Abstract
Gut radiation-induced injury is a concern during treatment of patients with cancer. Krüppel-like factor 4 (KLF4) is expressed in differentiated villous epithelial cells of the small intestine. We previously showed that KLF4 protects cells from apoptosis following γ-irradiation in vitro. We sought to determine whether KLF4 mediates the small intestinal response to γ-irradiation in vivo. Mice with intestinal epithelium-specific deletion of Klf4 (Klf4ΔIS) and control (Klf4fl/fl) mice were irradiated with total-body γ-radiation. Following irradiation, the Klf4ΔIS mice had significantly increased mortality compared with irradiated Klf4fl/fl mice. Immunohistochemistry and immunofluorescence staining were used to assess the morphological changes, levels of proliferation, and apoptosis in the intestinal epithelium. At 96 h following irradiation, there was a regenerative response manifested by an expansion of the proliferative zone in both mouse groups, with the control mice having a higher proliferative activity than the Klf4ΔIS group. In addition, there was a significant increase in the number of Klf4/Ki67-copositive cells in the irradiated control mice compared with unirradiated mice. Also, the irradiated Klf4ΔIS mice had a significantly higher number of crypt cells positive for apoptosis, p53, and p21 compared with irradiated Klf4fl/fl mice. Taken together, our data suggest that Klf4 may function as a radioprotective factor against gastrointestinal syndrome in mice following γ-irradiation by inhibiting apoptosis in the acute response to irradiation and contributing to crypt regeneration.
Keywords: Krüppel-like factor 4, intestinal epithelium, γ-irradiation, apoptosis, proliferation
radiation is widely used as a therapeutic treatment in almost two-thirds of all patients with cancer. The effects of this treatment include extensive DNA damage followed by apoptosis especially to the cells that undergo extensive proliferation, e.g., cancer cells and normal tissues [hematopoietic cells and epithelial cells of the gastrointestinal (GI) tract]. In a murine model of γ-radiation-induced gut injury, it has been shown that, in mice exposed to ≤8 Gy, the response is characterized by apoptosis of cells within crypt of intestinal epithelium and shortening of the villi compartment that is followed by complete recovery. At the same time radiation of ≥ 12 Gy causes death within 2 wk of treatment attributable to extended damage to intestinal epithelium, especially stem cells that cannot be rescued by any treatment (23, 26, 36, 54). Considering the high toxicity of the radiation treatment to the GI tract, studies revealing mechanisms that regulate the GI response to injury following irradiation are extremely beneficial. Because radiation causes DNA damage, the mechanisms that regulate the DNA damage response through cell-cycle arrest, damage repair, and apoptosis play a significant role in the cell response to injury. It has been shown that p53 is the main mediator of the cell response to DNA damage by involving mechanisms that contribute to cell-cycle arrest or death in epithelial tissues. The upregulation of p53 activity upon irradiation can lead to apoptosis through activation of p53-upregulated modulator of apoptosis or cell-cycle arrest and DNA repair through increased activity of cyclin-dependent kinase inhibitor p21 protein (p21) (23). The activity of p21 upon DNA damage is transcriptionally regulated by p53 and leads to inhibition of G1-to-S-phase transition of the cell cycle. It has been shown that the response of p53-deficient mice to radiation is characterized by inhibition of apoptosis, acceleration of proliferation, and increased animal lethality (27). In contrast, p21 knockout mice show lack of cell-cycle arrest, reduced DNA repair that leads to accumulation of DNA damage, reduced chromosomal integrity (29), enhanced crypt survival (18), and better mice survival than p53-deficient mice (29). In wild-type mice, the small intestinal epithelium exhibits a compensatory regenerative response that contributes to repopulation of the epithelium following radiation-induced injury (45). This response is designated by several consequential phases of upregulation and reduction of proliferation within the lower and upper part of the crypt of the intestinal epithelium. Despite these extensive studies, the mechanisms underlying protection of an intestinal niche from irradiation are not very well understood, and the potential regulators of p53 response to injury are not clearly identified.
The Krüppel-like factor (KLF) family of zinc-finger domain-containing transcription factors plays important roles in regulating a diverse range of cellular processes, including cellular proliferation, differentiation, reprogramming, and the cellular response to oncogenic signals and ionizing radiation (38). KLF4 is expressed in the villous compartment of the small intestinal epithelium and promotes cellular differentiation (37). Previously, we reported that KLF4 is an important factor in mediating the response of cells to stress following acute radiation exposure in vitro (19). We demonstrated that KLF4 exhibits antiapoptotic activity in vitro in response to γ-irradiation, via inhibition of p53-mediated apoptotic pathway. This occurs in conjunction with arrest of the cell cycle at the G1/S and G2/M transitions through the upregulation of the cyclin-dependent kinase inhibitor p21. More recently, we established an in vivo model with intestine-specific deletion of Klf4 and showed that Klf4 plays an important role in the regulation of homeostasis within intestinal epithelium (20).
In this study, we investigated the role of Klf4 in the response of small intestinal epithelium to radiation-induced injury in a mouse model. We used previously described mice with floxed Klf4 gene (Klf4fl/fl) and intestine-specific Klf4 knockout mice (Klf4ΔIS) (20) and evaluated their response to total-body irradiation of 12 Gy. Our data indicate that Klf4 acts to inhibit apoptosis and enhance crypt cell regeneration of the small intestine following γ-radiation-induced injury. Consequently, mice with intestinal epithelium-specific deletion of Klf4 had a higher mortality than control mice following irradiation. The results of this study suggest that Klf4 protects against radiation-induced gut injury in the mouse.
MATERIALS AND METHODS
Mouse strains and maintenance.
Mice with floxed Klf4 gene (Klf4fl/fl) and intestine-specific Klf4 deletion (Klf4ΔIS) were previously described (20). Klf4ΔIS were generated by crossing Klf4fl/fl with mice carrying a gene encoding Cre under the control of villin promoter. All animal studies were approved by the Stony Brook University Institutional Animal Care and Use Committee (IACUC).
γ-Irradiation procedure.
Mice were exposed to total-body γ-irradiation with a 137Cs source, with a dose rate of 0.8 Gy/min, for a total of 12 Gy. Another group of mice (sham) were placed in the room without being exposed to irradiation. Animals were either observed for survival postirradiation or were killed by CO2 asphyxiation followed by cervical dislocation at set times after irradiation, and the small intestine was removed for further analysis. For the survival experiment, we used the moribund state as the experimental endpoint, defined as an animal that lost more than 15% of its body weight and was unresponsive and immobile. For this purpose, the animals were monitored daily for body posture, eye appearance, and activity level. Animals reaching the moribund state were killed as mentioned above.
Tissue preparation.
The small intestines harvested from killed mice were flushed with modified Bouin's fixative (50% ethanol, 5% acetic acid) and cut open longitudinally for gross examination. The intestines were then Swiss rolled, fixed, and embedded in paraffin, and 5-mm sections were cut for histological hematoxylin and eosin (H and E) characterization and for immunofluorescence staining. H and E staining was performed by the Research Histology Core Laboratory, Department of Pathology, Stony Brook University.
Immunofluorescence and immunohistochemistry.
For immunostaining, sections were deparaffinized in xylene, incubated in 3% hydrogen peroxide in methanol for 30 min, rehydrated in ethanol gradient, and then treated with 10 mM Na citrate buffer, pH 6.0, at 120°C for 10 min in a pressure cooker [except for lipopolysaccharide-binding protein (LBP) staining for Paneth cells, where antigen retrieval was at 120°C for 1 min]. The histological sections were incubated with a blocking buffer [5% nonfat dry milk and 0.01% Tween 20 in 1× Tris-buffered PBS (TTBS)] for 1 h at room temperature. Sections were then stained using goat anti-KLF4 (1:300; R and D Systems), rabbit anti-cleaved caspase-3 (1:500; R and D Systems), rabbit anti-p53 (1:200; Leica Microsystems), rabbit monoclonal anti p21, rabbit anti-Ki67 (1:500; Biocare Medical), rabbit antiphosphorylated histone H2AX (γH2AX) (1:100; Cell Signaling), and goat anti-LBP (1:200; Santa Cruz Biotechnology) at 4°C overnight. Washes were done using TTBS, and detection of primary antibodies for immunofluorescence (IF) was carried out using appropriate Alexa Fluor-labeled secondary antibodies (Molecular Probes) at 1:500 dilutions in 3% BSA in TTBS for 30 min at 37°C, counterstained with Hoechst 33258 (2 μg/ml) (propidium iodide counterstain for γH2AX), mounted with Prolong gold (Molecular Probes), and coverslipped. For Klf4 and p21 detection by IF, secondary unconjugated rabbit anti-goat antibody (Jackson Immuno Research) and bovine anti-rabbit (Novus Biologicals), respectively, were added at 1:300 dilution in 3% BSA in TTBS for 30 min at 37°C. After being washed, goat anti-rabbit Alexa Fluor 548-labeled (Molecular Probes) and goat anti-bovine 548-labeled (Jackson Immuno Research) tertiary antibody were then added, respectively, at 1:500 dilutions in 3% BSA in TTBS for 30 min at 37°C, followed by counterstaining and mounting as described above. For LBP, horseradish peroxidase-conjugated secondary antibody was used at 1:500 dilution. Color was developed using substrate-chromogen solution, counterstained with hematoxylin, and then mounted. Images were acquired using a Nikon Eclipse 90i microscope (Nikon Instruments) equipped with DS-Qi1Mc and DS-Fi1, CCD cameras (Nikon Instruments). Representative images for γH2AX staining were captured using Zeiss 510 Meta NLO confocal microscope (Zeiss).
Senescence-associated endogenous β-galactosidase activity.
Freshly isolated intestines were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature and then immersed in 30% sucrose in PBS at 4°C overnight. The intestines were then embedded in optimal cutting temperature compound and frozen. Frozen sections were cut, placed on glass slides, and stained for endogenous β-galactosidase (β-gal) activity using Senescence Cells Histochemical Staining Kit (Sigma) according to the manufacturer's recommendations. Blue color development was done by incubating the slides in staining mixture at 37°C without CO2 for 9 h and washed in running tap water. The slides were then counterstained with Nuclear Fast Red, washed in running tap water, mounted with Prolong gold (Molecular Probes), and coverslipped.
Cell and crypt scoring.
