Abstract
Most retinal bipolar cells (BCs) transmit visual input from photoreceptors to ganglion cells using graded potentials, but some also generate calcium or sodium spikes. Sodium spikes are thought to increase temporal precision of light-evoked BC signaling; however, the role of calcium spikes in BCs is not fully understood. Here we studied how calcium spikes and graded responses mediate neurotransmitter release from Mb-type BCs, known to produce both. In dark-adapted goldfish retinal slices, light induced spikes in 40% of the axon terminals of intact Mbs; in the rest, light generated graded responses. These light-evoked membrane potentials were used to depolarize axotomized Mb terminals where depolarization-evoked calcium current (ICa) and consequent exocytosis-associated membrane capacitance increases (ΔCm) could be precisely measured. When evoked by identical dim light intensities, spiking responses transferred more calcium (QCa) and triggered larger exocytosis with higher efficiency (ΔCm/QCa) than graded potentials. QCa was translated into exocytosis linearly when transferred with spikes and supralinearly when transferred with graded responses. At the Mb output (ΔCm), spiking responses coded light intensity with numbers and amplitude whereas graded responses coded with amplitude, duration, and steepness. Importantly, spiking responses saturated exocytosis within scotopic range but graded potentials did not. We propose that calcium spikes in Mbs increase signal input-output ratio by boosting Mb glutamate release at threshold intensities. Therefore, spiking Mb responses are suitable to transfer low-light-intensity signals to ganglion cells with higher gain, whereas graded potentials signal for light over a wider range of intensities at the Mb output.
Keywords: bipolar cell, light response, calcium spike, graded potential, threshold
retinal bipolar cells (BCs) convey light information from photoreceptors to ganglion cells (GCs), connecting the input and output elements of the retina. While the classical view is that BCs operate with analog, graded potentials (Kaneko 1970; Werblin and Dowling 1969), a growing body of evidence suggests that certain BCs are capable of generating calcium (Ca2+) or sodium (Na+) action potentials (Baden et al. 2011, 2013a; Dreosti et al. 2011; Puthussery et al. 2013; Saszik and DeVries 2012) and employ digital signals to process information at the second stage of the visual pathway (Baden et al. 2013b). When present, voltage-gated Na+ currents amplify and speed up BC responses and subsequently enhance the temporal precision of postsynaptic GC signaling (Ichinose et al. 2005; Puthussery et al. 2013; Saszik and DeVries 2012). Less is understood concerning the role of Ca2+ spike-mediated digital BC signaling. In Mb-type BCs that lack voltage-gated Na+ currents (Zenisek et al. 2001), Ca2+ spikes promote larger and faster somatic light responses (Protti et al. 2000), suggesting similar roles for Ca2+ and Na+ spikes in BCs (Baden et al. 2013b). Importantly, in the vertebrate retina every BC expresses voltage-gated Ca2+ channels that are essential for glutamate release, but so far only a few types of BCs, in fish and mouse retinas, have been shown to generate light-evoked Ca2+ spikes (Baden et al. 2011, 2013a; Dreosti et al. 2011). When present, Ca2+ spikes in BCs originate at the site of presynaptic glutamate release (Baden et al. 2011; Burrone and Lagnado 1997; Dreosti et al. 2011; Palmer 2006; Protti et al. 2000), which implicates a potential role for Ca2+ spike-coded digital signaling in mediating BC output (Zenisek and Matthews 1998).
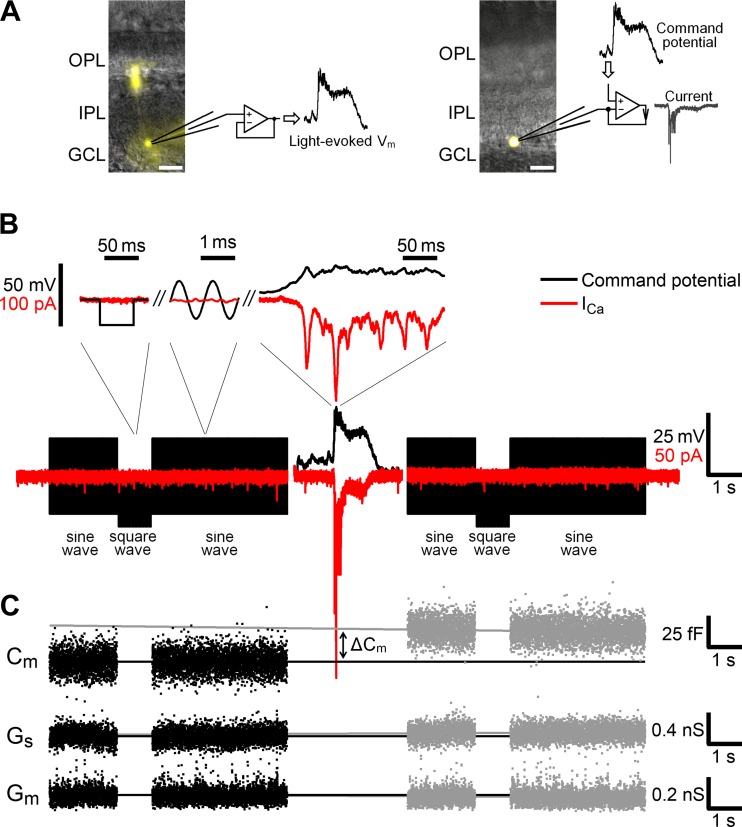
To investigate this notion, we sought to measure light-evoked glutamate release from ON-type Mb BCs, which have been shown to produce both Ca2+ spikes and graded potentials upon light stimulation of the retina (Baden et al. 2011; Joselevich and Kamermans 2007; Protti et al. 2000; Saito et al. 1979). Furthermore, Mb axon terminals are large enough for direct measurement of exocytosis-associated increases in their membrane capacitance (Cm) with high fidelity (von Gersdorff and Matthews 1999). Although the complex morphology of intact BCs can be considered during Cm measurements (Mennerick et al. 1997; Oltedal et al. 2007), the slowly decaying light-evoked synaptic currents (Saito et al. 1979) might prevent a reliable measurement of Cm changes (Gillis 2000) associated with light responses of Mbs. Therefore, first, light-evoked membrane potential (Vm) changes were recorded directly from the axon terminals of intact Mbs. Then, in consecutive experiments, these light-evoked signals were used to trigger exocytosis from axotomized Mb terminals (Fig. 1A) with compact, spherical morphology that favors precise Cm measurements (Palmer et al. 2003).
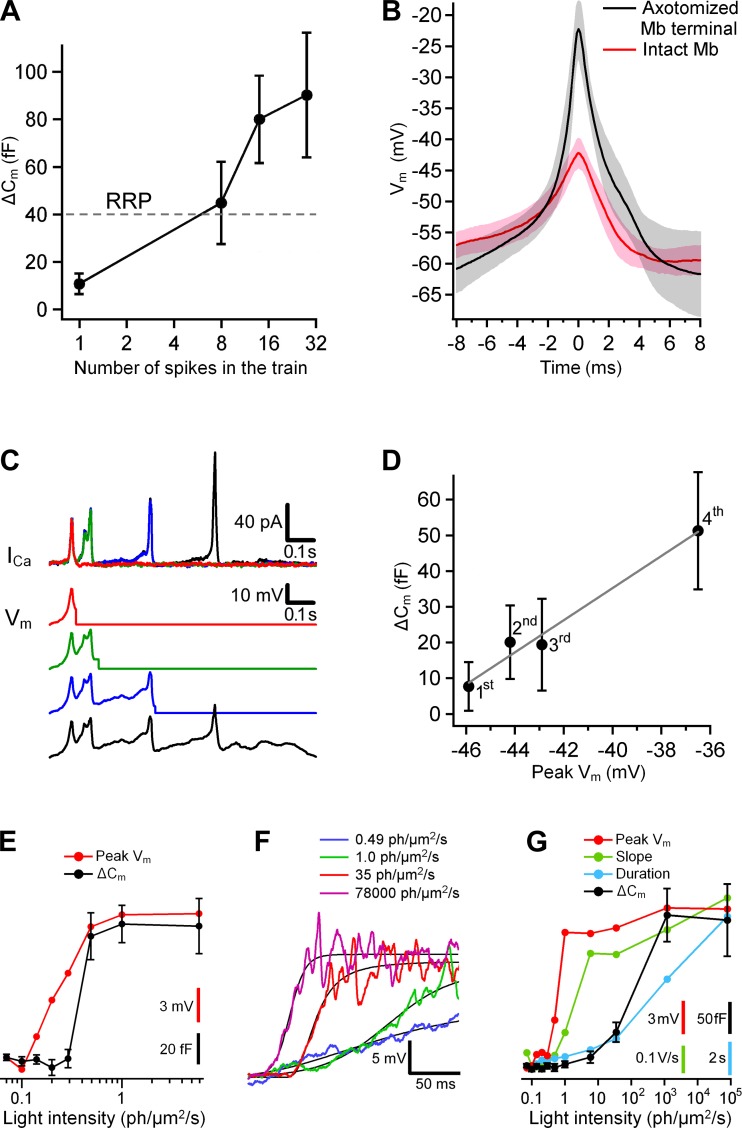
Fig. 1.
Experimental procedure. A: a light-evoked membrane potential (Vm) was recorded from an axon terminal of an intact Mb in current-clamp mode (left) and injected as a command potential into an axotomized terminal of another Mb in voltage-clamp mode (right). Alexa Fluor 488-filled Mbs (yellow) were imaged at the end of experiments. IPL, inner plexiform layer; OPL, outer plexiform layer; GCL, ganglion cell layer. Scale bars, 20 μm. B, bottom: injected light-evoked Vm (black, center) was flanked by sequences of sine and square waves. Top: the calculated Ca2+ current (ICa, red) in response to injected Vm (black) is shown on a fine timescale. Note that the ICa was noticeable when induced with light-evoked Vm but not with sine or square waves. C: trends of the membrane capacitance (Cm) before (black) and after (gray) injection of a light-evoked Vm were linearly extrapolated, and the difference in ΔCm at the peak magnitude of the ICa represented exocytosis. Trends of the series conductance (Gs, middle) and membrane conductance (Gm, bottom) were analyzed similarly.
We show that at low light intensities spiking Mb responses trigger an inward Ca2+ current (ICa) and exocytosis (ΔCm) more effectively than graded responses, ultimately conferring higher sensitivity to visual processing. However, graded Mb responses maintain the ability to code for luminance at bright intensities where spiking responses saturate the glutamate output from Mb terminals. We propose that spiking and graded responses in Mb BCs play a complementary role in coding luminance to maximize signal transfer to GCs over the physiologically relevant range of light intensities.
MATERIALS AND METHODS
Retinal slice preparation.
All experimental procedures were reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee, and all procedures met US Public Health Service guidelines. All procedures were performed under infrared (IR) light (940 nm) at room temperature (23 ± 1°C). Dark-adapted goldfish (Carassius auratus) of either sex, ∼10 cm long, were killed. The retina was dissected in Ames medium supplemented with 1 mM EGTA (osmolarity 260 mosM) and was incubated in the dissection medium supplemented with 0.73 mg/ml hyaluronidase for 25 min. Then, retinal pieces were attached to a filter paper (GSWP; Millipore) and cut into 250-μm-thick slices with a tissue chopper (model ST-20-S; Narishige). The retinal slices were transferred into a recording chamber and superfused by gravity at 1.5 ml/min with bicarbonate-buffered Ames medium supplemented with CaCl2 to contain a total of 2.3 mM Ca2+ (osmolarity 260 mosM). Both dissection and recording media were continuously gassed with 95% O2-5% CO2 throughout the entire experiment. The retinal slices were visualized with an upright microscope (Axioskop; Zeiss) with a custom-built infrared LED (940 nm; Osram) light source through a ×40 water-immersion objective coupled to a ×2.5 magnification Optovar (Zeiss) and camera (AxioCam; Zeiss).
Light stimulation.
The retinal slices were stimulated with 500-ms-long full-field green light (505 ± 5 nm) stimuli with an LED (American Bright Optoelectronics) positioned 3 cm above the preparation at a 30° angle. The LED voltage was controlled by the EPC-10 (HEKA Electronik) through D/A output. The intensity of light stimuli (in photons·μm−2·s−1) was 0.07, 0.10, 0.14, 0.20, 0.29, 0.49, 1.0, 5.9, 35, 1,200, and 78,000, calibrated with an optical meter (model 1918-C, Newport) with the sensor (918D-SL-OD3, Newport) positioned at the location of the specimen on the microscope stage. The time interval between consecutive light stimuli was 30 s at intensities of 0.07–0.20 photons·μm−2·s−1, 1 min at intensities of 0.20–1.0 photons·μm−2·s−1, and 2 min at intensities of 1.0–78,000 photons·μm−2·s−1. The time intervals between injections of the light-evoked waveforms into axotomized terminals matched those between corresponding light stimuli.
Electrophysiology.
