Abstract
Renal denervation has been shown to lower arterial pressure in some hypertensive patients, yet it remains unclear whether this is due to ablation of afferent or efferent renal nerves. To investigate the role of afferent renal nerves in arterial pressure regulation, previous studies have used methods that disrupt both renal and nonrenal afferent signaling. The present study was conducted to develop and validate a technique for selective ablation of afferent renal nerves that does not disrupt other afferent pathways. To do this, we adapted a technique for sensory denervation of the adrenal gland by topical application of capsaicin and tested the hypothesis that exposure of the renal nerves to capsaicin (renal-CAP) causes ablation of afferent but not efferent renal nerves. Renal-CAP had no effect on renal content of the efferent nerve markers tyrosine hydroxylase and norepinephrine; however, the afferent nerve marker, calcitonin gene-related peptide was largely depleted from the kidney 10 days after intervention, but returned to roughly half of control levels by 7 wk postintervention. Moreover, renal-CAP abolished the cardiovascular responses to acute pharmacological stimulation of afferent renal nerves. Renal-CAP rats showed normal weight gain, as well as cardiovascular and fluid balance regulation during dietary sodium loading. To some extent, renal-CAP did blunt the bradycardic response and increase the dipsogenic response to increased salt intake. Lastly, renal-CAP significantly attenuated the development of deoxycorticosterone acetate-salt hypertension. These results demonstrate that renal-CAP effectively causes selective ablation of afferent renal nerves in rats.
Keywords: afferent renal denervation, salt-sensitivity, hypertension
numerous uncontrolled, unblinded clinical trials have highlighted catheter-based renal nerve ablation (renal denervation, RDNX) as a possibly viable antihypertensive treatment in patients with drug-resistant hypertension (10, 26, 30). However, a recent multicenter, single-blind, randomized, sham-controlled trial failed to show a significant antihypertensive effect of the treatment (2). Despite the controversy surrounding these clinical data, RDNX has been consistently shown to reduce blood pressure in numerous animal models of hypertension (9), suggesting that when sufficient denervation is achieved, RDNX can have an antihypertensive effect in certain forms of hypertension. The failure of RDNX to reduce blood pressure in some cases highlights the importance of further understanding the mechanisms underlying the antihypertensive effect of RDNX. Specifically, since renal nerves contain both motor (efferent) and sensory (afferent) fibers, it is not known whether the antihypertensive response to RDNX is due to ablation of efferent nerves, afferent nerves, or both. Because elevated efferent renal nerve activity increases renal sodium reabsorption, renal vascular resistance, and renin release, one hypothesis is that RDNX lowers arterial pressure secondary to a reduction in one or more of these parameters (8). Alternatively, in acute studies, elevated afferent renal nerve activity has been shown to increase sympathetic nerve activity to renal and nonrenal vascular beds (36, 40), suggesting that RDNX may decrease arterial pressure by reducing sympathetic tone, not only to the kidney, but to other targets as well. Recent uncontrolled, unblinded studies in humans with drug-resistant hypertension support both of these hypotheses. Specifically, RDNX has been shown to decrease the renal resistive index, likely resulting from a loss of efferent neural control of renal vascular tone (28). It has also been reported that RDNX decreases skeletal muscle sympathetic activity (13, 38), plasma glucose (29), incidence of cardiac arrhythmias (37, 42), and the frequency of episodes of sleep apnea (51). These responses likely result from ablation of afferent renal nerves. However, it should be noted that at least one study has shown no reduction in skeletal muscle sympathetic activity following RDNX (3).
Surgical RDNX has been used for decades in animal models to study the role of renal nerves in the regulation of renal and cardiovascular function (9). However, as is true with catheter-based renal nerve ablation in humans, this method is nonselective, since it disrupts both afferent and efferent neural pathways. To study the functions of afferent renal nerves specifically, a procedure termed dorsal rhizotomy has been used (24, 25). This surgical technique interrupts afferent neural pathways from the kidney by sectioning the dorsal roots of the spinal cord at levels T9-L1. Although this procedure removes a large portion of the renal afferent input into the spinal cord, it is not specific to renal afferents, since it also disrupts all visceral, somatic, and cutaneous afferent inputs at these spinal levels. This is particularly important since osmosensitive and sodium-sensitive hepatoportal afferents, which are known to regulate efferent renal nerve activity, at least acutely (17, 31, 32), are also sectioned by this procedure. Moreover, we have previously shown that denervation of these afferents chronically increases arterial pressure in normal rats, although we did not establish the role of renal nerves in this response (4). Another method used to disrupt afferent renal nerve signaling is systemic administration of the transient receptor potential (TRP) V1 receptor agonist, capsaicin, to neonatal rat pups (16, 47, 48, 50). It is well documented that capsaicin ablates small, unmyelinated C-fibers (11, 15), and this method destroys afferent neurons in the kidney; however, it is not specific for renal afferents since systemic delivery of capsaicin has been shown to cause degeneration of TRPV1+ sensory fibers throughout the body. Nonetheless, it is interesting to note that both dorsal rhizotomy and systemic capsaicin treatment have been reported to cause salt-sensitive hypertension in the rat (24, 47), and these findings have been interpreted as evidence that disruption of renal afferent signaling may be the cause of this salt-sensitive hypertension (24). However, since both methods are not selective for renal afferents, the possibility remains that the observed salt-sensitive hypertension resulted from the ablation of other sensory afferents, such as hepato-portal osmoreceptors (32, 33).
There is clearly a strong need for a technique to selectively ablate afferent renal nerves experimentally. Such a technique would be a valuable tool for studying the role of renal afferent signaling in the regulation of renal and cardiovascular function and could also pave the way for development of more refined renal nerve ablation therapies in humans. The present study was conducted to address this need. The approach that we used was adapted from our previous findings that topical application of capsaicin to the adrenal gland causes selective ablation of adrenal afferent nerves in the rat (43–45). Because the majority of renal afferent fibers are unmyelinated (or thinly myelinated) and capsaicin-sensitive (23, 49), and TRPV1 receptors are localized along the axons of sensory fibers as well as nerve terminals (41), we hypothesized that periaxonal application of capsaicin to the renal nerves (renal-CAP) would cause selective ablation of afferent renal nerves.
We tested this hypothesis by quantifying the content of neuronal markers in the kidney at various time points following renal-CAP using the afferent nerve marker, CGRP, and the efferent nerve markers tyrosine hydroxylase (TH) and norepinephrine (NE). We also assessed the ability of renal-CAP to abolish the afferent renal nerve-dependent cardiovascular responses to intrarenal infusions of bradykinin in conscious rats. Additionally, since previous studies using nonselective methods to ablate afferent renal nerves have suggested that impaired renal afferent signaling may be a determinant of salt-sensitive hypertension, we assessed the effect renal-CAP on the regulation of arterial pressure and sodium/water balance in rats fed varying levels of dietary sodium. Finally, we tested whether the development of DOCA-salt hypertension, which has been proposed to be driven by afferent renal nerves (19), is attenuated by renal-CAP.
MATERIALS AND METHODS
Animals and General Procedures
With the exception of the experiments using intrarenal and intravenous bradykinin infusion, all experiments were performed at the University of Minnesota. For these studies, male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and were housed in pairs in a temperature- and light-controlled room until the beginning of the study. Rats were allowed access to standard rat chow and distilled water ad libitum during this preexperimental period. For all surgical procedures performed in these experiments, rats were anesthetized with 2.0% isoflurane and atropine sulfate (0.2 mg/kg ip), ketoprofen (5 mg/kg sc) and gentamicin sulfate (2.5 mg/kg im]) were administered prior to surgery. For 3 days following surgery, ketoprofen (2.5 mg/kg sc) was given once per day, and the drinking water was supplemented with amoxicillin (1 mg/ml). All procedures were approved by the University of Minnesota Animal Care and Use Committee and were conducted in accordance with the institutional and National Institutes of Health guidelines.
The experiments to assess cardiovascular responses to intrarenal and intravenous infusions of bradykinin were conducted at Boston University. For these studies, male Sprague-Dawley rats were purchased from Harlan Laboratories, (Indianapolis, IN) and housed individually in a temperature- and light-controlled room. Following completion of surgical procedures, rats received a standard rat chow and water ad libitum. All procedures were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Surgical Procedures
Renal-CAP.
