Abstract
Bone remodeling is the continuous process by which old bone is removed by bone-resorbing cells, the osteoclasts and replaced by new bone synthesized by bone forming cells, the osteoblasts. Osteoporosis is characterized by a progressive loss of bone mass and microarchitecture, which leads to increased fracture risk. Denosumab, a human monoclonal antibody resembling natural IgG2 immunoglobulin, has antiresorptive activity and is distinguished from other antiresorptive drugs. It mimics osteoprotegerin (OPG) that binds to RANKL and hence does not allow RANKL to bind with RANK receptor, thereby inhibiting osteoclast differentiation, activation and survival exerting primarily antiresorptive action. Denosumab trials have shown its efficacy in postmenopausal women with osteoporosis, unresectable giant cell tumor of bone and significant effect in non-metastatic prostate cancer and delay in the time-to-first skeletal related events (SRE) and subsequent SRE with denosumab than zoledronic acid in patients. It is available as 60 mg/ml in pre-filled syringes and approved for osteoporosis in postmenopausal women (60 mg s.c. twice yearly), unresectable giant cell tumor of bone in adults and skeletally mature adolescents (120 mh s.c. monthly), prevention of skeletal-related events and to increase bone mass in patients at high risk for fracture including androgen deprivation therapy for non-metastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer. Denosumab offers advantages of twice yearly dosing in osteoporosis and monthly dosing in giant cell tumor of bone with its novel mechanism of action and better tolerability.
Keywords: Antiresorptive, Osteoporosis, Giant cell tumor
Introduction
Bone remodeling is the continuous process by which old bone is removed by bone-resorbing cells, the osteoclasts and replaced by new bone synthesized by bone forming cells, the osteoblasts.1,2 This system is critical for skeletal health and its disruption leads to variety of pathologic conditions including osteoporosis, primary bone tumor and metastatic bone tumor.3
The receptor for activating nuclear factor-κB (RANK), the RANK ligand (RANKL), and osteoprotegerin (OPG) are the major regulators of bone metabolism.4,5 Bone loss occurs when there is an imbalance between the activity of osteoclasts (bone resorption) and osteoblasts (bone formation). RANKL/OPG ratio is a major determinant of bone mass. RANKL interacts with RANK expressed in osteoclast membrane and promotes differentiation, formation and survival of osteoclast.3
Osteoporosis is characterized by a progressive loss of bone mass and microarchitecture which leads to increased fracture risk.1,6 Bone is a major metastatic site for many solid tumors and bone metastasis. These tumors are responsible for pathologic fractures, spinal cord compression and intractable pain which are commonly referred as skeletal-related events (SREs).7,8 In the setting of osteoporosis and bone metastases in cancer, the interaction between RANKL, RANK and OPG is disrupted.
Currently the most commonly used drugs for osteoporosis and SREs consequent to bone metastasis are bisphosphonates.3,4 Bisphosphonates have rare but serious adverse events, such as osteonecrosis of the jaw, atypical fractures and oesophageal cancer.1,5,8
Human parathyroid hormone analogue teriparatide (the 1-34 N-terminal fragment of PTH) increases bone mineral density (BMD) in women with postmenopausal osteoporosis. It reduces the risk of vertebral and non-vertebral fractures but do not decrease the incidence of hip fractures in postmenopausal osteoporosis.2,9
Other available drugs that reduce bone resorption are selective estrogen receptor modulators like raloxifene and basedoxifene.2 However, these drugs increase the risk of venous thromboembolic events, fatal stroke in postmenopausal women.2,5,9
Denosumab, a human monoclonal antibody resembling natural IgG2 immunoglobulin, has been reported to have antiresorptive activity and is distinguished from other antiresorptive drugs.
Mechanism of action
Osteoblast produces RANKL which binds to RANK present on osteoclast membrane and stimulate differentiation, activation and survival of osteoclast.1,3,5 Osteoprotegerin (OPG) is a soluble RANKL-binding protein that binds RANKL, prevents it from combining with RANK on the osteoclast membrane. Denosumab by mimicking endogenous OPG binds to RANKL and hence does not allow RANKL to bind with RANK thereby inhibits osteoclast differentiation, activation and survival exerting primarily antiresorptive action (Fig. 1).3,10
Fig. 1.
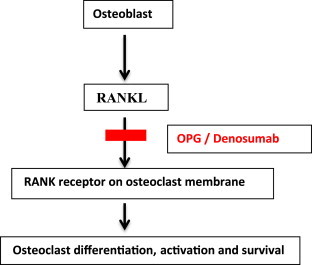
Mechanism of action of denosumab.