Cells showing immunoreactivity for cleaved caspase-3, Klf4, Ki67, p53, and γH2AX were counted and scored within the crypts of the small intestine. The numbers were either presented as total positive cells counted per crypt or were first normalized to the total number of cells per crypt and then presented as a ratio of positively stained cells to total number of cells in the crypt. To account for differences in crypt depth at different time points following irradiation, the crypt for every time point was identified as the region of the intestine staining positive for Ki67. A minimum of 20 crypts was counted from each mouse in every group. For Ki67-positive crypts and for Paneth cells, the number of crypts showing immunoreactivity for Ki67 and LBP, respectively, was scored per field at ×100 magnification. The number of Ki67-positive crypts per millimeter of length was counted. A minimum of 10 fields was counted from each mouse in every group.
Alcian blue staining.
Paraffin-embedded sections were deparaffinized in xylene, rehydrated in ethanol, and brought to distilled water for 5 min. Alcian Blue [AB, 1% in 3% glacial acetic acid (wt/vol) and then filtered] was applied to the sections for 15 min at room temperature, followed by a 2-min wash in running tap water, followed by dehydration (twice in 95% ethanol and twice in 100% ethanol), and they were coverslipped.
Statistical analysis.
The statistical analysis between groups for positively stained cells was performed using ANOVA. The cumulative survival days of irradiated mice were analyzed using Kaplan-Meier survival curve, and statistical significance was determined by log-rank test. A linear mixed model with position as random effect and compound symmetry as the dependence structure between Ki67 and Klf4 counts was used in SAS 9.3 (SAS Institute, Cary, NC) for regression analysis of the correlation between the temporal pattern of Ki67 and Klf4.
RESULTS
Mice with intestinal epithelium-specific deletion of Klf4 have increased mortality following total-body γ-irradiation.
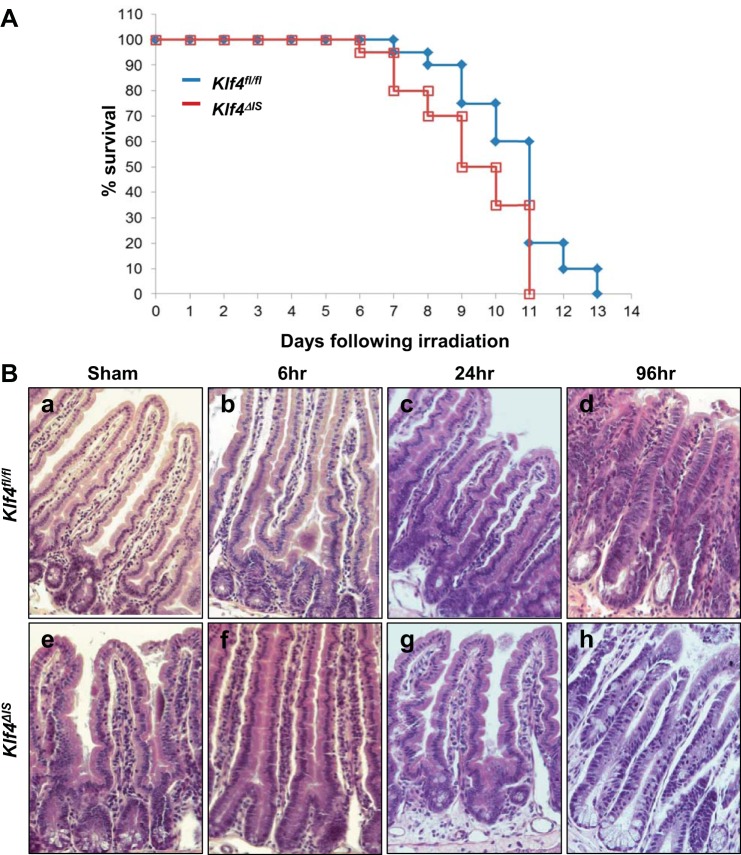
We sought to determine the role of Klf4 in the intestinal epithelium in a mouse model of intestinal γ-irradiation injury. Both Klf4fl/fl and Klf4ΔIS mice were exposed to a single total-body dose of 12 Gy, and their survival was followed. As shown in Fig. 1A, mice from both groups succumbed to death after γ-irradiation. However, irradiated Klf4fl/fl mice survived longer; that is statistically significant compared with irradiated Klf4ΔIS mice. This suggests that intestinal Klf4 might play a role in GI syndrome-related survival in response to γ-irradiation injury.
Fig. 1.
A: Kaplan-Meier survival curves of mice with floxed Krüppel-like factor 4 gene (Klf4fl/fl) (blue) and Klf4ΔIS (red) following 12-Gy total-body γ-irradiation; n = 20 per group, P = 0.0225, log-rank test. B: histological analysis of intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice following γ-irradiation. Tissues were collected from sham mice and mice after 6, 24, and 96 h following irradiation. a–d: Klf4fl/fl mice. e–h: Klf4ΔIS mice.
Morphological changes in the intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice following γ-irradiation.
To assess the morphological changes that may account for the different responses to γ-irradiation injury, we performed H and E staining on intestinal tissues collected from sham group and irradiated mice at 6, 24, and 96 h postirradiation. There was no distinction in morphology in both Klf4fl/fl and Klf4ΔIS sham groups and at 6 and 24 h postirradiation (Fig. 1B, a–c and e–g, respectively). However, at 96 h postirradiation, the intestinal epithelium in both Klf4fl/fl and Klf4ΔIS mice showed areas of normal-looking small intestinal epithelium interspersed with areas of altered morphology, wherein deep-set crypts and shorter villi were seen in lieu of the normal crypt-villus axis (Fig. 1B, d and h, respectively). This altered morphology phenomenon is described as a regenerative phenotype (39, 45, 64).
Regenerative response following γ-irradiation.
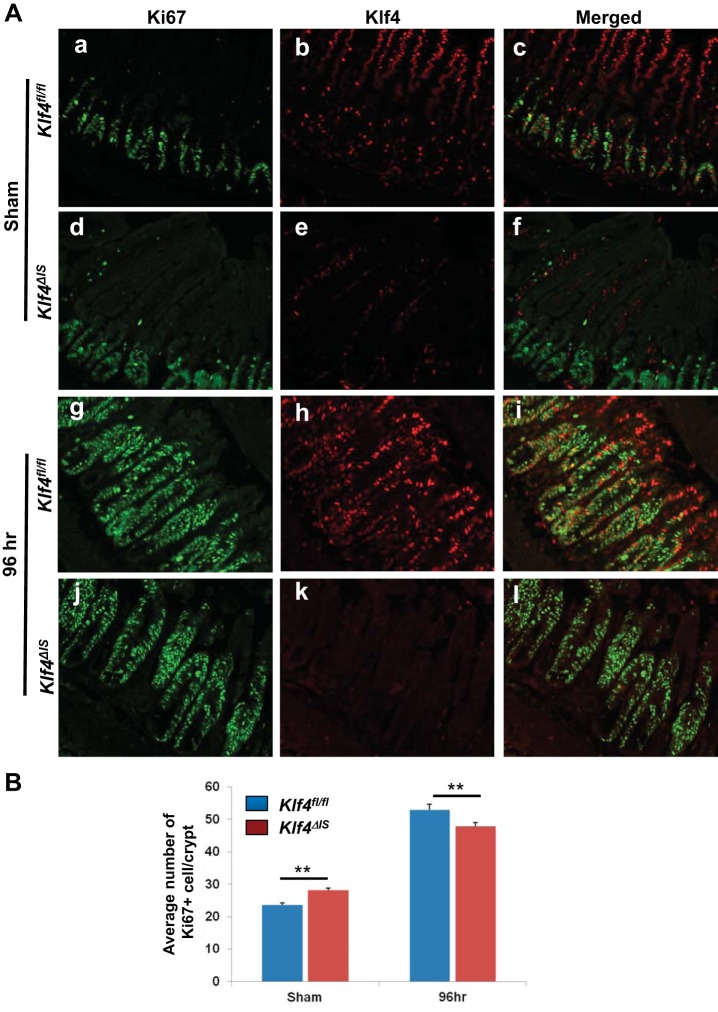
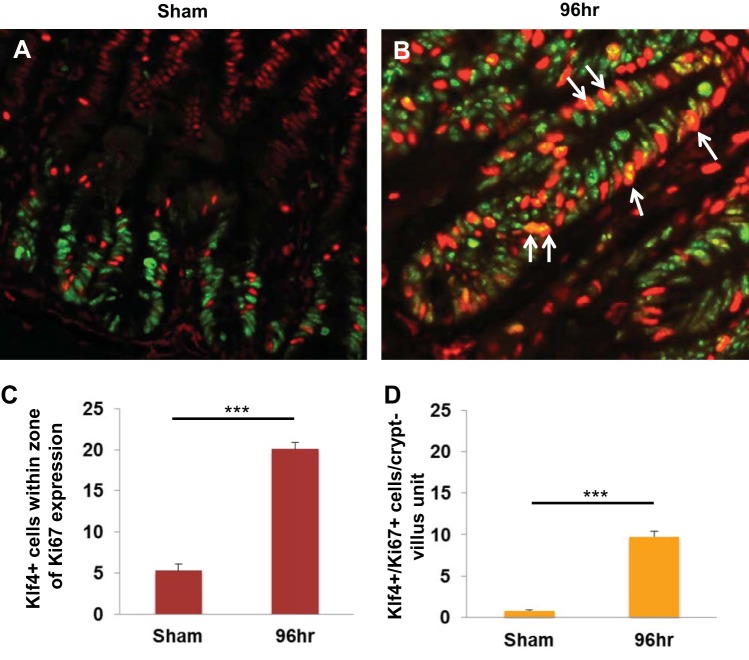
It has been previously shown that, after radiation injury, a regenerative response in the intestinal epithelium is characterized by increased levels of Ki67 expression around 96 h postirradiation (39, 45). To determine the extent of regeneration of small intestinal tissues, we performed immunofluorescence staining of Ki67 and Klf4 in Klf4fl/fl and Klf4ΔIS mice in the sham group and at 96 h following γ-irradiation (Fig. 2). As shown in Fig. 2A, a and d, and Fig. 2B, the numbers of proliferative cells in sham-treated Klf4ΔIS mice were significantly higher than in Klf4fl/fl mice. However, we observed that by 96 h after γ-irradiation in both Klf4fl/fl and Klf4ΔIS mice there were areas of regeneration within the small intestinal tissue, characterized by extended proliferative crypt region and identified by a higher number of Ki67-positive cells. In addition, the number of dividing cells marked by Ki67 expression was significantly higher in Klf4fl/fl compared with Klf4ΔIS mice (Fig. 2A, g and j, respectively, and Fig. 2B) although the differences were small. Simultaneously, the tissues were costained with an antibody against Klf4 (Fig. 2A, b, c, e, f, h, i, k, and l). In Klf4fl/fl mice, Klf4 expression at the basal level (sham) was mostly restricted to differentiated cells within the villous region of the small intestinal epithelium, with a few dispersed Klf4-positive cells (that were also Ki67 negative) in the crypt region (Fig. 2A, b and c). However, after γ-irradiation, the expression of Klf4 extended far into the crypt regions identified by Ki67-positive staining (Fig. 2A, h). Surprisingly, at 96 h postirradiation in Klf4fl/fl mice, we noticed that many of the cells within the Ki67-positive regenerating crypt-epithelium regions were concurrently stained for Klf4 and Ki67 (Fig. 2A, i). We validated our observation by quantifying Klf4- or Ki67-positive cells in Klf4fl/fl mice in sham and at 96 h postirradiation. As shown in Fig. 3, in sham there were on average five Klf4-positive cells within the zone of Ki67 expression (Fig. 3, A and C), and only a small fraction of these cells costained positively for Ki67 marker (Fig. 3D). On the other hand, at 96 h following γ-irradiation, the number of Klf4-positive cells within the Ki67 expression zone (Fig. 3, B and C) as well as the number of cells copositive for Klf4 and Ki67 within the regenerating crypt-epithelium unit increased considerably (Fig. 3, B and D). This observation suggests that Klf4, in contrast to its traditional role under normal conditions, may have a previously unrecognized role in the regenerative response to radiation-induced intestinal injury.
