Abstract
The development of the kidney arterioles is poorly understood. Mature arterioles contain several functionally and morphologically distinct cell types, including smooth muscle, endothelial, and juxtaglomerular cells, and they are surrounded by interconnected pericytes, fibroblasts, and other interstitial cells. We have shown that the embryonic kidney possesses all of the necessary precursors for the development of the renal arterial tree, and those precursors assemble in situ to form the kidney arterioles. However, the identity of those precursors was unclear. Within the embryonic kidney, several putative progenitors marked by the expression of either the winged-forkhead transcription factor 1 (Foxd1+ progenitor), the aspartyl-protease renin (Ren+ progenitor), and/or hemangioblasts (Scl+ progenitor) are likely to differentiate and endow most of the cells of the renal arterial tree. However, the lineage relationships and the role of these distinct progenitors in renal vascular morphogenesis have not been delineated. We, therefore, designed a series of experiments to ascertain the hierarchical lineage relationships between Foxd1+ and Ren+ progenitors and the role of these two precursors in the morphogenesis and patterning of the renal arterial tree. Results show that 1) Foxd1+ cells are the precursors for all the mural cells (renin cells, smooth muscle cells, perivascular fibroblasts, and pericytes) of the renal arterial tree and glomerular mesangium, and 2) Foxd1 per se directs the origin, number, orientation, and cellular composition of the renal vessels.
the kidney is a highly vascularized organ, which, in the adult animal, receives ∼20% of the cardiac output (21). Development of a functional kidney requires that each nephron be properly vascularized. Blood enters each glomerulus from an afferent arteriole and leaves it via an efferent arteriole. The spatial arrangement of the preglomerular and postglomerular vessels within each nephron is crucial for the regulation of renal blood flow, arterial blood pressure, glomerular filtration rate, urine concentration, acid-base balance, disposal of waste products, and other kidney functions that maintain the constancy of our internal milieu and whole body homeostasis. Therefore, proper assembly of the arterioles with their corresponding nephrons is a fundamental morphogenetic event that leads to the formation of a functioning kidney. The mechanisms involved in the development of the kidney vasculature are not well understood, but they are intimately linked to nephrogenesis, the formation of the epithelial nephron. Nephrogenesis of the definitive kidney in mammals occurs by the reciprocal inductive interaction of two mesoderm-derived structures: the ureteric bud, an outgrowth of the Wolfian duct, and the metanephric mesenchyme, a condensation of the paraxial, intermediate mesoderm. In mice, nephrogenesis begins around embryonic day (E) 11.5. The ureteric bud undergoes branching morphogenesis, and around each ureteric tip, it induces mesenchymal cells to arrange themselves into two distinct cell compartments (Fig. 1): 1) the cap mesenchyme, an inner, condensed cell population, which expresses Six2 and Cited1 and is in close contact with the ureteric bud. These cells undergo mesenchymal to epithelial transformation and generate most of the epithelial nephron, including glomerular epithelium, proximal tubules, and loops of Henle, distal and connecting tubules (whereas the ureteric bud, in turn, differentiates into the collecting ducts and ureter); and 2) an outer layer of loosely arranged mesenchymal cells, which express the transcription factor Foxd1 (stromal cells) (8) or cKit (endothelial precursors) (19). Within the loose mesenchyme compartment, we have previously identified renin precursors (24). These cells are strategically located to provide the necessary precursors for the formation of the kidney vasculature. However, the development of the kidney vasculature is poorly understood. Previous studies from our laboratory using immunostaining, in situ hybridization, and/or individual cell microisolation followed by nested RT-PCR indicated that the prevascular embryonic kidney possessed all of the necessary precursors (including renin, smooth muscle, and endothelial precursors) for the development of the renal arterial tree and that those precursors have the capability of assembling in situ to form the kidney arterioles (24). We also showed that renin progenitors differentiate to juxtaglomerular cells, a subset of arteriolar smooth muscle and mesangial cells, and that they are not related to the endothelial lineage (22). Thus, the stromal compartment contains these two progenitor cells (Foxd1+ and Ren1+), which are likely to endow most of the renal arterial tree. However, the lineage relationships and the role of these two distinct progenitors in renal arteriolar morphogenesis have not been delineated. We designed a series of experiments to define 1) whether Foxd1+ stromal cells are the precursors for the cells that compose the renal arterial tree and 2) whether Foxd1 per se regulates kidney vascular morphogenesis and orientation.
Fig. 1.
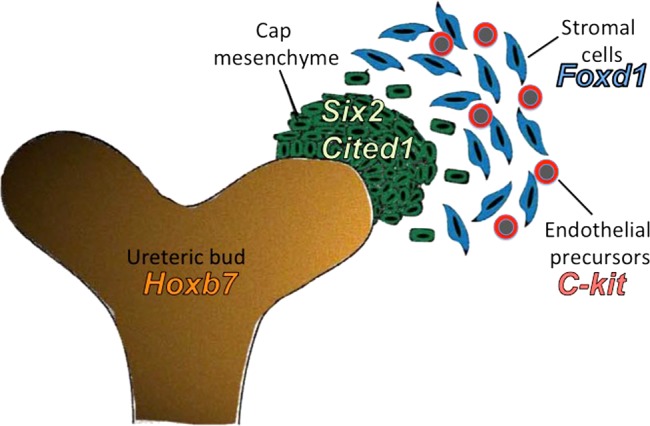
A schematic of the early metanephric kidney cells compartments is shown.
MATERIALS AND METHODS
Animals.
FoxD1-GFP-Cre mice (10) were crossed to reporter mice such as R26R-lacZ (26) and mT/mG (16) to trace the fate of Foxd1+ cells and isolate cells from the Foxd1 lineage and to B6.129-GT(ROSA)26Sortm1(DTA)Lky/J mice (27) (referred as R26RDTA) to investigate whether ablation of stromal cells would affect the development of the renal vasculature and mesangium.
All procedures were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Virginia Animal Care and Use Committee.
Genotyping.
Mice were genotyped from tail genomic DNA by standard PCR (22) performed in an Eppendorf thermocycler using Taq polymerase (Promega, Madison, WI). The primers used to detect Foxd1cre in genomic DNA are 5′ATA AGC AAT CCC CAG AAA TG (forward) and 5′AGG CGT TTT CTG AGC ATA CC (reverse), and to detect R26RDTA 5′CGA CCT GCA GGT CCT CG (forward) and 5′CTC GAG TTT GTC CAA TTA TGT CAC (reverse).
Histologic analysis and immunostaining.
