Summary
Epiblast stem cells (EpiSCs) in mice and rats are primed pluripotent stem cells (PSCs). They barely contribute to chimeric embryos when injected into blastocysts. Reprogramming of EpiSCs to embryonic stem cell (ESC)-like cells (rESCs) may occur in response to LIF-STAT3 signaling; however, low reprogramming efficiency hampers potential use of rESCs in generating chimeras. Here, we describe dramatic improvement of conversion efficiency from primed to naive-like PSCs through upregulation of E-cadherin in the presence of the cytokine LIF. Analysis revealed that blocking nuclear localization of β-CATENIN with small-molecule inhibitors significantly enhances reprogramming efficiency of mouse EpiSCs. Although activation of Wnt/β-catenin signals has been thought desirable for maintenance of naive PSCs, this study provides the evidence that inhibition of nuclear translocation of β-CATENIN enhances conversion of mouse EpiSCs to naive-like PSCs (rESCs). This affords better understanding of gene regulatory circuits underlying pluripotency and reprogramming of PSCs.
Highlights
-
•
E-cadherin overexpression considerably increases reprogramming efficiency of EpiSCs
-
•
E-cadherin overexpression negatively regulates β-catenin signaling in EpiSCs
-
•
Blocking nuclear localization of β-CATENIN enhances reprogramming of EpiSCs
In this article, Nakauchi and colleagues show that dramatic improvement of conversion efficiency from primed to naive-like mouse pluripotent stem cells through blocking nuclear localization of β-CATENIN in the presence of the cytokine LIF. This affords better understanding of gene regulatory circuits underlying pluripotency and reprogramming of primed pluripotent stem cells.
Introduction
Pluripotent stem cells (PSCs) can be classed as either naive or primed (Nichols and Smith, 2009). Mouse embryonic stem cells (ESCs) are naive PSCs derived from inner cell mass (ICM) of preimplantation blastocysts (Evans and Kaufman, 1981; Martin, 1981). Their naive state is maintained in an appropriate culture medium containing leukemia inhibitory factor (LIF) together with serum or with bone morphogenetic protein 4 (BMP4) (Smith et al., 1988; Ying et al., 2003). Media without LIF and supplemented with inhibitors of GSK3β and MAPK suffice to support long-term maintenance of naive PSCs (Ying et al., 2008). Epiblast stem cells (EpiSCs) are primed PSCs derived from postimplantation epiblasts; their self-renewal ability is maintained by activin A and basic fibroblast growth factor (bFGF) signaling (Brons et al., 2007; Tesar et al., 2007). Naive and primed PSCs are distinguished from one another by differences in signaling pathways that maintain pluripotency. In contrast to mouse ESCs, however, mouse EpiSCs are barely able to contribute to chimeras when injected into blastocysts, suggesting that a definitive difference between naive and primed PSCs exists with respect to ability to contribute to chimeras.
Genetic manipulation by overexpression of exogenous factors such as Nanog, Klf2, and Prdm14 enables conversion of mouse EpiSCs to ESC-like cells (rESCs) (Gillich et al., 2012; Silva et al., 2009). Furthermore, transition of mouse EpiSCs to rESCs rarely occurs even after stimulation with LIF-STAT3 signaling (Bao et al., 2009). However, the cellular mechanisms that limit reprogramming efficiency remain unclear.
Pluripotency in nonrodent PSCs is more like that in rodent primed-PSCs (Nichols and Smith, 2009), so that chimeric animals derived from PSCs are reported only in work with rodents (Nichols and Smith, 2009). Nonrodent PSCs thus are expected not to contribute to chimeras (one reason why knockout or transgenic studies have not been done using nonrodent mammals). We investigated the conditions for efficient conversion of primed PSCs to naive-like PSCs as part of generation of nonrodent naive PSCs.
Forced expression of E-cadherin in mouse EpiSCs under primed-PSC culture conditions promotes ICM development after blastocyst injection and results in generation of chimeric mice without reprogramming to the naive state (Ohtsuka et al., 2012). E-cadherin is a functional factor that can cooperate with reprogramming factors to promote generation of induced pluripotent stem cells (iPSCs) from somatic cells under naive-PSC culture conditions (Chen et al., 2010). These findings raised the possibility that E-cadherin upregulation under appropriate culture conditions might enhance reprogramming of primed PSCs. We therefore investigated the effects of E-cadherin upregulation in mouse EpiSCs under various culture conditions. We found that combining E-cadherin upregulation with LIF treatment dramatically improves rates of conversion of mouse EpiSCs to naive-like PSCs.
E-CADHERIN specifically binds β-CATENIN and regulates its nuclear translocation (Conacci-Sorrell et al., 2003; Sasaki et al., 2000; Stockinger et al., 2001). We found that nuclear translocation of β-CATENIN is negatively regulated by E-cadherin overexpression in mouse EpiSCs. Instead of upregulating E-cadherin expression, we used small-molecule inhibitors of Wnt signaling to study the role of such signaling in conversion of primed PSCs to naive-like PSCs. Interestingly, as did overexpression of E-cadherin, blocking nuclear localization of β-CATENIN significantly enhanced the efficiency of mouse EpiSCs conversion to naive-like PSCs in response to LIF. Our investigations thus provide insight into the significance of E-cadherin and β-CATENIN as well as into approaches for increasing efficiency of conversion of primed PSCs to naive-like PSCs.
Results
Overexpression of E-cadherin in the Presence of LIF Signaling Affects Pluripotency of Mouse EpiSCs
Culture conditions affect aspects of mouse EpiSC pluripotency (Bao et al., 2009) and artificial upregulation of E-cadherin enables chimera formation by mouse EpiSCs (Ohtsuka et al., 2012). We inferred that E-cadherin upregulation and appropriate culture conditions might in combination affect the pluripotentiality of primed PSCs (that is, their capacity to shift between primed-pluripotent and naive-pluripotent status). To test this hypothesis, we investigated the effect of upregulation of E-cadherin in mouse EpiSCs under various culture conditions.