Fig. 2.
Expression of Klf4 and Ki67 in the small intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice following γ-irradiation. Klf4fl/fl and Klf4ΔIS mice were costained with antibody against Klf4 and Ki67. A: immunofluorescence staining of Klf4 and Ki67. a–c: Klf4fl/fl mice, sham. d–f: Klf4ΔIS mice, sham. Klf4fl/fl mice (g–i) and Klf4ΔIS mice (j–l) at 96 h postirradiation; n = 4 mice per group. B: quantification of Ki67-positive cells based on the immunostaining presented in A. Shown are the average numbers of Ki67-positive cells in at least 20 crypts per mouse. **P < 0.01 ± SE.
Fig. 3.
Expression pattern of Ki67 and Klf4 in Klf4fl/fl sham mice and in mice 96 h following γ-irradiation. A: Klf4fl/fl mice, sham. B: Klf4fl/fl mice 96 h following γ-irradiation. Arrows mark examples of Klf4/Ki67 double-positive cells. Klf4 is indicated by green staining and Ki67 by red staining. C: graph depicting changes in the number of Klf4-positive cells within the proliferative region of Ki67 expression from sham mice and in mice 96 h following γ-irradiation. The number of positive Klf4 cells was averaged from ≥20 crypts per mouse. D: graph depicting changes in the number of Klf4/Ki67 double-positive cells in sham mice and in mice 96 h following γ-irradiation. The number of positive Klf4/Ki67 cells was averaged from ≥20 crypts per mouse. ***P < 0.001 ± SE.
Regression analysis of the temporal pattern of Ki67 and Klf4 showed that, in the analysis of the values of positive cell counts, Klf4 at 96 h on average was 42.66 ± 1.56 higher than at sham (P < 0.0001). Ki67 at 96 h on average was 27.91 ± 1.56 higher than at sham (P < 0.0001). The difference in Klf4 between the two time points was 14.75 ± 2.21 higher than the difference in Ki67 between the two time points. When analyzing the values of ratio of the positive cells to total number of cells in crypt, we found that Klf4 at 96 h on average was 0.4562 ± 0.0278 higher than at sham (P < 0.0001). Ki67 at 96 h on average was 0.0931 ± 0.0278 higher than at sham (P = 0.001). The difference in Klf4 between the two time points was 0.3631 ± 0.0392 higher than the difference in Ki67 between the two time points.
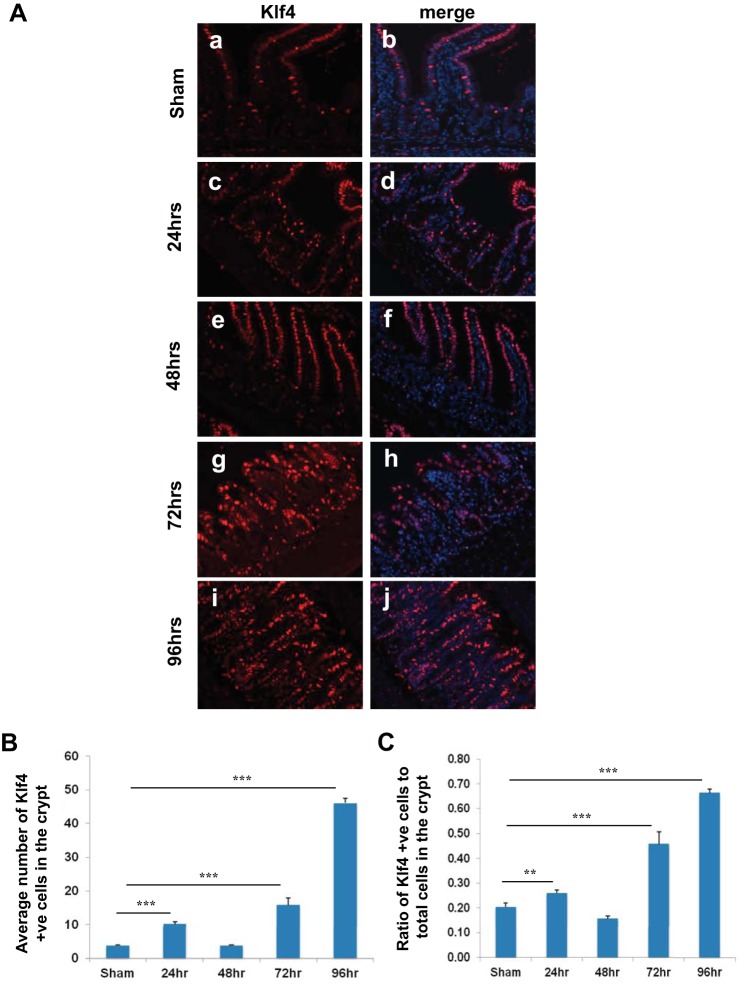
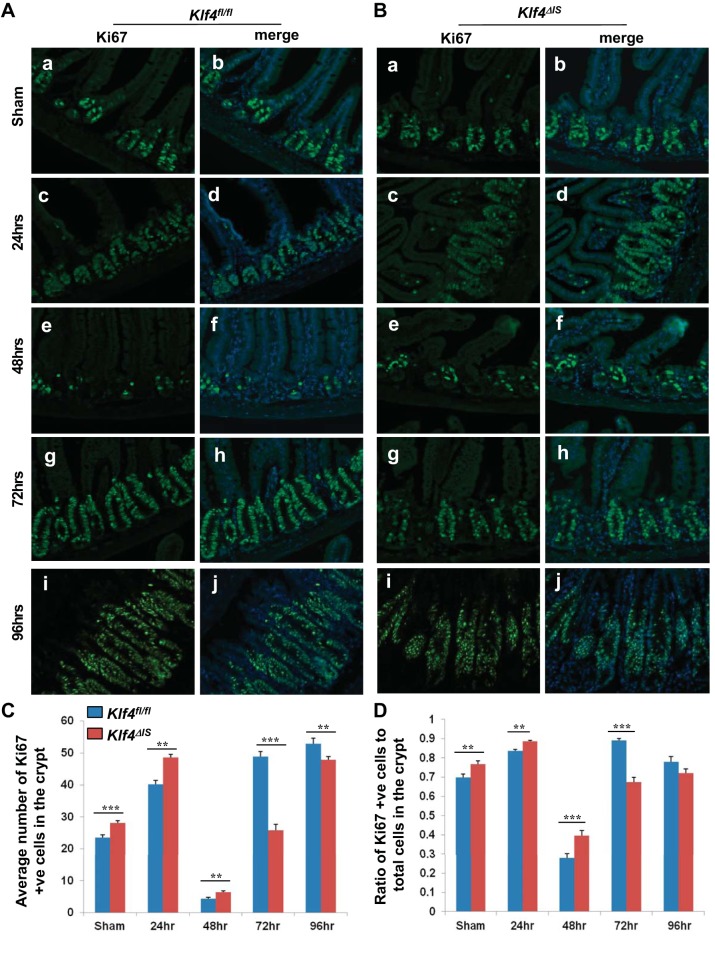
It has been previously shown that the peak of cell-cycle arrest in the intestinal crypt epithelium postirradiation is 24–48 h and that the cells begin to reenter the cell cycle by 48–72 h (30, 45). To determine whether Klf4 expression changes in the crypt epithelium between 24 and 96 h postirradiation, we compared Klf4 staining in Klf4fl/fl mice in sham and in mice 24, 48, 72, and 96 h postirradiation. Klf4 expression was shown to increase at 24 h, subside to near normal level at 48 h, and then increase progressively up to 96 h (Fig. 4, A–C). To determine whether there is any difference in the dynamics of the cell cycle postirradiation between Klf4fl/fl and Klf4ΔIS mice, we compared Ki67 staining in both genotypes in sham and in mice 24, 48, 72, and 96 h postirradiation. In Klf4fl/fl mice, Ki67 staining increased significantly by 24 h, cell-cycle arrest peaked at 48 h, and they reentered cell cycle by 72 h and continued to 96 h (Fig. 5, A, C, and D). In Klf4ΔIS mice, proliferation followed the same cell-cycle dynamics as Klf4fl/fl mice while being at significantly higher levels up to 48 h and then was significantly lower than Klf4fl/fl mice at the cell-cycle reentry phase at 72 h and up to 96 h (Fig. 5, B, C, and D).
Fig. 4.
A: expression pattern of Klf4 in Klf4fl/fl mice at sham (a and b) and 24 (c and d), 48 (e and f), 72 (g and h), and 96 h (i and j) following γ-irradiation. Klf4 is red, and nuclear staining is blue. B: graph depicting changes in the number of Klf4-positive cells within the crypt (region of Ki67 expression) from sham mice and in mice 24, 48, 72, and 96 h following γ-irradiation. The number of positive Klf4 cells was averaged from at least 20 crypts per mouse. C: graph depicting changes in the ratio in the number of Klf4-positive cells within the crypt in relation to the total number of cells in the crypt (region of Ki67 expression) from sham mice and mice 24, 48, 72, and 96 h following γ-irradiation. The number of positive Klf4 cells was averaged from at least 20 crypts per mouse; n = 4, **P < 0.01, ***P < 0.001 ± SE.