Axon terminals of Mbs were targeted in the inner plexiform layer (IPL) based on their proximity to the GC layer, size of ∼8 μm, and bulbous morphology. The axon terminals were patched with 9- to 12-MΩ microelectrodes pulled from 1.5-mm borosilicate glass capillaries (World Precision Instruments) on a horizontal puller (model P-97; Sutter Instrument) and coated with dental wax (Cavex) to reduce pipette capacitance. For current-clamp recordings, microelectrodes were filled with potassium-based internal solution containing (in mM) 106 potassium gluconate, 10 potassium chloride, 2 EGTA, 10 phosphocreatine-di(tris), 3 Mg-ATP, 0.5 Na3-GTP, 3 l-ascorbic acid, 0.04 Alexa Fluor 488, 13 NaOH (pH 7.25, osmolarity 260 mosM). For voltage-clamp recordings, we used a cesium-based internal solution containing (in mM) 102 cesium gluconate, 10 TEA-Cl, 2 EGTA, 10 phosphocreatine-di(tris), 3 Mg-ATP, 0.5 Na3-GTP, 3 l-ascorbic acid, 0.04 Alexa Fluor 488, 13 NaOH (pH 7.25, osmolarity 260 mosM). In both internal solutions, the concentration of EGTA was 2 mM, approximating the buffering capacity of soluble endogenous Ca2+ buffers (Burrone et al. 2002). In voltage-clamp recordings, Ames medium was supplemented with picrotoxin (PTX, 100 μM) to block GABA-gated chloride conductances in Mb terminals (Vigh et al. 2011; Vigh and von Gersdorff 2005).
To discriminate terminals of intact Mbs from axotomized terminals whose axon was severed during the slicing process, we analyzed the current response to a command voltage pulse from −60 mV to −80 mV according to Palmer et al. (2003). Mb morphology was also confirmed occasionally by fluorescent imaging (Fig. 1A). Recordings with series resistance (Rs) >30 MΩ were excluded from further analysis. If Rs did not exceed 30 MΩ, the maximal slope of ICa activation remained well below 1/Rs (Tooker et al. 2013), indicating a good voltage clamp (Marty and Neher 1995). Rs < 30 MΩ was not electronically compensated because it contributed <1% to membrane resistance (Rm) in axotomized Mb terminals (∼3–4 GΩ; Palmer et al. 2003); therefore the compensation did not offer significant advantage. Reported Vm values were corrected for liquid junction potentials (11.8 mV for potassium-based and 11.9 mV for cesium-based internal solutions).
Ames medium was purchased from USBiological, PTX from Tocris Bioscience, Alexa Fluor 488 from Invitrogen, and all other reagents from Sigma-Aldrich. To clamp either voltage or current, we used an EPC-10 USB patch-clamp amplifier controlled by PatchMaster software, version 2.30 (both HEKA Elektronik).
Data analysis.
The light-evoked potentials, injected into axotomized terminals, were flanked by sequences of sine waves (1 kHz, 30 mV peak to peak superimposed on the holding potential of −60 mV) and square pulses (20-Hz steps to −80 mV from the holding potential of −60 mV) (Fig. 1B). Current response to the sine wave was measured by the lock-in amplifier in PatchMaster to calculate Cm (Gillis 2000). The slow capacitance was compensated immediately before injection of the left flanking sequence. Exocytosis, triggered by a light-evoked voltage waveform, was calculated as the difference of linearly extrapolated capacitances from right and left flanks (ΔCm) (Fig. 1C). Extrapolation was necessary to exclude potential interference with (fast) endocytosis, especially when measuring ΔCm triggered by bright light-evoked voltage waveforms, lasting for several seconds. To exclude artifacts in the capacitance measurements, series and membrane conductance (Gs and Gm, respectively) were also analyzed (Gillis 1995, 2000; Neves and Lagnado 1999).
ICa was calculated by subtracting the leak current from the recorded current (Fig. 1B, top right). To calculate the leak current, which consists of resistive and capacitive components, we utilized the linear properties of a cell membrane demonstrated by Armstrong and Bezanilla (1974). First, we averaged the current response of the Mb terminal to the square-wave voltage pulses (Fig. 1B, top left) to get a step response; the averaging of 40 current responses reduced the noise power of the resulting step response to 1/40 or 2.5% of that of a single current trace. Second, we differentiated the step response to obtain the impulse response, since the step response of a linear system is the integral of its impulse response with respect to time (Mao et al. 2002). Third, we calculated the leak current by convolving the impulse response and injected command potential. The conventional P/n method of calculating the leak current requires n repetitive injections of a command potential downscaled by a factor of n (Armstrong and Bezanilla 1974). For example, at commonly used n = 4 and with duration of a command potential of 12 s, implementing the P/n method would require 48-s-long recordings and, in addition, would increase the noise power of the ICa by 400%. Comparatively, when using convolution with the impulse response, 1-s recordings only increase the noise power of the ICa by 2.5%. Furthermore, the electric properties of a cell may change during a long P/n protocol, resulting in an inaccurate calculation of the leak current. Thus the method we used for calculating the leak current has two distinct advantages over the P/n method: 1) shorter time requirement for testing the electrical properties of a cell and 2) less noise added to the ICa.
The transferred Ca2+ charge (QCa) was determined by integration of the leak-subtracted ICa over time. Note that the inward ICa traces were inverted in Figs. 5, 6, 7, and 10 for better comparison with the depolarizing command potentials that triggered them; QCa is presented as a positive value throughout this report.
Fig. 5.
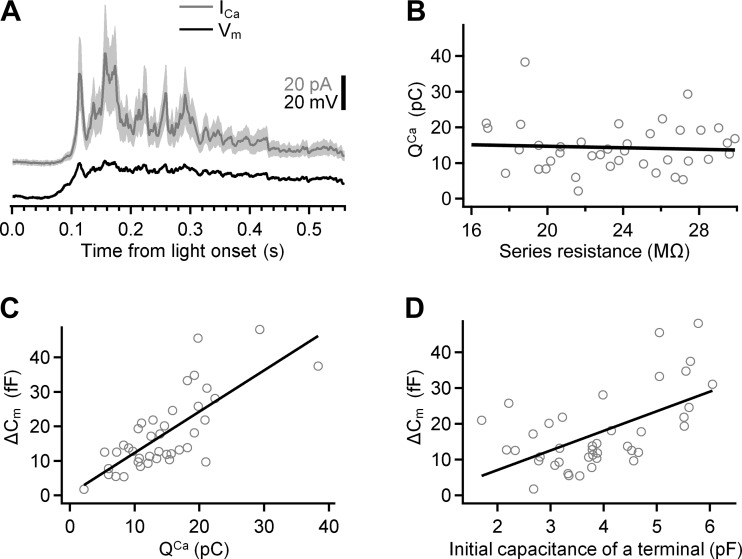
Variability in ICa and exocytosis in axotomized Mb terminals. A: mean ICa (gray) in response to injection of a light-evoked Vm (black) into axotomized Mb terminals (n = 40). The shaded region around the mean shows ±SD. The polarity of the inward ICa was reversed for easier side-by-side comparison with the Vm. The Vm was recorded from axon terminal of Mb10 in response to light with intensity of 5.9 photons·μm−2·s−1. The ratio of the mean ICa to its SD for the duration of the response was 2.1 ± 0.2. B: the transferred Ca2+ charge (QCa) did not correlate with the series resistance: the Pearson's correlation coefficient was −0.06 ± 0.16. C: the exocytosis (ΔCm) strongly correlated with QCa: the slope of the linear fit (black) was 1.20 ± 0.17 fF/pC; the Pearson's correlation coefficient was 0.75 ± 0.11. D: the exocytosis (ΔCm) strongly correlated with the initial capacitance of axotomized terminals: the slope of the linear fit (black) was 5.5 ± 1.3 fF/pF; the Pearson's correlation coefficient was 0.56 ± 0.14.
Fig. 6.
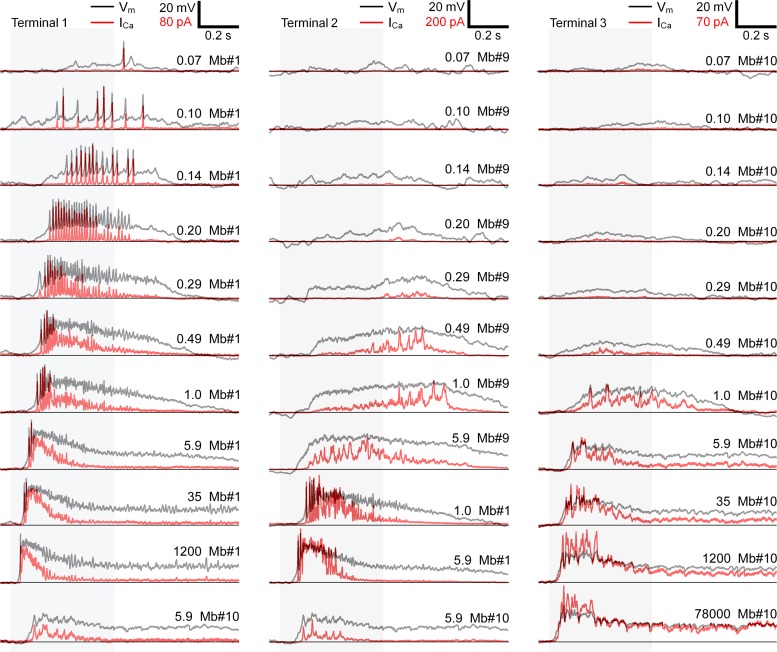
ICa of representative axotomized Mb terminals triggered by injection of light-evoked Vm changes. Light intensity corresponding to the shaded areas (in photons·μm−2·s−1) and Mbs' ID numbers are shown at top right of each Vm trace (black). The polarity of the inward ICa (red traces) was reversed for easier side-by-side comparison with the corresponding Vm. Terminal 1 (left) was tested with spiking responses of Mb1 (see Fig. 2) to light with intensities of 0.07–1,200 photons·μm−2·s−1 and with graded response of Mb10 to light with intensity of 5.9 photons·μm−2·s−1. Terminal 2 (center) was tested with graded responses of Mb9 to light with intensities of 0.07–5.9 photons·μm−2·s−1, 2 spiking responses of Mb1 to light with intensities of 1.0 and 5.9 photons·μm−2·s−1, and graded response of Mb10 to light with intensity of 5.9 photons·μm−2·s−1. Terminal 3 (right) was tested with graded responses of Mb10 to light with intensities of 0.07–78,000 photons·μm−2·s−1. Horizontal black lines show zero baseline for ICa and resting potential −63 mV for Vm.
Fig. 7.
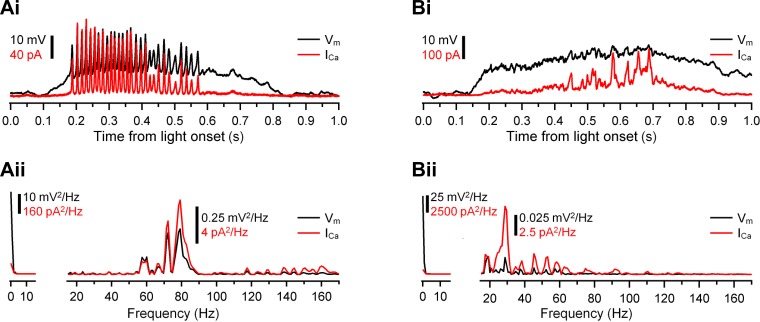
Ca2+ channels act as a high-pass filter of light-evoked Vm changes. Ai and Bi: Ca2+ transients (ICa, red) induced by injection of spiking (Ai) and graded (Bi) light-evoked Vm (black) into axotomized Mb terminals. Traces were reproduced from Fig. 6: Mb1 at 0.20 photons·μm−2·s−1 (Ai) and Mb9 at 0.49 photons·μm−2·s−1 (Bi). Note that ICa barely follows the sustained potentials. Aii and Bii: power spectrum of the Vm (black) and ICa (red) shown in Ai and Bi fell into 2 distinctly separated frequency bands: 0–16 Hz and 16–170 Hz. The 0–16 Hz band contained low-frequency components, whereas the 16–170 Hz band contained frequencies of the spike train (Aii) and Vm oscillations on top of graded potential (Bii). The normalized power of ICa was greater than that of Vm: 41.3 ± 0.5% vs. 6.4% (P = 0.001, n = 19) for the spiking response and 7.5 ± 0.7% vs. 0.2% (P = 0.02, n = 18) for the graded response.
Fig. 10.
Features of light-evoked spiking and graded Mb responses that affect exocytosis. A: the ΔCm triggered with light-evoked spike trains in axotomized terminals increased with the rise in the number of spikes in the trains: 10.8 ± 4.3 fF (1 spike) < 44.9 ± 17.3 fF (8 spikes) < 80.0 ± 18.4 fF (14 spikes) < 90.2 ± 26.1 fF (28 spikes); P ≤0.05 for each pair of means (Newman-Keuls test, n = 19). Horizontal dashed line shows the approximate size of the rapidly releasable pool (RRP). B: the 1st spike in a train induced by current injection in axotomized terminals (n = 10, black) and evoked by light in terminals of intact Mbs (n = 10, red). Shaded areas are within SD about means. C, bottom: the cuts of a light-evoked spike train recorded from an intact Mb. The cut of the 1st spike (red) overlies the cut of the 1st and 2nd spikes (green), which overlies the cut of the 1st, 2nd, and 3rd spikes (blue), which in turn overlies the cut of all 4 spikes in the train (black). Top: ICa in a representative axotomized Mb terminal in response to the cuts of the spike train shown below. D: the exocytosis per spike (black) triggered in axotomized Mb terminals (n = 16) vs. the amplitude of a spike in a train shown in C. The spikes' ordinal numbers are shown at the data points. The slope of the linear fit (gray) is 4.50 ± 0.48 fF/mV. E: the exocytosis (black) triggered in axotomized Mb terminals (n = 18) by the graded light-evoked potentials from Mb9 (see Fig. 6). Peak amplitude of the light-evoked potentials and its scale bar are shown in red. F: the rising phase of the graded potentials from Mb10 evoked by light with intensities of 0.49 (blue), 1.0 (green), 35 (red), and 78,000 (magenta) photons·μm−2·s−1. The fits (black) were done with the Hill equation. G: the exocytosis (black) triggered in axotomized Mb terminals (n = 10) by the graded light-evoked potentials from Mb10 (see Fig. 6). Peak amplitude of the light-evoked potentials and its scale bar are shown in red. The maximum slope of the potentials (from the Hill's fit) and its scale bar are shown in green. The duration of the light responses (above the threshold for ICa of −55 mV) and its scale bar are shown in blue.