To generate E-cadherin-overexpressing mouse EpiSCs, we constructed a E-cadherin inducible lentiviral vector. This was derived from a doxycycline (Dox)-dependent inducible vector (Yamaguchi et al., 2012) (Figure 1A). We introduced this lentiviral vector into a mouse EpiSC line obtained from DsRed-marked mouse EB3 ESCs (EB3DR ESCs) (Niwa et al., 2002; Ogawa et al., 2004). We injected EB3DR mouse ESCs into blastocysts and obtained a DsRed-expressing mouse EpiSC line (EB3DR EpiSC) from E6.5 epiblast. Established EB3DR-EpiSCs were confirmed not to form chimeras when injected into blastocysts (Table S2 available online). E-cadherin-inducible mouse EB3DR EpiSCs were purified for green fluorescent protein (GFP) expression by fluorescence-activated cell sorting (FACS).
Figure 1.
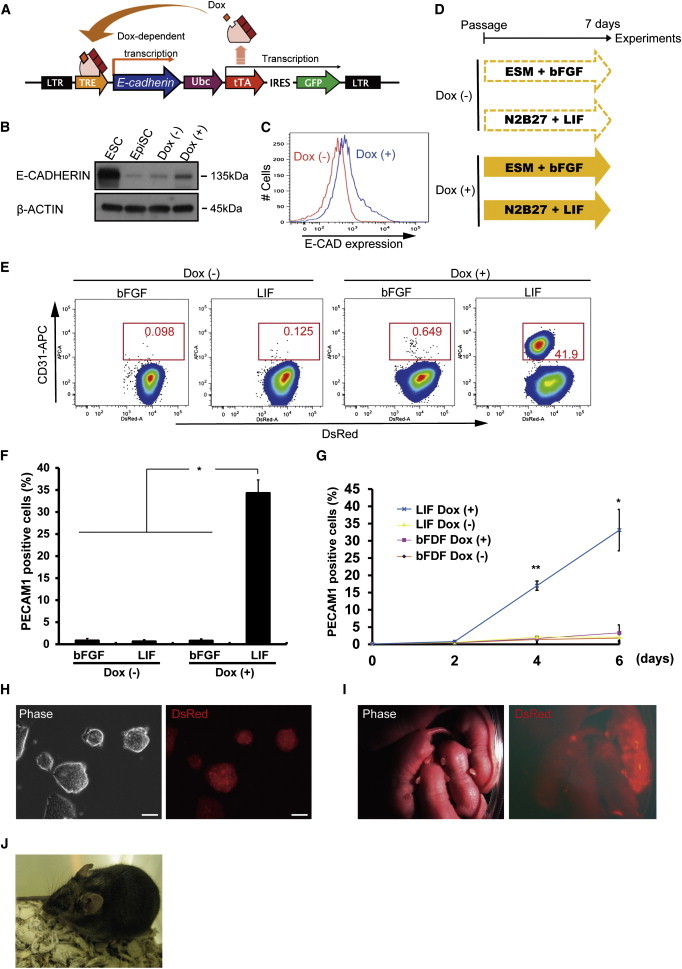
E-cadherin-Overexpressing Mouse EpiSCs Show Highly Efficient Conversion to Naive-like PSCs in the Presence of LIF
(A) Schematic diagram of doxycycline-inducible system for the expression of E-cadherin transgene.
(B) Western blotting analyses for E-CADHERIN in mouse EB3DR ESCs (ESC), mouse EB3DR epiblast stem cells (EpiSC), and transgenic mouse EB3DR EpiSCs cultured with or without doxycycline (2 μg/ml) for 2 days (Dox−, Dox+). β-ACTIN was used as a loading control.
(C) FACS analysis of E-CADHERIN expression levels in transgenic mouse EB3DR EpiSCs cultured with (blue line) or without (red line) doxycycline for 2 days.
(D) Time schedule of all experiments with or without doxycycline in mouse EB3DR EpiSCs. Culture conditions were defined as primed (ESM + bFGF) and naive (N2B27 + LIF).
(E) FACS analysis of CD31-positive cells in mouse EB3DR EpiSC (DsRed) under various conditions. Shown are the percentages of CD31-expressing cells after culturing with or without doxycycline for 7 days under primed (ESM + bFGF) and naive (N2B27 + LIF) conditions.
(F) Average of PECAM1-expressing cells associated with (E). The frequency of PECAM1-expressing cells is significantly increased in E-cadherin upregulated mouse EpiSCs in the presence of LIF (mean ± SEM of three independent experiments, ∗p < 0.05).
(G) Timing and efficiency of PECAM1 expression. In the presence of LIF, shifts of E-cadherin upregulated mouse EpiSCs to PECAM1-expressing status differ significantly from those seen under other conditions (mean ± SEM of three independent experiments, ∗p < 0.05, and ∗∗p < 0.01).
(H) Photomicrograph of PECAM1-expressing cells obtained from E-cadherin upregulated mouse EpiSCs in the presence of LIF. Almost all PECAM1-expressing cells occurred as dome-like compact colonies. Scale bar, 50 um.
(I) Live-born chimeric mice obtained from DsRed-marked mouse Ecad-rESCs.
(J) Chimeric mice obtained by injection of mouse E-cad-rESCs (agouti) into BDF1 × C57BL/6 blastocysts (black) show coat-color contribution from mouse E-cad-rESCs.
We next assessed E-CADHERIN expression in these mouse EpiSCs by western blotting and FACS analysis (Figures 1B and 1C). E-CADHERIN expression was upregulated by Dox compared to a control without Dox and to wild-type mouse EpiSCs (Figure 1B). FACS analysis also showed that E-CADHERIN expression levels increased after Dox treatment (Figure 1C).