Rats were anesthetized, a midline abdominal incision was made, and the visceral organs were externalized and reflected to expose the left kidney. A hole was made in the peritoneal membrane to expose the renal artery and vein. The fat surrounding the renal artery and vein was then gently dissected away from the vessels to expose the renal nerves. A small piece of gauze soaked in a capsaicin solution (33 mM in 5% ethanol, 5% Tween 80 and 90% normal saline) was wrapped around the renal artery and vein for 15 min. A small piece of parafilm was placed under the renal artery and vein prior to the placement of capsaicin-soaked gauze to prevent any nonrenal capsaicin exposure. Following 15 min of capsaicin exposure, the gauze and parafilm were removed, the area was dried, and the procedure was repeated on the contralateral side if called for in the experimental protocol. At the end of the procedure, the viscera were replaced, and the abdominal muscles and skin were closed separately with 3–0 silk suture. The sham control was performed by externalizing the viscera, visualizing the renal artery and vein, replacing the organs, and then closing the wound.
Complete RDNX.
Denervation of both afferent and efferent renal nerves was performed as previously described (19). Briefly, rats were anesthetized with 2% isoflurane, and the left renal nerves were then exposed through a midline abdominal incision. Using a dissecting microscope, the renal vein and artery were dissected out of the surrounding fascia and stripped of all visible renal nerve bundles. Following dissection, the renal artery was painted with 10% phenol in ethanol to ensure the destruction of any remaining renal nerve fibers. This procedure was then repeated on the contralateral side and the incision was closed.
Instrumentation for Acute Assessment of Afferent Renal Nerve Functionality
On the day of acute bradykinin experiments, rats were anesthetized with sodium methohexital (20 mg/kg ip; supplemented with 10 mg/kg, intravenous as required). To allow for intrarenal infusion of bradykinin, the left kidney was exposed through a small flank incision. A catheter [polyethylene (PE 10)] was then placed in a branch of the left renal artery and exteriorized through the flank incision, and the incision was closed. An additional incision was made in the left inguinal area. The left femoral vein was catheterized (PE 50) for intravenous infusion of saline or drugs, and the left femoral artery was catheterized (PE 50) for measurement of mean arterial pressure (MAP) and heart rate (HR). Both catheters were exteriorized at the incision site, and the incision was closed.
Validation of Renal-CAP by Immunohistochemistry
To test the efficacy of renal-CAP to cause selective ablation of afferent renal nerves and to determine whether reinnervation occurs over time, male Sprague-Dawley rats (58–65 days old; 220–330 g) were subjected to either bilateral renal-CAP or sham surgery and euthanized after 10 days or 4 wk. Kidneys were then collected for immunohistochemical labeling of the efferent nerve-specific marker TH and the afferent nerve-specific marker CGRP. Additionally, immunohistochemistry (IHC) data for a 7-wk postintervention time point were obtained from rats that underwent the salt-sensitivity protocol described below.
At the endpoint, rats were anesthetized with 2% isoflurane and euthanized by exsanguination; the kidneys were then immediately removed, rinsed in normal saline, and placed in 10% neutral buffered formalin until the time of sectioning. Kidneys were embedded in paraffin and cut longitudinally at a thickness of 5 μm. Sections were deparaffinized and subjected to heat-induced epitope retrieval in a Decloaking Chamber (model DC2008US; Biocare Medical, Concord, CA) using Antigen Decloaker solution (CB910M; Biocare Medical) at 95°C. Sections were incubated in Protein Block Serum-Free (Dako, Glostrup, Denmark; X0909) and then in a polyclonal CGRP antibody (BML-CA1134-0100; Enzo Life Science, Farmingdale, NY) diluted to 1:500 in DaVinci Green Universal Diluent (PD900; Biocare Medical). Endogenous peroxidases were blocked with Peroxidazed 1 (PX968M; Biocare Medical), and sections were incubated in an anti-rabbit:HRP polymer (87-9263; Invitrogen, Carlsbad, CA). Betazoid DAB Chromogen (BDB2004MM; Biocare Medical) was then applied to each slide, and sections were incubated with Protein Block Serum-Free. Sections were then incubated in a rabbit polyclonal TH antibody (ab112; Abcam, Cambridge, MA) diluted to 1:1,000 followed by Mach 3 Probe (Biocare Medical) and Mach 3 Rabbit AP-Polymer (Biocare Medical; M3R533L). Sections were then incubated in Warp Red Chromogen (WR806S; Biocare Medical) and counterstained with Mayer's hematoxylin (26043-06; Electron Microscopy Sciences, Hatfield, PA). Each slide was dipped in xylene, and a coverslip was applied with Surgipath Micromount permanent mounting media (3801731; Leica, Wetzlar, Germany).
To quantify the labeling of CGRP and TH, IHC slides were digitally photographed, and the images were analyzed using the Positive Pixel Count v9 algorithm in ImageScope software v11 (Aperio Technologies, Vista, CA). Because CGRP labeling was confined to the renal pelvis, CGRP content was quantified as the area of CGRP+ labeling (μm2) per length of pelvic wall analyzed (in mm). Because TH labeling was most robust in the large nerve bundles found in the hilum of the kidney, TH was quantified as the percent area of a major nerve bundle that was labeled positive for TH. Quantification was done by individuals blinded to the experimental group.
Validation of Renal-CAP by ELISA and HPLC
We also used an ELISA for CGRP and HPLC for NE to quantify the extent of selective afferent renal nerve ablation and the time course of reinnervation. Male Sprague-Dawley rats (59–74 days old, 230–375 g) were subjected to unilateral renal-CAP of the left kidney, with the right kidney serving as a sham-operated control. Rats were euthanized 10 days, 4 wk, or 7 wk after intervention, and kidneys were collected and assayed for content of CGRP and NE.
Kidneys were removed immediately after death; the renal artery, renal vein, ureter, and capsule were removed; and the kidneys were placed in a cold normal saline bath for further dissection. Renal parenchymal samples were taken from the poles and lateral portion of the kidney and flash frozen in liquid nitrogen. The renal pelvis was then carefully dissected from the remaining portion of kidney and flash frozen in liquid nitrogen. All frozen samples were stored at −80°C until being assayed.
The parenchymal samples were assayed for NE using HPLC, as previously described (27). The isolated renal pelvic samples were assayed for CGRP content using a commercially available ELISA kit (Cayman Chemicals, Ann Arbor, MI; item number 589001). Tissues were homogenized in 1 M acetic acid, and CGRP was extracted using C18 Sep-columns (Peninsula Laboratories; San Carlos, CA; item number Y-1000). To eliminate any interassay variance, all pelvic samples were run on single 96-well ELISA plates.
Functional Validation of Renal-CAP
A test of afferent renal nerve functionality was conducted in male Sprague-Dawley rats (275–300 g) 7–10 days after being subjected to either sham or bilateral renal-CAP (n = 6 per group). On the day of the acute experiment, rats were instrumented as described above. Rats were then placed in a Plexiglas holder, and an intravenous infusion of sterile isotonic saline (20 μl/min) was maintained for a 2-h surgical recovery period prior to experimentation to enable the animal to regain full consciousness and cardiovascular function to stabilize.
MAP and HR were continuously recorded via the surgically implanted femoral artery cannula using computer-driven BIOPAC data acquisition software (MP150 and AcqKnowledge 3.8.2, CA) connected to an external pressure transducer (P23XL; Viggo Spectramed, Oxnard, CA). MAP and HR were measured during a 20-min control period. Bradykinin was then infused, in a random order, at concentrations of 5, 10, 20, and 40 μg·kg−1·min−1 either intravenously or intrarenally for a period of 5 min per dose per administration route. MAP and HR were recorded as the average value of the last 2 min of each infusion period. To ensure standard recovery between bradykinin infusions, there was a 10-min recovery period between each infusion of bradykinin during which isotonic saline was infused via the venous line. This recovery period resulted in full recovery from the effects of bradykinin prior to the next infusion (i.e., cardiovascular parameters returned to baseline values).
Effect of Renal-CAP on Cardiovascular and Fluid Balance Responses to Increased Salt Intake
To determine whether renal-CAP adversely affects cardiovascular and fluid homeostasis under conditions of chronic sodium loading, rats were subjected to the following protocol. Male Sprague-Dawley rats (71–72 days old, 330–390 g) underwent bilateral renal-CAP (n = 8) or sham surgery (n = 8) and were implanted with radiotelemeters (model TA11PA-C40; Data Sciences, St. Paul, MN) to measure MAP and HR, as previously described (46). The rats were fed a 0.1% NaCl diet (Research Diets, New Brunswick, NJ) and were allowed to recover for 10 days. After a 4-day baseline period, the diet was increased to 4% NaCl (Research Diets) for 3 wk and then 8% NaCl (Research Diets) for 2 wk. The animals were housed in metabolic cages (Techniplast 3701M001, Buguggiate, Italy) for daily measurement of sodium intake, sodium excretion, water intake, and urine output. At the end of the protocol, rats were euthanized, and kidneys were collected for immunohistochemical analysis of TH and CGRP.
