Abstract
Tetrodotoxin-resistant (TTX-r) sodium channels are key players in determining the input-output properties of peripheral nociceptive neurons. Changes in gating kinetics or in expression levels of these channels by proinflammatory mediators are likely to cause the hyperexcitability of nociceptive neurons and pain hypersensitivity observed during inflammation. Proinflammatory mediator, tumor necrosis factor-α (TNF-α), is secreted during inflammation and is associated with the early onset, as well as long-lasting, inflammation-mediated increase in excitability of peripheral nociceptive neurons. Here we studied the underlying mechanisms of the rapid component of TNF-α-mediated nociceptive hyperexcitability and acute pain hypersensitivity. We showed that TNF-α leads to rapid onset, cyclooxygenase-independent pain hypersensitivity in adult rats. Furthermore, TNF-α rapidly and substantially increases nociceptive excitability in vitro, by decreasing action potential threshold, increasing neuronal gain and decreasing accommodation. We extended on previous studies entailing p38 MAPK-dependent increase in TTX-r sodium currents by showing that TNF-α via p38 MAPK leads to increased availability of TTX-r sodium channels by partial relief of voltage dependence of their slow inactivation, thereby contributing to increase in neuronal gain. Moreover, we showed that TNF-α also in a p38 MAPK-dependent manner increases persistent TTX-r current by shifting the voltage dependence of activation to a hyperpolarized direction, thus producing an increase in inward current at functionally critical subthreshold voltages. Our results suggest that rapid modulation of the gating of TTX-r sodium channels plays a major role in the mediated nociceptive hyperexcitability of TNF-α during acute inflammation and may lead to development of effective treatments for inflammatory pain, without modulating the inflammation-induced healing processes.
Keywords: tumor necrosis factor, nociceptor, sodium (Na+) current, DRG, inflammatory pain
primary nociceptive neurons are tuned to sense noxious endogenous and exogenous physical and chemical stimuli via specific high-threshold transducer channels (Basbaum et al. 2009; Dubin and Patapoutian 2010; Woolf and Ma 2007). Interestingly, in addition to noxious stimuli-mediated activation of transducer channels, nociceptive neurons can be activated, by modulation of the gating properties of TTX-resistant (TTX-r) sodium channels: voltage-gated sodium (Nav) 1.8 and Nav 1.9 (Binshtok et al. 2008). These channels are expressed mainly by nociceptive neurons where they play a major role in defining their output characteristics, by affecting the threshold and rate of action potential (AP) firing (Dib-Hajj et al. 2010). Immune factors, such as proinflammatory cytokine interleukin-1β (IL-1β), increase the number of functionally available TTX-r sodium channels, via p38 mitogen-activated protein kinase (p38 MAPK)-mediated relief of voltage dependence of slow inactivation, sufficiently to directly activate nociceptive neurons and produce acute pain hypersensitivity (Binshtok et al. 2008). Peripheral administration of another prototypic proinflammatory cytokine, tumor necrosis factor-α (TNF-α), has been shown to elicit a rapid significant enhancement of ongoing activity in nociceptive fibers (Sorkin et al. 1997), thereby causing acute mechanical (Sorkin and Doom 2000) and thermal hypersensitivity (Jin and Gereau 2006). In the present study, we sought to detail the mechanisms of the rapid TNF-α-induced hyperexcitability of nociceptor-like, dissociated rat dorsal root ganglion (DRG) neurons and nociceptive terminals in vivo. It has been demonstrated that TNF-α leads to upregulation of Nav 1.8 channels in DRG neurons, albeit indirectly, via the production of nerve growth factor (Cummins et al. 2000; Leffler et al. 2002). Furthermore, it has been shown that TNF-α leads to cyclooxygenase (COX)-2-dependent production of prostaglandin PGE2 (Maier et al. 1990). PGE2, in turn, increases Nav 1.8 current density by shifting the voltage dependence of the activation of Nav 1.8 channels in a hyperpolarized direction, in a PKA and PKC dependent manner (England et al. 1996; Gold et al. 1998). These COX-2-dependent mechanisms are expected to have a relatively slow onset and, therefore, cannot explain the above-mentioned rapid TNF-α-mediated mechanical and thermal hypersensitivity. Indeed, it has been demonstrated that, in addition to this indirect effect, TNF-α acts via TNF receptor 1 (TNFR1) and p38 MAPK to increase TTX-r sodium currents in DRG neurons, thus leading to pain hypersensitivity (Jin and Gereau 2006). This TNF-α-mediated increase in TTX-r current density is not accompanied by changes in voltage dependence of activation or steady-state inactivation of the channels (Jin and Gereau 2006). We thus decided to study the mechanism by which TNF-α affects TTX-r sodium current and hereby nociceptive excitability. To this end, 1) we detailed TNF-α-mediated changes in neuronal excitability; and 2) based on the character of the changes in the excitability, we studied the effects of TNF-α on availability and kinetics of the slow and persistent TTX-r currents. We found that the TNF-α mediated an acute increase in excitability of nociceptor-like, dissociated rat DRG neurons, which results from decreased AP threshold, increased gain and decreased accommodation. We demonstrated that TNF-α enhances the current density of persistent TTX-r current by shifting its voltage dependence of activation in a hyperpolarized direction, thereby boosting the cell excitability in subthreshold ranges. Moreover, we found that TNF-α acutely increases the availability of slow TTX-r sodium channels, via a p38 MAPK-dependent partial relief of TTX-r sodium channels from slow inactivation, thereby contributing to decrease in accommodation. Our results suggest that rapid gating modulation of the channels underlying slow and persistent TTX-r sodium currents plays a major role in TNF-α-mediated nociceptive hyperexcitability during acute inflammation.
MATERIALS AND METHODS
Animal procedures were approved by the Ethics Committee of the Hebrew University.
Behavioral tests.
Six- to seven-week-old, 250 g, Male Sprague-Dawley rats purchased from Harlan Laboratories, Jerusalem, Israel, were used in this study. The rats were habituated to handling and experimental procedures for 1 wk prior to injections. The behavioral baseline was obtained by three preliminary measurements. The experimenter was blind to the treatments. Recombinant rat TNF-α (10 ng/μl, Peprotech or R&D Systems) and lacosamide, enhancer of slow inactivation of Nav channels (5 μg/μl, AK Scientific), were prepared freshly in saline (0.9% NaCl, B. Braun, Melsungen). Ibuprofen, a nonspecific COX inhibitor (10 μg/μl, Sigma), and SB203580, a p38 MAPK inhibitor (5 μg/μl, Calbiochem), were prepared as a stock solution in DMSO. Final concentration of DMSO in saline was 0.1%.
Single intraplantar injections into the left hindpaw of 10 μl of saline, TNF-α, lacosamide or a combination of TNF-α and lacosamide were performed, and mechanical and thermal sensitivities were determined using von Frey hairs (Ugo Basile) and hot plate (52°C, Freid Instruments), respectively, at the times indicated, as described previously (Safieh-Garabedian et al. 1997; Tal and Bennett 1994). In another set of experiments, intraplantar injections of 10 μl vehicle (saline + 0.1% DMSO), ibuprofen or SB203580 were followed, 20 min later, by intraplantar injections to the same injection site of 10 μl saline or TNF-α, and mechanical and thermal sensitivities were determined at the times indicated. For experiments using von Frey hairs, 26-g force was defined as a cut-off. For experiments using the hot plate, lack of response after 30 s led to termination of the experiment.
Cell culture.
Primary DRG neuron cultures were prepared from 6- to 9-wk-old Sprague-Dawley male rats, as previously described (Binshtok et al. 2008).
General electrophysiology.
Recordings were performed from small (∼25 μm) dissociated rat DRG neurons with a membrane capacitance of 25.67 ± 0.58 pF (n = 163). These neurons have been described in the literature to be nociceptive, as they respond to application of capsaicin (see, for example, Cardenas et al. 1995). Whole-cell membrane currents and voltages were recorded in voltage clamp and fast current-clamp modes, respectively, using a Multiclamp 700B amplifier (Molecular Devices), at room temperature. Only the cells that demonstrated stable current amplitude or stable firing frequency during wash-in of vehicle and cells that showed less than 10% change in the access resistance during the entire recording period were analyzed. Data were sampled at 20 kHz and were low-pass filtered at 10 kHz (−3 dB, 8-pole Bessel filter). Patch pipettes were pulled using thick-walled borosilicate glass capillaries (1.5:1.1 mm, outer to inner diameter) on a P-1000 puller (Sutter Instruments) with a resistance of 2–6 MΩ when filled with Cs+-based internal solution. Access resistance was maintained at 4–8 MΩ. For voltage-clamp recordings, capacitive currents were minimized, and series resistance was compensated by 80–90%. The remaining capacitive transients and the linear leakage currents were digitally subtracted using a P4 protocol. Command voltage protocols were generated with a Digidata 1440A A/D interface (Molecular Devices). Data were digitized using pCLAMP 10.3 (Molecular Devices). Data averaging and peak detection were performed using Clampfit 10.3 software. Data were fitted using Origin 8 (OriginLab).
Voltage-clamp recordings.
To minimize space clamp errors, all voltage clamp recordings were performed no later than 12 h from plating.
Intracellular solution contained the following (in mM): CsCl, 110; CsOH, 25; NaCl 10, MgCl2, 2; CaCl2 1; EGTA 11; HEPES, 10 (pH = 7.4 with CsOH). Extracellular solution contained the following (extracellular solution 1, in mM): NaCl, 60; KCl, 4; MgCl2, 1; CaCl2, 2; HEPES, 10; CdCl2, 0.1; glucose, 10; choline chloride, 60; 4-AP, 5; TEA-Cl, 15 (pH = 7.4 with NaOH). To record TTX-r sodium currents, cells were preincubated with 300 nM TTX, for 20–30 min before the recording (Ogata and Tatebayashi 1993; Roy and Narahashi 1992), and 300 nM TTX were included in all extracellular solutions.
To analyze TTX-r-persistent sodium currents, the extracellular solution contained the following (extracellular solution 2, in mM): NaCl, 120; KCl, 4; MgCl2, 1; CaCl2, 2; HEPES, 10; CdCl2, 0.1; glucose, 10; 4-AP, 5; TEA-Cl, 15 (pH = 7.4 with NaOH). In some experiments, the extracellular solution was supplemented with 300 nM of TTX to block TTX-sensitive (TTX-s) current and 1 μM of A803467 to block Nav 1.8 current.
For quasi-steady-state current-voltage (I–V) curve recordings, external solution contained the following (extracellular solution 3, in mM): NaCl, 145; KCl, 5; MgCl2, 1; CaCl2, 2; HEPES, 10; CdCl2, 0.1; glucose, 10 (pH = 7.4 with NaOH).
Pipette potential was zeroed before seal formation, and membrane potential was not corrected for the small liquid junction potential (−1.8 mV for extracellular solution 1; −2.8 mV for extracellular solution 2 and −1.1 mV for extracellular solution 3, measured using 1 M KCl bridge).
The voltage dependence of the activation of sodium currents was determined by applying 150 ms depolarizing steps to a range of test potentials in 10-mV increments, from a holding potential of −70 mV. The voltage at which the sodium current was half-maximally activated (V1/2) was estimated by obtaining the macroscopic conductance (G) from peak current amplitudes recorded at different test potentials using the extended Ohm's law: G = Ipeak/(Vtest − Vrev), where Ipeak is the amplitude of the peak current, Vrev is the apparent reversal potential of sodium, measured for each cell (Vrev = 53.3 ± 0.9 mV, n = 30) and Vtest is the test potential. Data were fitted by a Boltzmann relationship: G = Gmax/{1 + exp [(V1/2 − Vm)/k]}, where Gmax is maximum conductance, V1/2 is the potential at which one-half of the channels are activated, Vm is membrane potential, and k is the slope factor (in mV).
Availability curves were generated from a double-pulse protocol consisting of one constant 20-ms pulse to 0 mV after 120-ms prepulses to voltages varying from −120 to +20 mV in steps of 10 mV. The peak sodium current during the test pulse was normalized and plotted against the prepulse voltage. Data were fitted by a Boltzmann relationship: Itest/Imax = 1/{1+ exp[(V − V1/2)/k]}, where Itest is the test current, Imax is maximum current, and V is the conditioning pulse (Vcond) potential. ΔV1/2 was defined as V1/2,Treatment − V1/2,before.
Rise time was defined and quantified as the time interval between 10% to 90% of the current peak. Decay time was defined and measured as the time interval between 90% of the current peak to the time when the current reached 10% of its peak value.
TTX-s currents were measured by digital subtraction of the currents evoked by 10-mV depolarizing steps applied after a 500-ms conditioning step to either −120 mV (for total sodium current) or −40 mV (for TTX-r current) (Binshtok et al. 2008; Flake et al. 2004; Gold et al. 1996).
To study voltage dependence of slow inactivation of TTX-r sodium channels, we used the following protocol: 5-s-long Vcond were applied from a holding potential of −70 mV. The amplitude of the Vcond varied systematically (in 10-mV steps) between −120 and +20 mV. Then a 20-ms step to −100 mV was applied to remove fast inactivation and was followed by a 10-ms test pulse (Vtest) to 0 mV. Resulting TTX-r current amplitude was normalized to the Imax amplitude (fraction available) and plotted (means ± SE) vs. voltage of Vcond. The data were fitted using Boltzmann equation Itest/Imax = 1/{1 + exp[(V − V1/2)/k]}.
Maximal current amplitude was obtained from a 150-ms depolarizing step to 0 mV from a holding at −120 mV, at least 5 s prior to the test pulse. In some experiments, 30-s Vcond from −90 mV to −50 mV with 10-mV increments were applied followed by a 40-ms test pulse to 0 mV.
The analysis of voltage dependence of activation, fast inactivation and slow inactivation as well as of the time kinetics were performed only from neurons that demonstrated more than 10% increase in current density following application of TNF-α (83% of the neurons). Control experiments were performed to monitor time-dependent changes in the current density of total and TTX-r current and voltage dependence of slow inactivation. To this end, only vehicle solution was washed in for 20–40 min.
