Abstract
Whether activation of afferent renal nerves contributes to the regulation of arterial pressure and sodium balance has been long overlooked. In normotensive rats, activating renal mechanosensory nerves decrease efferent renal sympathetic nerve activity (ERSNA) and increase urinary sodium excretion, an inhibitory renorenal reflex. There is an interaction between efferent and afferent renal nerves, whereby increases in ERSNA increase afferent renal nerve activity (ARNA), leading to decreases in ERSNA by activation of the renorenal reflexes to maintain low ERSNA to minimize sodium retention. High-sodium diet enhances the responsiveness of the renal sensory nerves, while low dietary sodium reduces the responsiveness of the renal sensory nerves, thus producing physiologically appropriate responses to maintain sodium balance. Increased renal ANG II reduces the responsiveness of the renal sensory nerves in physiological and pathophysiological conditions, including hypertension, congestive heart failure, and ischemia-induced acute renal failure. Impairment of inhibitory renorenal reflexes in these pathological states would contribute to the hypertension and sodium retention. When the inhibitory renorenal reflexes are suppressed, excitatory reflexes may prevail. Renal denervation reduces arterial pressure in experimental hypertension and in treatment-resistant hypertensive patients. The fall in arterial pressure is associated with a fall in muscle sympathetic nerve activity, suggesting that increased ARNA contributes to increased arterial pressure in these patients. Although removal of both renal sympathetic and afferent renal sensory nerves most likely contributes to the arterial pressure reduction initially, additional mechanisms may be involved in long-term arterial pressure reduction since sympathetic and sensory nerves reinnervate renal tissue in a similar time-dependent fashion following renal denervation.
Keywords: kidney, renal denervation, renal mechanosensory nerves, hypertension, angiotensin, substance P, prostaglandin E2
there has been a renewed interest in the neural control of renal function due to several studies on the effects of renal denervation on arterial pressure in patients with treatment-resistant hypertension. Although there are reports that failed to show a depressor effect of renal denervation in these patients, e.g., the Simplicity 3 study (8) and a study by Brinkmann et al. (10), the majority of the studies in treatment-resistant hypertensive patients show that renal denervation results in a long-term reduction in arterial pressure (e.g., 29, 95, 96). The apparent differences in the effects of the renal denervation procedure on arterial pressure in patients are currently the focus of much debate (e.g., 11). In this context, it is important to note the numerous studies in various species that show renal denervation reduces arterial pressure in many different models of experimental hypertension, and this lowering of the arterial pressure was documented to be related to removal of the renal nerves (24, 52).
Although, there is considerable evidence for increased efferent renal sympathetic nerve activity (ERSNA) in various pathological conditions, including hypertension, heart failure, chronic kidney disease, and diabetes (24, 110), it cannot be excluded that some of the reduction in arterial pressure could be related to removal of excitatory reflexes originating in the kidney in hypertensive patients and animals, since the renal denervation procedures involve denervation not only of the renal sympathetic nerves, but also of the afferent renal sensory nerves. A recent study in treatment-resistant hypertensive patients would support this hypothesis. This study showed that the reduction in arterial pressure produced by the renal ablation procedure was associated with reduction in muscle sympathetic nerve activity that lasted at least for 1 yr, i.e., as long as these patients were followed in this study (44). This suggests an important contributory role of the afferent renal nerves to the hypertension in these patients. Therefore, this review will focus on the afferent renal sensory nerves and to what extent activation of these nerves may contribute to the homoeostatic regulation of arterial pressure and sodium balance in health and disease and only briefly discuss the sympathetic innervation of the kidney, which has been the focus of many previous reviews (e.g., 24, 51, 52, 110).
Efferent Renal Sympathetic Nerves
The sympathetic nerves innervate all parts of the renal vasculature and the nephrons with the greatest density of innervation found along afferent arterioles (2, 6). Among the various portions of the nephron, the greatest number of sympathetic nerves are found along the proximal tubules. However, the greatest density of innervation is found along the thick ascending limbs and distal convoluted tubules followed by the collecting ducts and proximal tubules. The nerve fibers contain varicosities with dense cored vesicles facing the neuroeffector sites, i.e., the vasculature and the nephron or the interstitium. These findings suggest that norepinephrine (NE) released from the sympathetic nerve fibers may act on the vascular/nephron structures not only at the junctional site but also postjunctionally at a more distal site from the nephrons (2–6).
The renal functional responses to increases in ERSNA support the anatomical data, the main renal functional responses to increases in ERSNA being decreases in renal blood flow and urinary sodium excretion and increases in renin secretion rate. Importantly, the responsiveness of the various effector sites to increases in ERSNA varies with the stimulation frequency. At low-level stimulation frequencies, <1 Hz, there is a marked increase in renin secretion rate with no or minimal changes in urinary sodium excretion and renal blood flow. At higher frequencies, there is a further increase in renin secretion rate and a significant fall in urinary sodium excretion. At even higher stimulation frequencies, there is also a fall in renal blood flow. Stimulation frequencies at 5–7 Hz result in complete renal vasoconstriction in most species (24, 51, 52, 62).
Renal denervation will, of course, result in opposite functional responses. Importantly, in most physiological conditions that are characterized by low ERSNA, renal denervation will result in an increase in urinary sodium excretion and a reduction in renin secretion rate with no or minimal changes in renal hemodynamics (24).
Afferent Renal Sensory Nerves
Intrinsic innervation.
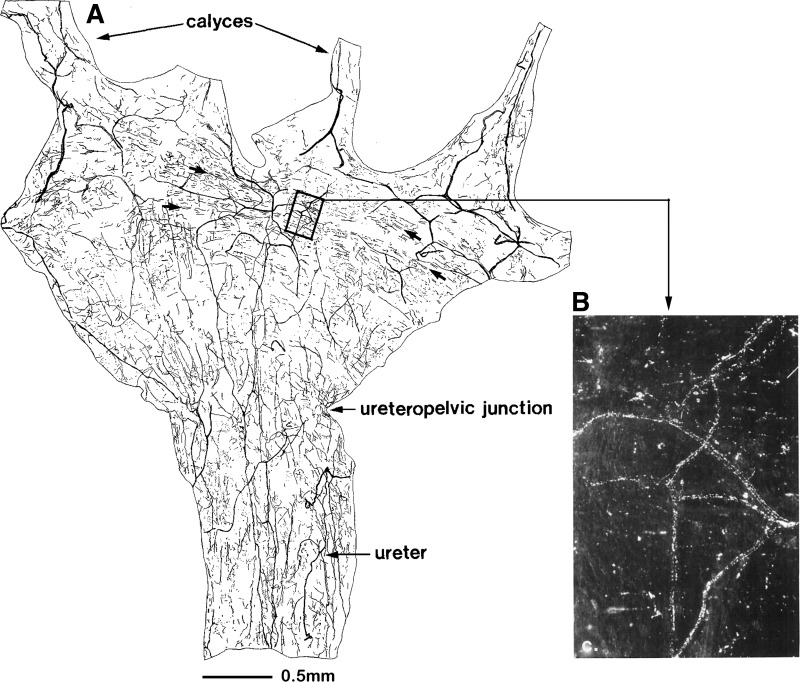
In contrast to the wide distribution of the sympathetic nerves in the renal tissue, the majority of the sensory nerves are located in the renal pelvic area with the greatest density in the renal pelvic wall, as shown by Marfurt and Echtenkamp (111) examining afferent renal sensory innervation by antegrade transport of wheat germ agglutinin horseradish peroxidase from dorsal root ganglia (DRG) (Fig. 1). The sensory nerves enter the renal pelvic area together with the renal artery or the proximal ureter. Many afferent renal sensory nerve fibers travel parallel to the long axis of the pelvis/ureter. There are also those that are oriented in a circumferential fashion. Many of the afferent nerve fibers are extremely fine and appear to terminate as free nerve endings. In addition to the renal pelvic wall, the renal artery and, to a lesser extent, the renal vein are also innervated with sensory nerves. There are few sensory nerve fibers in cortical tissue, and there are no fibers observed in the medulla. These findings are confirmed by immunohistochemical studies using antibodies against the neuropeptides calcitonin gene-related peptide (CGRP) and substance P (32, 33, 37, 68, 77, 78, 98, 104, 131), which are known to be present in sensory nerves (but not in sympathetic efferent nerves). The sensory nerves in the renal pelvic wall contain both substance P and CGRP. (Fig. 2).
Fig. 1.
A: camera lucida drawing of a whole mount renal pelvis and proximal ureter. In the kidney, the majority of the afferent sensory nerves are located in the renal pelvic wall. Most of the afferent fibers travel parallel to the long axis of the pelvis/ureter. There are also fibers that are oriented predominantly in a circumferential fashion, making them ideally located for sensing a stretch of the pelvic wall (arrows). The area enclosed by the box is illustrated in B. Many of the afferent nerve fibers are fine and appear to terminate as free nerve endings (B). [Modified from Ref. 111: Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol 311: 389–404, 1991.]
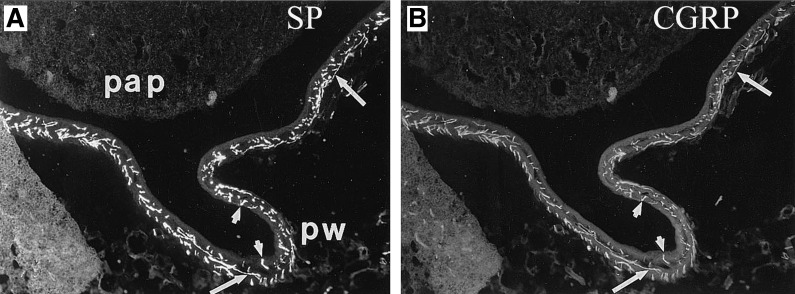
Fig. 2.
Renal tissue was double-labeled with antibodies against substance P (SP) (A) and calcitonin gene-related peptide (CGRP) (B). Substance P and CGRP are colocalized in all sensory nerves in the renal pelvic wall (pw) surrounding the papilla (pap). Arrows depict sensory nerves with both substance P-like immunoreactivity (-LI) and CGRP-LI. The majority of the sensory nerves are found in the subepithelial layer of the pelvic wall with few fibers penetrating into the uroepithelium (arrowheads). Magnification: ×120. [From Ref. 77].
The anatomical studies showing the majority of the sensory nerves being located in the renal pelvic wall are confirmed by functional studies showing that renal pelvic administration of capsaicin, which stimulates sensory nerves by an activation of the nonselective cation channel transient receptor potential vanilloid 1 (known as TRPV1), increased afferent renal nerve activity (ARNA) at 100-fold lower concentration when administered into the renal pelvis than into the renal interstitium at the level of the juxtamedullary border (90). Importantly, renal functional responses (see Activation of afferent renal sensory nerves modulates ERSNA) to renal pelvic administration of capsaicin are similar to those produced by substance P, CGRP, and increased renal pelvic pressure (90, 142).
The circumferential orientation of the renal sensory nerves makes them ideally located for sensing stretch of the renal pelvic wall, as demonstrated in numerous studies examining the effects of increasing renal pelvic pressure on ARNA (52, 62). Early studies in cats showed that short-term volume expansion resulted in parallel increases in renal pelvic pressure and ARNA with activation threshold being around 3 mmHg above baseline renal pelvic pressure (36). The low activation threshold was subsequently confirmed in studies in which the renal pelvic mechanosensory nerves were activated without concomitant changes in systemic hemodynamics (94). Together, these studies suggested that mechanosensitive renal pelvic nerves are activated by increases in renal pelvic pressure within the physiological range and well below that required for sensation of pain.