The radiotelemeter signal was monitored by a receiver (model RPC-1, Data Sciences International, St. Paul, MN) mounted on the side of the metabolic cage and connected to a Data Exchange Matrix (Data Sciences International). The arterial pressure signal was sampled at 500 samples/s for 10 s every 4 min using Dataquest A.R.T. Platinum Acquisition software (version 4.30, Data Sciences International). HR was determined from the arterial pressure profile using the same software. Twenty-four-hour averages of MAP and HR were determined and plotted for each day of the study.
Twenty-four-hour food intake, water intake, and urine output were measured gravimetrically. Twenty-four-hour sodium intake was calculated by multiplying food intake (grams) and sodium content of the diet (0.1% NaCl = 0.01711 mmol Na+/g food; 4.0% NaCl = 0.6844 mmol Na+/g food; 8.0% NaCl = 1.3688 mmol Na+/g food). Twenty-four-hour sodium excretion was calculated by multiplying 24-h urine output (ml) and urinary sodium concentration (mmol Na+/ml), which was measured using an ion-specific electrode (NOVA-5+ electrolyte analyzer; Nova Biomedical, Waltham, MA). Twenty-four-hour sodium and water balances were calculated as 24-h intake minus 24-h excretion.
Effect of Renal-CAP on the Development of DOCA-Salt Hypertension
We have previously reported that RDNX attenuates the development of hypertension in the DOCA-salt model by 50% and hypothesized that this was due to ablation of renal afferent nerves (19). Therefore, to test whether the antihypertensive effect of RDNX in the DOCA-salt model is due to ablation of afferent renal nerves, rats underwent either SHAM, RDNX, or renal-CAP and were subjected to DOCA-salt hypertension, as previously described (19). Briefly, male Sprague-Dawley rats (285–400 g) underwent left unilateral nephrectomy and were allowed 2 wk for compensatory renal hypertrophy. Rats were then subjected to sham surgery, RDNX, or renal-CAP and were implanted with a radiotelemeter (Data Sciences International) for measurement of MAP and HR. After 10 days of recovery and 3 days of baseline, silicone pellets containing a total of 100 mg of DOCA were implanted subcutaneously between the scapulas. Three weeks after the DOCA implant, rats were euthanized, and kidneys were harvested for tissue assays of CGRP and NE content, as described earlier.
DOCA implants were made by mixing 100 mg DOCA into 2 ml of silicone (Sylgard 184 silicone elastomer base; Dow Corning, Midland, MI). Once the DOCA was homogenously mixed into the silicone, the silicone elastomer curing agent (0.2 ml) was added. The DOCA implants were allowed to cure at room temperature for 24 h and were then stored at 4°C until implantation. Each DOCA implant was cut into 2- to 3-mm cubes that were then implanted subcutaneously between the scapulas.
Statistical Analysis
The magnitude of the changes in MAP and HR following intrarenal or intravenous infusion of bradykinin, as well as differences in daily measurements generated from the salt-sensitivity and DOCA-salt protocols, was analyzed by two-way ANOVA for repeated measures followed by the Bonferroni method for post hoc comparisons (SigmaPlot version 12.3; Systat Software, San Jose, CA). All body weight, IHC, ELISA, HPLC, as well as baseline MAP and HR, data were analyzed by one-way ANOVA followed by the Bonferroni method for post hoc comparisons (SigmaPlot version 12.3). For the time course of reinnervation, a linear regression (SigmaPlot version 12.3) was performed. For all statistical analyses, a P value less than 0.05 was considered to be statistically significant.
RESULTS
Validation of Renal-CAP by Immunohistochemistry
Figure 1 shows representative immunohistochemistry images of a sham kidney (top) and a renal-CAP kidney (bottom) 10 days postintervention. Robust labeling of TH is apparent in both kidneys, while the significant amount of CGRP labeling seen in the sham-operated kidney is absent in the renal-CAP kidney. Because careful examination of all sections revealed that CGRP labeling was observed in the renal pelvis exclusively, CGRP was quantified as area of CGRP+ labeling per length of pelvic wall. TH labeling was found throughout the kidney but was most robust in the large nerve bundles near the hilum of the kidney, so TH was quantified as percent area of a nerve bundle with TH+ labeling. Labeling of CGRP and TH was quantified in sham-operated and renal-CAP kidneys 10 days, 4 wk, and 7 wk postintervention (Fig. 2, A and B). TH labeling was not different between sham-operated and renal-CAP kidneys at any time point. In contrast, CGRP labeling was nearly abolished 10 days and 4 wk post renal-CAP. By 7 wk postintervention, CGRP labeling returned to ∼50% of that observed in sham-operated kidneys.
Fig. 1.
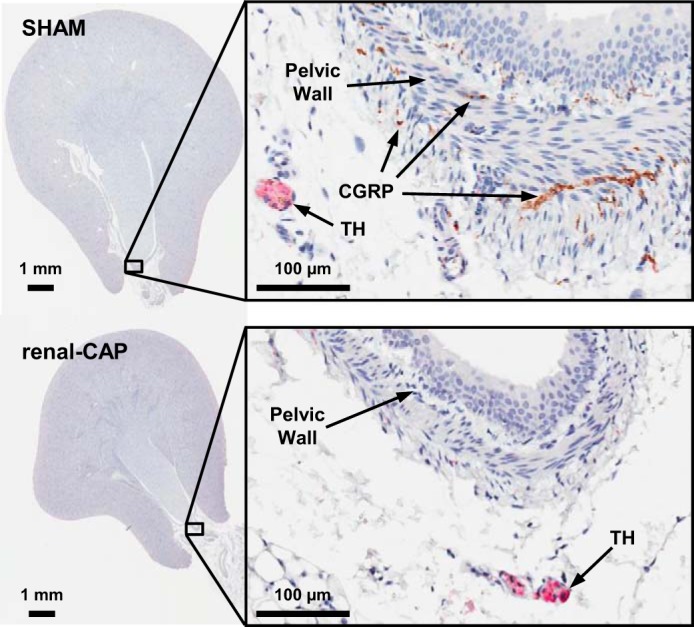
Representative immunohistochemistry (IHC) images stained for CGRP and tyrosine hydroxylase (TH) from a sham-operated kidney and renal-CAP kidney 10 days after intervention. The image on left shows the entire slice of the kidney. The boxed in portion of the left image is magnified in the image on the right. CGRP is shown in brown, while TH is shown in pink.
Fig. 2.
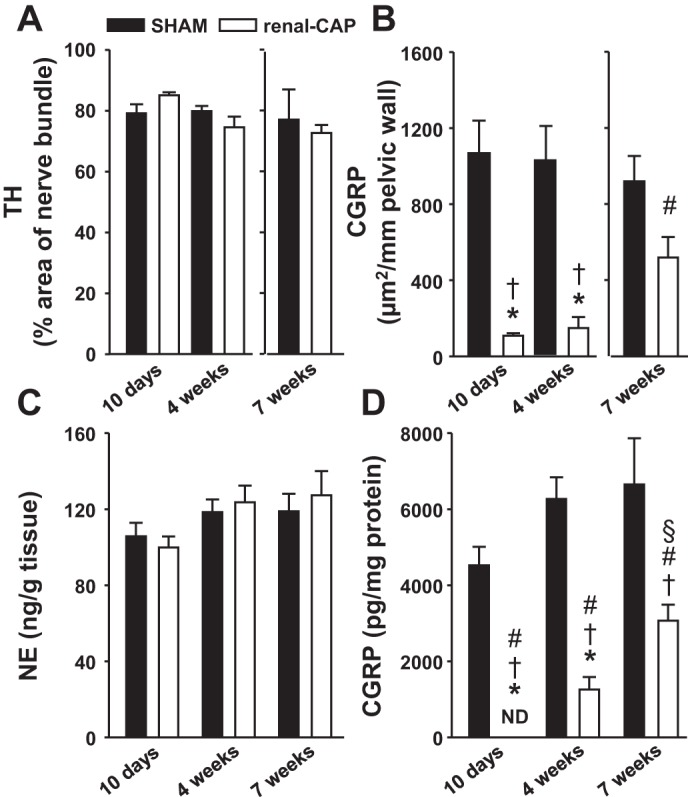
Tissue content of neurochemical markers 10 days, 4 wk, and 7 wk following sham operation or renal-CAP. A: IHC labeling of TH. B: IHC labeling of CGRP. C: NE content of the renal parenchyma by HPLC. D: CGRP content of the pelvic wall by ELISA. ND, not detectable. *P < 0.05 compared with the 10-day Sham. †P < 0.05 compared with the 4-wk Sham. #P < 0.05 compared with the 7-wk Sham group. §P < 0.05 compared with the 10-day renal-CAP.