Pharmacokinetics
Its approved route of administration is subcutaneous (s.c.).4,7,10 The bioavailability of denosumab after s.c. injection is 61% and its absorption is mediated by lymphatic system.2,4 Time to reach peak plasma concentration is 10 days after administration of 60 mg and its plasma half-life is 25–38 days.1 Its effect is reversible. Metabolism of denosumab is unknown and its elimination is through non-specific linear pathway via reticuloendothelial system.3,10
Clinical efficacy
Denosumab trial for prevention of fractures in postmenopausal women with osteoporosis (FREEDOM Trial) concluded that it is associated with a reduction in the risk of vertebral, non-vertebral and hip fractures in postmenopausal women with osteoporosis.5,9
Two multicentre open-label trials found improved objective response with denosumab in unresectable giant cell tumor of bone by using modified Response Evaluation Criteria in Solid Tumors (RECIST 1.1) which includes tumor shrinkage and time to the development of disease progression.5,9,11
Effects of denosumab on BMD and bone turnover in postmenopausal women transitioning from alendronate therapy (STAND Trial) shows that denosumab treatment results in significant increase in BMD in hip, lumbar spine, femoral neck, and distal 1/3rd radius than continued alendronate therapy at 12th month of study.5,6,8,11
Two international randomized double-blind, placebo-controlled trials in patients receiving adjuvant aromatase inhibitors therapy for breast cancer or androgen deprivation therapy for non-metastatic prostate cancer shows significant effect on BMD at 12 and 24 months respectively.9,11 In men with prostate cancer, denosumab also significantly reduced the incidence of new vertebral fractures at 36 months.11,12
Three international randomized double-blind double-dummy trials in patients with bone metastases found significant delay in the time-to-first SRE and subsequent SRE with denosumab than zoledronic acid in patients.9,11
Placebo controlled Phase-III FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis every 6 Months) trial, showed significant increase in BMD at the lumbar spine, hip, and distal radius over the three years duration. The mean increase in lumbar spine BMD in the denosumab trials ranged between 3.0% and 5.3% at 12 months, 6.5%–7.7% at 24 months, and 8.2%–10.1% at 36 months of treatment. The mean increase in total hip BMD was 1.6%–3.6% at 12 months, 3.4%–5.1% at 24 months, and 5.2%–6.7% at 36 months. The mean increase in distal radius BMD was 1.1%–1.3% at 12 months and 0.3%–1.4% at 24 months, compared to decrease in BMD of 2.1% in the placebo group.5,9
A randomized, double-blind, placebo-controlled, parallel-group, multicentre study (2011–2012) evaluated the efficacy and safety of denosumab in Korean postmenopausal women with osteoporosis. The study showed increased BMD at the lumbar spine.13
A phase-III randomized, double-blind, placebo-controlled, parallel-group, multicentre study (2012–2013) evaluated the efficacy and safety of denosumab in Indian postmenopausal women with osteoporosis. The study showed that denosumab is effective in increasing bone mineral density at the lumbar spine in Indian postmenopausal women with osteoporosis.14
An ongoing phase-III randomized, double-blind, double-dummy study is comparing denosumab with zoledronic acid in subjects of Asian ancestry with bone metastases from solid tumors. This study will provide data to support the regulatory approval for marketing and patient access to denosumab for the prevention of SREs. Study will be completed in 2017.15
Presently, it is also being studied for other indications like treatment of hypercalcemia due to malignancy and aneurysmal bone cysts.11,16,17
Comparative efficacy and cost of therapy of existing drugs and denosumab
Denosumab treatment is associated with a rapid, sustained and reversible reduction in bone turnover markers, a continuous significant increase in bone mineral density at all sites and a significant decrease of risk of vertebral, hip and non-vertebral fractures in women with postmenopausal osteoporosis.5 The magnitude of the risk reduction of vertebral fracture with denosumab is similar to that of zoledronic acid and teriparatide but is greater than other available oral drug therapies for osteoporosis. For reduction in non-vertebral and hip fracture, denosumab has not been found to be better than bisphosphonates and teriparatide. Among the most effective available drugs for osteoporosis treatment, zoledronic acid has not yet been approved for osteoporosis whereas teriparatide requires daily s.c. administration compared to denosumab requiring six monthly administration.10,18
Though the cost of annual treatment with denosumab appears to be higher but considering the efficacy and cost of treatment of vertebral fractures, it may be considered as more cost effective in comparison to other existing pharmacotherapies for postmenopausal women with high risk of osteoporotic fractures (Table 1).
Table 1.