Stretch is associated with an increase in muscle spindle cell membrane sodium permeability, resulting in an inward flux of sodium and depolarization (48, 119). Importantly, changes in intracellular sodium concentration may modulate the responsiveness of renal pelvic mechanosensitive nerves, as shown by the studies demonstrating reduction in the ARNA responses to increased renal pelvic pressure by renal pelvic administration of amiloride and lidocaine (86, 93) and enhancement of the ARNA responses by ouabain-induced inhibition of Na+-K+-ATPase (94).
Although the majority of the sensory nerves in the pelvic wall are located in the muscular layer, there are sensory nerve fibers penetrating into the uroepithelium potentially making these nerve endings accessible for sensing changes in the chemical composition of the urine. R2 chemosensitive nerve fibers, which have basal discharge and are activated by changes in the chemical composition of the urine, have been described by Recordati et al. (121). The R2 nerve fibers are also activated by renal ischemia. R1 chemosensitive nerve fibers have been described as having no basal activity and being activated by renal ischemia. Whether R1 and/or R2 chemosensitive nerve fibers are activated during physiological and/or pathophysiological conditions is currently not known.
Projection to the central nervous system.
Functional studies designed to examine whether the renorenal reflexes responses were integrated at a supraspinal or spinal level examined the effects of dorsal rhizotomy (DRX) at the T6 level on the contralateral renal responses to activation of renal mechanosensory nerves (93). However, the experiments failed to shed any light on this issue because ARNA did not increase in response to increases in renal pelvic pressure, suggesting that the responsiveness of the renal sensory nerves was modulated by ERSNA (see Activation of efferent renal nerves modulates ARNA).
However, there are numerous studies employing retrograde tract tracing of fluorescent dyes, horseradish peroxidase, or pseudorabies virus injected into kidneys that suggest a supraspinal integration of the afferent renal signals. The cell bodies of the afferent renal nerves are located in ipsilateral DRG from T6 to L4, with the majority of the renal sensory nerves deriving from DRG at the T12–L3 level (27, 137). Very few afferent renal nerves are derived from the contralateral DRG. The distribution of the cell bodies varies slightly among various species. Within the spinal cord, the afferent renal nerves project to the ipsilateral dorsal horn in laminae I, III–V (16), where they synapse with interneurons projecting to sites within the central nervous system associated with cardiovascular regulation, including nucleus tractus solitarius, rostral ventrolateral medulla, subfornical organ, and paraventricular nucleus of hypothalamus (130). There is also evidence for a monosynaptic projection of the afferent renal nerves to areas within the brain stem (141). Electrophysiological support for supraspinal integration of the input from the afferent renal nerves involving neurons in the medulla was derived from studies in rabbits showing that decreases in ERSNA produced by electrical stimulation of the afferent renal nerves were blocked by renal denervation or spinal cord transection at C2 but not by transection of the brain stem at the pontine-medullary junction (124).
Activation of afferent renal sensory nerves modulates ERSNA.
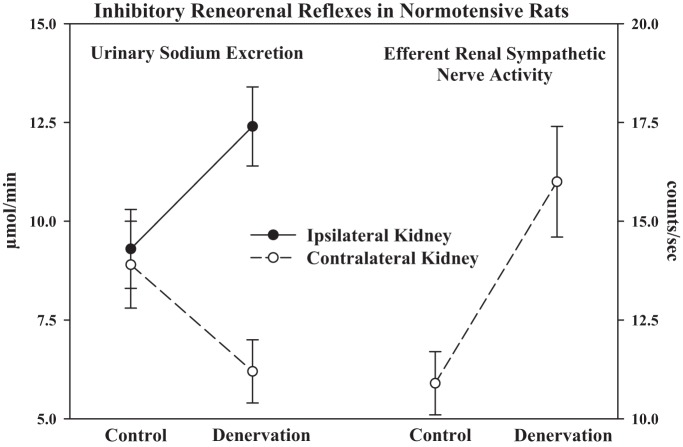
One of the first studies to suggest a functional role of the renal sensory nerves in normotensive healthy rats showed that unilateral renal denervation resulted, not only, in the expected increase in ipsilateral urinary sodium excretion but also in a decrease in contralateral urinary sodium excretion. The fall in contralateral urinary sodium excretion was produced by an increase in contralateral ERSNA, suggesting that the afferent renal nerves exert a tonic inhibition of ERSNA in healthy rats (17, 25). (Fig. 3).
Fig. 3.
In normotensive healthy rats, ipsilateral renal denervation increases ipsilateral urinary sodium excretion and decreases contralateral urinary sodium excretion. The fall in contralateral urinary sodium excretion is produced by an increase in contralateral efferent renal sympathetic nerve activity, suggesting that the afferent renal nerves exert a tonic inhibition of efferent renal sympathetic nerve activity. [Modified from Ref. 24].
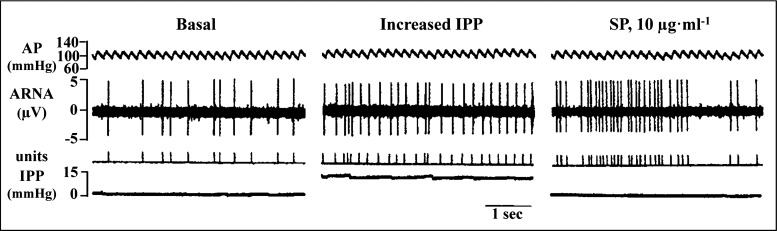
Single-unit recordings of ARNA showed that the same single unit that responded to increases in renal pelvic pressure also responded to renal pelvic administration of substance P, supporting an important role for substance P-containing nerve fibers, contributing to the activation of mechanosensory nerves (Fig. 4) (109). The electrophysiological studies have been supported by functional studies that showed that increases in renal pelvic pressure result in increases in ARNA that are associated with increases in renal pelvic release of substance P and are blocked by renal pelvic administration of substance P receptor antagonists (83, 89, 107, 109).
Fig. 4.
Single-unit recordings of afferent renal nerve activity (ARNA) showed that the same nerve fiber activated by increases in intrarenal pelvic pressure (IPP) can also be activated by substance P (SP) administered directly into the renal pelvic area. AP, arterial pressure. [Modified from Ref. 109 with permission from the American Society of Nephrology, Impaired renal sensory responses after renal ischemia in the rat, Ma MC, Huang HS, Wu MS, Chien CT, Chen CF. J Am Soc Nephrol 13: 1872–1883, 2002].
In normal healthy animals, the functional responses to activation of renal pelvic sensory nerves by various stimuli, including increased renal pelvic pressure, bradykinin, substance P, and capsaicin, consist of increases in ARNA, which cause a fall in contralateral ERSNA and increases in contralateral urine flow rate and urinary sodium excretion, which are blocked by ipsilateral renal denervation (87, 90, 91, 107, 109, 142), an inhibitory renorenal reflex response. These findings, together with the studies showing that activation of the chemosensitive renal nerves elicits excitatory reflex responses (122, 129), suggest that the tonic inhibitory effects produced by activation of afferent renal nerves and revealed by renal denervation (Fig. 3) are related to tonic activation of renal mechanosensory nerves in healthy normotensive rats. This hypothesis is further supported by the low activation threshold for the renal mechanosensory nerves (36, 94).
Activation of the efferent renal nerves modulates ARNA.
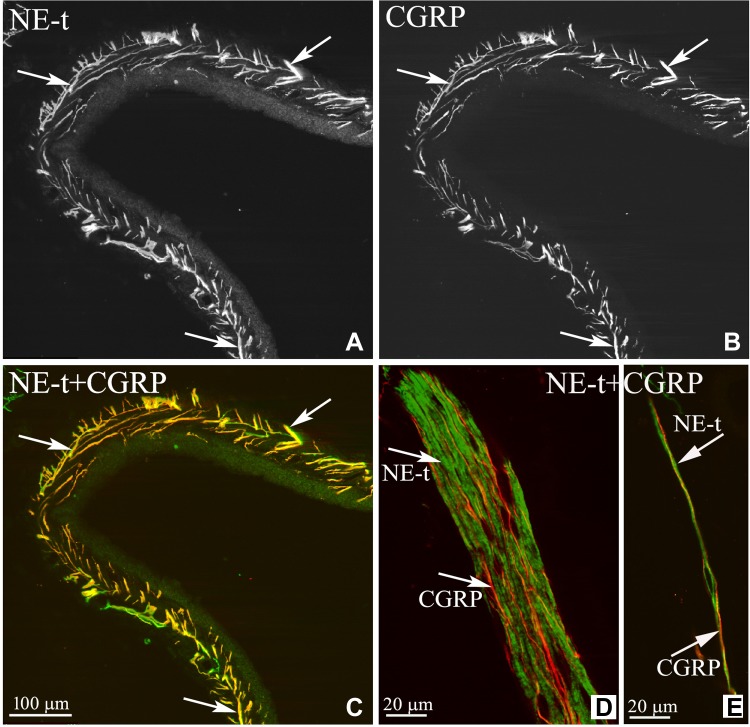
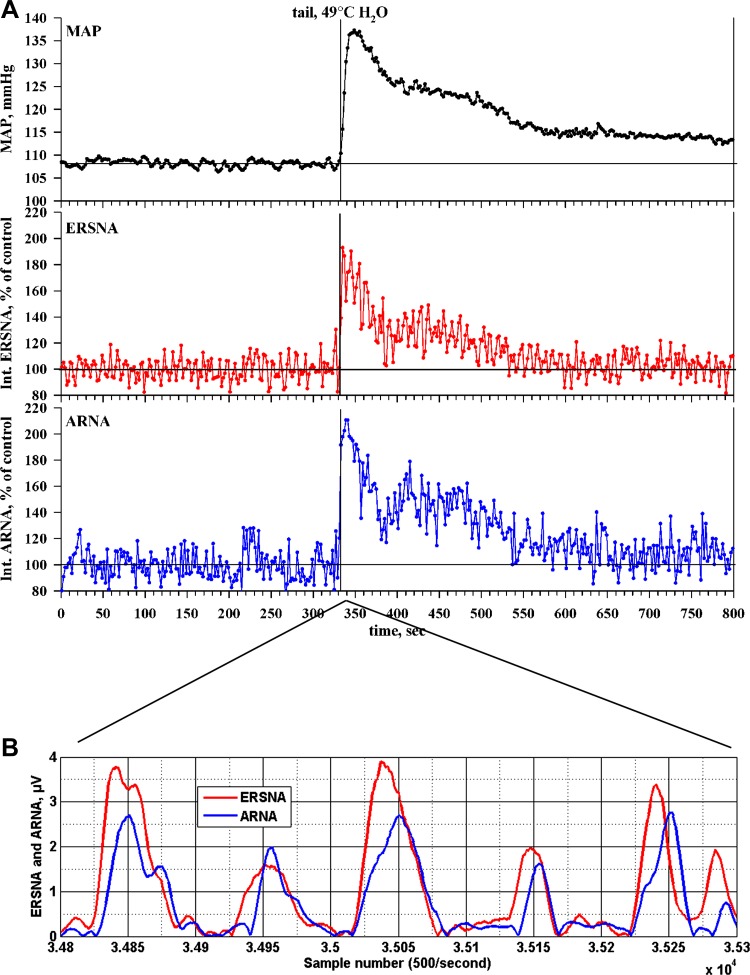
Early studies by Niijima (116) showed that electrical renal nerve stimulation or intra-arterial administration of NE increased ARNA in isolated kidney preparations. Although the results were interpreted to reflect increases in ARNA in response to activation of renal mechanosensory nerves (related to ERSNA-induced increases in renal perfusion pressure), the author speculated that “the possibility of direct sensitization of mechanoreceptors by noradrenaline cannot be eliminated”. Anatomical support for this hypothesis was subsequently first shown by Barajas and Wang (7), who demonstrated the presence of noradrenergic unmyelinated nerve fibers and myelinated, presumably afferent, nerve fibers in the same nerve bundle in the juxtamedullary region. Later studies have provided more substantial evidence for a close anatomical relationship between renal sympathetic and sensory nerves (33, 78, 104) (Fig. 5). In the renal pelvic wall, the sensory nerve fibers are more numerous than are the sympathetic nerve fibers, but where there are sympathetic nerve fibers, they are often running together with the afferent sensory nerve fibers intertwined in the same nerve bundles in the renal pelvic smooth muscle layer (78) (Fig. 5). These anatomical findings suggested that changes in ERSNA may modulate ARNA, as indicated by Niijima in 1972 (116). More recent functional studies have shown that reflex decreases and increases in ERSNA decrease and increase ARNA, respectively, (Fig. 6) (78). The changes in ERSNA may modulate ARNA independently of hemodynamic changes, as shown by the increases in ARNA and renal pelvic release of substance P in response to renal pelvic administration of NE (78). Taken together, there is strong evidence to support a negative feedback system, in which increases in ERSNA increase ARNA, which, in turn, decreases ERSNA via activation of the inhibitory renorenal reflex mechanism (Fig. 7).