To examine the effects of E-cadherin overexpression in the reprogramming process, we explored the conversion of mouse EpiSCs to rESCs (Bao et al., 2009) under various culture conditions such as ESM plus bFGF (typical expansion medium for primed PSCs) and N2B27 plus LIF including the MECK inhibitor PD0325901 (PD) combined with the GSK3β inhibitor CHIR99021 (CHIR) (typical expansion medium for naive PSCs) with or without Dox (Figure 1D). Surface marker expression by naive PSCs was analyzed by flow cytometry. Under naive-PSC culture conditions, around 40% of E-cadherin upregulated mouse EpiSCs converted to expression of the naive-PSC marker CD31 (PECAM1) in 7 days (Figure 1E). In the presence of LIF, the frequencies of PECAM1-expressing cells were significantly increased among E-cadherin upregulated mouse EpiSCs compared to those cultured under other conditions for 7 days (Figures 1F and 1G). These data suggest that overexpression of E-cadherin considerably increases the efficiency with which primed mouse EpiSCs convert to naive PSCs upon activation of the LIF signal. PECAM1-expressing cells obtained from E-cadherin upregulated EpiSCs formed dome-like compact colonies (Figure 1H). We call these cells mouse EpiSCs reprogrammed to ESC-like status by E-cadherin overexpression (mouse E-cad-rESCs).
We next examined the developmental potential of mouse E-cad-rESCs. After injection into mouse blastocysts, mouse E-cad-rESCs from mouse EB3DR-EpiSCs (DsRed-marked EpiSCs) contributed to chimeric live-born mice (Figure 1I). We also succeeded in generation of chimeric mice by injection of mouse E-cad-rESCs (normally giving rise to agouti-furred mice) into BDF1 × C57BL/6 mouse blastocysts (normally giving rise to black-furred mice). The chimeras showed coat-color contributions from the mouse E-cad-rESCs (Figure 1J; Table S2), indicating that mouse E-cad-rESCs contributed extensively to development.
We also tested effect of E-cadherin overexpression in a “standard” mouse EpiSC line (Tesar et al., 2007) to confirm reproducibility (Figure S1). The “standard” mouse EpiSCs were labeled with tdTomato to distinguish with mouse embryonic fibroblasts (MEFs). The cells were infected with lentiviral vector, which expresses tdTomato under CAG promoter. Flow-cytometry analyses showed that “standard” mouse EpiSCs overexpressing E-cadherin converted to CD31-expressing naive-like cells within 7 days (Figures S1B and S1C), as did EB3DR-EpiSCs. Because “standard” EpiSCs were a female line, we sorted CD31-expressing cells and established an E-cad-rESC line and then analyzed X chromosome inactivation (XCI) state immunocytochemically (Figure S1D). Although XCI was observed in nontreated EpiSCs (marked by dot-like expression of H3K27me3), no XCI was observed in converted cells.
These results suggest that upregulation of E-cadherin in the presence of LIF enables highly efficient derivation of naive-like PSCs.
Ohtsuka et al. (2012) reported that 2 days of E-cadherin overexpression enabled chimera formation with maintenance of primed-state pluripotency; however, the EpiSC lines that we used did not contribute to chimeras when cultured “ESM plus bFGF” medium or “N2B27 plus LIF” medium (Table S2). In our hands, reprogramming was necessary for EpiSCs into which the E-cadherin tet-on system had been introduced to form chimeras with preimplantation embryos.
Inhibition of Nuclear Translocation of β-CATENIN Enhances Reprogramming Efficiency
E-CADHERIN specifically binds to β-CATENIN and regulates its nuclear translocation (Conacci-Sorrell et al., 2003; Sasaki et al., 2000; Stockinger et al., 2001). Accumulation of nonphosphorylated β-CATENIN in the nucleus is required for β-CATENIN-mediated transcription (Staal et al., 2002). Thus, we hypothesized that E-CADHERIN overexpression trapped β-CATENIN in cytoplasm, thereby preventing intranuclear β-catenin signaling. To examine this, we quantitated β-CATENIN and nonphosphorylated β-CATENIN (active form) in cytoplasmic and nuclear fractions by western blotting (Figure 2A). Images and calculated relative expression results indicated that β-CATENIN in the nuclear fraction is decreased in Dox-treated EpiSCs into which the E-cadherin tet-on system had been introduced. (Such cells manifested an intermediate phenotype even without Dox treatment, reflecting leaky regulation of the tet-on system.) These results showed that overexpression of E-cadherin inhibits nuclear translocation of β-CATENIN in mouse EpiSCs.
Figure 2.
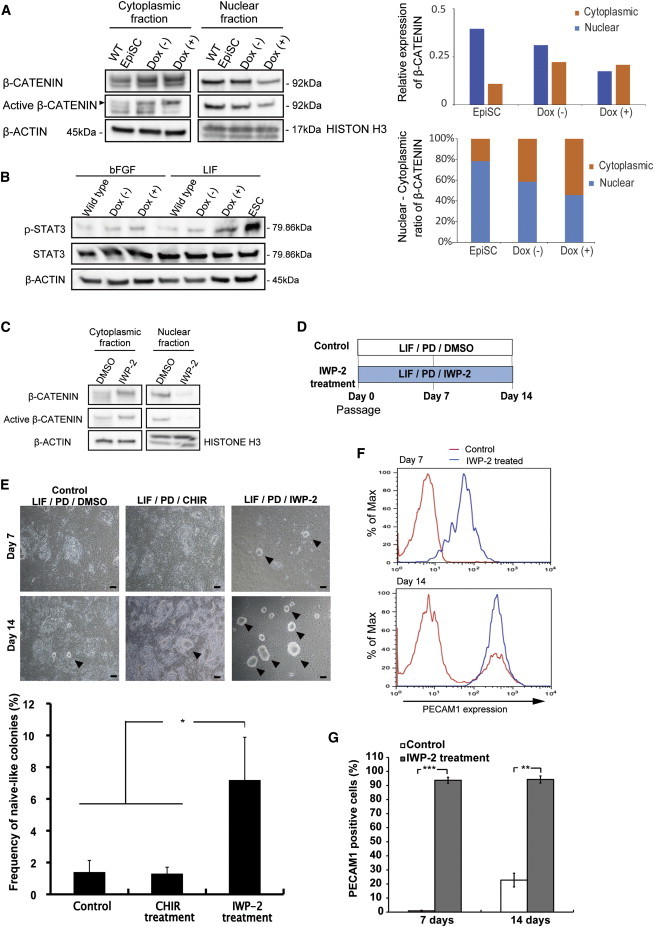
Wnt Signaling Inhibitor Facilitates Generation of Naive Pluripotency Marker-Positive Cells from Mouse EpiSCs
(A) Western blots of β-CATENIN and active β-CATENIN in wild-type mouse EpiSC and mouse EpiSC with inducible E-cadherin transgene cultured with or without doxycycline (2 μg/ml) for 2 days (Dox−, Dox+). Cytoplasmic and nuclear fractions were analyzed. β-ACTIN and HISTON H3 were used as loading controls in cytoplasmic and nuclear fractions, respectively (left panel). Relative expression of β-CATENIN (upper-right panel) and proportion of β-CATENIN in cytoplasmic and nuclear fractions (right-lower panel) in each condition are shown.