To measure the changes in the voltage dependence of activation of persistent TTX-r currents, a protocol of either 150- or 120-ms-long depolarizing steps from −50 mV to −20 mV in 10-mV increments was used from a holding potential of −90 mV (Amaya et al. 2006; Binshtok et al. 2008). To calculate the shift in voltage dependence of activation of TTX-r persistent sodium channels, we plotted the peak currents vs. the step voltages before and after TNF-α and used a computational algorithm to find the voltage shift which entails the minimum “distance” between the sum of current values after application of TNF-α [ITNF-α (V)] and their approximate equivalents before application of TNF-α [Ibefore (V + vstep)]. Accordingly, Ibefore values were shifted in increments of vstep = 0.01 mV, i.e., Ibefore (V) = Ibefore (V + vstep), while ITNF-α (V) was kept constant. We searched for the number of steps needed to minimize the sum of the absolute distances function, i.e., . To reach voltage shift value which gives the best fit between the two curves, the number of steps needed to minimize the absolute distances function was multiplied by vstep.
Quasi-steady-state I–V curves were generated from currents evoked by a slow 2.5-s depolarizing voltage ramp from −70 mV to +20 mV. The maximal currents of the early and late components were detected as first and second local minimums, respectively, and the membrane voltage at which the maximal currents occurred were measured. The onset of the early and late components were assessed manually from the low-pass-filtered (3 Hz) first derivative of dI/dt vs. Vm.
Charge densities of the non-inactivating currents were calculated as the area under the curve (AUC) of the quasi-steady-state I–V curve [a ∫ I(t)dt] with the time boundaries ranging with correlation of the voltages ramped from −40 to −70 mV according to dv = a·dt.
For the anisomycin experiments, only cells with membrane capacitance between 20 and 30 pF were analyzed, to reduce the variability between “anisomycin” and “vehicle” groups.
Current-clamp recordings.
Current-clamp recordings were performed 6 to 24 h after plating. Only neurons that had relatively long AP duration (Table 1) and prominent deflection on the falling phase (see Fig. 4A, inset) that was consistent with characteristics of nociceptive neurons (Abdulla and Smith 2001) were recorded. Among those, only neurons that demonstrated a stable resting potential and stable AP threshold with no significant change in the AP frequency-intensity (f-I) relationship during application of control solution (extracellular solution, 5 min), were analyzed. To evaluate possible time-dependent changes in neuronal excitability, we applied vehicle solution for an additional 15 min in some experiments. No changes in the neuronal excitability were observed during the period of vehicle application (n = 7).
Table 1.
Values and statistical analysis of differences in membrane excitability parameters, before and after application of TNF-α
| TTX Free |
TTX 250 nM |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | TNF-α | P value | n | Control | TNF-α | P value | n | |
| Threshold, mV | −35.29 ± 2.36 | −42.64 ± 2.85 | <0.01 | 11 | −24.29 ± 2.42 | −32.65 ± 4.14 | <0.01 | 8 |
| Duration of AP, ms | 3.58 ± 0.39 | 3.74 ± 0.39 | <0.05 | 11 | 4.04 ± 0.39 | 4.3 ± 0.43 | <0.05 | 8 |
| Repolarization velocity, mV/ms | −37.4 ± 4.5 | −37.67 ± 4.5 | ns | 11 | −35.1 ± 4.5 | −34.26 ± 4.8 | ns | 8 |
| AP amplitude, mV | 65.6 ± 5.3 | 69.4 ± 5.4 | <0.01 | 11 | 49.8 ± 4 | 56.7 ± 3 | <0.01 | 8 |
| AP count (depolarizing ramp) | 10.67 ± 2 | 14.56 ± 2.1 | <0.01 | 10 | 4.62 ± 1.45 | 8.62 ± 2.10 | <0.01 | 14 |
| FSL (depolarizing ramp), ms | 341.9 ± 85.7 | 235.9 ± 73.8 | <0.01 | 10 | 684.7 ± 141.3 | 491.1 ± 106.3 | <0.01 | 14 |
| AP accommodation (1st to last AP interval, depolarizing ramp), ms | 636.8 ± 105.1 | 775.4 ± 134.6 | <0.05 | 10 | 615.4 ± 84.3 | 923.1 ± 72.1 | <0.001 | 14 |
| f-I slope (depolarizing steps, 500 ms) | 2.26 ± 0.4 | 4.54 ± 0.8 | <0.001 | 11 | 1.86 ± 0.28 | 3.52 ± 0.32 | <0.05 | 13 |
| Input resistance, MΩ | 674 ± 60 | 652 ± 56 | ns | 22 | 748 ± 158 | 755 ± 144 | ns | 14 |
Values are means ± SE; n, no. of neurons. TTX, tetrodotoxin; TNF-α, tumor necrosis factor-α; AP, action potential; FSL, first spike latency; f–I, frequency-intensity. Data were analyzed using paired t-test. Data describing threshold, upstroke velocity and repolarization velocity were obtained from phase plots of changes in membrane voltage following application of 10-ms rectangular current steps. Data describing spike duration and spike peak voltage were obtained following application of 10-ms rectangular current steps. Data describing FSL and first to last spike interval (accommodation) were obtained following 1,500-ms duration of depolarizing ramps. Data describing the f-I slope were obtained following 500-ms rectangular current steps. Data describing the input resistance were obtained following a series of 500-ms rectangular hyperpolarized current steps. The parameters which changed significantly are bolded. ns, Nonsignificant: P > 0.05.
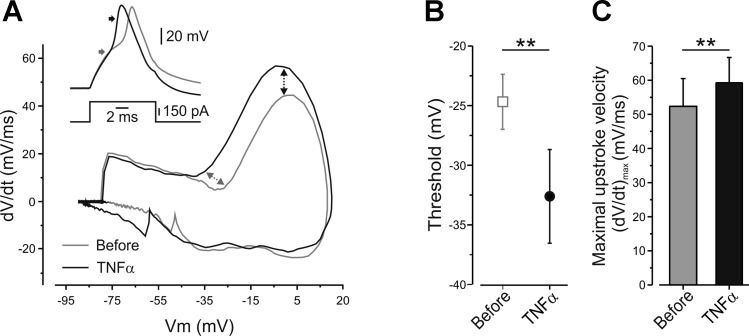
Fig. 4.
Application of TNF-α reduces the threshold and increases the maximal upstroke velocity of the AP, in the presence of TTX. A: representative phase plots of rate of change of the membrane potential (dV/dt) during an AP vs. membrane potential (Vm) recorded from the same neuron before (gray) and 10 min after application of TNF-α (black), in the presence of TTX. Note the leftward shift of the threshold voltage (gray arrows, quantified in B) and increase in maximal velocity, (dV/dt)max, of the upstroke of the AP (black arrows, quantified in C). Inset, traces of APs, before (gray) and after (black) application of TNF-α, which have been used for the generation of the phase plot, presented in A. The arrows indicate the estimated counterpart point presented in the phase plot graph. B: the thresholds for generation of APs, in the presence of TTX, calculated from the same neurons before and 10 min after application of TNF-α (means ± SE, **P < 0.01, paired t-test, n = 8). C: the maximal velocity (dV/dt)max of the upstroke of the AP before (gray) and after (black) application of TNF-α (means ± SE, **P < 0.01, paired t-test, n = 8).
Intracellular solutions contained the following (in mM): potassium-gluconate, 130; MgCl2, 2; KCl, 6; NaCl, 10; HEPES, 10; MgATP, 5; Li2GTP, 0.5 (pH = 7.4 with KOH). Extracellular solutions contained the following (in mM): NaCl, 145; KCl, 5; MgCl2, 1; CaCl2, 2; HEPES, 10; glucose, 10 (pH = 7.4 with NaOH). For TTX-r sodium channel-dependent excitability, cells were preincubated for 20–30 min with 250 nM TTX before recording, and 250 nM TTX were added to all extracellular solutions. Membrane potential was corrected for a liquid junction potential of −14.2 mV. Single APs were evoked by 10-ms depolarizing current pulses of 0.025-nA or 0.05-nA increments. The AP duration was measured at the half-maximal amplitude of the spike. The phase plots of dV/dt vs. membrane voltage were formed by plotting rate of change of membrane potential with respect to time (dV/dt), following a suprathreshold 10-ms current pulse, as a function of membrane potential. In control conditions, the voltage response to the first suprathreshold stimulus was analyzed. After the addition of TNF-α, the response to the current step of the same amplitude as in the control was analyzed. Maximum upstroke and repolarization velocities (V/s) were obtained from (dV/dt)max and (dV/dt)min, respectively. The AP thresholds were obtained by analyzing the phase plots (dV/dt) when plotted vs. time or vs. membrane voltage. The voltage of the threshold was measured using “first local minimum” of the function before the peak. The “first local minimum” was determined as the first minimal value of dV/dt after the peak, followed by an additional increase in the dV/dt, while analyzing the function from its positive peak to time “0”. The time for the first local minimum was defined as the time of threshold, and its voltage was then detected from the original trace. The rheobase (minimum current required to evoke an AP) and apparent input resistance (Rin) were measured using a series of 500-ms hyperpolarizing and depolarizing square pulses (1–2 nA in increments of 0.025–0.1 nA each). Rin was calculated as the slope of the voltage current curve in the linear part of the hyperpolarizing range. The AP amplitude was defined by subtraction of the threshold voltage from the peak AP voltage. The f-I relationship between applied stimuli and the frequency of AP firing following application of TNF-α gave best fit to the linear function y = mx, where m is a slope of the function that is referred to as the gain of a neuron (Kispersky et al. 2012). Time to discharge of the first AP was measured as the time from the ramp onset to the peak of the first AP. The AP accommodation was assessed as the time interval between first to last APs, generated during the ramp. For the lacosamide experiments only, the thresholds for the AP generation were determined from the response to the depolarizing ramps, as the potential at the onset of a clear, sharp deviation from passive response with subsequent AP generation.
Computer simulation.
The excitable properties of DRG neurons were simulated using NEURON simulation environment (Hines and Carnevale 1997). The simulations were performed in the single compartmental cylindrical model with diameter of 25 μm and length of 25 μm. The membrane capacitance value used in the model (1.275 μF/cm2) was derived from the measured averaged membrane capacitance from all recorded cells (25.7 pF) that was normalized to an area of the sphere of the model cell in (cm2) A = 1.96·10−5 cm2. Passive conductance value (Gpas) used in the simulation was calculated according to the actual Rin of the recorded neurons (650 MΩ) and equals 0.00005 S/cm2. The model includes slow-kinetic potassium current and fast-kinetic transient potassium current, TTX-s sodium current, TTX-s persistent sodium current, Nav 1.9 TTX-r sodium channels and Nav 1.8 TTX-r sodium channels. All kinetic parameters of the currents were adapted from Herzog et al. (2001) and Baker (2005). The voltage dependence of h-state of Nav 1.8 TTX-r sodium channels and channel conductances was slightly modified to match our experimental results.
Model of Nav 1.8 sodium current.
Since we assume that the slow inactive states are independent from other channel gating states, to model the slow inactivation of the Nav 1.8 channels we added s-variable to the Markovian Hodgkin-Huxley-like model (see Fig. 7A) where the transition between states is independent and occurs in a voltage-dependent manner. For simplicity, we assumed that transition to the slow inactive state is permitted from all fast states (see Fig. 7A) and occurs with similar rates. The transmembrane Nav 1.8 current is, therefore, described by the following equation:
where G1.8 represents the maximum conductance; ENa is the equilibrium potential for sodium; m and h are the voltage-dependent activation and inactivation gating dimensionless parameters for Nav 1.8 current, respectively; and s is the additional slow inactivation voltage-dependent variable. The entry and recovery from the slow inactivation state were modeled by using time constant τs as described in Blair and Bean (2003), where τs = (αs + βs)−1. Steady-state slow inactivation s∞ was calculated from the experimental data presented in Fig. 6E. Voltage-dependent rate constants αs and βs were calculated from τs and s∞, according to s∞ = αs/(αs + βs) and were fitted by the following rate constant equations:
and
where Aas = 0.00492, Bas = 44.86192, Cas = 5.9994, Abs = 0.00504, Bbs = 45.01948, and Cbs = −6.36602 are fitting constant parameter values.
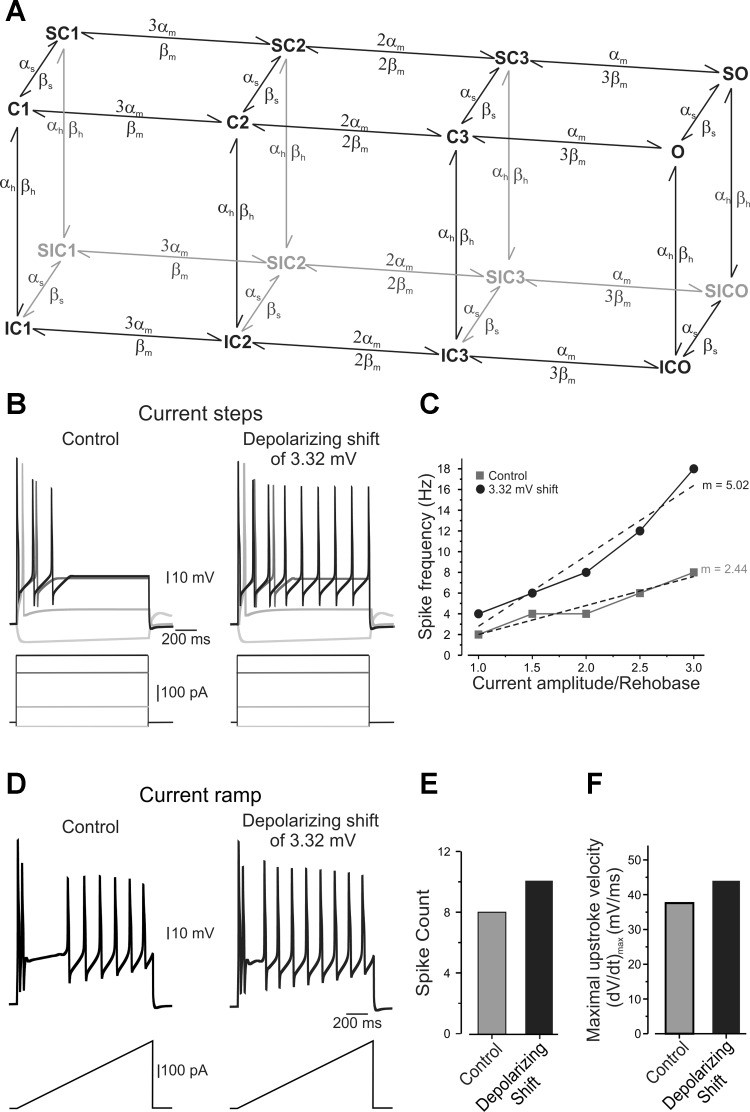
Fig. 7.