Fig. 5.
Renal tissue was double-labeled with antibodies against the norepinephrine transporter (NE-t) (A) and CGRP (B). C: the majority of the NE-t-immunoreactive (NE-t-ir) fibers (green) are close to the CGRP-ir fibers (red), (arrows) in the renal pelvic wall. D and E: confocal microscopy of two nerve bundles in the renal pelvic area showed that the sympathetic and sensory nerves are separate fibers (arrows). [Modified from Ref. 78].
Fig. 6.
A: thermal cutaneous stimulation produced by placing the anesthetized rat's tail in warm water produced a general activation of the sympathetic nervous system, as shown by the increases in mean arterial pressure (MAP; black lines), efferent renal sympathetic nerve activity (ERSNA; red lines) and ARNA (blue lines), recorded in the same rat. B: relationship between ERSNA and ARNA for the 1-s interval beginning 5.6 s after placing the rat's tail in warm water. The increases in ERSNA preceded the increases in ARNA by 17.1 ± 2.4 ms (n = 7). [Modified from Ref. 78].
Fig. 7.
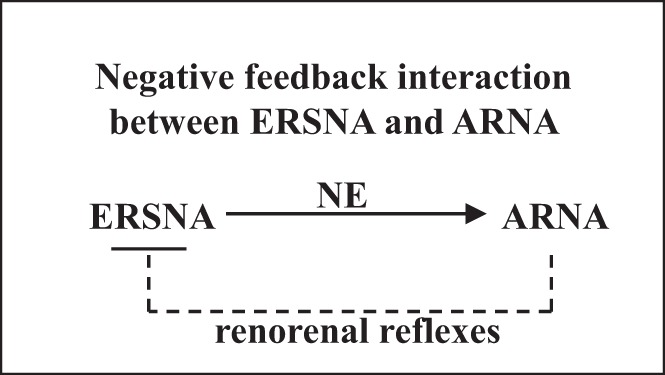
There is a reciprocal interaction between ERSNA and ARNA. Increases in ERSNA increase ARNA, the increase in ARNA will, in turn, decrease ERSNA via activation of the inhibitory renorenal reflexes in the overall goal of maintaining low ERSNA to minimize sodium retention. ERSNA modulates ARNA by release of norepinephrine (NE).
The dynamic relationship between ERSNA and ARNA was subsequently studied in more detail using transfer function analysis in seven rats. In the control period, ERSNA exhibited intermittent low-amplitude bursting, while ARNA bursts were fewer, random, and unrelated to ERSNA. During thermal cutaneous stimulation, ERSNA bursting behavior became regular with larger-amplitude bursts occurring uniformly in each cardiac cycle. ARNA exhibited an identical pattern with the ARNA burst following the ERSNA burst by 17.1 ± 2.4 ms (Fig. 6B). Transfer function analysis (ERSNA to ARNA) showed that transfer function gain was similar before and during stimulation. Phase angle was negative (0 to −0.5 rad), and calculated time delay indicated that ERSNA preceded ARNA by 15–20 ms. Maximal significant coherence was 0.27 ± 0.13 before and 0.46 ± 0.12 during stimulation. Cross-correlation showed significant correlation coefficients of 0.64 ± 0.02 at −12 ms lag before and 0.71 ± 0.02 at −10 ms lag during thermal cutaneous stimulation. There were cross-correlation peaks during thermal cutaneous stimulation but not during the control period at lag intervals equivalent to cardiac cycle length. The linear correlation coefficient between ERSNA and ARNA was 0.15 ± 0.05 before and 0.40 ± 0.06 during thermal cutaneous stimulation. The time delay between the ERSNA and subsequent ARNA bursts, 10–20 ms, is substantially greater than that to be expected with an ionotropic receptor (ligand-gated ion channel) and is more consistent with that observed for a metabotropic receptor involving a neurotransmitter molecule, which binds to a specific plasma membrane receptor with subsequent activation of signal transduction mechanisms.
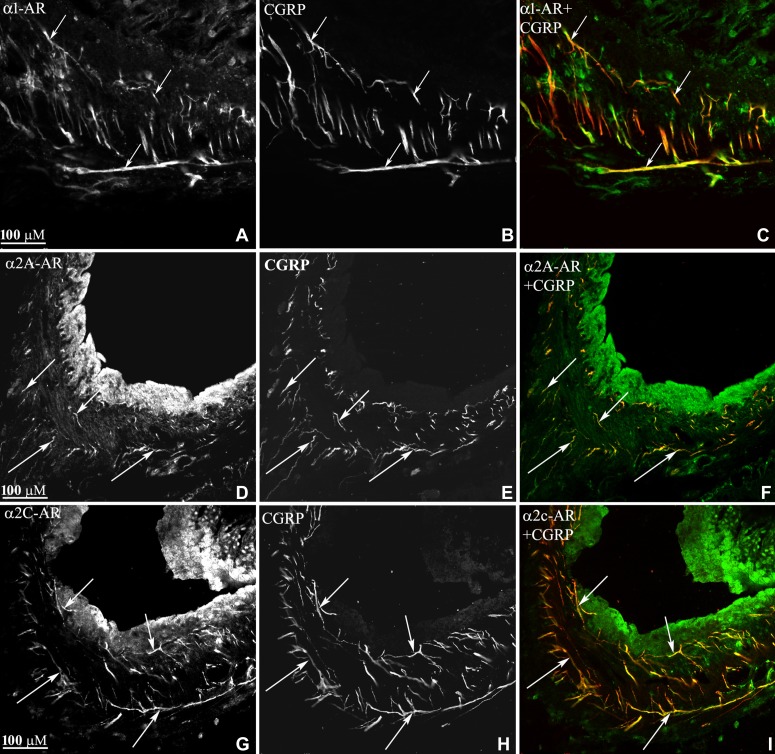
This notion was confirmed by our findings that the increases in ARNA produced by reflex-induced increases in ERSNA were blocked by renal pelvic perfusion of an α1 adrenoceptor antagonist and enhanced by an α2 adrenoceptor antagonist (79). These findings together with immunohistochemical studies demonstrating the presence of α1 and α2 adrenoceptors on/close to the renal pelvic sensory nerves (Fig. 8) provide evidence for a synaptic connection between efferent renal sympathetic nerves and afferent renal nerves in the renal pelvic wall (79). In this context, it is of interest that NE may also modulate ARNA at the level of the DRG. NE was found to decrease the activity of voltage-gated calcium channels in cultured DRG cells, retrogradely labeled from the kidney, by activating α2 adrenoceptors (26).
Fig. 8.
Renal tissue was double-labeled with antibodies against α1-adrencoceptors (AR) and CGRP (A–C), α2A-AR and CGRP (D–F) or α2C-AR and CGRP (G–I). In the renal pelvic wall, α1-AR-immunoreactive (ir) fibers, α2A-AR-ir fibers, and α2c-AR-ir fibers (all green) were close or on CGRP-ir fibers (red) as seen in C, F, and I (colocalization yellow). [Modified from Ref. 79].
It is important to note that an interaction between efferent and afferent nerves is not unique to ERSNA and ARNA. There is considerable evidence for a relationship between efferent and afferent nerve activity in the sinoaortic arterial baroreflexes and the carotid chemoreceptor reflexes (31). NE has been shown to increase baroreceptor afferent nerve activity by a direct action on the receptors (97). Also, there is considerable evidence for NE enhancing the activation of peripheral nociceptors (50).
Mechanisms Involved in Activation of Renal Sensory Nerves
Dietary sodium.
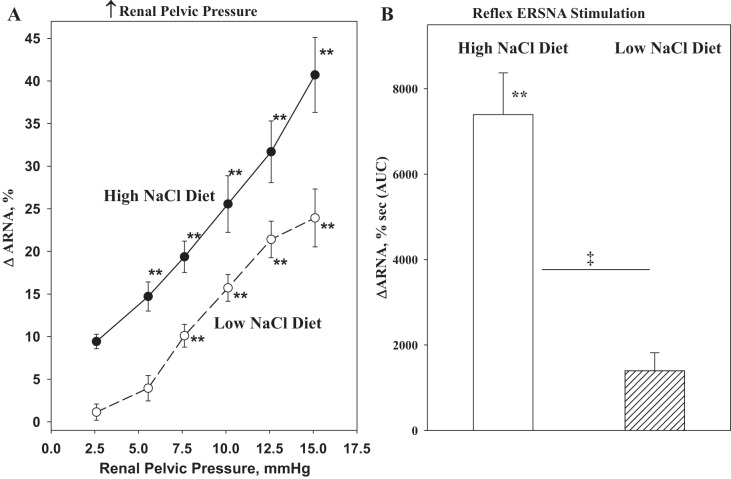
The functional importance of the natriuretic renorenal reflexes in the renal control of total body sodium was suggested by the findings that the responsiveness of the renal sensory nerves is modulated by dietary sodium. In rats fed a high-sodium diet, graded increases in renal pelvic pressure resulted in greater increases in ipsilateral ARNA and contralateral urinary sodium excretion at each level of increased renal pelvic pressure compared with those in rats fed a low-sodium diet (73). The ARNA responses to reflex changes in ERSNA were similarly modulated by dietary sodium (84) (Fig. 9). It is important to note that these are physiologically appropriate responses in the maintenance of sodium balance in various dietary sodium conditions. In high-sodium dietary conditions, enhancement of the ERSNA-induced increases in ARNA would increase the inhibitory renorenal reflex control of ERSNA, resulting in reduction of ERSNA to prevent or limit sodium retention. Conversely, in low-sodium dietary conditions, suppression of the ERSNA-induced increases in ARNA would result in increased ERSNA by reducing the renorenal reflex inhibition of ERSNA, eventually leading to sodium retention (Fig. 7).
Fig. 9.
A: high-sodium diet enhances the ARNA responses to increases in renal pelvic pressure. B: reflex increases in ERSNA produced by thermal cutaneous stimulation in healthy normotensive rats. **P < 0.01 vs. baseline control value. ‡P < 0.01 vs. low-NaCl diet. [Modified from Refs. 73 and 79].