Fig. 5.
A: expression pattern of Ki67 in Klf4fl/fl sham mice (a and b) and mice at 24 (c and d), 48 (e and f), 72 (g and h), and 96 h (i and j) following γ-irradiation. Ki67 is green, and nuclear staining is blue. B: expression pattern of Ki67 in Klf4ΔIS sham mice (a and b) and mice at 24 (c and d), 48 (e and f), 72 (g and h) and 96 h (i and j) following γ-irradiation. Ki67 is green, and nuclear staining is blue. C: graph depicting changes in the number of Ki67-positive cells within the crypt from sham mice and mice 24, 48, 72, and 96 h following γ-irradiation. The number of positive Ki67 cells was averaged from at least 20 crypts per mouse. D: graph depicting changes in the ratio in the number of Ki67-positive cells within the crypt in relation to the total number of cells in the crypt from sham mice and mice 24, 48, 72, and 96 h following γ-irradiation. The number of positive Ki67 cells was averaged from at least 20 crypts per mouse; n = 4, **P < 0.01, ***P < 0.001 ± SE.
Deletion of Klf4 from the intestinal epithelium leads to increased apoptosis in the intestinal crypts in response to γ-irradiation.
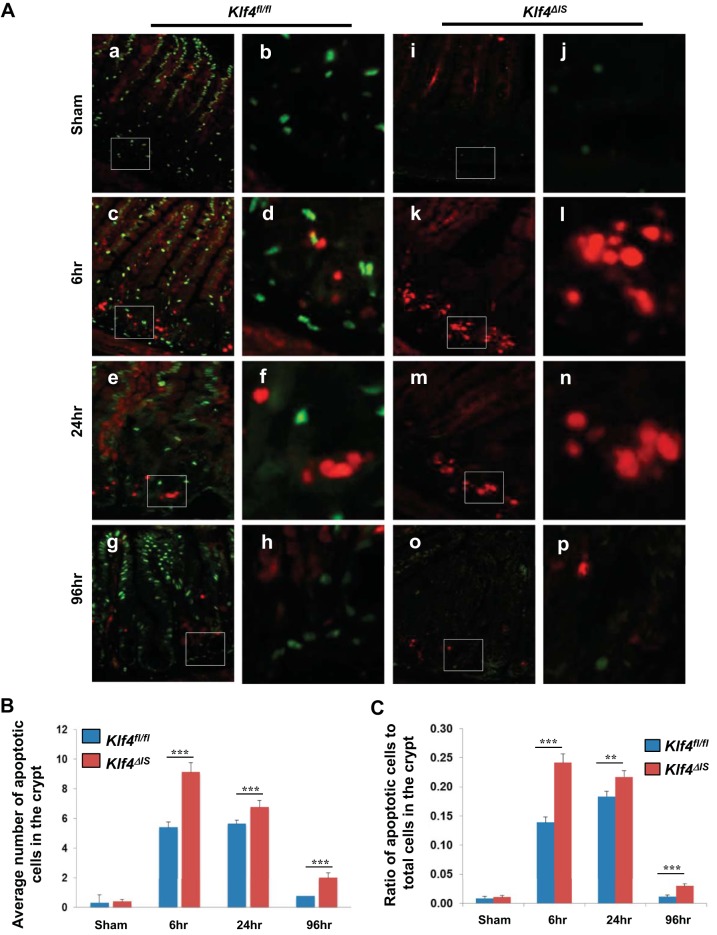
Because Klf4 is known to have antiapoptotic activity (19), we performed immunofluorescence staining for cleaved caspase-3 (a known effector of the apoptotic pathway) (13) and Klf4 in the small intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice in sham and at 6, 24, and 96 h postirradiation (Fig. 6A). There was no difference in the number of cells with cleaved caspase-3 staining between Klf4fl/fl and Klf4ΔIS mice in sham groups (basal level) (Fig. 6A, a–b and i–j, respectively). However, both groups showed increased levels of cleaved caspase-3 at 6, 24, and 96 h following irradiation (Fig. 6A, c–h and k–p, respectively) although significantly higher levels of apoptosis were observed in the crypts of small intestinal epithelium of Klf4ΔIS mice compared with Klf4fl/fl mice (Fig. 6, B and C) at these time points. This was indicated by considerably higher numbers of cleaved caspase-3-positive epithelial cells along the crypt-villus axis. These data are consistent with our previous in vitro studies indicating that Klf4 has antiapoptotic activity following γ-irradiation (19). It is worth mentioning that we did not observe costaining of cleaved caspase-3 and Klf4 in the small intestinal tissues of Klf4fl/fl and Klf4ΔIS mice at any tested time points.
Fig. 6.
Detection of apoptosis of intestinal epithelial cells after γ-irradiation. Klf4fl/fl and Klf4ΔIS mice were irradiated with 12 Gy, and small intestinal tissues were collected from sham mice and mice 6, 24, and 96 h following irradiation. A: immunofluorescence staining of small intestinal epithelium with antibody against Klf4 (green) and cleaved caspase-3 (red). a, c, e, and g (with insets shown in b, d, f, and h): staining of tissues from Klf4fl/fl mice. i, k, m, and o (with insets shown in j, l, n, and p): staining of tissues from Klf4ΔIS mice. B: quantification of the number of cleaved caspase-3-positive cells (apoptotic cells) in the crypt (region of Ki67 expression) in both mouse groups. C: graph depicting changes in the ratio in the number of cleaved caspase 3-positive cells within the crypt in relation to the total number of cells in the crypt (region of Ki67 expression) in both mouse groups following γ-irradiation. The number of positive cleaved caspase-3 cells was averaged from at least 20 crypts per mouse; n = 2, **P < 0.01, ***P < 0.001 ± SE.
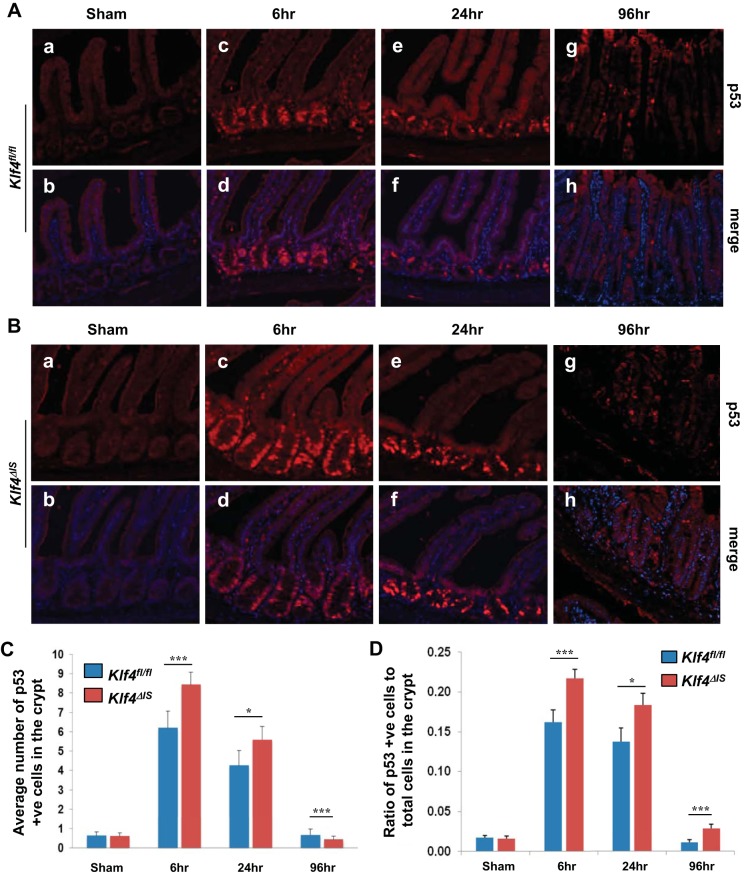
Cleaved caspase-3 is a known effector of the p53 apoptotic pathway, and previously we showed that, in vitro, Klf4 inhibits the transactivating function of p53 following γ-irradiation (19). To determine whether there is any correlation between Klf4 and p53 in the current in vivo model of γ-irradiation injury, we performed staining of p53 in the small intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice in sham and at 6, 24, and 96 h postirradiation (Fig. 7, A and B). Consistent with previous reports (60), nonirradiated mice from both groups had very low to undetectable levels of p53 (Fig. 7A, a–b, and Fig. 7B, a–b, respectively), and there was no significant difference in p53 expression in the crypts of the small intestinal epithelium between sham Klf4fl/fl and Klf4ΔIS mice (Fig. 7, C and D). At 6 and 24 h following γ-irradiation, there was an increase in p53 expression levels in both mouse groups, with Klf4ΔIS mice having significantly higher numbers of p53-positive cells per crypt than Klf4fl/fl mice (Fig. 7A, c–d and e–f, and Fig. 7B, c–d and e–f, respectively, and Fig. 7, C and D). This increase in p53-positive cells was transient in Klf4fl/fl mice, where, by 96 h postirradiation, the levels of p53 reverted to near basal level (Fig. 7A, g–h, and Fig. 7, C and D). In contrast, the number of p53-positive cells in Klf4ΔIS mice remained elevated even at 96 h postirradiation (Fig. 7B, g–h, and Fig. 7, C and D). The significantly increased levels of p53 at 6, 24, and 96 h following γ-irradiation in Klf4ΔIS mice compared with Klf4fl/fl mice were closely mirrored by the increased levels of apoptosis seen in these mice postirradiation as shown in Fig. 6.
Fig. 7.
Expression levels of p53 in the small intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice following γ-irradiation. Tissues were collected from sham mice and mice at 6, 24, and 96 h following irradiation. A: expression pattern of p53 in Klf4fl/fl mice at sham (a and b) and mice at 6 (c and d), 24 (e and f), and 96 h (g and h) following γ-irradiation. p53 is red, and nuclear staining is blue. B: expression pattern of p53 in Klf4ΔIS sham mice (a and b) and mice at 6 (c and d), 24 (e and f), and 96 h (g and h) following γ-irradiation. p53 is red, and nuclear staining is blue. C: quantification of the number of p53-positive cells in the crypt (region of Ki67 expression) in both mouse groups. D: graph depicting changes in the ratio in the number of p53-positive cells within the crypt in relation to the total number of cells in the crypt (region of Ki67 expression) in both mouse groups following γ-irradiation. The number of positive p53 cells was averaged from at least 20 crypts per mouse; n = 2, *P < 0.05, ***P < 0.001 ± SE.