(B) Western blotting analyses for phospho-STAT3 in mouse EB3DR ESCs (ESC), mouse EB3DR EpiSC (wild-type), and transgenic mouse EB3DR EpiSCs cultured with or without doxycycline (2 μg/ml) for 2 days (Dox−, Dox+). β-ACTIN was used as a loading control.
(C) Western blots of β-CATENIN and active β-CATENIN in mouse EpiSCs cultured with or without 5 nM IWP-2 for 2 days (DMSO, IWP-2). Cytoplasmic and nuclear fractions were analyzed. β-ACTIN and HISTONE H3 were used as loading controls in cytoplasmic and nuclear fractions, respectively.
(D) Timeline of naive-like cell induction using 5 nM IWP-2 and 1 μM PD0325901 in the presence of LIF. DMSO was used as control. Treatment was maintained for 7 and 14 days.
(E) Morphological observation of colonies derived from treated mouse EpiSCs (upper panel). Note the appearance of naive-like compact colonies (black arrowheads). Efficiency of generation of naive-like compact colonies at 14 days (lower panel). Efficiency of generation of naive-like compact colonies was calculated as the ratio of seeded cell number to the number of naive-like compact colonies (mean ± SEM of four independent experiments, ∗p < 0.05). Scale bar, 100 μm.
(F) FACS analysis of PECAM1 expression levels in mouse EpiSCs cultured with (blue line) or without (red line) IWP-2 for 7 and 14 days.
(G) Average of PECAM1-expressing cells associated with (F). The frequency of PECAM1-expressing cells increased significantly with IWP-2 treatment (mean ± SEM of three independent experiments, ∗∗p < 0.01, and ∗∗∗p < 0.001).
See also Figure S2.
E-CADHERIN reportedly also is responsible for LIF signal integration (del Valle et al., 2013). We therefore tested the effect of E-cadherin overexpression on LIF-STAT3 signaling in EpiSCs. We quantitated phospho-STAT3 (p-STAT3) by western blotting analysis with or without E-cadherin overexpression (Figure 2B). In the presence of LIF, EpiSCs overexpressing E-cadherin showed higher expression of p-STAT3 than control cells. This suggests that E-cadherin overexpression not only blocks nuclear translocation of β-CATENIN, but also enhances LIF-STAT3 signaling when EpiSCs are cultured with LIF.
We wondered whether attenuation of β-catenin signaling or enhancement of LIF-STAT3 signaling was mainly responsible for the promotion of reprogramming. To answer this question, we used the small-molecule inhibitor IWP-2, which interferes with the ability of cells to produce WNT proteins by blocking Porcupine, an enzyme essential for acylating WNT proteins, and results in suppression of accumulation of β-CATENIN (Chen et al., 2009). As was the case for E-cadherin overexpression, we found that IWP-2 inhibits nuclear translocation of β-CATENIN in mouse EpiSCs (Figure 2C). We then investigated whether IWP-2 can induce efficient reprogramming. For this purpose, we chose a combination of LIF and PD (Nichols and Ying, 2006), with DMSO as a control, and compared it with a combination of LIF, PD, and IWP-2 (Figure 2D). Morphological examination found dome-like compact colonies derived from mouse EpiSCs treated with IWP-2. After 14 days in culture, the frequency of naive-like colonies was significantly increased in the IWP-2-treated mouse EpiSCs compared with the DMSO-treated control and with CHIR-treated mouse EpiSCs (Figure 2E). Flow-cytometry analyses revealed that IWP-2-treated mouse EpiSCs convert to cells that express the naive-ESC marker CD31 (PECAM1-expressing cells) within 7 days (Figures 2F and 2G). PECAM1-expressing cells obtained from Wnt inhibitor-treated mouse EpiSCs formed mouse ESC-like colonies (Figure 3A). We called these cells Wnt inhibitor-treated mouse EpiSCs reprogrammed to ESC-like cells (mouse wit-rESCs). Next, we examined the developmental potential of mouse wit-rESCs. When injected into mouse blastocysts, mouse wit-rESCs from EB3DR-EpiSCs, which are DsRed-marked, contributed to chimeric embryos at E12.5 (Figure 3B). Furthermore, live-born and adult chimeric mice were obtained from mouse wit-rESCs (Figures 3C and 3D), indicating that mouse wit-rESCs contributed extensively to development. Those wit-rESC-derived chimeras also showed germline transmission (Figure 3E).
Figure 3.