The activation threshold for the renal mechanosensory nerves being <2 mmHg in rats fed a high-sodium diet (Fig. 9) suggested that the afferent renal nerves are tonically active in conditions of high-sodium dietary intake and contribute to the renal control of the homeostatic regulation of arterial pressure and sodium balance. This notion is supported by studies in rats, in which the afferent renal nerves were removed by DRX at T9–L1 (71, 85). Whereas arterial pressure was similar in DRX and sham-operated rats fed a normal-sodium diet, a high-sodium diet significantly increased mean arterial blood pressure in DRX rats but not in sham-operated litter mates. These data suggest that the afferent renal nerves are essential for achieving sodium balance during increased dietary sodium intake. Therefore, afferent renal denervation leads to salt-sensitive hypertension. These findings support the notion that the salt-sensitive hypertension in rats neonatally treated with capsaicin to destroy all sensory nerves is, at least in part, related to destruction of the renal sensory nerves (135). The DRX rats fed a high-sodium diet were characterized by increased basal ERSNA and increased ERSNA responsiveness to environmental and somatic stimulation, which is most likely due to impairment of the arterial baroreflex control of ERSNA (85). Early studies have provided strong evidence for convergence of afferent signals from the renal and carotid sinus nerves on neurons in various brain areas involved in cardiovascular control (12, 30, 130).
Prostaglandins.
Prostaglandins (PGs) are known to enhance the responsiveness of peripheral and central sensory nerves to various stimuli (e.g., 45, 115, 132). In the kidney, PGs play both a permissive and facilitatory role in the activation of the renal pelvic sensory nerves by various stimuli, including increased renal pelvic pressure, bradykinin, substance P, and capsaicin. Studies examining the various mechanisms involved in the activation of the renal mechanosensory nerves showed that bradykinin B2 receptors in the renal pelvic area contributes to the increases in ARNA produced by increased renal pelvic pressure by activating PKC. Activation of PKC eventually leads to increased renal pelvic synthesis of cyclooxygenase 2 (COX-2) and release of PGE2. PGE2 activates EP4 receptors on or close to the renal pelvic sensory nerves leading to induction of cAMP and activation of PKA. Activation of PKA results in a release of substance P from the sensory nerves in the renal pelvic wall (68, 76, 81, 82, 83). The release of substance P is a calcium-dependent mechanism that requires influx of calcium via N-type calcium channels (65) (Fig. 10).
Fig. 10.
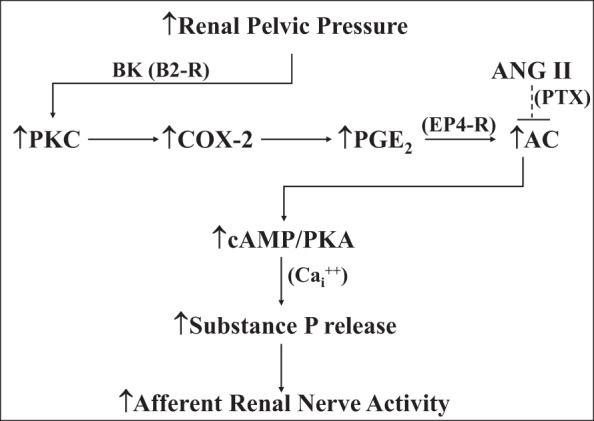
Stretching the renal pelvic wall by increases in renal pelvic pressure leads to activation of PKC by stimulation of bradykinin (BK) B2 receptors (B2-R), which leads to induction of cyclooxygenase-2 (COX-2) and an increase in renal pelvic release of PGE2. PGE2 activates EP4 receptors on or close to the renal pelvic sensory nerves, which, in turn, leads to activation of the adenylyl cyclase (AC)/cAMP/PKA transduction pathway. This results in a calcium (Cai2+)-dependent release of substance P and increases in afferent renal nerve activity. The mechanisms involved in the suppressed responsiveness of the renal sensory nerves in physiological and pathophysiological conditions of increased endogenous ANGII activity, include ANG II reducing the PGE2-mediated activation of AC by a pertussis toxin-sensitive (PTX) mechanism. [Data derived from Refs. 65, 68, 70, 73, 76, 81–83].
Angiotensin.
Angiotensin (ANG) type 1 (AT1) receptors have been localized in the renal pelvic wall by in situ hybridization and autoradiography (40, 112, 144). Similar to its inhibitory role on arterial and cardiopulmonary baroreflexes (23, 133), ANG II contributes to the suppressed responsiveness of the renal pelvic mechanosensory nerves in low-sodium dietary conditions. Renal pelvic administration of the AT1-receptor antagonist losartan restored the suppressed ARNA responses to increased renal pelvic pressure in rats fed a low-sodium diet toward those observed in rats fed a normal-sodium diet (73). Conversely, renal pelvic administration of ANG II suppressed the enhanced responsiveness of the renal mechanosensory nerves in rats fed a high-sodium diet. Subsequent studies showed that renal pelvic ANG II modulates the responsiveness of renal sensory nerves by suppressing PGE2-mediated activation of adenylyl cyclase via a pertussis toxin (PTX)-sensitive mechanism (Ref. 70; Fig. 10).
The mechanisms involved in the decreased responsiveness of the renal sensory nerves to increases in ERSNA in low-sodium dietary conditions are more complex and involve increased activation of both AT1-receptors and α2 adrenoceptors in the renal pelvic wall (79). The mechanisms by which losartan and the α2 adrenoceptor antagonist rauwolscine enhance the interaction between ERSNA and ARNA in low-sodium dietary conditions are currently unknown. Both AT1 receptors and α2 adrenoceptors are coupled to PTX-sensitive Gi/o proteins (41, 138), which may suggest a similar mechanism contributing to the ANG II and NE-mediated suppression of the responsiveness of the renal sensory nerves in low-sodium dietary conditions.
Endothelin.
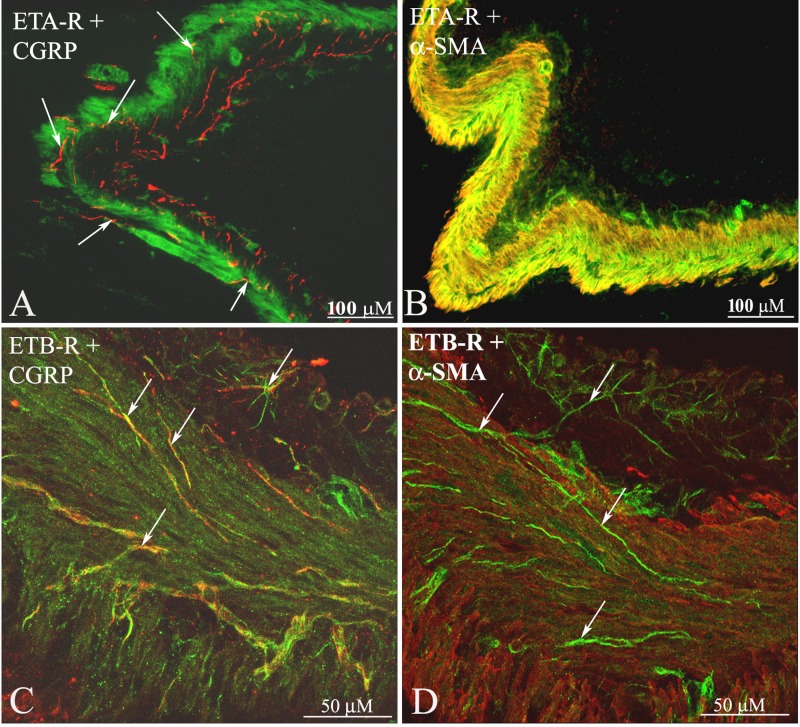
Endothelin (ET) is abundantly expressed throughout the body, including the brain and the kidney (42). ET exerts its effects by activating two G protein-coupled receptors, ETA and ETB (123). In the kidney, ET-1 is widely distributed, with the highest concentration in the inner medulla (123, 139). Whereas ETA-receptors (ETA-R) are predominantly localized to the renal vasculature, ETB-R are found in glomeruli, inner medullary collecting duct cells, and the renal pelvic area (84, 144). ETA-R and ETB-R are differentially distributed in the renal pelvic wall. Whereas, the ETB-R are found on or close to unmyelinating Schwann cells surrounding renal sensory nerves, the ETA-R are present on smooth muscle cells in the pelvic wall and small vessels in and adjacent to the renal pelvic wall and throughout the kidney (84) (Fig. 11). A similar distribution of ETA-R was found in DRG, T9-L1; ETA-R were mainly localized on smooth muscle cells in vessels distributed among CGRP containing neuronal cell bodies (84).
Fig. 11.
Renal tissue was double-labeled with antibodies against ETA-R (green) and CGRP (red) (A), ETA-R and α-smooth muscle actin (SMA; red) (B), ETB-R (green) and CGRP (red) (C), and ETB-R and α-SMA (red) (D). In the renal pelvic wall, ETA-R-ir fibers were found on smooth muscle cells and CGRP-ir nerve fibers (arrows) among the ETA-R-ir smooth muscle cells. Also, in the renal pelvic wall, ETB-R-ir fiber-like structures (arrows) were found close to CGRP-ir nerve fibers (arrows) among smooth muscle cells. [Modified from Ref. 84].
A possible role for ET-1 in the regulation of arterial pressure via the sensory nerves was suggested by studies in rats neonatally treated with capsaicin (143). The salt-sensitive hypertension in these rats was reduced by ETA receptor antagonists in a similar fashion as that seen in ETB receptor-deficient rats (34, 120). These findings together with the known modulatory role of ET-1 on nociceptors (20, 58, 59) and baroreceptors (14, 49, 101) and the salt-sensitive hypertension demonstrated in DRX rats (see Mechanisms Involved in the Activation of Renal Sensory Nerves; Dietary sodium and Refs. 71 and 85) led to the concept that ET-1 via activation of ETA-R and ETB-R may modulate the responsiveness of the renal sensory nerves. Interestingly, while blocking either renal pelvic ETA-R or ETB-R had no effect on the responsiveness of the renal mechanosensory nerves in rats fed a normal sodium diet, renal pelvic administration of ETB-R antagonists reduced the responsiveness of the renal mechanosensory nerves in rats fed a high-sodium diet. Conversely, the responsiveness of the renal mechanosensory nerves was enhanced by renal pelvic administration of an ETA-R antagonist (72). These findings suggest that activation of a renal pelvic ET-R contributes to the altered responsiveness of the renal mechanosensory nerves during a high- and low-sodium diet. Activation of ETA-R plays an important contributory role in the ANG II-induced suppression of the responsiveness of the renal mechanosensory nerves in low-sodium dietary conditions (see Mechanisms Involved in the Activation of Renal Sensory Nerves; Angiotensin and Ref. 69).
Renal ET-1 also contributes to the altered responsiveness of the renal sensory nerves to changes in ERSNA in high- and low-sodium diets. The ARNA responses to reflex increases in ERSNA were reduced by an ETB-R antagonist in a high-sodium diet and enhanced by an ETA-R antagonist in a low-sodium diet (84). In high-sodium dietary conditions, blocking renal ETB-R not only reduced the ARNA responses to increases in ERSNA and the NE-induced release of substance P, but also NE-induced release of PGE2. Because the NE release of substance P is dependent on intact PG syntheses (91), these data suggest that stimulation of ETB-R contributes to the enhanced activation of renal sensory nerves by a PGE2-dependent mechanism. The exact mechanism(s) by which activation of ETB-R on peripheral Schwann cells may modulate ARNA is unclear, but it is well known that glial cells can modulate neurotransmission by increasing intracellular Ca2+ in response to various neurotransmitters, including ET-1 (46, 134). Likewise, the mechanisms involved in the ETA-R-mediated decreased responsiveness of the renal sensory nerves is also unknown, but the presence of ETA-R on smooth muscle cells in small vessels in and adjacent to the renal pelvic wall may suggest that the ETA-R-induced modulation of renal sensory nerve activation is related to local vasoconstriction with ischemia, leading to an increase in oxygen free radicals (99, 105), which have been shown to impair carotid baroreceptor activity (102).
