, and stored at −80°C.
Cell culture and treatment.
HepG2 and RAW 264.7 cells obtained from the ATCC were cultured in DMEM supplemented with 10% fetal bovine serum and penicillin (100 U/ml)-streptomycin (100 μg/ml) and kept at 37°C in a humidified atmosphere of 95% O2, 5% CO2. Subconfluent cells were preincubated with or without 14,15-EET (1 μM); 14,15-EEZE [14,15-epoxyeicosa-5(Z)-enoic acid, a 14,15-EET antagonist, 10 μM] for 30 min, followed by treatment with 0.5 mM palmitic acid (PA) containing 1.0% acid-free BSA for 24 h.
Serum and urine analysis.
After overnight fasting, mice were anesthetized, and blood samples were collected from the cavernous sinus with a capillary tube. Serum was prepared and stored at −80°C. The serum triglyceride levels were measured with an automated clinical chemistry analyzer kit (Biosino Biotech, Beijing, China). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured on a clinical autoanalyzer (Beckman Coulter DX). 14,15-EET and 14,15-DHET (14,15-dihydroxyeicosatrienoic acid) were measured in mouse serum and urine using an ELISA kit (Detroit R&D, Detroit, MI) according to the manufacturer's instructions.
Triglyceride content in liver tissues and HepG2 cells.
Triglyceride concentrations were quantified in both liver tissue and HepG2 cells. Tissues or cells lysates were homogenized, and total lipids were extracted into a mixture of chloroform-methanol (2:1). Triglyceride content was analyzed using an automated clinical chemistry analyzer kit (Sigma).
Morphologic studies.
Liver tissues were excised and embedded in Tissue-Tek OCT compound or paraffin for histological analysis. OCT-embedded samples were stained with Oil red O. Formalin-fixed and paraffin-embedded sections were stained with hematoxylin and eosin (HE). Liver sections were incubated with the rat primary antibody against F4/80 overnight at 4°C and anti-rat horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 1 h at 37°C. The nuclei were counterstained with hematoxylin.
Determination of inflammatory cytokine levels in serum, liver extracts, and cell supernatants.
Levels of TNFα, IL-6, and IL-1β in serum, liver extracts, and cell supernatants were measured using ELISA kits (R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
Oxidative stress parameters.
Lipid peroxidation in liver and HepG2 cells was determined by measuring malondialdehyde (MDA) using a colorimetric assay kit (Bioxytech LPO-586; Oxis International, Foster City, CA). Protein carbonyl content in liver was determined as described previously (33). The levels of 8-hydroxydeoxyguanosine (8-OHdG), an additional marker of oxidative stress in livers, were measured using an Oxiselect Oxidative DNA Damage ELISA kit (Cell Biolabs, San Diego, CA). The glutathione in liver and HepG2 cells was assayed using commercially available ELISA kits (BioVision, Mountain View, CA).
Hepatic antioxidant enzyme activity assays.
Total hepatic superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) activities were measured on homogenized liver tissue using commercial assay kits according to the manufacturer's instructions (Abcam, San Francisco, CA).
Determination of mRNA expression levels in liver and cells by real-time RT-PCR.
Total RNA extracts were isolated from mouse livers or HepG2 and RAW 264.7 cell lysates using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's directions. Total RNA (1.0 μg) was reverse transcribed to cDNA in a reaction mixture using using EasyScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). The gene expression level was determined using the real-time PCR system (Applied Biosystems). Primer sequences used are shown in Table 1. The data were analyzed using a comparative 2−ΔΔCT method.
Table 1.
Primer sequences for real-time RT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| Mouse sequences | ||
| TNFα | 5′-CAGGCGGTGCCTATGTCTC-3′ | 5′-CGATCACCCCGAAGTTCAGTAG-3′ |
| IL-6 | 5′-TCTATACCACTTCACAAGTCGGA-3′ | 5′-GAATTGCCATTGCACAACTCTTT-3′ |
| IL-1β | 5′-TTCAGGCAGGCAGTATCACTC-3′ | 5′-GAAGGTCCACGGGAAAGACAC-3′ |
| CD68 | 5′-CCATCCTTCACGATGACACCT-3′ | 5′-GGCAGGGTTATGAGTGACAGTT-3′ |
| F4/80 | 5′-TGACTCACCTTGTGGTCCTAA-3′ | 5′-CTTCCCAGAATCCAGTCTTTCC-3′ |
| p22phox | 5′-AGCGATGTGGACAGAAGTACC-3′ | 5′-CAGCCCGGACGTAGTAATTCC-3′ |
| p47phox | 5′-ACACCTTCATTCGCCATATTGC-3′ | 5′-CCTGCCACTTAACCAGGAACA-3′ |
| gp91phox | 5′-AGTGCGTGTTGCTCGACAA-3′ | 5′-GCGGTGTGCAGTGCTATCAT-3′ |
| GAPDH | 5′-TGACCTCAACTACATGGTCTACA-3′ | 5′-CTTCCCATTCTCGGCCTTG-3′ |
| HepG2 sequences | ||
| SREBP-1c | 5-CACCGTTTCTTCGTGGATGG-3′ | 5′-CCCGCAGCATCAGAACAGC-3′ |
| FAS | 5′-CATCCAGATAGGCCTCATAGAC-3′ | 5′-CTCCATGAAGTAGGAGTGGAAG-3′ |
| CPT1α | 5′-CTGAGCACGGCAAGATGAGT-3′ | 5′-GGCAGCGATGTCTGGAAGC-3′ |
| PPARα | 5′-TCCATCGGCGAGGATAGTTC-3′ | 5′-GTCCCCGCAGATTCTACATTC-3′ |
| TNFα | 5′-GAGGCCAAGCCCTGGTATG-3′ | 5′-CGGGCCGATTGATCTCAGC-3′ |
| IL-6 | 5′-ACTCACCTCTTCAGAACGAATTG-3′ | 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ |
| IL-1β | 5′-ATGATGGCTTATTACAGTGGCAA-3′ | 5′-GTCGGAGATTCGTAGCTGGA-3′ |
| p22phox | 5′-CCCAGTGGTACTTTGGTGCC-3′ | 5′-GCGGTCATGTACTTCTGTCCC-3′ |
| p47phox | 5′-CAAGAGTACCGCGACAGACAT-3′ | 5′-AGGTCTTCTCGTAGTTGGCAAT-3′ |
| gp91phox | 5′-AACGAATTGTACGTGGGCAGA-3′ | 5′-GAGGGTTTCCAGCAAACTGAG-3′ |
| GAPDH | 5′-CTGGGCTACACTGAGCACC-3′ | 5′-AAGTGGTCGTTGAGGGCAATG-3′ |
See text for definitions.
Western blotting.
Western blots were performed as described previously (6). Intensity of each immunoreactive band was quantified by densitometry analysis using the Bio Image System (Syngene, Cambridge, UK).
Determination of ROS.
Cells (3 × 105 cells/ml) were incubated with 5 mmol/l DCFH-DA (Sigma) for 30 min at 37°C. DCF fluorescence was measured using fluorescent-activated cell sorting (FACS) analysis.
JC-1 staining for mitochondrial membrane potential.
Mitochondrial membrane potential was assessed using a cell-permeable, mitochondrial-specific fluorescent probe JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyaniniodide; Invitrogen), which is a lipophilic, cationic dye that can selectively enter into mitochondria and reversibly change color from green to red as the membrane potential increases. Cells were harvested and resuspended in DMEM at a density of 1 × 106 cells/ml and incubated with 10 μM JC-1 at 37°C for 30 min. Relative fluorescence intensities were monitored using FACS analysis. Healthy cells contain the aggregated JC-1 dye reagent within their mitochondria as a red fluorescent monomeric form. In unhealthy cells, the mitochondrial membrane potential (Δψm) collapses, and JC-1 remains in the cytoplasm as a green fluorescent monomeric form. The data are expressed as the percentage of ΔΨm collapsed cells (red fluorescence) of both red and green fluorescent cells.