Mice were anesthetized with tribromoethanol. The kidneys (and lungs) were removed, weighed, and either fixed in 2% paraformaldehyde (PFA) for 30 min for frozen sections or in Bouin's fixative overnight for paraffin sections. To evaluate β-gal expression, mouse organs fixed in 2% PFA were cryoprotected in 30% sucrose in PBS and then frozen in OCT (Miles, Elkhart, IL). Cryosections (7 μm) were cut using a Leica Cryocut 1800 cryostat, postfixed in 0.2% PFA in 0.1 M PIPES (pH 6.9) at 4°C for 10 min, washed in PBS plus 2 mM MgCl2, incubated in detergent rinse [0.1 M phosphate buffer (pH 7.4), containing 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% tergitol NP-40] for 10 min on ice, and placed in staining solution [detergent rinse, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6 3H2O, and 1 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal; Fisher Biotech)] overnight in the dark at 37°C. The slides were postfixed in 4% PFA in PBS at 4°C for 1 h, dehydrated in graded alcohols to xylenes, and mounted with xylene-based mounting medium (Cytoseal XYL; Richard-Allen Scientific, Kalamazoo, MI). To confirm the identity of cells that express β-gal in the Foxd1cre/+;R26R and Foxd1-DTA;R26R mice, slides were postfixed in Bouin's fixative for 5 min after the X-gal staining, rinsed in PBS, and then subjected to immunostaining (see below). To evaluate green fluorescent protein (GFP) or red fluorescent protein (RFP) expression, organs were processed, as previously described (17). Bouin's fixed paraffin sections were stained with hematoxylin and eosin (6). Immunostaining was performed on 5-μm-thick paraffin sections for renin (using a 1:500 dilution of rabbit anti-mouse renin polyclonal antibody), α-SMA (1:10,000 dilution of a mouse monoclonal anti-α-SMA-specific antibody isotype Ig2a; Sigma, St. Louis, MO), LYVE1 (1:1,000 dilution of a rabbit polyclonal anti-mouse LYVE1; Abcam, Cambridge, MA) and TUJ1 (anti-β-tubulin, 1:500 dilution, gift of Dr. A. Frankfurter), using the appropriate Vectastain ABC kit (Vector Laboratories, Burlingame, CA), as previously described (6, 7, 22). Immunostaining for PECAM-1 (1:400 dilution of a goat polyclonal antibody; Santa Cruz Biotechnology, Dallas, TX) was performed on 7-μm-thick frozen sections using an Alexa Fluor 350 donkey anti-goat IgG(H+L) secondary antibody (1:500 dilution; Life Technologies, Carlsbad, CA).
Quantification of renin-positive areas, total kidney areas, and α-SMA-positive vascular areas was performed on 50× magnification images from four kidney sections per animal (2 controls and 4 Foxd1−/−) using a Leica MM AF powered by MetaMorph (version 1.5; Leica Microsystems, Wetzlar, Germany).
Cell isolation and RT-PCR.
Foxd1-lineage cells were isolated using FACS, as previously described (2). Kidneys were dissected from mice anesthetized with tribromoethanol. Kidneys were decapsulated and minced into a fine mash (<1 mm2 pieces) using a razor blade. Kidney homogenate was dissolved into a single cell suspension using an enzymatic solution of 0.1% collagenase A (Roche, Indianapolis, IN), 0.25% trypsin (Sigma, St. Louis, MO), and 0.02% DNase (Life Technologies). Cells were filtered through successive cell strainers with pore diameters of 100 and then 40 μm (Millipore, Billerica, MA). Following RBC lysis, cells were sorted into GFP+ and RFP+ populations using an Influx cell sorter (Becton Dickinson, Franklin Lakes, NJ). RNA was isolated using the RNeasy mini kit (Qiagen, Germantown, MD), with 2 μg of RNA reverse transcribed to cDNA, as previously described (20). cDNA was probed using PCR for the presence of angiopoietin receptor (Tie2, forward 5′-TGTCAATCAGGCCTGGAAATAC-3′ and reverse 5′-GAGGAGGGAGAATGTCACTAAGG-3′) and β-globin (forward 5′-CACAACCCCAGAAACAGACA-3′ and reverse 5′-CTGACAGATGCTCTCTTGGG-3′).
Renal arterial trees dissection.
Kidneys attached to the aorta were dissected from embryos at E17.5 and from newborn mice and incubated in hydrochloric acid (6 M at 42°C) for 30 min. After incubation, the kidneys were washed several times with acidified water (pH 2.5). The arterial renal vasculature (arterial “tree”) was then carefully dissected from the aorta under direct stereoscopic visualization, using a pair of 20 × 11/2″ blunt needles attached to 1-ml insulin syringes at an angle of 120° (18).
Renal arterial trees perfusion.
Newborn mice were anesthetized, and the abdominal and thoracic cavities were opened to expose the kidneys and the heart. Each mouse was perfused through the left ventricle with a 0.5% of Evans blue in glycerol at 37°C and monitored until the blue dye was trapped in the glomeruli. The kidneys with the adrenals were excised maintaining their connections with the abdominal aorta and the iliac arteries and were photographed using a Leica DFC 480 color camera attached to a Leica MZ12.5 dissecting microscope.
Morphometric measurements.
The total kidney (or lung)-to-body weight ratio is a computed value of the total weight of both kidneys (or lungs) in grams divided by the body weight in grams.
Statistical analysis.
Results are presented as means ± SE. Statistical significance between two groups was determined by Student's t-test. A P value < 0.05 was considered significant.
RESULTS
Foxd1 cells are an early precursor for mesangial, vascular smooth muscle, renin cells, and pericytes.