Validation of Renal-CAP by ELISA and HPLC
While IHC is useful for visualizing neurons of interest, more accurate quantification of neuromarkers can be obtained by assaying tissues for content of neurotransmitters. Therefore, we measured pelvic CGRP content by ELISA and renal NE content by HPLC at 10 days, 4 wk, and 7 wk after unilateral renal-CAP, using the nontreated kidney as a sham control. As shown in Fig. 2C, renal NE content was not different between sham-operated and renal-CAP kidneys at any time point. Conversely, renal pelvic CGRP was undetectable 10 days after renal-CAP. Pelvic CGRP began to return to detectable levels at 4 wk, and by 7 wk, it had returned to roughly 50% of control levels (Fig. 2D). Fig. 3 shows CGRP content over time in renal-CAP kidneys plotted as a percentage of control (the ratio of CGRP content of the left renal pelvis to right renal pelvis × 100) and suggests that the time course of afferent reinnervation is roughly linear, i.e., once initiated, afferent reinnervation occurs at a fairly constant rate.
Fig. 3.
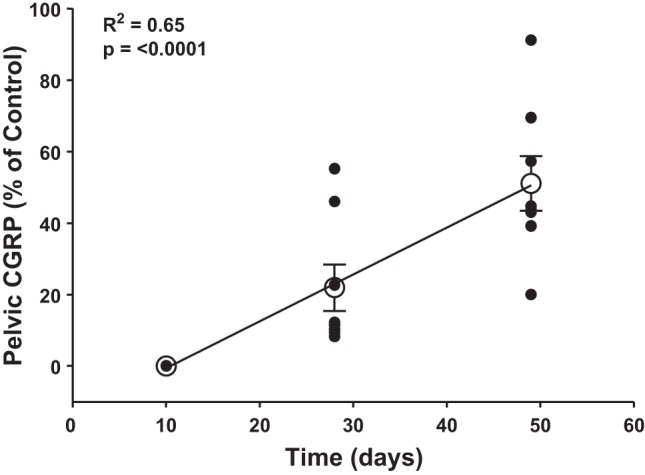
Time course of afferent reinnervation following renal-CAP. Renal pelvic CGRP content of renal-CAP (left) kidneys is expressed as % of within-animal control (right) kidneys. A linear regression was performed (R2 = 0.65, P < 0.0001) and group averages ± SE are overlaid.
Effect of Renal-CAP on Cardiovascular Responses to Activation of Afferent Renal Nerves in Conscious Rats
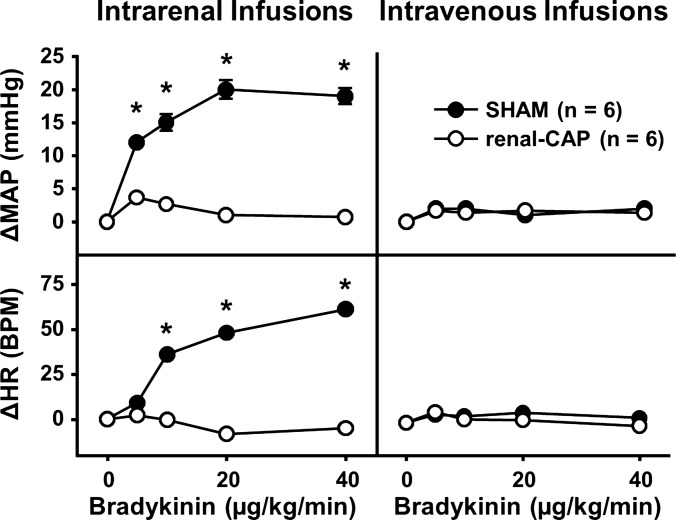
Infusion of bradykinin into the renal artery has been shown to activate afferent renal nerves (1, 52) and increase MAP and HR (40). Therefore, to determine whether renal-CAP causes functional ablation of afferent renal nerves, experiments were conducted in conscious rats in which the physiological responses to direct pharmacological activation of afferent renal nerves by bradykinin were assessed. There were no significant differences between groups in baseline MAP (sham = 124 ± 3 mmHg, renal-CAP = 121 ± 2 mmHg) or HR (sham = 414 ± 18 BPM, renal-CAP = 427 ± 16 beats per minute). More importantly, as shown in Fig. 4, bradykinin infused into the renal artery evoked a dose-dependent increase in MAP and HR in Sham, but not renal-CAP rats. The same doses of bradykinin administered intravenously had no effect on MAP or HR in either sham-operated or renal-CAP rats.
Fig. 4.
Physiological responses to pharmacological stimulation of afferent renal nerves in Sham and renal-CAP rats. Changes in MAP (ΔMAP; top) and HR (ΔHR; bottom) following intrarenal (left) and intravenous (right) infusions of bradykinin. *P < 0.05 for Sham vs. renal-CAP. Error bars not shown for SE <1.1 mmHg for ΔMAP and <3.6 beats per minute for ΔHR.
Effect of Renal-CAP on Cardiovascular and Fluid Balance Regulation During Chronic Salt Loading
An additional experiment was conducted to determine whether renal-CAP disrupts cardiovascular and fluid balance regulation during chronic increases in dietary salt intake. Such dysregulation could occur as a result of nonspecific effects of renal-CAP (i.e., ablation of efferent renal nerves) or secondary to disruption of normal dietary sodium-induced renal afferent signaling. Figure 5 shows MAP and HR in sham-operated and renal-CAP rats fed increasing levels of dietary salt. Renal-CAP had no effect on the basal level of MAP when rats were being fed a low-salt (0.1%) diet. Similarly, the response of MAP to a 40-fold (4.0% NaCl) and 80-fold (8.0% NaCl) increase in salt intake was not different between renal-CAP and sham-operated rats. Conversely, the progressive bradycardia observed in sham-operated rats in response to increasing dietary sodium was significantly attenuated in renal-CAP rats.
Fig. 5.
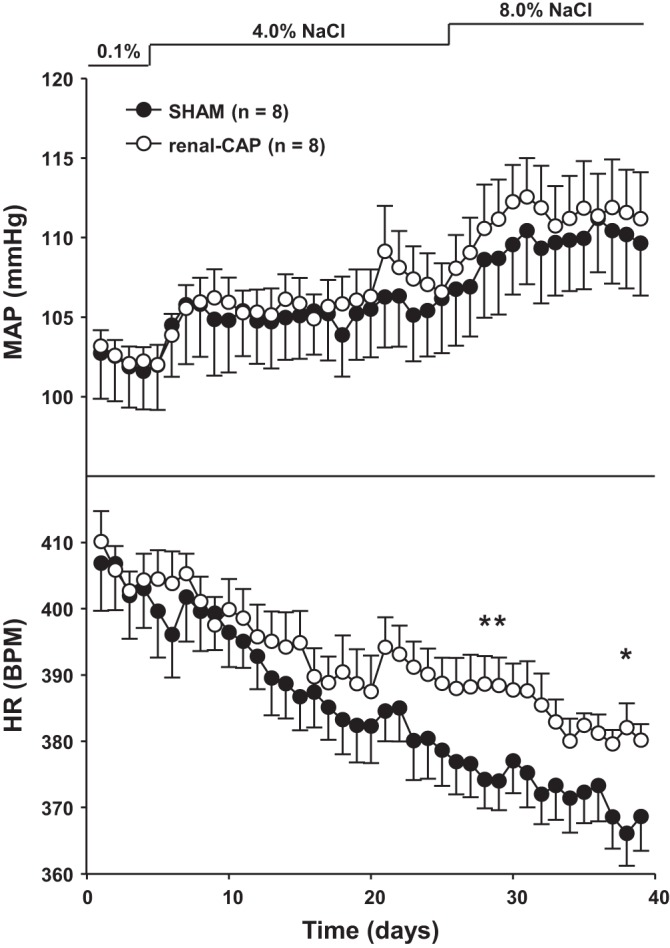
Mean arterial pressure (MAP) and heart rate (HR) in sham and renal-CAP rats subjected to the salt-sensitivity protocol. *P < 0.05 for Sham vs. renal-CAP.