To fit QCa and ΔCm, we used the Hill equation:
where R is the response (either QCa or ΔCm), Rmax is the maximal response, I is the intensity of the light stimulus, I1/2 is the intensity at half-maximal response, and h is the Hill coefficient. The exocytosis triggered by light-evoked single spikes in a spike train (see Fig. 10, C and D) was calculated as follows: from the light-evoked membrane potentials command potentials were generated to include the first, first and second, first, second, and third spikes, and so on. ΔCm triggered by the second spike in a train was calculated as a difference in the ΔCm triggered by the command potential including the first two spikes and the first spike only. Accordingly, ΔCm triggered by the kth spike in a train was calculated as a difference in the ΔCm triggered by command potentials including k and k − 1 spikes. Off-line analysis was performed in IGOR Pro 6.1 (WaveMetrics) with custom-designed procedures and PPT 2.13a (http://www3.mpibpc.mpg.de). The probability (P) that means are equal values was calculated with paired Student's t-test when measured on the same Mb terminal or unpaired Student's t-test when measured on different terminals. Data shown are means ± SD.
RESULTS
Light triggered both spiking and graded responses at axon terminals of Mb-type bipolar cells.
Whole cell current-clamp recordings were made directly from the large axon terminals of intact Mbs in a goldfish retinal slice preparation (Fig. 1A, left). Whole cell configuration was established in voltage-clamp mode at −60 mV. After switching to current-clamp mode, at zero holding current, the Vm of all tested Mb terminals (n = 7) underwent frequent spontaneous depolarizations with amplitudes up to −30 mV (data not shown). The presence of similar spontaneous, Ca2+-driven depolarizations/spikes has been reported in cultured, solitary Mbs (Burrone and Lagnado 1997; Zenisek and Matthews 1998) as well as in axotomized Mb terminals in a retinal slice preparation similar to ours (Palmer 2006), and the Vm threshold of the Ca2+ spikes of Mbs was determined to be −63 mV (Palmer 2006). Therefore, we set the liquid junction potential-corrected resting potential of Mbs to −63 mV (see materials and methods), which reliably prevented large spontaneous depolarizations and allowed us to identify light-evoked Vm in intact Mbs (n = 55 total). Note that the holding potential of Mbs is expected to influence their capability of producing Ca2+ spikes as well as spike amplitude: hyperpolarization eliminates spontaneous spikes, whereas depolarization facilitates spiking; however, gradually increasing the magnitude of membrane depolarization reduces spike amplitude until spiking responses are extinguished (Baden et al. 2011; Palmer 2006; Zenisek and Matthews 1998). The resting potential of −63 mV (achieved by appropriate holding current injection calibrated for every recorded intact Mb) remained within the range of Vm in which light-evoked spike activity has been observed in BCs (Baden et al. 2011). Coincidentally, this resting potential was also close to the resting potential values measured at the soma from dark-adapted mouse rod bipolar cells (RBCs) (−59 to −57 mV: Pang et al. 2004; −58 mV: Dunn et al. 2006; −61 mV: Arman and Sampath 2012), suggesting that it was a realistic resting potential value for ON BCs that process rod information in the vertebrate retina.
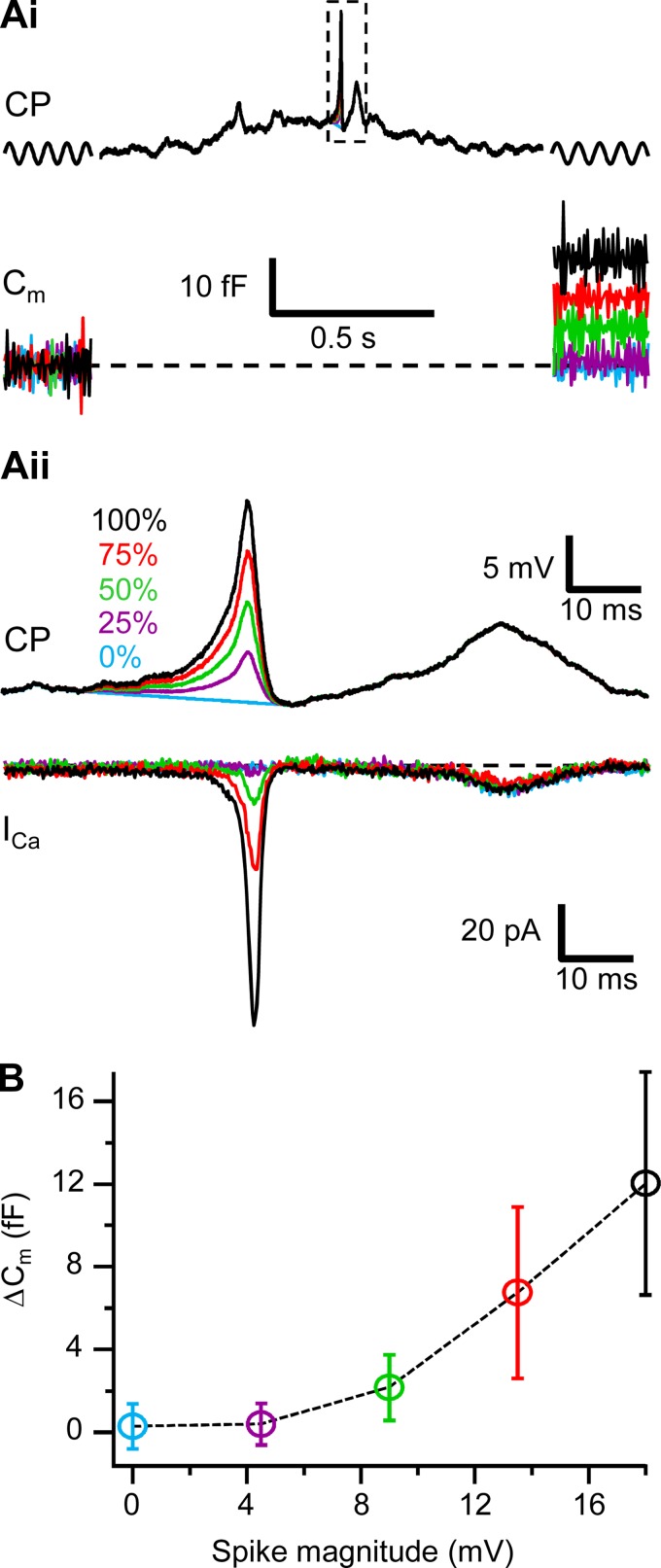
When dark-adapted retina slices were exposed to 500-ms stimuli of full-field green light (λ = 505 nm) in the range of 0.07–78,000 photons·μm−2·s−1 (see materials and methods), responses of Mb terminals to identical light stimulations showed a great variability, particularly at low intensities. Representative light responses are shown in Fig. 2. Overall, the light responses of Mb terminals contained a mixture of slow, graded, and transient spikelike Vm increases. Some of the transient Vm increases were large and appeared as spikes (Fig. 2; Mb1). However, these light-evoked spikes were clearly not as stereotypical as most all-or-nothing axonal Na+ spikes in CNS neurons: they varied in amplitude across cells, across intensities for a given cell, or even within a response of a cell to a single light stimulus. To sort these into somewhat objective categories, we defined spikes by setting an amplitude threshold on a functional basis. Accordingly, we sought to term a light-evoked transient (with a duration ≤5 ms at half-magnitude) Vm increase a “spike” if it was large enough to trigger exocytosis from Mb terminals. Note that in Mb terminals, light-evoked transient Vm increases most often arise on top of a depolarized (graded) platform (Fig. 2), supporting the notion that small depolarizations arising via synaptic inputs at the dendrites trigger self-propelling, robust Ca2+ channel opening at the axon terminal (Zenisek and Matthews 1998). Therefore, to determine the “functional spike threshold” we selected a representative light response with a single spike (Fig. 2, top left, Mb1, response to 0.07 photons·μm−2·s−1) and scaled down only the spike portion of it to 75% 50%, 25%, or 0% (no spike). These scaled waveforms, along with the original light response (100%), were injected into seven axotomized Mb terminals to determine the minimal spike amplitude that produced measurable ΔCm. The data are presented in Fig. 3. We found that single, light-evoked spikes in Mb terminals that produced small but measurable glutamate release, evidenced by ΔCm of 2.2 ± 1.6 fF (n = 7; Fig. 3B), had a minimal amplitude of 9 mV.
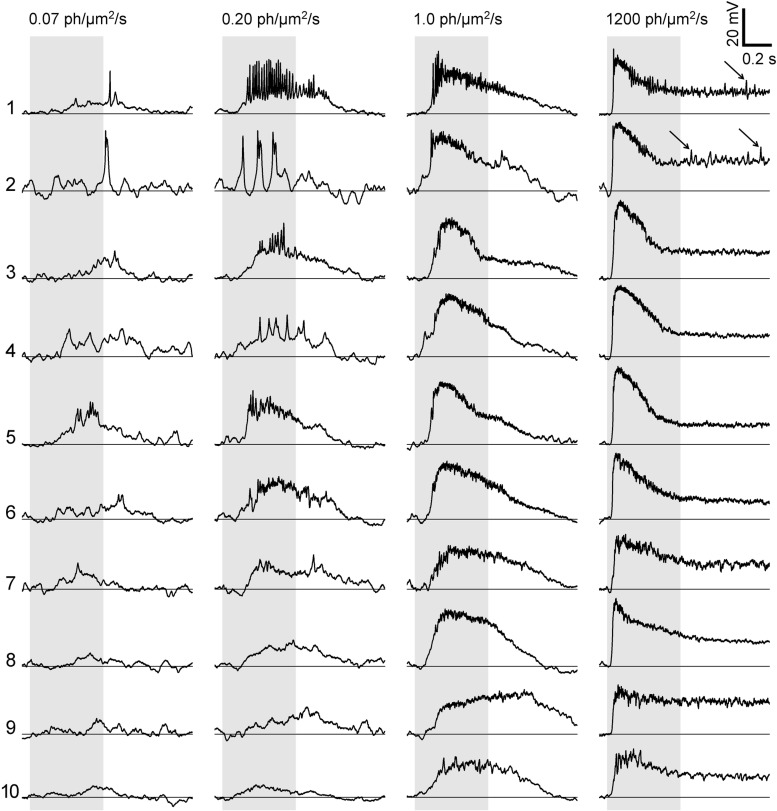
Fig. 2.
Light responses of Mb-type bipolar cells in dark-adapted retinas varied from spiking to graded. Vm in response to 500-ms-long light stimuli (shaded) recorded from axon terminals are shown for 10 representative Mbs. Light intensities (0.07, 0.20, 1.0, and 1,200 photons·μm−2·s−1) are shown at top. The Mbs' ID numbers (1–10) are shown on left. Light responses with voltage transients >9 mV were considered as spiking (Mb1–Mb7) and those with transients ≤9 mV as graded (Mb8–Mb10). Arrows point to small voltage transients during plateau phase of the light response (Mb1 and Mb10, 1,200 photons·μm−2·s−1).
Fig. 3.
Determining the functionally relevant spike magnitude. Ai, top: a light-evoked response with spike downscaled to 75%, 50%, 25%, and 0% of its original magnitude (dashed box, shown in detail in Aii), flanked with sine waves (shown schematically) was injected as a command potential (CP) into axotomized Mb terminals. Bottom: the exocytosis triggered in axotomized Mb terminals by the command potentials shown at top. Capacitance traces were averaged over 10 samples for better visibility. Noticeable exocytosis was triggered by the spike downscaled to 50% of its magnitude (green) but not with spikes downscaled to 25% (purple) or 0% (blue). Aii, top: expanded command potentials from dashed box shown in Ai. Bottom: ICa induced in axotomized Mb terminals by the command potentials shown at top. Note that the noticeable ICa is induced with spike downscaled to 50% (green) but not to 25% (purple) or 0% (blue). B: exocytosis triggered with the downscaled spikes (shown in Ai) in axotomized Mb terminals (n = 7). Note that noticeable exocytosis is triggered with spike with magnitude downscaled to 50% (green) but not 25% (purple) or 0% (blue).