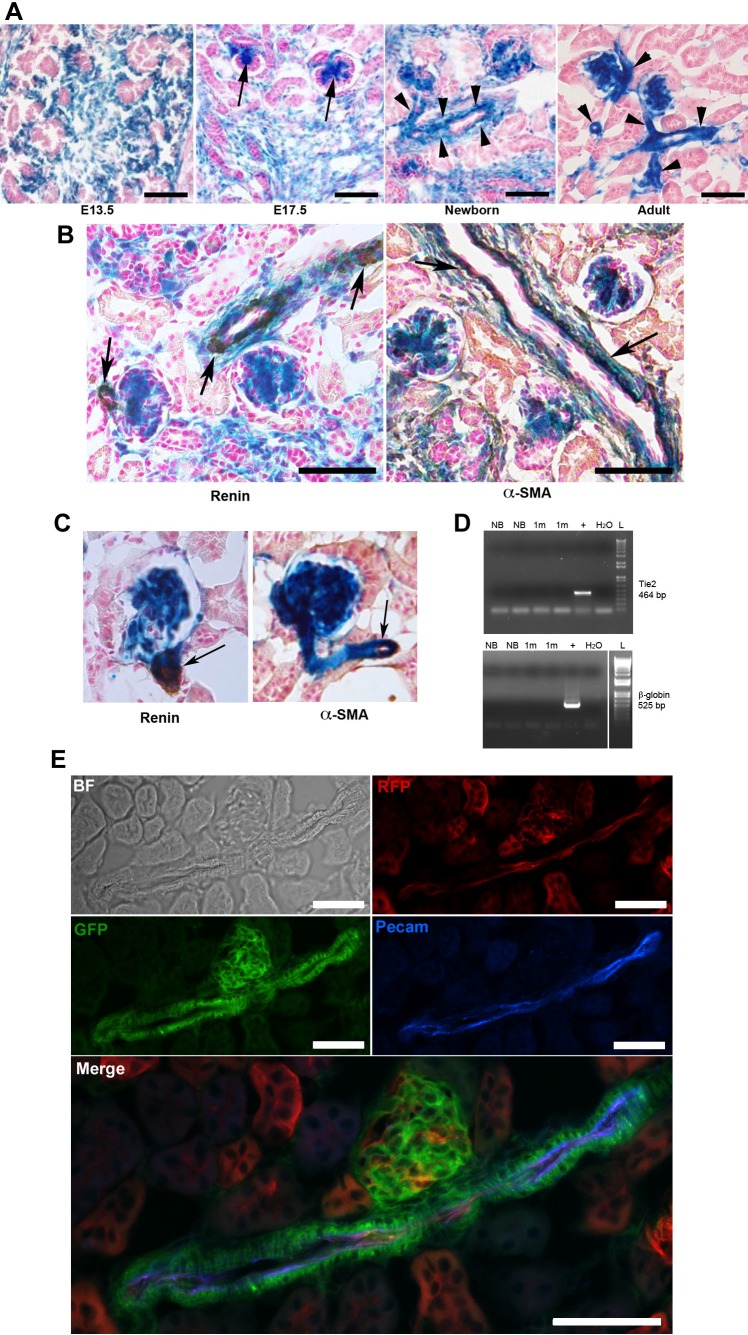
Foxd1 is a marker of stromal cells during kidney development (8). To investigate the fate of Foxd1 cells within the kidney, we crossed Foxd1-Cre knock-in mice (FoxD1-GFP-Cre, referred to as Foxd1cre mice) (10) to R26R (26) or mTmG (16) reporter mice, and followed the distribution of β-galactosidase-positive (β-gal+) or green fluorescent protein-positive (GFP+) cells within the kidneys during embryonic (E13.5–E17.5) and postnatal life (at 1 day, 1 mo, and 2 mo). As shown in Fig. 2A, β-gal+ cells were restricted to the undifferentiated stroma at E13.5 with no expression among the developing/branching epithelial cells. By E17.5, there was expression in mesangial cells of the developing glomeruli, afferent and efferent arterioles and within the renal interstitium. In the newborn kidney, the walls of the developing arterioles and arteries were covered by β-gal+ cells. The endothelial cells (ECs), however, were not positive for β-gal. In the adult kidney, when development is completed and, therefore, mature arterioles and arteries can be easily discerned, β-gal+ cells are present throughout their walls, including the adventitia. Again, ECs were not labeled by the blue Xgal reaction product. Within the peritubular interstitium, β-gal+ pericytes are present surrounding the ECs that form the peritubular capillaries, as previously described (10).
Fig. 2.
Foxd1+ cells are an early precursor for mesangial, vascular smooth muscle, renin cells, and pericytes but not for endothelial cells. A: Foxd1cre;R26R kidneys sections at different developmental stages showing extensive expression of β-gal within the undifferentiated stromal compartment (E13.5), with progressive differentiation into glomerular mesangium (E17.5, arrows), and cells within the wall of the arterial tree (newborn and adult, arrowheads). Endothelial cells within the arterioles do not express β-gal. B: Foxd1cre;R26R newborn kidneys sections showing coexpression of β-gal with immunostaining for renin (left, brown) and with α-SMA (right, brown). Endothelial cells do not express β-gal. C: immunostaining for renin (left) and α-SMA (right) in kidney sections from adult mice show coincidence of the DAB product (brown) with the X-gal staining (blue) (arrows). D: RT-PCR for Tie2 (top) and β-globin (bottom) was positive in samples of whole kidneys (+) and negative in FACS isolated Foxd1-lineage cells from Foxd1cre;mTmG newborn (NB) and 1-mo-old (1m) mouse kidneys. L, 1KB ladder; H2O, negative control. E: immunofluorescence for Pecam in kidney section from an adult Foxd1cre;mTmG mouse shows lack of coincidence of the blue fluorescent marker with cells derived from the Foxd1 precursors (GFP). However, ECs identified by Pecam expression are also positive for RFP, which marks cells not derived from Foxd1 progenitors. Scale bars: 50 μm.
Double-labeling studies [X-gal staining and immunostaining for α-smooth muscle actin (α-SMA) and renin] confirmed that Foxd1-derived cells (stained blue) correspond to both renin and vascular smooth muscle cells (SMCs) within the developing (Fig. 2B) and mature vessels (Fig. 2C).
Isolation of Foxd1-lineage cells from Foxd1cre;mTmG newborn and 1-mo-old mice (GFP+) by FACS followed by RT-PCR for Tie2 (EC marker) and β-globin (EC and blood cell marker) confirmed that cells from the Foxd1 lineage do not differentiate into cells expressing the endothelial marker Tie2 or β-globin (Fig. 2D). Immunofluorescence for the endothelial marker PECAM (Fig. 2E) further shows no coincidence between ECs and cells derived from Foxd1 precursors.
Altogether, these studies suggest that Foxd1+ cells are progenitors for all the mural cells of the arteriole. ECs, however, are not derived from Foxd1+ cells.
Foxd1 is necessary for the proper development of the renal arterial tree.