In compartmental simulations, rightward shift in voltage dependence of slow inactivation, equivalent to one produced by TNF-α, leads to increase in AP firing rate. A: a Markovian state model for TTX-r sodium channel represents two parallel planes: the first, a classic Hodgkin-Huxley like kinetic scheme with fast voltage-dependent transition; and the second, describing the slow inactivation states with much slower kinetics (see materials and methods). O denotes the open state; C denotes the closed states; IC and SC represent the fast and slow inactivation states, respectively; and SIC is the state in which the channel is inactivated by both slow and fast inactivation mechanisms. Activation occurs with the independent movement of gates, with voltage-dependent transitions between the multiple closed states. B: responses of simulated neuron to 2,000-ms hyperpolarizing and depolarizing suprathreshold current steps without (left) and with (right) 3.32-mV rightward shift in the voltage dependence of slow inactivation of slow Nav 1.8 channels (see materials and methods). A 3.32-mV shift was used to match the shift in voltage dependence in slow inactivation produced by TNF-α (Fig. 6 and Table 2). Note the increase in AP firing following the same stimuli (shown below) when rightward shift is applied. C: f–I curves calculated with (black circles) and without (gray squares) a 3.32-mV rightward shift in the voltage dependence of slow inactivation of Nav 1.8 channels, expressed as spike frequency evoked by suprathreshold stimulus and plotted against stimuli intensity normalized to rheobase. Note the significantly increased spike frequency and increase in slope (m) of the f–I curve following the shift. D: rightward shift of 3.32 mV in voltage dependence of slow inactivation in simulated neuron produced increased firing rate in response to 1.5-s-long depolarizing current ramps (333 pA/s, shown below). E and F: histograms plotting the spike count (E) and the maximal velocity (dV/dt)max of the upstroke of the AP (F) with (black) and without (gray) 3.32-mV depolarizing shift in the voltage dependence of slow inactivation of Nav 1.8 channels.
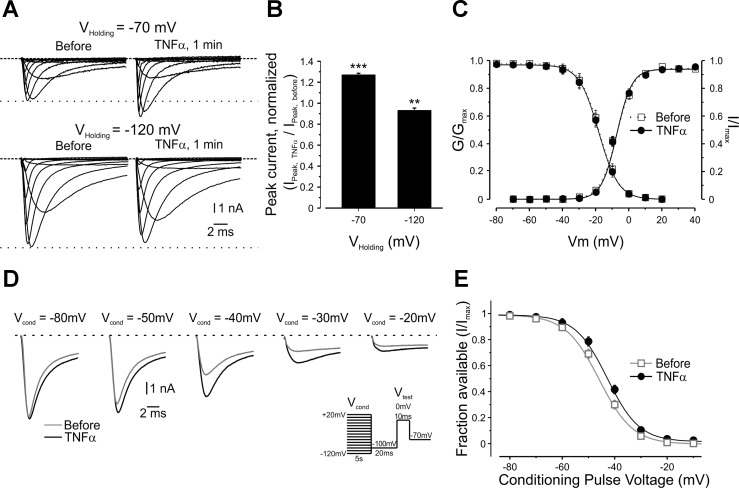
Fig. 6.
TNF-α increases TTX-r sodium currents in nociceptor-like DRG neurons by modulating voltage dependence of slow inactivation. A: representative families of TTX-r sodium currents evoked by a series of 150-ms, 10-mV depolarizing voltage steps from a holding potential of −70 mV (top) or −120 mV (bottom), recorded from the same neurons before and 1 min after application of TNF-α. The dashed lines indicate the zero current level. The dotted lines indicate the peak current level before application of TNF-α. B: histogram plotting the ratio of the peak sodium currents 1 min after TNF-α application (Ipeak,TNF-α) to the peak current before application (Ipeak,before) when elicited from a holding potential (Vholding) of −70 mV or −120 mV (means ± SE, ***P < 0.001, **P < 0.01, Student's t-test, n = 30). For A and B, currents are adjusted according to the baseline before the activation step. Note, significant increase in current at holding of −70 mV and significant decrease in current at holding of −120 mV. C: activation [conductance/maximum conductance (G/Gmax)] and availability [current/maximal current (I/Imax)] curves show no change in voltage dependence of activation (P = 0.607, paired t-test between the values of V1/2,before and V1/2,TNF-α, n = 30) and fast inactivation (P = 0.718, paired t-test between the values of V1/2,before and V1/2,TNF-α, n = 14), respectively, following TNF-α. D: superimposed representative traces of TTX-r sodium currents before (open squares) and 3 min after application of TNF-α (closed circles). Currents were elicited by 10-ms test steps to 0 mV, after 5-s conditioning pulses (Vcond) to the indicated voltages. Note that the substantial current reduction, following depolarized Vcond, was diminished by application of TNF-α. Currents are adjusted according to the baseline before the activation step. The dashed line indicates the zero current level. Inset: stimulus protocol (see materials and methods). The values of the current following the test step (Itest) were divided by the maximal current (Imax) and plotted as means ± SE against Vcond to calculate the fraction of channels available for activation at each holding voltage (E) (n = 25). The lines are the best fits to the Boltzmann function (see materials and methods). Note the increase in fraction of available channels following TNF-α [refer to Table 2 for values of the midpoints of voltage dependence (V1/2), values of slope and statistical analysis].
All the fast kinetics of Nav 1.8 channels were adapted from Herzog et al. (2001) and Baker (2005) as αm = 3.83/{1 + exp[(V + 2.58)/(−11.47)]}, βm = 6.894/{1 + exp[(V + 61.2)/19.8]}, αh = 0.013 exp[−(V + 80)/46.33], βh = 0.617/{1 + exp[−(V − 46.8)/11.99]}.
Modeling the response of Nav 1.8 sodium current to TNF-α.
For simulating the excitable properties of the neuron, we used the following fixed conductance parameters: GNav1.8 = 0.0125 s/cm2, GNav1.9 = 0.0008 s/cm2, GNaP = 0.00005 s/cm2, GTTXs = 0.001425 s/cm2, GKF = 0.000125 s/cm2, GKDR = 0.0013 s/cm2. ENa (+68 mV) and equilibrium potential for potassium (EK) (−85 mV) were calculated from the concentrations of sodium and potassium used in the external and internal solutions for current clamp recordings. As our results state, TNF-α caused a 3.32-mV rightward shift in the voltage dependence of slow inactivation of the slow TTX-r sodium currents. To model the effect of this shift on the excitable properties of the simulated neuron, we introduced a rightward shift modification in αs and βs in which we assigned V + vshift to V, where vshift is a parameter introduced to parallel shift the inactivation toward a more positive value. All other above-mentioned parameters were fixed at the initial values.
Drugs application.
All cells were perfused using a mini-valve perfusion system (Warner Instruments) with controlled pressure of 1–2 psi at a rate of ∼10 chamber volumes/min. “Vehicle” or “before” refer to bath solutions applied through the drug delivery system while recording, before application of the tested drugs. TNF-α (100 ng/ml) was diluted with PBS containing 0.1% BSA. For in vitro whole cell recordings, the fast-acting p38 MAPK inhibitor, SB202190 (100 μM, Sigma), and its inactive analog SB202474 (100 μM, Calbiochem), were used. Lacosamide (100 μM) and brilliant blue G (BBG), enhancer of slow inactivation of Nav channels (100 μM, Sigma), were first prepared as 10 mM stock solutions using extracellular solution. In the anisomycin experiments, neurons were divided into two groups and preincubated for 30–40 min prior to the recording with either vehicle (extracellular solution with 0. 1% of DMSO) or anisomycin, a nonspecific p38 MAPK activator (10 μg/ml, Sigma).
Statistical analysis.
Statistical analysis was performed using different modifications of Student's t-test appropriate to each type of experiment, and one- or two-way ANOVA, followed by Bonferroni posttest. Data are presented as means ± SE.
RESULTS
Acute TNF-α-mediated hypersensitivity is COX independent.
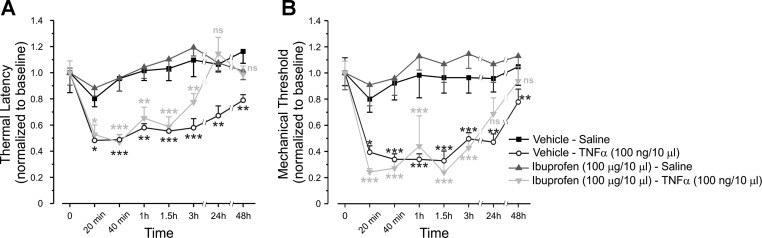
It is well documented that TNF-α mediates acute and long-lasting increase in pain sensitivity (Jin and Gereau 2006; Mannion et al. 1999; Woolf et al. 1997). To further explore the mechanisms of TNF-α's acute effect, we first examined whether COX-2 is involved in rapid TNF-α-mediated hypersensitivity to noxious mechanical and thermal stimuli in vivo. We found that preinjection of the nonselective COX inhibitor, ibuprofen, did not prevent the short-onset (20 min to 3 h after injection) decrease in the response latency to noxious heat and decrease in mechanical withdrawal threshold, which followed intraplantar injection of TNF-α (Fig. 1, A and B, respectively). However, it did abolish the delayed-onset, long-lasting (3 to 48 h after injection) TNF-α-mediated effect on thermal and mechanical thresholds, raising them to levels comparable to those of control animals (Fig. 1, A and B). These data suggest that TNF-α produces a rapid, COX-2-independent decrease in nociceptive thresholds, in addition to the long-lasting COX-2-dependent decrease.
Fig. 1.
Tumor necrosis factor (TNF)-α produces acute cyclooxygenase (COX)-2-independent hypersensitivity to noxious mechanical and thermal stimuli. Latency for paw withdrawal from hot plate (52°C), normalized to baseline (A) and mechanical threshold for paw withdrawal, normalized to baseline, measured by von Frey filaments (B), after intraplantar injection of vehicle, followed, 20 min later, by intraplantar injection of saline (Vehicle-Saline group, black squares); vehicle followed, 20 min later, by intraplantar injection of TNF-α (Vehicle-TNF-α group open circles); COX antagonist, ibuprofen, followed, 20 min later, by intraplantar injection of saline (Ibuprofen-Saline group, gray triangles); or ibuprofen followed by intraplantar injection of TNF-α (Ibuprofen-TNF-α group, light gray inverted triangles). The values are means ± SE; n = 6 for each group. Time point “0” refers to the baseline. Black asterisks, comparison between Vehicle-TNF-α group and Vehicle-Saline group; gray asterisks, comparison between Ibuprofen-TNF-α group and Ibuprofen-Saline group. Nonsignificant (ns), P > 0.05; ***P < 0.001; **P < 0.01; *P < 0.05; two-way ANOVA followed by Bonferroni post-test.
Excitability of nociceptor-like DRG neurons is acutely increased in the presence of TNF-α.
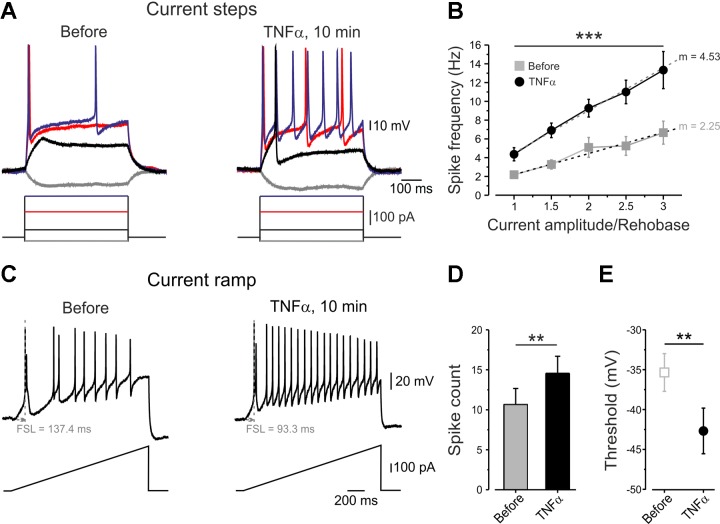
To study the mechanisms underlying the acute phase of TNF-α-mediated hyperexcitability, we compared the excitable properties of the same dissociated rat nociceptive-like DRG neurons (see materials and methods) before and shortly after bath application of TNF-α. Prolonged application of vehicle on these neurons (15 min, n = 7) did not produce any changes in the excitability parameters measured. However, application of TNF-α leads to rapid and significant decrease of rheobase in the majority (85%) of analyzed neurons (n = 11), such that previously subthreshold current steps became suprathreshold (Fig. 2A). TNF-α also produced an increase in the gain of nociceptor-like neurons (see materials and methods, Kispersky et al. 2012) (Fig. 2B; Table 1). Accordingly, suprathreshold current steps elicited more APs (Fig. 2, A and B). TNF-α significantly shortened the latency to the first AP (FSL, Fig. 2C, Table 1), increased the number of APs and decreased AP accommodation, as measured by applying slow depolarizing current ramps (Fig. 2, C and D, Table 1). This effect was first detected after 2.5 min and peaked at 10 min, with a stable plateau until washout (data not shown). Moreover, application of TNF-α produced a robust decrease in AP threshold (Fig. 2E, Table 1) and increase in single AP duration and single AP amplitude (Table 1). Together, these data suggest that TNF-α leads to acute increase in nociceptor excitability.
Fig. 2.
Application of TNF-α increases the excitability of nociceptor-like dorsal root ganglion (DRG) neurons. A: representative traces of membrane voltage responses to current steps, recorded from the same small nociceptor-like DRG neurons, before and 10 min after bath application of TNF-α. Note that, following the application of TNF-α, subthreshold stimuli become suprathreshold (Rheobasebefore = 187.3 ± 61.6 pA; RheobaseTNF-α = 139.1 ± 52.8 pA, P < 0.01, n = 11). B: frequency-intensity (f–I) curves calculated from the same neurons, before (gray squares) and 10 min after application of TNF-α (black circles), expressed as spike frequency (means ± SE) evoked by suprathreshold stimulus and plotted against stimuli intensity normalized to rheobase of each analyzed neuron. Note the significantly increased spike frequency (***P < 0.001, two-way ANOVA, n = 10) and increase in slope (m) of the f–I curve following application of TNF-α (P < 0.001, paired t-test, n = 11). C: representative traces of the membrane voltage responses to 1.5-s depolarizing current ramps (333 pA/s) recorded from the same neuron before and after application of TNF-α. Note that TNF-α reduced first spike latency (FSL) and increased action potential (AP) frequency. D: histogram plotting the spike count before and 10 min after application of TNF-α measured from traces as in C. Note the increase in the number of spikes (means ± SE, **P < 0.01, paired t-test, n = 10). E: AP thresholds for generation of APs, obtained by the phase plot analysis of AP, before and 10 min after application of TNF-α (means ± SE, **P < 0.01, paired t-test, n = 11).