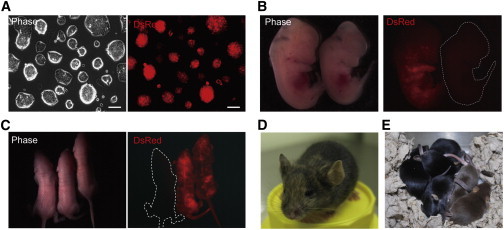
Developmental Potential of Mouse wit-rESCs
(A) Photomicrograph of PECAM1-expressing cells obtained from IWP-2-treated mouse EpiSCs in the presence of LIF (wit-rESCs). Almost all PECAM1-expressing cells occurred as dome-like compact colonies. Scale bar, 100 μm.
(B) DsRed-marked mouse wit-rESCs contribute to chimeric embryos at E12.5. An embryo lacking DsRed expression is shown as a nonchimera.
(C) Live-born chimeric mice were obtained from DsRed-marked mouse wit-rESCs. A pup lacking DsRed expression is shown as a nonchimera.
(D) Chimeric mice obtained from injection of mouse wit-rESCs (agouti) into BDF1 × C57BL/6 blastocysts (black) show coat-color contribution from mouse wit-rESCs.
(E) Germline-transmitted agouti coat color is shown in pups born to mice obtained from wit-rESCs.
To assess reprogramming quantitatively, we next used Rex1GFPd2 cells, in which GFP is expressed via the Rex1 (Zfp42) locus (Wray et al., 2011). Rex1 is specifically expressed in naive-state pluripotent cells and is downregulated at the onset of differentiation (Toyooka et al., 2008; Wray et al., 2010). We injected Rex1-GFP mouse ESCs into tetraploid (4N) embryos and obtained a GFP-negative Rex1-GFP mouse EpiSC line from postimplantation embryos (E6.5) (Figures S2A–S2C). In the reprogramming process, the frequency of cells expressing both GFP and PECAM1 (“double-positive” cells) was significantly increased among IWP-2-treated mouse EpiSCs compared to the DMSO-treated control (Figures S2D and S2E). Furthermore, we inhibited tankyrase via XAV939, another small-molecule inhibitor that promotes degradation of β-CATENIN (Huang et al., 2009), to confirm that enhancement of reprogramming efficiency was reproducible. Upon XAV939 treatment in the presence of LIF, the configuration of mouse EB3DR EpiSCs indeed changed to that of ESC-like compact colonies expressing PECAM1 (Figures S3A and S3B). Moreover, PECAM1-expressing cells reprogrammed by XAV939 treatment contributed to chimeras (Figure S3C). These results clearly indicate that blocking nuclear localization of β-CATENIN significantly enhances conversion efficiency of mouse EpiSCs to naive-like PSCs.
Mouse E-Cad-rESCs and wit-rESCs Express Naive Pluripotency Genes
To gain insight into the processes by which reprogramming occurs in mouse EpiSCs, we examined gene-expression profiles by RT-PCR analysis. We found that the naive pluripotency genes Stella and Rex1 are expressed in all lines of mouse E-cad-rESC and wit-rESC that we established (Figure 4A). Furthermore, real-time PCR analysis revealed that mouse E-cad-rESCs and wit-rESCs showed high expression of the naive pluripotency genes Pecam1, Rex1, and Kit (also called c-kit) (Figure 4C). However, expression of the primed pluripotency genes Lefty1 and Fgf5 was scant (Figure 4B). Expression of the major pluripotency genes Nanog and Sox2 was also increased in mouse E-cad-rESCs and wit-rESCs compared to expression in mouse EpiSCs (Figure 4C). These results indicate that the gene expression profiles of mouse E-cad-rESCs and wit-rESCs are similar to those of mouse ESCs and differ from those of mouse EpiSCs.
Figure 4.
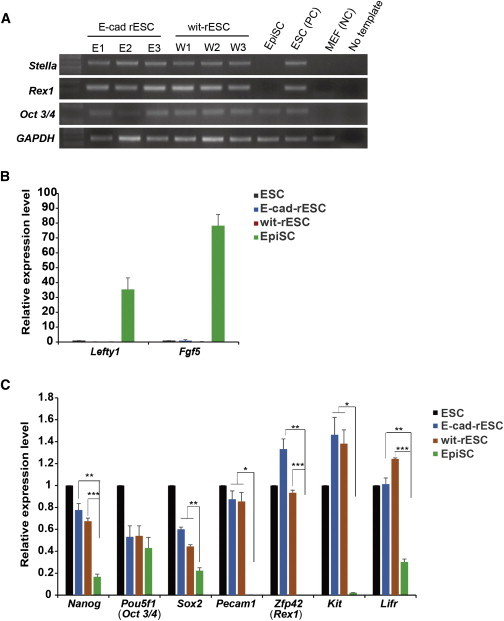
Gene Expression Profiling of Various Mouse Stem Cell Lines
(A) RT-PCR of pluripotency genes. Note naive marker gene expression by mouse E-cad rESC and wit-rESC lines.
(B) Real-time PCR of primed pluripotency genes in mouse E-cad-rESCs, wit-rESCs, and EpiSCs. Expression levels of pluripotency genes are compared to those in mouse ESCs. Expression values are normalized to Gapdh. Data are represented as mean ± SEM of three independent experiments.
(C) Real-time PCR analysis for naive pluripotency genes in mouse E-cad-rESCs, wit-rESCs, and EpiSCs. Expression levels of pluripotency genes are compared to those in mouse ESCs. Relative expression values are normalized to Gapdh.
Data represent mean ± SEM of three independent experiments, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Note that expression patterns in mouse E-cad-rESCs and wit-rESCs resemble those in mouse ESCs, but not those in mouse EpiSCs.