Statistical analysis.
Results are expressed as means ± SE. Multiple comparisons between two groups were performed with unpaired t-tests. Differences between three or more groups were determined with ANOVA analyses. P < 0.05 was considered statistically significant.
RESULTS
Metabolic characteristics and liver function in HFD-fed mice.
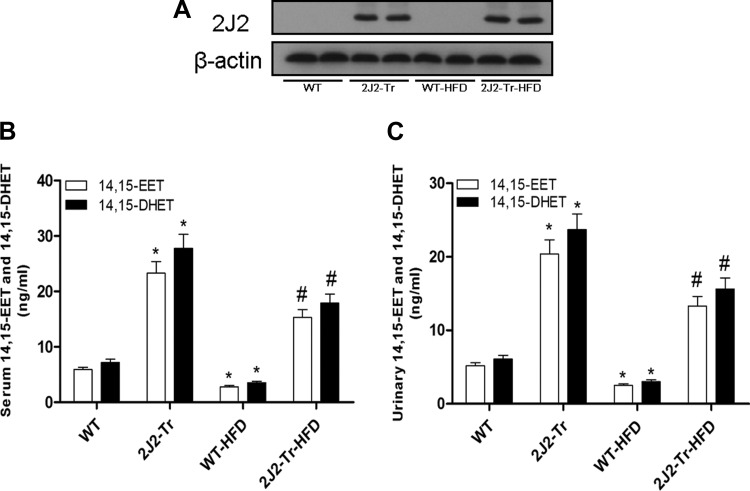
Western blots carried out with CYP2J2 antibodies show no CYP2J2 protein expression in livers of WT mice. However, Tie2-CYP2J2-Tr mice show abundant CYP2J2 liver protein expression. Induction of NAFLD with HFD had no significant effect on CYP2J2 protein expression in WT or Tie2-CYP2J2-Tr mice (Fig. 1A). We found that serum and urinary 14,15-EET and 14,15-DHET levels in Tie2-CYP2J2-Tr mice were higher than in WT mice. HFD feeding significantly reduced 14,15-EET and 14,15-DHET levels in serum and urine compared with WT controls. However, relative to WT HFD-fed mice, Tie2-CYP2J2-Tr HFD-fed mice exhibited significantly higher levels of 14,15-EET and 14,15-DHET in serum and urine(Fig. 1, B and C). These results indicate that CYP2J2 overexpression induces production of EETs in vivo. Table 2 lists the biochemical and physiological parameters of WT and Tie2-CYP2J2-Tr mice in both normal chow- and HFD-fed groups. Administration of HFD to mice significantly increased serum triglycerides, ALT activity, and AST activity compared with control mice (P < 0.05). Interestingly, the increases serum triglycerides, ALT activity, and AST activity were lowered in Tie2-CYP2J2-Tr mice (P < 0.05).
Fig. 1.
Expression of CYP2J2 in liver and detection of 14,15-EET (14,15-epoxyeicosatrienoic acid) and 14,15-DHET (14,15-dihydroxyeicosatrienoic acid) levels in serum and urine. HFD, high-fat diet; WT, wild type. A: representative CYP2J2 protein expression levels in liver tissues from the 4 treatment groups. B: 14,15-EET and 14,15-DHET levels in serum. C: 14,15-EET and 14,15-DHET levels in urine. Data are shown as means ± SE (n = 8 per group). *P < 0.05 vs. WT control mice; #P < 0.05 vs. WT-HFD mice.
Table 2.
Physiological parameters of WT and Tie2-CYP2J2-Tr mice in control and HFD-fed cohorts
| Genotype | WT | 2J2-Tr | WT-HFD | 2J2-Tr-HFD |
|---|---|---|---|---|
| Body weight, g | 28.9 ± 0.9 | 28.5 ± 1.1 | 41.5 ± 2.1* | 40.8 ± 1.8* |
| Serum triglyceride, mg/dl | 38.7 ± 2.7 | 38.3 ± 2.9 | 67.3 ± 4.5* | 45.8 ± 3.6# |
| Serum ALT activity, U/l | 41.6 ± 3.6 | 42.5 ± 3.2 | 132.5 ± 8.7* | 65.7 ± 5.5# |
| Serum AST activity, U/l | 62.4 ± 4.8 | 61.9 ± 5.2 | 165.8 ± 11.2* | 81.4 ± 6.5# |
Values are means ± SE (n = 8 per group).
WT, wild type; Tie2-CYP2J2-Tr, endothelial-specific CYP2J2 overexpression transgenic; HFD, high-fat diet; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
P < 0.05 vs. WT control mice,
P < 0.05 vs. WT-HFD mice.
Effects of CYP2J2 overexpression on hepatic steatosis.
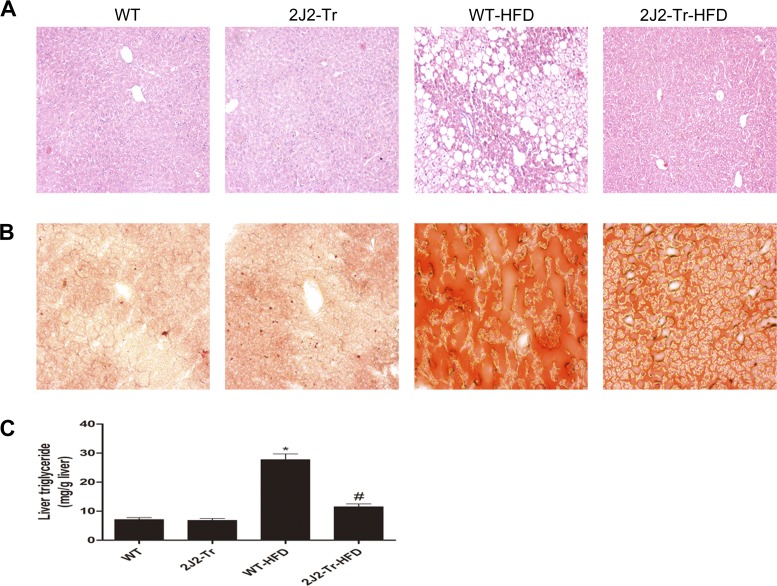
We examined the effect of CYP2J2 overexpression on liver steatosis by histological staining and quantification of hepatic triglyceride content. As shown in Fig. 2A, compared with the normal-chow group, moderate centrilobular microvesicular steatosis, scattered infiltration of neutrophils and lymphocytes, and ballooning degeneration were observed in the livers of WT HDF-fed mice. These effects were attenuated in Tie2-CYP2J2-Tr HFD-fed mice. Liver sections from WT HFD-fed mice displayed a clearly visible positive signal for oil red O, which stains neutral lipids in tissues. Oil red O staining was markedly lowered in the Tie2-CYP2J2-Tr HFD-fed mice (Fig. 2B). Results of hepatic triglyceride content analysis support the histological differences in steatosis observed between WT and Tie2-CYP2J2-Tr mice fed a HFD. Triglyceride content was equivalent for both groups fed the control-chow diet (Fig. 2C). The fourfold increase in hepatic triglyceride content observed after 24 wk of HFD in WT mice was attenuated by in Tie2-CYP2J2 Tr mice (Fig. 2C). Together, these data show that CYP2J2 overexpression has a protective effect against HFD-induced hepatic steatosis in mice in vivo.
Fig. 2.
Liver histology and triglyceride content of mice. Hematoxylin and eosin (A) or Oil red O staining (B) of liver sections from representative WT or Tie2-CYP2J2-Tr (2J2-Tr) mice fed normal chow or HFD. C: liver triglyceride content is expressed per gram of tissue. Data are shown as means ± SE (n = 8 per group). *P < 0.05 vs. WT control mice; #P < 0.05 vs. WT-HFD mice.