Summary of pharmacological agents used in osteoporosis.2,3,5,10,12,18,19,21
| Drugs | Route of administration | Dose | % of fracture reduction in comparison to placebo |
Limitations | Annual cost (Rs) | ||
|---|---|---|---|---|---|---|---|
| Vertebral | Non-vertebral | Hip | |||||
| Alendronate | Oral | 5 mg daily for 2 years; 10 mg daily or 70 mg once weekly for 5–10 years | 50 | 20 | 50 | Esophagitis, osteonecrosis of jaw, atypical femoral fractures, myalgia | 1300 |
| Risedronate | Oral | 5 mg daily or 35 mg once weekly or 150 mg once monthly | 50 | 40 | 40 | Esophagitis, flu-like symptoms, hypocalcaemia | 2600 |
| Zoledronic acid | I.V. | 5 mg as single 15–30 min infusion once yearly | 70 | 25 | 40 | Renal toxicity, flu-like symptoms, severe dizziness, anemia | 19,516 |
| Raloxifene | Oral | 60 mg daily | 30 | NR | NR | Worsen vasomotor symptoms, venous thrombosis, peripheral edema, vaginal bleeding, breast pain, fatal strokes | 3650 |
| Strontium ranelate | Oral | 2 g daily | 41 | 36 | 36 | Risk of myocardial infarct, venous thromboembolism, DRESS syndrome | 19,345 |
| Estrogen replacement therapy | Oral or transdermal | Oral 0.3 mg/day esterified estrogen or 5 μg/day ethinyl estradiol; transdermal 50 μg/day estradiol | 34 | NR | 34 | Risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, deep vein thrombosis | 2400 |
| Teriparatide | S.C. | 20 mcg daily for 2 years | 65 | 53 | 53 | Dizziness, muscle cramps, infrequent hypercalcemia, increase risk of osteosarcoma, daily subcutaneous injections | 144,000 |
| Denosumab | S.C. | 60 mg every 6 months for 3 years | 68 | 20 | 40 | Arthralgia, hypocalcaemia, eczema, cataract | 120,000 |
*NR: Not reported.
Adverse effects
Common adverse effects include nausea, fatigue, asthenia, dyspnea, cataract, eczema and flu-like syndrome.2,3,5,18
Severe hypocalcaemia (serum calcium less than 7 mg/dL or 1.75 mmol/L) is one of the serious adverse effects which may be controlled with calcium and vitamin D supplementation.5,9,12,18
Other rare but serious adverse effects include severe hypophosphatemia (serum phosphorous less than 2 mg/dL or less than 0.6 mmol/L)5,12 and osteonecrosis of the jaw. To prevent osteonecrosis of jaw, regular dental checkup is advised before starting and during the treatment.1,5,8,12
Precautions & contraindications
It is contraindicated in pre-existing hypocalcaemia which should be corrected before therapy. Serum calcium concentration should be monitored in patient with renal impairment. It should be used cautiously in children as it leads to postnatal impairment of dentition and bone growth.1,3 It should also be avoided in patient with creatinine clearance <30 ml/min or chronic kidney disease stage IV or V.8,9 Hypersensitivity is another contraindication for injectable denosumab.8
Safety in pregnancy
Denosumab is a type C category drug as adequate information in pregnant women is not available. However, in animal studies, it has been shown to interfere with the development of lymph nodes in the fetus and impairment of lactation in mother in postpartum period.10
Approved indications and dose
It is available as single use vial of 1.7 ml containing 120 mg (i.e.70 mg/ml) and approved for following conditions:
-
1)
Treatment of osteoporosis in postmenopausal women who are at high risk for fracture. Denosumab 60 mg subcutaneous injection twice a year is highly effective in reducing the risk of vertebral, non-vertebral and hip fractures 1,2,6,8 and was approved in June 2010 by the FDA for it.4
-
2)
Prevention of skeletal-related events i.e. pathological fractures, spinal cord compression and intractable pain in patients with bone metastases from solid tumors at a dose of 120 mg s.c. every 4 weeks.9–11 It was approved in Nov 2010 by FDA.4
-
3)
To increase bone mass in patients at high risk for fracture including androgen deprivation therapy for non-metastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer.3,10–12 This indication was approved in Sept 2011 by FDA.4
-
4)
Treatment of unresectable giant cell tumor of bone in adults and skeletally mature adolescents. Denosumab 120 mg s.c. every 4 weeks with additional dose 120 mg on day 8 and 15 of first month of therapy is used which was approved in June 2013 by FDA.4
Denosumab has already been approved in India and license for manufacturing and import has been authorized to Pharmaceutical Company on 2013.20
Conclusion
Denosumab has become first line agent for treatment of osteoporosis in postmenopausal women by decreasing bone resorption and increasing bone mineral density by binding with RANKL and inhibiting it from binding to its receptor.3 It is also used for unresectable giant cell tumor of bone, bone metastases from solid tumors and bone metastases from urological cancers. Denosumab offers advantages of twice yearly dosing in osteoporosis and monthly dosing in giant cell tumor of bone with its novel mechanism of action and better tolerability.