Using our 9-mV amplitude threshold definition, we recorded spiking responses in 22 of 55 (40%) intact Mbs (Fig. 2, Mb1–Mb7); the rest (n = 33) did not produce voltage transients that met the criteria for a spike at any intensity (e.g., Mb8–Mb10, Fig. 2). The largest spikes were triggered particularly at intensities between 0.07 and 0.20 photons·μm−2·s−1. At light intensities ≥ 1.0 photons·μm−2·s−1, the number of spikes decayed as if several spikes converged to create a wide transient initial depolarization followed by a lower-magnitude sustained plateau response (best seen in Fig. 2 in responses triggered by 1,200 photons·μm−2·s−1). In some cells, small membrane oscillations were seen during this plateau phase of the response (Fig. 2, Mb1 and Mb2, arrows).
The presence or absence of the light-evoked spikes with magnitude ≥ 9 mV could not be explained by apparent differences in Mb morphology revealed by fluorescent imaging of Mbs that were loaded with Alexa Fluor 488 via the recording pipette (Fig. 4A). Note, however, that the resolution of these fluorescent images did not allow for the analysis of fine morphological parameters (i.e., size of dendritic field, axonal thickness, primary dendrite diameter) that could enable us to sort Mbs into distinct morphological subpopulations unequivocally (i.e., b1, b2, and b3; Ishida et al. 1980).
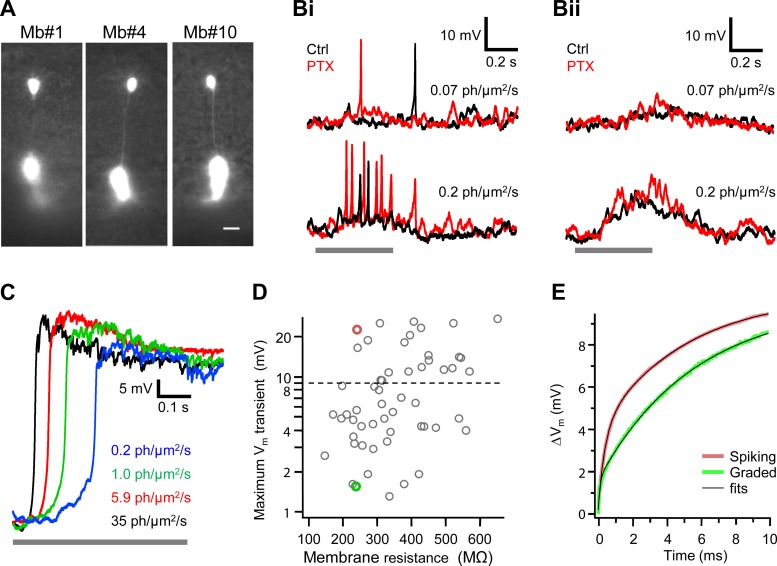
Fig. 4.
Mb-type bipolar cells with spiking and graded responses. A: superimposed fluorescent and infrared images of Mb-generated spiking (Mb1 and Mb4) and graded (Mb10) responses shown in Fig. 2. Scale bar, 10 μm. Note the similarity in morphology of Mb-generated spiking and graded responses. Bi and Bii: responses of Mb with spiking (Bi) and graded (Bii) membrane voltage (Vm) to light with intensities of 0.07 photons·μm−2·s−1 (top) and 0.2 photons·μm−2·s−1 (bottom) in control Ames medium (Ctrl, black) and in the presence of 100 μM picrotoxin (PTX, red). Note that although PTX potentiated light-evoked responses, it did not turn spiking into graded (Bi) or graded into spiking (Bii) Vm. C: Mb light responses with cesium-based internal solution in current-clamp mode. Under the block of potassium channels with Cs+ and TEA, Mbs responded to light with nonspiking sustained depolarization up to −9.7 ± 9.9 mV (n = 7) with duration exceeding that of the light stimulus applied between 0 and 0.5 s. D: maximum magnitude of light-evoked Vm transient weakly correlated with Mb membrane resistance at command voltage −60 mV (Pearson's correlation coefficient 0.34 ± 0.13). Horizontal dashed line shows 9 mV threshold for spiking potentials. The input resistance of the dark-adapted Mbs was 365 ± 140 MΩ (n = 55), similar to ∼444 MΩ measured at the soma by Protti et al. (2000). Red and green circles represent Mbs selected for further analysis shown in E. E: mean response (n = 20) to a current step (42 pA) of 2 Mbs with the same input resistance but generating different maximum light-evoked Vm transients: 22.5 mV (red) and 1.54 mV (green). Note that the Vm depolarization was consistently larger for the Mb generating spiking responses (red), reaching the maximal difference of 2 mV at 1.6 ms after current onset. Black traces show double-exponential fits. Based on the fit parameters, capacitances of the axon terminals of Mbs with spiking and graded responses were similar, 3.3 ± 0.1 pF vs. 3.1 ± 0.2 pF, whereas the axon resistance was larger for the Mb with spiking responses, 87.6 ± 0.5 MΩ vs. 36.0 ± 1.9 MΩ.
It is possible that the spiking light responses observed in some intact Mbs and the graded responses in others are a result of varying levels of inhibitory feedback from amacrine cells to Mb terminals in the inner retina, due to variation within our slices. Supporting this notion are the findings that GABA puff eliminated spontaneous Ca2+ spikes in solitary, cultured Mbs (Zenisek and Matthews 1998) and that the magnitude of lateral and reciprocal GABAergic feedback to Mb1 terminals in retina slice preparation showed a great variability (Vigh et al. 2011; Vigh and von Gersdorff 2005). However, blocking inhibitory GABAergic signaling by blockade of GABAA/C receptors with 100 μM PTX (Vigh et al. 2011; Vigh and von Gersdorff 2005) could not switch spiking light responses to graded ones and did not turn graded responses into spikes (Fig. 4, Bi and Bii, respectively). Note, though, the apparent changes in spiking and graded light responses upon eliminating inner retinal feedback: application of PTX that blocked amacrine cell feedback to the Mb terminals increased excitability of Mbs in terms of the number of spikes and amplitude of the graded depolarization similar to what was found for mammalian BCs (Euler and Masland 2000).
Voltage-gated K+ (IK) and big-conductance (BK) Ca2+-dependent K+ [IK(Ca)] currents are expressed by Mbs (Burrone and Lagnado 1997; Kaneko and Tachibana 1985; Palmer 2006; Sakaba et al. 1997; Zenisek and Matthews 1998) and were shown to influence the shape of spontaneous Ca2+ spikes in cultured, solitary Mbs (Burrone and Lagnado 1997) and in axotomized Mb terminals in a slice preparation (Palmer 2006). We found that in the presence of intracellular cesium, which blocks IK as well as IK(Ca), the light responses of intact Mbs were uniform: at every light intensity tested, after a steep rise up to −9.7 ± 9.9 mV (n = 7), all continued in a sustained depolarized plateau with a duration far exceeding the light stimulation (n = 7; Fig. 4C), similar to what has been reported for spontaneous depolarizations of solitary Mbs after blocking K+ conductances (Burrone and Lagnado 1997). Thus the propensity for generating light-evoked spiking responses appears to be heavily influenced by IK and/or IK(Ca) in Mbs. Nonetheless, it is not known whether or not the expression pattern of various types of voltage-gated K+ channels (Yazulla and Studholm 1998) and/or BK channels varies across ON type Mbs, and in the present study this was not investigated further.
Whether an intact Mb fires spikes or generates graded potentials may be related to the Rm. This biophysical parameter provides insight into how much leak is attributed to the Mb, beyond the site of the patch electrode. The holding current at the command voltage of −60 mV was −116.1 ± 44.4 pA for Mbs with graded responses (n = 33) and −71.9 ± 28.1 pA for those producing spiking responses (n = 22). Comparatively, these two mean holding current values were significantly different (P = 0.01). The maximal spike magnitude negatively correlated with holding current at the command voltage of −60 mV, as reflected by the Pearson's correlation coefficient of −0.38 ± 0.13 and the linear fit coefficient of −0.057 ± 0.019 mV/pA. Thus it appears that there is an inherent feature of spiking Mbs that results in more efficient handling of current flow through the cell and less current loss compared with those Mbs that exhibited graded responses. Coincidentally, the correlation between Rm of intact Mbs and the amplitude of spikelike transients (Fig. 4D) was weak (Pearson's correlation coefficient: 0.34 ± 0.13). Although these data collectively suggested that the presence and amplitude of spikes in the light responses of Mbs might depend on the Rm, clearly, some intact Mbs produced large spikes with low Rm (∼250 MΩ) and, vice versa, some intact Mbs with Rm of ∼500 MΩ responded to light with graded potentials (Fig. 4D). Therefore, whether an intact Mb produced spiking or nonspiking light responses could not be explained and/or predicted simply by this stationary biophysical parameter of the Mb membrane.
The generation of spikes is a dynamic process on a millisecond timescale. To test whether the temporal properties of the membranes of Mbs generating spikes differ from those producing graded potentials, we analyzed the time course of the Vm in response to 20 current steps with duration of 30 ms. Current injections were used to mimic the natural excitation of neurons and to minimize the effect of the Rs on the temporal properties of the cell's membrane. The amplitude of the current steps was set to depolarize the Vm of selected intact Mbs from −80 mV by ∼10 mV.
The two representative Mbs that were selected for this analysis generated different maximum light-evoked Vm transients, 22.5 mV vs. 1.54 mV (i.e., spiking vs. graded responses, respectively), but had similar Rm, 238 MΩ vs. 240 MΩ (shown in Fig. 4D as red and green circles, respectively). In these two cells, accordingly, the stationary membrane depolarization in response to a 42-pA current step was the same (10 mV). However, on the timescale matching the time course of a Ca2+ spike (with half-width of <5 ms), the initial membrane depolarization of the spiking Mb was consistently larger, by up to 2 mV at 1.6 ms after the current onset (Fig. 4E). Taking into account a sharp, e-fold increase in ICa magnitude for each 4.5–6.6 mV increase in Vm (Burrone and Lagnado 2000; Rieke and Schwartz 1996; Taylor and Morgans 1998), this 2-mV difference in Vm predicts up to 50% larger ICa for Mb with spiking responses that might facilitate generation of Ca2+ spike.
The Vm depolarization induced by the current steps was fitted with double exponentials. In the two-compartment electrical model of Mb developed by Mennerick et al. (1997), the fast time constant in current-clamp mode is the product of the axonal resistance and the capacitance of a terminal. We found the fast time constant larger for the Mb with spiking responses, 0.29 ms vs. 0.11 ms, although the capacitances of the axon terminals of the selected Mbs with spiking and graded responses were similar, 3.3 pF vs. 3.1 pF, respectively. This implies that the axon resistance was larger for the selected Mb with spiking responses compared with that with graded responses (88 MΩ vs. 36 MΩ, respectively). Indeed, the axonal resistance and magnitude of the maximal Vm transient across Mbs showed strong correlation: the Pearson's correlation coefficient was 0.91 ± 0.08 (n = 55). Therefore, the ability to generate spikes might be attributed to the Mb's intrinsic electrical properties. Intuitively, the larger axon resistance the greater electrical separation between the soma and axon terminal, resulting in less damping of the spike by the leak in the somatic compartment. This is in concert with the observation that in the axotomized Mb terminal (which is an extreme case of the infinite axonal resistance), current injection-evoked Ca2+ spikes are large (Palmer 2006). These results suggest that the axon resistance might be the best biophysical parameter to predict the propensity of Ca2+ spike production in Mbs. Whether or not this parameter defines different subpopulations of Mbs requires further investigation.
Despite the substantial variability, there were several features of the light responses that were common across all recorded Mb terminals (n = 55). The first was that every Mb terminal produced responses to intensities that activate rod photoreceptors (≤1.0 photons·μm−2·s−1), as is expected because of the extensive rod input Mbs reportedly receive (Ishida et al. 1980; Joselevitch and Kamermans 2007). It is important to point out that the overall light sensitivity of the dark-adapted Mbs in our hands (Fig. 2, left) matched previous reports addressing scotopic threshold intensities that evoked measurable graded Vm changes at the soma of ON Mbs and rod-driven horizontal cells in goldfish retina slices (Joselevitch and Kamermans 2007). The second shared feature was that responses at high photopic intensities (≥1,200 photons·μm−2·s−1; Fig. 2) were very similar across all Mbs and matched the shape of light responses obtained from Mbs via sharp electrode recording (Saito and Kujiraoka 1982). Third, responses triggered by the brightest intensity used in our experiments (78,000 photons·μm−2·s−1) only differed from those evoked by 1,200 photons·μm−2·s−1 in the length of the sustained plateau (16.8 ± 2.5 s vs. 7.3 ± 1.2 s, respectively; n = 24). Finally, the latency of the light responses, measured by the delay between light onset and the half-maximum magnitude, decreased with the increase in light intensity for spiking and graded responses (Fig. 2). However, even though the latency decreased with the increase of light intensity for both spiking and graded potentials, when compared, the latency was significantly lower (P = 0.01) for spiking responses than for graded ones: 124.1 ± 14.6 ms vs. 172.6 ± 26.1 ms at 1.0 photons·μm−2·s−1, 44.0 ± 5.4 ms vs. 67.7 ± 11.7 ms at 1,200 photons·μm−2·s−1, and 38.1 ± 2.9 ms vs. 44.3 ± 4.6 ms at 78,000 photons·μm−2·s−1. These data support the role of Ca2+ channels expressed at the axon terminals in mediating somatic BC responses (Euler and Masland 2000); specifically, our data show explicitly that spikes decrease the latency of light responses at the BC's axon terminal, similar to what was predicted for somatic BC responses (Protti et al. 2000; Zenisek and Matthews 1998).