Summary: normotensive healthy rats.
ARNA is predominantly inhibitory in normal rats. Activation of the afferent renal nerves by various stimuli, including increased renal pelvic pressure, bradykinin, PGE2, substance P, and NE, exerts an inhibitory effect on ERSNA with the overall goal of maintaining low ERSNA to minimize sodium retention. Importantly, the responsiveness of the afferent renal nerves is modulated by dietary sodium. High dietary sodium enhances, and low dietary sodium reduces, the responsiveness of the renal sensory nerves in the overall goal to maintain sodium balance during various dietary sodium intakes. Important mechanisms contributing to the altered responsiveness of the renal sensory nerves include ANG II, ET, and NE; increased activation of AT1-R, ETA-R, and α2 adrenoceptors suppresses the responsiveness of the renal sensory nerves in the low-sodium diet, and increased activation of ETB-R enhances the responsiveness of these nerves in the high-sodium diet.
Pathophysiology
There is considerable evidence for increased activity of the sympathetic nervous system in various pathological conditions involving renal injury, including hypertension, heart failure, chronic renal failure, diabetes, and obesity (24, 38, 43, 103, 118, 127). Renal inflammation is prevalent in many of these pathological conditions and may contribute to the increased ERSNA via activation of afferent renal nerves. Indirect support for this hypothesis may be derived from the recent studies in patients with treatment-resistant hypertension, which showed that renal denervation decreases arterial pressure in association with decreases in muscle sympathetic nerve activity, suggesting that the renal denervation procedure interrupted excitatory reflexes originating in the kidneys (44). This hypothesis would appear to be in an apparent contrast to the considerable evidence (see Activation of afferent renal sensory nerves modulates ERSNA) for inhibitory reflexes originating in the kidney. However, it is important to note that the studies demonstrating the presence of inhibitory renorenal reflexes were performed in healthy normotensive animals. In various pathological states, available data would suggest that activation of the afferent renal nerves elicits a different reflex pattern.
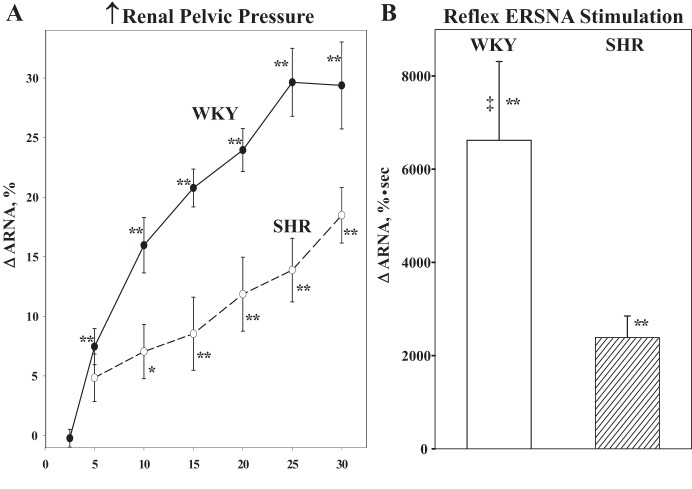
In the spontaneously hypertensive rat (SHR), accumulating evidence indicates that the renal nerves contribute to the pathogenesis of hypertension (24, 51, 52, 110). Peripheral sympathetic nerve activity and, in particular, ERSNA is enhanced. In SHRs, there is decreased responsiveness of the renal sensory nerves to various stimuli, including increased renal pelvic pressure and renal pelvic administration of bradykinin and substance P (66, 88, 92) (Fig. 12). The decreased responsiveness of renal mechanosensitive nerves cosegregated with hypertension in a backcross of SHR and WKY (22). The reduced responsiveness of the renal mechanosensory nerves in SHR is due, at least in part, to a peripheral defect at the level of the sensory receptors in the renal pelvis involving endogenous ANG II suppressing the PGE2-mediated release of substance P via a PTX-sensitive mechanism (64). The ERSNA-induced increase in ARNA is also suppressed in SHR, which is partly due to increased activation of AT1-R and partly to increased NE-mediated activation of α2 adrenoceptors on the peripheral sensory nerve endings (74). The findings that losartan only enhanced the responsiveness of the renal sensory nerves to NE in the presence of α2 adrenoceptor blockade suggest a powerful inhibitory effect of activation of α2 adrenoceptors on the responsiveness of the renal sensory nerves. This may be related to an increased density of the α2 adrenoceptors in peripheral renal (pelvic) tissue (125). Thus, one possible mechanism contributing to the hypertensive process in SHR is an impairment of the inhibitory renorenal reflexes that would lead to increased ERSNA and water and sodium retention, factors known to contribute to the hypertensive process.
Fig. 12.
The ARNA responses to graded increases in renal pelvic pressure (A) and reflex increases in ERSNA (B) produced by thermal cutaneous stimulation are suppressed in SHR vs. WKY. [Data in B are from Ref. 74]. *P < 0.05, **P < 0.01 vs. baseline control value; ‡P < 0.01 vs. SHR.
Increased activation of endogenous ANG also contributes to the impaired renorenal reflexes in other pathological states characterized by increased ERSNA and sodium retention, including diabetes type I and congestive heart failure (75, 80). In congestive heart failure, increased activation of ETA-R together with increased activation of AT1-Rs contributes to the reduced responsiveness of the renal sensory nerves (67). Further, numerous studies by Chen and coworkers (15, 107–109) have presented evidence for reduced responsiveness of the renal pelvic mechanosensory nerves in ischemia-induced acute renal failure, obstructive nephropathy, cirrhosis, and hypoxia—pathological conditions characterized by increased endogenous ANG II activity (61).
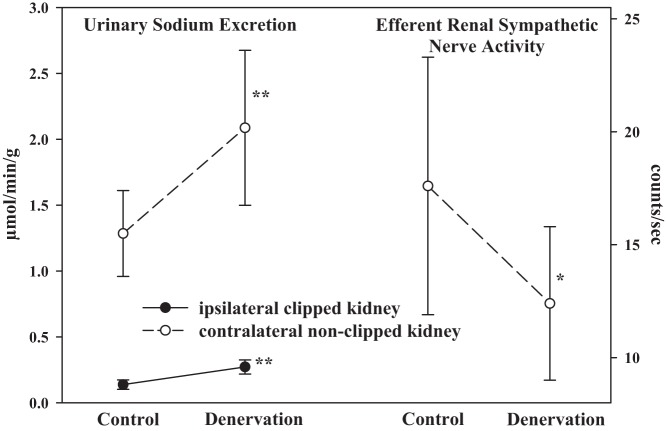
There is evidence to suggest that when the inhibitory renorenal reflexes are suppressed, excitatory reflexes may prevail, especially in conditions of renal injury and damage. In two-kidney, one-clip hypertensive rats, increasing renal pelvic pressure in the clipped ischemic kidney failed to elicit a contralateral inhibitory renorenal reflex response. On the other hand, denervation of the ipsilateral clipped kidney in two-kidney, one-clip hypertensive rats increased urinary sodium excretion not only from the ipsilateral ischemic kidney but also from the contralateral kidney (63). The increase in contralateral urinary sodium excretion was associated with a fall in contralateral ERSNA (Fig. 13), suggesting that the afferent renal nerves from clipped ischemic kidneys exert an excitatory influence on contralateral ERSNA—in contrast to normal healthy rats, in which the renorenal reflexes are inhibitory (Fig. 3). There is considerable support for excitatory reflexes deriving from ischemic kidneys. Measurements of systolic arterial pressure in conscious two-kidney, one-clip hypertensive rats showed that denervation of the ischemic clipped kidney reduced systolic arterial pressure, almost to the same level as removing the clip from the renal artery (57). Studies in rabbits and dogs showing that intrarenal adenosine increases ARNA (53, 106) and arterial pressure, plasma NE, and ERSNA (56) have led to the hypothesis that adenosine plays an important role in the excitatory reflexes originating in the ischemic kidneys. Support for this hypothesis is derived from studies in one-kidney one-clip hypertensive rats, which showed that intrarenal administration of adenosine deaminase reduced arterial pressure in association with marked decreases in urinary adenosine concentration (54). Altogether, these studies would suggest that excitatory reflexes may originate in ischemic kidneys and involve adenosine, possibly activating chemosensitive afferent renal nerve fibers. Whether these chemosensitive nerve fibers are those previously identified as CR2 is currently unknown.
Fig. 13.
In two-kidney, one-clip hypertensive rats, renal denervation of the ipsilateral clipped kidney increases both ipsilateral and contralateral urinary sodium excretion. The increase in contralateral urinary sodium excretion is produced by a fall in contralateral efferent renal sympathetic nerve activity, suggesting that the afferent renal nerves from the clipped ischemic kidney exert an excitatory influence on efferent renal sympathetic nerve activity. [Data are derived from Ref. 63]. *P < 0.05, **P < 0.01 vs. Control.
Further evidence for excitatory reflexes originating in diseased kidneys is derived from studies in patients with renal failure. Comparing arterial blood pressure and muscle sympathetic nerve activity in hemodialysis patients with and without their native diseased kidneys intact showed markedly reduced arterial pressure and muscle sympathetic nerve activity in patients with bilateral nephrectomy compared with the patients who had their kidneys intact (18, 39). In a subgroup of transplant patients, muscle sympathetic nerve activity was measured before and after the second kidney was removed and was found to be reduced following removal of the second diseased kidney (39). These studies provide strong evidence that the diseased kidneys exert an excitatory effect on sympathetic nerve activity. Studies in rats with chronic renal failure would support the notion that excitatory reflexes may originate in diseased kidneys and contribute to increased activity of the sympathetic nervous system. Chronic renal failure produced by 5/6 nephrectomy resulted in increased arterial pressure and NE turnover in posterior hypothalamus and locus coeruleus that were prevented by prior DRX to remove the afferent renal innervation (13). Taken together, these studies suggest that local renal injury may result in sympathoexcitatory reflexes involving activation of the afferent renal nerves and central cardiovascular regulatory areas, resulting in increased efferent systemic and renal sympathetic nerve activity that eventually leads to hypertension.
Chronic heart failure is characterized by impairment of the inhibitory renorenal reflexes (see above) and increased ERSNA (28). Recent animal studies in which heart failure was induced by coronary ligation in rats (47) or rapid ventricular pacing in dogs and rabbits (126, 136) showed that renal denervation performed prior to the induction of heart failure improved cardiac function. Further, a pilot study in chronic heart failure patients showed improved exercise capacity following bilateral renal denervation (21). Taken together, these studies suggest an important role for the renal nerves in chronic heart failure. The contributory roles of the efferent vs. the afferent renal nerves to the renal and cardiac dysfunction in chronic heart failure are currently unclear.
Summary: pathophysiological conditions.
In pathophysiological sodium-retaining states, including hypertension and renal edema-forming diseases, such as congestive heart failure, nephrotic syndrome, and cirrhosis, ERSNA is inappropriately increased in the presence of sodium retention. It has long been known that impairment of the aortic and arterial baroreflexes contributes to the inappropriately increased ERSNA in these pathological conditions. In addition, more recent evidence supports a role for an impairment of inhibitory reflexes originating in the kidneys, per se, contributing to the increased ERSNA in these conditions. In conditions of renal disease, available data would suggest that there is a shift from inhibitory to excitatory renorenal reflexes possibly due to activation of renal chemosensitive nerves, one activator being adenosine. The excitatory renorenal reflexes would contribute to the increased ERSNA, leading to increased sodium retention and arterial pressure prevalent in these pathophysiological conditions.