Conflicts of interest
All authors have none to declare.
References
- 1.Paul D.M. A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis: a review. Ther Adv Musculoskelet Dis. 2011;3(6):271–282. doi: 10.1177/1759720X11424220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachner T.D., Khosla S., Hofbauer L.C. New horizons in osteoporosis. Lancet. 2011 April 9;377(9773):1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanley D.A., Adachi J.D., Bell A., Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66(12):1139–1146. doi: 10.1111/ijcp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA Approval for Denosumab. [Cited 2013 June 13]. Available from: http://www.fda.gov/informationOnDrugs/ApprovedDrugs/ucm356667.htm.
- 5.Athanasios A.D., Toulis K.A., Polyzos S.A., Anastasilakis C.D., Mkras P. Long-term treatment of osteoporosis: safety and efficacy appraisal of denosumab. Ther Clin Risk Manag. 2012;8:295–306. doi: 10.2147/TCRM.S24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J.P., Prince R.L., Deal C. Comparison of the effect of denosumab and alendronate on bone Mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009 Dec 14:1–34. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 7.Lewiecki E.M., Miller P.D., McClung M.R. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007 Dec;22(12):1832–1841. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- 8.Cummings S.R., San Martin J., McClung M.R. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 9.Yuasa T., Yamamoto S., Urakami S., Fukui I., Yonese J. Denosumab: a new option in the treatment of bone metastases from urological cancers. Onco Targets Ther. 2012;5:221–229. doi: 10.2147/OTT.S30578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippuner Kurt. The future of osteoporosis treatment – a research update. Swiss Med Wkly. 2012;142:w13624. doi: 10.4414/smw.2012.13624. [DOI] [PubMed] [Google Scholar]
- 11.Karim F., Linda B., Guozhi G., Tomas S., Richard M. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: result of a randomized phase II trial. J Urol. 2013;189(1):S51–S58. doi: 10.1016/j.juro.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 12.McClung M.R., Lewecki E.M., Geller M.L. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int. 2013;24:227–235. doi: 10.1007/s00198-012-2052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A Six Month Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Study With A Six Month Open-label Extension To Evaluate The Efficacy And Safety Of Denosumab In Korean Postmenopausal Women With Osteoporosis. [Cited on 2013 Dec 8]. Available from: http://www.gsk clinicalstudyregister.com/study/114163#rs.
- 14.A Six-month Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicentre Study To Evaluate The Efficacy And Safety Of Denosumab In Indian Postmenopausal Women With Osteoporosis. [Cited on 2013 Dec 8]. Available from: http://www.gsk-clinicalstudyregister.com/study/114161#ps.
- 15.A Study Comparing Denosumab With Zoledronic Acid In Subjects Of Asian Ancestry With Bone Metastasis From Solid Tumours. [Cited on 2013 Dec 8]. Available from: http://www.gsk-clinicalstudyregister.com/study/114273#ps.
- 16.Lange T., Stehling C., Frohlich B. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(3):593–601. doi: 10.1007/s00586-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakarun C.J., Forrester D.M., Gottsegen C.J., Patel D.B., White E.A., Matcuk G.R., Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Musculoskeletal imaging. Radiographics. 2013;33:197–211. doi: 10.1148/rg.331125089. Published online http://dx.doi.org/10.1148/rg.331125089. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay R., Cosman F. 18th ed. The McGraw Hill; 2012. Harrison’s Principles of Internal Medicine: Osteoporosis; pp. 3131–3136. [Google Scholar]
- 19.Licata A.A. Discovery, clinical development and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39:668–677. doi: 10.1345/aph.1E357. [DOI] [PubMed] [Google Scholar]
- 20.File no.6–8/FF-08/2012-BD(Biotech). Form 41. Registration certificate no.FFR-27. Dtd 28 Feb 2013. Available from: [Cited on 2013 Dec 28]. http://www.cdsco.nic.in/biological09.07.../recombinant%20RC%202013.pdf.
- 21.Hiligsmann M., Boonen A., Dirksen C.D., Ben Sedrine W., Reginster J.Y. Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporotic women. Expert Rev Pharmacoecon Outcomes Res. 2013 Feb;13(1):19–28. doi: 10.1586/erp.12.76. [DOI] [PubMed] [Google Scholar]