Deletion of Klf4 from the intestinal epithelium leads to differential regulation of p21 expression in the terminally differentiated intestinal epithelial cells vs. cells in transient amplification zone of the crypt in the response to γ-irradiation.
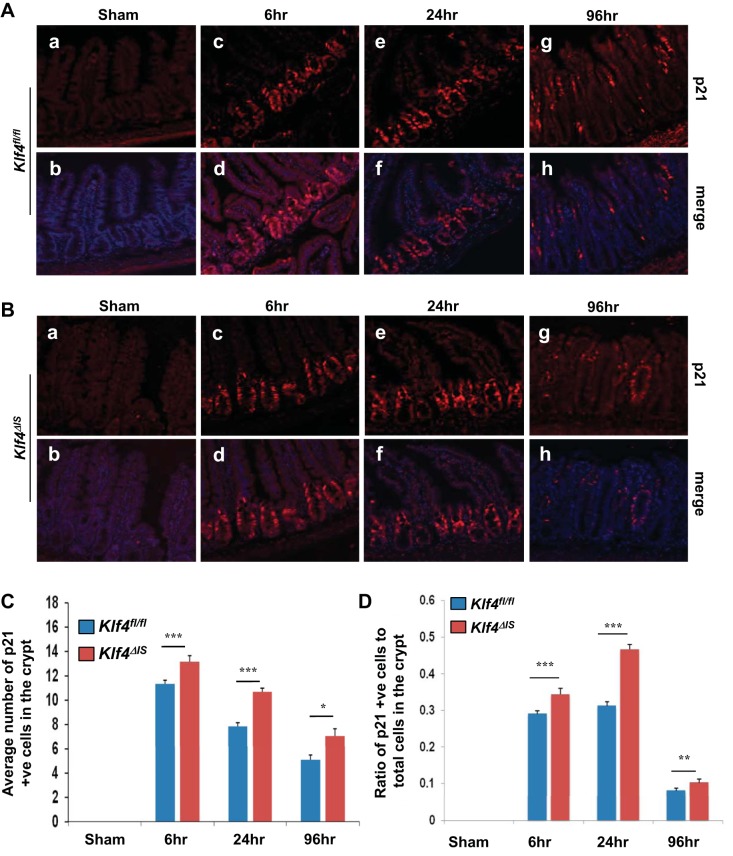
The cyclin-dependent kinase inhibitor, p21Waf1/Cip1/Sdi1 (p21), plays an essential role in p53-mediated growth arrest following DNA damage (6, 8). After DNA damage, expression of p21 is transcriptionally regulated by the tumor suppressor p53 and by other p53-independent mechanisms (17). Additionally, we have previously shown that Klf4 mediates the p53-dependent expression of p21 following γ-irradiation in vitro (19, 63). Consequently, we sought to determine whether p21 expression in vivo is also dependent on Klf4 following γ-irradiation (Fig. 8, A and B). At basal level, both Klf4fl/fl and Klf4ΔIS mice had undetectable p21 level in their intestinal epithelium (Fig. 8A, a–b, and Fig. 8B, a–b, respectively). At 6 h postirradiation, p21 expression was limited to the crypt region in Klf4fl/fl (Fig. 8A, c and d). Contrary to the previous in vitro finding, p21 was expressed in the crypt cells in the absence of Klf4 in the intestinal epithelium in Klf4ΔIS mice (Fig. 8B, c and d). By 24 h postirradiation, the p21 expression was sustained in the crypt region in both genotypes (Fig. 8A, e–f, and Fig. 8B, e–f, respectively). At 96 h postirradiation, p21-positive cells were detected scattered along the regenerative crypt epithelium in both groups with some regenerative crypts staining negative for p21 in both groups (Fig. 8A, g–h, and Fig. 8B, g–h, respectively). A closer examination of the villus-crypt axis in both mouse groups revealed that, whereas in Klf4fl/fl mice there was absence of p21 expression in the nonproliferating terminally differentiated epithelial cells in the villi at 6 and 24 h postirradiation (Fig. 8A, c–d, and Fig. 8A, e–f), Klf4ΔIS mice had p21-positive staining in the villi region at 24 h postirradiation, albeit weaker than the staining in the crypt region in general (Fig. 8B, c–d and e–f).
Fig. 8.
Expression level of p21 in the small intestinal epithelium of Klf4fl/fl and Klf4ΔIS mice. A: expression pattern of p21 in Klf4fl/fl sham mice (a and b) and mice at 6 (c and d), 24 (e and f), and 96 h (g and h) following γ-irradiation. p21 is red, and nuclear staining is blue. B: expression pattern of p21 in Klf4ΔIS sham mice (a and b) and at 6 (c and d), 24 (e and f), and 96 h (g and h) following γ-irradiation. p21 is red, and nuclear staining is blue. C: quantification of the number of p21-positive cells in the crypt (region of Ki67 expression) in both mouse groups. D: graph depicting changes in the ratio in the number of p21-positive cells within the crypt in relation to the total number of cells in the crypt (region of Ki67 expression) in both mouse groups following γ-irradiation. The number of positive p21 cells was averaged from at least 20 crypts per mouse; n = 2, *P < 0.05, **P < 0.01, ***P < 0.001 ± SE.
Consistent with previous reports in wild-type mice (60), Fig. 8, C and D, shows that, in Klf4fl/fl mice, quantification of p21-positive cells in the proliferative regions of the intestine revealed a significant increase in p21 staining at 6 h postirradiation that then slowly tapered off and reduced by 96 h. In Klf4ΔIS mice, quantification of p21-positive cells showed a similar pattern as that observed in Klf4fl/fl mice, except that there was a significantly higher number of p21-positive cells in the proliferative regions of Klf4ΔIS mice from 6 to 96 h postirradiation.
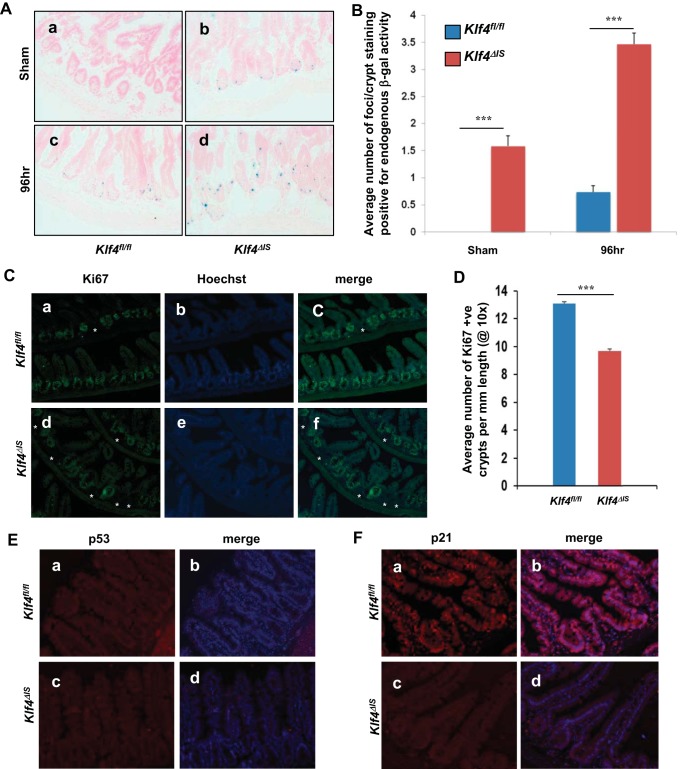
p53 and its downstream effector, p21, have been previously implicated in senescence (33). Some recent reports show that the p21 activation of senescence is context dependent (3, 47). In systems where it is involved in promoting senescence, p21 accumulates in senescent cells and has been used as a marker reflecting the activation of p53 pathway in senescence (28). Activity of endogenous β-gal is a commonly used senescence biomarker (7, 9). To determine whether senescence is induced in intestinal tissue postirradiation, we stained for senescence-associated β-Gal (SA β-Gal) activity. No SA β-Gal could be detected in sham-irradiated Klf4fl/fl (Fig. 9A, a, and Fig. 9B). However, Klf4ΔIS mice had detectable basal senescence of about two foci per crypt (Fig. 9A, b, and Fig. 9B). At 96 h postirradiation, there were some low levels of SA β-Gal in Klf4fl/fl mice (Fig. 9A, c, and Fig. 9B). In contrast, there was significantly higher β-Gal staining in Klf4ΔIS mice 96 h postirradiation compared with sham Klf4ΔIS mice and compared with 96 h Klf4fl/fl mice (Fig. 9A, d, and Fig. 9B).
Fig. 9.
A: staining for senescence-associated endogenous β-galactosidase (β-gal) activity in the intestines of Klf4fl/fl (a and c) and Klf4ΔIS (b and d) mice. B: quantification of β-gal foci per crypt in Klf4fl/fl and Klf4ΔIS mice at sham and 96 h following γ-irradiation. The number of positive β-gal foci per crypt was averaged from at least 20 crypts per mouse. C: analysis of mice intestines at 7–9 days postirradiation of Klf4fl/fl (a–c) and Klf4ΔIS (d–f) mice. Atrophied crypts are marked by asterisks. D: quantification of the percentage of atrophied crypts per field in Klf4fl/fl and Klf4ΔIS mice at 7–9 days following γ-irradiation. The number of atrophied crypts was averaged from at least 10 fields per mouse. E: p53 staining of Klf4fl/fl (a and b) and Klf4ΔIS (c and d) mice. p53 is red, and nuclear staining is blue. F: p21 staining of Klf4fl/fl (a and b) and Klf4ΔIS (c and d) mice. p21 is red, and nuclear staining is blue; n = 4, ***P < 0.001 ± SE.
Deletion of Klf4 in the intestinal epithelium is associated with increased intestinal crypt atrophy and absence of p21 expression in the intestinal epithelial cells during late chronic phase after γ-irradiation.
Because mice started dying around 7 days after total-body irradiation, we examined the intestines of mice at 7–9 days postirradiation. As seen in Fig. 9C, the hyperproliferative crypt architecture seen at 96 h postirradiation (Fig. 2A, g–i) is no longer observed, and Ki67 staining was limited to normal crypt region with the rare exception of crypts that lacked Ki67 staining (Fig. 9C, a–c). As observed in Klf4fl/fl mice, hyperproliferative crypts are not observed anymore in Klf4ΔIS mice, and Ki67 staining was limited to normal crypt region (Fig. 9C, d–f). Notably, compared with Klf4fl/fl mice, there was a significantly higher frequency of atrophied crypts (marked by absent Ki67 staining) in the intestines of Klf4ΔIS mice (Fig. 9C, a–c and d–f, respectively, and Fig. 9D).