CYP2J2 overexpression reduces HFD-induced serum and liver inflammatory cytokine levels.
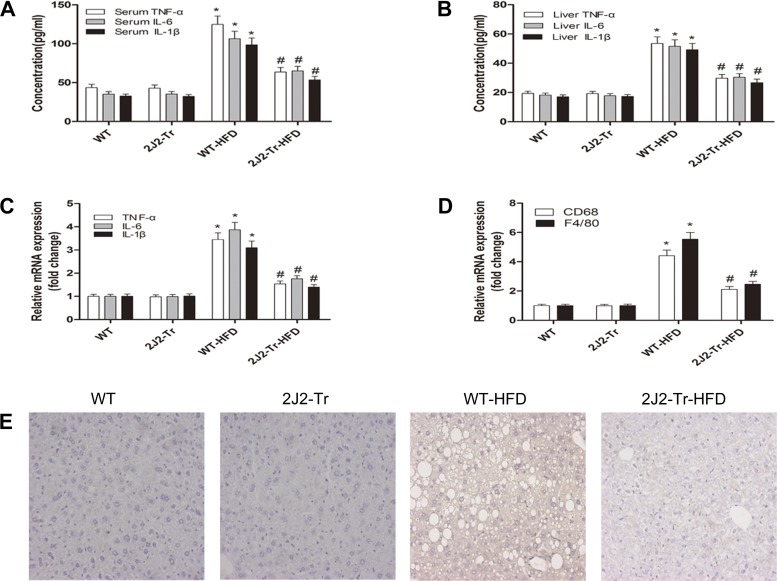
To evaluate the signaling mechanisms responsible for the liver-protective effect of the CYP2J2 overexpression, we quantified cytokines that might influence the extent of liver damage caused by NAFLD. HFD upregulated the proinflammatory cytokines TNFα, IL-6, and IL-1β in serum and liver from WT mice. This upregulation was significantly attenuated in Tie2-CYP2J2-Tr mice (Fig. 3, A and B). Similarly, real-time RT-PCR analysis revealed that CYP2J2 overexpression significantly suppressed the hepatic mRNA levels of TNFα, IL-6, IL-1β, F4/80, and CD68 compared with WT HFD-fed mice (Fig. 3, C and D). Immunohistochemical analysis was performed to identify inflammatory cell infiltration. Macrophage marker F4/80, which recognizes specialized macrophages in the liver called Kupffer cells, revealed staining of cells mainly trafficked to interstitial areas after 24 wk of HFD (Fig. 3E). CYP2J2 overexpression lowered macrophage infiltration after HFD. These data are consistent with the ameliorated fat accumulation in Tie2-CYP2J2-Tr NAFLD mouse livers. Thus, hepatic steatosis-associated inflammatory cell infiltration in the liver is relieved by CYP2J2 overexpression.
Fig. 3.
CYP2J2 overexpression reduces HFD-induced increase markers of serum and liver inflammation. WT or Tie2-CYP2J2-Tr (2J2-Tr) mice were fed normal chow or a high-fat diet (HFD). A: serum levels of proinflammatory cytokines TNFα, IL-6, and IL-1β were determined. Liver levels of proinflammatory cytokine protein (B) or mRNA (C) of TNFα, IL-6, and IL-1β were also determined. D: relative mRNA expression of macrophage markers F4/80 and CD68 was shown. E: immunohistochemical staining of F4/80 in liver was performed (original magnification, ×200). Representative pictures of immunohistochemical detection of F4/80-positive cells are shown. Data are shown as means ± SE (n = 8 per group). *P < 0.05 vs. WT control mice; #P < 0.05 vs. WT-HFD mice.
CYP2J2 overexpression prevents NF-κb and JNK activation.
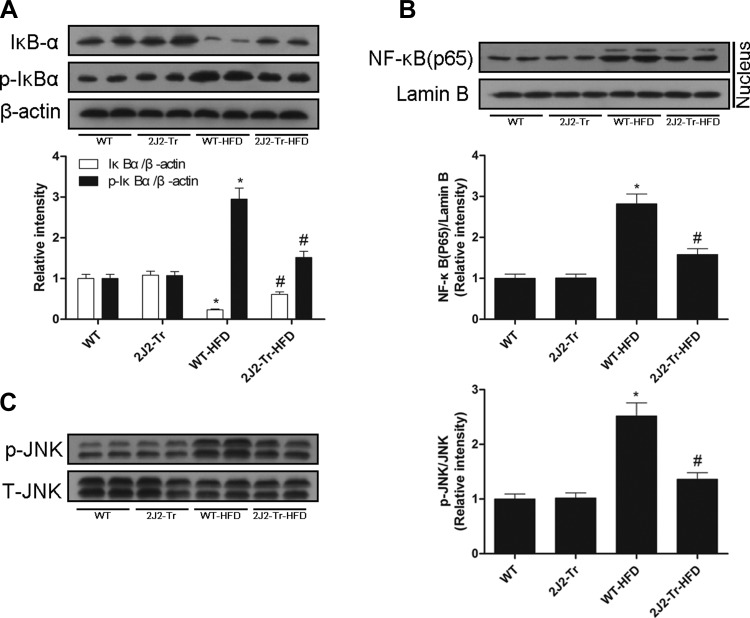
The degradation and phosphorylation of IκB are necessary to release NF-κB from the cytoplasmic NF-κB/IκB complex and allow NF-κB translocation to the cell nucleus. HFD increased the phosphorylation and degradation of IκBα and nuclear translocation of the NF-κB p65 subunit. These effects were attenuated in Tie2-CYP2J2-Tr mice (Fig. 4, A and B). Since JNK may regulate induction of inflammatory cytokines implicated in NAFLD, we determined the phosphorylation status of JNK in liver lysates. HFD increased JNK phosphorylation in livers in WT mice. In contrast, HFD-induced hepatic activation of JNK was suppressed in Tie2-CYP2J2-Tr mice (Fig. 4C). These data suggest that CYP2J2 overexpression attenuated HFD-induced hepatic inflammation via the NF-κB/JNK signaling pathways.
Fig. 4.
CYP2J2 overexpression prevents NF-κB and JNK activation. Western blots of total liver expression of IκBα, p-IκBα (A) or nuclear protein levels of NF-κB(p65) (B) of the 4 treatment groups were described. C: hepatic levels of activated JNK (p-JNK) protein were shown. Data are shown as means ± SE (n = 8 per group). *P < 0.05 vs. WT control mice; #P < 0.05 vs. WT-HFD mice.