Axotomized Mb terminals form a homogeneous population in terms of glutamate release in response to depolarization that is mimicking physiological signals.
Axotomized BC terminals offer a perfect presynaptic structure for voltage-clamped current and Cm measurements with high temporal resolution (Mennerick et al. 1997; Oltedal et al. 2007; Palmer et al. 2003). We used the light-evoked Vm waveforms, previously recorded from the axon terminal of intact Mbs, as command potentials in voltage-clamp protocols to trigger ICa and consecutive glutamate release in axotomized Mb terminals. Axotomized Mb terminals with round morphology (Palmer et al. 2003) were targeted in the innermost layer of the IPL (Fig. 1A). Extensive studies describing depolarization-evoked ICa and transmitter release, performed on dissociated, solitary Mbs, did not report multiple populations with regard to various aspects of the release (Burrone and Lagnado 2000; Grabner and Zenisek 2013; Heidelberger et al. 1994; Mennerick and Matthews 1996; Midorikawa et al. 2007; Neves and Lagnado 1999; von Gersdorff et al. 1996; von Gersdorff and Matthews 1994). However, a recent RT-PCR study of Ca2+ channel subunits of dissociated large-terminal, ON-type BCs revealed that they express transcripts of CaV1.3a- and/or CaV1.3b-type α-subunits (LoGiudice et al. 2006). Uneven expression pattern of Ca2+ channel subunits across Mb terminals might be responsible for the variability we found in the light responses (Fig. 2) and, in turn, might lead to differences in the depolarization-evoked transmitter release across terminals.
Therefore, first we tested whether the round, axotomized Mb terminals in slice preparation form a homogeneous population with regard to physiologically relevant depolarization-evoked Ca2+ influx (ICa) and subsequent glutamate release. To address this question, we selected a previously recorded nonspiking Vm, evoked by a bright scotopic stimulation (5.9 photons·μm−2·s−1, from Mb10; Fig. 5A). Using this waveform as a command potential in a voltage-clamp experiment, in the presence of Cs-based internal solution, we analyzed ICa and ΔCm triggered in 40 axotomized Mb terminals (see materials and methods and Fig. 1). In these experiments and every experiment presented from this point on, the GABA-mediated chloride current associated with reciprocal feedback from amacrine cells to depolarized Mb terminals was blocked with PTX (100 μM) (Vigh et al. 2011; Vigh and von Gersdorff 2005).
In response to the selected depolarizing potential, the amplitude of the ICa varied across the tested axotomized terminals (Fig. 5A, gray trace), as did the transferred Ca2+ charge (QCa) of 14.3 ± 6.8 pC (n = 40). QCa showed no correlation to the Rs: the Pearson's correlation coefficient was −0.06 ± 0.16, indicating that the variability did not result from a recording artifact introduced by the Rs (Fig. 5B). However, we found a strong correlation between QCa and the triggered ΔCm; the Pearson's correlation coefficient was 0.75 ± 0.11 (Fig. 5C). This strong correlation was entirely similar to what has been reported for the given range of ΔCm (∼50–100 fF) triggered by square depolarizing voltage steps driving QCa of 15–50 pC (von Gersdorff et al. 1998). The size of the Mb terminals, measured by the initial Cm before depolarization, correlated well with the ΔCm: the Pearson's correlation coefficient was 0.56 ± 0.14 (Fig. 5D). In simple terms, larger terminals produced larger exocytosis in response to the same depolarization (von Gersdorff et al. 1996) even if the depolarization closely mimicked physiologically relevant light responses.
Together, these results indicated that the properties of ICa and the exocytosis machinery are similar across all Mb terminals and that the axotomized Mb terminals can be used as a reliable, homogeneous model system to study exocytosis (Burrone and Lagnado 2000; Palmer 2006; von Gersdorff et al. 1998).
Voltage-gated Ca2+ channels high-pass filter light-evoked membrane potentials.
Next we sought to quantify the glutamate release from light-evoked spiking and graded Mb responses by voltage clamping axotomized Mb terminals with representative Mb responses. Among the spiking responses, we chose the light responses of Mb1 because this cell produced the most spikes at the intensities tested (see Fig. 2). For graded command potentials, we used the light responses from Mb9 and Mb10 (see Fig. 2). Mb9 produced the largest graded response at the dimmest stimulus intensity (0.07 photons·μm−2·s−1), which we interpreted as the most sensitive graded Mb response encountered in this study. Furthermore, when the light-induced depolarization was quantified by integrating the area between the line representing −63 mV (dark resting potential) and the actual light-evoked Vm, among all graded responses, the response of Mb9 was closest to that calculated for Mb1 at 0.07 photons·μm−2·s−1: 2.36 mV·s vs. 2.55 mV·s, respectively. In other words, Mb1 and Mb9 had similar magnitudes of depolarization, regardless of whether the response was spiking or graded. Mb10, on the other hand, was the least sensitive Mb encountered in this study with graded responses, producing the smallest depolarizations at dim intensities. Nonetheless, at 1.0 photons·μm−2·s−1 the overall light-induced depolarizations of Mb10 and Mb1 were remarkably similar: 10.31 mV·s vs. 11.36 mV·s. Note that 1.0 photons·μm−2·s−1 is still below the activation threshold of cones in the goldfish (Joselevitch and Kamermans 2007), indicating that both Mb1 and Mb10 received substantial rod input.
We used the light-evoked potentials of Mb1, Mb9, and Mb10 as command potentials to depolarize axotomized Mb terminals (n = 19, 18, and 10, respectively) and first analyzed the triggered ICa. Light-evoked spiking potentials of Mb1 induced transients in ICa closely following the pattern of voltage spikes (Fig. 6, terminal 1). Surprisingly, injection of graded potentials of Mb9 and Mb10 also induced fast transients in ICa that appeared to correlate with small oscillating components of graded potentials (Fig. 6, terminals 2 and 3). To analyze this interesting phenomenon quantitatively, we compared the Fourier transforms of representative spiking and graded light-evoked potentials (responses of Mb1 at 0.20 photons·μm−2·s−1 and Mb9 at 0.49 photons·μm−2·s−1, respectively) to the spectra of ICa they triggered (Fig. 7, Ai and Bi). The Fourier spectrums of the selected spiking and graded Vm responses contained most of the power (99.9%) in two clearly separated frequency bands: 0–16 Hz and 16–170 Hz. In the low frequency band (0–16 Hz), the normalized power of both spiking and graded Vm was greater than that of ICa: 93.6% vs. 58.7 ± 0.5% (P = 0.001, n = 19; Fig. 7Aii, left) and 99.8% vs. 92.5% ± 0.7% (P = 0.02, n = 18; Fig. 7Bii, left). On the contrary, in the high frequency band (16–170 Hz), the normalized power of ICa was greater than that of Vm: 41.3 ± 0.5% vs. 6.4% (P = 0.001, n = 19; Fig. 7Aii, right) for the spiking response and 7.5% ± 0.7% vs. 0.2% (P = 0.02, n = 18; Fig. 7Bii, right) for the graded response. These data provide strong quantitative support for the observation that sustained components of the light-induced Mb responses were underrepresented in ICa whereas the transient components (including spikes) were overrepresented. This overrepresentation can be interpreted as a preferential conversion of high-frequency light-evoked Vm changes into ICa rather than harmonic distortion by nonlinear Ca2+ channels. The presence of ICa transients is in concert with the steep rise of L-type ICa with a Vm increase: an e-fold increase in ICa magnitude was reported for each 4.5–6.6 mV increase in Vm (Burrone and Lagnado 2000; Rieke and Schwartz 1996; Taylor and Morgans 1998). Importantly, Rs in all recordings was kept below 30 MΩ (materials and methods). This ensured that erroneous clamping of ICa (Marty and Neher 1995) did not cause unwarranted sudden, sharp activation of ICa in response to gradual Vm changes of Mb terminals (Tooker et al. 2013).
The release machinery at the Mb axon terminals was shown to respond to a step depolarization in a manner similar to a high-pass filter (Burrone and Lagnado 2000). The results presented above mechanistically extend this notion by demonstrating that Ca2+ channels act as a high-pass filter for light-evoked Vm recorded at the presynaptic Mb terminal.
At the level of transmitter release, spiking bipolar cell responses code low light intensity with higher sensitivity, whereas graded ones code over a wider intensity range.
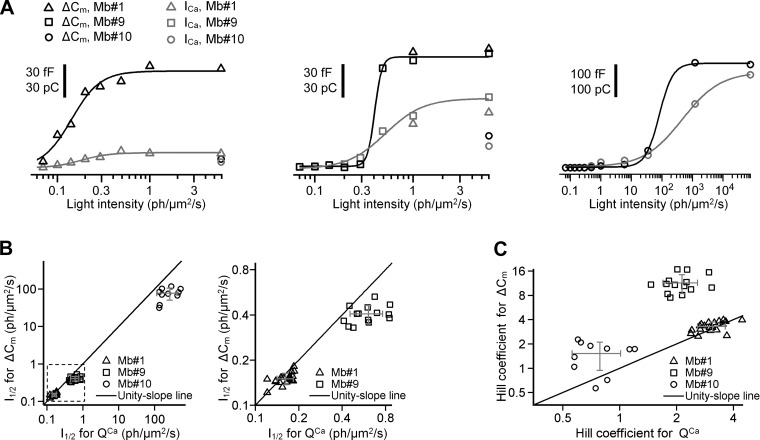
Next we analyzed the glutamate-releasing capability of spiking and graded Mb responses with respect to light intensity. Ca2+ charge (QCa) and exocytosis (ΔCm) triggered by selected representative light-evoked potentials (from Mb1, Mb9, and Mb10) were plotted as a function of light intensity and fit to the Hill equation. For each axotomized terminal (n = 19 for spiking Mb1, n = 18 for graded Mb9, and n = 10 for graded Mb10), we processed QCa and ΔCm (see materials and methods) to calculate the Hill coefficient (h) and the light intensity at the half-maximal response (I1/2) (Fig. 8A).
Fig. 8.
Exocytosis and Ca2+ influx triggered by light-evoked potentials. A: ΔCm (black) and QCa (gray) triggered by light-evoked potentials in axotomized Mb terminals 1 (left), 2 (center), and 3 (right). The initial capacitance of axotomized Mb terminals 1, 2, and 3 was 3.29, 4.38, and 2.71 pF, respectively. Fit was done with the Hill equation. For ΔCm, the intensity at half-maximal response (I1/2, in photons·μm−2·s−1) was 0.15 ± 0.01 for spiking potentials from Mb1, 0.41 ± 0.02 for graded potentials from Mb9, and 82 ± 25 for graded potentials from Mb10, whereas the Hill coefficients were 2.79 ± 0.50, 11.7 ± 2.9, and 1.9 ± 0.7, respectively. For QCa, I1/2 (in photons·μm−2·s−1) was 0.18 ± 0.02 for spiking potentials from Mb1, 0.52 ± 0.06 for graded potentials from Mb9, and 462 ± 70 for graded potentials from Mb10, whereas the Hill coefficients were 2.89 ± 0.83, 2.25 ± 0.50, and 0.70 ± 0.06, respectively. Note that spiking light-evoked potentials from Mb1 and the most light-sensitive graded potentials from Mb9 almost saturated exocytosis at similar level (∼90 fF) at similar light intensity (0.49 photons·μm−2·s−1). B, left: I1/2 (in photons·μm−2·s−1) for ΔCm vs. that of for QCa. Right: expanded area inside the dashed box shown on left. Note that for spiking potentials mean values of I1/2 for ΔCm and QCa were almost equal (P = 0.1). For graded potentials mean value of I1/2 for ΔCm was less than that of for QCa. C: Hill coefficient for ΔCm vs. that for QCa. Note that for spiking potentials mean values of the Hill coefficient for ΔCm and QCa were not significantly different (P = 0.1). For graded potentials, the mean value of the Hill coefficient for the ΔCm was greater than that for the QCa (P = 10−7 for Mb10 and P = 0.05 for Mb10). Gray error bars in B and C show SD about the mean.
In an intensity-response relation, h describes the power law of processing at the presynaptic terminal; h values > 2 indicate supralinear processing (Odermatt et al. 2012). I1/2, on the other hand, inversely reflects the light sensitivity—the less the I1/2 the higher the sensitivity (Odermatt et al. 2012). We found that spiking responses initiated ICa (QCa) at a significantly lower light intensity than the graded potentials (P = 10−12); I1/2 was 0.16 ± 0.02 photons·μm−2·s−1 for responses recorded from Mb1 vs. 0.61 ± 0.16 photons·μm−2·s−1 (Mb9) and 267 ± 152 photons·μm−2·s−1 (Mb10) (Fig. 8B). As expected, I1/2 for QCa of the most sensitive graded response, Mb9, was significantly less than that of the least sensitive graded response, Mb10: 0.61 ± 0.16 photons·μm−2·s−1 vs. 267 ± 152 photons·μm−2·s−1 (P = 10−5), representing a sensitivity difference of 2.6 log units. The nonlinearity in coding light intensity with Ca2+ influx at the Mb terminal was greater for the spiking responses of Mb1 compared with that of graded responses produced by Mb9 and Mb10, with h of 3.29 ± 0.46 (Mb1) vs. 2.14 ± 0.45 (Mb9) and 0.79 ± 0.23 (Mb10) (P = 0.001).