Figure 6 shows sodium intake, excretion, and balance throughout the protocol. There were no differences in sodium intake between sham-operated and renal-CAP rats at any level of dietary salt; 0.1%, (∼0.4 mmol/day), 4.0% (∼17 mmol/day), and 8.0% (∼31 mmol/day). Furthermore, the renal sodium excretory responses to these large increases in salt intake were not different between renal-CAP and sham-operated rats throughout the protocol. As a result, there were no differences in sodium balance between groups over the 40-day protocol. Likewise, water intake and urine output were largely similar between groups throughout the protocol with the exception that renal-CAP rats tended to drink more water and excrete more urine than sham-operated rats initially when diets were changed from 0.1% to 4.0% and from 4.0% to 8.0% (Fig. 7). Water balance was similar between groups on all days of the protocol.
Fig. 6.
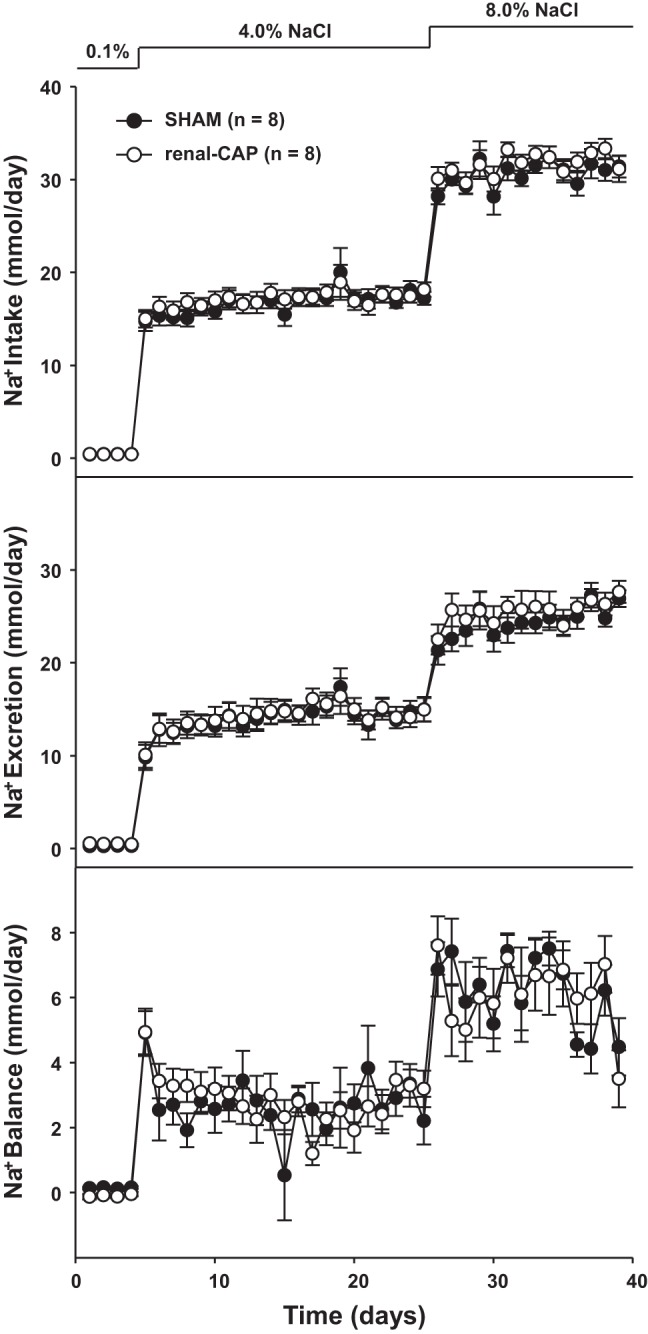
Sodium intake, excretion, and balance in sham and renal-CAP rats subjected to the salt sensitivity protocol. There were no significant differences between groups.
Fig. 7.
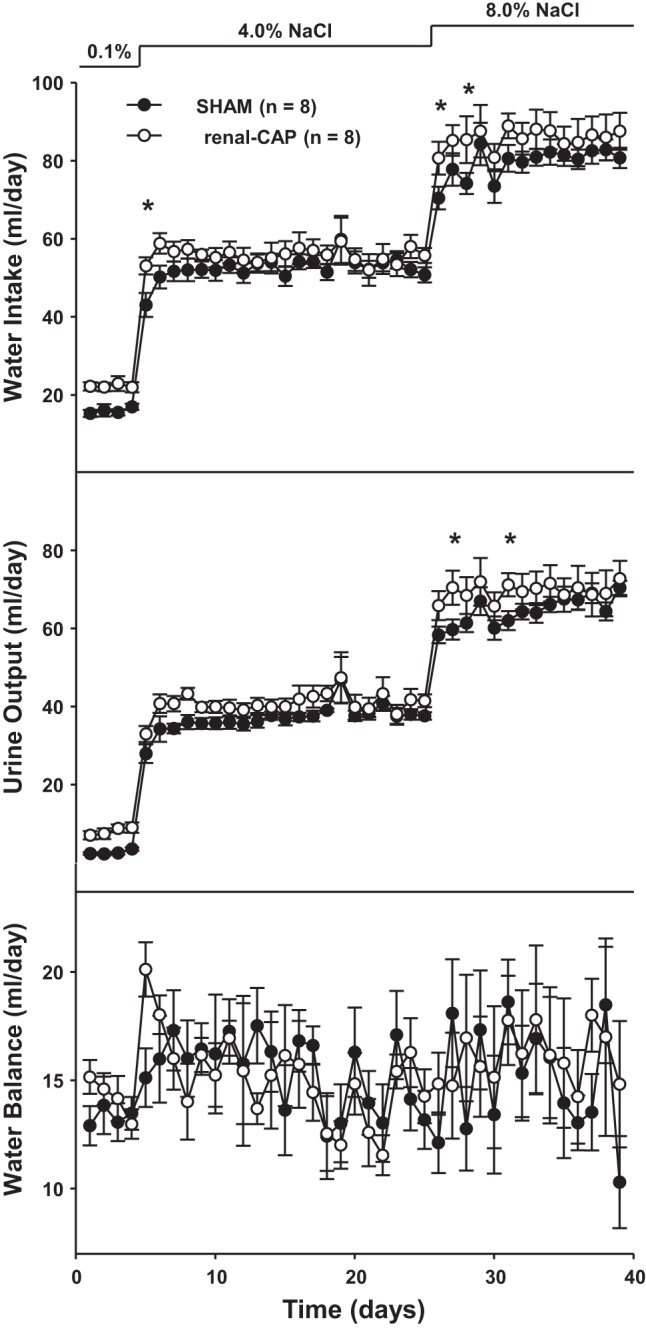
Water intake, urine output, and water balance in Sham and renal-CAP rats subjected to the salt-sensitivity protocol. *P < 0.05 for renal-CAP vs. Sham.
Table 1 shows body weight at the time of intervention (bilateral renal-CAP or Sham) and at death 10 days, 4 wk, or 7 wk postintervention, as well as the total weight gain from time of intervention to the time of death. Consistent with our findings that renal-CAP does not adversely affect food intake or maintenance of body fluid balance during chronic dietary salt loading, these data demonstrate that renal-CAP does not affect the rate of body weight gain over the same period of time.
Table 1.
Body weight at the time of intervention, body weight at euthanasia, and weight gain from intervention to euthanasia in Sham and renal-CAP rats that underwent a 10-day, 4-wk, or 7-wk protocol
| 10 day |
4 wk |
7 wk |
||||
|---|---|---|---|---|---|---|
| Sham | renal-CAP | Sham | renal-CAP | Sham | renal-CAP | |
| BW at Tx, g | 242.0 ± 4.2 | 250.2 ± 4.3 | 307.7 ± 5.9 | 309.2 ± 5.2 | 357.5 ± 7.5 | 355.7 ± 4.1 |
| Final BW, g | 294.0 ± 3.4 | 280.8 ± 5.5 | 462.8 ± 14.5 | 451.3 ± 16.0 | 512.9 ± 25.8 | 513.2 ± 17.5 |
| ΔBW, g | 52.0 ± 6.7 | 30.6 ± 8.8 | 155.1 ± 10.6 | 142.0 ± 13.0 | 155.5 ± 21.7 | 157.5 ± 16.3 |
Values are expressed as means ± SE. BW at Tx, body weight at time of intervention; final BW, body weight at time of euthanasia; DBW, weight gain from intervention to euthaniasia. No significant differences were found between Sham and renal CAP rats that underwent the same protocol.