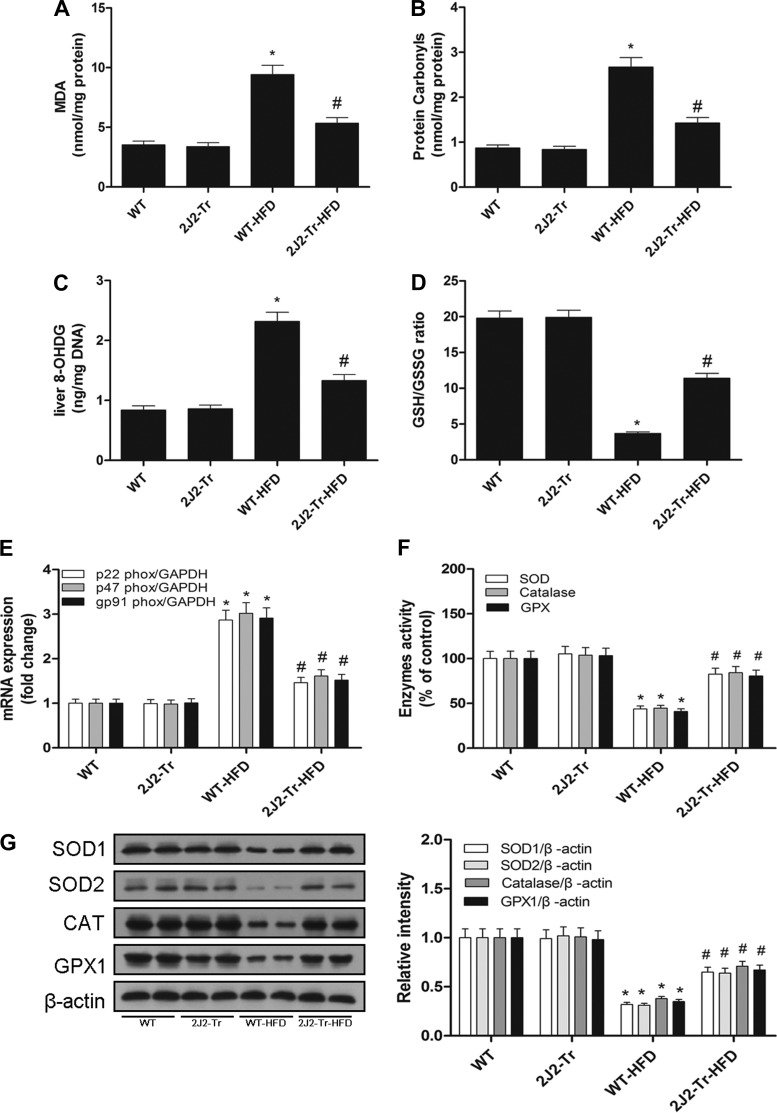
CYP2J2 overexpression attenuates HFD-induced hepatic oxidative stress.
CYP2J2 overexpression can exert protective effects by increasing antioxidant enzymes and reducing oxidative stress (22). Livers from WT NAFLD mice had increased accumulation of lipid peroxides (Fig. 5A), protein carbonyls (Fig. 5B), and 8-OHdG levels (Fig. 5C). Expression of mRNA of various ROS-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (p22phox, p47phox, gp91phox; Fig. 5E) was also elevated in livers from WT mice fed a HFD. These results were concordant with a decrease in the reduced/oxidized glutathione ratio (Fig. 5D) and lower expression and activity of antioxidant enzymes (SOD1, SOD2, CAT, and GPX1; Fig. 5, F and G). These changes were attenuated in livers from Tie2-CYP2J2-Tr mice fed a HFD (Fig. 5). These results are consistent with the other findings that CYP2J2 overexpression exerts a hepatoprotective effect, at least in part by reducing oxidative stress.
Fig. 5.
CYP2J2 overexpression suppresses HFD-induced oxidative stress in liver. Oxidative stress in the hepatic tissues from WT and 2J2-Tr mice were determined by measuring malondialdehyde (MDA; A), protein carbonyl content (B), 8-OhdG levels (C), GSH/GSSG ratio (D), and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunit (p22phox, p47phox, gp91phox) mRNA (E). Antioxidant enzyme [superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX1)] activity (F) and expression (G) were shown. Data are shown as means ± SE (n = 8 per group). *P < 0.05 vs. WT control mice; #P < 0.05 vs. WT-HFD mice.
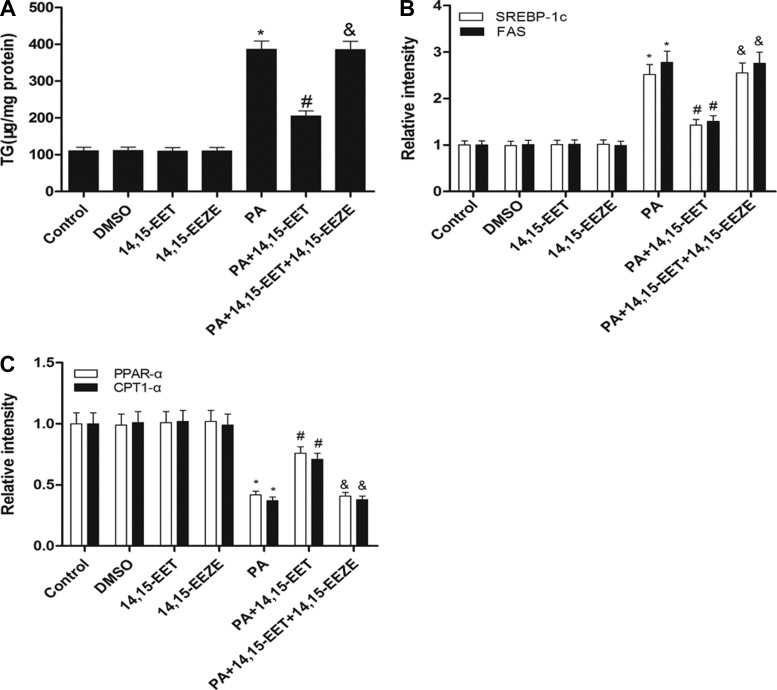
Effects of 14,15-EET treatment on PA-induced hepatic lipid accumulation in vitro.
Palmitic acid treatment of HepG2 cells was used as an in vitro model to investigate the role of EETs in triglyceride accumulation. HepG2 cells were exposed to 0.5 mM PA for 24 h, and intracellular triglyceride content was determined. Compared with untreated cells, PA-treated cells accumulated greater than 2.5-fold more triglycerides (Fig. 6A). 14,15-EET suppressed triacylglycerol accumulation in PA-treated HepG2 cells, which was blocked by the putative EET receptor antagonist 14,15-EEZE (Fig. 6A). To determine the mechanism by which EETs decreased the PA-induced lipid accumulation in HepG2 cells, real-time RT-PCR analysis was performed to evaluate the mRNA expression levels of of genes involved in lipogenesis (SREBP-1c, FAS) and fatty acid β-oxidation (PPARα, CPT1α), respectively. As shown in Fig. 6, A and B, we confirmed that 14,15-EET decreased mRNA levels of SREBP-1c and FAS in PA-treated cells. Conversely, 14,15-EET increased mRNA levels of PPARα and CPT1α in PA-treated cells. These effects were also significantly inhibited by pretreatment with 14,15-EEZE (Fig. 6, B and C). These results suggest that 14,15-EET reduces hepatic lipid accumulation in two ways: downregulating lipogenesis genes and upregulating genes in the β-oxidation pathway.
Fig. 6.
Effects of 14,15-EET treatment on palmitic acid (PA)-induced hepatic lipid accumulation in vitro. HepG2 cells were treated for 24 h in DMEM containing 1.0% free acid-free BSA, or for 24 h in DMEM containing 0.5 mM PA and 1.0% free acid-free BSA with or without 14,15-EET or 14,15-EEZE [14,15-epoxyeicosa-5(Z)-enoic acid]. HepG2 cells were lysed, and triglyceride concentrations were evaluated by biochemical test kits. Triglyceride level was corrected by total protein. Relative mRNA expression of lipogenesis genes (SREBP-1c, FAS; B) and fatty acid β-oxidation genes (PPARα, CPT1α; C) were determined. Data were normalized to GAPDH. Data are presented as means ± SE (n = 6). *P < 0.05 vs. untreated control. #P < 0.05 vs. PA-treated cells; &P < 0.05 vs. 14,15-EET and PA-treated cells.