Total sodium current in nociceptor-like DRG neurons increases in response to TNF-α.
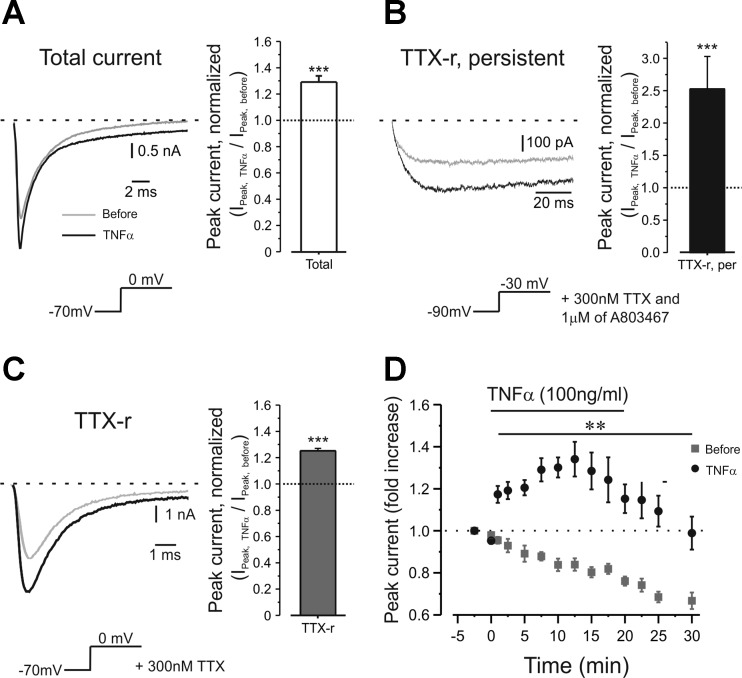
The observed TNF-α-mediated hyperexcitability could be explained by modulation of passive membrane properties, e.g., increase in the membrane Rin. However, we did not observe any changes in membrane Rin following application of TNF-α (Table 1, see also Fig. 2A). This implies that TNF-α affects neuronal excitability via modulation of voltage-dependent ion conductances. To further characterize the mechanism of TNF-α-induced nociceptive hyperexcitability, we used whole-cell voltage-clamp recordings and examined the amplitude of sodium currents in the same nociceptor-like DRG neurons before and after a short bath application of TNF-α. In the majority (78%) of the recorded neurons (n = 18), TNF-α produced a rapid (within 1 min) increase in total sodium current density (peak current density before = 203.1 ± 36.5 pA/pF; peak current density 1 min after TNF-α = 251.5 ± 39.1 pA/pF; n = 12, P < 0.0001; Fig. 3A, left and right), contrary to application of vehicle, for which current density decreased by about 4% (P < 0.05, n = 5), probably due to current rundown.
Fig. 3.
TNF-α increases the amplitude of tetrodotoxin-resistant (TTX-r) slow and TTX-r persistent sodium currents in nociceptor-like DRG neurons. A–C, left: representative traces of total (A), persistent TTX-r (B) and slow TTX-r (C) sodium currents recorded from the same neuron before (gray traces) and 1 min after application of TNF-α (black traces). The currents were elicited by protocols represented in the inset beneath the traces (for the detailed protocols, see materials and methods). The dashed line indicates the zero current level. Currents are adjusted according to the baseline before the activation step. Traces (150 μs) were blanked to remove residual uncompensated capacitance transient currents. Note the different scale bars between A, B and C. Right: ratio of the peak of total (A), persistent TTX-r (B) and slow TTX-r (C) sodium currents after TNF-α application (Ipeak,TNF-α) to the peak current before (Ipeak,before) from all measured cells (means ± SE, nTotal = 14, nTTX-r per = 5, nTTX-r = 30, paired t-test, ***P < 0.001). Dotted lines indicate the level of Ipeak,before. D: time course of changes of the peak amplitude of TTX-r sodium current assessed by 150-ms depolarizing steps from −70 mV to 0 mV during a 20-min application of TNF-α (black circles) or vehicle (gray squares) (n = 11 for time points −2.5 to 17.5; n = 7 for time point 20; n = 5 for time points 25 to 30; means ± SE). Time point −2.5 refers to data collected shortly after establishing the whole cell configuration when vehicle was applied. Time points 0 to 20 indicate time of application of TNF-α. Note fast onset of the effect of TNF-α (1 min) and decrease in sodium current amplitude when recorded without TNF-α (n = 9 for time points −2.5 to 10; n = 6 for time point 12.5 to 30; n = 5 for time points 25 to 30; means ± SE). Peak current normalized to peak current amplitude detected at −2.5 time point (3.1 ± 1.1 nA for the TNF-α group, 3.2 ± 0.6 nA, for the vehicle group, **P < 0.01, two-way ANOVA, n = 30) is marked by dashed line.
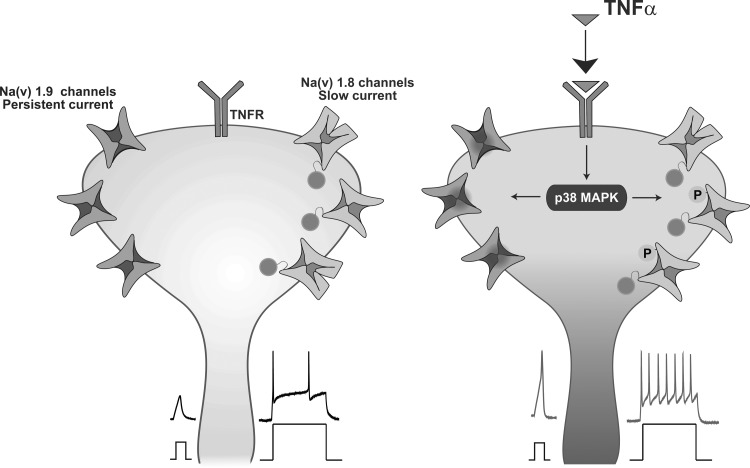
“Persistent” and “slowly activating and inactivating” components of TTX-r sodium current are enhanced by TNF-α.
The total sodium current in nociceptive DRG neurons is composed from a fast, TTX-s component and from a TTX-r component, which can be further subdivided into “slowly activating and inactivating” and “persistent” components. Each of these currents has distinctive kinetics and, therefore, contributes to different excitability parameters (Blair and Bean 2002; Herzog et al. 2001; Rush et al. 2007). TTX-s currents contribute to the “near-the-threshold” properties of the AP (Blair and Bean 2002), TTX-r persistent current controls subthreshold excitability (Baker et al. 2003; Cummins et al. 1999; Herzog et al. 2001) and slowly activating and inactivating TTX-r current contributes to most of the sodium current underlying APs (Blair and Bean 2002), also during repetitive firing (Blair and Bean 2003). To explain TNF-α-mediated acute changes in AP threshold and firing properties, we asked which of the fractions of the sodium currents are modulated by TNF-α. We first analyzed the amplitude and kinetics of the TTX-s component before and shortly after application of TNF-α. TNF-α produced a ∼10% increase in the amplitude of TTX-s current accompanied by a 4-mV leftward shift in the voltage dependence of activation from V1/2,control = −13.2 ± 1.9 mV to V1/2,TNF-α = −17.2 ± 2 mV.
To isolate the TTX-r persistent component, mediated by Nav 1.9 channels, from the total sodium current, we used the protocol described by Amaya et al. (2006) (see materials and methods) in the presence of 300 nM TTX, to block all TTX-s currents (Ogata and Tatebayashi 1992; Roy and Narahashi 1992) and 1 μM A803467 to minimize the residual persistent component of Nav 1.8 (Ramachandra et al. 2012; Scroggs 2011), which might be present considering 120 mM of NaCl used in external solution. The TTX-r persistent current amplitude was substantially increased shortly after application of TNF-α (Fig. 3B). This increase in persistent current likely underlies the TNF-α-mediated decrease in AP threshold (Baker 2005; Baker et al. 2003) and can also be associated with increase in firing rate (Baker 2005; Baker et al. 2003).
We examined the changes in amplitude and kinetics of the slowly activating and inactivating TTX-r sodium current (slow TTX-r current) in the presence of TNF-α. Most neurons (83%, n = 36) demonstrated a rapid increase in slow TTX-r sodium current density after a short exposure to TNF-α (Fig. 3, C and D, see also Fig. 6A). This increase was not accompanied by changes in reversal potential (Vrev,before = 53.3 ± 0.9 mV; Vrev,TNF-α = 53.8 ± 1, P = 0.51, paired t-test, n = 30). The effect of TNF-α was first detected after 1 min, peaked at 10 min and gradually decreased 10 min after TNF-α was washed out (Fig. 3D). This effect might be associated with the observed increase in the AP amplitude and increased gain (Blair and Bean 2003).
In summary, the substantial relative increase in TTX-r persistent and slowly activating and inactivating currents (Fig. 3) portray TTX-r currents as the major contributor to the observed TNF-α-mediated hyperexcitability.
To examine whether the effect on TTX-r sodium currents is sufficient to explain the changes in the excitability, we assessed the effect of TNF-α on excitable parameters of the DRG neurons in the presence of 250 nM TTX for which TTX-s channels are blocked and only TTX-r channels are functional (Catterall et al. 2005; Ogata and Tatebayashi 1993; Roy and Narahashi 1992). In the majority (91%) of recorded cells, application of TNF-α leads to an increase in AP firing and increase in the slope of the f-I curve (Table 1), qualitatively, in a similar manner as in the absence of TTX.
We further analyzed the mechanism underlying TNF-α-mediated changes in AP properties by comparing phase plots (the rate of change of membrane voltage as a function of membrane voltage, dV/dt vs. Vm) of single APs before and after application of TNF-α in the presence of 250 nM TTX (Fig. 4A). We found that TNF-α produced a robust decrease in the AP threshold (Fig. 4B, Table 1), similarly to what we observed in the absence of TTX (P = 0.65, n = 8, two-sampled t-test comparing between TTX and TTX-free groups), lending to the idea that TTX-s currents have a small effect on AP threshold in the presence of TNF-α.
The analysis of the phase plots also allowed us to determine maximal upstroke velocity (dV/dt)max which is solely dependent on the maximal availability of sodium channels during an AP, since at this phase the contribution of conductances of other ions is negligible (Bean 2007; Jenerick 1963). Application of TNF-α led to a significant increase in upstroke velocity (Fig. 4, A and C), suggesting that TNF-α increases the number of available TTX-r sodium channels.
Collectively, these results suggest that modulation of persistent and slow TTX-r sodium currents has a substantial contribution to the acute TNF-α-mediated nociceptive hyperexcitability, such that the increase in the slow and persistent components may underlie the increased gain and augmentation of the persistent component contributes to decreased threshold.
Leftward shift in the voltage dependence of activation underlies the effect of TNF-α on TTX-r persistent current.
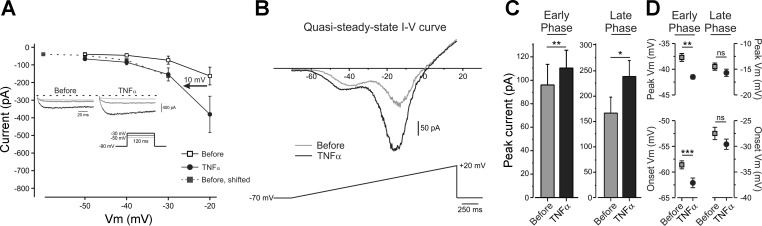
We next examined the mechanism of TNF-α-mediated increase in the persistent TTX-r current. We performed an analysis of voltage dependence of activation of TTX-r persistent currents in the presence of 300 nM TTX and 1 μM A803467 (see materials and methods). TNF-α produced a significant hyperpolarizing shift of about 10 mV in the voltage dependence of activation of TTX-r persistent current (Fig. 5A). The effect of TNF-α on the kinetics and the amplitude of the “persistent” component of the sodium currents was also observed when we monitored currents evoked by applying slow (0.036 mV/ms, 2.5 s, Fig. 5B) voltage ramps, which inactivate nonpersistent components of the sodium current (Binshtok et al. 2008; Fleidervish and Gutnick 1996; Maingret et al. 2008). The majority of cells exhibited a “W” shaped slow ramp current with an early small inward component (peak amplitude = 95.9 ± 17.8 pA, detected at −37.7 ± 1 mV, n = 6) and a larger late inward component (peak amplitude = 166.6 ± 30 pA, detected at 14.5 ± 1 mV n = 6) (Fig. 5, B–D, black). Based on their voltage dependence, the early component is likely to be mediated by TTX-r persistent current, whereas the late component represents a non-inactivating fraction of slow TTX-r current (Maingret et al. 2008). Application of TNF-α produced a substantial increase in the amplitude of both early and late components (Fig. 5, B, black, and C) and significantly shifted the onset and peak of the early component in a hyperpolarized direction (Fig. 5D), in agreement with the shift in the activation curve observed. Moreover, analysis of charge density, measured as the area under the I–V curves in the “−70 mV to −40 mV” range of membrane potentials (see materials and methods), revealed that application of TNF-α led to a 63% increase in charge density at the subthreshold potentials (AUCbefore = 485.1 ± 117.1; AUCTNF-α = 790.1 ± 139.5; P < 0.0001; n = 6, paired t-test). This likely contributes to the decrease in threshold.
Fig. 5.