Staining for p53 showed negative staining in both mouse genotypes at 7–9 days postirradiation (Fig. 9E, a–d). Interestingly, at 7–9 days postirradiation Klf4fl/fl mice had low-intensity staining for p21 in cells of both the villus and the crypt but no high-intensity staining for p21 observed (Fig. 9F, a and b). On the contrary, Klf4ΔIS mice had no detectable high- or low-intensity staining for p21 at 7–9 days postirradiation (Fig. 9E, c and d). There was no detectable SA β-Gal staining in Klf4fl/fl or Klf4ΔIS mice at 7–9 days postirradiation (data not shown).
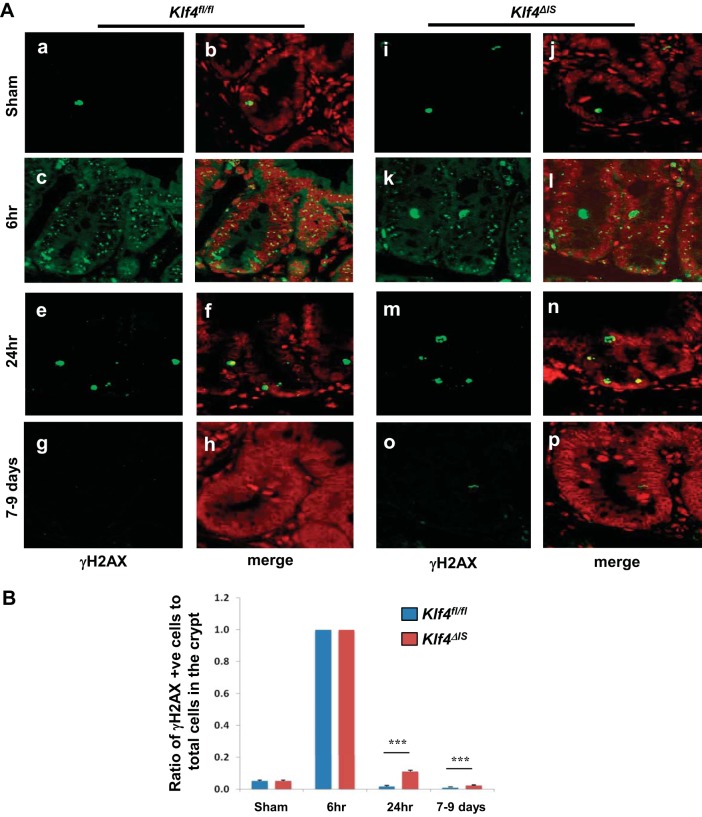
To determine what effect Klf4 deletion has on DNA damage repair following irradiation, we stained for phosphorylated histone H2AX (γH2AX), a marker for DNA breakage. In Klf4fl/fl mice, there was a low basal number of γH2AX-positive cells in sham, peaking at 6 h postirradiation and then subsiding to near normal levels at 24 h and up to 7–9 days postirradiation (Fig. 10A, a–h, and Fig. 10B). Similar to Klf4fl/fl mice, Klf4ΔIS mice had a low basal number of γH2AX-positive cells in sham mice that peaked at 6 h postirradiation to the same level as in Klf4fl/fl mice (Fig. 10A, i–l, and Fig. 10B). The number of γH2AX-positive cells then subsided at 24 h and up to 7–9 days postirradiation; however, they were maintained at significantly higher numbers compared with Klf4fl/fl mice (Fig. 10A, m–p, and Fig. 10B).
Fig. 10.
A: expression pattern of phosphorylated histone H2AX (γH2AX) in Klf4fl/fl (a–h) and Klf4ΔIS (i–p) sham mice (a and b, i and j) and mice at 6 h (c and d, k and l), 24 h (e and f, m and n), and 7–9 days (g and h, o and p) following γ-irradiation. γH2AX is green, and nuclear staining is red. B: graph depicting changes in the ratio in the number of γH2AX-positive cells within the crypt in relation to the total number of cells in the crypt (region of Ki67 expression) in Klf4fl/fl and Klf4ΔIS sham mice and mice at 6 h, 24 h, and 7–9 days following γ-irradiation. The number of γH2AX-positive cells was averaged from at least 20 crypts per mouse; n = 4, ***P < 0.001 ± SE.
Defective Paneth cell lineage allocation during regeneration after γ-irradiation in mice with intestinal epithelium deletion of Klf4.
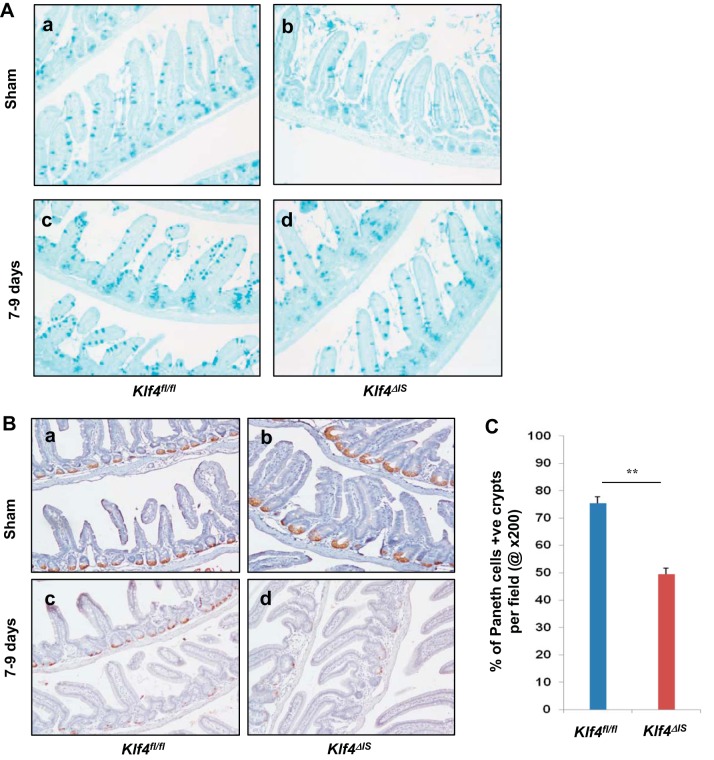
We have previously shown that Klf4 deletion causes perturbation of both goblet and Paneth cells (20). To assess whether Klf4 has any role in cell lineage allocation during regeneration postirradiation, we stained for goblet cells and Paneth cells in sham mice and in mice 7–9 days postirradiation. Compared with Klf4fl/fl sham mice (Fig. 11A, a), Klf4ΔIS sham mice had reduced staining for goblet cells (Fig. 11A, b), consistent with our previous report. However, at 7–9 days postirradiation, there was no significant difference in goblet cell staining between the two groups (Fig. 11A, c and d). Staining for LBP as a marker for Paneth cells showed that, compared with Klf4fl/fl sham mice (Fig. 11B, a), Klf4ΔIS sham mice had perturbed Paneth cells localization (Fig. 11B, b). However, in the regenerative crypt 7–9 days postirradiation, Klf4fl/fl mice had a significantly higher number of LBP-positive crypts compared with Klf4ΔIS mice (Fig. 11B, c and d, respectively, and Fig. 11C).
Fig. 11.
A: goblet cell staining in Klf4fl/fl and Klf4ΔIS sham mice (a and b) and mice at 7–9 days (c and d) following γ-irradiation. B: Paneth cell staining in Klf4fl/fl and Klf4ΔIS sham mice (a and b) and mice at 7–9 days (c and d) following γ-irradiation. C: quantification of the percentage of Paneth cell-positive crypts per field (at ×200) in Klf4fl/fl and Klf4ΔIS mice at 7–9 days following γ-irradiation. The number of Paneth cell-positive crypts was averaged from at least 10 fields per mouse; n = 4, **P < 0.01 ± SE.
DISCUSSION
The mechanisms that regulate the response of the intestinal epithelium upon radiation-induced injury are largely unknown. In the current study, we demonstrate that KLF4 plays an important role in protecting the small intestinal epithelial cells against such injury. KLF4 is expressed in the differentiated villus epithelial cells and plays an essential role in regulating intestinal epithelial homeostasis (20). Previously, we have also demonstrated that, in vitro, KLF4 has an important function in response to γ-irradiation injury (19, 62). To further investigate the in vivo role of KLF4 in response to radiation-induced injury in the intestine, we examined a mouse model with intestine epithelium deletion of Klf4 (Klf4ΔIS). Here, we determined that both Klf4fl/fl and Klf4ΔIS mice were susceptible to a high dose of 12-Gy total-body γ-irradiation and died within 2 wk of treatment although Klf4ΔIS mice had significantly poorer survival compared with Klf4fl/fl mice (Fig. 1A). Death upon high doses of irradiation was previously described (23, 26, 27) and is attributed to the long-term deleterious effects of irradiation on the stem cell compartment of the intestinal epithelium because mice exposed to whole-body irradiation still die within 10 days postirradiation even after the radiation-induced hematopoietic syndrome is rescued by bone marrow transplantation (36, 54).
It has been previously reported that, in the mouse intestine, apoptosis and cell-cycle arrest of epithelial cells are closely followed by a regenerative response after irradiation (39, 45). Our data show that, by 96 h postirradiation, both Klf4fl/fl and Klf4ΔIS mice exhibited signs of regenerative response in the small intestinal epithelium, as shown by H and E staining and Ki67 expression levels (Figs. 1B and 2, respectively, and Fig. 5). It is worth emphasizing that, at sham and up to 48 h postirradiation, Klf4ΔIS mice had significantly higher proliferation than Klf4fl/fl mice (Figs. 2 and 5). This was reversed starting at 72 h postirradiation and up to 96 h (Figs. 2 and 5). Moreover, our results show that the Klf4 expression pattern was altered upon γ-irradiation in Klf4fl/fl mice. In sham Klf4fl/fl mice, Klf4 staining was mostly confined to the differentiated cells within the villous compartment and a few cells in the crypt zone, both of which rarely costained with Ki67 (Fig. 3). However, at 96 h postirradiation, Klf4 staining extended into the proliferating crypt zone and considerably overlapped with Ki67-positive cells (Fig. 3). Also, its expression level in Klf4fl/fl mice was dynamic in response to irradiation (Fig. 4), where it mimicked that of Ki67. The close similarity between Klf4 and Ki67 expression dynamics postirradiation in the proliferative regions (Figs. 4 and 5) suggests a potential role for Klf4 in the regenerative crypts. Given the differential proliferative responses between Klf4fl/fl and Klf4ΔIS mice at sham to 48 h and after 48 h up to 96 h (Fig. 5), and that Klf4ΔIS mice still exhibited a regenerative response in the absence of Klf4, albeit with fewer Ki67-positive cells (Fig. 2B), we propose that, after irradiation, Klf4 might not be responsible for the initiation of the regeneration but instead plays a role in sustaining the regeneration of the small intestinal epithelium.