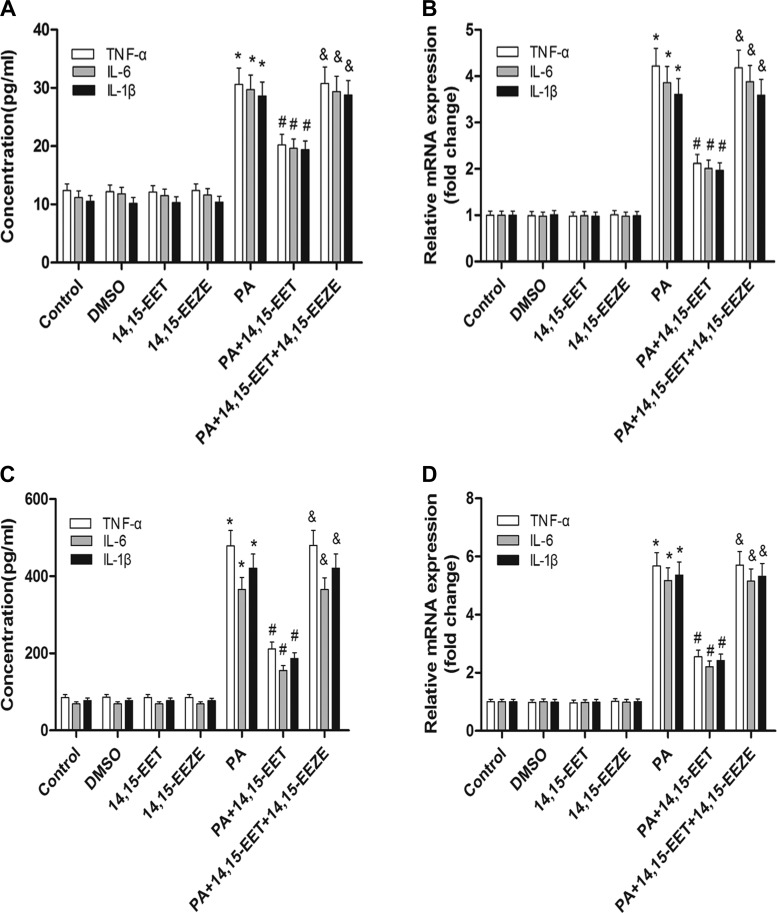
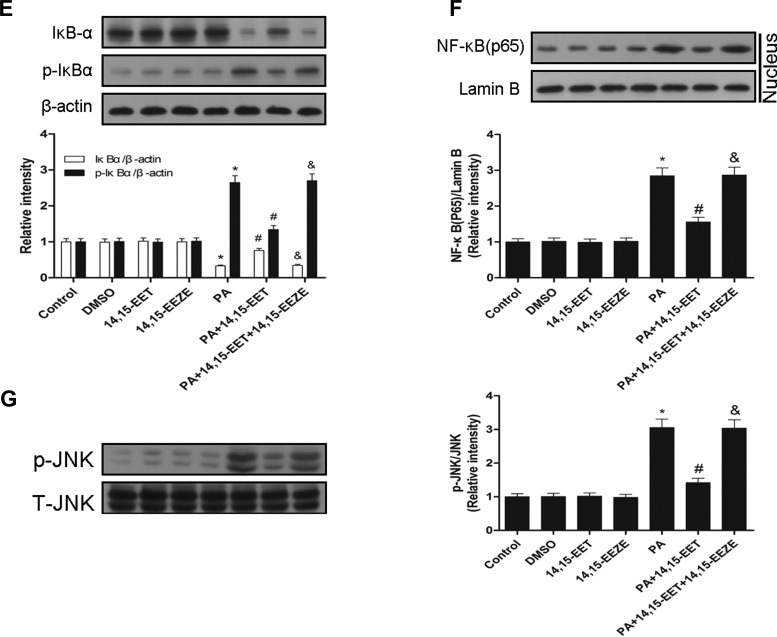
14,15-EET inhibits PA-mediated inflammatory cytokine expression and signal pathways activation in cells.
EETs reduce NF-κB activation mediated by many stimuli, such as TNFα and LPS (15, 28). Therefore, we sought to determine whether 14,15-EET would similarly reduce PA-dependent inflammation in cells. Pretreatment with 14,15-EET significantly reduced PA-dependent increase of TNFα, IL-6, and IL-1β levels in supernatants compared with vehicle-treated cells (Fig. 7A). To validate the changes in supernatants, cells were then harvested for mRNA analysis. Real-time RT-PCR analysis showed that the expression of TNFα, IL-6, and IL-1β increased significantly after incubation with PA, but this was reversed by 14,15-EET (Fig. 7B). Similarly, treatment with 14,15-EET significantly attenuated the palmitate-stimulated upregulation of TNFα, IL-6, and IL-1β protein secretion as well as their increased mRNA levels in RAW 264.7 cells (Fig. 7, C and D). Pretreatment with 14,15-EET also attenuated PA-mediated NF-κB and JNK activation in HepG2 cells, as assessed by Western blotting (Fig. 7, E–G). Interestingly, protection by 14,15-EET was abolished by administration of the putative selective EET antagonist 14,15-EEZE (Fig. 7, A–G). Therefore, 14,15-EET is able to attenuate PA-mediated inflammatory responses in cells in vitro.
Fig. 7.
14,15-EET inhibits PA-induced inflammatory cytokine expression and signal pathway activation in HepG2 cells. HepG2 and RAW 264.7 cells were exposed to PA pretreatment with 14,15-EET or 14,15-EEZE for 24 h. A: levels of TNFα, IL-6, and IL-1β in supernatants were quantified with ELISA kits in HepG2 cells. B: TNFα, IL-6, and IL-1β mRNA expression from HepG2 cells as analyzed by real-time RT-PCR. Levels of TNFα, IL-6, and IL-1β in supernatants were quantified with ELISA kits in RAW 264.7 cells. D: TNFα, IL-6, and IL-1β mRNA expressions from RAW 264.7 cells were analyzed by real-time RT-PCR. Total protein levels of IκBα and p-IκBα (E) and nuclear protein levels of NF-κB(p65) (F) from HepG2 cells were assessed by Western blot. G: immunoblot analysis of p-JNK and JNK was performed in HepG2 cell extracts. Data are presented as means ± SE (n = 6). *P < 0.05 vs. untreated control; #P < 0.05 vs. PA-treated cells; &P < 0.05 vs. 14,15-EET and PA-treated cells.
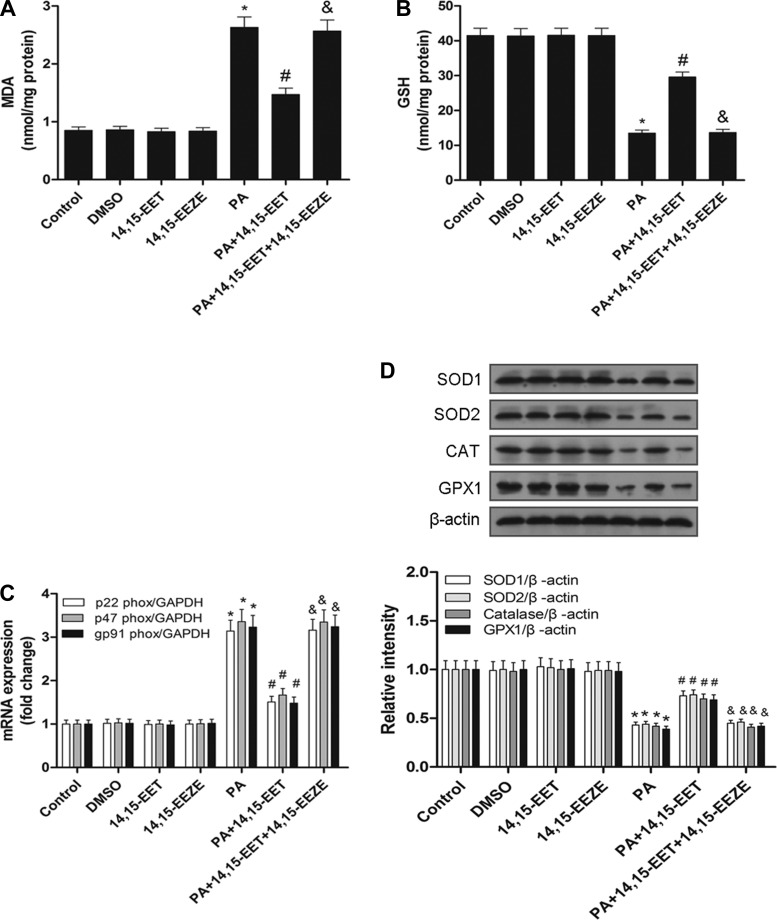
14,15-EET mitigates PA-induced oxidative damage in HepG2 cells.