Effect of Renal-CAP on the Development of DOCA-Salt Hypertension
An additional experiment was conducted to test whether renal-CAP attenuates a model of hypertension that is thought to be mediated by afferent renal nerves, the DOCA-salt model. As shown in Fig. 8A, baseline MAP trended lower in RDNX rats compared with sham-operated and renal-CAP rats, and baseline HR was not different between groups. After 3 wk of DOCA implantation, MAP in the Sham group had increased ∼30 mmHg above baseline (Fig. 8B). In confirmation with data we have previously reported (19), this increase was attenuated by ∼50% in RDNX rats. Importantly, renal-CAP had a nearly identical antihypertensive effect as RDNX. Neither RDNX nor renal-CAP had an effect on the change in HR observed with DOCA implantation (Fig. 8B). As shown in Fig. 9, both RDNX and renal-CAP caused significant reductions in the renal pelvic content of the afferent nerve marker, CGRP, whereas RDNX and not renal-CAP drastically reduced renal content of the efferent nerve marker, NE in DOCA-salt rats.
Fig. 8.
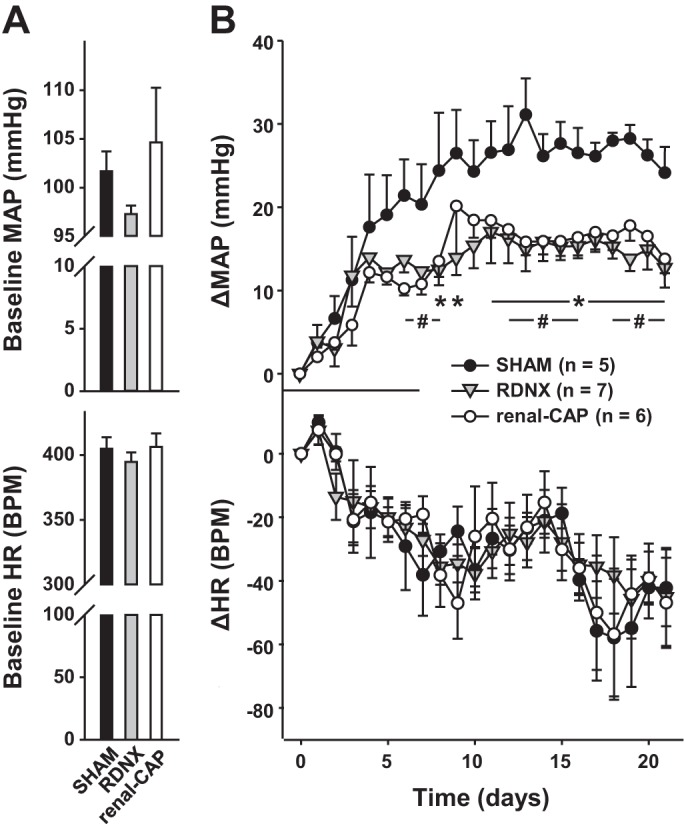
MAP and HR responses in sham, RDNX, and renal-CAP rats that underwent the DOCA-salt protocol. A: baseline MAP and HR. B: change in MAP from baseline (ΔMAP) and change in HR from baseline (ΔHR) upon DOCA implantation. *P < 0.05 for RDNX vs. Sham. #P = 0.05 for renal-CAP vs. Sham.
Fig. 9.
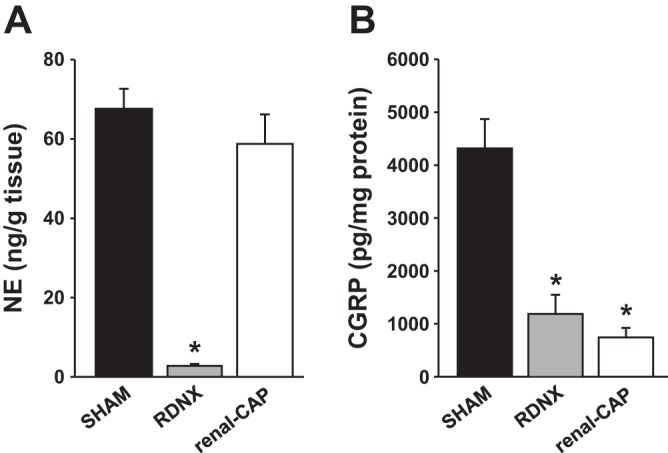
Tissue content of neurochemical markers in the kidneys of rats that underwent the DOCA-salt protocol A: NE content in the renal parenchyma. B: CGRP content in the renal pelvic wall. *P < 0.05 compared with Sham.
DISCUSSION
The role of renal nerves in arterial pressure regulation and hypertension has been investigated in various animal models for years (9, 12, 19). Furthermore, recent technological advances have raised interest in targeting the renal nerves for the treatment of human hypertension (10, 26, 30) and possibly other diseases, such as diabetes (29) and sleep apnea (51). However, the clinical trials using RDNX have sparked many questions that need to be answered to optimize the effectiveness of renal nerve ablation. One commonly raised question is whether the antihypertensive effect of RDNX is due to ablation of afferent or efferent renal nerves. Clarification of this point is important to our understanding of the pathophysiological mechanisms involved and to the development of more targeted nerve ablation approaches.
In the present study, we investigated the ability of periaxonal application of the TRPV1 receptor agonist capsaicin on renal nerves to selectively ablate afferent renal nerves, while leaving efferent nerves intact. While capsaicin is known to activate sensory neurons acutely, it is also neurotoxic following prolonged exposure (15, 41). Systemic administration of capsaicin to neonatal rat pups results in whole body sensory nerve denervation (21), and local periaxonal application of capsaicin has been shown to selectively ablate sensory fibers of specific organs, such as the adrenal gland (43–45). Moreover, since the TRPV1 receptor is expressed along the axons of sensory fibers but is not expressed in sympathetic efferent neurons (41), we hypothesized that periaxonal application of capsaicin could be used to selectively ablate sensory fibers in the kidney, while leaving efferent fibers intact.
Renal-CAP Causes Temporary Selective Loss of Afferent Nerve Markers from the Kidney as Assessed by IHC and Tissue Assays
Confirmation of sympathetic denervations has typically been achieved by assessing the content of NE in the denervated tissue, and measurement of renal NE content by HPLC is the most commonly used and widely accepted method to confirm RDNX as successful in animals. We have performed numerous studies investigating the effect of RDNX on the pathogenesis of experimental hypertension and, using HPLC, typically report renal NE content to be reduced by 90–95% in renal denervated rats compared with control animals (12, 18–20, 22). While useful, it is important to note that this method does not assess ablation of afferent renal nerves, which is typically done by measurement of the neurotransmitters CGRP and substance P (SP).
In the present study, we quantified the content of neurochemical markers of efferent (TH and NE) and afferent (CGRP) nerves in SHAM or renal-CAP kidneys. We found that renal-CAP virtually eliminated renal pelvic CGRP content without affecting renal NE or TH content. Pelvic CGRP was undetectable 10 days postintervention by ELISA but did recover over time. This recovery occurred linearly over time with levels ∼20% and ∼50% of control at 4 and 7 wk postintervention, respectively.
The time course of afferent reinnervation following renal-CAP observed in the present study is strikingly similar to a recent investigation of the reinnervation pattern following complete surgical RDNX in rats (34). In this study, Mulder et al. (34) used IHC to label markers of efferent nerves [TH and neuropeptide Y (NPY)] and afferent nerves (CGRP and SP) in renal denervated and sham-operated kidneys at various time points after intervention. Labeling for the efferent makers TH and NPY in the renal cortex was nearly eliminated 4–5 days after RDNX, but returned to ∼50% by 4 wk. A very similar pattern was observed for the afferent markers CGRP and SP in the pelvic wall. In the present study, IHC revealed that renal-CAP reduced pelvic CGRP labeling to ∼10 and 20% of control at 10 days and 4 wk postintervention, respectively. In addition, using an ELISA for CGRP, we found that renal-CAP reduced renal pelvic content to undetectable levels at 10 days postintervention and 20% of control at 4 wk. In our study, both methods (IHC and ELISA) demonstrated that pelvic CGRP returns to ∼50% of control levels by 7 wk postintervention. Although this time point was not measured in the study by Mulder et al. (34), they did report that NPY, TH, CGRP, and SP returned to 100% of control by 12 wk postintervention. In the present study, linear regression analysis of pelvic CGRP vs. time postintervention suggests that pelvic GGRP would return to 100% of control 14 wk postintervention. On the basis of the results of two independent measurements of the presence of afferent renal nerves, IHC for CGRP, and ELISA for CGRP content, we conclude that renal-CAP causes ablation of afferent renal nerves as effectively as, if not more effectively, than surgical RDNX. Moreover, in contrast to surgical RDNX, this technique does not cause ablation of efferent renal nerves, as assessed by renal NE and TH content.