TNF-α shifts the activation of TTX-r persistent in hyperpolarized direction. A: averaged peak current-voltage (I–V) characteristics of TTX-r persistent sodium current, before (open squares) and 1 min after application of TNF-α (black circles). To estimate the change in the voltage dependence of activation, we calculated the voltage shift, which entails the minimum “distance” between the sum of control current values [Ibefore (V)] and their approximate equivalents after TNF-α [ITNF-α (V + vstep)]. To this end, Ibefore (V) values were shifted in steps with 0.01-mV increments towards ITNF-α (V), while ITNF-α (V) was kept constant. The shift (arrow) of 10 mV gave the best fit (see materials and methods). Inset: representative traces of TTX-r persistent sodium currents, recorded in the presence of TTX and a blocker of voltage-gated sodium (Nav) 1.8 channels, A803467, elicited by 120-ms steps from a holding potential of −90 mV to −50 mV (light gray), −40 mV (gray) and −30 mV (black) before and 1 min after application of TNF-α. Currents are adjusted according to the baseline before the activation step. The dashed line indicates the zero current level. B: representative instantaneous I–V curve during a slow (36 mV/s) depolarizing ramp from −70 to +20 mV before (gray) and 1 min after application of TNF-α (black). Note “W” shaped inward current with clear early and late components. TNF-α increased the amplitude of both early and late components of the inward current (C) (**P < 0.01, *P < 0.05, paired t-test, n = 6) and shifted the onset (circles) and peak activation (squares) of early, but not the late, component to more negative potentials (D) (***P < 0.001, **P < 0.01, *P > 0.05, paired t-test, n = 6).
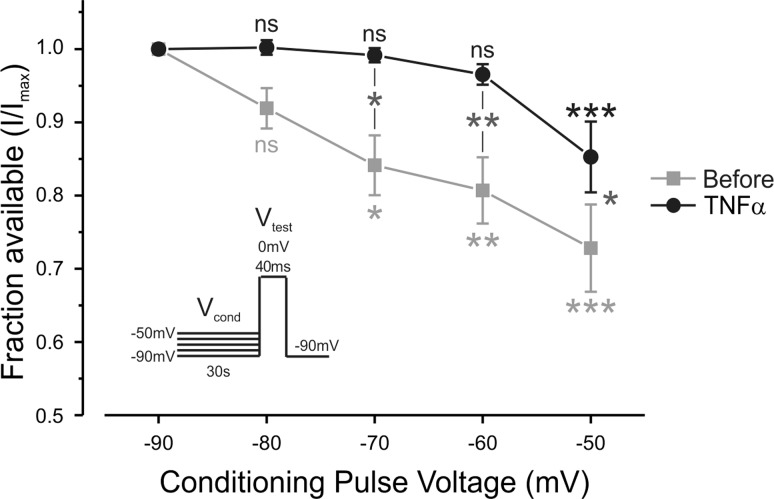
Partial relief of resting slow inactivation of TTX-r sodium channels underlies the effect of TNF-α on their availability.
The voltage clamp experiments and phase plot analysis suggest that TNF-α's effect is mediated by increased availability of TTX-r sodium channels. We first examined the possibility that TNF-α leads to increase in the amount of functional TTX-r sodium channels on the plasma membrane. To this end, we compared the maximal TTX-r current (see materials and methods) before and after application of TNF-α. The maximal current amplitude, evoked from a holding potentials of −120 mV (where all channels are available), did not increase but rather decreased following application of TNF-α, most probably due to the rundown of the sodium current (Fig. 6, A, bottom traces, and B), suggesting that TNF-α does not lead to increase in channel density at the plasma membrane. The TNF-α-mediated increase in TTX-r current assayed from a holding potential of −70 mV also did not result from acute changes in current kinetics and voltage dependence of activation or fast inactivation (Fig. 6C).
We raised the possibility that TNF-α enhances TTX-r current density by increasing the functional availability of the existing TTX-r channels, defined by properties of slow inactivation of these channels. Prolonged holding of the membrane at depolarized potentials resulted in substantial decrease in the amplitude of the TTX-r currents (Fig. 6D, black), revealing the impact of slow inactivation on the availability of TTX-r channels (Fig. 6E, black). Application of TNF-α reversed the decrease in the amplitude of TTX-r sodium currents to a certain extent (Fig. 6D, black), by partial relief of the voltage dependence of slow inactivation (Fig. 6E, black, Table 2), thus increasing the availability of TTX-r sodium channels. Application of TNF-α caused a small rightward shift in the voltage dependence of slow inactivation of the TTX-r sodium currents [ΔV1/2,TNF-α (V1/2,Control − V1/2,Treatment) = 3.32 ± 0.53 mV; P < 0.0001, n = 25, Table 2], without affecting the steepness of the curve (a Boltzmann slope factor). The change in availability became statistically significant at −70 mV (P < 0.05, paired t-test, n = 25) and reached a peak difference in availability at −40 mV (Fig. 6, D and E), leading to the addition of about 15% available TTX-r sodium channels at this potential. All of the examined cells demonstrated a rightward shift following application of TNF-α, some of the cells demonstrated a prominent ∼10 mV shift and other cells demonstrated a less prominent (∼2 mV) shift.
Table 2.
Values and statistical analysis of differences in voltage dependence of slow inactivation (ΔV1/2 and slope) following treatments
| ΔV1/2 |
ΔSlope |
|||||
|---|---|---|---|---|---|---|
| Means ± SE | P value | n | Means ± SE | P value | n | |
| Vehicle | −1.9 ± 0.4 | <0.05 | 6 | −0.07 ± 0.1 | ns | 6 |
| TNF-α | +3.32 ± 0.5 | <0.0001 | 25 | −0.27 ± 0.1 | ns | 25 |
| Lacosamide | −6.05 ± 2.1 | <0.05 | 4 | −0.84 ± 1.0 | ns | 4 |
| Lacosamide + TNF-α | −0.49 ± 0.7 | ns | 8 | 0.39 ± 0.3 | ns | 8 |
| BBG | −7.77 ± 2.0 | <0.05 | 4 | 1.2 ± 0.4 | ns | 4 |
| BBG + TNF-α | −3.29 ± 2.5 | ns | 4 | 0.51 ± 0.3 | ns | 4 |
| SB202190 | −3.26 ± 1.1 | <0.05 | 6 | 0.08 ± 0.1 | ns | 6 |
| SB202190 + TNF-α | −1.03 ± 0.2 | ns | 14 | 0.05 ± 0.1 | ns | 14 |
| SB202474 + TNF-α | +2.41 ± 0.4 | <0.0001 | 18 | −0.25 ± 0.1 | ns | 18 |
Values are means ± SE; n, no. of neurons. Change in voltage at which the sodium current was half-maximally activated (ΔV1/2) is defined as V1/2,Treatment − V1/2,Control, where V1/2,Control is the value measured during application of vehicle, and V1/2,Treatment is the value recorded from the same neuron after application of treatment. Minus sign describes shift in hyperpolarized direction. Plus sign describes shift in depolarized direction. Data were analyzed using paired t-test. SB202190 + TNF-α, 2 min of application of SB202190 followed by a 1-min coapplication with TNF-α; SB202474 + TNF-α, 2 min of application of SB202474 followed by a 1-min coapplication with TNF-α; Lacosamide + TNF-α, lacosamide following 1-min coapplication with TNF-α; BBG + TNF-α, brillian blue G following 1-min coapplication with TNF-α. The parameters that changed significantly are bolded. ns, P > 0.05.
Next we examined whether this shift is sufficient to change the excitable properties of DRG neurons. To this end, we built a simplified single compartment numerical model of a nociceptive-like DRG neuron using NEURON simulating environment (Hines and Carnevale 1997). To simulate electrical properties of DRG neurons, we based our model on previously described models of nociceptive-like DRG neurons (Baker 2005; Herzog et al. 2001). We adapted from these models the kinetic properties of the delayed rectifier potassium current and fast-kinetic transient potassium current, TTX-s sodium current, TTX-s persistent sodium current, Nav 1.9 TTX-r sodium channels and Nav 1.8 TTX-r sodium channels (see materials and methods). To explore the influence of slow inactivation on the availability of slow TTX-r sodium channels, we modified the kinetic properties of Nav 1.8 by introducing an additional slow inactivation state s (Fig. 7A). To model this state, we used the Markovian Hodgkin-Huxley-like model which assumes, for simplicity, independent interstate transitions. The kinetic properties of s were derived from our experimental data, τs was adapted from Blair and Bean (2003) (see materials and methods). The simulated responses to current steps (Fig. 7B, left) and depolarizing ramps (Fig. 7D, left) replicated experimental findings quite well (see Fig. 2). We then shifted the voltage dependence of slow inactivation by exactly the same numerical value as it observed experimentally, following TNF-α application (a rightward shift of 3.32 mV), while keeping all other parameters fixed. This shift caused no significant alteration in either resting potential (Vrest,control = −66.5 mV, Vrest,shift = −66.5 mV) or in AP threshold (Vthreshold,control = −35 mV, Vthreshold,shift = −35 mV), but led to a 6 mV/ms increase in (dV/dt)max (Fig. 7F) and substantially increased AP firing rate during the prolonged current steps (Fig. 7, B, right, and C) and ramps (Fig. 7, D, right, and E), similarly to the change in AP firing shown experimentally following application of TNF-α (see Fig. 2). These simulated results suggest that the TNF-α-mediated small rightward shift in voltage dependence of slow inactivation is sufficient to partially explain TNF-α-induced increase in excitability, namely increase in neuronal gain.
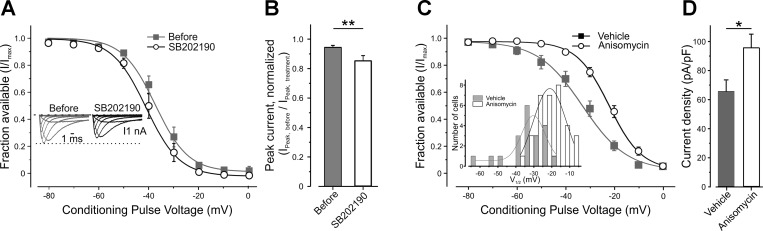
p38 MAPK modulates gating of TTX-r sodium channels.
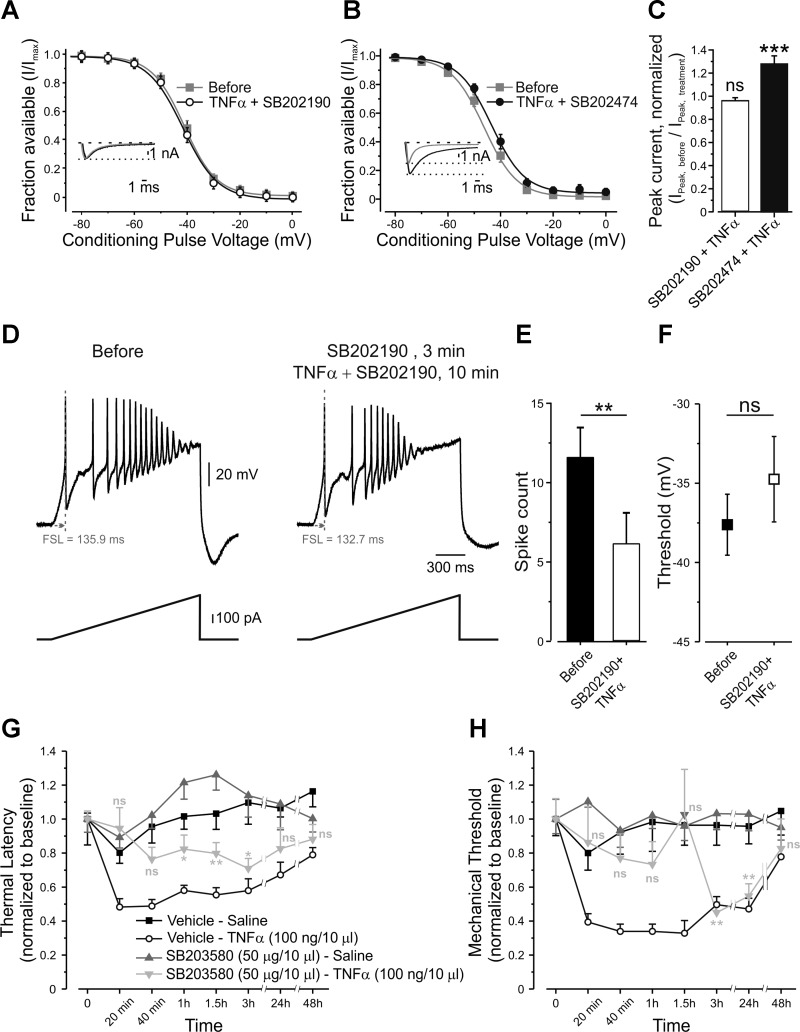
We further explored the pathway linking TNF-α to its effect on slow inactivation of TTX-r sodium channels. It has been demonstrated that TNF-α induces increased activation of p38 MAPK in DRG neurons (Jin and Gereau 2006; Pollock et al. 2002). Furthermore, p38 MAPK mediates TNF-α-induced enhancement of TTX-r sodium currents (Jin and Gereau 2006). Based on these results, we investigated whether the activation of p38 MAPK by TNF-α leads to gating modulation of persistent and slow TTX-r currents. Pretreatment of nociceptor-like DRG neurons with fast-acting p38 MAPK inhibitor SB202190 (10 μM, 3 min) substantially reduced the TNF-α-mediated increase in persistent inward current and prevented the leftward shift in its activation (Table 3). Moreover, pretreatment with SB202190, but not with its inactive analog SB202474 (10 μM, 3 min), abolished the TNF-α-mediated relief in slow inactivation of TTX-r sodium channels (Fig. 8, A and B, Table 2). Inhibition of p38 MAPK by SB202190 also prevented increase in TTX-r current amplitude (Fig. 8C) and prevented TNF-α-mediated changes in neuronal excitability (Fig. 8, D, E, and F, Table 3). Moreover, intraplantar injection of the slow-acting p38 MAPK inhibitor, SB203580, 20 min before intraplantar injection of TNF-α, delayed the onset of TNF-α-mediated decrease in thermal latency by 1 h and mechanical withdrawal threshold by 3 h, after injection (Fig. 8, G and H, respectively, light gray inverted triangles). On the other hand, injection of TNF-α after vehicle produced an immediate (20 min) effect (Fig. 8, G and H, open circles).
Table 3.