TCF/LEF Activity in E-cadherin-Overexpressing or Inhibitor-Treated EpiSCs
To investigate whether blocking nuclear localization of β-CATENIN result in downregulation of TCF transcriptional activity, we measured TCF/LEF activity by TOPFlash assay (Veeman et al., 2003). Upon overexpression of E-cadherin, luciferase activity did not differ significantly between the tet-on E-cadherin construct-carrying EpiSCs and their parental EB3DR EpiSCs, regardless of Dox treatment (Figure S4A). Furthermore, we examined the effect of luciferase activity on EB3DR EpiSCs treated with Wnt signaling inhibitors (Figure S4B). Luciferase activity did not differ significantly in IWP-2- or XAV939-treated cells from that in DMSO-treated controls. Although E-cadherin overexpression or IWP-2 treatment blocked β-CATENIN localization in nuclei (Figures 2A and 2C), downregulation of TCF/LEF activity was not detected. These results may suggest that improvement of reprogramming efficiency (Figures 1E, 2E, and 2G) was achieved via an unknown signaling pathway that was stimulated by blocking β-CATENIN translocation.
Discussion
Conversion of mouse EpiSCs to ESC-like cells in response to LIF-STAT3 signaling rarely occurs under ordinary cell culture conditions (Bao et al., 2009). Indeed, a recent study has shown that upregulation of E-cadherin expression does not induce conversion from primed to naive state under culture in media containing bFGF and activin A (Ohtsuka et al., 2012). However, we demonstrated that overexpression of E-cadherin in combination with the cytokine LIF yields highly efficient derivation of cells that express naive-PSC markers and that can contribute to chimeras. We then extended these data by confirming E-cadherin-dependent induction of naive PSC markers and repression of primed PSC markers in the presence of LIF. These results suggest that overexpression of E-cadherin induces conversion of mouse EpiSCs toward the ESC-like naive state.
Ohtsuka et al. (2012) showed that 2 days of E-cadherin overexpression enabled chimera formation by EpiSCs without any evident conversion to ESC-like cell status. However, the EpiSC lines that we used did not acquire the ability to form chimeras after short-term (2 days) overexpression of E-cadherin. To explain this discrepancy, two possibilities can be considered. One is differences in culture conditions. Ohtsuka et al. maintained EpiSCs with activin and FGF2 under feeder-free conditions, whereas we maintained EpiSCs in a different medium supplemented with bFGF and on feeder cells. Different culture conditions might set EpiSCs at slightly different stages within the primed pluripotent state and might result in different outcomes with respect to chimera formation after E-cadherin overexpression. The other is use of different vector systems to induce E-cadherin overexpression. We used the all-in-one tet-on lentiviral vector system (Yamaguchi et al., 2012), whereas Ohtsuka et al. used the piggyBack transposon system. Of importance may be that they described E-cadherin expression levels in EpiSC-line cells into which the tet-on system had introduced E-cadherin as “slightly higher” than those in ESCs, whereas cells in our line showed much higher expression than did ESCs after Dox treatment in a “standard” mouse EpiSC line. This may suggest that E-cadherin expression levels must be similar to those in ESCs to permit integration into the ICM.
As with reprogramming factors (Oct4, Klf2, Sox2, and c-Myc), overexpression of E-cadherin enhances the efficiency of iPSC generation from mouse embryonic fibroblasts (Chen et al., 2010). We demonstrated that E-cadherin overexpression affects both attenuation of β-catenin signaling and enhancement of LIF-Stat3 signaling. These changes might underlie efficient reprogramming. We consider blocking nuclear localization of β-CATENIN to be a major factor because Wnt inhibitors also promoted reprogramming.
We also demonstrated that although innate E-cadherin expression levels vary among EpiSC lines, E-cadherin overexpression supported reprogramming independent of innate expression levels. This may suggest that, rather than absolute β-catenin signal intensity, the relative change in β-catenin signaling is important.
Consistent with reports indicating such a role for E-CADHERIN (Conacci-Sorrell et al., 2003; Sasaki et al., 2000; Stockinger et al., 2001), our data confirmed negative regulation of nuclear translocation of β-CATENIN through E-cadherin overexpression in mouse EpiSCs. As expected, we could also demonstrate that blocking nuclear localization of β-CATENIN by the small-molecule inhibitors IWP-2 or XAV939 confers high efficiency in conversion of mouse EpiSCs to naive-like PSCs that can contribute to chimeras. This suggests that, as was the case with upregulation of E-cadherin expression, the combination of blocking nuclear localization of β-CATENIN and LIF signaling activation primes mouse EpiSCs for reprogramming. Absent upregulation of E-cadherin, small-molecule inhibitors of Wnt signaling can significantly amplify reprogramming frequency. One can infer that upregulation of E-cadherin leads efficient reprogramming by blocking nuclear localization of β-CATENIN. In this way, we succeeded in establishing culture conditions for efficient conversion of primed PSCs to naive-like PSCs. To our surprise, we demonstrated that E-cadherin overexpression and Wnt inhibitor treatments did not affect to TCF/LEF-mediated transcriptional activity though nuclear localization of β-CATENIN was significantly blocked. Kim et al. (2013) previously reported that subcellular localization of β-CATENIN does not implicate Wnt-downstream transcriptional activity but has some role in maintenance of self-renewal. Our results suggested that TCF/LEF-independent β-catenin signaling is stimulated by blocking nuclear localization or subcellular localization of β-CATENIN, thereby also promoting conversion from primed state to naive state pluripotency.
Pluripotentiality in primate PSCs resembles pluripotentiality in mouse EpiSCs; primate PSCs are not thought to contribute to chimeras (Nichols and Smith, 2009). For this reason, the use of primate PSCs for knockout and transgenic studies is rare. Although our work was done only in mice, our findings, with efficient conversion of primed to naive PSCs, may provide the key to establishing naive PSCs derived from other animals. We consider that requirements for β-catenin signaling must be reassessed, because the culture conditions employed in reports of establishment of human naive PSCs all were set to promote β-catenin signaling (Gafni et al., 2013; Hanna et al., 2010; Li et al., 2009; Ware et al., 2014). Further study would be required to clarify the effect of β-catenin signaling for the conversion to naive-like state in other animals and human PSCs.