Palmitic acid induced lipid peroxidation, as illustrated by increased MDA generation, and a remarkable decrease in GSH levels. These changes were significantly blunted by addition 14,15-EET. Importantly, the protective role of 14,15-EET against lipid peroxidation and prevention of GSH depletion were abolished by 14,15-EEZE (Fig. 8, A and B). 14,15-EET treatment attenuated the increase in NADPH oxidase subunit (p22phox, p47phox, and gp91phox) mRNA levels and the decrease in antioxidant enzyme (SOD1, CAT, GPX1) expression after PA treatment in HepG2 cells. These effects were also significantly inhibited by pretreatment with 14,15-EEZE (Fig. 8, C and D). Collectively, these findings demonstrate that 14,15-EET mitigates PA-induced oxidative damage by modulating MDA generation, GSH levels, NADPH oxidase subunits, and antioxidant enzyme expression in HepG2 cells.
Fig. 8.
14,15-EET mitigates PA-induced oxidative damage in HepG2 cells. Oxidative damage in HepG2 cells was evaluated by measuring MDA (A) and GSH (B). C: nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunit (p22phox, p47phox, gp91phox) mRNA detection was shown. D: antioxidant enzyme (SOD1, CAT, GPX1) protein expression was shown and quantified by densitometry. Data are presented as means ± SE (n = 6). *P < 0.05 vs. untreated control; #P < 0.05 vs. PA-treated cells; &P < 0.05 vs. 14,15-EET and PA-treated cells.
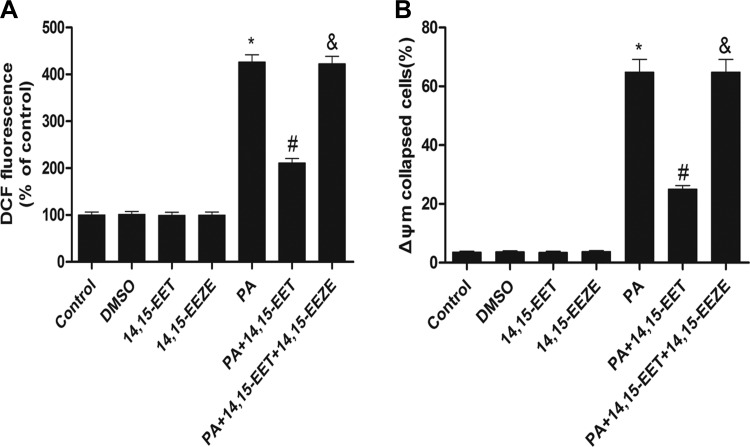
14,15-EET decreases PA-induced ROS generation and loss of Δψm in HepG2 cells.
After PA treatment with or without the receptor antagonist, intracellular ROS was determined in HepG2 cells by flow cytometry using the nonfluorescent probe DCFH-diacetate, which is converted to highly fiuorescent dichlorofluorescein (DCF) by ROS. Exposure of HepG2 cells to PA for 24 h led to a marked increase in ROS production, and pretreatment with 14,15-EET (1μM) attenuated the ROS generation induced by PA (Fig. 9A). Moreover, Δψm estimated by JC-1 showed that 14,15-EET preserved PA-mediated the loss of Δψm in HepG2 cells (Fig. 9B). Decreased intracellular ROS and increased Δψm by 14,15-EET treatment were also significantly reversed by coincubation with 10 μM 14,15-EEZE (Fig. 9, A and B). These results further confirm the essential protective role of EETs against oxidative damage by inhibiting ROS production and preserving Δψm in HepG2 cells.
Fig. 9.
Effects of 14,15-EET on ROS generation and mitochondrial potential (Δψm) in HepG2 cells. A: fold changes in ROS levels was measured by DCFH-DA and flow cytometry after incubation with PA, 14,15-EET, 14,15-EEZE, or both. HepG2 cells were incubated with 10 mM DCFH-DA and PA in the presence or absence of 14,15-EET or 14,15-EEZE and analyzed by flow cytometry. Quantification of ROS generation was assessed by flow cytometry per each treatment as indicated. B: cells were pretreated with 14,15-EET or 14,15-EEZE for 30 min followed by treatment with 0.5 mM PA for 24 h. After 24-h exposure, media were removed, and Δψm's were measured using JC-1 dye, as described in materials and methods. Data are presented as means ± SE (n = 5). *P < 0.05 vs. untreated control; #P < 0.05 vs. PA-treated cells; &P < 0.05 vs. 14,15-EET and PA-treated cells.
DISCUSSION
In this study, we describe the protective effect of endothelial-specific CYP2J2 overexpression in a mouse model of NAFLD induced by HFD. Our transgenic mice exhibited constitutive endothelial expression of the human CYP2J2 and increased EET biosynthesis. Our findings demonstrate that CYP2J2 overexpression decreased liver damage, improved liver function, ameliorated NAFLD progression, reduced proinflammatory mediator levels in serum and injured livers, attenuated lipid peroxidation, and upregulated antioxidant levels in livers of NAFLD mice. The mechanism of protection against HFD-induced NAFLD involved anti-inflammatory and antioxidant effects of the primary CYP2J2 epoxygenase products, EETs. CYP2J2 overexpression and EET production inhibit inflammation activation, macrophage infiltration, and release of proinflammatory cytokines induced by HFD and attenuate oxidative stress by preventing upregulation of prooxidant NADPH oxidase subunits and the loss of antioxidant enzymes such as SOD, CAT, and GSX. Our data validate the utility of transgenic mice as a model system to characterize the functional impact of endothelial CYP epoxygenase-derived EETs on the regulation of this pathophysiological process. Together, these findings suggest that CYP2J2 overexpression protects against several pathogenic events leading to liver damage and NAFLD induced by HFD in a murine model.
The rising rates of NAFLD make addressing the underlying causes of this serious condition a pressing concern. A major problem in the management of NAFLD is the lack of effective therapy. In the present study, the mouse model of NAFLD successfully reproduced typical pathogenetic and histopathological features of NAFLD in humans, such as abnormal aminotransferase, increased plasma TG and FFA, upregulated levels of inflammatory factors in serum and liver, histological evidence of steatosis and inflammation, elevated lipid peroxidation, and oxidative stress. This rodent model of NAFLD was consistent with a previous study that a chronic HFD led to fatty liver and steatohepatitis (1, 30). We showed that CYP2J2 overexpression decreased the plasma levels of TG, improved liver function, and prevented the development of inflammation in NAFLD mice. Thus, CYP2J2 overexpression successfully slowed down the development of steatohepatitis via both histopathological and biochemical improvements.