Reinnervation of Renal Sympathetic and Renal Sensory Nerves Following Denervation
Although it has long been known that ERSNA is increased in hypertensive patients (28) and renal denervation reduces arterial pressure in various experimental models of hypertension (24), it was not thought that removing or decreasing renal sympathetic nerve activity would result in a long-lasting reduction in arterial blood pressure because of the considerable evidence for sympathetic nerve reinnervation of renal tissue following renal denervation.
An early study in a limited number of conscious dogs showed that electrical stimulation of the peripheral renal nerves to a kidney autotransplanted to the neck resulted in marked reductions in urine flow rate 4–6 mo post-transplantation, suggesting functional renal reinnervation (100). Further evidence for renal reinnervation of canine autografts is derived from histological studies showing some reinnervation at 2–3 mo with more complete reinnervation observed 4–6 mo after transplantation (19, 128). Likewise, measurements of renal cortical NE levels and plasma renin activity (PRA) in dogs on a low-sodium diet showed a gradual return of both NE and PRA toward predenervation levels 4 mo after renal denervation (113). Although the majority of the studies in dogs suggest renal reinnervation following renal transplantation and renal denervation, there are immunohistochemical studies in dogs that show only partial or no renal reinnervation 3–12 mo after renal denervation (1, 117). On the other hand, studies in normotensive rats have shown partial return of renal cortical NE content and renal vasoconstrictor responses to electrical renal nerve stimulation 4 wk following renal denervation (60) with a complete restoration of the renal vasoconstrictor responses at 8–9 wk after renal denervation. Similar findings have been demonstrated in hypertensive rats. Renal denervation delayed the onset of hypertension in both SHR and DOCA-salt hypertensive rats in association with marked decreases in renal tissue NE content. Arterial pressure increased gradually and was similar to that in sham-denervated hypertensive rats 6–7 wk following renal denervation at which time renal tissue NE content in the denervated kidneys was 60–80% of that in the sham-operated rats (55, 140). There are few studies reported in humans. However, there is anatomical evidence for gradual reinnervation of the human kidney starting 4 wk after renal transplantation with extensive reinnervation at 8 mo (35). Thus, available data in various species suggest that the sympathetic nerves reinnervate renal tissue following denervation, with differences among the species in the time required for sympathetic reinnervation being weeks in rats, months in dogs, and months to year(s) in humans.
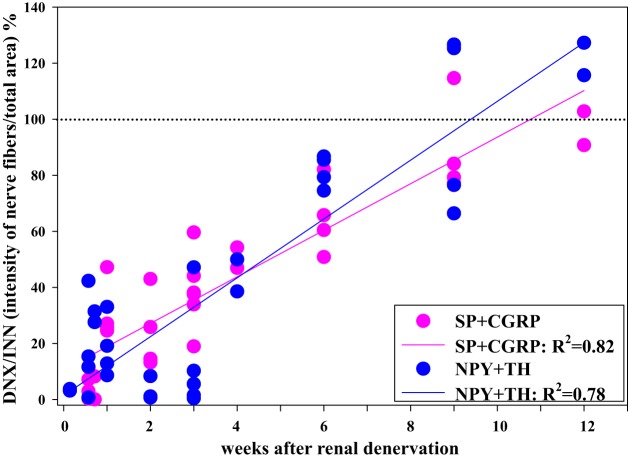
Because of the considerable evidence for renal sympathetic reinnervation (see above), one of the possible mechanisms suggested to contribute to the long-term reduction in arterial pressure in treatment-resistant hypertensive patients following bilateral renal denervation is interruption of excitatory reflexes originating in the kidney, as suggested by the long-term reduction in muscle sympathetic nerve activity (44). This hypothesis is based on the assumption that the afferent renal nerves would not reinnervate renal tissue, at least not to the same extent as the sympathetic nerves. Although there is currently a shortage of data in humans concerning the issue of sensory nerve reinnervation following renal denervation, studies in rats comparing the reinnervation of the sympathetic nerves and sensory nerves following surgical denervation would not support such a hypothesis. Immunohistochemistry studies examined the density of labeled nerve fibers with antibodies against tyrosine hydroxylase (TH) and NPY (markers of sympathetic nerves) and substance P and CGRP (markers of sensory nerves) in the denervated and contralateral innervated kidney at various time points after ipsilateral renal denervation. The findings showed that the sympathetic and sensory nerves reinnervate renal tissue along a similar time axis with complete reinnervation of both types of nerves at 9–12 wk following renal denervation (114, Fig. 14). Although previous studies have shown normal functional responses to activation of the renal sympathetic nerves at 8–9 wk after renal denervation (60), it is currently unknown whether the functional responses to activation of the afferent renal nerves are restored toward normal at this time point in the rat. Of importance in this context is a recent study in sheep. This study presents both anatomical and functional evidence for reinnervation of the efferent and afferent renal nerves 5.5 and 11 mo following catheter-based renal denervation using the Simplicity Flex catheter (9).
Fig. 14.
Adjacent slides of rat renal tissue from the denervated and contralateral innervated kidneys were labeled with antibodies against tyrosine hydroxylase (TH) and neuropeptide Y (NPY) and SP and CGRP. The optical density of the nerve fibers in renal tissue was determined by ImageJ software. The optical density of sympathetic nerve fibers containing NPY and TH (blue symbols) and sensory nerves containing SP and CGRP (pink symbols) in the denervated kidney was compared with those in the contralateral innervated kidney in each rat at various time points following unilateral renal denervation. The data derived from the optical density curves of the NPY, TH, CGRP, and SP-containing fibers suggest that the sensory nerves and sympathetic nerves reinnervate renal pelvic and peripelvic cortical area with a similar time course in normal healthy rats. [Data are derived from Ref. 114].
Perspectives and Significance
There is now considerable evidence for both efferent and afferent renal nerves contributing to the maintenance of water and sodium homeostasis. Increases in ERSNA decrease renal blood flow and increase sodium retention and renin secretion. Increases in ERSNA also increase ARNA, which, in turn, decreases ERSNA by a negative feedback mechanism in the overall goal of maintaining low ERSNA to minimize sodium retention. The interaction between ERSNA and ARNA in the control of sodium balance is especially important during various dietary sodium intakes. During high-sodium dietary conditions, the inhibitory renorenal reflex control of ERSNA is enhanced. Conversely, during low-sodium dietary conditions, the renorenal reflex control of ERSNA is reduced. It is important to note that these are physiologically appropriate responses to maintain sodium balance during various dietary sodium conditions. Increased activation of the renin angiotensin system contributes importantly to the reduced responsiveness of the afferent renal nerves in low-sodium dietary conditions.
In pathological conditions characterized by increased ANG II activity—including hypertension, heart failure, diabetes and renal ischemia—the inhibitory renorenal reflexes are suppressed. It is likely that the absence of an inhibitory renorenal reflex control of ERSNA contributes to the inappropriately increased activity of the sympathetic nervous system in these pathological conditions. When the inhibitory renorenal reflexes are suppressed, there is evidence for a prevalence of excitatory reflexes originating from the kidney in various pathological conditions involving renal injury, including hypertension, heart failure, chronic renal failure, diabetes, and obesity. Renal inflammation, prevalent in many of these pathological conditions, would lead to activation of renal sensory nerves, presumably chemosensitive nerve fibers, resulting in increases in sympathetic nerve activity leading to a vicious cycle in which increased ERSNA will result in further aggravation of the renal injuries/damage, resulting in further increases in ARNA. Supporting this hypothesis are the numerous studies in various species with experimental hypertension and in patients with treatment-resistant hypertension in which renal denervation reduced arterial pressure. Importantly, the reduction in arterial pressure following bilateral renal denervation was associated with a reduction in muscle sympathetic nerve activity, suggesting an important role for excitatory reflexes originating in the diseased or injured kidney.
Removal of both renal sympathetic and renal sensory nerves most likely contributes to the arterial pressure reduction in patients following renal denervation, at least initially. Although available data suggest that renal reinnervation will take months to year(s) in humans versus weeks in rats, the finding that the renal sensory nerves reinnervate the renal tissue in a similar time-dependent fashion as the sympathetic nerves following renal denervation in normal healthy rats suggests that additional mechanisms, possibly related to the initial removal of the renal sympathetic/sensory nerves, are likely to contribute to the long-term arterial pressure reduction observed in patients with drug-resistant hypertension following renal denervation.
GRANTS
The work performed in the author's laboratory was supported by the National Institutes of Health, Heart, Lung and Blood Institute (Grant RO1 HL-66068) and by research grants from the Department of Veterans Affairs and the American Heart Association, Heartland Affiliate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: U.C.K. conception and design of research; U.C.K. analyzed data; U.C.K. interpreted results of experiments; U.C.K. prepared figures; U.C.K. drafted manuscript; U.C.K. edited and revised manuscript; U.C.K. approved final version of manuscript.