Values and statistical analysis of differences in membrane excitability parameters and quasi-steady-state I–V curve parameters before (control) and after application of SB-202190 together with TNF-α
| Control | TNF-α + SB202190 | P Value | n | |
|---|---|---|---|---|
| Membrane excitability parameters (assessed by current clamp) | ||||
| Threshold, mV | −37.6 ± 1.4 | −34.74 ± 2.69 | ns | 8 |
| Rheobase, pA | 230.6 ± 49.6 | 238.8 ± 52.5 | ns | 8 |
| FSL (depolarizing ramp), ms | 508.1 ± 98.5 | 559.1 ± 108.53 | ns | 8 |
| (dV/dt)max, mV/ms | 76.52 ± 10.2 | 58.6 ± 9.64 | <0.01 | 8 |
| AP count (depolarizing ramp) | 11.37 ± 1.89 | 6 ± 1.95 | <0.01 | 8 |
| Quasi-steady state I–V curve parameters (assessed by voltage clamp) | ||||
| Onset early phase, mV | −62.6 ± 0.9 | −63.4 ± 1.5 | ns | 6 |
| Peak early phase, mV | −38.6 ± 0.9 | −40.9 ± 1 | <0.05 | 6 |
| Charge density from −70 mV to −40 mV | 625 ± 88 | 725 ± 116 | <0.05 | 6 |
Values are means ± SE; n, no. of neurons. Data describing threshold and rate of change of membrane potential with respect to time [(dV/dt)max] were obtained from phase plots of changes in membrane voltage following application of 10-ms rectangular current steps. Data describing FSL and AP count were obtained following 1,500-ms depolarizing ramps. Data describing the rheobase were obtained following 500-ms rectangular current steps. Data describing the parameters of quasi-steady-state current-voltage (I–V) curves were obtained in voltage clamp recording, following application of slow (0.036 mV/ms, 2.5 s, Fig. 3D) voltage ramps. Data were analyzed using two-tail paired t-tests. The parameters which changed significantly are bolded. Note that application of SB202190 together with TNF-α lead to a significant decrease in (dV/dt)max and AP count. ns, P > 0.05.
Fig. 8.
TNF-α-mediated relief of voltage dependence of slow inactivation and increase in nociceptive excitability requires p38 MAPK. A: voltage dependence of slow inactivation of TTX-r sodium currents, expressed as peaks of TTX-r sodium currents during a step to 0 mV, normalized to Imax (fraction available, means ± SE) and plotted against conditioning voltages, before (gray squares) and 2 min after application of p38 MAPK antagonist SB202190, followed by a 1-min coapplication with TNF-α (open circles, paired t-test between the values of V1/2,before and V1/2,TNF-α, P > 0.05, n = 14, see also Table 2). Inset: representative traces of TTX-r sodium currents, recorded in the presence of TTX, elicited by 150-ms steps from a holding potential of −70 mV to 0 mV before (gray) and 2 min after application of p38 MAPK antagonist SB202190, followed by a 1 min coapplication with TNF-α (black). Currents are adjusted according to the baseline before the activation step. The dashed line indicates the zero current level. The dotted line indicates the peak current level before application of TNF-α. B: voltage dependence of slow inactivation of TTX-r sodium currents, assessed as explained in A, before (gray squares) and 2 min after application of the inactive analog, SB202474, followed by a 1-min coapplication with TNF-α (black, paired t-test between the values of V1/2,before and V1/2,TNF-α, P < 0.0001, n = 18; see also Table 2). Inset: representative traces of TTX-r sodium currents, recorded in the presence of 300 nM TTX, elicited by 150-ms steps from a holding potential of −70 mV to 0 mV before (gray) and 2 min after application of the inactive analog SB202424, followed by 1-min coapplication with TNF-α (black). The dashed line indicates the zero current level. The dotted line indicates the peak current level before application of TNF-α. C: the ratio of peak TTX-r sodium current before application of treatment (INa,before) to the peak current after application (INa,treatment) of SB202190 + TNF-α (n = 14) and SB202474 + TNF-α (n = 18). The values are means ± SE; ns, P > 0.05, ***P < 0.001, one-sample t-test comparing the values before and after the application of treatment. D: representative traces of current clamp recordings from the same nociceptor-like DRG neuron, following 500 pA, 1.5-s depolarizing ramps before (left) and 3 min after application of p38 MAPK antagonist SB202190, followed by a 10-min coapplication with TNF-α (right). FSL, first spike latency. E: SB202190 prevents the effect of TNF-α on spike count (means ± SE, **P < 0.01, paired t-test, n = 8) and threshold for generation of AP (F) (means ± SE, ns, P > 0.05, paired t-test, n = 8). G: latency for paw withdrawal from hot plate (5°C), normalized to baseline, after intraplantar injection of vehicle followed, 20 min later, by intraplantar injection of saline (Vehicle-Saline, black squares); intraplantar injection of vehicle followed, 20 min later, by intraplantar injection of TNF-α (Vehicle-TNF-α, open circles); intraplantar injection of p38 MAPK inhibitor, SB203580, followed, 20 min later, by intraplantar injection of saline (SB203580-Saline, gray triangles); or intraplantar injection of SB203580 followed by intraplantar injection of TNF-α (SB203580-TNF-α, light gray inverted triangles). The values are means ± SE. Time point “0” refers to the baseline. Gray asterisks, comparison between SB203580-Saline group to SB203580-TNF-α group. ns, P > 0.05; **P < 0.01; *P < 0.05; two-way ANOVA followed by Bonferroni post-test, n = 6 for each group. H: mechanical threshold for paw withdrawal, normalized to baseline, obtained by von Frey filaments. Time point “0” refers to the baseline. Symbols are as in G.
These data suggest that activation of p38 MAPK is necessary for the TNF-α-mediated hyperpolarized shift in activation of TTX-r persistent channels and for modulation of voltage dependence of slow inactivation and thereby for acute TNF-α-induced hypersensitivity.
Interestingly, inhibition of p38 MAPK by SB202190 leads to enhancement of resting slow inactivation of TTX-r sodium channels (Fig. 9A, Table 4) and to decrease in the TTX-r current, which was significantly lower than the decrease as a result of the current rundown (Fig. 9B). This suggests that the degree of slow inactivation of TTX-r sodium channels can be adjusted by modulating the activity of p38 MAPK, and that p38 MAPK has sustained basal activity, which constantly affects the degree of slow inactivation. This would suggest that activation of p38 MAPK leads to relief of voltage dependence of slow inactivation. It has been demonstrated that activation of p38 MAPK leads to increase in TTX-r sodium current, without affecting voltage dependence of activation or fast inactivation (Hudmon et al. 2008). We sought to explore whether activation of p38 MAPK is sufficient for changing voltage dependence of slow inactivation of TTX-r sodium channels. To this end, we pretreated DRG neurons with either anisomycin, a cell-permeable activator of p38 MAPK (Ogawa et al. 2004; Wittmack et al. 2005), or with vehicle (0.1% DMSO). Voltage dependence of slow inactivation measured 30 min after pretreatment with anisomycin was dramatically right shifted to depolarized voltages (Fig. 9C, V½,vehicle: −32.9 ± 2.5 mV, n = 20; V½,anisomycin: −21.62 ± 1.3 mV, n = 26; P < 0.001), indicating that activation of p38 MAPK relieves the voltage dependence of slow inactivation. TTX-r sodium current density was also significantly increased after pretreatment with anisomycin (P < 0.05, two-sampled t-test, n = 20; Fig. 9D).
Fig. 9.
p38 MAPK modulates voltage dependence of slow inactivation. A: voltage dependence of slow inactivation of TTX-r sodium currents, expressed as peaks of TTX-r sodium currents during a step to 0 mV, normalized to Imax (fraction available, means ± SE) and plotted against conditioning voltages before (gray squares) and 3 min after application of SB202190 (open circles, paired t-test, P < 0.05, n = 7. See Table 2 for the values of the V1/2 and slope and statistical analysis). Inset: representative families of TTX-r sodium currents, recorded in the presence of TTX, elicited by 150-ms, 10-mV steps from a holding potential of −70 mV before (gray) and 3 min after application of SB202190 (black). Currents are adjusted according to the baseline before the activation step. The dashed line indicates the zero current level. The dotted line indicates the peak current level before application of SB202190. B: the ratio of peak TTX-r sodium current before application of treatment (INa,before) to the peak current after application (INa,treatment) of vehicle (n = 11) or SB202190 alone (n = 6). Values are means ± SE; **P < 0.01, one sample t-test comparing the values before and after the application of treatment. C: voltage dependence of slow inactivation of TTX-r sodium currents, expressed as peaks of TTX-r sodium currents during steps to 0 mV, normalized to Imax (fraction available, means ± SE) and plotted against conditioning voltages, measured 30 min after pretreatment with the p38 MAPK activator anisomycin (open circles, n = 26) or vehicle (black squares, n = 20), P < 0.01, two-sampled t-test; see also Table 3 for numerical data. Inset: histograms of the distribution of the values of V1/2 of voltage dependence of slow inactivation of TTX-r sodium currents, recorded form neurons preincubated for 30 min with vehicle (gray, n = 20) or 10 μg/ml anisomycin (white, n = 26). Fitting of the distribution with the amplitude version of Gaussian peak function, y = y0 + A × exp(−0.5 × {[V1/2 − V1/2(c)]/w})2, where A is the peak number of cells and V1/2(c) is the value of V1/2 which corresponds to A; black solid line for vehicle; gray solid line for anisomycin, reveals that anisomycin produced substantial rightward shift of population of V1/2 values [V1/2(c),Vehicle = −30.3 ± 1.5 mV; V1/2(c),Anisomycin = −21.2 ± 0.9 mV]. D: the peak TTX-r current density, measured from currents elicited by depolarizing steps to 0 mV from a holding potential of −70 mV, in cells pretreated with anisomycin or vehicle. Values are means ± SE, *P < 0.01, two sampled t-test, n = 26 for anisomycin group, n = 20 for vehicle group.
Table 4.
Values and statistical analysis of differences in membrane excitability parameters before application of TNF-α, 10 min after application of TNF-α and 5 min after application of lacosamide
| TTX Free |
TTX 250 nM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | TNF-α | TNF-α + Lacosamide | P value | n | Control | TNF-α | TNF-α + Lacosamide | P value | n | |
| Threshold (depolarizing ramp), mV | −37.8 ± 2.7 | −48 ± 3.8 | −35.8 ± 5 | P1 < 0.01, P2 < 0.01, P3 = 0.29 | 5 | −21.29 ± 5.15 | −31.43 ± 5.05 | −22.71 ± 3.93 | P1 < 0.01, P2 < 0.05, P3 = 0.4 | 6 |
| AP count (depolarizing ramp) | 9.67 ± 2.6 | 15.67 ± 2.8 | 9.33 ± 2.9 | P1 < 0.001, P2 < 0.001, P3 = 0.8 | 5 | 6.17 ± 0.54 | 10.83 ± 0.87 | 5.5 ± 0.67 | P1 < 0.001, P2 < 0.01, P3 = 0.36 | 6 |
| First AP latency (depolarizing ramp), ms | 263.9 ± 96 | 231.83 ± 101.2 | 420.08 ± 122.2 | P1 < 0.05, P2 = 0.14, P3 = 0.2 | 5 | 612.42 ± 107.5 | 397.38 ± 70.4 | 569.63 ± 78.32 | P1 < 0.01, P2 < 0.01, P3 = 0.58 | 6 |
| 1st to lastAP interval (depolarizing ramp), ms | 983.2 ± 52.4 | 1242 ± 96.8 | 718.8 ± 81 | P1 < 0.05, P2 = 0.064, P3 = 0.989 | 5 | 717.98 ± 94.6 | 1014.92 ± 50.3 | 547.82 ± 99.4 | P1 < 0.01, P2 < 0.05, P3 = 0.58 | 6 |
Values are means ± SE; n, no. of neurons. Data were obtained following 1,500-ms depolarizing ramps. P1, comparison between control and TNF-α group; P2, comparison between TNF-α and TNF-α + Lacosamide group; P3, comparison between control and TNF-α + Lacosamide group. Data were analyzed using paired t-test.
These data suggest that p38 MAPK activity regulates the level of voltage dependence of slow inactivation of TTX-r channels.
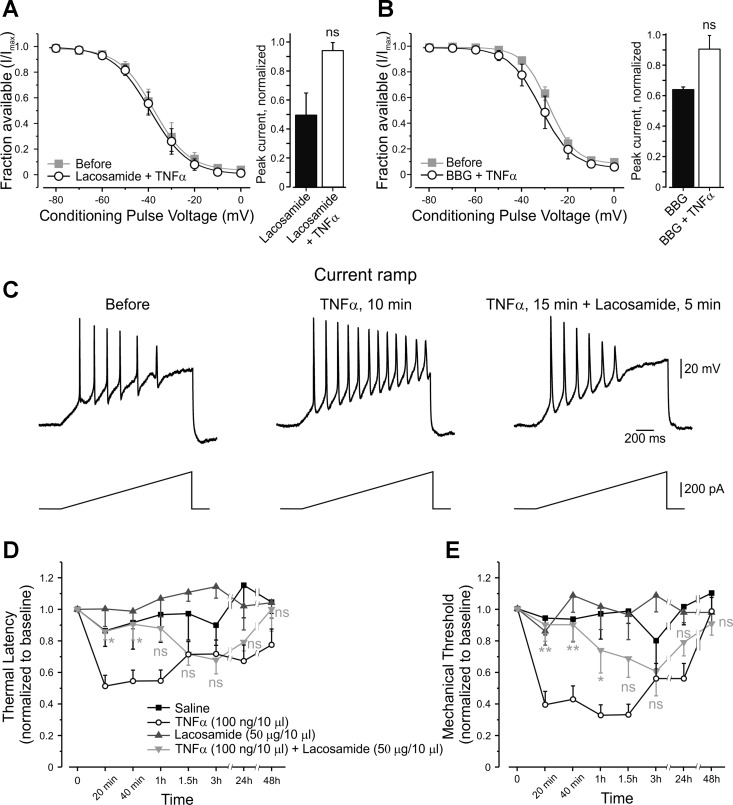
Pharmacological enhancement of TTX-r sodium channel slow inactivation eliminates the effect of TNF-α.
We next examined the effect of pharmacologically induced enhancement of slow inactivation on TNF-α-mediated neuronal hyperexcitability. A short (3–5 min) application of lacosamide, which has been shown to produce a hyperpolarizing shift of voltage dependence of slow inactivation of sodium channels (Sheets et al. 2008), prevented the TNF-α-mediated rightward shift in voltage dependence of slow inactivation (Fig. 10A, left, Table 2) and TNF-α-mediated increase in sodium current density (Fig. 10A, right). To control for the effect of lacosamide on slow inactivation, we used BBG, which also enhances slow inactivation of sodium channels (Jo and Bean 2011). Application of BBG mimicked the effects of lacosamide (Fig. 10B, Table 2).