As mechanisms involved in the development of NAFLD in humans are not yet fully understood, therapeutic and preventive options are still quite limited. The proinflammatory cytokines TNFα, IL-1β, and IL-6 are considered to have a critical role in the progression of hepatic steatosis to more advanced stages of liver damage (7). TNFα, IL-1β, IL-6, and other proinflammatory factors also showed high expression levels in NAFLD patients (32). Experimental depletion of Kupffer cells prevents HFD-induced hepatic steatosis and inflammation in rodents (17). CYP2J2 treatment inhibited MCT-induced elevation of serum IL-6 concentrations in rats (46). Overexpression of CYP2J2 significantly attenuated serum levels of proinflammatory cytokines, including sVCAM-1, sE-selectin, IL-1β, and IL-6 after TNFα injection in rats (45). In the present study, CYP2J2 overexpression significantly decreased serum TNFα, IL-6, and IL-1β levels in NAFLD Tie2-CYP2J2-Tr mice induced by HFD. The transcription of genes encoding TNFα, IL-6, and IL-1β and the secretion of TNFα, IL-6, and IL-1β are barely detectable in control mouse livers. However, TNFα, IL-6, and IL-1β expression in the liver is elevated in NAFLD mice. Interestingly, the upregulation of proinflammatory cytokines was reversed by CYP2J2 overexpression in the liver of Tie2-CYP2J2-Tr mice induced by HFD. The effect of CYP2J2 overexpression was also observed histologically in hepatic expression of genes such as F4/80 that involved the inflammatory response. Immunohistochemical analysis showed a reduced number of F4/80-positive cells in livers of Tie2-CYP2J2-Tr mice induced by HFD. Thus, our data clearly showed that CYP2J2 overexpression attenuated inflammatory factors accompanied by the reduction of macrophage accumulation and liver injury.
We investigated the mechanism by which CYP2J2 overexpression inhibited the production of inflammatory mediators. Among the intracellular signaling systems involved in the regulation of inflammatory and immune responses, NF-κB is of special interest (35). Expression of NF-κB is significantly increased in NAFLD patients, and activation of NF-κB is an important step in regulating gene expression of various kinds of proinflammatory factors (34, 43). Others have found that blockade of NF-κB transcriptional activation substantially protected against development of steatohepatitis, with significant reductions in liver injury and hepatic inflammation in NAFLD mice (11). Similar to NF-κB, increased JNK activation has been identified as a critical mechanism in the development and progression of murine steatohepatitis. In diet-treated Jnk1 knockout mice, steatosis and liver injury were markedly decreased, displaying a pivotal role for JNK1 in the development of steatohepatitis (37). Antisense oligonucleotide-induced knockdown of JNK1 in established steatohepatitis significantly decreased the degree of steatosis and liver injury, which suggested that JNK1 function was also essential for the maintenance and progression of fat accumulation and hepatitis (38). EETs are known to regulate JNK in other models. CYP2C9 overexpression in endothelial cells results in increased intracellular EET levels and a concomitant dephosphorylation and inactivation of JNK, suggesting that EETs inhibit JNK activity (31). EETs decrease palmitate-induced phosphorylation of JNK in hepatocytes (39). Our study demonstrated the activation of NF-κB and JNK during development of NAFLD induced by HFD. Consequently, the anti-inflammatory effects observed in the current investigation occurred in the presence of physiological EET levels and demonstrated an important role for the CYP epoxygenase-EET system in the regulation of NAFLD inflammation in vivo. Collectively, CYP2J2 overexpression attenuated inflammatory reactions and meditated hepatoprotection in NAFLD mice, at least in part, through inhibition of NF-κB/JNK activation.
To evaluate the effect of CYP2J2 overexpression on fatty liver, the effects of exogenous supplementation of 14,15-EET were investigated in an in vitro fatty liver model. Adding PA to HepG2 cells simulated the condition of high plasma levels of FFA in the animal experiment. Palmitic acid significantly increased the intracellular TG content in liver cells, similarly to that observed often when plasma FFA is elevated in animal and human studies (5, 41). Interestingly, treatment with 14,15-EET reduced intracellular TG in HepG2 cells. Lipotoxicity is implicated in the pathogenesis of nonalcoholic steatohepatitis (NASH). Palmitic acid is known as a lipotoxic fatty acid that induces inflammatory responses and increases oxidative stress in hepatocytes (24). FFAs can induce the activation of the key regulator of inflammation, NF-κB, as well as the inflammatory cytokine TNFα in hepatocytes while simultaneously causing JNK-dependent lipotoxicity (13). In the present study, 14,15-EET reduced PA-mediated inflammatory cytokine expression and NF-κB/JNK pathway activation in HepG2 and RAW 264.7cells. These results are in line with previous reports indicating that EETs mitigate inflammatory responses in lung tissue and vascular cells following various insults (12, 46). These data suggest that attenuation of inflammatory responses may be an important mechanism by which CYP2J2 overexpression reduces liver injury and slows down fatty liver progression.
Oxidative stress is an imbalance between oxidants and antioxidant defenses systems. Overproduction of mitochondrial and cytoplasmic superoxide anion and other reactive oxygen species (ROS) plays pivotal roles in NAFLD development (20). Oxidative stress increases lipid peroxidation and oxidative damage to proteins and DNA. ROS activates Kupffer cells toward production of inflammatory cytokines and initiation of signal transduction, which further increase oxidative stress through effects on the mitochondria (18). The development of steatohepatitis is associated with increased lipid peroxidation, as indicated by the increased levels of lipid peroxidation products such as 4-HNE in the plasma and urine of patients with NAFLD and the liver of rodents with steatohepatitis (42). Increased ROS generation could contribute to HFD-induced liver lesions through the formation of reactive and biologically active peroxidation products such as MDA, protein carbonyls, and oxidative DNA damage (36). NADPH oxidase is an important source of ROS production, and increased expression of NADPH oxidase proteins has been observed in liver from animal model of NAFLD (8). Thus, NADPH oxidase inhibition represents an attractive and common treatment target for NAFLD. Antioxidant defenses (SOD, CAT, and GPX1) play an important role in protecting the cells against oxidative stress. Decreased expression and activity in antioxidant defenses is also a major factor promoting oxidative stress in NASH patients, which correlates with the severity of liver disease (4). The involvement of oxidative stress in the pathogenesis of NAFLD suggests that antioxidants might have beneficial effects in the treatment of NAFLD. Restoration of hepatic GSH by treatment with S-adenosylmethionine (SAMe) improves diet-induced steatohepatitis in rats (29), suggesting an important protection role for GSH against oxidative stress. In present study, we found mice fed with high fat diet were characterized by enhanced expression of NADPH oxidase isoforms p22phox, p47phox, and gp91phox, attenuated antioxidant defenses (decreased SOD, CAT, and GPX1 expression and activity) coupled with increased lipid peroxidation, oxidative modification of proteins, and DNA damage in livers. Interestingly, CYP2J2 overexpression significantly reversed these pathological changes and strengthened the antioxidant defense system. Indeed, specific targeting of CYP2J2, a major EET-producing enzyme, to the vascular endothelium affected not only the vasculature, attenuating HFD-induced endothelial dysfunction (2), but also liver, ameliorating the HFD-induced NAFLD by a mechanism that lowers lipotoxicity-related inflammation and oxidative stress. These findings provide direct evidence that CYP2J2-derived EETs may act through critical intracellular signaling pathways that lead to the protective effects in liver.
Treatment with 14,15-EET reduced ROS accumulation, lipid peroxidation (MDA) levels, expression of NADPH oxidase subunits p22phox, p47phox, and gp91phox, and preserved Δψm in HepG2 cells induced by PA. In aerobic cells, the antioxidant defense enzymes are endowed with endogenous antioxidant enzymes such as SOD, CAT, GPXs, as well as nonenzymatic substance-glutathione serving as antioxidant defense mechanisms to counteract the harmful effects of ROS (14). Overexpression of SOD or CAT protected against hepatic oxidative injury in HepG2 cells (3). We found that 14,15-EET upregulated the protein expression of antioxidant enzyme (SOD, CAT, GPX) in HepG2 cells. Thus, the decreased oxidative damage in 14,15-EET-treated cells may be attributed to the improved antioxidant capacity. These data are consistent with the in vivo data from Tie2-CYP2J2-Tr NAFLD mice.