Renal-CAP Causes Functional Ablation of Afferent Renal Nerves
Because depletion of neural markers does not necessarily correspond with a loss of neuronal function, we performed experiments in conscious rats to determine whether renal-CAP causes functional ablation of afferent renal nerves. In these experiments, we measured the cardiovascular responses to intrarenal infusions of bradykinin, which has been shown to stimulate afferent renal nerves (1, 52) and increase MAP and HR (14, 40). To control for nonrenal effects of bradykinin, intravenous infusions were given at the same doses.
Bradykinin infused into the renal artery of sham-operated rats caused a dose-dependent increase in MAP and HR, and these responses were absent in renal-CAP rats. Since intravenous infusions at the same doses caused no changes in MAP or HR, we conclude that the responses to intrarenal infusion were, indeed, due to intrarenal actions of bradykinin. More importantly, abolition of these responses by renal-CAP confirms previous reports that these responses are mediated by activation of afferent renal nerves and that renal-CAP results in complete functional ablation of afferent renal nerves. These results suggest that, in addition to causing loss of the neurotransmitter CGRP, renal-CAP results in a complete loss of afferent neuronal function.
Renal-CAP Does not Affect the Regulation of Arterial Pressure, Fluid Balance, or Weight Gain During Chronic Dietary Salt Loading
In addition to examining the effectiveness of renal-CAP in selectively ablating afferent renal nerves, we sought to determine whether renal-CAP causes a disruption in the regulation of fluid balance and arterial pressure during chronic increases in dietary salt intake. This could potentially occur as a result of nonspecific effects of renal-CAP (i.e., ablation of renal efferent nerves) or secondary to disruption of normal renal afferent signaling, as suggested by earlier studies using nonselective methods of afferent renal denervation, such as dorsal rhizotomy (24) and systemic administration of capsaicin (47, 48, 50). In the present study, renal-CAP did not affect the regulation of arterial pressure or sodium and water balance in response to increasing dietary salt intake 40- and 80-fold from baseline over a period of 5 wk. Additionally, renal-CAP rats gained weight to a similar extent as rats in the Sham group. We conclude that, while renal-CAP is an effective method for targeted ablation of CCRP+ afferent renal nerves, this intervention does not interfere with regulation of arterial pressure, fluid balance, or body weight gain in normotensive Sprague-Dawley rats subjected to dietary sodium loading.
Previous studies have shown that nonselective renal “deafferentation” causes salt-sensitive hypertension (24, 47, 48, 50). This was not observed in the present study using a more selective method for ablation of afferent renal nerves. In addition, we have previously reported that complete surgical RDNX (ablation of both afferents and efferents) also has no effect on the salt sensitivity of arterial pressure or regulation of sodium and water balance in normotensive Sprague-Dawley rats (18, 20). There are at least two explanations for the discrepancy between the results of the present study and those of previous studies. The first is that the nonspecific methods used previously also target nonrenal visceral afferent pathways that are salt-sensitive, such as hepatoportal osmoreceptors (32, 33). These sodium sensors have been shown to be important in the inhibition of renal sympathetic nerve activity following hypertonic saline infusion into the portal vein and cause suppression of efferent renal nerve activity following ingestion of meals high in sodium content (17, 31, 35). Therefore, it is possible that any method that disrupts afferent signaling from these receptors may result in sodium retention and hypertension. The second possible explanation for the differences between studies is the method of arterial pressure measurement. In the current study, arterial pressure was measured continuously by radio telemetry in unrestrained rats. In contrast, previous studies employed methods that are known to induce acute stress responses, such as indirect tail cuff measurements in restrained rats (47, 48, 50), acute direct recordings in anesthetized rats (47, 48), or acute direct recordings in conscious rats days after catheter implantation (24). Thus, it is possible that in the earlier studies, afferent renal nerve ablation led to exaggerated arterial pressure responses to acute stress in rats consuming a high-salt diet. Both of these possibilities remain to be investigated. It is important to note that, whereas renal-CAP had no effect on the basal level of arterial pressure in rats consuming a normal-salt diet, we have consistently found that complete surgical RDNX decreases arterial pressure ∼10 mmHg (18, 20). This suggests that the chronic hypotensive response to complete RDNX in nonhypertensive Sprague-Dawley rats is due to loss of efferent renal nerves exclusively.
Renal-CAP Amplifies the Dipsogenic Response and Attenuates the Bradycardic Response to Increased Dietary Sodium Intake
Another interesting finding in this study was that renal-CAP rats drank more water than control rats during the initial increases in salt intake. This occurred when dietary salt was increased from 0.1% to 4.0% NaCl and from 4.0% to 8.0% NaCl. Although we did not measure plasma vasopressin (AVP) in this study, it has been suggested that afferent renal nerves influence AVP release (5–7, 39). One explanation for our findings is that renal-CAP rats had an impaired AVP response to acute increases in plasma osmolality and compensated for this by increasing water intake. Additionally, it is possible that afferent renal nerves play a direct regulatory role in the dipsogenic response to increases in dietary sodium load. While this is an interesting finding, the mechanism underlying this observation remains to be investigated.
Finally, while we saw no differences in MAP between rats in the Sham and renal-CAP groups, we did observe an effect of renal-CAP on chronic regulation of HR during increased dietary salt intake. Specifically, the bradycardic response to dietary sodium loading that was observed in Sham rats was significantly attenuated by renal-CAP. This suggests a role of afferent renal nerve signaling in the regulation of HR under conditions of high-salt intake. This hypothesis remains to be tested by further studies.
Afferent Renal Nerves Mediate the Development of DOCA-Salt Hypertension
Having shown that afferent renal nerves play a minimal role in the regulation of arterial pressure in normotensive rats, we performed one additional experiment to explore the role of these nerves in hypertension. We have previously reported that RDNX attenuates the development of DOCA-salt hypertension by 50% and hypothesized that this was mediated by, at least in part, ablation of afferent renal nerves (19). This hypothesis was based on the difference in magnitudes of the bradycardic response to DOCA-salt treatment in sham-operated and RDNX rats. Although HR decreased in both groups, it was significantly higher in sham-operated rats despite the fact that arterial pressure was also higher in these rats. We hypothesized that afferent renal nerves modulate baroreflex control of HR in DOCA-salt rats and that RDNX blocks this modulation secondary to ablation of renal afferent nerves (19). Moreover, we hypothesized that the antihypertensive effect of RDNX was due to ablation of afferent renal nerves.
To test this hypothesis, we subjected DOCA-salt rats to sham surgery, RDNX, or renal-CAP. The results of the present experiment show clearly that selective ablation of afferent renal nerves by renal-CAP attenuates the development of DOCA-salt hypertension over the same time course and by the same magnitude as RDNX, suggesting that the antihypertensive effect of RDNX in the DOCA-salt model is due to ablation of afferent rather than efferent renal nerves. Further studies will be needed to elucidate the mechanisms underlying this antihypertensive effect.
Neither renal-CAP nor RDNX had a significant effect on the HR response to DOCA implantation over the 21-day protocol, which is consistent with our previous report in which we observed no significant difference in HR between sham-operated and RDNX rats until after 4 wk of DOCA (19). This observation is also consistent with the salt sensitivity study, in which renal-CAP had no effect on HR compared with the Sham group until after 3 wk of high salt administration. Although the results of our previous DOCA-salt study and the current salt-sensitivity study suggest a role of afferent renal nerves in HR regulation, this role seems to be minor and only apparent during long-term protocols. Further studies will be needed to fully elucidate the role of afferent renal nerves in HR regulation.
Limitations
The primary limitation to the present study is that renal-CAP is effective in temporarily ablating CGRP containing afferent renal neurons, it is not clear to what extent this procedure is effective in total “deafferentation” of the kidney, as it does not target sensory neurons in the kidney that lack the TRPV1 receptor. Although the conventional view is that the vast majority of renal afferents are indeed TRPV1+, this is open to further investigation.
Additionally, we cannot entirely exclude the possibility that the antihypertensive effect of renal-CAP in the DOCA-salt model was due, in part, to nonspecific actions of capsaicin. Although application of the capsaicin solution is well contained to the treatment area, it is theoretically possible that a small amount came in contact with nearby structures, such as the adrenal gland or the kidney itself. Therefore, the antihypertensive effect of renal-CAP may be due to an alteration in adrenal function or an afferent renal nerve-independent alteration in renal excretory function. We feel this is highly unlikely since the capsaicin solution was well contained to the treatment area and renal-CAP had no effect on sodium or water balance in the salt-sensitivity study.