Fig. 10.
Pharmacological enhancement of voltage dependence of slow inactivation reverses the TNF-α-mediated relief of slow inactivation and diminishes the effect of TNF-α on nociceptive excitability. A, left: voltage dependence of slow inactivation of TTX-r sodium currents, expressed as peaks of TTX-r sodium currents during a step to 0 mV, normalized to Imax (fraction available, means ± SE) and plotted against conditioning voltages before (gray squares) and after application of TNF-α, together with lacosamide (open circles). Note: there was no change in voltage dependence of slow inactivation (see Table 2 for V1/2,slope values and statistical analysis, n = 8). A, right: the ratio of peak TTX-r sodium current before application of treatment (INa,control) to the peak current after the treatment (INa,treatment) with lacosamide and lacosamide + TNF-α. Values are means ± SE, n = 10; P = 0.32, compared with the control, one-sample t-test. B, left: voltage dependence of slow inactivation of TTX-r sodium currents, before (gray squares) and after application of TNF-α, together with brilliant blue G (BBG) (open circles). Note: there was no change in voltage dependence of slow inactivation (see Table 2 for V1/2,slope values and statistical analysis, n = 4). B, right: the ratio of peak TTX-r sodium current before application of treatment (INa,control) to the peak current after the treatment (INa,treatment) with BBG and BBG + TNF-α. Values are means ± SE, n = 4, P = 0.6, compared with the control, one-sample t-test. C, top: representative traces of current clamp recordings from the same nociceptor-like DRG neuron, following 500 pA, 1.5-s depolarizing ramps in control condition (left trace), 10 min after application of TNF-α (middle trace) and 5 min after application of lacosamide on top of 10 min application of TNF-α (right trace). See Table 4 for the numerical data and statistical analysis. Bottom: stimulation protocol. D: latency for paw withdrawal from hot plate (52°C), normalized to baseline, after intraplantar injection of saline (black squares); TNF-α alone (open circles); lacosamide alone (gray triangles); or TNF-α applied together with lacosamide (light gray inverted triangles). The values are means ± SE. Time point “0” refers to the baseline. Gray asterisks, comparison between the TNF-α group to TNF-α + Lacosamide group; ns, P > 0.05, *P < 0.05, two-way ANOVA followed by Bonferroni posttest, n = 6 for each group. E: mechanical threshold for paw withdrawal, normalized to baseline, obtained by von Frey filaments. The values are means ± SE, n = 6 for each group. Time point “0” refers to the baseline. Symbols are as in E; **P < 0.01.
We performed current clamp recordings in the whole cell configuration, in the presence of lacosamide and TNF-α to examine whether the enhancement of slow inactivation would prevent the effect of TNF-α on neuronal excitability. Lacosamide, when applied 10 min after TNF-α, reversed TNF-α-mediated increase in total (without TTX) (Fig. 10C, Table 4) and TTX-r-dependent membrane excitability (Table 4).
We further investigated whether lacosamide would also prevent TNF-α-mediated pain hypersensitivity in vivo. Coapplication of lacosamide, together with TNF-α, abolished TNF-α's effect on mechanical threshold and thermal latency, but only during the first hour after the injection (Fig. 10, D and E, light gray inverted triangles).
These results demonstrate that pharmacological enhancement of slow inactivation reverses TNF-α-mediated changes in sodium current density, membrane excitability and sensitivity to noxious stimuli in vivo.
DISCUSSION
Primary nociceptive neurons detect noxious stimuli and encode them in the form of AP firing rate. The coding is determined by the gain of nociceptive neurons, which defines the firing pattern, following incoming stimuli (Chance et al. 2002; Kispersky et al. 2012; Prescott et al. 2006; Prescott and De Koninck 2003). Along with the potassium channels, quantity and gating kinetics of the sodium channels expressed by nociceptors play a major role in defining the gain (Blair and Bean 2003; Dib-Hajj et al. 2010). Here we found that TNF-α acutely increases the gain of nociceptive neurons and decreases their threshold for AP generation. These results, together with previous findings, demonstrating that TNF-α evokes ectopic nociceptive activity (Sorkin et al. 1997), provide a platform for explaining the acute TNF-α-induced hypersensitivity in vivo (Jin and Gereau 2006; Junger and Sorkin 2000; Sorkin and Doom 2000). Moreover, our results support the notion that there are at least two distinct phases for TNF-α's effect on nociceptive excitability, and that these two phases are governed by different mechanisms. During the early, COX-2-independent phase, TNF-α acts rapidly, via p38 MAPK, to increase nociceptive excitability, thus swiftly relaying the onset of inflammation as well as its severity. In the second, delayed-onset, COX-2-dependent phase, TNF-α continuously sensitizes nociceptors via multiple indirect mechanisms, involving transcription and de novo protein synthesis (Hucho and Levine 2007), thus transmitting the information regarding the presence and intensity of the inflammatory process. Unlike the late phase, the mechanism underlying the early acute phase has not been fully detailed. Here we show that the rapid TNF-α-mediated increase in persistent and slow TTX-r sodium currents could underlie TNF-α-dependent acute hypersensitivity. We demonstrated that TNF-α increases the firing rate and decreases the threshold also when TTX-s sodium channels are blocked. Moreover, the magnitude of increase in neuronal excitability was similar in the presence and absence of TTX. Since the excitability of nociceptive neurons primarily depends on TTX-s and TTX-r sodium channels, the robust TNF-α-mediated hyperexcitability in the presence of TTX implies that the modulation of TTX-r sodium channels accounts for the majority of TNF-α's effect on excitability. This notion is supported by the highly similar values of increase in sodium channel availability in the −60-mV to −20-mV potential range, calculated directly from the shift in slow inactivation curve (assessed in voltage clamp, Fig. 6, D and E), to predicted increase in availability of TTX-r sodium channels, assessed from current clamp data, by calculating the maximal rate of change of the membrane voltage (dV/dt)max of the AP (Fig. 4). However, we cannot rule out the possibility that TNF-α's effect on excitability also originates from direct modulation of other ion conductances. Considering the changes in the excitability which we observed, specifically the broadening of the AP, decrease in threshold and increase in firing rate, the best candidates for these changes, apart from the sodium channels, would be the potassium channels. There is disagreement in the literature as to whether TNF-α suppresses (Liu et al. 2008) or has no effect (Czeschik et al. 2008) on voltage-dependent potassium currents; thus a more detailed study is needed to examine the effect of TNF-α on different potassium conductances. Modulation of voltage-dependent K+ (Kv) 3 channels in DRG neurons, for example, leads to broadening of the AP (Ritter et al. 2012). Delayed rectifier Kv2 channels, which affect firing rate (Du et al. 2000) and are expressed by nociceptive neurons (Bocksteins et al. 2009), are regulated by phosphorylation (O'Connell et al. 2010) and therefore could also be a good target for TNF-α-mediated modulation. Yet our observations showing that the maximal repolarization velocity (dV/dt)min, which is partially governed by Kv2 channels (Tsantoulas et al. 2014), did not change (see Table 1) following TNF-α application negates such a possibility. In addition, modulation of voltage-gated calcium channels (Hagenacker et al. 2010) and transducer channels (Jin and Gereau 2006; Nicol et al. 1997) could partially account for the excitatory effect of TNF-α.
It has been previously demonstrated that TNF-α acutely increases TTX-r current density (Jin and Gereau 2006). We found that this rapid increase in slow TTX-r current results from TNF-α-mediated modulation of TTX-r sodium channel voltage dependence of slow inactivation, which leads to increase in sodium channel availability. This modulation primarily affects the availability of TTX-r sodium channels at relatively depolarized potentials with the peak effect at the range of −50 to −30 mV (Fig. 6, D and E). The resting potential of nociceptive neurons measured utilizing sharp electrodes, which interfere less with the intracellular milieu, is averaged at around −45 mV (Matsuda et al. 1978; McCarthy and Lawson 1997). Thus the TNF-α-mediated depolarized shift of voltage dependence of slow inactivation, reported here, primarily takes place at physiologically relevant potentials, such that it leads to about a 15% increase in the TTX-r sodium channel availability at the subthreshold potentials. The latter is likely to contribute to the fast sodium influx mediated by transducer channel-mediated depolarization, thus boosting nociceptive excitability, leading to a decrease in the threshold for AP generation. Furthermore, as we demonstrated using simulation of a numerical model, a TNF-α-mediated shift in voltage dependence of slow inactivation of slow TTX-r current is sufficient to explain the substantial TNF-α-mediated increase in AP firing, reported here.
The shift in voltage dependence of slow inactivation reported here leads to a statistically significant, but small (about 1.5%) increase in availability at a holding potential of −70 mV (Fig. 6E). Such an increase cannot explain the 25% increase in current evoked from −70 mV observed here. This discrepancy could be explained by differences in the time of holding the membrane at −70 mV, prior to the test step in different protocols we used. In the protocol used to obtain channel availability (slow inactivation protocol), the membrane is held at −70 mV for 5 s only. In the activation protocol, the holding potential is −70 mV, such that the (considering P4 protocol used, see materials and methods) membrane is held at −70 mV for tens of seconds prior to the activation step. It is possible that the prolonged holding at −70 mV leads to decreased availability of TTX-r channels, via the slow inactivation mechanism with an ultraslow time constant (Goldin 2003; Todt et al. 1999; Zarrabi et al. 2010) compared with the shorter 5-s protocol, and that TNF-α acts on this ultraslow inactivation by increasing the availability at −70 mV. To check this hypothesis, we increased the duration of the conditioning step to 30 s. We were able to do it only to the conditioning voltages of −90 mV to −50 mV, since the prolonged holding at more depolarized and hyperpolarized voltages led to seal disruption. Indeed, 5-s holding at −70 mV produce significant but small changes in channel availability (Fig. 6D); however, when we increased the duration of the conditional step to 30 s, some neurons showed a significant decrease in current evoked from −70 mV compared with the current evoked from −90 mV (Fig. 11, black asterisks, n = 6). In this case, application of TNF-α prevented the decrease in current evoked from −70 mV (Fig. 11, light gray asterisks, n = 6), significantly increasing the channel availability by 15% at −70 mV (Fig. 11, gray asterisks, n = 6). This could readily explain the observed increase in slow TTX-r current evoked from −70 mV. Several other mechanisms could also potentially account for the rapid increase in slow TTX-r sodium current. For example, a leftward shift in activation of TTX-r channels underlies PGE2- and nerve injury-mediated increase in TTX-r sodium current (Gold et al. 1998; Huang and Song 2008), as well as increased excitability of DRG neurons in small-fiber neuropathy (Huang et al. 2013). However, it is less likely to explain the acute TNF-α effect, since we and others have demonstrated that the rapid increase in TTX-r sodium currents is not accompanied by changes in fast channel gating (Jin and Gereau 2006) (see also Fig. 6 here). Basic fibroblast growth factor leads to increase in TTX-r sodium currents without affecting activation and fast inactivation kinetics (Andres et al. 2013). Insertion of submembranous sodium channels into the plasma membrane was suggested as a possible mechanism responsible for the increase in current. This mechanism is also less likely to underlie the TNF-α effect described here, since an increase in the number of channels on the membrane should lead to increased maximal TTX-r sodium current. We showed here a decrease in the maximal current following application of TNF-α, most probably due to a rundown of the current. We, however, cannot exclude that TNF-α acts on other parameters of slow TTX-r sodium channels.
Fig. 11.
TNF-α relieves TTX-r channels from ultraslow inactivation at hyperpolarized potentials. A fraction of channels available for activation (peaks of TTX-r sodium currents during a step to 0 mV, normalized to Imax, means ± SE) following prolonged 30-s holding at voltages, as indicated in the x-axis, before (gray squares) and 3 min after application of TNF-α (black circles). Currents were elicited by 10-ms test steps to 0 mV, after 30-s conditioning pulses (Vcond) to the indicated voltages. Note that the substantial current reduction, following prolonged holding at Vcond of −70 mV, −60 mV and −50 mV (black asterisks, comparison within Before group, ns, P > 0.05, **P < 0.01, ***P < 0.001 one-way ANOVA with post hoc Bonferroni, n = 6), was diminished by application of TNF-α (gray asterisks, comparison within TNF-α group, ns, P > 0.05, ***P < 0.001, one-way ANOVA with post hoc Bonferroni, n = 6). Note also that TNF-α leads to increase of available channels at −70 mV by about 15%. Light great asterisks, comparison between Before and TNF-α groups. *P < 0.05; **P < 0.01, two-way ANOVA with post hoc Bonferroni, n = 6. Inset: stimulation protocol.
One of the major effects of TNF-α, shown here is a significant current increase and a shift in the activation of Nav 1.9-mediated persistent TTX-r current. Enhancement of persistent TTX-r current, either by a soup of proinflammatory mediators (Maingret et al. 2008), PKC (Baker 2005), or GTP (Baker et al. 2003), has been shown to produce an increase in nociceptive gain and decrease in threshold for APs, similar to what we report here. Moreover, considering that both Nav 1.8, which underlies TTX-r slow currents, and Nav 1.9, which mediates TTX-r persistent currents, are expressed (Baker 2005) by nociceptive terminals (Black and Waxman 2002; Persson et al. 2010; Zimmermann et al. 2007), TNF-α, which is released acutely during inflammation, could modulate the TTX-r slow and persistent currents in the same nociceptive terminals, similarly to what was described for the another proinflammatory agent, serotonin (Scroggs 2010). Thus the effect on Nav 1.9-mediated persistent TTX-r current, together with the increase in Nav 1.8 current shown here, substantially amplify sodium flux at sub- and suprathreshold potentials, leading to decrease in threshold, increase in repetitive firing, burst firing and reduction of conductance block (Baker 2005; Blair and Bean 2003; Maingret et al. 2008; Tripathi et al. 2006). The resulting overall increase in nociceptive output likely contributes to TNF-α-mediated increase in excitability.