Previously, we showed that, in colorectal cancer cell lines and mouse embryonic fibroblasts, loss of Klf4 leads to extended apoptosis upon γ-irradiation treatment (19). Klf4 exerts its antiapoptotic role by inhibiting the transactivating activity of p53, thus reducing cell death. To validate the role of Klf4 in regulating the p53-dependent apoptotic pathway in vivo, we analyzed small intestinal tissues of Klf4fl/fl and Klf4ΔIS mice following γ-irradiation with antibodies against cleaved caspase-3 and p53. Whereas there was no difference in the levels of apoptosis between Klf4fl/fl and Klf4ΔIS mice at the baseline level (sham), there was a significant increase in apoptosis in Klf4ΔIS mice compared with Klf4fl/fl mice at 6, 24, and 96 h following γ-irradiation (Fig. 6). These data indicate that Klf4 plays a role in limiting the extent of apoptosis that occurs in intestinal epithelium following irradiation. This is consistent with our previous findings that Klf4 exhibits antiapoptotic activity following γ-irradiation in vitro (19). It has been previously established that, in wild-type mice, the levels of p53 increase at 6 and 24 h following γ-irradiation of 12-Gy-exposed mice compared with nonirradiated mice (40). We analyzed the expression of p53 in both Klf4fl/fl and Klf4ΔIS mice. Our results show that, after irradiation, the number of p53-positive cells increased at 6 and 24 h in both mouse groups (Fig. 7), and. in the case of Klf4ΔIS, the increased number prevailed until 96 h postirradiation. Furthermore, the levels of p53 were significantly higher in Klf4ΔIS than in Klf4fl/fl mice at all time points following irradiation (Fig. 7, C and D). p53 has been associated with cell death in the gastrointestinal epithelium by triggering an intrinsic apoptotic pathway (25, 46, 57) as well as via nonapoptotic pathways (26). Our results show that, in the absence of Klf4, both apoptosis and p53 expression were increased in the small intestinal epithelium following γ-irradiation, indicating that endogenous Klf4 plays a role in limiting apoptosis. These results are consistent with our previous findings in vitro (19) and other published data indicating p53 involvement in small intestinal epithelial cell death and survival following γ-irradiation (58). Our data also suggest that the role of p53 in DNA damage response is limited to the early phase after irradiation. On the basis of our results, Klf4 may play a role in reducing p53-dependent apoptosis in mice following radiation-induced gut injury, and the reason that p53 is lower in Klf4fl/fl than in Klf4ΔIS mice is because Klf4 normally inhibits p53, as shown before (49, 50).
Following γ-irradiation, p21 plays an important role in survival, as p21-deficient mice exhibited reduced survival compared with controls (29). In contrast to our previous finding in vitro (19), the initiation of p21 expression in the intestinal crypts in the acute postirradiation phase was not dependent on Klf4; instead, it was the dynamics of p21 expression that was affected by Klf4 during the acute and the chronic phases postirradiation (Figs. 8 and 9F).
In mice with intact Klf4 (Klf4fl/fl), p21 was observed to persist in all cells up to 7–9 days postirradiation although seemingly with a lower intensity (Fig. 9F). Klf4ΔIS mice had undetectable p21 by 7–9 days postirradiation. Thus our data suggest that crypt survival during the chronic phase postirradiation (7–9 days) is dependent on both Klf4 and p21 (Fig. 9). Absence of both in Klf4ΔIS mice led to an increased frequency of atrophied crypts (Fig. 9, C and D). Additionally, the expression of p21 following DNA damage has been shown to be both p53 dependent and p53 independent (2, 10, 11, 16, 34, 59) and to be p53 and Klf4 dependent in vitro (19, 63). Also, p21 levels have been shown to have a dose-dependent and a cell-cycle dependent effect on the apoptotic or antiapoptotic cell fate (1, 32, 35, 41). Furthermore, results of DNA-damage repair (Fig. 10) strongly support a role for Klf4 in DNA-damage repair response in vivo, consistent with its role in this response that was previously shown in vitro (12, 24). Our data suggest a plausible model where, in the absence of Klf4 and following DNA damage, p21 could be driving the cell toward apoptosis and senescence, probably attributable to a defect in the DNA repair mechanism in Klf4ΔIS mice, as evident by the γH2AX staining. While in the presence of Klf4, p21 is driven more toward a cell-cycle arrest and DNA-repair functions during the acute phase following irradiation. In the presence of Klf4, p21 might be the main player for the long-term cell survival postirradiation. Thus, in our model, Klf4 could be the key player in the decision of what role p21 undertakes following DNA damage. However, a limitation of this study is that quantification is based on immunofluorescence (e.g., p53 and p21) and lacks biochemical validation. In our hands, some of the biochemical methods, e.g., Western blotting, proved to be inconclusive. This is possibly due to factors such as 1) nuclear localization and/or modification of the protein such as with p53 and 2) the fact that we are analyzing only a subset of cells, the intestinal crypt epithelium, where the difference in staining between the two groups of mice is, although significant, usually in the range of 5–10 cells, a difference that might not be sufficient to clearly detect by methods such as Western blot.
Several studies have focused on the regeneration of intestinal crypts following high-dose irradiation and the role of intestinal stem cells (ISC) in the regeneration process. To date, two main ISC populations have been identified to take over the mission of repopulating the intestinal crypts and epithelium (42–44). One is a slower cycling or quiescent cell population that stains positive for Bmi1 at the +4 position (31, 51, 61), and the other is the crypt base columnar (CBC) cells that are actively cycling population and stain positive for Lgr5 (4, 5, 31, 61). Of other markers that have been identified to differentially mark the two populations of ISC, Sox9 has been shown to be uniquely expressed at two different intensities when using reporter system, to differentially mark the CBCs (Sox9-low) vs. +4 cells (Sox9-high) (15, 56). Also, cross-regulatory interaction between Sox9 and Klf4 in the intestinal epithelium (14) and in colon cancer cells (53) has been reported. Staining for Klf4 in the intestine consistently shows very few Klf4-positive cells in the crypt, and some of them are peculiarly at position +4 (Fig. 4A, b and c). The identity of these crypt Klf4-positive cells is yet to be determined, in particular those at the +4 position. Because our data demonstrate a critical role for Klf4 in crypt survival postirradiation, we may raise the question of whether the Klf4-positive cells at the +4 position are also marking the Bmi1/Sox9-high +4 cells and whether Klf4 plays a role in maintaining the quiescent state and/or the stemness of these cells under normal conditions and following radiation injury.
Analysis of cell lineage allocation during intestinal epithelium regeneration postirradiation suggests an important role for Klf4 in the survival and/or the regeneration of the Paneth cell lineage (Fig. 11). Paneth cells have been previously shown to constitute the niche for Lgr5 stem cells in the intestinal crypts (52), to be relatively resistant to ionizing irradiation (22), and to play an important role in the regenerative process and remodeling following tissue injury such as chronic inflammation (48) and irradiation (21, 22). Also, intestinal Lgr5 stem cells have been shown to be relatively more sensitive to irradiation, and they are regenerated from the radiation resistant +4 stem cells (55). Our results suggest that Klf4 plays a role in Paneth cell survival postirradiation and that the defect in Paneth cell population observed in Klf4ΔIS mice postirradiation could likely have a negative effect on the Lgr5 stem cell niche postirradiation and consequently be contributing to the increased crypt atrophy and to the overall decreased survival of Klf4ΔIS mice postirradiation.
Our current study suggests that the absence of Klf4 from the intestinal epithelium may limit cell-cycle arrest and elevate the rate of apoptotic cell death, resulting in the induction of senescence and increased crypt atrophy following radiation-induced intestinal damage. To further investigate the role of Klf4 in this system, it would be of interest to elucidate the exact mechanism by which Klf4 interacts with p21, how it regulates the p53-dependent apoptotic pathway, how it may regulate the pro- and antiapoptotic functions of p21, and what role, if any, it plays in the ISC survival and the regeneration of the crypt. Even though we have not investigated it in this study, we cannot rule out the role of nonapoptotic cell death and the role that Klf4 might have in it.
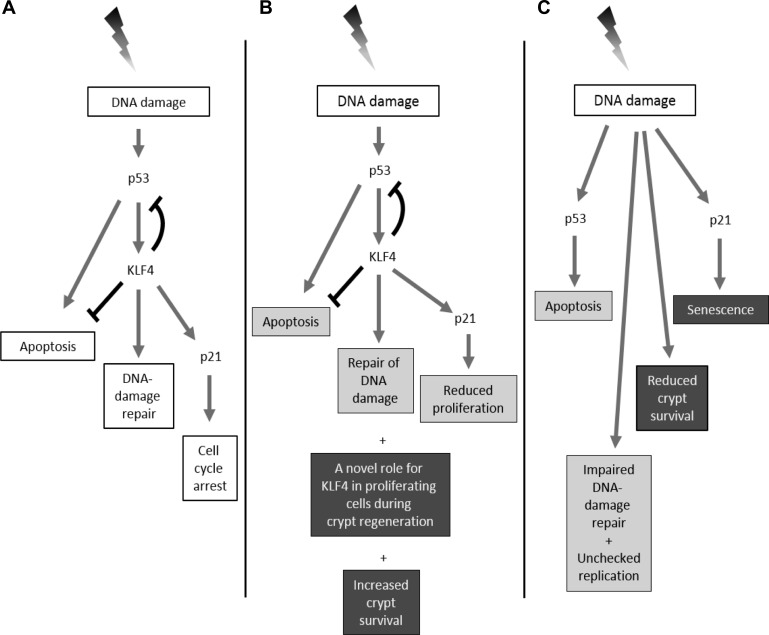
Accordingly, we propose a model (Fig. 12) for the role of KLF4 in response to irradiation-induced DNA damage in the intestinal epithelium in vivo compared with our previous finding in vitro (22). In vitro, p53 is upregulated following irradiation and activates KLF4, which plays a dual function, to induce the expression of p21 leading to cell-cycle arrest and to suppress apoptosis by inactivating p53 expression (Fig. 12A). However, in the proliferative crypts in vivo (Fig. 12B), the dependence of Klf4 expression on p53 following irradiation might still occur, while Klf4 may still inhibit apoptosis by suppressing p53 expression. Also, Klf4 might be required but not necessary for regulating the expression of p21 in the intestinal epithelium. Our results suggest that Klf4 might have short-term and long-term roles in the intestinal epithelium following radiation injury. During the short term, Klf4 suppresses apoptosis and proliferation and is involved in DNA-repair response. During the long term, Klf4 plays a role in increased crypt survival and surprisingly might have a novel role in proliferation during the regenerative phase of the intestinal epithelium. Upon Klf4 deletion and following irradiation (Fig. 12C), there is elevated p53 expression and increased apoptosis and proliferation, while DNA repair is suppressed. Also, there is overall reduced crypt survival. Additionally, p21 expression shifts to a Klf4-independent pathway that may be linked to increased senescence.