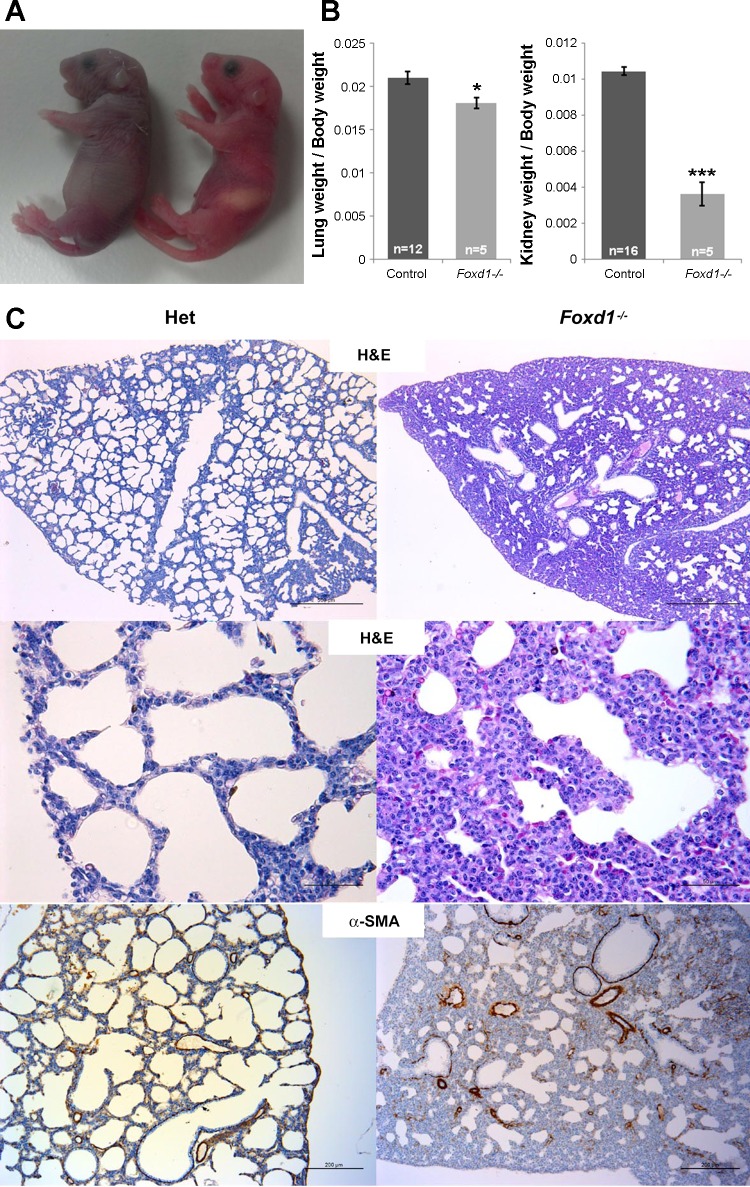
To address whether Foxd1 is necessary for the development of the kidney vasculature, we studied Foxd1−/− mice. Mice with deletion of the Foxd1 gene died within the first hours of postnatal life. Shortly after birth, they displayed prominent cyanosis, gasping, and abdominal distention (their stomachs were filled with ∼150 mm3 of air instead of milk), indicating severe respiratory distress (Fig. 3A). Histological analysis of the lungs showed thickened interstitial mesenchyme with infrequent septation and markedly reduced airway space, suggesting that the cause of perinatal death was respiratory failure due to abnormal lung development (Fig. 3C). Furthermore, the lung weight-to-body weight ratio was significantly decreased (Fig. 3B).
Fig. 3.
Foxd1 is necessary for normal lung development. A: newborn Foxd1−/− mouse (left) shows cyanosis, abdominal distention, an empty stomach, and the mouth wide open, indicating respiratory distress; a littermate control (right) shows normal pink skin color and a stomach full of milk. B: lung weight-to-body weight and kidney weight-to-body weight ratios were significantly reduced in Foxd1−/− mice (*P < 0.05, ***P < 0.001). C: lung sections stained with hematoxylin and eosin (top and middle) and immunostained for α-SMA (bottom) show normal lung septation and alveolar development in a Foxd1 heterozygous mouse and abnormal thickened interstitial mesenchyme with infrequent septation and markedly reduced airway space in a Foxd1−/− mouse. Scale bars: 500 μm (top), 50 μm (middle), and, 200 μm (bottom).
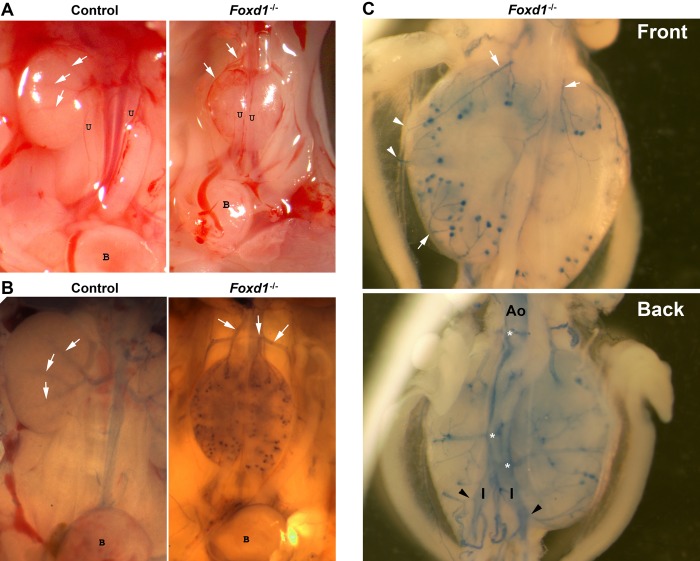
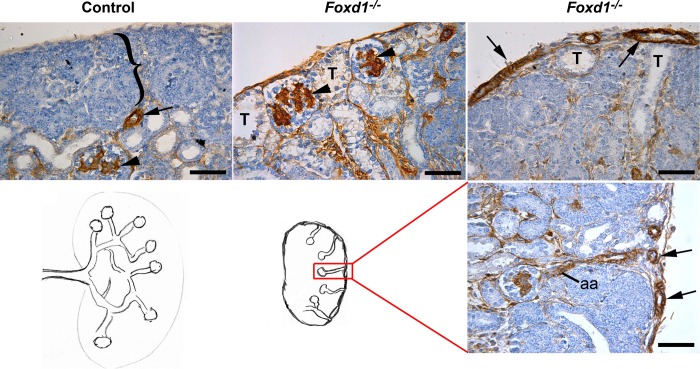
In agreement with previous studies (using mice were Foxd1 was replaced by lacZ) (8), the kidneys of Foxd1−/− mice were smaller [about one-third of the size of the heterozygous or wild-type (WT) littermates and with a significantly decreased kidney weight-to-body weight ratio (Fig. 3B)], were fused in the midline and to the retroperitoneal wall, and failed to ascend; the ureters displayed an abnormal ventral orientation (Fig. 4A). The renal parenchyma was underdeveloped, and instead of the normal centrifugal gradient of nephron differentiation, it consisted of multiple disorganized lobular areas containing few abnormally distributed subcapsular glomeruli. Furthermore, as shown in Fig. 5, immunostaining for α-SMA at E17.5 revealed an aberrant distribution of the renal vessels, with subcapsular and capsular arterioles entering the kidney directly from the capsule and connecting to glomeruli in a centripetal rather than in the normal centrifugal fashion. In addition, the nephrogenic zone was disorganized, and replaced in some areas with subcapsular distal tubules in addition to subcapsular mature glomeruli.
Fig. 4.
Foxd1 is necessary for the proper origin and development of the renal arterial tree. A: gross morphology of kidneys of control mice show normal intrarenal branches of the renal artery (white arrows) and ureters (u) facing to the midline whereas Foxd1−/− mice display capsular arteries (white arrows) originating from the aorta above smaller fused kidneys with ureters oriented ventrally (B, bladder). B: Evans blue glycerol-labeled perfusion of the aorta and abdominal branches showing the normal arterial branching pattern (white arrows) from a single hiliar renal artery in control mice and aberrant arteries (white arrows) originating from the aorta above the kidneys in Foxd1−/− mice. C: higher magnification of Foxd1−/− dissected kidneys area from the same animal shown in B (front and back) attached to the abdominal aorta (Ao) and to the iliac (I) arteries showing in more detail that there are several subcapsular arteries (white arrows) originating from the aorta at multiple levels (white asterisk), including the iliac arteries (black arrowheads).
Fig. 5.