Perspectives and Significance
While RDNX has been shown to lower arterial pressure in some humans with resistant hypertension, it is not known whether the antihypertensive effect is due to ablation of afferent or efferent renal nerves. To this point, it is possible that there are some patients for whom RDNX will work by disrupting renal efferent signaling and some for whom it will work by reducing renal afferent signaling. Therefore, it is crucially important to address 1) whether afferent renal nerves are involved in hypertension, 2) if they are involved, whether we can target afferent renal nerves specifically to optimize the efficacy and reduce the possible side effects of RDNX, and 3) whether we can identify patients who would benefit from afferent- or efferent-specific renal nerve ablation. As a first step toward addressing these points, we have developed a method for selective ablation of afferent renal nerves in the rat. This procedure, termed renal-CAP, effectively ablates afferent renal nerves, leaving efferent renal nerves intact. This experimental tool may be valuable in elucidating the role of afferent renal nerves in animal models of hypertension and other cardiovascular diseases and may establish a rationale for developing a means for permanent ablation of afferent renal nerves in humans. Moreover, the results of our DOCA-salt experiment suggest that afferent renal nerves may be involved in some forms of hypertension, making afferent renal nerves both an important subject for future basic science research, as well as a possible target for human hypertension. Further studies will be needed to determine the mechanisms underlying this antihypertensive effect, but some possibilities include decreased sympathetic outflow to renal and/or nonrenal targets or decreased plasma AVP levels. Understanding the mechanisms underlying this antihypertensive effect may be critical to our understanding of the therapeutic effects of RDNX and to the development of more refined treatments.
GRANTS
This research was supported by National Institutes of Health Grants R01HL076312 (JWO), RO1HL107330, and K02HL112718 (RDW) and by National Science Foundation Grant IOS1025119 (to W. C. Engeland).
DISCLOSURES
J. W. Osborn is a paid consultant of Medtronic CardioVascular, Inc., Santa Rosa, CA.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.F., W.C.E., and J.W.O. conception and design of research; J.D.F. and R.D.W. performed experiments; J.D.F., R.D.W., W.C.E., and J.W.O. analyzed data; J.D.F., R.D.W., W.C.E., G.D.F., and J.W.O. interpreted results of experiments; J.D.F. and R.D.W. prepared figures; J.D.F. drafted manuscript; J.D.F., R.D.W., W.C.E., G.D.F., and J.W.O. edited and revised manuscript; J.D.F., R.D.W., W.C.E., G.D.F., and J.W.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Stefan Tunev and Lori Garcia of Medtronic CardioVascular, for their work in performing immunohistochemistry and their help in analyzing the resulting images. We thank Ruijun Han and Xinying Wang for performing the ELISA analysis of pelvic CGRP, as well as Robert Burnett for performing the tissue NE measurements. We also thank Dusty Van Helden, Jeff Larson, and Marcos Kuroki for their help with the collection of metabolic data.
REFERENCES
- 1.Ammons WS. Spinoreticular cell responses to intrarenal injections of bradykinin. Am J Physiol Regul Integr Comp Physiol 255: R994–R1001, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370: 1393–1401, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension 60: 1485–1490, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Carlson SH, Osborn JW, Wyss JM. Hepatic denervation chronically elevates arterial pressure in Wistar-Kyoto rats. Hypertension 32: 46–51, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Caverson MM, Ciriello J. Effect of stimulation of afferent renal nerves on plasma levels of vasopressin. Am J Physiol Regul Integr Comp Physiol 252: R801–R807, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol 275: R1745–R1754, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Day TA, Ciriello J. Afferent renal nerve stimulation excites supraoptic vasopressin neurons. Am J Physiol Regul Integr Comp Physiol 249: R368–R371, 1985. [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol 298: R245–R253, 2010. [DOI] [PubMed] [Google Scholar]
- 9.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for intervention of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 126: 2976–2982, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald M. Capsaicin and sensory neurones—a review. Pain 15: 109–130, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61: 806–811, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61: 457–464, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Hoagland KM, Maddox DA, Martin DS. Intrarenal infusion of bradykinin elicits a pressor response in conscious rats via a B2-receptor mechanism. Can J Physiol Pharmacol 77: 563–570, 1999. [PubMed] [Google Scholar]
- 15.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991. [PubMed] [Google Scholar]
- 16.Huang Y, Wang DH. Role of renin-angiotensin-aldosterone system in salt-sensitive hypertension induced by sensory denervation. Am J Physiol Heart Circ Physiol 281: H2143–H2149, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Ishiki K, Morita H, Hosomi H. Reflex control of renal nerve activity originating from the osmoreceptors in the hepato-portal region. J Auton Nerv Syst 36: 139–148, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol 284: H2302–H2310, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jacob F, LaBine BG, Ariza P, Katz SA, Osborn JW. Renal denervation causes chronic hypotension in rats: Role of β1-adrenoceptor activity. Clin Exp Pharmacol Physiol 32: 255–262, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270: 741–743, 1977. [DOI] [PubMed] [Google Scholar]
- 22.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Knuepfer MM, Schramm LP. The conduction velocities and spinal projections of single renal afferent fibers in the rat. Brain Res 435: 167–173, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 42: 968–973, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kopp UC, Jones SY, DiBona GF. Afferent renal denervation impairs baroreflex control of efferent renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 295: R1882–R1890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krum H, Schlaich MP, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with intervention-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383: 622–629, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci 154: 66–73, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Boehm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60: 419–424, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Bohm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 123: 1940–1946, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Bohm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 128: 132–140, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Morita H, Ishiki K, Hosomi H. Effects of hepatic NaCl receptor stimulation on renal nerve activity in conscious rabbits. Neurosci Lett 123: 1–3, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Morita H, Matsuda T, Furuya F, Khanchowdhury MR, Hosomi H. Hepatorenal reflex plays an important role in natriuresis after high-NaCl food intake in conscious dogs. Circ Res 72: 552–559, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Morita H, Yamashita Y, Nishida Y, Tokuda M, Hatase O, Hosomi H. Fos induction in rat brain neurons after stimulation of the hepatoportal Na-sensitive mechanism. Am J Physiol Regul Integr Comp Physiol 272: R913–R923, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Mulder J, Hokfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 304: R675–R682, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida Y, Sugimoto I, Morita H, Murakami H, Hosomi H, Bishop VS. Suppression of renal sympathetic nerve activity during portal vein infusion of hypertonic saline. Am J Physiol Regul Integr Comp Physiol 274: R97–R103, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Patel KP, Knuepfer MM. Effect of afferent renal nerve stimulation on blood pressure, heart rate and noradrenergic activity in conscious rats. J Auton Nerv Syst 17: 121–130, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 60: 1163–1170, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Simon JK, Kasting NW, Ciriello J. Afferent renal nerve effects on plasma vasopressin and oxytocin in conscious rats. Am J Physiol Regul Integr Comp Physiol 256: R1240–R1244, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Smits JF, Brody MJ. Activation of afferent renal nerves by intrarenal bradykinin in conscious rats. Am J Physiol Regul Integr Comp Physiol 247: R1003–R1008, 1984. [DOI] [PubMed] [Google Scholar]
- 41.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999. [PubMed] [Google Scholar]
- 42.Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, Sobotka PA, Gawaz M, Bohm M. Renal sympathetic denervation for intervention of electrical storm: first-in-man experience. Clin Res Cardiol 101: 63–67, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290: R1128–R1135, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Ulrich-Lai YM, Fraticelli AI, Engeland WC. Capsaicin-sensitive nerve fibers: a potential extra-ACTH mechanism participating in adrenal regeneration in rats. Microsc Res Tech 61: 252–258, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich-Lai YM, Marek DJ, Engeland WC. Capsaicin-sensitive adrenal sensory fibers participate in compensatory adrenal growth in rats. Am J Physiol Regul Integr Comp Physiol 283: R877–R884, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Veitenheimer B, Osborn JW. Role of spinal V1a receptors in regulation of arterial pressure during acute and chronic osmotic stress. Am J Physiol Regul Integr Comp Physiol 300: R460–R469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension 32: 649–653, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Wang DH, Wu W, Lookingland KJ. Degeneration of capsaicin-sensitive sensory nerves leads to increased salt sensitivity through enhancement of sympathoexcitatory response. Hypertension 37: 440–443, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Wang DH, Galligan JJ. P2Y2 receptors mediate ATP-induced resensitization of TRPV1 expressed by kidney projecting sensory neurons. Am J Physiol Regul Integr Comp Physiol 298: R1634–R1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Chen AF, Wang DH. ETA receptor blockade prevents renal dysfunction in salt-sensitive hypertension induced by sensory denervation. Am J Physiol Heart Circ Physiol 289: H2005–H2011, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 58: 559–565, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Wu YM, He RR. Biphasic activation of renal afferent by intrarenal artery injection of bradykinin in anesthetized rabbits. Acta Physiol Sinica 51: 651–659, 1999. [PubMed] [Google Scholar]