In summary, CYP2J2 overexpression both decreased HFD-induced liver dysfunction, lipid accumulation, and inflammatory signaling and strengthened the antioxidant defense system in vivo. Similarly, we also demonstrated that 14,15-EET protects HepG2 cells against PA-induced inflammation and oxidative stress in vitro. These results highlight a new role for CYP2J2 and EETs in lipotoxicity-related inflammation and oxidative stress. These findings reveal a new molecular mechanism underlying EETs-mediated anti-inflammatory and antioxidant effects, which could be potentially useful in the design of new therapies for the treatment or prevention of NAFLD disease.
GRANTS
This work was supported by NSFC Grant (nos. 81400369 and 31130031) and in part by the intramural research program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 025034 to D. C. Zeldin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.C., R.X., Y.W., and D.W.W. conception and design of research; G.C., R.X., S.Z., and Y.W. performed experiments; G.C., R.X., S.Z., and P.W. analyzed data; G.C., R.X., and P.W. interpreted results of experiments; G.C. and R.X. prepared figures; G.C. drafted manuscript; G.C., M.L.E., D.C.Z., and D.W.W. edited and revised manuscript; M.L.E., D.C.Z., and D.W.W. approved final version of manuscript.
REFERENCES
- 1.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr 141: 603–610, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, Lee C, Zeldin DC, Schwartzman ML. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension 64: 1352–1361, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem 274: 26217–26224, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin Biochem 40: 776–780, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, Beilfuss A, Schmitt J, Hannivoort RA, Rust C, Berr F, Tschopp O, Gerken G, Friedman SL, Geier A, Canbay A. Free fatty acids repress SHP activation and adiponectin counteracts bile acid induced liver injury in super-obese patients with NASH. Hepatology 57: 1394–1406, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Wang P, Zhao G, Xu G, Gruzdev A, Zeldin DC, Wang DW. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins Other Lipid Mediators 96: 63–71, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S, Diehl AM. Role of inflammation in nonalcoholic steatohepatitis. Curr Opin Gastroenterol 21: 702–707, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, Mandrekar P, Dolganiuc A, Kurt-Jones E, Szabo G. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol 300: G433–G441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 142: 711–725e716, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 129: 1663–1674, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, DeGraff LM, Lih FB, Foley J, Bradbury JA, Graves JP, Tomer KB, Falck JR, Zeldin DC, Lee CR. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J 25: 703–713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 40: 185–194, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gambino R, Musso G, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities. Antioxid Redox Signal 15: 1325–1365, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Feng J, Ma K, Zhou Z, Zhu Y, Xu Q, Wang X. 8,9-Epoxyeicosatrienoic acid inhibits antibody production of B lymphocytes in mice. PLoS One 7: e40258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol 21, Suppl 3: S3–S6, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59: 347–357, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta 412: 1297–1305, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Kotzka J, Knebel B, Haas J, Kremer L, Jacob S, Hartwig S, Nitzgen U, Muller-Wieland D. Preventing phosphorylation of sterol regulatory element-binding protein 1a by MAP-kinases protects mice from fatty liver and visceral obesity. PLoS One 7: e32609, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurent A, Nicco C, Tran Van Nhieu J, Borderie D, Chereau C, Conti F, Jaffray P, Soubrane O, Calmus Y, Weill B, Batteux F. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology 39: 1277–1285, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Chen L, Hu L, Liu Y, Sun HY, Tang J, Hou YJ, Chang YX, Tu QQ, Feng GS, Shen F, Wu MC, Wang HY. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology 54: 1620–1630, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Chen C, Gong W, Li Y, Edin ML, Zeldin DC, Wang DW. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther 339: 451–463, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One 7: e39165, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaka T, Atsumi A, Matsumori R, Nie T, Shinozaki H, Suzuki-Kemuriyama N, Kuba M, Nakagawa Y, Ishii K, Shimada M, Kobayashi K, Yatoh S, Takahashi A, Takekoshi K, Sone H, Yahagi N, Suzuki H, Murata S, Nakamuta M, Yamada N, Shimano H. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology 56: 2199–2208, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Milic S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis 30: 158–162, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. EET displays anti-inflammatory effects in TNF-alpha stimulated human bronchi: putative role of CPI-17. Am J Resp Cell Mol Biol 38: 192–201, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Moshal KS, Zeldin DC, Sithu SD, Sen U, Tyagi N, Kumar M, Hughes WM Jr, Metreveli N, Rosenberger DS, Singh M, Vacek TP, Rodriguez WE, Ayotunde A, Tyagi SC. Cytochrome P450 (CYP) 2J2 gene transfection attenuates MMP-9 via inhibition of NF-kappabeta in hyperhomocysteinemia. J Cell Physiol 215: 771–781, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oz HS, Im HJ, Chen TS, de Villiers WJ, McClain CJ. Glutathione-enhancing agents protect against steatohepatitis in a dietary model. J Biochem Mol Toxicol 20: 39–47, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathil A, Mueller J, Warth A, Chamulitrat W, Stremmel W. Ursodeoxycholyl lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology 55: 1369–1378, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem 277: 15671–15676, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Rabelo F, Oliveira CP, Faintuch J, Mazo DF, Lima VM, Stefano JT, Barbeiro HV, Soriano FG, Alves VA, Carrilho FJ. Pro- and anti-inflammatory cytokines in steatosis and steatohepatitis. Obes Surg 20: 906–912, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horvath B, Mukhopadhyay B, Becker L, Hasko G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher P. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56: 2115–2125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro PS, Cortez-Pinto H, Sola S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol 99: 1708–1717, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Robinson SM, Mann DA. Role of nuclear factor kappaB in liver health and disease. Clin Sci (Lond) 118: 691–705, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52: 59–69, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 43: 163–172, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology 49: 87–96, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skepner JE, Shelly LD, Ji C, Reidich B, Luo Y. Chronic treatment with epoxyeicosatrienoic acids modulates insulin signaling and prevents insulin resistance in hepatocytes. Prostaglandins Other Lipid Mediators 94: 3–8, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol 7: 456–465, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Vaidyanathan V, Bastarrachea RA, Higgins PB, Voruganti VS, Kamath S, DiPatrizio NV, Piomelli D, Comuzzie AG, Parks EJ. Selective cannabinoid-1 receptor blockade benefits fatty acid and triglyceride metabolism significantly in weight-stable nonhuman primates. Am J Physiol Endocrinol Metab 303: E624–E634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valle A, Catalan V, Rodriguez A, Rotellar F, Valenti V, Silva C, Salvador J, Fruhbeck G, Gomez-Ambrosi J, Roca P, Oliver J. Identification of liver proteins altered by type 2 diabetes mellitus in obese subjects. Liver Int 32: 951–961, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Videla LA, Tapia G, Rodrigo R, Pettinelli P, Haim D, Santibanez C, Araya AV, Smok G, Csendes A, Gutierrez L, Rojas J, Castillo J, Korn O, Maluenda F, Diaz JC, Rencoret G, Poniachik J. Liver NF-kappaB and AP-1 DNA binding in obese patients. Obesity (Silver Spring) 17: 973–979, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Wierzbicki AS, Oben J. Nonalcoholic fatty liver disease and lipids. Curr Opin Lipidol 23: 345–352, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Zhao G, Wang J, Xu X, Jing Y, Tu L, Li X, Chen C, Cianflone K, Wang P, Dackor RT, Zeldin DC, Wang DW. Epoxyeicosatrienoic acids protect rat hearts against tumor necrosis factor-alpha-induced injury. J Lipid Res 53: 456–466, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng C, Wang L, Li R, Ma B, Tu L, Xu X, Dackor RT, Zeldin DC, Wang DW. Gene delivery of cytochrome p450 epoxygenase ameliorates monocrotaline-induced pulmonary artery hypertension in rats. Am J Resp Cell Mol Biol 43: 740–749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]