Importantly, spiking potentials triggered measurable exocytosis (ΔCm) at significantly (P = 10−12) lower light intensity than the graded ones: I1/2 was 0.15 ± 0.02 photons·μm−2·s−1 (Mb1) vs. 0.41 ± 0.06 photons·μm−2·s−1 (Mb9) and 77 ± 27 photons·μm−2·s−1 (Mb10) (Fig. 8B). The sensitivity difference seen in the light responses between the graded responses of Mb9 and Mb10 was expressed at the level of exocytosis: the I1/2 of the most sensitive graded Mb9 was significantly (P = 10−8) less than that of the least sensitive graded Mb10 (0.41 ± 0.06 vs. 77 ± 27 photons·μm−2·s−1, respectively). In other words, the difference in light sensitivity between the most sensitive (Mb9) and least sensitive (Mb10) graded responses was 2.3 log units at the level of glutamate output, similar to that found for their capability of inducing Ca2+ influx (2.6 log units).
I1/2 of QCa and ΔCm triggered by spiking potentials were similar: 0.15 ± 0.02 photons·μm−2·s−1 vs. 0.16 ± 0.02 photons·μm−2·s−1 (P = 0.06). In contrast, the I1/2 of the exocytosis for the graded potentials was significantly less than that of QCa: 0.41 ± 0.06 photons·μm−2·s−1 vs. 0.61 ± 0.16 photons·μm−2·s−1, P = 0.0002 (Mb9) and 77 ± 27 photons·μm−2·s−1 vs. 267 ± 152 photons·μm−2·s−1, P = 0.002 (Mb10). Note that graded potentials of Mb9 did not evoke measurable ΔCm at intensities < 0.3 photons·μm−2·s−1 but then at the next light intensity tested (0.49 photons·μm−2·s−1), the exocytosis (ΔCm) nearly saturated (Fig. 8A, center). However, QCa continued to increase with the light intensity through the intensity of 5.9 photons·μm−2·s−1, resulting in a shallower Hill slope and larger I1/2 (Fig. 8A, center). A similar phenomenon was observed in the data obtained using the graded light responses of Mb10, except that both QCa and ΔCm intensity/response curves are shifted to the right (Fig. 8A, right). Accordingly, when we compared nonlinearity of the exocytosis and transferred Ca2+ charge triggered by spiking and graded potentials, the Hill coefficients of ΔCm and QCa were not significantly different for spiking potentials: 3.29 ± 0.46 vs. 3.10 ± 0.52, P = 0.1. However, for the graded potentials, the Hill coefficient of exocytosis was significantly greater than that of the transferred Ca2+ charge: 11.4 ± 3.0 vs. 2.14 ± 0.45, P = 10−7 (Mb9) and 1.52 ± 0.58 vs. 0.79 ± 0.23, P = 0.05 (Mb10). This finding suggests that Ca2+ influx evoked by spiking Mb responses is translated into exocytosis linearly, whereas the Ca2+ signal evoked by graded Mbs undergoes supralinear amplification at the level of exocytosis (Fig. 8C).
Calcium spikes boost efficiency of exocytosis at low calcium influx.
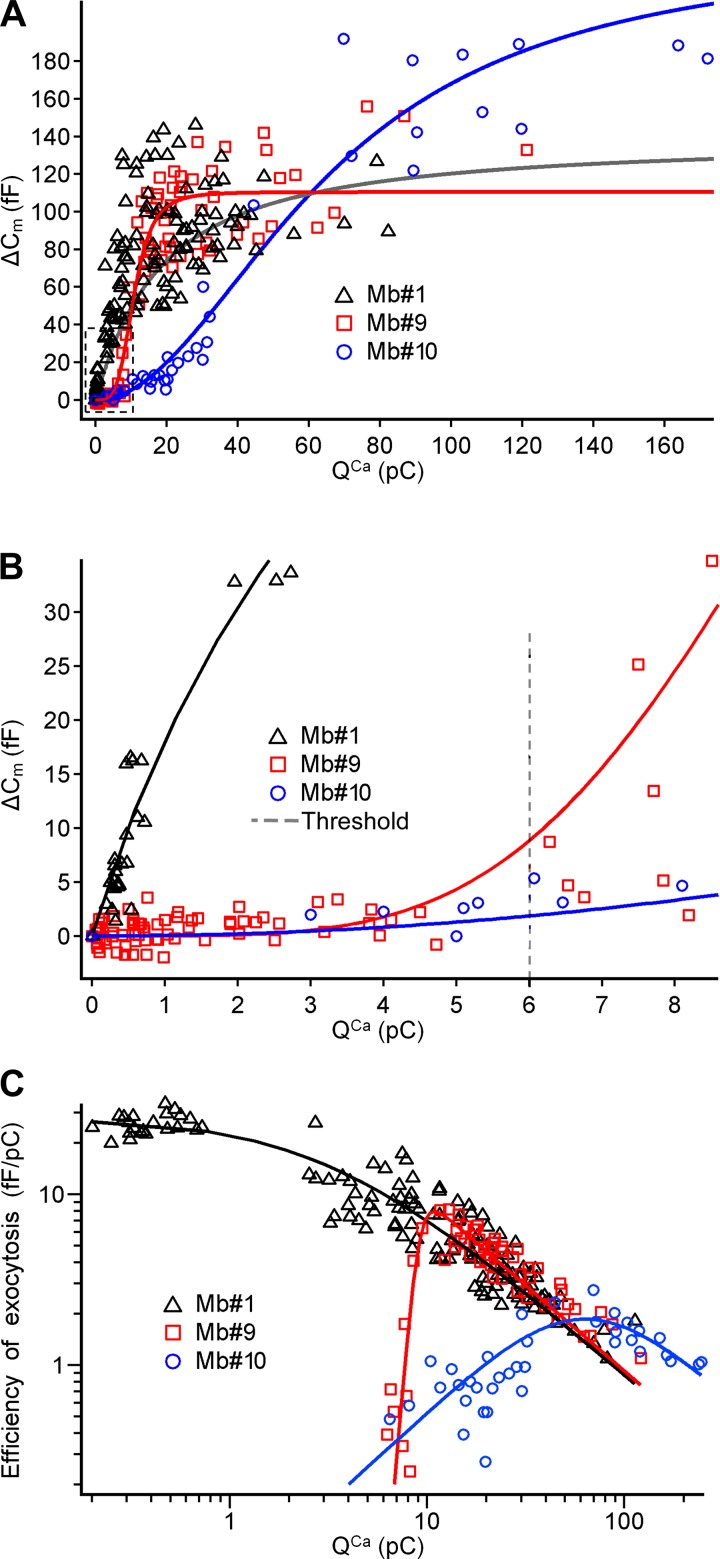
The quantitative relationship between the increase in intracellular Ca2+ concentration ([Ca2+]i) and the number of released synaptic vesicles in Mb terminals has been studied with a variety of techniques from a variety of angles (Burrone and Lagnado 2000; Grabner and Zenisek 2013; Heidelberger et al. 1994; Midorikawa et al. 2007; Tachibana et al. 1993; von Gersdorff et al. 1998; Zenisek and Matthews 2000). Here we revisited this relationship by triggering the Ca2+ influx and subsequent exocytosis with depolarizing commands that mimic light-evoked spiking (Mb1) and graded (Mb9, Mb10) Vm in response to 500-ms light stimuli (Fig. 9A). We found that the QCa transferred by spiking potentials triggered measurable exocytosis with a threshold of ∼0.2 pC (Fig. 9B) and was translated into exocytosis linearly, with a Hill coefficient of 0.95 ± 0.19. This is similar to what was found with short (5–20 ms) step depolarization of the Mb terminals from −60 mV to −10 mV (von Gersdorff et al. 1998), except that the single stepwise depolarizations resulted in slightly larger ΔCm than the light-evoked spike patterns used in our experiments. On the contrary, the QCa transferred with graded potentials was translated into exocytosis supralinearly, with Hill coefficients 4.14 ± 0.52 for Mb9 and 2.02 ± 0.17 for Mb10. Note that QCa transferred with the graded potentials did not trigger exocytosis below a threshold of ∼6 pC (Fig. 9B). The largest ΔCm value recorded in this study triggered by a spiking light response (Mb1, evoked by 5.9 photons·μm−2·s−1, causing QCa of 28.1 pC) was 145 fF, whereas it was 156 fF and 191 fF for graded responses (Mb9, evoked by 5.9 photons·μm−2·s−1 causing QCa of 76.3 pC, and Mb10, evoked by 78,000 photons·μm−2·s−1, causing QCa of 69.7 pC) (Fig. 9A). These values are in agreement with studies estimating that the total number of synaptic vesicles in the releasable pool is between 5,300 and 7,000 in Mbs: assuming the Cm of a single vesicle to be 26.4 aF, releasing all vesicles translates into a ΔCm value between 140 and 185 fF (Gomis et al. 1999; Neves and Lagnado 1999; Tachibana 1999; von Gersdorff et al. 1996), matching the data presented here.
Fig. 9.
Exocytosis triggered by Ca2+ influx in response command potentials generated from light responses. A: ΔCm triggered in axotomized Mb terminals by QCa transferred with light-evoked potentials recorded at axon terminals of Mb1 (black), Mb9 (red), and Mb10 (blue). Fits were done with the Hill equation. Hill coefficients were 0.95 ± 0.19, 4.14 ± 0.52, and 2.02 ± 0.17 for Mb1, Mb9, and Mb10, respectively. I1/2s were 6.2 ± 1.8, 10.8 ± 0.4, and 65.9 ± 5.4 pC for Mb1, Mb9, and Mb10, respectively. B: expanded region shown in dashed box in A. Vertical dashed line marks the threshold for QCa transferred with graded potentials. Note that below this threshold the QCa transferred with spikes triggered exocytosis, whereas that transferred with graded potentials did not. C: efficiency of the exocytosis for Mb1 (black), Mb9 (red), and Mb10 (blue) as a ratio of ΔCm to QCa using the Hill fits from A. For spiking potentials from Mb1, exocytosis was the most efficient when triggered with QCa of 0.34 ± 0.08 pC transferred with a single spike. For graded potentials from Mb9 and Mb10, exocytosis was the most efficient when triggered with QCa of 14.3 ± 1.9 and 66.7 ± 7.9 pC, respectively.
Next we calculated the efficiency of exocytosis triggered by spiking and graded light responses, by dividing each capacitance jump (ΔCm) by the corresponding Ca2+ charge (QCa) (Hull et al. 2006b). For the spiking potentials recorded from Mb1, the efficiency of the exocytosis reached its maximum of 25.7 ± 3.5 fF/pC at 0.34 ± 0.08 pC, corresponding to QCa that was transferred with a single spike (Fig. 9C). For spiking responses, the efficiency of exocytosis decreased with the increase in QCa or, by extension, with the increase in the number of spikes triggered by light. As a single spike in Mb1 was evoked with minimum light intensity (see Fig. 6, left), the efficiency of the exocytosis in spiking Mb was maximal at stimulations with minimal light intensity and decreased when light intensity increased. Note that the efficiency of exocytosis for a single light-evoked spike recorded from an intact Mb (25.7 ± 3.5 fF/pC) was slightly below that of a current-evoked or spontaneous Ca2+ spike recorded in axotomized Mb terminals (38 fF/1.24 pC or 30.6 fF/pC, from Palmer 2006).
For the light-evoked graded potentials of Mb9 and Mb10, the efficiency of exocytosis reached its maximum of 5.9 ± 0.9 fF/pC at QCa of 14.3 ± 1.9 pC and 1.8 ± 0.2 fF/pC at QCa of 66.7 ± 7.9 pC, respectively (Fig. 9C). Interestingly, the QCa that triggered exocytosis with the maximal efficiency in graded Mbs was transferred by potentials evoked by light intensities similar to those that triggered half-maximal exocytosis (Fig. 8A): 0.42 ± 0.10 vs. 0.41 ± 0.06 photons·μm−2·s−1 (P = 0.75) in Mb9 and 81 ± 32 vs. 77 ± 27 photons·μm−2·s−1 (P = 0.7) in Mb10.
Parameters of light-evoked spiking and graded potentials that determine exocytosis.
A single (Ca2+) spike that was induced by depolarizing current injection in an axotomized Mb terminal was shown to trigger substantial exocytosis, reflected by ΔCm of ∼38 fF (Palmer 2006), indicating the release of ∼1,500 vesicles. This is similar to the size of the rapidly releasable pool (RRP) of synaptic vesicles in Mb terminals, deployed within 10 ms of the ICa activation (Heidelberger et al. 1994; Mennerick and Matthews 1996; Neves and Lagnado 1999; Tachibana 1999; von Gersdorff et al. 1996). These observations raise the question: if the first spike releases all docked vesicles, can multiple light-evoked spikes, in close succession, influence the exocytosis from Mbs?
We analyzed the exocytosis from axotomized Mb terminals (n = 19) triggered by the spiking responses of Mb1, which were evoked by 500-ms green light stimuli with intensities from 0.07 to 0.20 photons·μm−2·s−1. In this intensity range, the Ca2+ spikes were clearly separated from each other and their number increased from 1 to 24 (Fig. 6, left, top 4 traces). We found that the exocytosis (ΔCm) triggered by the light-evoked spikes was greater for the larger number of spikes (Fig. 10A): 10.8 ± 4.3 fF (1 spike) < 44.9 ± 17.3 fF (8 spikes) < 80.0 ± 18.4 fF (14 spikes) < 90.2 ± 26.1 fF (28 spikes) (P ≤ 0.05 in each case, Newman-Keuls test). These results also indicated that a single light-evoked spike recorded from intact Mbs did not deplete the RRP of axotomized Mb terminals.