REFERENCES
- 1.Almgård LE, Ljungqvist A, Ungerstedt U. The reaction of the intrarenal sympathetic nervous system to renal transplantion. Scand J Urol Nephrol 5: 65–70, 1971. [DOI] [PubMed] [Google Scholar]
- 2.Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol 70: 735–749, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Barajas L, Powers K. Innervation of the renal proximal convoluted tubule of the rat. Am J Anat 186: 378–388, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Barajas L, Powers K. Monoaminergic innervation of the rat kidney: a quantitative study. Am J Physiol Renal Fluid Electrolyte Physiol 259: F503–F511, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. Am J Physiol Renal Fluid Electrolyte Physiol 247: F50–F60, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Barajas L, Wang P. Localization of [3H]norepinephrine in the renal arteriolar nerves. Anat Rec 195: 525–34, 1979. [DOI] [PubMed] [Google Scholar]
- 7.Barajas L, Wang P. Myelinated nerves of the rat kidney. A light and electron microscopic autoradiographic study. J Ultrastruct Res 65: 148–162, 1978. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, David Kandzari DE E, O'Neill WW, D'Agostino R, Flackn JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370: 1393–1401, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN. Reinnervation of renal afferent and efferent nerves at 5 ½ and 11 months after catheter-based radio-frequency renal denervation in sheep. Hypertension HYPERTENSIONAHA.114.04176. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension 60: 1485–1490, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Böhm M, Linz D, Ukena C, Esler M, Mahfoud F. Renal denervation for the treatment of cardiovascular high-risk hypertension or beyond? Circ Res 115: 400–409, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst 3: 311–320, 1981. [DOI] [PubMed] [Google Scholar]
- 13.Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis 26: 861–865, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Chapleau MW, Hajduczok G, Abboud FM. Suppression of baroreceptor discharge by endothelin at high carotid sinus pressure. Am J Physiol Regul Integr Comp Physiol 263: R103–R108, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Chien CT, Fu TC, Wu MS, Chen CF. Attenuated response of renal mechanoreceptors to volume expansion in chronically hypoxic rats. Am J Physiol Renal Physiol 273: F712–F717, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat an antegrade transport study of horseradish peroxidase. J Auton Nerv Syst 8: 273–285, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Colindres RE, Spielman WS, Moss NG, Harrington WW, Gottschalk CW. Functional evidence for renorenal reflexes in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 239: F265–F270, 1980. [DOI] [PubMed] [Google Scholar]
- 18.Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Couch NP, McBride RA, Dammin GJ, Murray JE. Observations on the nature of the enlargement, the regeneration of the nerves and the function of the canine renal autograft. Br J Exp Pathol 42: 106–113, 1961. [PMC free article] [PubMed] [Google Scholar]
- 20.Da Cunha JM, Rae GA, Ferreira SH, Cunha de QF. Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase C. Eur J Pharmacol 501: 87–94, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in man safety evaluation of renal denervation for chronic systolic heart failure: Primary outcome from REACH-Pilot study. Int J Cardiol 162: 189–192, 2013. [DOI] [PubMed] [Google Scholar]
- 22.DiBona GF, Jones SY, Kopp UC. Renal mechanoreceptor dysfunction: an intermediate phenotype in spontaneously hypertensive rats. Hypertension 33: 472–475, 1999. [DOI] [PubMed] [Google Scholar]
- 23.DiBona GF, Jones SY, Sawin LL. Effect of endogenous angiotensin II on renal nerve activity and its arterial baroreflex regulation. Am J Physiol Regul Integr Comp Physiol 271: R361–R367, 1996. [DOI] [PubMed] [Google Scholar]
- 24.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. [DOI] [PubMed] [Google Scholar]
- 25.DiBona GF, Rios LL. Renal nerves in compensatory renal response to contralateral renal denervation. Am J Physiol Renal Fluid Electrolyte Physiol 238: F26–F30, 1980. [DOI] [PubMed] [Google Scholar]
- 26.Ditting T, Linz P, Freisinger W, Heinlein S, Reeh PW, Fiedler C, Siegel K, Scrogin KE, Neuhuber W, Veelken R. Norepinephrine reduces ω-conotoxin-sensitive Ca2+ currents in renal afferent neurons in rats. Am J Physiol Renal Physiol 302: F350–F357, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Donovan MK, Wyss JM, Winternitz SR. Localization of renal sensory neurons using the fluorescent dye technique. Brain Res 259: 119–122, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11: 3–20, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet 376: 1903–1909, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Felder RB. Excitatory and inhibitory interactions among renal and cardiovascular afferent nerves in dorsomedial medulla. Am J Physiol Regul Integr Comp Physiol 250: R580–R588, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Felder RB, Heesch CM, Thames MD. Reflex modulation of carotid sinus baroreceptor activity in the dog. Am J Physiol Heart Circ Physiol 244: H437–H443, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson M, Bell C. Substance P-immunoreactive nerves in the rat kidney. Neurosci Lett 60: 183–188, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson M, Bell C. Ultrastructural localization and characterization of sensory nerves in the rat kidney. J Comp Neurol 274: 9–16, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Gariepy CE, Ohuchi T, Williams C, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazdar AF, Dammin GJ. Neural degeneration and regeneration in human renal transplants. N Engl J Med 283: 222–224, 1970. [DOI] [PubMed] [Google Scholar]
- 36.Genovesi S, Pieruzzi F, Wijnmaalen P, Centonza L, Golin R, Zanchetti A, Stella A. Renal afferents signaling diuretic activity in the cat. Circ Res 73: 906–913, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Geppetti P, Baldi E, Castellucci A, Del Bianco E, Santiciolij P, Maggi CA, Lippe IT, Amann R, Skofitsch G, Theodorsson E, Manzini S. Calcitonin gene-related peptide in the rat kidney: occurrence, sensitivity to capsaicin, and stimulation of adenylate cyclase. Neuroscience 30: 503–513, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Giamouzis G, Butler J, Triposkiadis F. Renal function in advanced heart failure. Congest Heart Fail 17: 180–188, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation 106: 1974–1979, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Healy DP, Ye MQ, Troyanovskaya M. Localization of angiotensin II type 1 receptor subtype mRNA in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F220–F226, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res 326: 541–551, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Hemsen A, Lundberg JM. Presence of endothelin-1 and endothelin-3 in peripheral tissues and central nervous system of pig. Regul Pept 36: 71–83, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Henegar JR, Zhang Y, Rama RD, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens 10: 1285–1292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 64: 118–124, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Hintgen CM, Vasko MR. Prostacyclin enhances the evoked-release of substance P and calcitonin gene-related peptide from rat sensory neurons. Brain Res 655: 51–60, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Hori S, Komatsu Y, Shigemoto R, Mizuno N, Nakanishi S. Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology 130: 1885–1895, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Li Y, Cheng W, Yang Z, Wang F, Lv P, Niu C, Hou Y, Yan Y, Ge J. A comparison of the efficacy of surgical renal denervation and pharmacological therapies in post-myocardial infarction heart failure. PLos One 7: e96996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol 71: 683–698, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh S, van den Buuse M. Sensitization of baroreflex by central endothelin in conscious rats. Am J Physiol Heart Circ Physiol 260: H1106–H1112, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Jänig W, Levine JD, Michaelis M. Interaction of sympathetic and primary afferent neurons following nerve injury and tissue trauma. In: Progress in Brain Research, edited by Kumasawa T, Kruger L, Mizumura K. New York: Elsevier Science, 1996; vol 113, p. 162–184. [DOI] [PubMed] [Google Scholar]
- 51.Johns EJ, DiBona GF, Kopp UC. Neural control of renal function. Compr Physiol 1: 731–767, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Johns EJ, Kopp UC. Neural control of renal function. In: Seldin and Giebisch's The Kidney: Physiology and Pathophysiology, 5th ed., edited by Alpern RJ, Moe OW, and Caplan M. San Diego, CA: Academic, 2013, p. 451–486. [Google Scholar]
- 53.Katholi RE, Hageman GR, Whitlow PL, Woods WT. Hemodynamic and afferent renal nerve responses to intrarenal adenosine in the dog. Hypertension 5 Suppl I: I-149–I-154, 1983. [DOI] [PubMed] [Google Scholar]
- 54.Katholi RE, McCann WP, Woods WT. Intrarenal adenosine produces hypertension via renal nerves in the one-kidney, one-clip rat. Hypertension 7: I88–I93, 1985. [DOI] [PubMed] [Google Scholar]
- 55.Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 2: 266–273, 1980. [DOI] [PubMed] [Google Scholar]
- 56.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens 2: 349–359, 1984. [PubMed] [Google Scholar]
- 57.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one-clip Goldblatt hypertension. Hypertension 4 Suppl II: II-166–II-174, 1982. [PubMed] [Google Scholar]
- 58.Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci 22: 7788–7796, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klass M, Hord A, Wilcox M, Denson D, Csete M. A role for endothelin in neuropathic pain after constriction injury of the sciatic nerve. Anesth Analg 101: 1757–1762, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Kline RL, Mercer PF. Functional reinnervation and development of supersensitivity to NE after denervation. Am J Physiol Regul Integr Comp Physiol 238: R353–R358, 1980. [DOI] [PubMed] [Google Scholar]
- 61.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Kopp UC. Neural control of renal function. In: Colloquium Series in Integrated Systems Physiology: From Molecule to Function, edited by Granger DN and Granger J. San Rafael, CA: Morgan & Claypool Life Sciences, 2011, p. 1–98. [PubMed] [Google Scholar]
- 63.Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one-clip hypertensive rats. Hypertension 14: 445–452, 1989. [DOI] [PubMed] [Google Scholar]
- 64.Kopp UC, Cicha MZ. Impaired substance P release from renal sensory nerves in SHR involves a pertussis toxin-sensitive mechanism. Am J Physiol Regul Integr Comp Physiol 286: R326–R333, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Kopp UC, Cicha MZ. PGE2 increases substance P release from pelvis sensory nerves via activation of N-type calcium channels. Am J Physiol Regul Integr Comp Physiol 276: R1241–R1248, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Kopp UC, Cicha MZ, Farley DM, Smith LA, Dixon BS. Renal substance P-containing neurons and substance P receptors impaired in hypertension. Hypertension 31: 815–822, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Kopp UC, Cicha MZ, Jones SY. Activation of endothelin A receptors contributes to impaired responsiveness of renal mechanosensory nerves in congestive heart failure. Can J Physiol Pharmacol 88: 622–629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kopp UC, Cicha MZ, Nakamura K, Nüsing RM, Smith LA, Hökfelt T. Activation of EP4 receptors contributes to prostaglandin E2-mediated stimulation of renal sensory nerves. Am J Physiol Renal Physiol 287: F1269–F1282, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Kopp UC, Cicha MZ, Smith LA. Activation of endothelin-A receptors contributes to angiotensin-induced suppression of renal sensory nerve activation. Hypertension 49: 141–147, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Kopp UC, Cicha MZ, Smith LA. Angiotensin blocks substance P release from renal sensory nerves by inhibiting PGE2-mediated activation of cAMP. Am J Physiol Renal Physiol 285: F472–F483, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 42: 968–973, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Kopp UC, Cicha Smith LA MZ. Differential effects of endothelin on the activation of renal mechanosensory nerves: stimulatory in high and inhibitory in low-sodium diet. Am J Physiol Regul Integr Comp Physiol 291: R1545–R1556, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Kopp UC, Cicha MZ, Smith LA. Endogenous angiotensin modulates PGE2-mediated release of substance P from renal mechanosensory nerve fibers. Am J Physiol Regul Integr Comp Physiol 282: R19–R30, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Kopp UC, Cicha MZ, Smith LA. Impaired interaction between efferent and afferent renal nerve activity in SHR involves increased activation of α2-adrenoceptors. Hypertension 57: 640–647, 2011. [DOI] [PubMed] [Google Scholar]