Foxd1−/− kidneys display abnormal topological orientation of the renal vessels and disorganized renal structure. Immunostaining for α-SMA (brown) of E17.5 kidneys show that control mice present a normal outer undifferentiated nephrogenic zone (bracket) and a further developed inner cortex with arterioles (arrow) and more mature glomeruli expressing α-SMA in the mesangium (arrowhead), whereas Foxd1−/− mice present abnormal subcapsular glomeruli (arrowheads) and tubules (T) and capsular arteries (arrows). Lower panel shows a drawing of the vascular pattern in control and Foxd1−/− mice, as seen in the section stained with α-SMA antibody to highlight the abnormal arteries (arrows) and afferent arterioles (aa). Scale bar: 50 μm.
Foxd1 is necessary for the proper orientation and branching of the renal arterial tree.
To define whether Foxd1 is important for the appropriate orientation and branching pattern of the renal arterial tree, we harvested kidneys from newborn mice and manually microdissected the kidney vasculature. The renal arterial tree of WT newborn kidneys displayed normal centrifugal branching of arteries originating from a single renal artery, whereas in Foxd1−/− mice, the vasculature consisted of multiple independent arterial and arteriolar branches originating directly from the aorta and not from the renal artery. In turn, those vessels connected with capsular vessels that gave rise to the intrarenal arterioles. Those intrarenal vessels were not properly interconnected with one another. To understand their intrarenal orientation and their connections with their respective nephrons, we performed histological analysis of consecutive sections immunostained for α-SMA to more easily label arterioles (Fig. 6). Next, we developed a new perfusion technique using 0.5% of Evans blue in glycerol to directly visualize the abnormal preglomerular arterial supply and identify its precise origin. Kidneys from Foxd1−/− mice receive their blood supply from anomalous branches originating from the aorta above and below the kidneys (including branches from the iliac arteries) that surround the superior and inferior poles and give rise to subcapsular branches that enter the kidney laterally, often ending as a single afferent arteriole (Figs. 4–6). Lymphatic vessels in the Foxd1−/− kidneys followed the aberrant capsular arteries and veins, as evidenced by immunostaining for the specific marker for lymphatic vascular endothelium (LYVE; Fig. 7). Immunostaining for neuron-specific class III β-tubulin (TUJ1) demonstrated that the capsular vessels were innervated, whereas nerve fibers within the kidney were scarce (not shown).
Fig. 6.
Foxd1−/− kidneys are irrigated by multiple branches from the aorta instead of a single renal artery. A–C: transversal sections of E17.5 Foxd1−/− kidneys and vertebral area show midline fusion of the kidneys (arrows), ventrally oriented ureters (shown as U in C), and multiple capsular arteries (arrowheads). D–L: sagittal sections of E17.5 Foxd1−/− kidneys show several capsular arteries entering the kidneys and some of them originating from the aorta at different levels. M: sagittal section of E17.5 littermate control kidneys shows a single renal artery entering the kidney on the left side and normal midline orientation of the ureters (U). Scale bars: 500 μm.
Fig. 7.
Foxd1−/− kidneys display abnormal subcapsular lymphatics. Immunostaining for the specific marker for lymphatic vascular endothelium (LYVE, brown) show in newborn kidneys of control mice (left) few lymphatic capillaries within the renal capsule and lymphatic vessels adjacent to a large vein (V) and artery (A), whereas in the Foxd1−/− kidneys, there are multiple large lymphatic vessels adjacent to aberrant capsular arteries and veins.
Mosaic ablation of Foxd1 progenitors phenocopies the Foxd1−/− phenotype.
To explore whether deleting or injuring stromal cells would affect the development of the renal vasculature and mesangium, Foxd1 cells were ablated with diphtheria toxin. Foxd1cre heterozygous mice were crossed with mice containing the Rosa-DTA allele (R26RDTA) (27), which expresses diphtheria toxin upon recombination by cre. These mice are referred to as “Foxd1-DTA” mice.
At all ages, mice lacking cre-recombinase or the Rosa-DTA allele were indistinguishable from control animals. Foxd1-DTA animals died prior to birth. At E13.5 in control animals, the kidneys have normally rotated and separated from each other. However, in Foxd1-DTA animals, the kidneys were fused at the midline (Fig. 8A). In addition, the distribution of the arterioles, delineated by smooth muscle staining, was markedly abnormal in mutant mice: whereas in control animals, the arteries and arterioles were located within the developing and still poorly defined corticomedullary junction, in Foxd1-DTA animals, they were located at the periphery of the kidney (Fig. 6B, black arrows).
Fig. 8.
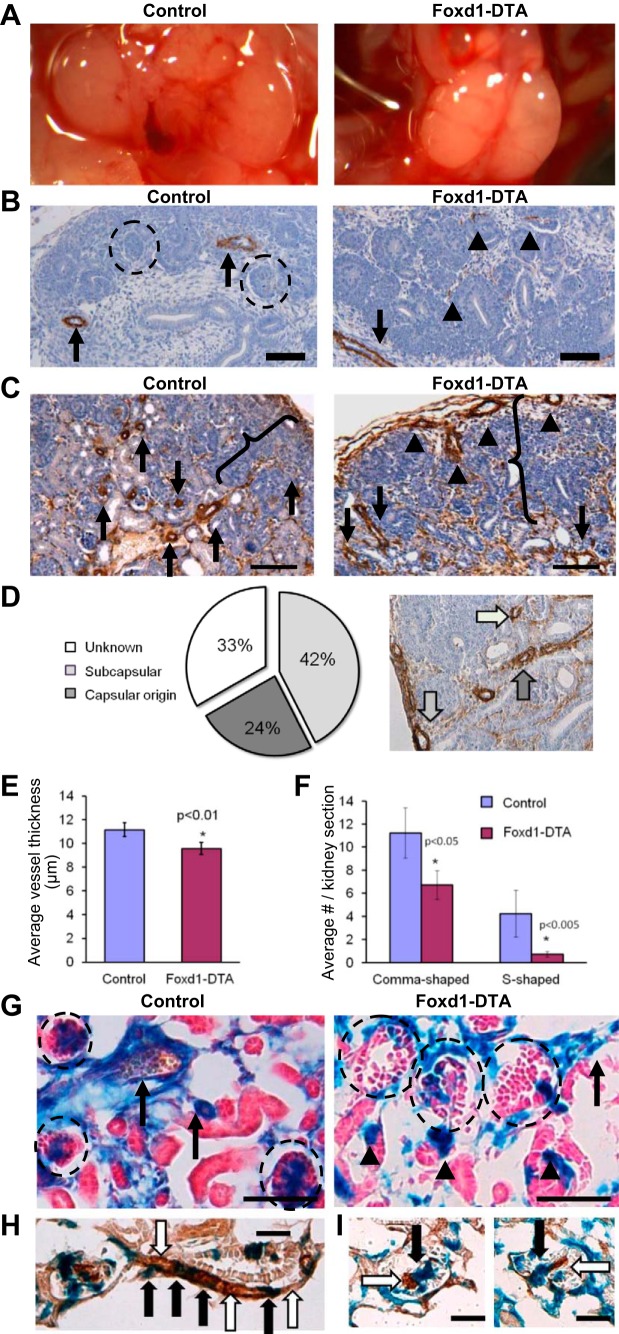
Ablation of Foxd1 cells using diphtheria toxin chain A (DTA) results in significant renal abnormalities. A: gross morphology of kidneys from mice expressing DTA upon Foxd1-cre-mediated recombination (Foxd1-DTA) reveals midline fusion, a failure to separate from the retroperitoneum, and blood vessels visible on the periphery of the kidney cortex. B and C: immunohistochemistry for α-SMA (brown) in control and Foxd1-DTA kidneys. B: at E15.5, control kidneys have well-defined SMC layers surrounding major arteries (arrows) in the interior of the kidney. By contrast, Foxd1-DTA kidneys display areas of diffuse and poorly organized staining within the kidney (arrowheads). However, large, fully coated vessels can be observed along the periphery of the kidney (arrows). At this age, control kidneys also display all of the stages of nephrogenesis, up to and including glomeruli (circles). Meanwhile, Foxd1-DTA kidneys lack mature glomeruli or any of the earlier stages of nephrogenesis, such as comma-shaped or s-shaped bodies. Scale bars: 100 μm C: at E18.5, control kidneys have multiple α-SMA-positive arterioles (arrows) throughout the kidney cortex. Meanwhile, Foxd1-DTA animals have comparatively fewer arterioles. In addition, a large subset of arteries appears to originate from the periphery of the kidney (arrowheads). In contrast to E15.5, Foxd1-DTA kidneys now possess all of the different stages of nephron development, but we note that the nephrogenic zone (brackets) is broader in Foxd1-DTA animals. Scale bars: 50 μm. D: quantification of the orientation of arteries seen in E18.5 Foxd1-DTA kidney demonstrates that nearly two-thirds of vessels observed lay directly underneath or emerge from the capsule. E: average thickness of the smooth muscle layer of renal arteries and arterioles is reduced in Foxd1-DTA animals at E18.5. F: E18.5 Foxd1-DTA kidneys have relatively fewer comma-shaped and s-shaped bodies. G: lineage tracing of Foxd1 cells upon ablation. Foxd1-DTA mice were crossed with mice carrying the R26R allele, which expresses β-galactosidase upon cre-mediated recombination, labeling Foxd1-expressing cells and their derivatives. In control animals at E18.5, the Foxd1-lineage marker labels arterial SMCs and mesangial and interstitial cells. The same cell types are labeled in Foxd1-DTA animals. However, a decrease in the thickness of the Foxd1-lineage SMC layer (arrows) and a marked decrease in the complement of mesangial cells is observed. We also note increased ectopic expression of the lineage marker within tubules (arrowheads). Scale bars: 50 μm. H–I: double staining of Foxd1-DTA kidneys, with LacZ reaction (blue) for Foxd1-lineage cells and immunohistochemistry (brown) for α-SMA. As seen in this afferent arteriole extending from the glomerulus, a subset of arteriolar SMCs is positive for both the lineage marker and α-SMA (black arrows), while other SMCs are positive only for α-SMA and not the lineage marker (white arrows). I: some mesangial cells are double-positive for both markers (black arrows), while others are only positive for α-SMA but negative for the lineage marker (white arrows). Scale bars: 50 μm.
In addition, in contrast to WT vessels which possess a thick layer of SMCs, the few observable deeper intrarenal vessels found in Foxd1-DTA animals had few, small, and poorly defined segments of smooth muscle that did not support a proper vascular lumen (Fig. 8B, arrowheads). Subsequently, at E17.5, the kidneys had a significant number of smooth muscle-coated arteries (arrows) in both controls and Foxd1-DTA mice (Fig. 8C). Interestingly, at this stage, the total number of vessels was not different between WT and mutant kidneys (not shown). However, similar to the phenotype seen in Foxd1−/− mice, the orientation of vessels in Foxd1-DTA kidneys was reversed: the blood vessels laid directly underneath the capsule, turned inward toward the renal parenchyma, and made connections with glomeruli (Fig. 8C, arrowheads). Quantification of this phenotype indicated that two-thirds of Foxd1-DTA vessels either rested alongside or originated directly from the kidney capsule (Fig. 8D). As in the Foxd1−/− mice, the subcapsular vessels also connect with aberrant and accessory arteries. There was also a significant decrease in the thickness of the vascular smooth muscle layer in Foxd1-DTA animals compared with controls (Fig. 8E).
As a lack of developing nephrons at E13.5 implied either delayed or interrupted nephrogenesis and glomerulogenesis in Foxd1-DTA animals, we examined Foxd1-DTA kidneys at E17.5 to discriminate between these two possibilities. At E17.5, we observed nephrons at all stages of development, including comma-shaped, s-shaped bodies, and mature glomeruli (Fig. 8C). However, the nephrogenic zone (Fig. 8C, brackets), an area characterized by rapid cellular proliferation and tissue condensation, was broader in Foxd1-DTA animals, an observation consistent with a delay in nephrogenesis. Supporting this hypothesis, the number of comma-shaped and S-shaped bodies was markedly decreased in mutant animals (Fig. 8F).
Although diphtheria toxin is purported to be lethal to cells at extremely low concentrations, we noted that some of the cells derived from Foxd1 cells persisted. To examine the subsequent fate of Foxd1 cells upon DTA expression, the Rosa-26 reporter allele was bred into Foxd1-DTA mice to label Foxd1 cells and their descendants. Whereas in control mice, labeling of mural cells and mesangial cells was uniform, encompassing all the cells descended from the Foxd1 lineage, labeling in Foxd1-DTA animals was patchy. To identify the labeled and unlabeled cells in Foxd1-derived structures, we stained for markers of smooth muscle and mesangium. Staining for α-SMA showed mosaic labeling of SMCs, whereas some cells stained double-positive for both α-SMA and the blue lineage marker, other cells positive for α-SMA were negative for β-gal expression (Fig. 8H). A similar result was found using staining for PDGFR-B (Fig. 8I), a marker for mesangial and vascular SMCs, showing again the presence of double-positive cells, as well as cells positive for the mesangial marker but negative for the lineage reporter. These findings indicate that the cell ablation was mosaic: whereas some cells were depleted, others survived and yet others were replaced by adjacent cells. In essence, the Foxd1-DTA mice behaved like a Foxd1 hypomorph, due in great part to the mosaic deletion of cells expressing Foxd1. Overall, the findings of Foxd1-DTA animal phenocopied those of the Foxd1−/− mice, underscoring the importance of Foxd1 per se in the development of the kidney vasculature.
Deletion of Foxd1 resulted in decreased renin expression.