A possible explanation for this apparent discrepancy between our results and earlier observations (Palmer 2006) is that the amplitude of spikes induced by current injections in axotomized Mb terminals was larger than those evoked by light in intact Mbs. Indeed, this was the case: in axotomized Mb terminals, depolarizing current-evoked single spikes peaked at −22.3 ± 4.6 mV (n = 11), which was significantly larger (P = 10−12) than the peak potentials of those spikes evoked by light at the terminals of intact Mbs, which varied from −45.9 mV to −36.3 mV, averaging −42.3 ± 4.2 mV (n = 20) (Fig. 10B). Phosphocreatine is thought to facilitate the generation of Ca2+ spikes in BCs, presumably by preventing rundown of ICa (Baden et al. 2011). Therefore, the presence of phosphocreatine in our experiments might explain why the depolarizing current-evoked single spikes in axotomized Mb terminals were slightly (∼4–5 mV) larger than previously reported with internal solutions lacking phosphocreatine (Palmer 2006). Nonetheless, these results collectively suggest that the exocytosis of glutamate in intact Mbs is more dependent upon the amplitude of spikes than was previously predicted in studies of axotomized Mb terminals.
The durations of the light-evoked trains of 8, 14, and 28 spikes we selected for these studies were 414.7 ms, 320.7 ms, and 383.0 ms, respectively. Note that the train of eight light-evoked spikes triggered ΔCm that was roughly the size of the RRP, or, in other words, eight light-evoked spikes depleted the RRP. Still, trains of 14 and 28 spikes, over a shorter duration, released more vesicles, which strongly indicated that the fast refilling of the RRP must have taken place. The refilling of the RRP in Mbs is estimated to be relatively fast: ∼4,500 vesicles/s (Gomis et al. 1999), which predicts the refilling of ∼1,800 vesicles/terminal over 400 ms. Our data suggest that a train of 28 spikes fully exhausted the RRP as well as the newly refilled vesicles; the release of 1,500 + 1,800 vesicles (RRP + refilled) would be associated with an estimated maximal ΔCm of ∼87 fF, which matches our results (90.2 ± 26.1 fF).
To determine how the amplitude of a spike affects the exocytosis, we selected a light-evoked spike train with a large variability of spike amplitudes (Fig. 10C, bottom) and injected this waveform into axotomized Mb terminals (n = 16). The peak Vm of the first, second, third, and fourth spikes in the train were −45.9 mV, −44.2 mV, −42.9 mV, and −36.5 mV, respectively. We made a series of cuts to the spike train to create individual waveforms that included the first spike only, the first and second spikes, the first, second, and third spikes, and all four spikes and injected the cuts separately. Although the second spike appears to be a voltage duplet, it was counted as a single spike because the calcium current was continuous within the duplet (Fig. 10C, top). The time intervals between the first and second, second and third, and third and fourth spikes were 73.2 ms, 228.6 ms, and 247.8 ms, respectively. The ΔCm almost linearly depended upon the magnitude of the light-evoked spikes, with a slope of 4.50 ± 0.48 fF/mV (Fig. 10D); the Pearson's correlation coefficient was 0.99. These data suggest that the magnitude of exocytosis triggered by a light-evoked spike train depended on the number of spikes as well as on the amplitudes of spikes.
Next we analyzed different characteristics of the graded light-evoked potentials with regard to triggering exocytosis. The graded potentials recorded from Mb9, evoked by light intensities up to 0.29 photons·μm−2·s−1, did not trigger exocytosis consistently from axotomized Mb terminals (n = 18; Fig. 10E). The graded potential recorded from Mb9 evoked by light with the intensity of 0.49 photons·μm−2·s−1 triggered large exocytosis (81.3 ± 15.2 fF) that, on average, was 91% of maximal value of the ΔCm that could be evoked by the graded responses of Mb9. This almost all-or-none type increase in the exocytosis was associated with an 4.3-mV increment of the light response amplitudes from −48.8 mV to −44.5 mV. In the case of the graded responses recorded from Mb10 (Fig. 10G), the exocytosis was not different from zero when triggered with potentials evoked by light intensities up to 0.49 photons·μm−2·s−1 (0.5 ± 6.9 fF, P = 0.8) and became significant at responses evoked by 1.0 photon·μm−2·s−1 (6.3 ± 5.5 fF, P = 0.002). This increase in the exocytosis resulted from an increase in the light response amplitude from −52.7 mV to −44.8 mV.
Interestingly, the amplitudes of the graded potentials that triggered significant exocytosis, −44.5 mV for Mb9 (at 0.49 photons·μm−2·s−1) and −44.8 mV for Mb10 (at 1.0 photon·μm−2·s−1), were about equal to the amplitude of the first spike in a train from the spiking Mbs: −43.1 ± 1.6 mV for Mb1 and −45.9 mV for the train shown in Fig. 10C. These data suggest a critical role of ICa activation threshold in relation to exocytosis from the Mb terminal. The amplitude of the Vm from Mb10, in response to light with a larger intensity of 5.9 photons·μm−2·s−1, was not larger than that of the responses to light with intensity of 1.0 photon·μm−2·s−1, −44.9 mV vs. −44.8 mV, yet the former triggered significantly larger exocytosis: 11.7 ± 8.3 fF vs. 6.3 ± 5.5 fF (P = 0.04). To understand how the Vm with almost the same amplitude triggers larger exocytosis, we analyzed other parameters of the light responses as well (Fig. 10G). First we calculated the maximal slope of the Hill's fit of the rising phase of the light responses (Fig. 10F) and the duration of light responses above the ICa activation threshold. We found that the higher the intensity of the light stimulus, the steeper the rising phase of the graded response (Fig. 10F). For Mb10, the increase of light intensity from 1.0 to 5.9 photons·μm−2·s−1 did not increase the peak amplitude of responses but increased the slope from 0.127 V/s to 0.413 V/s (Fig. 10F). Although the duration of the light response also increased from 0.67 s to 1.09 s (Fig. 10F), the larger slope seemed to be an important factor for the exocytosis mediated by light responses. If it were not, the exocytosis in response to slow rising graded potential from Mb10 evoked by light with intensity of 1.0 photon·μm−2·s−1 would be much larger than the response to the first spike in a light-evoked train shown in Fig. 10C with lower amplitude, −45.9 mV vs. −44.8 mV, shorter duration, 0.02 s vs. 0.67 s, but ∼10-fold larger slope: 1.123 V/s vs. 0.127 V/s. However, the exocytoses triggered by the different responses are practically the same, 7.7 ± 6.8 fF vs. 6.3 ± 5.5 fF (P = 0.5), emphasizing the role of the slope.
The amplitude and slope of the Vm from Mb10 in response to light with larger intensity, 35 photons·μm−2·s−1, were not much different from those in response to light with intensity of 5.9 photons·μm−2·s−1, −44.4 mV vs. −44.9 mV and 0.413 V/s vs. 0.409 V/s, yet the triggered exocytosis was significantly larger, 64.1 ± 17.9 fF vs. 11.7 ± 8.3 fF (P = 0.001). In this case, the larger exocytosis resulted from the increased duration of the light response from 1.09 s to 1.94 s. Further increase in the intensity of the light stimulus up to 1,200 photons·μm−2·s−1 did not drastically affect the amplitude and slope, but it did increase the duration of the response from 1.75 s to 6.13 s. These results collectively suggest that amplitude, slope, and duration are the main features of light-evoked graded potentials influencing the magnitude of exocytosis from Mb terminals.
DISCUSSION
In the vertebrate retina, photoreceptors use analog Vm changes to code for the intensity of visual stimulus, whereas GC signals at the retinal output are digital, formed by trains of Na+ action potentials with uniform magnitude, encoding information in their pattern. BCs, interposed between photoreceptors and GCs, produce both analog and digital light responses (Baden et al. 2013a). Na+ action potentials in certain mammalian BCs are thought to increase temporal precision of coding (Saszik and DeVries 2012). However, the role of Ca2+ spike-mediated digital signaling in BCs is not well understood. Here we studied how light-evoked Ca2+ spikes (Protti et al. 2000) and graded responses (Joselevitch and Kamermans 2007) mediate glutamate output of ON-type Mb BCs.
The major findings of our investigation are as follows: 1) compared to graded responses, light-evoked spikes confer higher sensitivity and gain for processing light information in Mbs at the cost of saturating exocytosis at lower light intensities; 2) within their intensity range of operation, spiking light responses trigger more linear glutamate release than graded potentials; 3) at low light intensities, at the visual threshold of Mb signaling, a single Ca2+ spike ensures coding for light detection at the Mb output more efficiently than graded responses; however, 4) at these low light intensities, Ca2+ spikes do not improve temporal precision of Mb signaling.
Light responses recorded at Mb axon terminal.
During somatic recordings, amplitude and rise time of fast (synaptic) currents, generated at the axon terminal of mammalian RBCs, are significantly reduced because of filtering by the axonal resistance and somatic Cm (Oltedal et al. 2007). This might explain why fast Ca2+ spikes were not reported during decades of electrophysiological investigations of BCs in mammalian retinas. A recent study, however, reported at least three types of mouse BCs that produce Ca2+ spikes at their axon terminals upon light stimulation (Baden et al. 2013a). Coincidentally, in vivo imaging of transgenic zebrafish retinas expressing Ca2+ reporters described multiple types of Ca2+ spike-producing BCs in addition to Mbs (Baden et al. 2011; Dreosti et al. 2011). Although light-triggered Ca2+ spikes in Mbs are robust enough for electrophysiological detection via somatic recordings (Baden et al. 2011; Protti et al. 2000), we recorded light responses directly from Mb axon terminals to minimize filtering (Baden et al. 2011; Oltedal et al. 2007). By voltage clamping axotomized Mb terminals, free of space-clamp errors (Mennerick et al. 1997; Oltedal et al. 2007), at previously recorded light-evoked Vm, we obtained precise and realistic measures of ICa and glutamate release, reflected by Cm increase, from Mb terminals in response to a natural stimulus: light.
Spiking responses confer hypersensitivity to Mb signaling.
Ca2+ spikes recorded from BCs in fish retinas code for high contrast and bright light with millisecond precision (Baden et al. 2011; Tooker et al. 2013). However, Ca2+ spikes are much slower (Baden et al. 2011; Protti et al. 2000; Fig. 9B) than Na+ spikes described in mammalian BCs (Puthussery et al. 2013; Saszik and DeVries 2012), suggesting that Ca2+ spikes might be less suitable for signaling rapid changes within the visual field than Na+ spikes (Saszik and DeVries 2012). Although analysis of the temporal precision of spikes was not performed here, our data support this view: in Mbs, the jitter in the first spike delay not only increased with the reduction of light intensity (Tooker et al. 2013; Fig. 8D) but at light intensities corresponding to the threshold of Mb signaling Ca2+ spikes initiated after the termination of stimuli (Fig. 2, Mb1, Mb2, 0.07 photons·μm−2·s−1). Thus at Mb signaling threshold intensity Ca2+ spikes ensure information transfer and stimulus detection by cells postsynaptic to Mbs but, clearly, with poor temporal precision. Although all intact Mbs encountered in this study produced identifiable responses (without averaging) to the lowest intensity of light (0.07 photons·μm−2·s−1, λ = 505 nm, for 500 ms), only spiking responses evoked by light intensities up to ∼0.3 photons·μm−2·s−1 triggered substantial exocytosis (Cm increase) from axotomized Mb terminals.
As shown by investigations of the behavioral visual threshold, the goldfish retina is at least as sensitive as the human retina (Powers and Easter 1978). The mammalian retina utilizes a dedicated circuitry for processing light signals around the absolute visual threshold, the rod→RBC→AII amacrine→cone BC→GC pathway (Bloomfield and Dacheux 2001), which allows for gain control/amplification at multiple stages (Schwartz and Rieke 2013). AII amacrine cells amplify RBC output, set the visual threshold for GCs, and also provide a gain control mechanism to ensure luminance encoding over a wide range of light intensities (Arman and Sampath 2012; Dunn et al. 2006). However, the fish retina does not have an AII-equivalent cell that collects outputs of dozens of Mbs and amplifies the signal before forwarding it toward GCs. Instead, Mbs synapse directly onto GCs (Marc and Liu 2000; Palmer 2010; Witkovsky and Dowling 1969). Interestingly, the scotopic threshold, or, in other words, the minimal stimulus intensity that activated dark-adapted mouse RBCs, was found to be ∼0.03 rhodopsin photoisomerization (Rh*)/rod (Dunn et al. 2006; Saszik et al. 2002). This stimulus intensity is the equivalent of 0.07 photons·μm−2·s−1 presented for 500 ms (Pang et al. 2004) and is similar to the scotopic threshold of Mb signaling (Fig. 2 in the present study; also Joselevitch and Kamermans 2007). Therefore, we propose that the spike-mediated hypersensitivity of luminance coding in Mbs extends the absolute light sensitivity of the fish retina by lowering the intensity threshold for glutamate output at Mb level of the rod pathway and it might be critical in setting the visual threshold of the AII-less fish retina below or equal to that of humans (Powers and Easter 1978).