Our studies indicate that blocking nuclear localization of β-CATENIN can enhance conversion of primed mouse PSCs to naive-like PSCs. Further studies of this phenomenon may not only provide better understanding of gene regulatory circuits underlying pluripotency, but perhaps also provide processes to induce reprogramming in primed PSCs.
Experimental Procedures
Lentiviral Vector Construction and Preparation
An E-cadherin inducible lentiviral vector was derived from the self-inactivating lentiviral vector CS-TRE-PRE-Ubc-tTA-I2G (Yamaguchi et al., 2012). Mouse E-cadherin was amplified by PCR using the primers 5′-CGTACGCCACCATGGGAGCCCGGTGCCGCAG-3′ and 5′-GAATTCCTAGTCGTCCTCACCACCGC-3′ (restriction sites are underlined). Amplified E-cadherin was cloned into the PCR-Blunt II-TOPO cloning vector (Invitrogen), and its incorporation was confirmed by sequencing. A BsiWI-EcoRI fragment of TOPO E-cadherin was then inserted into BsiWI-EcoRI sites of CS-TRE-PRE-Ubc-tTA-I2G, resulting in CS-TRE-mouse E-cadherin-PRE-Ubc-tTA-I2G.
Derivation of DsRed-Marked EpiSC Lines and Culture Conditions of PSCs
This study used the three mouse EpiSC lines EB3DR EpiSC, Rex1-GFP EpiSC, and the EpiSC reported by Tesar et al. (2007) and the two mouse ESC lines EB3DR ESC (Niwa et al., 2002; Ogawa et al., 2004) and Rex1-GFP ESC (Toyooka et al., 2008; Wray et al., 2010, 2011). To generate DsRed-marked mouse EpiSCs, we injected EB3DR ESCs into blastocyst embryos and established an EpiSC line from E6.5 embryos as described (Tesar et al., 2007). The endodermal layers of E6.5 embryos collected from the uterus were peeled off manually with glass needles and were transferred onto mitomycin-C-treated mouse embryonic fibroblasts (MEFs) in primed PSCs medium consisting of DMEM-F12, 20% knockout serum replacement, 1 mM sodium pyruvate, 1 × nonessential amino acids, 0.66 mM 2-mercaptoethanol (all Gibco), and 5 ng/ml human basic FGF (bFGF, PeproTech) (“ESM plus bFGF”). Growing colonies were observed within a few days. Colonies expressing DsRed were picked up and passaged with 10 μM Y-27632. After several passages, mouse EpiSCs usually were stably self-renewing. All mouse ESC lines were cultured on gelatin-coated plates in N2B27 medium (Ying et al., 2008) containing 1,000 U/ml of mouse leukemia inhibitory factor (LIF; Millipore) (“N2B27 plus LIF”). The effects of 1 μM PD0325901 (Axon), of 3 μM CHIR99021 (Axon), of 5 nM IWP-2 (Wako), and of 10 μM XAV939 (Sigma-Aldrich) on the conversion of mouse EpiSCs to naive-like status were examined.
Flow-Cytometry Analysis
For FACS analysis, single-cell suspensions were stained with APC-conjugated anti-mouse CD31 antibody (BD Biosciences) and anti-CD324 (E-CADHERIN) antibody (BD Biosciences). The stained cells were analyzed and sorted by FACSCalibur (BD Biosciences) and FACSAria II (BD Biosciences), respectively.
Western Blot Analysis
Whole-cell lysates were prepared using lysis buffer (complete Lysis-M, EDTA-free; Roche), and nuclear and cytoplasmic fractions were prepared by using a Nuclear/Cytosol Fractionation Kit (BioVision Technologies) in accordance with the manufacturer’s instructions. After centrifugation, the supernatants were dissolved in Laemmli Sample Buffer (Bio-Rad) for SDS-PAGE. Cell lysates were separated by SDS-PAGE, electroblotted onto polyvinylidene fluoride (PVDF) transfer membranes (Millipore), and probed with primary antibodies. After incubation with horseradish-peroxidase-conjugated secondary antibody (GE Healthcare UK), labeled proteins were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Primary antibodies against β-CATENIN (1:1000), nonphospho (active) β-CATENIN (1:1,000), E-CADHERIN (1:1,000), STAT3 (1:2,000), p-STAT3 (1:2,000), β-ACTIN (1:1,000), and HISTONE H3 (1:2,000) were employed (all Cell Signaling Technology). For quantification of protein expression levels, blotted images were captured by LAS 4000 equipment (GE Healthcare) and determined by ImageQuant TL software (GE Healthcare). Relative value was calculated the ratio of signal intensity of loading control to signal intensity of β-CATENIN in cytoplasmic and nuclear fractions, respectively.
Embryo Manipulation for Chimera Formation Assay
Chimera formation was assayed as described (Kobayashi et al., 2010). Mouse 8-cell/morula-stage embryos collected in M2 medium (Millipore) from the oviduct and the uterus of BDF1 × C57BL/6 mice 2.5 days postcoitum (dpc) were transferred into KSOM-AA medium (Millipore) and were cultured for 24 hr before blastocyst injection. For micromanipulation, trypsinized PSCs were suspended in PSC culture medium. A piezo-driven micromanipulator (Prime Tech) was used to drill zona pellucida and trophectoderm under the microscope, and ten PSCs were introduced into blastocyst cavities near the inner cell mass. After blastocyst injection, embryos underwent follow-up culture for 1–2 hr, after which they were transferred into the uteri of pseudopregnant recipient ICR mice (2.5 dpc).
RT-PCR and Quantitative Real-Time PCR Analysis
Total RNA was isolated using an RNAeasy kit (QIAGEN) followed by cDNA synthesis using SuperScript III reverse transcriptase (InvitrogenCA). PCR was performed using rTaq (Takara Bio) with conditions of 94°C for 1 min, followed by 30 cycles of 94°C for 30 s, annealing temperature (55°C) for 30 s, and 72°C for 1 min, with a final extension at 72°C for 7 min. The primer sequences are listed in Table S1. Pluripotency gene profiles were assayed by quantitative real-time PCR using TaqMan Mouse Stem Cell Pluripotency Array v2.0 (Applied Biosystems) according to the manufacturer’s instructions.