- 75.Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: role of endogenous angiotensin. Am J Physiol Regul Integr Comp Physiol 284: R116–R124, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Kopp UC, Cicha MZ, Smith LA. PGE2 increases release of substance P from renal sensory nerves by activating the cAMP-PKA transduction cascade. Am J Physiol Regul Integr Comp Physiol 282: R1618–R1627, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Kopp UC, Cicha MZ, Smith LA, Hökfelt T. Nitric oxide modulates renal sensory nerve fibers by mechanisms related to substance P receptor activation. Am J Physiol Regul Integr Comp Physiol 281: R279–R290, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Kopp UC, Cicha MZ, Smith LA, Mulder J, Hökfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of α1- and α2-adrenoceptors on renal sensory nerve fibers. Am J Physiol Regul Integr Comp Physiol 293: R1561–R1572, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Kopp UC, Cicha MZ, Smith LA, Ruohonen S, Scheinin M, Fritz Hökfelt T. Dietary sodium modulates the interaction between efferent and afferent renal nerve activity by altering activation of α2-adrenoceptors on renal sensory nerves. Am J Physiol Regul Integr Comp Physiol 300: R298–R310, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopp UC, Cicha MZ, Yorek MA. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: role of angiotensin. Am J Physiol Regul Integr Comp Physiol 294: R858–R866, 2008. [DOI] [PubMed] [Google Scholar]
- 81.Kopp UC, Farley DM, Cicha MZ, Smith LA. Activation of renal mechanosensitive neurons involves bradykinin, protein kinase C, PGE2, and substance P. Am J Physiol Regul Integr Comp Physiol 278: R937–R946, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Kopp UC, Farley DM, Smith LA. Bradykinin-mediated activation of renal sensory neurons due to prostaglandin-dependent release of substance P. Am J Physiol Regul Integr Comp Physiol 272: R2009–R2016, 1997. [DOI] [PubMed] [Google Scholar]
- 83.Kopp UC, Farley DM, Smith LA. Renal sensory receptor activation causes prostaglandin-dependent release of substance P. Am J Physiol Regul Integr Comp Physiol 270: R720–R727, 1996. [DOI] [PubMed] [Google Scholar]
- 84.Kopp UC, Grisk O, Cicha MZ, Smith LA, Steinbach A, Schlüter T, Mähler N, Hökfelt T. Dietary sodium modulates the interaction between efferent renal sympathetic nerve activity and afferent renal nerve activity: role of endothelin. Am J Physiol Regul Integr Comp Physiol 297: R337–R351, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kopp UC, Jones SY, DiBona GF. Afferent renal denervation impairs baroreflex control of efferent renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 295: R1882–R1890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kopp UC, Matsushita K, Sigmund RD, Smith LA, Watanabe S, Stokes JB. Amiloride-sensitive Na+ channels in pelvic uroepithelium involved in renal sensory receptor activation. Am J Physiol Regul Integr Comp Physiol 275: R1780–R17 92, 1998. [DOI] [PubMed] [Google Scholar]
- 87.Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol Renal Fluid Electrolyte Physiol 246: F67–F77, 1984. [DOI] [PubMed] [Google Scholar]
- 88.Kopp UC, Smith LA. Bradykinin and protein kinase C activation fail to stimulate renal sensory neurons in hypertensive rats. Hypertension 27: 607–612, 1996. [DOI] [PubMed] [Google Scholar]
- 89.Kopp UC, Smith LA. Effects of the substance P receptor antagonist CP-96,345 on renal sensory receptor activation. Am J Physiol Regul Integr Comp Physiol 264: R647–R653, 1993. [DOI] [PubMed] [Google Scholar]
- 90.Kopp UC, Smith LA. Inhibitory renorenal reflexes: a role for substance P or other capsaicin-sensitive neurons. Am J Physiol Regul Integr Comp Physiol 260: R232–R239, 1991. [DOI] [PubMed] [Google Scholar]
- 91.Kopp UC, Smith LA. Role of prostaglandins in renal sensory receptor activation by substance P and bradykinin. Am J Physiol Regul Integr Comp Physiol 265: R544–R551, 1993. [DOI] [PubMed] [Google Scholar]
- 92.Kopp UC, Smith LA, DiBona GF. Impaired renorenal reflexes in spontaneously hypertensive rats. Hypertension 9: 69–75, 1987. [DOI] [PubMed] [Google Scholar]
- 93.Kopp UC, Smith LA, DiBona GF. Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am J Physiol Renal Fluid Electrolyte Physiol 249: F507–F517, 1985. [DOI] [PubMed] [Google Scholar]
- 94.Kopp UK, Smith LA, Pence AL. Na+-K+-ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol Regul Integr Comp Physiol 267: R1109–R1117, 1994. [DOI] [PubMed] [Google Scholar]
- 95.Krum H, Schlaich M, Sobotka P. Renal sympathetic nerve ablation for treatment-resistant hypertension. Br J Clin Pharmacol 76: 495–503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383: 622–629, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Kunze DL, Krauhs JM, Orlea CJ. Direct action of norepinephrine on baroreceptors of rat adventitia. Am J Physiol Heart Circ Physiol 247: H811–H816, 1984. [DOI] [PubMed] [Google Scholar]
- 98.Kurtz A, Muff R, Born W, Lundberg JM, Millberg BI, Gnidinger MP, Uehlinger DE, Weidmann P, Hökfelt T, Fischert JA. Calcitonin gene-related peptide is a stimulator of renin secretion. J Clin Invest 82: 538–543, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laplante MA, Wu R, Moreau P, de Champlain J. Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic Biol Med 38: 589–596, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Lebedev AA. Efferent nerves of the transplanted reinnervated kidney. Bull Exp Biol Med 51: 396–399, 1961. [Google Scholar]
- 101.Li DP, Fan MZ, He RR. Effects of endothelin on carotid baroreceptor activity in anesthetized rats. Sheng Li Xu Bao 50: 532–538, 1998. [PubMed] [Google Scholar]
- 102.Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radical contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res 79: 802–811, 1996. [DOI] [PubMed] [Google Scholar]
- 103.Linz D, Hohl M, Schütze J, Mahfoud F, Speer T, Linz B, Hübschle T, Juretschke HP, Dechend R, Geisel J, Rütten H, Böhm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: The role of renal sympathetic innervation. Am J Hypertension In press. [DOI] [PubMed] [Google Scholar]
- 104.Liu L, Barajas L. The rat renal nerves during development. Anat Embryol (Berl) 188: 345–361, 1993. [DOI] [PubMed] [Google Scholar]
- 105.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther 315: 1058–1064, 2005. [DOI] [PubMed] [Google Scholar]
- 106.Ma HJ, Ma HJ, Liu YX, Wang QS. Injection of adenosine into the renal artery activates spontaneous activity of renal afferent nerve fibers. Sheng Li Xue Bao 56: 192–197, 2004. [PubMed] [Google Scholar]
- 107.Ma MC, Huang HS, Chen CF. Impaired renal sensory responses after unilateral ureteral obstruction in the rat. J Am Soc Nephrol 13: 1008–1016, 2002. [DOI] [PubMed] [Google Scholar]
- 108.Ma MC, Huang HS, Chien CT, Wu MS, Chen CF. Temporal decrease in renal sensory responses in rats after chronic ligation of the bile duct. Am J Physiol Renal Physiol 283: F164–F172, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Ma MC, Huang HS, Wu MS, Chien CT, Chen CF. Impaired renal sensory responses after renal ischemia in the rat. J Am Soc Nephrol 13: 1872–1883, 2002. [DOI] [PubMed] [Google Scholar]
- 110.Malpas SC. Sympathetic nervous system overactivity and its role in the develpoment of cardiovascular disease. Physiol Rev 90: 513–557, 2009. [DOI] [PubMed] [Google Scholar]
- 111.Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol 311: 389–404, 1991. [DOI] [PubMed] [Google Scholar]
- 112.Miyazaki Y, Tsuchida S, Nishimura H, Pope JC, Harris RC, McKanna JM, Inagami T, Hogan BLM, Fogo A, Ichikawa I. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J Clin Invest 102: 1489–1497, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mogil RA, Itskovitz HD, Russell JH, Murphy JJ. Renal innervation and renin activity in salt metabolism and hypertension. Am J Physiol 216: 693–697, 1969. [DOI] [PubMed] [Google Scholar]
- 114.Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 304: R675–R682, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicol GD, Cui M. Enhancement of prostaglandin E2 of bradykinin activation of embryonic rat sensory neurones. J Physiol 480: 485–492, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Niijima A. The effect of efferent discharges in renal nerves on the activity of arterial mechanoreceptors in the kidney in rabbit. J Physiol 222: 335–343, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nomura G, Kurosaki M, Takabatake T, Kibe Y, Takeuchi J. Reinnervation and renin release after unilateral renal denervation in the dog. J Appl Physiol 33: 649–655, 1972. [DOI] [PubMed] [Google Scholar]
- 118.Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, Ewen S, Ukena C, Uder M, Böhm M, Schmieder RE. Improvement of albuminuria after renal denervation. Int J Cardiol 173: 311–315, 2014. [DOI] [PubMed] [Google Scholar]
- 119.Ottoson D, Shepherd GM. Transducer characteristics of the muscle spindle as revealed by its receptor potential. Acta Physiol Scand 82: 545–554, 1971. [DOI] [PubMed] [Google Scholar]
- 120.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Recordati G, Moss NG, Genovesi S, Rogenes P. Renal chemoreceptors. J Auton Nerv Syst 3: 237–251, 1981. [DOI] [PubMed] [Google Scholar]
- 122.Rogenes PR. Single-unit and multiunit analyses of renorenal reflexes elicited by stimulation of renal chemoreceptors in the rat. J Auton Nerv Syst 6: 143–156, 1982. [DOI] [PubMed] [Google Scholar]
- 123.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology and pathophysiology. Pharmacol Rev 46: 325–415, 1994. [PubMed] [Google Scholar]
- 124.Saeki Y, Terui N, Kumada M. Participation of ventrolateral medullary neurons in the renal-sympathetic reflex in rabbits. Jpn J Physiol 38: 267–281, 1988. [DOI] [PubMed] [Google Scholar]
- 125.Sanchez A, Pettinger WA. Dietary sodium regulation of blood pressure and renal α1 and α2-receptors in WKY and SH rats. Life Sci 29: 2795–2802, 1981. [DOI] [PubMed] [Google Scholar]
- 126.Schiller AM, Haack KK, Pellegrino PR, Curry PL, Zucker IH. Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 305: R886–R892, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 20: 933–939, 2009. [DOI] [PubMed] [Google Scholar]
- 128.Shvalev VN, Shioshvili TI. Effect of reinnervation on the state of transplanted kidneys in dogs. Arkh Anat Gistol Embriol 74: 42–50, 1978. [PubMed] [Google Scholar]
- 129.Smits JF, Brody MJ. Activation of afferent renal nerves by intrarenal bradykinin in conscious rats. Am J Physiol Regul Integr Comp Physiol 247: R1003–R1008, 1984. [DOI] [PubMed] [Google Scholar]
- 130.Solano-Flores LP, Rosa-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal stimulation. Brain Res 753: 102–119, 1997. [DOI] [PubMed] [Google Scholar]
- 131.Su HC, Wharton J, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, Morrison JFB, Ballizsta J, Brooru SR. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience 18: 721–747, 1986. [DOI] [PubMed] [Google Scholar]
- 132.Vasko MR, Campbell WB, Waite KJ. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from the rat sensory neurons in culture. J Neurosci 14: 4987–4997, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Veelken R, Hilgers KF, Scrogin KE, Mann JFE, Schmieder RE. Endogenous angiotensin II and the reflex response to stimulation of cardiopulmonary serotonin 5-HT3 receptors. Br J Pharmacol 125: 1761–1767, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Verkhratsky A, Orkand RA, Kettenmann H. Glial calcium: homeostasis and signalling function. Physiol Rev 78: 99–141, 1998. [DOI] [PubMed] [Google Scholar]
- 135.Wang DH, Li JP, Qiu JX. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension 32: 649–653, 1998. [DOI] [PubMed] [Google Scholar]
- 136.Wang X, Zhao Q, Deng H, Wang X, Guo Z, Dai J, Xiao J, Wan P, Huang C. Effects of renal sympathetic denervation on atrial electrophysiology in dogs with pacing-induced heart failure. Pacing Clin Electrophysiol 37: 1357–1366, 2014. [DOI] [PubMed] [Google Scholar]
- 137.Weiss ML, Chowdhury SI. The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res 812: 227–241, 1998. [DOI] [PubMed] [Google Scholar]
- 138.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 85: 1159–1204, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Wilkes BM, Susin M, Mento PF, Macica CM, Girardi EP, Boss E, Nord EP. Localization of endothelin-like immunoreactivity in rat kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 260: F913–F920, 1991. [DOI] [PubMed] [Google Scholar]
- 140.Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest 66: 971–978, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wyss JM, Donovan K. A direct projection from the kidney to the brainstem. Brain Res 298: 130–134, 1984. [DOI] [PubMed] [Google Scholar]
- 142.Xie C, Sachs JR, Wang DH. Interdependent regulation of afferent renal nerve activity and renal function: role of transient receptor potential vanilloid type 1, neurokinin 1, and calcitonin gene-related peptide receptors. J Pharmacol Exp Ther 325: 751–757, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ye DZ, Wang DH. Function and regulation of endothelin-1 and its receptors in salt sensitive hypertension induced by sensory nerve degeneration. Hypertension 39: 673–678, 2002. [DOI] [PubMed] [Google Scholar]
- 144.Zhuo JL. Renomedullary interstitial cells: a target for endocrine and paracrine actions of vasoactive peptides in the renal medulla. Clin Exp Pharmacol Physiol 27: 465–473, 2000. [DOI] [PubMed] [Google Scholar]