Coding light intensity with Ca2+ spikes and graded responses.
In Mbs, ICa is mediated by L-type Ca2+ channels (Tachibana 1999) expressed at the axon terminal with properties reminiscent of CaV1.3 (Burrone and Lagnado 1997; Heidelberger and Matthews 1992; Mennerick and Matthews 1998; Tachibana et al. 1993; von Gersdorff and Matthews 1996; Zenisek and Matthews 1998). The nonlinearity reflected by the Hill coefficient in coding light intensity with Ca2+ influx was greater during spiking than graded Mb responses. Counterintuitively, at the level of ΔCm, low light intensities were coded more linearly (i.e., with lower Hill coefficient) by spiking Mb responses than by graded ones with comparable sensitivity (Mb1 vs. Mb9, Fig. 9C). The cooperative action of Ca2+ required for vesicle fusion (Heidelberger et al. 1994) and soluble endogenous Ca2+ buffers that influence RRP size in Mb terminals (Burrone and Lagnado 2000) introduce nonlinearity during the translation of QCa into exocytosis. Soluble endogenous buffers, with an estimated capacity equal to 2 mM EGTA (Burrone and Lagnado 2000), might prevent gradually increasing [Ca2+]i to reach the vesicle fusion threshold of ∼10 μM (Heidelberger et al. 1994) during graded Mb responses over a range of intensities (Mb9 vs. Mb10, Fig. 8B). When Ca2+ is conveyed with spikes, quick Ca2+ influx overwhelms the endogenous buffering; each single spike delivered a “quantum” of QCa (0.34 ± 0.08 pC; Fig. 9C) triggered sizable exocytosis. The interspike intervals (train of 8 spikes: 59.2 ± 26.3 ms, train of 14 spikes: 24.7 ± 9.1 ms, train of 28 spikes: 14.2 ± 2.4 ms; Fig. 10A) were much greater than the Ca2+ binding time of 0.33 ms estimated for 2 mM EGTA (Adler et al. 1991) but much shorter than the timescale on which other presynaptic Ca2+ handling mechanisms function (Wan et al. 2012; Zenisek and Metthews 2000). Therefore, we propose that during spike trains soluble endogenous buffers might promote spatiotemporal separation of spike-evoked [Ca2+]i domain formation (Neher 1998) so that consecutive Ca2+ spikes trigger exocytosis rather independently from each other (Fig. 10A). Consequently, Ca2+ spike trains at low light intensities linearize the Mb output, while spikes also ensure a high probability of propagating dim light information onto GCs. Importantly, a single light-evoked spike did not exhaust the RRP (Fig. 10A), making the spike magnitude an important factor in determining the triggered ΔCm (Fig. 10D). Synaptic conductances that attenuate spike magnitude in Mbs (Hull et al. 2006a) can further decrease exocytosis per spike and, in conjunction with fast RRP replenishment (Burrone and Lagnado 2000), increase the capability of coding for the light intensity with the number of spikes.
Longer depolarizations transfer larger QCa and trigger larger exocytosis from Mbs (Burrone et al. 2002; Burrone and Lagnado 2000; Mennerick and Matthews 1996; Neves and Lagnado 1999). Nonetheless, fast “tail” ICa, associated with repolarization of Mb terminals from +60 mV to −60 mV, triggered larger exocytosis than depolarization from −60 to −10 mV, even if the latter transferred much larger QCa (Tachibana 1999; von Gersdorff et al. 1996), emphasizing the importance of ICa activation kinetics over QCa (Mennerick and Matthews 1996) and suggesting that Ca2+ spikes might drive exocytosis more efficiently than graded responses (Protti et al. 2000; Zenisek and Matthews 1998). Indeed, we found that in Mbs the calculated efficiency of exocytosis (ΔCm/QCa) (Hull et al. 2006b) was maximal at the lowest QCa transferred with a single light-evoked spike, exceeding that of any graded responses (Fig. 9C). Although light-evoked spikes have not been reported from mammalian RBCs, tail ICa in RBCs (Singer and Diamond 2003) triggered the fastest excitatory postsynaptic currents (EPSCs) in AII amacrine cells (Singer et al. 2004). Taken together, mammalian BCs capable of Ca2+ spike production (Baden et al. 2013a) might have accelerated glutamate release at higher efficiency compared with those that produce graded responses. Our results suggest that the mechanisms by which BCs filter visual information into separate channels during parallel processing may not be restricted to differential expression of dendritic glutamate receptors to sense photoreceptor output with distinct kinetics (DeVries 2000) and might include differences in glutamate release based on the BC's propensity for Ca2+ spike generation.
GRANTS
This work was supported by a grant from National Eye Institute R01 EY-019051 (J. Vigh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.Y.L. performed experiments; M.Y.L. and J.V. analyzed data; M.Y.L. and J.V. interpreted results of experiments; M.Y.L. prepared figures; M.Y.L. drafted manuscript; M.Y.L. and J.V. approved final version of manuscript; J.V. conception and design of research; J.V. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Ryan E. Tooker for comments and critical reading of the manuscript.
REFERENCES
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci 11: 1496–1507, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman AC, Sampath AP. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J Neurophysiol 107: 2649–2659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol 63: 533–552, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Berens P, Bethge M, Euler T. Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr Biol 23: 48–52, 2013a. [DOI] [PubMed] [Google Scholar]
- Baden T, Esposti F, Nikolaev A, Lagnado L. Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision. Curr Biol 21: 1859–1869, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Euler T, Weckström M, Lagnado L. Spikes and ribbon synapses in early vision. Trends Neurosci 36: 480–488, 2013b. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–384, 2001. [DOI] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Electrical resonance and Ca2+ influx in the synaptic terminal of depolarizing bipolar cells from the goldfish retina. J Physiol 505: 571–584, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci 20: 568–578, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron 33: 101–112, 2002. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28: 847–856, 2000. [DOI] [PubMed] [Google Scholar]
- Dreosti E, Esposti F, Baden T, Lagnado L. In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat Neurosci 14: 951–952, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci 26: 3959–3970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol 83: 1817–1829, 2000. [DOI] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Single-Channel Recording, edited by Sakmann B, Neher E. New York: Plenum, 1995, p. 155–198. [Google Scholar]
- Gillis KD. Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Pflügers Arch 439: 655–664, 2000. [DOI] [PubMed] [Google Scholar]
- Gomis A, Burrone J, Lagnado L. Two actions of calcium regulate the supply of releasable vesicles at the ribbon synapse of retinal bipolar cells. J Neurosci 19: 6309–6317, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner CP, Zenisek D. Amperometric resolution of a prespike stammer and evoked phases of fast release from retinal bipolar cells. J Neurosci 33: 8144–8158, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371: 513–515, 1994. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol 447: 235–256, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Li GL, von Gersdorff H. GABA transporters regulate a standing GABAC receptor-mediated current at a retinal presynaptic terminal. J Neurosci 26: 6979–6984, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol 96: 2025–2033, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Shields CR, Lukasiewicz PD. Sodium channels in transient retinal bipolar cells enhance visual responses in ganglion cells. J Neurosci 25: 1856–1865, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT, Stell WK, Lightfoot DO. Rod and cone inputs to bipolar cells in goldfish retina. J Comp Neurol 191: 315–335, 1980. [DOI] [PubMed] [Google Scholar]
- Joselevitch C, Kamermans M. Interaction between rod and cone inputs in mixed-input bipolar cells in goldfish retina. J Neurosci Res 85: 1579–1591, 2007. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol 207: 623–633, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol 358: 131–152, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice L, Henry D, Matthews G. Identification of calcium channel alpha1 subunit mRNA expressed in retinal bipolar neurons. Mol Vis 12: 184–189, 2006. [PMC free article] [PubMed] [Google Scholar]
- Mao BQ, MacLeish PR, Victor JD. Relation between potassium-channel kinetics and the intrinsic dynamics in isolated retinal bipolar cells. J Comput Neurosci 12: 147–163, 2002. [DOI] [PubMed] [Google Scholar]
- Marc RE, Liu W. Fundamental GABAergic amacrine cell circuitries in the retina: nested feedback, concatenated inhibition, and axosomatic synapses. J Comp Neurol 425: 560–582, 2000. [DOI] [PubMed] [Google Scholar]
- Marty A, Neher E. Tight-seal whole-cell recording. In: Single-Channel Recording, edited by Sakmann B, Neher E. New York: Plenum, 1995, p. 31–52. [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17: 1241–1249, 1996. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Rapid calcium-current kinetics in synaptic terminals of goldfish retinal bipolar neurons. Vis Neurosci 15: 1051–1056, 1998. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zenisek D, Matthews G. Static and dynamic membrane properties of large-terminal bipolar cells from goldfish retina: experimental test of a compartment model. J Neurophysiol 78: 51–62, 1997. [DOI] [PubMed] [Google Scholar]
- Midorikawa M, Tsukamoto Y, Berglund K, Ishii M, Tachibana M. Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cells. Nat Neurosci 10: 1268–1276, 2007. [DOI] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399, 1998. [DOI] [PubMed] [Google Scholar]
- Neves G, Lagnado L. The kinetics of exocytosis and endocytosis in the synaptic terminal of goldfish retinal bipolar cells. J Physiol 515: 181–202, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Nikolaev A, Lagnado L. Encoding of luminance and contrast by linear and nonlinear synapses in the retina. Neuron 73: 758–773, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Mørkve SH, Veruki ML, Hartveit E. Patch-clamp investigations and compartmental modeling of rod bipolar axon terminals in an in vitro thin-slice preparation of the mammalian retina. J Neurophysiol 97: 1171–1187, 2007. [DOI] [PubMed] [Google Scholar]
- Palmer MJ. Modulation of Ca2+-activated K+ currents and Ca2+-dependent action potentials by exocytosis in goldfish bipolar cell terminals. J Physiol 572: 747–762, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ. Characterisation of bipolar cell synaptic transmission in goldfish retina using paired recordings. J Physiol 588: 1489–1498, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H. Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J Neurosci 23: 4831–4841, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol 558: 897–912, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers M, Easter SS Jr. Absolute visual sensitivity of the goldfish. Vision Res 18: 1137–1147, 1978. [DOI] [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, von Gersdorff H. Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron 25: 215–227, 2000. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Venkataramani S, Gayet-Primo J, Smith RG, Taylor WR. NaV1.1 channels in axon initial segments of bipolar cells augment input to magnocellular visual pathways in the primate retina. J Neurosci 33: 16045–16059, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol 493: 1–8, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Kondo H, Toyoda JI. Ionic mechanisms of two types of on-center bipolar cells in the carp retina. I. The responses to central illumination. J Gen Physiol 73: 73–90, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Kujiraoka T. Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol 205: 161–170, 1982. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Ishikane H, Tachibana M. Ca2+-activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neurosci Res 27: 219–228, 1997. [DOI] [PubMed] [Google Scholar]
- Saszik S, DeVries SH. A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J Neurosci 32: 297–307, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol 543: 899–916, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, Rieke F. Controlling gain one photon at a time. Elife 2: e00467, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci 23: 10923–10933, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Lassová L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci 7: 826–833, 2004. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Regulation of transmitter release from retinal bipolar cells. Prog Biophys Mol Biol 72: 109–133, 1999. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Okada T. Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J Neurosci 11: 2199–2208, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Okada T, Arimura T, Kobayashi K, Piccolino M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci 13: 2898–2909, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Morgans C. Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci 15: 541–552, 1998. [DOI] [PubMed] [Google Scholar]
- Tooker R, Lipin M, Leuranguer V, Rozsa E, Bramley J, Harding J, Reynolds M, Vigh J. Nitric oxide mediates activity-dependent plasticity of retinal bipolar cell output via S-nitrosylation. J Neurosci 33: 19176–19193, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh J, Vickers E, von Gersdorff H. Light-evoked lateral GABAergic inhibition at single bipolar cell synaptic terminals is driven by distinct retinal microcircuits. J Neurosci 31: 15884–15893, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh J, von Gersdorff H. Prolonged reciprocal signaling via NMDA and GABA receptors at a retinal ribbon synapse. J Neurosci 25: 11412–1423, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature 370: 652–655, 1994. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J Neurosci 16: 115–122, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Electrophysiology of synaptic vesicle cycling. Annu Rev Physiol 61: 725–752, 1999. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron 21: 1177–1188, 1998. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron 16: 1221–1227, 1996. [DOI] [PubMed] [Google Scholar]
- Wan QF, Nixon E, Heidelberger R. Regulation of presynaptic calcium in a mammalian synaptic terminal. J Neurophysiol 108: 3059–3067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol 32: 339–355, 1969. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Dowling JE. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat 100: 60–82, 1969. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Differential distribution of Shaker-like and Shab-like K+-channel subunits in goldfish retina and retinal bipolar cells. J Comp Neurol 396: 131–140, 1998. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Henry D, Studholme KM, Yazulla S, Matthews G. Voltage-dependent sodium channels are expressed in nonspiking retinal bipolar neurons. J Neurosci 21: 4543–4550, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. Calcium action potentials in retinal bipolar neurons. Vis Neurosci 15: 69–75, 1998. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron 25: 229–237, 2000. [DOI] [PubMed] [Google Scholar]