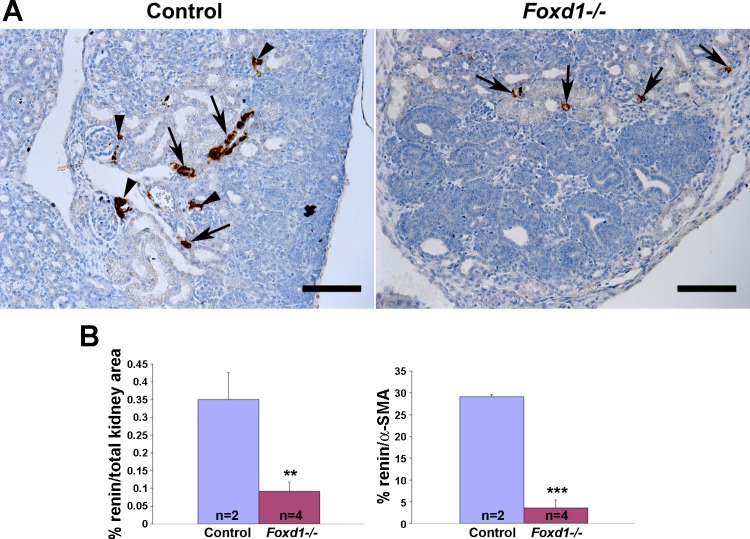
To investigate whether deletion of Foxd1 altered the expression of renin or distribution of renin-expressing cells, we performed renin immunostaining throughout embryonic development. The expression of renin in the kidneys from Foxd1−/− mice was markedly diminished at all embryonic developmental points and on the day of birth (Fig. 9). Because the size of the kidneys from the Foxd1−/− mice was smaller than that of the WTs, we corrected the areas of renin expression per total kidney area and per total arterial/arteriolar areas (as elicited by α-SMA staining), and as shown in Fig. 9, expression of renin was still markedly reduced.
Fig. 9.
Foxd1 is necessary for normal renin expression. A: immunostaining for renin (brown) of E17.5 kidneys show that control mice present a normal distribution of renin along developing arterioles and arteries (arrows), as well as in juxtaglomerular areas (arrowheads), whereas in Foxd1−/− mice there is a marked reduction of renin expression (arrows). Scale bar: 100 μm. B: quantification of areas immunostained for renin per total kidney area (left) and per total vascular areas positive for α-SMA on a consecutive section (right). **P = 0.01. ***P = 0.004.
DISCUSSION
Results from these studies show that 1) Foxd1+ renal stromal cells are the progenitors for renin cells, arteriolar SMCs, pericytes, and mesangial cells, and 2) stromal cells, via their defining transcription factor, Foxd1, are essential for proper morphogenesis and orientation of a single renal arterial tree per kidney.
We have previously suggested that early in gestation, the embryonic kidney possesses all the necessary precursors that compose the kidney vasculature well before arteriolar vessels can be discerned (24). We proposed that those as yet unidentified precursors differentiated into all the cell types necessary for the development of the kidney arterioles, including ECs, SMCs, and renin cells (12, 24). In fact, cross-transplantation studies of embryonic prevascular kidneys under the kidney capsule showed that those precursor cells had the capacity to differentiate, acquire the right positional information, and fully assemble to form the kidney arterioles (24). It was not clear, however, whether those various cell types arose from one or multiple progenitors. Work from our laboratory indicated that embryonic renin cells differentiate not only into juxtaglomerular cells but also into a subset of mesangial and arteriolar SMCs (22, 24). Although those studies suggested the hypothesis that a single progenitor may give rise to all of the cells of the kidney vasculature, the fact that renin cells did not differentiate into ECs and only gave rise to a subset of mesangial and SMCs suggested that a much earlier and upstream precursor was, indeed, the progenitor for all the mural arteriolar cells. Indeed, in a review article and on the basis of our early preliminary work, we suggested that Foxd1+ cells were progenitors for the mural cells of the renal arterioles (14, 21). Here, we show that Foxd1 cells not only give rise to SMCs, mesangial cells, and pericytes but also to the renin progenitor cell. Although these two progenitors (Foxd1 and renin progenitor) partially overlap in the generation of descendants, the Foxd1 progenitor is hierarchically upstream of the renin cell: when Foxd1 cells or the Foxd1 gene are deleted, renin cells are frankly diminished. Our results are in agreement with studies demonstrating that Foxd1+ cells differentiate into renal interstitial pericytes, which in pathological circumstances, also give rise to myofibroblasts (11).
It has recently been reported that a small subset of ECs located in the renal interstitium may originate from Foxd1 cells (25). However, in our study, we did not find interstitial ECs colabeled with the lineage tracer or expression of EC markers in sorted cells derived from Foxd1 precursors. Interestingly, and in agreement with the same study, we find that ECs from the glomeruli and arteriolar vessels are unrelated to the Foxd1 lineage (25). Thus, arteriolar ECs are likely to originate from a more primitive endothelial precursor. Our results are in agreement with work showing that within the developing renal interstitium, stromal cells expressing Foxd1 and endothelial precursors expressing c-kit constitute two distinct populations originating from different intraembryonic sites (19). A subset of c-kit+ cells also expresses angioblast markers, such as podocalyxin and Flk1 (19), suggesting that at least a subpopulation of c-kit+ cells may be the precursor for the renal ECs. Thus, the available evidence suggests that most, if not all, of the ECs of the renal interstitium, glomeruli, and arterioles originate from a separate precursor unrelated to the stromal Foxd1+ cell. The identity of such a precursor as a primitive renal hemangioblast has recently been suggested (21). Further work will be needed to test such a hypothesis.
Our results show that Foxd1 cells are necessary for the appropriate morphogenesis, composition, and orientation of the kidney vasculature. Foxd1−/− mice and Foxd1-DTA mice show very similar phenotypes, suggesting that most of the effects exerted by Foxd1+ cells are accomplished via their transcription factor Foxd1. The kidneys of Foxd1−/− mice displayed numerous developmental defects: they were smaller, they were fused in the midline, and they failed to ascend; the ureters were ventrally oriented as previously described (8, 13). Multiple accessory and aberrant arteries originating from the abdominal aorta and iliac arteries identified in this study are likely the cause of the kidneys' failure to ascend. Whether mutations of the FOXD1 gene in humans are associated to accessory or aberrant renal arteries remains to be investigated. Given that Foxd1 is expressed in stromal cells early in development, these findings suggest that Foxd1+ stromal cells affect the development of adjacent nephron precursors in a yet to be defined manner. In agreement with this hypothesis, elegant work from two laboratories has begun to unravel some the mechanisms involved: first, Das et al. (4) have suggested that stromal cells, via their production of the cadherin Fat4, inhibits nephron progenitor expansion/renewal and cooperates with Wnt9b produced by the ureteric bud to promote differentiation of progenitors, and more recently, Fetting et al. (5) have suggested the interesting possibility that lack of Foxd1, which under normal circumstances inhibits decorin, may lead to premature differentiation of stromal cells and progenitor retention in the epithelial nephron compartment. It is also possible that the alterations in epithelial nephron development are due to the role of Foxd1 cells in vascular development. Given the striking vascular abnormalities seen in Foxd1-null mice and Foxd1-DTA animals, inadequate tissue perfusion may have further contributed to impaired development of both compartments. Clearly, the available evidence from those and the current study indicates that the stromal compartment not only regulates vascular-interstitial development, but it plays a fundamental role in the development and maintenance of the epithelial nephron as well (4, 5). In our studies, we found occasional labeling of renal tubular structures when crossing Foxd1cre mice to either R26R or mT/mG reporter mice, suggesting a residual activity of Foxd1 in Six2 nephron precursors after the delimitation of epithelial nephron and interstitial progenitors from a common mesenchymal progenitor (15). This unexpected mosaic labeling of nephrons was recently reported when activating EGFP-L10a expression using Foxd1cre mice (15). However, in Foxd1-DTA animals, we observed increased ectopic renal tubular expression of the lineage marker, suggesting either an attempt to increase the expression of Foxd1 in the common mesenchymal progenitor, resulting in further differentiation into more Six2 lineage cells, or a de novo expression of Foxd1 in an attempt to compensate for the lack of Foxd1 in stromal derivatives.