Fig. 12.
Model comparing the role of Klf4 in vitro (A) and in vivo (B and C) after γ-irradiation. A: in in vitro models, the expression of KLF4 is activated in a p53-dependent manner after γ-irradiation that then leads to increased p21 and decreased BAX expression. A net effect is to steer cells away from apoptosis and toward cell-cycle arrest. B: in vivo the interaction between p53, Klf4, and p21 might still hold in the intestinal epithelium following radiation injury; however, Klf4 might be required but not necessary for regulating the expression of p21. Klf4 is proposed to have short-term and a long-term roles in the intestinal epithelium following radiation injury. During the short term (gray boxes), Klf4 suppresses apoptosis and proliferation and is involved in DNA-repair response. During the long term (black boxes), Klf4 plays a role in increased crypt survival and might have a novel role in proliferation during the regenerative phase of the intestinal epithelium. C: in vivo deletion of Klf4 leads in the short term (gray boxes) to elevated p53, increased apoptosis, impaired DNA-damage repair, and unchecked proliferation. The expression of p21 shifts to a Klf4-independent pathway. In the long term (black boxes), p21 might divert crypt-cell fate toward senescence, and there is overall reduced crypt survival.
Taken together, our data show that, in vivo, deletion of intestinal epithelial Klf4 was associated with limited cell-cycle arrest in the first 48 h, increased apoptosis and senescence, high p53, and high-intensity p21 levels following γ-irradiation. In addition, Klf4 expression was associated with a regenerative response after 48 h, in which the normally quiescent Klf4-expressing cells in unirradiated mice became proliferative at 96 h following irradiation. On the basis of these results, Klf4 may aid in cell survival secondary to irradiation through either its association with DNA repair, reduced cell death, increased regeneration, or all three. Klf4 may also influence cell fate by limiting p53-dependent apoptosis in lieu of cell-cycle arrest through the protein p21 and/or by modulating the stem cell niche in the crypt by promoting Paneth cell survival. Detailed biochemical studies are necessary to establish the exact role of Klf4 in p53-independent apoptosis, nonapoptotic cell death, and crypt survival. Furthermore, the mechanism by which Klf4 mediates the proliferative and regenerative response merits further investigation.
GRANTS
This work was supported by grants from the National Cancer Institute (CA084197 and DK052230) (V. Yang) and by an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship (D. Talmasov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.T., V.W.Y., and A.M.G. conception and design of research; D.T., Z.X., B.Y., M.O.N., E.E., J.K., and A.M.G. performed experiments; D.T., Z.X., V.W.Y., and A.M.G. analyzed data; D.T., Z.X., V.W.Y., and A.M.G. interpreted results of experiments; D.T., Z.X., A.B.B., and A.M.G. prepared figures; D.T., Z.X., A.B.B., and A.M.G. drafted manuscript; D.T., Z.X., B.Y., M.O.N., A.B.B., E.E., J.K., V.W.Y., and A.M.G. edited and revised manuscript; D.T., Z.X., M.O.N., A.B.B., E.E., J.K., V.W.Y., and A.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jie Yang from the Department of Preventive Medicine, Stony Brook University, for assistance regarding biostatistical analysis of the survival experiment and for carrying out the regression analysis.
REFERENCES
- 1.Adiga SK, Toyoshima M, Shiraishi K, Shimura T, Takeda J, Taga M, Nagai H, Kumar P, Niwa O. p21 provides stage specific DNA damage control to preimplantation embryos. Oncogene 26: 6141–6149, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Aliouat-Denis CM, Dendouga N, Van den Wyngaert I, Goehlmann H, Steller U, van de Weyer I, Van Slycken N, Andries L, Kass S, Luyten W, Janicot M, Vialard JE. p53-independent regulation of p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res 3: 627–634, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep 3: 1164–1174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betaGal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4: 1798–1806, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82: 675–684, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacock M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013–1023, 1994. [DOI] [PubMed] [Google Scholar]
- 11.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825, 1993. [DOI] [PubMed] [Google Scholar]
- 12.El-Karim EA, Hagos EG, Ghaleb AM, Yu B, Yang VW. Kruppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts. Mol Cancer 12: 89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Biochim Biophys Acta 37: 719–727, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Flandez M, Guilmeau S, Blache P, Augenlicht LH. KLF4 regulation in intestinal epithelial cell maturation. Exp Cell Res 314: 3712–3723, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 296: G1108–G1118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1: 639–649, 2002. [PubMed] [Google Scholar]
- 17.Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res 246: 280–289, 1999. [DOI] [PubMed] [Google Scholar]
- 18.George RJ, Sturmoski MA, May R, Sureban SM, Dieckgraefe BK, Anant S, Houchen CW. Loss of p21Waf1/Cip1/Sdi1 enhances intestinal stem cell survival following radiation injury. Am J Physiol Gastrointest Liver Physiol 296: G245–G254, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaleb AM, Katz JP, Kaestner KH, Du JX, Yang VW. Kruppel-like factor 4 exhibits antiapoptotic activity following gamma-radiation-induced DNA damage. Oncogene 26: 2365–2373, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol 349: 310–320, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorbunov NV, Garrison BR, Kiang JG. Response of crypt Paneth cells in the small intestine following total-body gamma-irradiation. Int J Immunopathol Pharmacol 23: 1111–1123, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Gorbunov NV, Kiang JG. Up-regulation of autophagy in small intestine Paneth cells in response to total-body gamma-irradiation. J Pathol 219: 242–252, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 3: 117–129, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Hagos EG, Ghaleb AM, Dalton WB, Bialkowska AB, Yang VW. Mouse embryonic fibroblasts null for the Kruppel-like factor 4 gene are genetically unstable. Oncogene 28: 1197–1205, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4: 321–328, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kirsch DG, Santiago PM, di Tomaso E, Sullivan JM, Hou WS, Dayton T, Jeffords LB, Sodha P, Mercer KL, Cohen R, Takeuchi O, Korsmeyer SJ, Bronson RT, Kim CF, Haigis KM, Jain RK, Jacks T. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327: 593–596, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, Gudkov AV. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 23: 3265–3271, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 24: 2463–2479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leibowitz BJ, Qiu W, Liu H, Cheng T, Zhang L, Yu J. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res 9: 616–625, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Hassan GS, Williams TM, Minetti C, Pestell RG, Tanowitz HB, Frank PG, Sotgia F, Lisanti MP. Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle 4: 1817–1825, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 327: 542–545, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Bishop WR, Liu M. Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist Updat 6: 183–195, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature 432: 307–315, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 9: 935–944, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, Tulchinsky E, Jones GD, Roninson IB, Macip S. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem 287: 9845–9854, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res 117: 480–488, 1989. [PubMed] [Google Scholar]
- 37.McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays 29: 549–557, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev 90: 1337–1381, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: Evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 14: 2759–2766, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 54: 614–617, 1994. [PubMed] [Google Scholar]
- 41.Piccolo MT, Crispi S. The dual role played by p21 may influence the apoptotic or anti-apoptotic fate in cancer. J Can Res Updates 1: 189–202, 2012. [Google Scholar]
- 42.Potten CS, Ellis JR. Adult small intestinal stem cells: Identification, location, characteristics, and clinical applications. Ernst Schering Res Found Work 2006: 81–98, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Potten CS, Gandara R, Mahida YR, Loeffler M, Wright NA. The stem cells of small intestinal crypts: Where are they? Cell Prolif 42: 731–750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110: 1001–1020, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Potten CS, Owen G, Roberts SA. The temporal and spatial changes in cell proliferation within the irradiated crypts of the murine small intestine. Int J Radiat Biol 57: 185–199, 1990. [DOI] [PubMed] [Google Scholar]
- 46.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2: 576–583, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romanov VS, Abramova MV, Svetlikova SB, Bykova TV, Zubova SG, Aksenov ND, Fornace AJ Jr, Pospelova TV, Pospelov VA. p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle 9: 3945–3955, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, Sansom O, Fodde R. Paneth cells in intestinal homeostasis and tissue injury. PloS One 7: e38965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol 7: 1074–1082, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer 6: 11–23, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sellak H, Wu S, Lincoln TM. KLF4 and SOX9 transcription factors antagonize beta-catenin and inhibit TCF-activity in cancer cells. Biochim Biophys Acta 1823: 1666–1675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terry NH, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys 17: 569–573, 1989. [DOI] [PubMed] [Google Scholar]
- 55.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–G1132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302: 1036–1038, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, Cheng J, Liu D, Sun H, Zhao J, Wang J, Chen J, Su Y, Zou Z. P53-participated cellular and molecular responses to irradiation are cell differentiation-determined in murine intestinal epithelium. Arch Biochem Biophys 542C: 21–27, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Su ZZ, Fisher PB, Wang S, VanTuyle G, Grant S. Evidence of a functional role for the cyclin-dependent kinase inhibitor p21(WAF1/CIP1/MDA6) in the reciprocal regulation of PKC activator-induced apoptosis and differentiation in human myelomonocytic leukemia cells. Exp Cell Res 244: 105–116, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Wilson JW, Pritchard DM, Hickman JA, Potten CS. Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am J Pathol 153: 899–909, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon HS, Ghaleb AM, Nandan MO, Hisamuddin IM, Dalton WB, Yang VW. Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene 24: 4017–4025, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem 275: 18391–18398, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou WJ, Geng ZH, Spence JR, Geng JG. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature 501: 107–111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]