Statistical Analysis
The results are presented as the mean ± SEM. Student’s two-tailed nonpaired t test was used to determine the statistical significance of differences. Significant differences were defined as ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
All experiments were performed under institutional guidelines.
Animal experiments were performed with approval of the Institutional Animal Care and Use Committee of the Institute of Medical Science, University of Tokyo.
Author Contributions
H. Murayama and H. Masaki performed experiments, data analysis, interpretation, and manuscript writing. H.S. and T.H. performed experiments. T.Y. and H.N. designed and supervised the study.
Acknowledgments
We thank H. Niwa for providing the EB3DR ESC line; A. Smith for providing the Rex1-GFP ESC line; Randall Moon for providing the Super 8x TOPFlash plasmid; and M. Kasai, A. Knisely, Y. Yamazaki, Y. Ishii, and M. Watanabe for supporting this work. This work was supported by grants from Japan Science and Technology Agency (JST), Exploratory Research for Advanced Technology (ERATO), Nakauchi Stem Cell and Organ Regeneration Project, Tokyo, Japan. H.N. is a founder and shareholder of iCELL and a founder, shareholder, and a scientific advisor for Megakaryon Corporation and ReproCELL.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Bao S., Tang F., Li X., Hayashi K., Gillich A., Lao K., Surani M.A. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M.E., Tang W., Lu J., Ma Z., Fan C.W., Wei S., Hao W., Kilgore J., Williams N.S. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Yuan D., Wei B., Jiang J., Kang J., Ling K., Gu Y., Li J., Xiao L., Pei G. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010;28:1315–1325. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M., Simcha I., Ben-Yedidia T., Blechman J., Savagner P., Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J. Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle I., Rudloff S., Carles A., Li Y., Liszewska E., Vogt R., Kemler R. E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development. 2013;140:1684–1692. doi: 10.1242/dev.088690. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Gillich A., Bao S., Grabole N., Hayashi K., Trotter M.W., Pasque V., Magnúsdóttir E., Surani M.A. Epiblast stem cell-based system reveals reprogramming synergy of germline factors. Cell Stem Cell. 2012;10:425–439. doi: 10.1016/j.stem.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J., Cheng A.W., Saha K., Kim J., Lengner C.J., Soldner F., Cassady J.P., Muffat J., Carey B.W., Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.M., Mishina Y.M., Liu S., Cheung A., Stegmeier F., Michaud G.A., Charlat O., Wiellette E., Zhang Y., Wiessner S. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Kim H., Wu J., Ye S., Tai C.I., Zhou X., Yan H., Li P., Pera M., Ying Q.L. Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat. Commun. 2013;4:2403. doi: 10.1038/ncomms3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Yamaguchi T., Hamanaka S., Kato-Itoh M., Yamazaki Y., Ibata M., Sato H., Lee Y.S., Usui J., Knisely A.S. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Li W., Wei W., Zhu S., Zhu J., Shi Y., Lin T., Hao E., Hayek A., Deng H., Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nichols J., Ying Q.L. Derivation and propagation of embryonic stem cells in serum- and feeder-free culture. Methods Mol. Biol. 2006;329:91–98. doi: 10.1385/1-59745-037-5:91. [DOI] [PubMed] [Google Scholar]
- Niwa H., Masui S., Chambers I., Smith A.G., Miyazaki J. Phenotypic complementation establishes requirements for specific POU domain and generic transactivation function of Oct-3/4 in embryonic stem cells. Mol. Cell. Biol. 2002;22:1526–1536. doi: 10.1128/mcb.22.5.1526-1536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Matsui H., Ohtsuka S., Niwa H. A novel mechanism for regulating clonal propagation of mouse ES cells. Genes Cells. 2004;9:471–477. doi: 10.1111/j.1356-9597.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka S., Nishikawa-Torikai S., Niwa H. E-cadherin promotes incorporation of mouse epiblast stem cells into normal development. PLoS ONE. 2012;7:e45220. doi: 10.1371/journal.pone.0045220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C.Y., Lin H., Morin P.J., Longo D.L. Truncation of the extracellular region abrogrates cell contact but retains the growth-suppressive activity of E-cadherin. Cancer Res. 2000;60:7057–7065. [PubMed] [Google Scholar]
- Silva J., Nichols J., Theunissen T.W., Guo G., van Oosten A.L., Barrandon O., Wray J., Yamanaka S., Chambers I., Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.G., Heath J.K., Donaldson D.D., Wong G.G., Moreau J., Stahl M., Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Staal F.J., Noort Mv Mv., Strous G.J., Clevers H.C. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger A., Eger A., Wolf J., Beug H., Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J. Cell Biol. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Toyooka Y., Shimosato D., Murakami K., Takahashi K., Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- Veeman M.T., Slusarski D.C., Kaykas A., Louie S.H., Moon R.T. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Ware C.B., Nelson A.M., Mecham B., Hesson J., Zhou W., Jonlin E.C., Jimenez-Caliani A.J., Deng X., Cavanaugh C., Cook S. Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J., Kalkan T., Smith A.G. The ground state of pluripotency. Biochem. Soc. Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- Wray J., Kalkan T., Gomez-Lopez S., Eckardt D., Cook A., Kemler R., Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat. Cell Biol. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Hamanaka S., Kamiya A., Okabe M., Kawarai M., Wakiyama Y., Umino A., Hayama T., Sato H., Lee Y.S. Development of an all-in-one inducible lentiviral vector for gene specific analysis of reprogramming. PLoS ONE. 2012;7:e41007. doi: 10.1371/journal.pone.0041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