To examine the anatomy and distribution of the renal arterial tree we used two different techniques: 1) microdissection under direct stereoscopic visualization and 2) Evans blue glycerol-labeled perfusion. Both techniques, as well as immunostaining for markers of the mural cells of the kidney arterioles, yielded similar results: Foxd1 −/− mice display a disrupted origin (multiple) and topological orientation of the renal vessels. Whereas in WT animals the arteries and arterioles arise from the renal artery, which enters the kidney through the hilum and divides successively into smaller branches in a centrifugal fashion, in mutant mice, there are multiple accessory and aberrant arteries that originate at various levels from the aorta and iliac arteries that surround the superior and inferior poles within the renal capsule and wherefrom they traverse toward the center of the kidney in a total reversal of the renal circulatory pattern. These results indicate that Foxd1 is necessary for the proper origin, number, and orientation of the renal arterial tree and that Foxd1 governs a set of guidance cues involved in the appropriate targeting and directionality of the renal vessels. The results agree with previous work showing that loss of Foxd1 results in defects in ureter branching and equally important, loss of appropriate capsular morphogenesis and cellular composition: in Foxd1−/− animals, the capsule contains a variety of cell types not normally found in the WT mice (8, 13). The above results also suggest that Foxd1 is important in the differentiation of progenitor cells in the capsule. Using immunostaining against an endothelial marker (PECAM), a recent study suggested that in Foxd1-DTA mice, the blood vessels were ”thickened“ as a result of an overgrowth of ECs (9). We could not confirm the presence of a thicker endothelium but verified instead the presence of subcapsular and capsular arterioles and arteries, which normally possess a thick muscle wall composed of SMCs. The presence of capsular vessels in our study indicates that Foxd1 normally inhibits the ectopic differentiation and assembly of the renal vasculature. The mechanisms and molecules involved are unclear and remain to be defined. Our preliminary unpublished data indicate that Foxd1 inhibits a genetic program involved in the acquisition of the muscular layers of the renal arterial tree. Further, the results suggest that Foxd1 participates in the proper orientation of the kidney vasculature. Given that Foxd1 has been shown to be required for the specification and targeting of ganglion cells in the temporal retina of mammals (3), it is possible that Foxd1 may exert a similar influence on the orientation and targeting of renal vessels. Whether this is achieved with the participation of axon-guiding molecules, such as ephrins, semaphorins, plexins, slits, and netrins (1) will need to be determined. Further studies will be needed to dissect the mechanism underlying the complex nephron-vascular phenotype ensuing from lack of Foxd1 cells or their transcription factor, Foxd1. Furthermore, in this study, we also identified that the cause of death of Foxd1−/− mice is abnormal lung development; whether Foxd1 is also required for normal branching morphogenesis of the epithelial and vascular compartment of the lung is a possibility that remains to be determined.
Perspectives and Significance
Identification of the Foxd1+ stromal cells as the precursors for the morphogenesis of the renal arterial tree may open new avenues for understanding the mechanisms that govern tissue vascularization and remodeling in normal and disease states. Further, it is becoming evident that the renal vasculature is a major determinant of tissue organization in the developing mammal, as well as appropriate preservation of function and structure in the growing and adult individual.
GRANTS
This work was supported by National Institutes of Health Grants DK-091330 to M. L. S. Sequeira-Lopez, DK-096373 and HL-096735 to R. A. Gomez and HL-048058, HL-061446, HL-084207 to C. D. Sigmund, the American Heart Association 13PRE16920043 to E. E. Lin and the University of Virginia Children's Hospital Grant-in Aid to M. L. S. Sequeira-Lopez.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.L.S.S.-L. and R.A.G. conception and design of research; M.L.S.S.-L., E.E.L., M.L., and Y.H. performed experiments; M.L.S.S.-L. and E.E.L. analyzed data; M.L.S.S.-L., C.D.S., and R.A.G. interpreted results of experiments; M.L.S.S.-L., E.E.L., and M.L. prepared figures; M.L.S.S.-L. drafted manuscript; M.L.S.S.-L., C.D.S., and R.A.G. edited and revised manuscript; M.L.S.S.-L., E.E.L., M.L., Y.H., C.D.S., and R.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We greatly thank the technical assistance of Danielle Stumbo for the mouse work. We thank Akio Kobayashi and Andrew McMahon for providing the Foxd1cre mice.
REFERENCES
- 1.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2: a001875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreres MI, Escalante A, Murillo B, Chauvin G, Gaspar P, Vegar C, Herrera E. Transcription factor Foxd1 is required for the specification of the temporal retina in mammals. J Neurosci 31: 5673–5681, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, Olson EN, Perantoni AO, Carroll TJ. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15: 1035–1044, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development 141: 17–27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira-Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol 296: H1255–H1262, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257: F850–F858, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged helix transcription factor BF-2. Genes Dev 10: 1467–1478, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9: e88400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyink DP, Tucker DC, St John PL, Leardkamolkarn V, Accavitti MA, Abrass CK, Abrahamson DR. Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 270: F886–F899, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Lin EE, Sequeira-Lopez ML, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, McMahon AP. Cell-specific translational profiling in acute kidney injury. J Clin Invest 124: 1242–1254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA. Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics 6: 45–55, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 9: 63–71, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol 299: 238–249, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Sequeira-Lopez ML, Chernavvsky DR, Nomasa T, Wall L, Yanagisawa M, Gomez RA. The embryo makes red blood cell progenitors in every tissue simultaneously with blood vessel morphogenesis. Am J Physiol Regul Integr Comp Physiol 284: R1126–R1137, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Sequeira-Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sequeira-Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Sequeira-Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, Gittes G, Bates CM. Endothelial progenitors exist within the kidney and lung mesenchyme. PLoS One 8: e65993, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development 133: 581–590, 2006. [DOI] [PubMed] [Google Scholar]