The rapid effect of TNF-α on TTX-r channels is likely mediated by its binding and activation of TNFR1 (Jin and Gereau 2006). This receptor is present on nociceptive neurons expressing transient receptor potential vanilloid type 1 and neuropeptides SP and CGRP, as well as on the majority of other nociceptive and nonnociceptive DRG neurons (Li et al. 2004). This expression pattern could potentially account for the relatively high percentage rate of effect of TNF-α we observed in vitro and also implies that it could potentially affect the excitability of nonnociceptive neurons. These neurons express primarily TTX-s sodium channels. We demonstrated here about a 10% increase in the amplitude of INattxS following application of TNF-α. Although the method of TTX-s current isolation we used here is not perfect, and the extrapolated current might be contaminated by the increased TTX-r current, our data indicate that that a prepulse to −120 mV leads to maximal current and that, according to the fast inactivation availability curve, TTX-r channels are still available after 500 ms, after a prepulse to −40 mV, whereas most of the TTX-s channels become inactive (Cummins and Waxman 1997). We, therefore, cannot rule out the possibility that TNF-α has an effect on TTX-s current. Further investigation, using medium-sized DRG neurons that primarily express TTX-s sodium channels (Cardenas et al. 1997; Tripathi et al. 2011), is needed to clarify the role of TTX-s channels in cytokine-induced increase in excitability.
TNFRs activate p38 MAPK (Pollock et al. 2002), which increases TTX-r sodium current density (Hudmon et al. 2008; Jin and Gereau 2006) and, as we have shown here, act to relieve slow inactivation of TTX-r channels. Administration of a p38 MAPK blocker prevented hyperexcitable effects of TNF-α in vitro and the acute effect of TNF-α in vivo, without interfering with the late onset TNF-α-mediated pain hypersensitivity. Blockade of p38 MAPK leads to a decrease in sodium current density (Hudmon et al. 2008; Ostenfeld et al. 2013) and to a leftward shift in slow inactivation (Fig. 9A). This observation may account for a previously reported analgesic effect of p38 MAPK inhibition (Smith et al. 2013). However, this effect was not observed in our behavior assays, which might be explained by their inadequacy as tools suitable for detection of local analgesia.
It has been shown that activation of p38 MAPK increases TTX-r sodium currents by phosphorylation of L1 loop serines of Nav 1.8 channels, without affecting fast-gating properties of these channels (Hudmon et al. 2008). Future work should examine whether phosphorylation of L1 serines of Nav 1.8 channels is responsible for the p38 MAPK-mediated robust relief in voltage dependence of TTX-r sodium channel slow inactivation, which we have reported here.
Another proinflammatory cytokine, interleukin-1β, produced similar, albeit more robust, modulation of the gating of TTX-r sodium channels, also in a p38 MAPK-dependent manner (Binshtok et al. 2008). Thus p38 MAPK-mediated modulation of sodium channel availability may provide a convergence site for proinflammatory cytokines to affect the excitability of nociceptive neurons. Together, these data suggest that p38 MAPK might serve as a “tuning knob” for excitability of nociceptive neurons.
In summary, proinflammatory cytokine TNF-α, when released peripherally, rapidly boosts nociceptive excitability by modulating the activity of p38 MAPK, which in turn leads to a hyperpolarizing shift of activation of the persistent component and a rightward shift of voltage dependence of slow inactivation of the slow component of TTX-r sodium current (Fig. 12). Thus, by increasing the gain of nociceptive neurons and reducing their threshold, TNF-α affects the pattern of the output of nociceptive peripheral neurons to the central nervous system, thereby producing acute pain hypersensitivity. By showing that a slight modulation of voltage dependence of slow inactivation strongly affects gain of nociceptive neurons, our results also suggest that enhancement of slow inactivation shortly after the onset of tissue injury or inflammation could be beneficial in treating acute inflammatory pain, without affecting the inflammation-induced healing processes.
Fig. 12.
Scheme depicting TNF-α-mediated nociceptive hyperexcitability. Left: in normal conditions, only a fraction of Nav 1.8 channels, which underlie slow TTX-r sodium current, are available for activation, due to slow inactivation (denoted by collapsed outer pore of the channel). This, together with the voltage dependence of activation of Nav 1.9 channels, which underlies persistent TTX-r sodium current, defines a threshold for AP initiation and the ability to fire repetitively after threshold is achieved. TNFR, TNF receptor. Right: TNF-α binds to its receptor and, via p38 MAPK, leads to a hyperpolarization shift in the activation of Nav 1.9 channels. Moreover, TNF-α-mediated activation of p38 MAPK, probably via direct phosphorylation of Nav 1.8 channels (see Hudmond et al. 2008 and also Fig. 9 here) increases the availability of Nav 1.8 channels, by modulating their slow inactivation. These processes lead to decrease in threshold, such that previously subthreshold stimuli now evoke APs, and to increase in gain, enhancing the ability of nociceptive neurons to fire repetitively, altogether producing nociceptive hyperexcitability.
GRANTS
B. Katz received the Teva NNE postdoctoral fellowship. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 260914.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G., O.B., Y.C., and A.M.B. performed experiments; S.G., O.B., and A.M.B. analyzed data; S.G., B.K., S.L., and A.M.B. interpreted results of experiments; S.G. and A.M.B. prepared figures; S.G., O.B., B.K., S.L., and A.M.B. drafted manuscript; S.G., Y.C., B.K., S.L., and A.M.B. edited and revised manuscript; S.G., O.B., Y.C., B.K., S.L., and A.M.B. approved final version of manuscript; S.G. design of research; A.M.B. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Ilya Fleidervish and Dr. Efrat Katz for help with the compartmental model and for useful discussions.
REFERENCES
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol 85: 630–643, 2001. [DOI] [PubMed] [Google Scholar]
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral. J Neurosci 26: 12852–12860, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres C, Hasenauer J, Ahn HS, Joseph EK, Isensee J, Theis FJ, Allgower F, Levine JD, Dib-Hajj SD, Waxman SG, Hucho T. Wound-healing growth factor, basic FGF, induces Erk1/2-dependent mechanical hyperalgesia. Pain 154: 2216–2226, 2013. [DOI] [PubMed] [Google Scholar]
- Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol 567: 851–867, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol 548: 373–382, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 18–20, 2007. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 28: 14062–14073, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Molecular identities of two tetrodotoxin-resistant sodium channels in corneal axons. Exp Eye Res 75: 193–199, 2002. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Role of tetrodotoxin-resistant Na current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci 23: 10338–10350, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na current, TTX-resistant Na current, and Ca current in the action potentials of nociceptive sensory neurons. J Neurosci 22: 10277–10290, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocksteins E, Raes AL, Van de Vijver G, Bruyns T, Van Bogaert PP, Snyders DJ. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am J Physiol Cell Physiol 296: C1271–C1278, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Cooper BY, Scroggs RS. 5HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. J Neurosci 17: 7181–7189, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol 74: 1870–1879, 1995. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII nomenclature and structure-function. Pharmacol Rev 57: 397–409, 2005. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron 35: 773–782, 2002. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Black JA, Dib-Hajj SD, Waxman SG. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci 20: 8754–8761, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci 19: RC43, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 17: 3503–3514, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett 434: 293–298, 2008. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci 33: 325–347, 2010. [DOI] [PubMed] [Google Scholar]
- Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol 522: 19–31, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest 120: 3760–3772, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol 495: 429–440, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake NM, Lancaster E, Weinreich D, Gold MS. Absence of an association between axotomy-induced changes in sodium currents and excitability in DRG neurons from the adult rat. Pain 109: 471–480, 2004. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Gutnick MJ. Kinetics of slow inactivation of persistent sodium current in layer V neurons of mouse neocortical slices. J Neurophysiol 76: 2125–2130, 1996. [DOI] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci 18: 10345–10355, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A 93: 1108–1112, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol 13: 284–290, 2003. [DOI] [PubMed] [Google Scholar]
- Hagenacker T, Czeschik JC, Schafers M, Busselberg D. Sensitization of voltage activated calcium channel currents for capsaicin in nociceptive neurons by tumor-necrosis-factor-alpha. Brain Res Bull 81: 157–163, 2010. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol 86: 1351–1364, 2001. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput 9: 1179–1209, 1997. [DOI] [PubMed] [Google Scholar]
- Huang J, Yang Y, Zhao P, Gerrits MM, Hoeijmakers JG, Bekelaar K, Merkies IS, Faber CG, Dib-Hajj SD, Waxman SG. Small-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neurons. J Neurosci 33: 14087–14097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Song XJ. Differing alterations of sodium currents in small dorsal root ganglion neurons after ganglion compression and peripheral nerve injury. Mol Pain 4: 20–35, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55: 365–376, 2007. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)18 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 28: 3190–3201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenerick H. Phase plane trajectories of the muscle spike potential. Biophys J 3: 363–377, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26: 246–255, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Bean BP. Inhibition of neuronal voltage-gated sodium channels by brilliant blue G. Mol Pharmacol 80: 247–257, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 85: 145–151, 2000. [DOI] [PubMed] [Google Scholar]
- Kispersky TJ, Caplan JS, Marder E. Increase in sodium conductance decreases firing rate and gain in model neurons. J Neurosci 32: 10995–11004, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler A, Cummins TR, Dib-Hajj SD, Hormuzdiar WN, Black JA, Waxman SG. GDNF and NGF reverse changes in repriming of TTX-sensitive Na(+) currents following axotomy of dorsal root ganglion neurons. J Neurophysiol 88: 650–658, 2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci 24: 9623–9631, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BG, Dobretsov M, Stimers JR, Zhang JM. Tumor necrosis factor-alpha suppresses activation of sustained potassium currents in rat small diameter sensory neurons. Open Pain J 1: 1–16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem 265: 10805–10808, 1990. [PubMed] [Google Scholar]
- Maingret F, Coste B, Padilla F, Clerc N, Crest M, Korogod SM, Delmas P. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J Gen Physiol 131: 211–225, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson Ga, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 96: 9385–9390, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Yoshida S, Yonezawa T. Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells cultured in vitro. Brain Res 154: 69–82, 1978. [DOI] [PubMed] [Google Scholar]
- McCarthy PW, Lawson SN. Differing action potential shapes in rat dorsal root ganglion neurones related to their substance P and calcitonin gene-related peptide immunoreactivity. J Comp Neurol 388: 541–549, 1997. [PubMed] [Google Scholar]
- Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci 17: 975–982, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell KM, Loftus R, Tamkun MM. Localization-dependent activity of the Kv2.1 delayed-rectifier K+ channel. Proc Natl Acad Sci U S A 107: 12351–12356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Tatebayashi H. Kinetic analysis of two types of Na+ channels in rat dorsal root ganglia. J Physiol 466: 9–37, 1993. [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Tatebayashi H. Slow inactivation of tetrodotoxin-insensitive Na+ channels in neurons of rat dorsal root ganglia. J Membr Biol 129: 71–80, 1992. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Hayashi T, Kyoizumi S, Kusunoki Y, Nakachi K, MacPhee DG, Trosko JE, Kataoka K, Yorioka N. Anisomycin downregulates gap-junctional intercellular communication via the p38 MAP-kinase pathway. J Cell Sci 117: 2087–2096, 2004. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Krishen A, Lai RY, Bullman J, Baines AJ, Green J, Anand P, Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: a double-blind, placebo-controlled study. Eur J Pain 17: 844–857, 2013. [DOI] [PubMed] [Google Scholar]
- Persson AK, Black JA, Gasser A, Cheng X, Fischer TZ, Waxman SG. Sodium-calcium exchanger and multiple sodium channel isoforms in intra-epidermal nerve terminals. Mol Pain 6: 84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases. Neuropharmacology 42: 93–106, 2002. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc Natl Acad Sci U S A 100: 2076–2081, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Sejnowski TJ, De Koninck Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: towards a biophysical basis for neuropathic pain. Mol Pain 2: 32, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol 108: 2230–2241, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DM, Ho C, O'Leary ME, Covarrubias M. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J Physiol 590: 145–161, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci 12: 2104–2111, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579: 1–14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Kanaan SA, Haddad JJ, Jaoude PA, Jabbur SJ, Saade NE. Involvement of interleukin-1 beta, nerve growth factor and prostaglandin E2 in endotoxin-induced localized inflammatory hyperalgesia. Br J Pharmacol 121: 1619–1626, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS. Serotonin upregulates low- and high-threshold tetrodotoxin-resistant sodium channels in the same subpopulation of rat nociceptors. Neuroscience 165: 1293–1300, 2010. [DOI] [PubMed] [Google Scholar]
- Scroggs RS. Up-regulation of low-threshold tetrodotoxin-resistant Na+ current via activation of a cyclic AMP/protein kinase A pathway in nociceptor-like rat dorsal root ganglion cells. Neuroscience 186: 13–20, 2011. [DOI] [PubMed] [Google Scholar]
- Sheets PL, Heers C, Stoehr T, Cummins TR. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther 326: 89–99, 2008. [DOI] [PubMed] [Google Scholar]
- Smith MT, Woodruff TM, Wyse BD, Muralidharan A, Walther T. A small molecule angiotensin II type 2 receptor [AT(2)R] antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen-activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. Pain Med 14: 1557–1568, 2013. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst 5: 96–100, 2000. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81: 255–262, 1997. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy. Pain 57: 375–382, 1994. [DOI] [PubMed] [Google Scholar]
- Todt H, Dudley SC Jr, Kyle JW, French RJ, and Fozzard HA. Ultra-slow inactivation in mu1 Na+ channels is produced by a structural rearrangement of the outer vestibule. Biophys J 76: 1335–1345, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi PK, Cardenas CG, Cardenas CA, Scroggs RS. Up-regulation of tetrodotoxin-sensitive sodium currents by prostaglandin E(2) in type-4 rat dorsal root ganglion cells. Neuroscience 185: 14–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi PK, Trujillo L, Cardenas Ca Cardenas CG, de Armendi AJ, Scroggs RS. Analysis of the variation in use-dependent inactivation of high-threshold tetrodotoxin-resistant sodium currents recorded from rat sensory neurons. Neuroscience 143: 923–938, 2006. [DOI] [PubMed] [Google Scholar]
- Tsantoulas C, Zhu L, Yip P, Grist J, Michael GJ, McMahon SB. Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Exp Neurol 251: 115–126, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD. Voltage-gated sodium channel Nav16 is modulated by p38 mitogen-activated protein kinase. J Neurosci 25: 6621–6630, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 121: 417–424, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron 55: 353–364, 2007. [DOI] [PubMed] [Google Scholar]
- Zarrabi T, Cervenka R, Sandtner W, Lukacs P, Koenig X, Hilber K, Mille M, Lipkind GM, Fozzard HA, Todt H. A molecular switch between the outer and the inner vestibules of the voltage-gated Na+ channel. J Biol Chem 285: 39458–39470, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi Ji Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447: 855–858, 2007. [DOI] [PubMed] [Google Scholar]